Abstract
Background
Length of stay after non–ST-segment elevation myocardial infarction (NSTEMI) continues to decrease, but information to guide duration of hospitalization is limited.
Methods
We used landmark analyses, in which the landmark defined potential days of discharge, to estimate complication rates on the first day the patient would have been out of the hospital, and estimated associations between timing of discharge and 30-day and 1-year event-free survival after discharge among NSTEMI patients.
Results
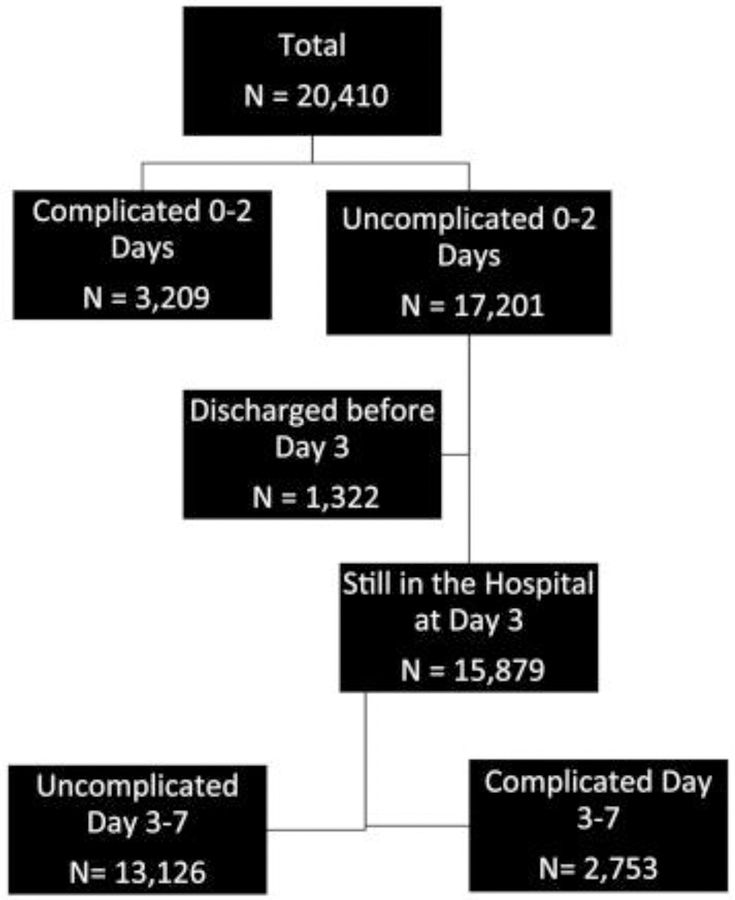
Among 20,410 NSTEMI patients, median length of stay was 7 (4, 12) days; 3,209 (15.7%) experienced a cardiac complication on days 0 to 2 and 1,322 (6.5%) were discharged without complications during hospital days 0 to 2. At the start of day 3, 15,879 patients (77.8%) were still hospitalized without complications. Of these, 1,689 (10.6%) were discharged event-free on day 3. Adjusted event-free survival rates of death or myocardial infarction from day 4 to 30 days after among the 1,689 patients was 99.1% compared with 93.1% for the 14,190 who remained hospitalized at the end of day 3. For 1-year mortality, these rates were 98.1% and 96.4%, respectively. Among 13,334 patients hospitalized without complications at the start of day 4, 1,706 were discharged event-free that day. Adjusted survival rates among these patients, compared with those still hospitalized at the end of day 4, were 98.0% versus 93.7% for 30-day death or myocardial infarction and 97.8% versus 96.1% for 1-year mortality.
Conclusions
Patients with NSTEMI who had no serious complications during the first 2 hospital days were at low risk of subsequent short- and intermediate-term death or ischemic events.
Non–ST-segment elevation myocardial infarction (NSTEMI) accounts for approximately 900,000 to 1.2 million hospitalizations in the United States each year,1, 2 and health care expenditures for management and treatment of NSTEMI were estimated to be $75.2 billion in 2010.3, 4 Use of evidence-based medical therapies and early invasive strategies has improved short-term mortality and reduced cardiovascular complications immediately after NSTEMI.3 At the same time, hospital length of stay (LOS) has decreased, driven both by earlier intervention and by economic pressures to reduce hospital stay and related costs.3–6. In the United States, from 1990 to 99, the median hospital stay related to acute myocardial infarction (MI) decreased from 8.3 to 4.3 days.3 In a recent analysis from the ACTION Registry–Get With the Guidelines (GWTG) database, overall LOS after NSTEMI was a median of 3 (2, 5) days.7 Little is known about the relationship between timing of discharge after NSTEMI and subsequent outcomes after discharge. We used landmark analyses, in which the landmark defined potential days of discharge, to estimate rates of complications on the first day the patient would have been out of the hospital, and estimated associations between timing of actual discharge and 30-day and 1-year event-free survival after discharge among 20,410 NSTEMI patients enrolled in 5 randomized trials of antithrombotic therapy.
Methods
Trial selection
We performed a patient-level pooled analysis of outcomes in 5 acute coronary syndrome clinical trials published from 1996 to 2004 for which we had the complete final database available at the Duke Clinical Research Institute: Superior Yield of the New Strategy of Enoxaparin, Revascularization, and Glycoprotein IIb/IIIa Inhibitors (SYNERGY),8 Platelet IIb/IIIa Antagonism for the Reduction of Acute coronary syndrome events in a Global Organization Network (PARAGON)-A,9 PARAGON-B,10 Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT),11 and Global Use of Strategies To Open occluded coronary arteries in acute coronary syndromes (GUSTO)-IIb.12 Key elements of each trial are shown in Table I, Table II.
Table I.
Summary of clinical trials used for pooled analysis
| Total N (NSTEMI) | Patient population | Treatment tested | Primary end point | 6-mo outcomes (yes/no) | |
|---|---|---|---|---|---|
| SYNERGY (2004) | 10,027 (8170) | Unstable angina/NSTEMI | Subcutaneous enoxaparin vs intravenous unfractionated heparin | 30-d all-cause death or nonfatal MI during the first 30 d after randomization | Yes |
| PARAGON-B (2002) | 5225 (2970) | Unstable angina/NSTEMI | Bolus and 72-h infusion of lamifiban vs placebo | 30-d death, MI, or severe recurrent ischemia | Yes |
| PARAGON-A (1998) | 2282 (825) | Unstable angina/NSTEMI | Lamifiban (low-dose with and without heparin vs high-dose) with and without heparin) or standard therapy (placebo and heparin) | 30-d death or nonfatal MI | Yes |
| PURSUIT (1998) | 10,948 (4927) | Unstable angina/NSTEMI | Bolus and infusion of eptifibatide vs placebo | 30-d death or nonfatal MI | Yes |
| GUSTO-IIb (1996) | 12,142 (3518) | Unstable angina/NSTEMI/STEMI | Intravenous heparin vs hirudin | 30-d death or nonfatal MI or reinfarction | Yes |
Table II.
Characteristics of clinical trials used for pooled analysis
| Definition of MI | Rates of ventricular tachycardia/ventricular fibrillation | Concomitant antithrombotic therapy and procedures | |
|---|---|---|---|
| SYNERGY (2004) | Ischemic symptoms >10 min within 24 h before enrollment and >2 of age >60 y, troponin or CK elevation >ULN, or ST-segment changes on ECG | Enoxaparin, 4.8% Heparin, 4.9% |
Aspirin, clopidogrel 46.9% PCI; 18.7% CABG |
| PARAGON-B (2002) | Ischemic symptoms at rest >10 min and <12 h before randomization, and either (1) ECG changes (transient or persistent ST-segment depression >0.5 mm, transient [<30 min] ST-segment elevation >0.5 mm, or definite T-wave inversion) or (2) CK-MB or troponin T or I >ULN | Not given | Aspirin, thienopyridine, heparin 28% PCI (76% stented) 15% CABG |
| PARAGON-A (1998) | Chest discomfort within the previous 12 h associated with transient or persistent ST-segment depression >0.5 mm, T-wave inversion or transient (30 min) ST-segment elevation >0.5 mm | Not given | Aspirin, heparin 47–53% coronary angiography (10–15% PTCR) 10–12% CABG |
| PURSUIT (1998) | Ischemic symptoms at rest, >10 min, within previous 24 h, and transient ST-segment elevation >0.5 mm, transient or persistent ST-segment depression, T-wave inversion >1 mm or CK-MB >ULN | Not given | Aspirin, heparin 23%–24% PTCA (50% stented) 14% CABG |
| GUSTO-IIb (1996) | Chest discomfort within previous 12 h associated either with transient or persistent ST-segment elevation or depression >0.5 mm or persistent, definite T-wave inversion >1 mm | Not given | Aspirin 19%–31% PTCA 8%–14% CABG |
Abbreviations: ULN, upper limit of normal; ECG, electrocardiogram; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; CK, creatine kinase.
Study population
The pooled trial population comprised 39,615 patients with unstable angina, NSTEMI, and STEMI. We included only patients who presented with NSTEMI (n = 20,438), from which we excluded individuals with hospitals stays <0 and >365 days and those with insufficient data to determine discharge timing (n = 28), leaving an analysis population of 20,410 patients.
All patients enrolled in each trial gave written informed consent to participate in that trial, and each trial was approved by local institutional review board or ethics committee. The current analyses were approved by the institutional review board of Duke University Medical Center with a waiver of consent and HIPAA authorization. The authors had full access to the data, designed and conducted the analyses, and assume responsibility for the contents of the article.
In-hospital complications
For our analyses, we categorized patients as uncomplicated or complicated through various hospital days. The date of admission was considered hospital day 0 and subsequent in-hospital days were numbered sequentially. On the basis of our previous work in STEMI populations, “uncomplicated” was defined as the absence of death, MI, recurrent ischemia, shock, heart failure, or stroke through the start of the hospital day of interest.13–15. Rates of these complications after each of the first 7 hospitalization days were summarized. We were particularly interested in the rate of events on the following day (eg, events on day 4 when evaluating day 3) as a reflection of the potential risk of discharging too early.
Short- and long-term outcomes
We assessed death or MI through 30 days, and death at 6 months and 1 year after discharge. Although there were subtle differences in the definitions of MI applied by the individual trials, for the purposes of our current analyses, MI was used as adjudicated by the blinded clinical events classification committee for the original trial.
Statistical methods
Descriptive statistics are presented for baseline characteristics of patients with and without complications during days 0 to 2. The group that was uncomplicated from days 0 to 2 was further split into those who remained uncomplicated from days 3 to 7 and those who experienced their first complication on days 3 to 7. Continuous variables were summarized using medians (25th, 75th percentiles) and discrete variables by frequencies (percentages). No statistical testing of differences in baseline characteristics was performed.
Rates of complications were summarized among complication-free patients not yet discharged by the start of days 3, 4, and 5 for the subsequent days to day 7. In other words, for day 3, the rate of death from 4 to 7 days is displayed. For day 4, the rate of death from days 5 to 7 is displayed. This illustrates the additional events one may expect in subsequent days in each cohort of patients who have remained complication free up to each new hospitalization day.
There are 2 sources of confounding we address in our analyses. One is the issue of timing. A patient enrolled hours after the index NSTEMI event is at a different level of risk than a patient who has remained in the hospital complication free for 3 days after NSTEMI. Landmark analyses were applied to address this issue. The second is differences in the comparator groups, in this case, the patient characteristics for those who are discharged versus those who remain in the hospital. Adjustment models were applied accordingly.
Cox proportional hazards models along with landmark methods were used to evaluate the adjusted association between potential timing of discharge and subsequent clinical outcomes. For each landmark of days 3, 4, and 5, this method moves the start of the follow-up forward to the landmark time point and, among those who are in the hospital and complication-free, compares those who are discharged with those who are not on that day. Thus, the landmarks represented time points in the follow-up period from which survival analyses were started, the purpose of which was to establish a cohort of patients who were “eligible” (still in the hospital and complication free) to be discharged at the beginning of the landmark period. Landmarks were prespecified at the beginning of hospital days 3, 4, and 5 on the assumption that uncomplicated patients would generally qualify for discharge within this time frame. Patients who were discharged before the next landmark were compared with those still hospitalized on the subsequent day. Thus, for the first landmark, patients in the hospital and without complications at the beginning of day 3 made up the cohort. Those discharged on day 3 were compared with those not yet discharged. Predicted outcome values are the average predicted value across all patients. Predicted values are generated across the entire cohort assuming they were discharged, and again across the entire cohort assuming they remained in the hospital. Averages are calculated across these 2 sets of predicted values.
Predictor variables from models previously developed for 6-month death (trial, region, age, sex, weight, smoking status, chronic renal insufficiency, Killip class, ST-segment depression on baseline electrocardiogram [ECG], diabetes mellitus, peripheral vascular disease, history of heart failure, heart rate), death or MI (trial, region, age, sex, height, diabetes mellitus, smoking status, heart rate, chronic renal insufficiency, prior MI), and stroke (trial, region, history of heart failure, hypertension, age, prior stroke or transient ischemic attack, heart rate, smoking status, diabetes mellitus, prior MI), and 1-year death (trial, region, age, sex, weight, smoking status, chronic renal insufficiency, Killip class, ST-segment depression on baseline ECG, diabetes mellitus, peripheral vascular disease, history of heart failure, heart rate) were used to adjust for differences in baseline characteristics.8–12, 16, 17.
Considering the exploratory nature of the analyses, statistical significance was declared at a 2-sided α < .01; however, because it is unlikely that all of information that goes into a discharge decision is captured in clinical trials databases, caution should remain when interpreting the results. No further adjustments were made for multiple comparisons. All analyses were performed with SAS software package version 9.2 (SAS Institute, Cary, NC).
No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, and drafting and editing of the manuscript.
Results
Baseline patient characteristics
Figure 1 provides a diagram of patients grouped by occurrence or absence of complications by the beginning of hospital day 3. Table III displays baseline characteristics for all NSTEMI patients (n = 20,410), stratified according to occurrence or absence of complications by the beginning of hospital day 3. Compared with patients who were uncomplicated during days 0–2 (n = 15,879), those who experienced complications (n = 3,209) were more frequently female and from North America. They also more frequently had hypertension, diabetes, and a history of a prior stroke or prior MI.
Figure 1.
Cohort diagram of patients grouped by occurrence or absence of complications by the beginning of hospital day 3.
Table III.
Baseline characteristics, overall and according to whether or not there were complications before day 3 (total analysis cohort = 20,410)
| At least one complication, days 0–2 (n = 3209) | Uncomplicated, days 0–2 (n = 17,201) | ||||
|---|---|---|---|---|---|
| Uncomplicated, discharged days 0–2 (n = 1322) | Uncomplicated, not discharged, days 0–2 (n = 15,879) | ||||
| Overall (n = 15,879) | Complications, days 3–7 (n = 2753) | Uncomplicated, days 3–7 (n = 13,126) | |||
| Demographics | |||||
| Age (y) | 67.0 (58.4, 74.0) | 63.5 (55.0, 71.0) | 66.0 (57.0, 73.0) | 67.0 (59.0, 74.0) | 65.0 (56.0, 73.0) |
| Female sex (%) | 31.2 | 31.6 | 29.9 | 28.7 | 30.2 |
| Race (%) | |||||
| White | 90.4 | 85.9 | 89.4 | 90.7 | 89.1 |
| Black | 4.2 | 7.5 | 4.5 | 3.6 | 4.7 |
| Other | 5.4 | 6.6 | 6.1 | 5.7 | 6.2 |
| Region (%) | |||||
| North America | 58.1 | 91.6 | 49.7 | 55.6 | 48.4 |
| Western Europe | 23.6 | 3 | 30.9 | 28.1 | 31.5 |
| Eastern Europe | 7.4 | 1.1 | 6.9 | 5.8 | 7.1 |
| Latin America | 2.7 | 1.1 | 3.3 | 3.1 | 3.3 |
| Medical history (%) | |||||
| Diabetes mellitus | 25.2 | 22.6 | 24.4 | 27.8 | 23.7 |
| Hypertension | 57.5 | 62.5 | 56.9 | 58 | 56.7 |
| Hypercholesterolemia | 46.8 | 56.9 | 48.5 | 47.7 | 48.6 |
| Prior MI | 30.6 | 26.5 | 29 | 30.8 | 28.7 |
| Prior PCI | 12.4 | 21.4 | 12.5 | 12.2 | 12.6 |
| Cigarette smoking | |||||
| Current | 25.8 | 32.7 | 29.3 | 26.4 | 29.9 |
| Past | 36.5 | 32.4 | 33.8 | 36.7 | 33.2 |
| Never | 37.7 | 35 | 36.9 | 36.9 | 36.9 |
| Peripheral vascular disease | 9 | 7.5 | 8.8 | 10.5 | 8.4 |
| Chronic renal insufficiency | 15.4 | 35 | 18.7 | 17.6 | 18.9 |
| Prior stroke or TIA | 6.6 | 6.6 | 6.2 | 6.7 | 6.1 |
| Severe COPD | 4.3 | 4.4 | 3.4 | 4.3 | 3.2 |
| Clinical characteristics at presentation | |||||
| Weight (kg) | 79.0 (70.0, 90.0) | 84.0 (73.0, 96.8) | 79.0 (70.0, 90.0) | 79.0 (70.0, 90.0) | 79.0 (69.6, 90.0) |
| Systolic blood pressure (mm Hg) | 130.0 (116.0, 146.0) | 131.0 (117.0, 148.0) | 131.0 (120.0, 150.0) | 130.0 (118.0, 150.0) | 132.0 (120.0, 150.0) |
| Heart rate (beats/min) | 74.0 (64.0, 85.0) | 71.0 (62.0, 80.0) | 72.0 (64.0, 84.0) | 74.0 (64.0, 84.0) | 72.0 (64.0, 84.0) |
| Killip class (%) | |||||
| I | 82.3 | 87.5 | 87 | 84.2 | 87.6 |
| II | 14.7 | 10.5 | 10.9 | 13.8 | 10.3 |
| III/IV | 3 | 2.1 | 2.1 | 2.1 | 2.1 |
| Qualifying event ECG (%) | |||||
| ST-segment depression | 61 | 39.2 | 52.1 | 58.2 | 50.7 |
| Transient ST-segment elevation | 15.4 | 14.4 | 15.7 | 14.5 | 16 |
| T-wave inversion | 35.2 | 33.8 | 43.3 | 34 | 32.5 |
Continuous variables are presented as median (25th, 75th percentile), and categorical variables are presented as percentages.
Abbreviations: PCI, percutaneous coronary intervention; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease.
Among 15,879 patients who had no complications from days 0 to 2 and remained hospitalized at the start of day 3, 2,753 (17.3%) experienced subsequent in-hospital clinical events through day 7. Compared with the 13,126 (82.7%) patients who remained uncomplicated from days 3 to 7, those who experienced subsequent complications before 7 days were older and more often had diabetes mellitus or ST-segment depression on presentation ECG. They were less often current cigarette smokers and were more frequently from North America.
Of all patients, 1,322 (6.5%) were discharged without complications from days 0 to 2. Compared with uncomplicated patients who remained hospitalized on day 3 (n = 15,879), these patients were younger, heavier, more often nonwhite, and more frequently from North America. They more often had hypertension, hyperlipidemia, chronic renal insufficiency, and prior revascularization. At presentation, they were more likely to be Killip class 1 and less often had ST-segment depression ECG.
Complications according to hospital day landmarks
The median LOS for all NSTEMI patients was 7.0 (4.0, 12.0) days. Among those with complications (including death) from days 0 to 2, the median LOS overall was 5.0 (2.0, 10.0) days, and after excluding those who died from days 0 to 2, the median LOS was 6.0 (2.0, 10.0) days. The median LOS for all patients without complications from days 0 to 2 was 7.0 (4.0, 12.0) days, and after excluding those who were discharged from days 0 to 2, the median LOS was 8.0 (5.0, 12.0) days. The median LOS varied by trial and region (Table IV).
Table IV.
LOS according to trial and geographic region
| NSTEMI patients (n) | LOS, median (IQR) | |
|---|---|---|
| Clinical trial | ||
| PARAGON-A | 825 | 9 (7, 14) |
| PARAGON-B | 2970 | 8 (5, 12) |
| PURSUIT | 4927 | 9 (6, 14) |
| GUSTO-IIb | 3518 | 9 (6, 13) |
| SYNERGY | 8170 | 5 (3, 9) |
| Geographic region | ||
| North America | 9659 | 5 (3, 9) |
| Western Europe | 4965 | 10 (7, 15) |
| Eastern Europe | 1175 | 12 (8, 16) |
| Arab Region | 117 | 6 (5, 11) |
| Latin America | 543 | 8 (5, 13) |
| Australia/New Zealand | 1159 | 6 (4, 9) |
| Asia | 48 | 8 (6, 13) |
| Other (Israel, South Africa) | 220 | 7 (5, 10) |
| All patients | 20,410 | 7 (4, 12) |
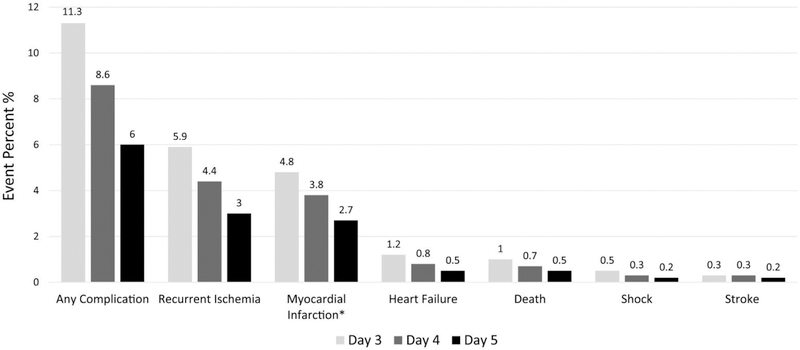
The rate of the first occurrence of a specific short-term complication or the composite of any of the events on a specified hospital day landmark among patients without any complications before that hospital day landmark is shown in Figure 2, and is a surrogate for the out-of-hospital rate through day 7 of that event had the patient actually been discharged on that landmark day.
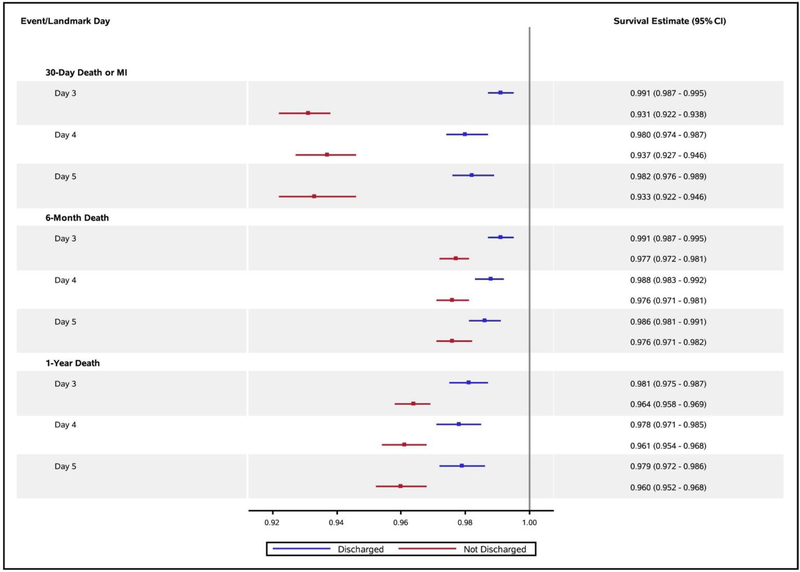
Figure 3 displays adjusted survival estimates (95% CIs) free from prespecified events according to landmark day for uncomplicated patients actually discharged on that day and those who remained hospitalized. After adjustment for clinical factors known to influence the occurrence of these events, survival to 6 months or 1 year was similar to or slightly higher among uncomplicated patients discharged earlier.
Figure 3.
Adjusted event-free survival estimates by landmark day for uncomplicated patients to that hospital day.
Discussion
In our exploration of timing of discharge and subsequent outcomes among NSTEMI patients, more than three quarters of all NSTEMI patients were complication-free from presentation through hospital day 2, and nearly two thirds overall (or 83% of those without complications through day 2) had no complications through 7 days. We demonstrated that NSTEMI patients who did not have serious complications during days 0 to 2 were at low risk of complications on landmark day 3 and for subsequent short- and intermediate-term death and recurrent ischemic events. Adjusted survival rates were high for patients without complications from 0 to 2 days who were actually discharged on day 3 and were similar to those for uncomplicated patients who remained hospitalized for additional days. These observations suggest that many NSTEMI patients without complications through hospital day 2 could be suitable candidates for earlier hospital discharge.
Trends in discharge timing
Both clinical trials databases and population registries confirm that LOS after admission for unstable angina and NSTEMI has steadily decreased. In multicenter, international clinical trials of antithrombotic therapy, LOS fell from 8 days in trials enrolling from 1994 to 1998 to 6 days in trials enrolling from 1999 to 2003 to 5 days from 2004 to 2008.6 This occurred despite enrollment of higher-risk patients into the trials across these periods and concurrent with decreasing in-hospital, 30-day, and 6-month mortality. In the United States, from 2007 to 2009 among 351 hospitals in the ACTION-GWTG registry, the median LOS after unstable angina or NSTEMI was 3 (2, 5) days.7 This evolution toward shorter hospital stays has occurred in parallel with increasing and earlier use of percutaneous coronary intervention (PCI) and stenting and improved acute antithrombotic options and long-term secondary prevention strategies.6, 18, 19.
The question of why LOS was shorter for complicated than uncomplicated patients remains. We do not know for sure why the median LOS was longer for uncomplicated patients. However, we postulate that the shorter stays among complicated patients likely reflect not only the early (days 0–2) deaths but also in-hospital deaths on later days that were not subsequently studied in our analysis. Furthermore, although we do not have enough information from the case report form to assess this adequately, there is also the potential of early transfer to more advanced centers that were not trial sites and for which the subsequent hospital days not captured. We also note the substantial regional variation in LOS among the uncomplicated patients, and note that in some countries, particularly during the trials conducted earlier in this series, inpatient cardiac rehabilitation was considered part of the index hospitalization and could have contributed to the longer median stay among uncomplicated patients.
Several of the ACS trials used in our pooled analysis required a minimum duration of study drug, which could potentially influence LOS, but was unlikely to have influenced whether a patient remained uncomplicated by a given hospitalization day landmark. In addition, although many of the ACS trials we used were older than the GWTG data, the heterogeneity in timing of discharge within this pooled cohort renders it particularly valuable for our landmark analyses as detailed complications data are not collected in these trials after discharge. Furthermore, one might expect that the estimates we generated of complication rates on a landmark day would be conservative relative to current care given advances in antithrombotic therapy and earlier use of the invasive strategy over more recent years as reflected in GWTG.
Clinical outcomes with earlier discharge
Multiple observational studies and small randomized trials of early discharge in STEMI populations have supported the safety of discharge as early as 3 days after admission in both thrombolytic-treated patients and those treated with primary PCI if they had not had major complications to that point,13, 15, 20–23. and have questioned the cost-effectiveness of hospital stays >3 days in STEMI patients.14 In a recent study from the American College of Cardiology Foundation’s CathPCI Registry, mean LOS after primary PCI fell from 4 days in 2005 to 3.6 days in 2009, at which time 45.6% of registry hospitals discharged at least 50% of their low-risk, uncomplicated patients within 2 days.24 However, patients with NSTEMI tend to be older and have more extensive coronary artery disease and more comorbidities than STEMI patients. Thus, observations about timing of discharge and outcomes from STEMI populations may not translate directly to patients with NSTEMI.
Our analyses suggest that as for STEMI, NSTEMI patients who have remained complication-free through 2 hospital days are at low risk for subsequent in-hospital complications, and as a group, they have excellent survival, free from death or recurrent ischemic events, through 30 days, 6 months, and 1 year. These observations are consistent with earlier studies in NSTEMI patients. Before the era of early invasive treatment, one small (n = 458) randomized trial, in which approximately 20% of patients had NSTEMI, showed that outcomes were similar among patients (n = 233) randomized to early (days 3–5) discharge and those (n = 225) randomized to standard discharge (7–9 days) if a pharmacologic stress test result was negative.25
Larger population-based studies have revealed similar results. In one study of more than 4,100 patients, despite declining duration of hospitalization among uncomplicated MI patients (both NSTEMI and STEMI) over a 10-year period, there were no increases in risk of hospital readmission at 7 or 30 days after hospital discharge or in mortality at 30 or 90 days after discharge.26 In a large mixed STEMI and NSTEMI population of more than 20,000 Medicare patients across 4 states, LOS declined from just more than 10 days in 1992 to 6.9 days in 2011, with no change in 1-month mortality rates.
Implications of earlier discharge
Ultimately, cost may be the prime motivation for earlier discharge, in particular for uncomplicated acute MI patients. A prior analysis from the GUSTO-1 trial demonstrated that a fourth hospital day in uncomplicated STEMI patients up to the 72-hour time point would cost an additional $105,629 per quality-adjusted year of life; thus, hospitalization of uncomplicated STEMI patients beyond 72 hours was economically unattractive.14 In an analysis from the VALIANT registry, 50.7% of the variation in cost of MI care among 9 countries was explained by hospital LOS compared with only 31.9% by procedure intensity.27
In prior STEMI studies, discharge of uncomplicated MI patients on the third hospital day seemed ideal when weighing timing of complications, incremental clinical benefit, and incremental expense.14, 28. However, several challenges and concerns have been cited for not discharging uncomplicated MI patients earlier: concurrent management of concomitant medical factors, poor discharge planning and transition of care, and limited/poor outpatient social support.14, 29, 30. These factors may be particularly relevant for the older NSTEMI population that also has more baseline comorbidities. Higher rates of readmission among countries with lower lengths of stay may in part point to the need for hospitals to maximize the efficiency and availability of discharge and postdischarge services to manage the tension between earlier discharge and limiting readmission.31
Strengths and limitations
The pooling of 5 large trials conducted over several years allowed us to analyze a large patient population with enough variation in timing of discharge to examine multiple timing landmarks and subsequent complications rates. Also, by pooling large patient data sets from international trials, we were able to study a population representative of various regions and take advantage of regional variation in timing of discharge. Although the pooled trials reflected patients enrolled in trials published from 1996 through 2004 and LOS in practice has since declined, 18% of patients in our analysis were discharged after 2 or fewer uncomplicated days compared with 25% in the recent ACTION Registry–GWTG analysis. We recognize the age of some of the trials used in this pooled analysis, and that clinical practice has evolved to include more intensive medical and invasive management over this period. We would expect from our previous work in this group of trials that with more intensive medical and interventional care that has evolved over the time frame in which these trials were conducted, complication rates (for those we defined: death, MI, recurrent ischemia, heart failure, shock, and stroke) would generally be lower (despite higher predicted risk),32 although there may be some trade-offs in bleeding and renal dysfunction with greater use of invasive therapy that our databases did not systematically capture.
The use of a clinical trials data set does not entirely reflect less selected populations encountered in clinical practice, even when applying a classification scheme based on major in-hospital complications, and our observations are not intended to replace clinical judgment when concurrent clinical characteristics or social situations dictate longer stays among uncomplicated MI patients. Furthermore, we recognize that the art of medicine is alive, and physicians may keep patients in the hospital longer than clinically necessary to catch potential “low-frequency” events. In addition, although we adjusted for differences in baseline characteristics, there may be variables that influence timing of discharge, complications, or clinical events for which we did not or could not account with our statistical methods. For example, the development of acute kidney injury, which could appropriately lead to delayed discharge, was not captured in the databases we used or in our analysis.
In our study, we could not systematically assess transfusion or major bleeding in relation to complication status due the variation in treatments and intervention (use and timing) over time and among the clinical trials. Bleeding complications may be an important component in considering when to discharge a post-NSTEMI patient. However, in one of the few randomized trials of early discharge after elective PCI that included NSTEMI patients (19%), and in which all patients were treated with abciximab, the rates of major bleeding were very low in both groups (0.8% in the same-day discharge group, 0.2% in the overnight stay group).33
Conclusions
Patients with NSTEMI who had no serious complications during hospital days 0 to 2 seemed to be at low risk for major complications on day 3 and for subsequent short- and intermediate-term death or recurrent ischemic events. Earlier discharge could be reasonable for many NSTEMI patients.
Figure 2.
Crude rate (in percent; y axis) of a specified complication (x axis, from left to right: any complication, recurrent ischemia, MI, heart failure, death, shock, stroke) that occurred through day 7 among individuals who were uncomplicated at the start of a given day (day 3, day 4, or day 5).
Acknowledgments
This work was supported in part by a Barton F. Haynes Resident Research Award, Department of Medicine, Duke University Medical Center, and from academic discretionary resources of the senior investigator. Dr Rymer was supported by a training grant to Duke University from the National Heart Lung and Blood Institute (5 T32 HL 69749).
Disclosures
Dr Rymer, Dr Templehof, Mr Clare, Ms Pieper, Dr Granger, and Dr Moliterno have no relevant conflicts of interest. Dr Van de Werf reports research grants from Merck, and advisory board membership, DSMB, and lecture fees from Merck. Harrington reports research grants from Merck, Astra, BMS, J&J, and consulting honoraria from CSL, BMS, Daiichi, Gilead, J&J, Merck, and MyoKardia. Dr White reports research grants from Sanofi Aventis, Eli Lilly, Medicines Company, NIH, Roche, Merck Sharpe & Dohme, Astra Zeneca, GSK, and Daiichi Sankyo Pharma Development, as well as consulting from Astra Zeneca, Merck Sharpe & Dohme, Roche, and Regado Biosciences. Dr Armstrong reports research grants from Merck & Company, and consulting honoraria from Eli Lilly. Dr Lopes reports research grants from BMS, Pfizer, Bayer, Jansen, and Astra-Zeneca. Dr Mahaffey reports research grants from AstraZeneca, Boehringer Ingleheim, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Johnson & Johnson, Merck, Portola, Regado Biotechnologies, Sanofi, Schering-Plough (now Merck), and The Medicines Company, as well as consulting honoraria from Bayer, Daiichi Sankyo, and Johnson & Johnson. Dr Newby reports no relevant conflicts of interest. Her relationships with industry are reported at: https://www.dcri.org/about-us/conflict-of-interest.
References
- 1.A.G. TurpieBurden of disease: medical and economic impact of acute coronary syndromes Am J Manag Care, 12 (2006), pp. S430–S434 [PubMed] [Google Scholar]
- 2.Leesar MA, Abdul-Baki T, Akkus NI, et al. Use of fractional flow reserve versus stress perfusion scintigraphy after unstable angina. Effect on duration of hospitalization, cost, procedural characteristics, and clinical outcome. J Am Coll Cardiol, 41 (2003), pp. 1115–1121 [DOI] [PubMed] [Google Scholar]
- 3.Adabag AS, Therneau TM, Gersh BJ, et al. Sudden death after myocardial infarction JAMA, 300 (2008), pp. 2022–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers WJ, Canto JG, Lambrew CT, et al. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the U.S. from 1990 through 1999: the national registry of myocardial infarction 1, 2 and 3. J Am Coll Cardiol, 36 (2000), pp. 2056–2063 [DOI] [PubMed] [Google Scholar]
- 5.Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirudin during primary PCI in acute myocardial infarction. New Engl J Med, 358 (2008), pp. 2218–2230 [DOI] [PubMed] [Google Scholar]
- 6.Chan MY, Sun JL, Newby LK, et al. Trends in clinical trials of non–ST-segment elevation acute coronary syndromes over 15 years. Int J Cardiol, 167 (2013), pp. 548–554 [DOI] [PubMed] [Google Scholar]
- 7.Vavalle JP, Lopes RD, Chen AY, et al. Hospital length of stay in patients with non–ST-segment elevation myocardial infarction. Am J Med, 125 (2012), pp. 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson JJ, Califf RM, Antman EM, et al. Enoxaparin vs unfractionated heparin in high-risk patients with non–ST-segment elevation acute coronary syndromes managed with an intended early invasive strategy: primary results of the SYNERGY randomized trial. JAMA, 292 (2004), pp. 45–54 [DOI] [PubMed] [Google Scholar]
- 9.The PARAGON Investigators International, randomized, controlled trial of lamifiban (a platelet glycoprotein IIb/IIIa inhibitor), heparin, or both in unstable angina. Circulation, 97 (1998), pp. 2386–2395 [DOI] [PubMed] [Google Scholar]
- 10.Global Organization Network (PARAGON)-B Investigators Randomized, placebo-controlled trial of titrated intravenous lamifiban for acute coronary syndromes. Circulation, 105 (2002), pp. 316–321 [DOI] [PubMed] [Google Scholar]
- 11.The PURSUIT Trial Investigators Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. N Engl J Med, 339 (1998), pp. 436–443 [DOI] [PubMed] [Google Scholar]
- 12.The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO-IIb) Investigators A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. N Engl J Med, 335 (1996), pp. 775–782 [DOI] [PubMed] [Google Scholar]
- 13.Newby LK, Califf RM, Guerci A, et al. Early discharge in the thrombolytic era: an analysis of criteria for uncomplicated infarction from the Global Utilization of Streptokinase and tPA for Occluded Coronary Arteries (GUSTO) Trial. J Am Coll Cardiol, 27 (1996), pp. 625–632 [DOI] [PubMed] [Google Scholar]
- 14.Newby LK, Eisenstein EL, Califf RM, et al. Cost effectiveness of early discharge after uncomplicated acute myocardial infarction. N Engl J Med, 342 (2000), pp. 749–755 [DOI] [PubMed] [Google Scholar]
- 15.Newby LK, Hasselblad V, Armstrong PW, et al. Time-based risk assessment after myocardial infarction: implications for timing of discharge and application to medical decision-making. Eur Heart J, 24 (2003), pp. 181–188 [DOI] [PubMed] [Google Scholar]
- 16.Anderson JL, Adams CD, Antman EM, et al. American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction); American College of Emergency Physicians; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; American Association of Cardiovascular and Pulmonary Rehabilitation; Society for Academic Emergency Medicine. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non–ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, American College of Physicians, Society for Academic Emergency Medicine, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol, 50 (2007), pp. e1–e157 [DOI] [PubMed] [Google Scholar]
- 17.Cohen M, Demers C, Gurfinkel EP, et al. A comparison of low-molecular-weight heparin with unfractionated heparin for unstable coronary artery disease. Efficacy and safety of subcutaneous enoxaparin in non–Q-wave coronary events study group. N Engl J Med, 337 (1997), pp. 447–452 [DOI] [PubMed] [Google Scholar]
- 18.Fox KA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999–2006. JAMA, 297 (2007), pp. 1892–1900 [DOI] [PubMed] [Google Scholar]
- 19.Katritsis DG, Siontis GC, Kastrati A, et al. Optimal timing of coronary angiography and potential intervention in non–ST-elevation acute coronary syndromes. Eur Heart J, 32 (2011), pp. 32–40 [DOI] [PubMed] [Google Scholar]
- 20.Topol EJ, Burek K, O’Neill WW, et al. A randomized controlled trial of hospital discharge three days after myocardial infarction in the era of reperfusion. N Engl J Med, 318 (1988), pp. 1083–1088 [DOI] [PubMed] [Google Scholar]
- 21.Grines CL, Marsalese DL, Brodie B, et al. Safety and cost-effectiveness of early discharge after primary angioplasty in low risk patients with acute myocardial infarction: PAMI-II investigators: primary angioplasty in myocardial infarction. J Am Coll Cardiol, 31 (1998), pp. 967–972 [DOI] [PubMed] [Google Scholar]
- 22.Mark DB, Sigmon K, Topol EJ, et al. Identification of acute myocardial infarction patients suitable for early hospital discharge after aggressive interventional therapy. Results from the Thrombolysis and Angioplasty in Acute Myocardial Infarction Registry. Circulation, 83 (1991), pp. 1186–1193 [DOI] [PubMed] [Google Scholar]
- 23.Kotowycz MA, Cosman TL, Tartaglia C, et al. Safety and feasibility of early hospital discharge in ST-segment elevation myocardial infarction—a prospective and randomized trial in low-risk primary percutaneous coronary intervention patients (the Safe-Depart Trial). Am Heart J, 159 (2010), pp. 117.e1–117.e6 [DOI] [PubMed] [Google Scholar]
- 24.Chin CT, Weintraub WS, Dai D, et al. Trends and predictors of length of stay after primary percutaneous coronary intervention: a report from the CathPCI Registry. Am Heart J, 162 (2011), pp. 1052–1061 [DOI] [PubMed] [Google Scholar]
- 25.Desideri A, Fioretti PM, Cortigiani L, et al. Cost of strategies after myocardial infarction (COSTAMI): a multicentre, international, randomized trial for cost-effective discharge after uncomplicated myocardial infarction. Eur Heart J, 24 (2003), pp. 1630–1639 [DOI] [PubMed] [Google Scholar]
- 26.Saczynski J, Lessard D, Spencer F, et al. Declining length of stay for patients hospitalized with AMI: impact on mortality and readmissions. Am J Med, 123 (2010), pp. 1007–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kauf TL, Velazquez EJ, Crosslin DR, et al. The cost of acute myocardial infarction in the new millennium: Evidence from a multinational registry. Am Heart J, 151 (2006), pp. 206–212 [DOI] [PubMed] [Google Scholar]
- 28.Mark DB, Newby LK Early hospital discharge after uncomplicated myocardial infarction: are further improvements possible? Eur Heart J, 24 (2003), pp. 1613–1615 [DOI] [PubMed] [Google Scholar]
- 29.Bogaty P, Dumont S, O’Hara GE, et al. Randomized trial of a noninvasive strategy to reduce hospital stay for patients with low-risk myocardial infarction. J Am Coll Cardiol, 37 (2001), pp. 1289–1296 [DOI] [PubMed] [Google Scholar]
- 30.Newby LK, Califf RM, Guerci A, et al. Early discharge in the thrombolytic era: an analysis of criteria for uncomplicated infarction from the Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO) Trial. JACC, 27 (1996), pp. 625–632 [DOI] [PubMed] [Google Scholar]
- 31.Kociol RD, Lopes RD, Clare R, et al. International variation in and factors associated with hospital readmission after myocardial infarction. JAMA, 307 (2012), pp. 66–74 [DOI] [PubMed] [Google Scholar]
- 32.Kragholm K, Goldstein SA, Yang Q, et al. Trends in enrollment, clinical characteristics, treatment, and outcomes according to age in non–ST-SEGMENT-elevation acute coronary syndromes clinical trials. Circulation, 133 (2016), pp. 1560–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertrand OF, De Larochellière R, Rodés-Cabau J, et al. A randomized study comparing same-day home discharge and abciximab bolus only to overnight hospitalization and abciximab bolus and infusion after transradial coronary stent implantation. Circulation, 114 (2006), pp. 2636–2643 [DOI] [PubMed] [Google Scholar]