Abstract
This chapter begins with a brief review of descriptions and definitions of mystical-type experiences and the historical connection between classic hallucinogens and mystical experiences. The chapter then explores the empirical literature on experiences with classic hallucinogens in which claims about mystical or religious experiences have been made. A psychometrically validated questionnaire is described for the reliable measurement of mystical-type experiences occasioned by classic hallucinogens. Controlled laboratory studies show that under double-blind conditions that provide significant controls for expectancy bias, psilocybin can occasion complete mystical experiences in the majority of people studied. These effects are dose-dependent, specific to psilocybin compared to placebo or a psychoactive control substance, and have enduring impact on the moods, attitudes, and behaviors of participants as assessed by self-report of participants and ratings by community observers. Other studies suggest that enduring personal meaning in healthy volunteers and therapeutic outcomes in patients, including reduction and cessation of substance abuse behaviors and reduction of anxiety and depression in patients with a life-threatening cancer diagnosis, are related to the occurrence of mystical experiences during drug sessions. The final sections of the chapter draw parallels in human neuroscience research between the neural bases of experiences with classic hallucinogens and the neural bases of meditative practices for which claims of mystical-type experience are sometimes made. From these parallels, a functional neural model of mystical experience is proposed, based on changes in the default mode network of the brain that have been observed after the administration of classic hallucinogens and during meditation practices for which mystical-type claims have been made.
Keywords: psilocybin, hallucinogens, meditation, mystical experiences, neural model, default mode network, medial prefrontal cortex, posterior cingulate, angular gyrus, inferior parietal lobule
1. Introduction
Reports of mystical-type experiences date back many centuries (e.g. in the case of Rumi or St. Teresa of Avila), if not millennia (i.e. in the case of Plotinus) (Stace, 1960a). Mystical experiences have occurred in the course of structured spiritual or religious practices as well as in cases in which there was no direct intention to have such an experience. Mystical experiences are uniquely interesting and important to study because they are sometimes associated with abrupt, substantial, and sustained changes in behavior and perception (Miller & C’de Baca, 2001). Furthermore, the authoritative sense of interconnectedness and sacredness that defines such experiences suggest that mystical experiences may be foundational to the world’s ethical and moral systems (Huxley 1947). Despite their apparent importance, the unpredictability and low probability of “naturally occurring” mystical-type experiences, whether they occur in religious or non-religious contexts, has made them inherently difficult to study in controlled empirical research.
While there are countless reports of profound spiritual and mystical experiences that have occurred in the absence of psychoactive substances, historical evidence abounds for the role of psychoactive substances in ceremonial approaches to facilitating such experiences (Schultes, Hofmann, & Rätsch, 2001). Further, descriptions of naturally occurring mystical experiences (Stace, 1960b) are strikingly similar to profound spiritual experiences occasioned by hallucinogenic substances (Roberts, 2001). Experimental investigations have begun to utilize classic hallucinogens to study the reliability, characteristics, subjective nature, and behavioral consequences of mystical-type experiences (Pahnke, 1963; Griffiths, Richards, McCann, & Jesse, 2006; Griffiths et al., 2011; Garcia-Romeu et al., 2015). Classic hallucinogens are a structurally diverse group of compounds that bind at 5-HT2A serotonin receptors and produce a unique profile of changes in thoughts, emotions, and perceptions, often including profound alterations in the perception of reality, that are rarely experienced except in dreams, naturally occurring mystical experiences, and acute psychosis. The use of classic hallucinogens makes the study of mystical experiences more tractable because classic hallucinogens can be administered under double-blind conditions and can occasion mystical experiences with high probability (Griffiths et al., 2006; Griffiths et al., 2011). Classic hallucinogens allow for prospective and controlled exploration of such experiences, and provide a degree of neurobiological specificity and mechanistic understanding that is not possible in correlational or descriptive studies, or in reviews of present-day or historical case reports.
The following section of this chapter reviews descriptions and definitions of mystical-type experiences. Evidence of the historical connection between classic hallucinogens and mystical experiences is then presented. The chapter then reviews empirical evidence of mystical experiences occasioned by classic hallucinogens and the potential therapeutic benefits of such experiences. The chapter ends with an exposition of a functional neural model of mystical experience that is based on changes in the default mode network of the brain that have been observed after the administration of classic hallucinogens and during meditation practices which are sometimes associated with mystical-type experiences.
2. What are Mystical Experiences?
“[Mystical experiences are] those peculiar states of consciousness in which the individual discovers himself to be one continuous process with God, with the Universe, with the Ground of Being, or whatever name he may use by cultural conditioning or personal preference for the ultimate and eternal reality”
The theological and philosophical literatures have primarily taken descriptions of experiences as their source data for the exploration of mystical experiences. The theological literature on mystical experiences is based on accounts of prophets, saints, and the practices of mystic sects within religious traditions. Mystic sects exist within all major world religions. These sects are lineages of individuals who have organized around a spiritual practice within their culture and religion, with characteristic lifestyles, teachings, and traditions that instruct the individual and provide a supportive setting for the individual to achieve a deeper connection with the God of their understanding. In this fashion, mystics may be considered “empirical” theologians (Huxley, 1947). Sufism is a mystical sect within the religion of Islam, while Kabbalists explore mysticism in the context of Judaism. Individuals from various religious traditions, including Christian (e.g. Meister Eckhart from the 13th century, St. Teresa of Avila and St. John of the Cross from the 16th century), Islamic (e.g. Rumi and Saadi from the 13th century), and Jewish (e.g. Maimonides of the 12th century) traditions, are considered to be mystics. Some Hindu and Buddhist meditation practices that focus on non-dual experiences likely increase the probability of mystical-type experiences (Stace, 1960a).
The most definitive philosophical treatise on mystical experiences was compiled by Walter Stace (1960a). Stace identified, collated, and distilled descriptions of mystical experiences from a variety of sources. From this literature, he argued that mystical experiences have a “common core” of phenomenological features that are independent from the interpretation of those experiences. His thesis in this regard was that differences in the descriptions of mystical experiences given by mystics of different faiths amount not to a difference in the core experience, but rather a difference in the interpretational frame through which those experiences are viewed. The idea of a “common core” of mystical experience is consistent with the notion of a “perennial philosophy” in which an “immemorial and universal” substrate underlies all religions and spiritual paths and is reflected in “every religious tradition and in all the principal languages of Asia and Europe” (Huxley, 1947).
A critical definitional feature of the mystical experience is a sense of unity, or the experience of becoming one with all that exists. Stace (1960b) described this mystical unity as “the apprehension of an ultimate nonsensuous unity in all things”. Stace made a distinction between the qualitative nature of unity in extrovertive mystical experiences and the qualitative nature of unity in introvertive mystical experiences. Unity in the extrovertive mystical experience involves recognition of the oneness of all, in which one finds unity at the core of the inner subjectivity or inner reality of all things despite the diversity or apparent individual identity and separation of all things. Unity in the introvertive mystical experience, on the other hand, involves an experience of the complete dissolution of the self, loss of the notion of “I” and loss of all boundaries, such that there is no separation or individual identity. Introvertive mystical experience involves an experience of unity that is otherwise devoid of content, sometimes referred to as “the void”. While both extrovertive and introvertive types are considered experiences of mystical unity, Stace considered extrovertive unity as an “incomplete kind of experience which finds its completion and fulfillment in the introvertive kind of experience” (Stace, 1960a).
In addition to the experience of introvertive or extrovertive types of unity, Stace described six other dimensions of mystical experience: 1) sacredness: a sense that what is encountered is holy or sacred; 2) noetic quality: the experience is imbued with an aspect of meaning and a sense of encountering ultimate reality that is more real than usual everyday reality; 3) deeply felt positive mood: joy, ecstasy, blessedness, peace, tenderness, gentleness, tranquility, awe; 4) ineffability: the experience is difficult to put into words; 5) paradoxicality: to explain the experience, one seems to have to describe the co-existence of mutually exclusive states or concepts; 6) transcendence of time and space: Introvertive mystical experiences may have a non-spatial and non-temporal aspect, such that the traditional notions of time and space have no meaning.
As described in more detail later in this chapter, mystical experiences may be encountered following the ingestion of classic hallucinogens. The following is a written description of an experience reported by a volunteer who received 20 mg/70 kg of psilocybin in a study conducted at Johns Hopkins. Bold typeface indicates portions of the description that directly align with dimensions of mystical experience that were identified by Stace:
“In my mind’s eye, I felt myself instinctively taking on the posture of prayer in my head. I was on my knees, hands clasped in front of me and I bowed to this force. I wasn’t scared or threatened in any way. It was more about reverence. I was showing my respect. I was humbled and honored to be in this presence. This presence was a feeling, not something I saw or heard. I only felt it, but it felt more real than any reality I have experienced. And it was a familiar place too. One I had felt before. It was when I surrendered to this, that I felt like I let go. I was gone…or I should say this earthly part of me was. It was still on the couch in some sort of suspended animation awaiting my return. I was in the void. This void had a strange and indescribable quality to it in that there was nothing to it but this feeling of unconditional and undying Love. It felt like my soul was basking in the feeling of this space. I have no idea how long this lasted. Time and space did not exist there …it was all different manifestations of this Love feeling I found myself wrapped in.”
Mystical experiences have been an active area of investigation in the experimental psychology literature, particularly within the psychology of religion (Hood, 2009). Research in this domain has frequently used the Mysticism Scale, a psychometric instrument that codifies the descriptive definition of mystical experience provided by Stace (Hood, 1975; Hood et al., 2001). The Hood Mysticism Scale is scored into three factors: extrovertive mysticism, introvertive mysticism, and religious interpretation. While the precise factor structure of items of the Hood Mysticism Scale may nominally vary between different cultures and different religious backgrounds (Chen, Hood, Qi, & Watson, 2011a; Chen, Hood, Yang, & Watson, 2011b; Chen, Zhang, Hood, & Watson, 2012), the basic concepts that underlie the factor structure of this instrument are reliable and align with Stace’s model. The extrovertive mysticism factor typically includes items related to external unity (unity in diversity) and inner subjectivity. The introvertive mysticism factor typically includes items related to internal unity (contentless unity), ego loss, ineffability, timelessness and spacelessness. The religious interpretation factor typically includes items that measure reverence or sacredness, noetic quality, and positive mood. Paradoxicality, though identified by Stace as a characteristic of mystical experiences, was not included as a criterion dimension in the Hood Mysticism Scale (Hood, 1975).
The Hood Mysticism Scale has been used in survey research and to assess retrospective accounts of mystical experiences in other psychological studies. With tools such as the Mysticism Scale, Ralph Hood and colleagues have vigorously defended the thesis of the “common core” (Hood & Williamson, 2000; Hood, 2006; Anthony, Hermans, & Sterkens, 2010) by demonstrating the overall reliability and reproducibility of the factor structure of mystical experiences across various participant samples, across cultures, and across a number of different religious traditions. Iranian Muslims (Hood et al., 2001), American Christians (Hood et al., 2001), Chinese Christians (Chen et al., 2012), Chinese Buddhists, (Chen et al., 2011a), and Tibetan Buddhists (Chen et al., 2011b) were assessed using the Hood Mysticism Scale and the instrument was shown to validly measure mystical experiences in each sample. The common features of mystical experiences shared across cultures and religious traditions are consistent with idea that there is a common core to such religious experiences (i.e. the perennial philosophy; Huxley, 1947).
It is important to acknowledge that the most extreme interpretation of the common core hypothesis, which holds that mystical experience is a direct encounter with the divine, and from which claims of a perennial philosophy are made, has been criticized by some scholars of religion. These scholars argue that the cross-religion and cross-cultural generality of such experiences is impossible from a constructionist position that asserts that all such experiences are necessarily and significantly shaped by language and culture, the differences in which obviate the potential for a common core to these experiences (Katz, 1978; 1983; Proudfoot, 1985; Sharf, 1998). Although debating the conceptual extreme interpretations of mystical experience has provided a platform for academic scholarship, it may not be a productive strategy for advancing a scientific basis for exploring the immediate causes and consequences of such experiences (Hood, 2003). The research discussed throughout this chapter is focused on an empirical description and analysis of mystical type experiences, which we believe has documented impressive generality and replicability of such experiences.
Mystical experiences have not been restricted to any particular path, canon, or dogmatic viewpoint, and experiences identified as mystical have been encountered in secular contexts outside of the framework of any traditional religious or spiritual practice or interpretation (Hood, Morris, & Watson, 1990). Experiences that fit the mystical-type framework have a wide range of apparent etiologies, including meditation and prayer (d’Aquili & Newberg, 2000; Newberg & Iversen, 2003; Newberg et al., 2010; Josipovic, 2014), sensory deprivation/isolation (Hood et al., 1990), music listening by “deep listeners” (Gabrielsson, 2010; Penman & Becker, 2009), breathwork (Grof et al 2010), and ingestion of classic hallucinogens such as psilocybin (Griffiths et al., 2006; Griffiths, Richards, Johnson, McCann, & Jesse, 2008; Griffiths et al., 2011).
While certain examples of mystical experience may be accompanied by quite dramatic and seemingly paranormal events (e.g. some of the experiences described by prophets in the Old Testament), these are exceptions rather than exemplars of mystical-type experiences. Likewise, abrupt experiences of “rapture” as described by mystics such as St. Teresa of Avila may be atypical (Stace, 1960a). Rapture in the case of St. Teresa involved not only feelings of positive mood (ecstasy or pleasure) but also feelings of fear, and abnormal body changes (Underhill, 1911 [2009]). Seeing visions and hearing voices are likewise not core elements of mystical experiences (Stace, 1960a). Prophetic experiences per se, although heavily associated with classic religious and scriptural encounters with God, may not contain all the key features of mystical experience (Hood, 2009).
Mystical experiences are not similar to the altered states of consciousness associated with intoxication of many common psychoactive drugs such alcohol or opiates (Aaronson & Osmond, 1970). Nor are mystical experiences necessarily associated with religious experiences such as glossolalia, or speaking in tongues (Newberg, Wintering, Morgan, & Waldman, 2006). Mystical experiences may be accompanied by spiritual insights, but the mystical experience is not, in and of itself, simply the experience of religious or spiritual insight. Mystical experiences are also not simply an interesting aesthetic and/or euphoric experience, an experience of an archetypal construct, or a psychodynamic or intellectual insight (Richards, 2014). Mystical experiences are defined as a self-reported experience of unity accompanied by the additional dimensions of experience as outlined by Stace.
3. Historical Use of Indigenous Hallucinogens
The ingestion of naturally occurring classic hallucinogens in ceremonial contexts has a long history. While there is clear anthropological evidence of the ceremonial consumption of hallucinogenic plant and fungal matter in the past few centuries (Dobkin de Rios, 1984; Guzmán, 2008), there is speculation that ceremonial use of similar psychoactive compounds dates back many thousands of years (Schultes, 1969; Westermeyer, 1988; Wasson, Hofman, & Ruck, 1998; Schultes et al., 2001). The reasons for ceremonial use of these substances by indigenous people included use for medicinal and divination purposes, but a prominent goal of ceremonial consumption of classic hallucinogens has also likely been to occasion primary spiritual experiences that fit a mystical-type description (Roberts, 2001). Psychoactive plants and fungi for which there is substantive knowledge of ceremonial use include peyote, ayahuasca, and psilocybin mushrooms. While double-blind studies have not rigorously compared the psychoactive effects of these and other classic hallucinogens (including synthetic hallucinogens such as LSD), the alterations in consciousness produced by these compounds are generally considered to be quite similar, putatively because they share a common mechanism of action initiated through agonism at the 5HT2A receptor and likely involving downstream glutamate effects (Nichols, 2004, 2016; Vollenweider & Kometer, 2010). Under appropriate set and setting conditions, these substances may produce mystical type experiences.
Peyote is a cactus that contains the hallucinogenic alkaloid mescaline (3,4,5-trimethoxy-phenethylamine), which was used historically by Mexican indigenous people, including the Chichimeca, Huichol, and Tarahumara tribes, for thousands of years (El-Seedi, De Smet, Beck, Possnert, & Bruhn, 2005; Schultes et al., 2001). Historically, peyote has been used both for medicinal and ceremonial purposes (Schultes et al., 2001). Peyote is currently used for religious purposes by the Native American Church (Halpern, Sherwood, Hudson, Yurgelun-Todd, & Pope, 2005; Osmond, 1970). Humphrey Osmond described an experience that he had with peyote as a participant in a ceremony of the Native American Church, and concluded: “We had wrestled with the angel. We had grappled with the Heavenly Father” and “Peyote acts not by emphasizing one’s own self but by expanding it into the selves of others, with a deepening empathy or in-feeling. The self is dissolved and, in being dissolved, enriched” (Osmond, 1970)
Psilocybin (4-phosphoryloxy-N,N-dimethyl-tryptamine), another hallucinogenic alkaloid, occurs naturally in mushrooms of the Psilocybe genus that are found on all continents (Guzman, 1983). The ceremonial use of psilocybin-containing mushrooms by indigenous tribes in Mexico can be traced back at least to the 15th century. At least nine indigenous groups in Mexico have been identified that still consume psilocybin mushrooms in a ceremonial context (Guzmán, 2008). R. Gordon Wasson used language suggestive of mystical-type experiences when describing his first exposure to psilocybin-containing mushrooms during a ceremony led by the now famous mushroom shaman, Maria Sabina: “… your soul is free, loses all sense of time … you know what the ineffable is, and what ecstasy means … the flight of the soul from the body” (Wasson et al., 1998).
DMT (N,N-dimethyltryptamine) is another hallucinogenic tryptamine alkaloid that has been used in various forms for medicinal, prophetic, and divination purposes by groups in South America since pre-Columbian times (Dobkin de Rios, 1984; Schultes et al., 2001). Ayahuasca is a plant admixture made from two or more plants, including a plant (e.g. Psychotria viridis) that contains DMT and other plants that contain monoamine oxidase inhibitors (e.g. Banisteriopsis caapi). Ayahuasca is currently used for religious purposes in South America, the United States, and Europe by Uniao do Vegetal and the Santo Daime Church (Dobkin de Rios, 1996; Grob et al., 1996; Tupper, 2008). While not as widely used as ayahuasca, snuffs made from DMT-containing plants also have a history of ceremonial use in South America, with more frequent use in pre-Columbian than in modern times (Dobkin de Rios, 1984).
4. Experimental Evidence of Mystical Experiences with Psilocybin
The classic hallucinogens produce a unique profile of cognitive, perceptual, and emotional changes that have similarities with various states of consciousness, including mystical experiences, psychoses, and liminal sleep states. During the first phase of modern human scientific research with classic hallucinogens, from roughly the 1950s to the 1970s, classic hallucinogens were primarily studied as models of psychosis and as therapeutic agents in a variety of disorders. In 1962, Walter Pahnke conducted the remarkable Good Friday experiment, administering either 30 mg psilocybin (10 subjects) or 200 mg nicotinic acid (10 subjects) with the intent of studying the incidence and character of psilocybin-induced mystical experiences (Pahnke, 1963).
In an effort to maximize the effects of set and setting, Pahnke conducted the study with seminary students in a private chapel on Good Friday during the broadcast of the traditional Good Friday religious service. After the experience, and at a 6 month follow-up, participants completed a questionnaire that assessed eight dimensions of mystical experience that were based on the model of mystical experience developed by Stace (Stace, 1960a): introvertive unity, extrovertive unity, transcendence of time and space, deeply felt positive mood, sacredness, objectivity (noetic quality), ineffability, and paradoxicality. In addition, the transiency of the experience was assessed for a total of nine dimensions of mystical experience.
Pahnke considered a “complete” mystical experience as one in which ratings of at least 60 percent of the total possible score for each of the nine categories of mystical experience were provided (Pahnke, 1969). By this criterion, 30–40% of participants in the psilocybin condition achieved a complete mystical experience during the study, whereas none of the control participants who received nicotinic acid achieved a complete mystical experience (Pahnke, 1967). In a 25-year follow-up to the Good Friday experiment, Doblin (Doblin, 1991) was able to contact 16 of the 20 original participants and collect additional retrospective ratings on the nine dimensions of mystical experience measured by Pahnke. Doblin found little change between the 6-month retrospective ratings and the 25-year retrospective ratings of mystical experience. Moreover, both Pahnke (at the 6 month follow-up) and Doblin (at the 25 years) found that participants in the psilocybin condition rated persisting positive changes in attitudes and behavior that they attributed to their psilocybin experience, while participants in the control condition indicated no such changes.
While groundbreaking, the Good Friday experiment had significant limitations, including limited generality due to the highly selective demographics of the participants (seminary students), conduct of the study in a group setting that allowed interactions among participants (thus resulting in non-independence of individual subject data), explicit instructions to participants that some would and some would not receive psilocybin (thus creating powerful expectancy effects), and the fact that half of the researchers present during the study also received psilocybin. Not surprisingly, under these conditions, the blind was broken shortly after drug administration, which likely contributed to the assessed differences between groups (Doblin, 1991; Wulff, 1991; Smith, 2000).
In a replication and extension of the Good Friday experiment, Griffiths and colleagues conducted a double-blind crossover comparative pharmacology study of psilocybin (30 mg/70 kg) and methylphenidate (40 mg/70 kg), which were administered in separate sessions to each of 36 participants individually, with at least two months between sessions (Griffiths et al., 2006; Griffiths et al., 2008). Participants in this study were well educated, psychiatrically and medically healthy, had no prior hallucinogen use, and represented a more general sample of the population than those used in the Good Friday experiment. The study reduced expectancy and group confounding effects by studying participants without personal histories of hallucinogen use, by studying only a single participant at a time, and by using an experimental design and instructions that obscured the range of drug conditions that would be administered as well as the total possible number of sessions. The study also utilized a stronger control condition (methylphenidate) than the Good Friday experiment (nicotinic acid). Methylphenidate and psilocybin can both induce strong subjective effects with some similarities, and with a reasonably similar time course. Nicotinic acid, in contrast, has a relatively short time course and a profile of subjective effects that is very different from psilocybin. Finally, in addition to using a revised and updated version of the mystical experience questionnaire used in the Good Friday experiment, Griffiths and colleagues used two psychometrically validated questionnaires that assessed mystical and spiritual effects (the Hood Mysticism Scale and the Spiritual Transcendence Scale) as well as ratings of changes in participant’s attitudes and behavior by community observers (family members and friends of participants).
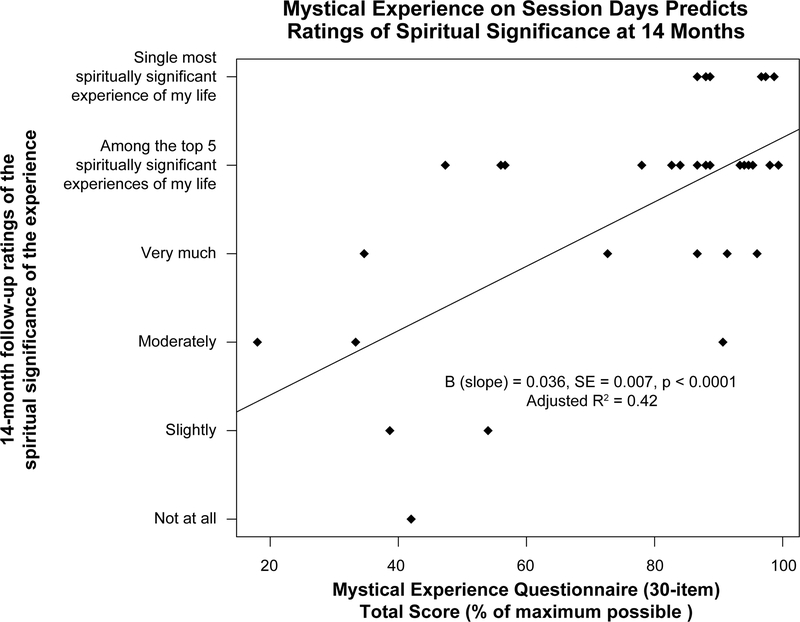
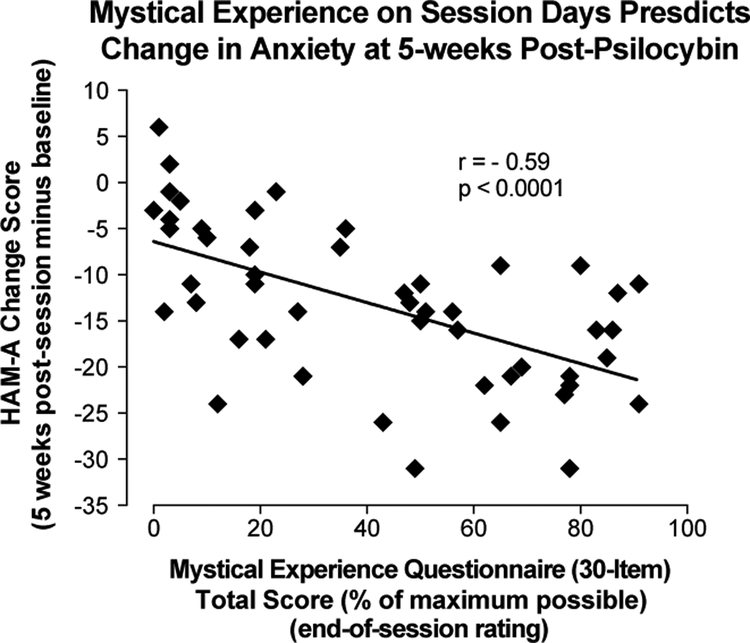
In this study, Griffiths and colleagues demonstrated a fairly high frequency of “complete mystical experiences” during psilocybin sessions (61% of participants), but not during methylphenidate sessions (11% of participants). The criterion for a complete mystical experience was a score of 60% of the total possible score on each dimension of the Mystical Experience Questionnaire, including scores on either internal or external unity factors (whichever was greater) and sacredness, noetic quality, transcendence of time and space, ineffability, and positive mood factors (Griffiths et al., 2006). This is a scoring system is similar to the one used by Pahnke in the Good Friday Experiment. Two months after the session, most participants rated their psilocybin session as among the top five (71%) or single most (33%) spiritually significant experience of their lives, compared to 8% of participants who rated the methylphenidate experience to be among the top 5 spiritually significant experiences of their lives, with no one rating it as the single most. Ratings of positive attitudes about life and self, positive mood, positive behaviors, and positive social effects two months after psilocybin sessions were significantly greater than those provided two months after methylphenidate sessions. Negative ratings of these same dimensions were low and did not differ between the psilocybin and methylphenidate conditions. Further, community observers rated small but significant changes in participants’ positive attitudes and behaviors two months after the psilocybin sessions, but no changes were found two months after methylphenidate sessions. In a 14-month follow-up report, 67% of participants rated their psilocybin session as among the top five most spiritually significant experiences of their lives, and 58% of participants rated their psilocybin session as among the top five most personally meaningful experiences of their lives (Griffiths et al., 2008). Ratings of positive behavior, mood, attitude, and social changes associated with the psilocybin session at the 14-month follow-up were not significantly different from those provided two months post-session. Correlation and regression analyses indicated a central role of mystical experience assessed on the session day (but not intensity of psilocybin experience) in predicting the high ratings of spiritual significance and personal meaning assessed at 14 months (Griffiths et al. 2008). Figure 1 illustrates the significant relationship between mystical experience score and follow-up ratings of spiritual significance.
Figure 1.
Mystical experience on session days predicts ratings of spiritual significance at 14 months. Total score on the 30-item Mystical Experience Questionnaire (MEQ30) is expressed as the percent of the maximum possible score. Data points represent individual participants (n=36). B = slope of the regression of spiritual significance on mystical experience questionnaire total score. SE = standard error of the slope (B). Adjusted R2 indicates the amount of variance in spiritual significance ratings that is explained by the mystical experience questionnaire total score. MEQ30 data have been rescored from MEQ43 responses reported in Griffiths et al., 2008.
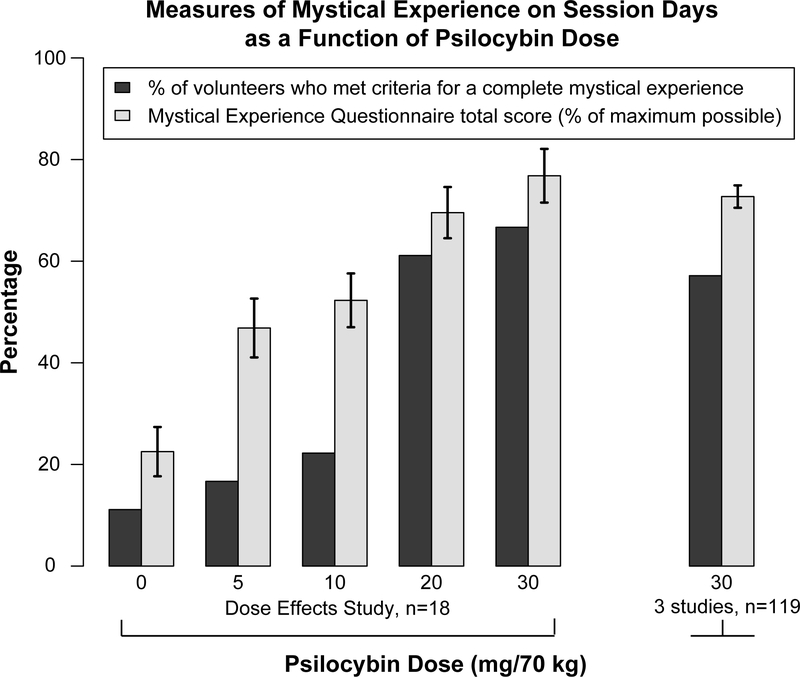
A further extension of this line of research was published by Griffiths and colleagues in 2011. This study utilized a double-blind placebo-controlled design that assessed the effects of placebo and a range of psilocybin doses on ratings of spiritual significance and meaningfulness of psilocybin sessions, and on the likelihood of complete mystical experience. Participants in this study were well educated, medically and psychiatrically healthy, and most were hallucinogen naïve. Participants received 5, 10, 20, and 30 mg/70 kg of psilocybin in separate sessions in either ascending or descending order, with at least one month between each session and a placebo session randomly placed within the sequence. Participants were not informed of the ascending or descending nature of the dose sequence. 61% of participants met criteria for a “complete” mystical experience during the 20 mg/70 kg session, whereas 67% of participants met criteria for a “complete mystical experience during the 30 mg/70 kg session (Figure 2, “Dose Effects Study”, dark grey bars). Although “complete” mystical experience was coded in the original publication using items from the 7-factor, 43-item MEQ (MEQ43), the above percentages were derived using the psychometrically validated 4-factor, 30-item MEQ (MEQ30). The MEQ30 has been validated in both retrospective accounts of mystical experiences with psilocybin (MacLean, Leoutsakos, Johnson, & Griffiths, 2012) and in prospective, experimental laboratory studies with psilocybin (Barrett, Johnson, & Griffiths 2015). The criteria for complete mystical experiences in the MEQ30 are a score of at least 60% of the total possible score on each of four factors of the MEQ30. The MEQ30 will be discussed in more detail later in this chapter.
Figure 2.
Measures of mystical experience on session days as a function of psilocybin dose. “Dose Effects Study” data (5 pairs of bars at the left) show the dose-related effect of psilocybin on two measures of mystical experience (Griffiths et al 2011). “3 studies” observations (bars at the right) show post-session ratings from the first high-dose (30 mg/70 kg) session administered in each of 3 studies (Griffiths et. al. 2006, 2011, unpublished study in beginning meditators). Dark bars show the percentage of volunteers who met criteria for having a complete mystical experience. Lighter bars show the mean Mystical Experience Questionnaire total score expressed as a percentage of maximum possible score; brackets show ± 1 S.E.M. Categorizations of complete mystical experiences and calculations of total scores were based on the 30-item Mystical Experience Questionnaire (MEQ30).
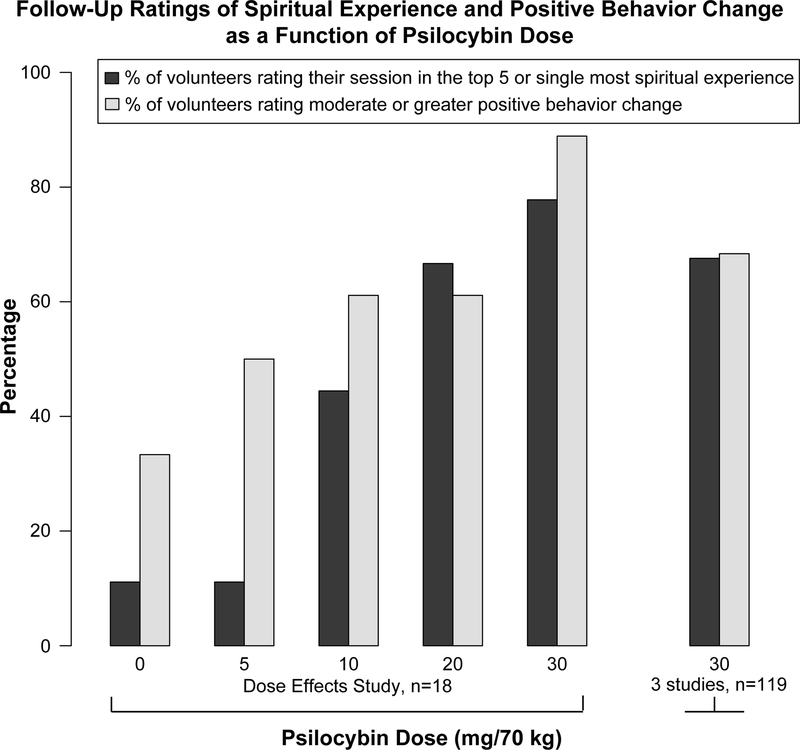
Figure 2 (“Dose Effects Study”) shows that the mean total score on the MEQ30 (light grey bars) as well as the percentage of volunteers who met criteria for a complete mystical experience (dark grey bars) increased as a function of psilocybin dose. Overall, 72% of volunteers had a “complete” mystical experience during the 20 mg/70kg session and/or the 30 mg/70 kg session (using MEQ30 scoring). Follow-up ratings one month after each session of the spiritual significance of the experience (Figure 3, “Dose Effects Study”, dark grey bars), as well as ratings of positive behavior change attributed to the experience (Figure 3, “Dose Effects Study”, light grey bars), increased in a dose-dependent fashion. Eighty-three percent of participants rated the session experiences after 20 and/or 30 mg/70 kg as among the five most spiritually significant experiences of their life; 61% also rated at least one of these as the single most spiritually significant experience of their life. Likewise, one month follow-up ratings of positive attitudes about life and self, positive behavior, positive social effects, and increased spirituality generally increased as a function of psilocybin dose. One month follow-up ratings after the 20 or 30 mg/70 kg sessions did not differ from follow-up ratings 14 months after study completion. Finally, compared to pre-study ratings, community observers rated significant positive change in the attitudes and behaviors of participants three to four weeks after the final session and 14 months after the final session.
Figure 3.
Follow-up ratings of spiritual experience and positive behavior change as a function of psilocybin dose. “Dose Effects Study” data (5 pairs of bars at the left) show the dose-related effect of psilocybin on retrospective ratings assessed 1 month after sessions (Griffiths et al., 2011). “3 studies” observations (bars at the right) show retrospective ratings assessed 3 to 8 weeks after the first high-dose (30 mg/70 kg) session administered in each of 3 studies (Griffiths et. al. 2006, 2011, unpublished study in beginning meditators). Dark bars show the percentage of volunteers who rated their session as within the top five or single most spiritual experience of their life. Lighter bars show the percentage of volunteers who rated a moderate or greater degree of positive behavior change at follow-up.
In addition to showing psilocybin dose effects on mystical experience and follow-up ratings, the rightmost columns of Figures 2 and 3 (“3 studies”) present data from 119 volunteers after a high dose of psilocybin (30 mg/70 kg) (three studies: Griffiths et al., 2006, 2011 and an unpublished study in beginning meditators). Fifty-seven percent met criteria for a complete mystical experience (Figure 2, “3 Studies”). More than 65% of high-dose sessions were retrospectively rated in the top 5 if not the single most personally meaningful (66%) or spiritually significant (68%) experience that volunteers have had in their lives, with most participants (70%) rating at least moderate positive behavior change that they attributed to their session experience (Figure 3, “3 Studies”). The overall rate of complete mystical experiences achieved in these studies exceeds the rate achieved by Pahnke in the original Good Friday Experiment (30 to 40%; Pahnke, 1967). Various factors likely contributed to this difference, with the most important being the greater volunteer preparation before and support during the sessions provided in the more recent studies.
Overall, the psilocybin studies reviewed in this section (Pahnke, 1963; Griffiths et al., 2006, 2008, 2011, unpublished) make important contributions to the understanding of mystical experiences. These studies show that under double-blind conditions that provided significant controls for expectancy bias, psilocybin can occasion complete mystical experiences in the majority of people studied. These effects are dose-dependent, specific to psilocybin compared to a psychoactive control substance (methylphenidate), and have enduring impact on the moods, attitudes, and behaviors of participants as assessed by self-report of participants and ratings by community observers.
5. The Mystical Experience Questionnaire
A primary tool for the study of mystical experiences with classic hallucinogens is the Mystical Experience Questionnaire. The 43-item Mystical Experience Questionnaire (MEQ43), also known as the Pahnke-Richards Mystical Experience Questionnaire (Griffiths et al., 2006), was comprised of 43 items that were similar, but not identical, to the original item set used by Pahnke in the Good Friday experiment. The MEQ43 was scored into seven scales based on seven descriptive dimensions of mystical experience described by Stace (1960): internal unity, external unity, noetic quality, sacredness, positive mood, transcendence of time and space, and a final factor that combined ineffability and paradoxicality scores. Items from a transiency scale used by Pahnke were not included. Thus, the MEQ43 dimensions are theoretically derived, not empirically derived. The MEQ43 was used in the psilocybin vs. methylphenidate comparison study (Griffiths et al., 2006; Griffiths et al., 2008), the psilocybin dose effects study (Griffiths et al., 2011), and the psilocybin smoking cessation study (Johnson et al., 2014; Garcia-Romeu et al., 2015). The factor structure of this instrument was recently evaluated through analysis of retrospective accounts of mystical experiences with psilocybin-containing mushrooms, collected in two internet surveys (MacLean et al., 2012) (n1 = 1602, n2 = 440). Psychometric analysis of the MEQ43 responses provided in these samples yielded a four-factor structure using a subset of 30 of the original 43 items. The four factors of the 30-item MEQ (MEQ30) are: 1. mystical (including items from the previously proposed internal unity, external unity, noetic quality, and sacredness factors); 2. positive mood; 3. transcendence of time and space; and 4. ineffability. The MEQ30 items and factor structure are presented in Figure 4. This factor structure differs from that of the Hood Mysticism Scale, which has three factors: introvertive mysticism (typically including items measuring contentless unity, timelessness, and spacelessness), extrovertive mysticism (typically including items measuring unity in diversity and inner subjectivity), and religious interpretation (typically including items that measure sacredness, noetic quality, positive affect, paradoxicality, and ineffability). Although some items on the Hood Mysticism Scale were shown to load onto different factors in different subject populations (Chen et al., 2011a; Chen et al., 2011b), the underlying conceptual framework of the three-factor structure of the items of the Hood Mysticism Scale has been repeatedly replicated. The four factor structure for the MEQ30 was found to fit responses to the MEQ30 items better than two variations of a three-factor model that included the model proposed by Hood (MacLean et al., 2012).
Figure 4.
Revised 30-item Mystical Experience Questionnaire (MEQ30). The MEQ30 is a psychometrically-validated retrospective measure of mystical experience. The 4 factors of the psychometrically validated questionnaire are derived from a total of 30 items that probe 7 dimensions (designated by underlines) of mystical experience that were identified by Stace (1960). The Mystical factor is composed of 15 items probing four dimensions of the Stace model (internal unity, external unity, noetic quality, and sacredness). Positive Mood (6 items), Transcendence of Time and Space (6 items) and Ineffability (3 items) factors correspond to three separate dimensions of the Stace model.
The MEQ30 has more recently been validated using data collected after psilocybin administration in the context of prospective experimental laboratory psilocybin studies conducted at Johns Hopkins (Barrett, Johnson, & Griffiths, 2015). MEQ data from the first moderate to high dose session (20 mg/70 kg or greater) for 184 volunteers from a total of 5 psilocybin studies (Griffiths et al., 2006; Griffiths et al., 2011; Johnson et al., 2014; Griffiths et al., in press; and an unpublished study in beginning meditators) were pooled and submitted to confirmatory factor analysis. The analysis confirmed the internal validity of the MEQ30 by demonstrating acceptable fit of the four-factor structure of the MEQ30. This analysis also demonstrated the external validity of the instrument by showing that the four factors of the MEQ30 and a higher-level MEQ total score significantly predicted subsequent ratings (from 3 to 8 weeks after psilocybin administration) of the meaningfulness, spiritual significance, impact on well-being, and positive change in behavior attributed to each psilocybin session, while also controlling for rated strength of drug effects (Barrett et al., 2015). Another recent study suggested a 2-factor solution to the MEQ30, based on ratings (N=158) given by individuals who had just completed an ayahuasca ceremony, however this solution was not validated with either a separate or a large sample (Bouso et al., 2016). The limitations of this study include details of the set and setting as well as the actual dose of dimethyltryptamine consumed in any given instance. Without proper control of these elements of experience or experimental design, and without validation of the 2-factor structure with rigorous methods in separate samples, we must caution readers to treat the 2-factor structure tentatively (Barrett & Griffiths, 2016).
The MEQ30 has also been utilized in studies of LSD and MDMA. A controlled laboratory experiment involving administration of psychedelics compared MEQ30 scores provided in reference to experiences with LSD (200 μg), MDMA (75 mg), methylphenidate (40mg), and placebo (Liechti, Dolder & Schmid, 2016). While MEQ30 scores for those who received LSD were significantly higher than for those in any other experimental condition, rates of complete mystical experience were lower for LSD (12.5%) than for previous reports of mystical experience after methylphenidate (23–33%) or psilocybin (up to 67% for a high dose of psilocybin; Barrett, Johnson, & Griffiths, 2015). It must be noted, however, that the set and setting involved in previous reports of mystical experience with methylphenidate and psilocybin (Barrett, Johnson, & Griffiths, 2015; Griffiths et al., 2006; Griffiths et al., 2011) were highly optimized to support the emergence of mystical experience, involving an experimental room modeled after a living room, a continuous musical accompaniment, art on the walls and soft lighting, and continual interpersonal preparation, support and integration of the experience. The set and setting reported for LSD administration was significantly different, occurring in a standard hospital patient room with optional music listening (Liechti, Dolder, & Schmid, 2016).
The MEQ30 and the Hood Mysticism Scale are psychometrically validated measures of mystical experience that have been derived from the same conceptual frame (Stace, 1960a), but they differ in specific items and underlying factor structure. The MEQ30 and the Hood Mysticism Scale also differ in the timeframe over which the dimensions of mystical experiences are assessed. The MEQ30 assesses phenomena occurring during a single discrete experience, while the Hood Mysticism Scale typically assesses phenomena occurring over a lifetime. In addition, the MEQ30 has only been used in studies of classic hallucinogens (Griffiths et al., 2006; Griffiths et al., 2011; Johnson et al., 2014; Garcia-Romeu et al., 2015), whereas the Hood Mysticism Scale has been used most often in the context of survey research, in which the etiology of mystical experience is unknown, but generally not assumed to be due to classic hallucinogens. Future research is needed to validate the MEQ30 in assessing mystical experiences that occur in experimental and non-experimental contexts in absence of drug administration. The psychometrically and experimentally validated MEQ30 may serve as a useful tool to facilitate investigation of both the determinants and consequences of mystical experience, including a wide variety of behavioral, pharmacologic, neurophysiological, genetic, personality, psychological, and therapeutic outcome measures.
While it has been suggested that the oceanic boundlessness (OAV) sub-scale of the Altered States of Consciousness (APZ; Dittrich, 1998) questionnaire or the spiritual sub-scale of the derivative 5-Dimensional Altered States of Consciousness (5D-ASC; Dittrich, Lamparter, & Maurer, 2010; Studerus, Gamma, & Vollenweider, 2010) questionnaire may provide a measure mystical experience (Majic, Schmidt, & Gallinat, 2015), the items of these scales have only limited conceptual overlap with proposed (Stace, 1960a) and empirically tested (Hood, 1975; Griffiths et al., 2006; Hood, 2009; Griffiths et al., 2011) models of mystical experience. Unity and positive mood (assessed in the “Blissful State” and “Experience of Unity” factors) are scored in the 11-dimension scoring of the 5D-ASC, and may be considered more suitable measures of some aspects of mystical experience. However, none of the proposed scales of 5D-ASC uniquely assess the constructs of ineffability or transcendence of time and space. While the experience oceanic boundlessness, unity, and positive mood may be necessary for a complete mystical experience, they may not be sufficient. One could expect to experience these effects after ingesting other drugs that would not be expected to reliably occasion a mystical experience. For instance, MDMA may be expected to increase feelings of unity and positive mood, but it may not be expected to occasion mystical experience (Lyvers & Meester, 2012).
The newly developed Ego-Dissolution Inventory (EDI) measures a construct that is shown to correlate highly with a select number of MEQ items that assess unity (Nour et al., 2016), however this scale does not measure positive affect, ineffability, or transcendence of time and space, nor does this scale assess other aspects of mystical experience that are assessed by items that load onto the Mystical factor of the MEQ30 (e.g. external unity, noetic quality, reverence, or sacredness). It is possible that one might report having experienced ego dissolution (e.g. might endorse items from the EDI including “All notion of self and identity dissolved away”, “I lost all sense of ego”, “I felt far less absorbed by my own issues and concerns”, and “I experienced a [dissolution or disintegration] of my ‘self’ or ego”) under the effects of anesthesia (e.g. propofol), but this experience would not include other dimensions of mystical experience described by Stace or included in either the MEQ30 or Hood Mysticism Scale. Further, some psychedelic experiences of ego dissolution are psychologically challenging, are devoid of positive affect, and may have enduring negative psychological effects (Carbonaro et al., 2016). As constructed, the EDI will not differentiate such experiences from mystical type experiences.
A complete mystical experience, as described by Stace (1960), which is codified in the MEQ30, includes not only just ego dissolution (as assessed by the EDI; Nour et al., 2016), unity (as assessed by the 5D-ASC), or positive mood (as assessed by the 5D-ASC), but also transcendence of space and time, ineffability, noetic quality, and reverence or sacredness. While other inventories have been used to measure and quantify important aspects of the subjective of psychedelics, such as experiences of unity and ego dissolution, none but the MEQ contains an array of items and subscales that adequately evaluates the construct of a complete mystical experience in relation to discrete psychedelic experiences.
6. Mystical Experiences, Classic Hallucinogens, and Therapeutic Interventions
Although mystical experiences are an intrinsically fascinating target of research in their own right in the study of consciousness, the correlates and consequences of mystical experiences may have relevance to therapeutic interventions. Research first conducted in the 1960s and continued in the present day suggests the possible efficacy of the classic hallucinogens in treating anxiety and depression (Grob et al., 2011; Carhart-Harris et al., 2016; Griffiths et al., 2016; Ross et al., 2016) and in treating addiction disorders such as alcohol dependence (Bogenschutz and Pommy, 2012; Bogenschutz and Johnson, 2016) and nicotine dependence (Johnson, Garcia-Romeu, Cosimano, & Griffiths, 2014). Relevant to the present review, drug-occasioned mystical experiences have been suggested as a mediating mechanism underlying possible therapeutic effects (Richards et al., 1977; Garcia-Romeu et al., 2015; Griffiths et al., 2016; Ross et al., 2016).
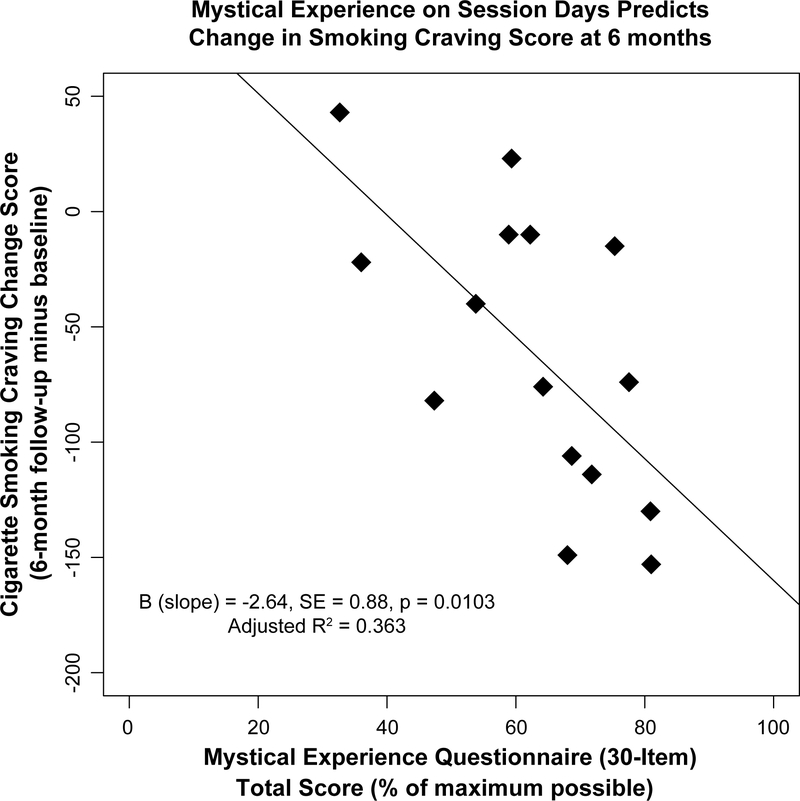
A recent open-label, proof of concept study investigated the value of psilocybin as an adjunct to a smoking cessation intervention (Johnson, Garcia-Romeu, Cosimano, & Griffiths, 2014). Fifteen participants received up to three sessions with either a 20 mg/70 kg or 30 mg/70 kg dose of psilocybin. The authors reported a striking clinical outcome of successful smoking cessation in 80% of the sample (12/15 participants), with biologically verified abstinence 6 months after each participant’s planned quit date. Seventy-three percent of participants rated at least one of their sessions as among the top five most spiritually significant experiences of their lives. Scores on a measure of individual mystical experience (the States of Consciousness Questionnaire, which contains the 43-item Mystical Experience Questionnaire, or MEQ43) correlated strongly, negatively, and significantly with a validated measure of cigarette craving (the Questionnaire on Smoking Urges). The MEQ43 data for this study have been re-scored using the MEQ30 scoring, and are presented in Figure 5. This suggests a link between strength of mystical experience during psilocybin sessions and clinical change in subjective effects that drive addictive behavior (Garcia-Romeu et al., 2015).
Figure 5.
Mystical experience scores on session days predicts change in smoking craving score at 6 months. X-Axis: total score on the 30-item version of the Mystical Experience Questionnaire (MEQ30) expressed as the percent of the maximum possible score. Y-Axis: difference in score on the Questionnaire on Smoking Urges between study intake and 6-month follow-up. Data points represent individual participants (n=15). B = slope of the regression of cigarette smoking craving change score on mystical experience questionnaire total score, SE = standard error of the slope (B). Adjusted R2 indicates the amount of variance in spiritual significance ratings that is explained by mystical experience questionnaire total score. MEQ30 data have been rescored from MEQ43 responses reported in Garcia-Romeu et al. 2015.
In a separate study, a similar finding was demonstrated in individuals with alcohol dependence (Bogenschutz et al., 2015). Ten volunteers were administered either a moderate (0.3 mg/kg) or high (0.4 mg/kg) dose of psilocybin in each of two experimental sessions during the course of treatment for alcohol dependence. While the exact number of participants who attained complete mystical experience was not reported, and while total scores on measures of mystical experience were lower than those attained in previous studies with psilocybin, patients in the study exhibited a significant improvement in drinking after their first psilocybin session and scores on ratings of psilocybin-occasioned mystical experience correlated strongly with change in drinking behavior.
Similar findings have been demonstrated in studies of the effect of psilocybin on anxiety and depression. A recent open-label pilot study consisting of an experimental session involving a 10 mg dose of psilocybin followed by a second experimental session involving a 25 mg dose of psilocybin reported sharp declines in depressive symptoms of patients with treatment-resistant depression at one week and three months after the second psilocybin session (Carhart-Harris et al., 2016). A separate series of investigations have demonstrated significant reduction in both anxiety and depression symptoms related to a life-threatening cancer diagnosis (Grob et al., 2011; Griffiths et al., 2016; Ross et al., 2016). Significant negative correlations were found between total mystical experience scores (scored using the MEQ30) and outcome measures assessed 5 weeks after a high-dose psilocybin session, including measures of anxiety and depression symptoms. Figure 6 shows the relationship between total score on the MEQ30 and change from baseline to 5 weeks post-psilocybin on the Hamilton Anxiety Rating Scale (HAM-A). Importantly, total score on the MEQ30 was shown to mediate the effect of psilocybin on anxiety- and depression-related outcome measures (Griffiths et al., 2016; Ross et al., 2016). Initial findings from small-scale open label studies (Johnson et al., 2015; Carhart-Harris et al., 2016) along with findings from studies including more rigorous scientific controls (Grob et al., 2011; Griffiths et al., 2016; Ross et al., 2016) demonstrate the potential for therapeutic effects of psilocybin, and are suggestive of a link between mystical experience and therapeutic efficacy.
Figure 6.
Mystical experience on session days predicts change in anxiety at 5 weeks post-psilocybin. X-Axis: total score on the 30-item version of the Mystical Experience Questionnaire (MEQ30) expressed as the percent of the maximum possible score. Y-Axis: difference in score on the Hamilton Anxiety Rating Scale between baseline and 5-week follow-up. Data points represent individual participants (n=51). r = Pearson product moment correlation between HAM-A and MEQ30 scores. Data are from Griffiths et al., 2016.
7. Are Mystical Experiences Reducible to Neural Processes?
Whether experience itself is wholly reducible to neural processes is an open question. There are psychological abilities and processes, such as perception and cognition, that are amenable to neuroscientific investigation (Gazzaniga, Ivry, & Mangun, 2014), and that could be considered the contents of consciousness (Chalmers, 1995). Some theorists have argued that while abilities and processes such as these are amenable to investigation, the nature of experience itself is nonreducible and should be taken as a fundamental property of consciousness (Chalmers, 1995). However, this position is not universally accepted (Dennett, 1995). Both of these positions acknowledge that the nature of experience itself is difficult to approach from a reductive perspective, and the nature of mystical experience is no different.
Some of psychological processes, such as the experience of positive mood or alterations in the perception of time and space, are elements of mystical experiences which can be likely associated with primary neural processes. However, in the same way that memories or visual percepts do not wholly constitute consciousness itself but rather constitute the contents of consciousness, explanations of the individual neural elements of mystical experience may not provide a complete account of a mystical experience. Yet, there is still value in identifying and understanding neural and psychological processes that relate to mystical experience.
Investigation of mystical experiences may reveal valuable information about brain functioning. Much in the tradition of the brain lesion model of psychology and neuroscience, systematic perturbation of a neural system permits better understanding of that system. Thus, the study of the neural correlates of mystical experiences may lead to a better understanding of the possible brain mechanisms underlying self-referential, spatial, and temporal processing, as well as complex emotions such as reverence or sacredness. Further, the study of therapeutic outcomes of mystical experiences may advance the understanding of the neural basis of addiction and mood disorders.
8. Hallucinogens and Meditation as Tools to Investigate the Neural Correlates of Mystical Experiences
While little is currently known about the neural basis of discrete mystical experiences, we have a basic framework from which hypotheses can be generated. Stace’s work provides a phenomenological model of mystical experiences. Griffiths and colleagues provide a pharmacological model for prospectively investigating mystical experiences that fit the Stace model. The reliability and pharmacological specificity of a selective 5HT2A receptor agonist (psilocybin) to occasion mystical experiences can be offered as initial evidence in favor of some neurobiological component of mystical experiences. Recent neuroimaging research with classic hallucinogens provides a basis for beginning to develop a neural model of mystical experiences.
Over the past two and a half decades, a wide range of neuroimaging methods, including molecular, magnetic, and electrocortical modalities, have been used to study the effects of classic hallucinogens in humans. Multiple neural processes in nearly every major cortical and subcortical division of the brain have been reported to be modulated by classic hallucinogens. Obviously, hallucinogen-related changes in these brain areas cannot be taken as absolute markers of mystical experience, since not all experiences with classic hallucinogens are mystical. Substances such as psilocybin are neither necessary nor sufficient for producing such experiences, and 5HT2A receptor agonism may play only an initiatory role in the brain processes that account for or correlate with mystical experiences. Thus, the literature on the neural correlates of classic hallucinogens alone will be insufficient to provide a complete model of the neural basis of mystical experiences.
One approach to exploring the neural basis of mystical experience is to consider brain states produced by other approaches to occasioning mystical experiences, such as meditation practices. Although meditation encompasses a broad range of practices (Nash & Newberg, 2013), a few specific practices have been the focus of brain imaging studies. These include focused awareness, open awareness or open monitoring (Lutz, Slagter, Dunne, & Davidson, 2008), and non-dual awareness (Josipovic, 2010). Focused awareness practice typically involves attentional focus on an explicit object of consciousness (e.g. breath exhalation). In contrast, the intention of an open awareness practice is effortless sustaining of awareness without explicit selection of a discrete object of focus (Lutz et al, 2008). In both cases, a goal is to cultivate non-attachment to thoughts or distractions that may arise, with the further goal of stabilizing the mind. Such practices may ultimately lead to experiences of unity, with more specific objects of consciousness receding into the background. Non-dual awareness practices are a more subtle and perhaps a more direct technique of cultivating the experience of unity or pure awareness. In these practices, the object of attention is awareness itself or, as some have described it, awareness of awareness. The non-dual or transcendental unitive state that can arise from such practices appears to be descriptively identical to those described in peak mystical experiences, especially in terms of the dissolution of the conventional sense of personhood or the lack of differentiation between the sense of self and other (i.e. mystical unity).
9. Hallucinogens, Meditation, Mystical Experience, and the Default Mode Network
This section provides a review of hallucinogen studies and meditation studies that identify neural changes that may be relevant to mystical experiences – more specifically, changes in the default mode network (DMN) of the brain as they relate to the experiences of unity and transcendence of time and space, which are core dimensions of mystical experiences. The “default mode network” (DMN) consists of a number of brain areas in which task-related decreases in activity were reliability identified in early studies of human brain activity using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) (Raichle et al., 2001). The pattern of activity that is typically found in the DMN is hypothesized to reflect intrinsic patterns of communication in the brain (Raichle & Snyder, 2007). Activity in the DMN (Power et al., 2011) (also called the “task-negative network”) generally correlates with internally directed attention and is typically negatively correlated with activity in task-positive networks (i.e. networks that support externally directed attention and behavioral response) (Fox et al., 2005).
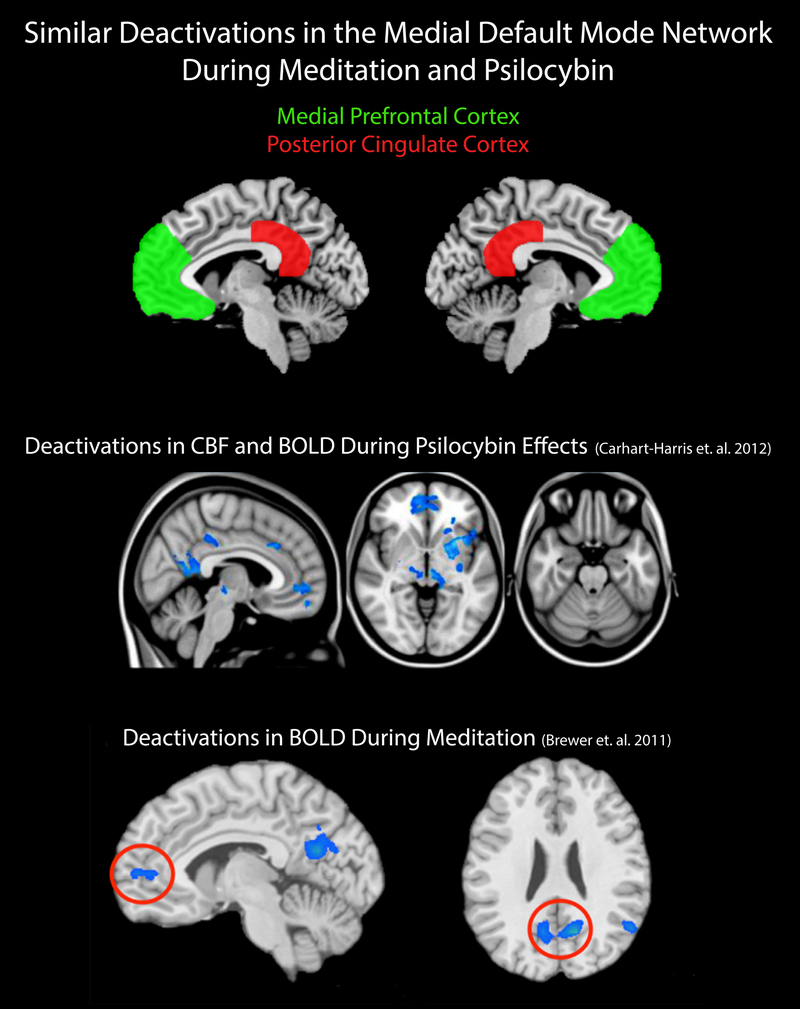
As is described in more detail below, the medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), and parahippocampal cortex (PHC), which densely express 5HT2A receptor sites (the cellular targets of classic hallucinogens), display altered functioning in response to classic hallucinogens (Figure 7, “Deactivations During Psilocybin Effects”). Activity and connectivity in these brain regions is also altered by acute and long-term meditation practice (Figure 7, “Deactivations During Meditation”). It can be argued that alteration of neural activity within these brain regions is consistent with the decreases in self-referential processing (i.e. dissolution of a sense of self, likely related an experience of unity) that accompanies introvertive mystical experience. Neural functioning in the lateral default mode network, more specifically in the angular gyrus region of the inferior parietal lobule (IPL), is also implicated in meditation, self-transcendence, and experiences with classical hallucinogens. It is argued that change in neural activity in this region is consistent with spacelessness and timelessness that often accompanies introvertive mystical experience.
Figure 7.
Similar deactivations in the medial default mode network during meditation and experience with psilocybin. The upper portion of the figure illustrates approximate locations of the medial prefrontal cortex (labeled in green) and posterior cingulate cortex (labeled in red). The center portion of the figure (adapted from Carhart-Harris et al 2014, Figure 4, with permission), shows regions in the medial default mode network, including the medial prefrontal cortex and posterior cingulate, where both deactivation in blood-oxygenation-level-dependent (BOLD) data and decrease in cerebral blood flow (CBF) were observed after intravenous injection of psilocybin. The lower portion of the figure (adapted from Brewer et al 2011, Figure 1, with permission) shows regions in the medial default mode network, including the medial prefrontal cortex and posterior cingulate, where decreases in BOLD data were observed during meditation. The decreased activity within the medial prefrontal cortex and posterior cingulate which is observed after psilocybin and during meditation is consistent with decreased self-referential processing that accompanies introvertive mystical experience.
9.1. The Medial Default Mode Network
The medial prefrontal cortex (MPFC), the posterior cingulate cortex (PCC), and parahippocampal cortex (PHC) have been identified as major nodes of the medial aspect of the DMN (Fox et al., 2005). This has been confirmed in multiple large samples of volunteers (N between 180 and 1000), using a variety of analysis techniques (e.g. seed-based connectivity analysis, independent component analysis, graph-theoretic analysis, and clustering-based analysis) (Doucet et al., 2011; Power et al., 2011; Yeo et al., 2011). The psychological processes ascribed to the MPFC and PCC include various types of self-referential processing including mentalizing (i.e. thinking about your own or others’ thoughts) (Gilbert et al., 2006), internal dialogue (Northoff et al., 2006; Denny, Kober, Wager, & Ochsner, 2012), self-related judgments (Northoff et al., 2006; Denny et al., 2012), and autobiographical memory retrieval (Svoboda, McKinnon, & Levine, 2006). The process ascribed to the PHC is primarily the coding of episodic memory content, as opposed to semantic memory content which is coded by the perirhinal cortex (Ranganath & Ritchey, 2012). The MPFC and PCC have been implicated in self-recognition and self-awareness in studies using tasks such as recognition of a face as either “you” or “not-you” (van Veluw & Chance, 2014), tasks requiring the participant to identify adjectives as traits of themselves or others (Kelley et al., 2002), tasks assessing self-agency (Renes, van Haren, Aarts, & Vink, 2014), and tasks that test false beliefs and theory of mind (van Veluw & Chance, 2014). The PHC, and more broadly the hippocampal complex, has been implicated in supporting consciousness (Behrendt, 2013) by supporting the moment-to-moment binding of sensory information into coherent memory representations, including those thought to be related to a sense of self. The PHC is involved in maintaining and recalling memories of self and self-relevant information.
Molecular (PET and single photo emission computed tomography, or SPECT) and fMRI (measuring blood-oxygenation-level-dependent, or BOLD, response) brain imaging methods have been used to show that the MPFC, PCC, and PHC are modulated by classic hallucinogens. Glucose metabolism after psilocybin ingestion (Vollenweider et al., 1997; Gouzoulis-Mayfrank et al., 1999) and cerebral blood flow after ingestion of either ayahuasca (Riba et al., 2006) or mescaline (Hermle et al., 1992) have been shown to increase in medial frontal areas that include or overlap with the MPFC. After intravenous administration of psilocybin or LSD, cerebral blood flow and BOLD activity in MPFC and PCC decreased substantially (Figure 7, “Deactivations During Psilocybin Effects”), as did effective connectivity between these areas (Carhart-Harris et al., 2012, 2016). In the same studies, decreased cerebral blood flow correlated with increased intensity of drug effects and decreased sense of self (or increased “ego-dissolution”). The discrepancy between Vollenweider, Riba, Gouzoulis-Mayfrank, and Hermle’s work (showing increases in glucose metabolism and cerebral blood flow with classic hallucinogens) and the findings of Carhart-Harris and colleagues (showing decrease in BOLD activity and cerebral blood flow with classic hallucinogens) may be due to various factors including differing rates of drug CNS penetration, drug metabolic differences due to differing routes of administration (oral vs. intravenous, respectively), and differences in the specific neuronal process being measured by the different techniques (molecular techniques with PET and SPECT in the cases of Vollenweider, Riba, and Hermle, and magnetic resonance imaging techniques in the case of Carhart-Harris).
With injected psilocybin, decreased low frequency power and long-range temporal correlations in BOLD data were observed in the PCC and MPFC, suggesting a breakdown in communication between these areas (Tagliazucchi, Carhart-Harris, Leech, Nutt, & Chialvo, 2014). Increased spectral power and a greater diversity of functional connectivity was observed in the PHC after injected psilocybin, suggesting a substantial shift in communication involving the PHC (Tagliazucchi et al., 2014). After injected LSD, increased global connectivity was shown to correlate with increases in “ego dissolution” (Tagliazucchi et al., 2016) and decreases in mental time travel to the past (Speth et al., 2016). Both psilocybin (Lebedev et al., 2015) and LSD (Lebedev et al., 2016) were shown to increase global brain entropy. These findings all support a breakdown of long-distance communication in brain regions involved in the medial DMN.
Additional evidence for the breakdown of long-distance communication in the brain comes from studies using electroencephalography (EEG) or magnetoencephalography (MEG) that demonstrate changes in electro-cortical activity induced by classic hallucinogens. Slow or low-frequency electro-cortical oscillations (e.g. delta: 1–4 Hz; theta: 4–8 Hz, alpha: 8–16Hz) are often shown to coordinate communication of distant neural areas, whereas faster or higher-frequency oscillations (e.g. gamma: 32+ Hz) are often shown to coordinate local synchronization of a small number of neurons within limited populations (von Stein & Sarnthein, 2000; Buzsaki, Logothetis, & Singer, 2013). An absolute decrease in oscillatory power in all frequency bands was observed in frontal areas using EEG during the administration of ayahuasca (Riba et al., 2002), and with MEG during the administration of psilocybin (Muthukumaraswamy et al., 2013). With ayahuasca, this decrease was accompanied by an increase in the ratio of high to low frequency oscillatory power (Riba et al., 2002), suggesting that low-frequency oscillatory power is more strongly decreased than high-frequency oscillatory power. With psilocybin, despite the decrease in power, stimulus-induced high frequency oscillations were preserved (Muthukumaraswamy et al., 2013). Reduced synchronization of cortical oscillations in the PCC and PHC has been shown to correlate with a measure of spiritual experience during the effects of oral psilocybin (Kometer et al., 2015). Taken together, evidence in BOLD and electro-cortical signals suggest an alteration of long-distance communication in the brain and a relative preservation of local and basic perceptual processing.
After psilocybin, oscillatory power decreases in all frequency bands in the PCC correlated with ratings of “disintegration of the self”, with alpha power decreases correlating most strongly (Muthukumaraswamy et al., 2013). These power decreases in the PCC were consistent with the excitation of layer 5 pyramidal neurons, which densely express 5HT2A receptors, the primary site of action of classic hallucinogens. The negative correlation between alpha power and ratings of disintegration of self is notable because alpha power is positively correlated with perceptual framing, self-reflection, and introspection (Carhart-Harris et al., 2014). These findings may be relevant to mystical experiences insofar as a sense of disintegration of self is a primary aspect of the experience of introspective unity, a defining feature of the mystical experience.
With regard to meditation, amplitude of spontaneous fluctuations in the DMN have been shown to be reduced during mindfulness meditation (Berkovich-Ohana et al., 2016), and consistent deactivations of the MPFC, PCC, and IPL have been found during both focused awareness and open monitoring practices (Fox et al., 2016). Josipovic et al. (2011) demonstrated that while the typically observed negative correlations between the DMN (associated with internally directed attention) and task-positive networks (associated with externally directed attention) are maintained during a focused awareness practice and during a simple visual perception task, this negative correlation is significantly decreased during a non-dual awareness meditation practice, which may be associated with mystical-type experiences. Decreased negative correlation between the DMN and task-positive networks, as well as decreased activations and decreased spontaneous fluctuations in BOLD signal, are changes in brain dynamics that one might expect to accompany an experience of unity (a critical dimension of mystical experiences) in which representations of internal and external objects of consciousness blend together. Interestingly, decreased negative correlations between DMN and task positive network areas in meditation are consistent with reports of increased resting-state functional connectivity between the DMN and various task-positive networks after intravenous psilocybin administration (Roseman, Leech, Feilding, Nutt, & Carhart-Harris, 2014), and increased global connectivity after LSD administration (Tagliazucchi et al., 2016). These decreases are also possibly consistent with both a breakdown of within-network functional connectivity in the DMN during the effects of psilocybin and LSD (i.e. between MPFC and PCC regions; Carhart-Harris et al., 2012; Carhart-Harris et al., 2014; Tagliazucchi et al., 2014; Carhart-Harris et al., 2016), increased brain entropy during the effects of psilocybin and LSD (Lebedev et al., 2015, 2016), and the development of new homological scaffolds in functional brain networks identified during the effects of psilocybin (Petri et al., 2014).
A very intriguing parallel between neuroimaging research on meditation (Brewer & Garrison, 2014) and neuroimaging research with classic hallucinogens (Carhart-Harris et al., 2014) involves the functional relevance that is attributed to change in the activity of the PCC. The PCC is implicated in “internally directed cognition” (Leech & Sharp, 2014), “‘getting caught up in’ one’s experience” (Brewer, Garrison, & Whitfield-Gabrieli, 2013), and “ego integrity” (Carhart-Harris et al., 2014). One could argue that activity in the PCC may reflect self-referential processing (i.e. an internal dialogue or chatter) that is quieted by meditation training. This is supported by research demonstrating that mind-wandering is associated with PCC activity (Mason et al., 2007). Meditators with many years of experience in mindfulness practice showed decreased activity in the DMN (specifically the MPFC and PCC; Figure 7, “Deactivations During Meditation”) compared to non-meditators when data were collapsed across practices including focused awareness and open monitoring meditations (Brewer et al., 2011). Subsequent studies used a neuro-feedback paradigm to demonstrate that decreased activity in the PCC correlated with decreased mind-wandering during focused awareness practice (Garrison, Santoyo, et al., 2013; Garrison, Scheinost, et al., 2013). This is generally consistent with observations after intravenous psilocybin administration showing decreased low frequency power and long-range temporal correlations in BOLD data in the PCC and MPFC (Tagliazucchi et al., 2014), substantially decreased effective connectivity in BOLD data between the PCC and MPFC (Carhart-Harris et al., 2012), and decreases in alpha oscillatory power in the PCC correlated with ratings of ego disintegration (Muthukumaraswamy et al., 2013). Considering all of the findings summarized above, it could be hypothesized that decreased activity and functional connectivity in the PCC and MPFC (medial nodes of the DMN) mediate aspects of the experience of introvertive unity (including decreased self-referential processing and a loss of sense of self) that is a key component of mystical experience, be that occasioned by classic hallucinogens or by meditation.
9.2. The Lateral Default Mode Network
The inferior parietal lobule (IPL) is one of the only lateral regions of the brain that is consistently identified as a node of the DMN across various techniques used to identify the DMN (Buckner, Andrews-Hanna, & Schacter, 2008). The IPL is a complex region that contains the anatomical locations of the angular gyrus (in the posterior IPL) and supramarginal gyrus (in the anterior IPL). The angular gyrus is variously implicated in spatial cognition (Amorapanth, WIdick, & Chatterjee, 2009), semantics and memory (Humphreys & Lambon Ralph, 2014), empathy, (Kubit & Jack, 2013), and identifying and tracking the intentions of others (Scholz, Triantafyllou, Whitfield-Gabrieli, Brown, & Saxe, 2009; Kubit & Jack, 2013). The supramarginal gyrus is implicated more generally in categorical information processing (Amorapanth et al., 2009), phonological information processing, attentional processing (Scholz et al., 2009; Humphreys & Lambon Ralph, 2014), target detection, and reorienting to salient stimuli (Kubit & Jack, 2013). Distributed regions in the IPL have also been implicated in the processing of time and temporal relationships (Battelli et al 2007, Bueti & Walsh, 2009).
The angular gyrus is the region of the IPL most consistently included in the DMN, whereas the supramarginal gyrus most consistently overlaps with task-positive networks (Kubit & Jack, 2013; Humphreys & Lambon Ralph, 2014). The precise functional role of the angular gyrus may depend on the regions that are co-active with it in a given context (Seghier, 2013). In the context of the DMN and self-referential processing, the angular gyrus may support spatial and temporal processing and the representation of the self as an individual in space and time.
With regard to meditation and mystical experience, d’Aquili and Newberg (2000) hypothesized that the experience of unity encountered in meditation and religious experiences is related to decreased activity in the posterior superior parietal lobe and IPL, consistent with alterations of the subjective experience of space and time. This relationship during meditation was confirmed using SPECT imaging in studies of Tibetan Buddhist meditators (Newberg et al., 2001). The IPL has also been implicated in the experience of unity outside of meditative practice. Patients undergoing parietal lobe surgery for resection of brain tumors completed a self-transcendence scale that included items that assess aspects of the mystical experience such as unity, timelessness, and spacelessness. The investigators reported that resection of the inferior posterior parietal lobes (the IPL in the left hemisphere, and specifically the angular gyrus in the right hemisphere) increased the experience of self-transcendence (Urgesi, Aglioti, Skrap, & Fabbro, 2010).
With regard to the classic hallucinogens, neuroimaging studies have reported changes in activity and functional connectivity of the IPL in response to these compounds. A decrease in regional cerebral blood flow in the inferior parietal cortex was identified after mescaline ingestion (Hermle et al., 1992), and an increase in glucose metabolism was identified in the lateral parietal cortex after psilocybin ingestion (Vollenweider et al., 1997). Decreased low frequency power in BOLD signal in the angular gyrus but not the supramarginal gyrus (Tagliazucchi et al., 2014) and decreased functional connectivity between the PCC and the IPL (likely the angular gyrus, though exact coordinates were not provided) was demonstrated (Carhart-Harris et al., 2012) after psilocybin injection.
With regard to electro-cortical findings, ayahuasca ingestion led to decreased current density in delta, alpha and beta frequency bands localized to both the angular gyrus and the supramarginal gyrus (Riba, Anderer, Jane, Saletu, & Barbanoj, 2004). Psilocybin ingestion led to a decrease in pre-stimulus alpha band oscillations in parieto-occipital regions as detected using EEG (Kometer, Schmidt, Jancke, & Vollenweider, 2013). Decreased power in delta, theta, alpha, and beta bands was detected with MEG over lateral parietal regions after psilocybin injection, specifically over the supramarginal gyrus, but the angular gyrus was not identified (Muthukumaraswamy et al., 2013).
Decreased activity in the IPL (and specifically in the angular gyrus), and decreased communication between the IPL and other areas involved in maintaining a sense of self (such as the PCC) is observed both in studies of meditation and in studies of classic hallucinogens. These observations are consistent with the experience of altered sense of time and space that has been reported with both meditation (d’Aquili & Newberg, 2000) and classic hallucinogens (Griffiths et al., 2006). Considering the findings summarized above, it could be hypothesized that decreased activity and functional connectivity in the IPL (a lateral node of the DMN) mediates the experience of timelessness and spacelessness that accompanies introvertive mystical experience, be that occasioned by meditation or by classic hallucinogens.
10. Toward a Neural Model of Mystical Experiences
The experience of unity that is central to mystical experiences involves a decrease in self-referential processing. There is compelling evidence for a network of brain areas (i.e. the nodes of the DMN) that are involved in self-referential processing and maintenance of a sense of the self in space and time. Decreased activity in these areas has been observed using multiple imaging modalities, both after administration of classic hallucinogens and during meditation practices. After classic hallucinogens, BOLD and electrocortical data show decreased activity and low-frequency power localized to DMN areas (Riba et al., 2002, 2004; Carhart-Harris et al., 2012, 2016; Muthukumaraswamy et al., 2013), which and may be related to a decrease in long-range neural communication between these and other brain areas. Alteration of the efficiency or fidelity of long-distance communication between nodes of the DMN after classic hallucinogens may be a neural mechanism underlying changes in activity in the DMN, and it may be a mechanistic change that is crucial to supporting mystical experiences.
Consistent with the general neural principle of low-frequency oscillations supporting long-range neural communication (von Stein & Sarnthein, 2000; Buzsaki et al., 2013), decreased long-distance and increased short-distance connections may reduce “small world” features of brain networks and increase either “random graph” features or “regular lattice” features (Bullmore & Sporns, 2009). In a “small world” network, most nodes only have a small number of connections to other nodes, and are generally not many connections away from any other node in the network. These features support efficient communication between nodes in large networks and they are nearly ubiquitous in natural complex systems (including healthy brains). Movement away from this small world architecture (e.g. toward “random graph” architecture, in which each node has equal probability of being connected directly to every other node) would represent a notable departure from typical brain organization. This is consistent with idea that psychedelics induce disintegration and desegregation of functional brain networks (Carhart-Harris et al., 2016). The PCC, MPFC, and IPL can be considered connector hubs that support the small-world architecture of the brain; thus, breakdown of long-distance connections between these nodes, or decreased integration and segregation of these nodes of the DMN, that is observed with meditation and classic hallucinogens may be a marker of altered brain state that is consistent with mystical experiences.
Despite the lack of a ground truth against which to definitively validate a mystical experience, states engendered by classic hallucinogens and meditation share phenomenological descriptions that are consistent with mystical experiences, and it is tempting to interpret the neural correlates of hallucinogens and meditation as a model of the neural correlates of mystical experiences. While a number of observations have been made with classic hallucinogens and meditation that are consistent with aspects of mystical experiences, these observations have not been explicitly linked to “complete” mystical experiences. Thus, we conclude with the following hypotheses that are not specific not to meditation or experience with classic hallucinogens per se, but rather are specific to mystical experiences occasioned by meditation or classic hallucinogens: 1) activity within the medial nodes of the DMN will decrease, and communication between these nodes and other cortical targets in associative and sensory processing cortex will be fundamentally altered, in support of decreased self-referential processing during introvertive mystical experiences; 2) activity within the lateral nodes of the DMN will decrease, and communication between these nodes and other cortical targets will be fundamentally altered, in support of the experience of timelessness and spacelessness experienced during introvertive mystical experiences; 3) long-distance cortical communication will generally decrease, while local sensory and associative processing is maintained to some degree, during mystical experiences; 4) “small-world” properties of the brain will decrease during mystical experiences.
11. Conclusion
Naturally-occurring mystical experiences (Stace, 1960a) as well as the ceremonial use of classic hallucinogens (Schultes et al., 2001) have long histories that in some cases are complementary and may be intertwined (Osmond, 1970; Dobkin De Rios, 1984; Schultes et al., 2001). Certainly, not all experiences with classic hallucinogens are of the mystical type. However, with the proper setting, preparation, support, and dose of a classic hallucinogen, mystical experiences are occasioned at high probability (Griffiths et al., 2011). Post-session and follow-up ratings of mystical experience and positive impact of psilocybin session experiences are dose dependent (Griffiths et al., 2011) and sustained at follow-up periods ranging from several weeks to 25 years (Pahnke, 1963; Doblin, 1991; Griffiths et al., 2006, 2008, 2011). Hallucinogen-occasioned mystical experiences are pharmacologically specific (i.e. occur at much higher frequency after a high dose of psilocybin than after placebo, low doses of psilocybin, methylphenidate, or nicotinic acid; Griffiths et al., 2006; Pahnke, 1963) and not generally due to expectancy (i.e. demonstrated under conditions that obscured the range of drug conditions administered and not shown with placebo or a low dose of psilocybin Griffiths et al., 2006, 2011). Mystical experiences have a clear operational definition (MacLean et al., 2012; Barrett, Johnson, & Griffiths, 2015), and the positive outcomes associated with mystical experiences have been empirically demonstrated (Griffiths et al 2006, 2008, 2011; Bogenschutz et al 2015; Garcia-Romeu et al 2015).
Although the most fundamental questions regarding mystical experiences presently evade a reductive neuroscientific explanation, analysis of the biological correlates suggestive of underlying mechanisms of mystical experiences are tractable. We have highlighted an intriguing overlap in neural findings on classic hallucinogens and neural findings on meditative practices that may occasion mystical experiences. More specifically, changes in activity, connectivity, and neural oscillatory processes in regions of the default mode network may underlie dimensions of mystical experience, especially decreased self-referential processing and altered sense of time and space that accompany introvertive mystical experiences. Further research with classic hallucinogens as experimental tools can be expected to provide significant insights into both the neural mechanisms and well as the biological and behavioral consequences of mystical experiences. Such information may have important implications for developing a science of moral and ethical behavior (Shermer, 2015) as well as for developing novel therapeutic interventions for producing persisting positive behavioral and psychological changes.
Acknowledgements
This work was supported by R01DA003889 (PI: Griffiths) and a grant from the Heffter Research Institute. Dr. Barrett was also supported by T32DA007209 (PI: Bigelow). Dr. Griffiths is a board member of the Heffter Research Institute. The funding sources had no role in the writing of this report or the interpretation or communication of scientific findings summarized in this report.
References
- Aaronson BS & Osmond H (1970). Introduction: Psychedelics, Technology, Psychedelics In Aaronson BS & Osmond H (Eds.), Psychedelics: The Uses and Implications of Hallucinogenic Drugs (pp. 3–20). New York: Anchor Books. [Google Scholar]
- Amorapanth PX, WIdick P, & Chatterjee A (2009). The Neural Basis for Spatial Relations. Journal of Cognitive Neuroscience, 22(8), 1739–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony FV, Hermans CAM, & Sterkens C (2010). A Comparative Study of Mystical Experience Among Christian, Muslim, and Hindu Students in Tamil Nadu, India. Journal for the Scientific Study of Religion, 49(2), 264–277. [Google Scholar]
- Barrett FS, Johnson MJ, & Griffiths RR (2015). Validation of the revised mystical experiences questionnaire in experimental sessions with psilocybin. [DOI] [PMC free article] [PubMed]
- Battelli L, Pascual-Leone A, & Cavanagh P (2007). The ‘when’ pathway of the right parietal lobe. Trends in Cognitive Sciences, 11(5): 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogenschutz MP & Pommy JM (2012). Therapeutic mechanisms of classic halluciongens in the treatment of addictions: from indirect evidence to testable hypotheses. Drug Testing and Analysis, 4(543–555), 543. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa PCR, & Strassman RJ (2015). Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. Journal of Psychopharmacology. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang YY, Weber J, & Kober H (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci U S A, 108(50), 20254–20259. doi: 10.1073/pnas.1112029108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Garrison KA, & Whitfield-Gabrieli S (2013). What about the “Self” is Processed in the Posterior Cingulate Cortex?. Front Hum Neurosci, 7, 647. doi: 10.3389/fnhum.2013.00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA & Garrison KA (2014). The posterior cingulate cortex as a plausible mechanistic target of meditation: findings from neuroimaging. Ann N Y Acad Sci, 1307, 19–27. doi: 10.1111/nyas.12246 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci, 1124, 1–38. doi: 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Bueti D & Walsh V (2009). The parietal cortex and the representation of time, space, number and other magnitudes. Phil Trans R Soc B, 364, 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E & Sporns O (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci, 10(3), 186–198. doi: 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Logothetis N, & Singer W (2013). Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron, 80(3), 751–764. doi: 10.1016/j.neuron.2013.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, Tyacke RJ, Leech R, Malizia AL, Murphy K, Hobden P, Evans J, Feilding A, Wise RG, & Nutt DJ (2012). Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A, 109(6), 2138–2143. doi: 10.1073/pnas.1119598109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Leech R, Hellyer PJ, Shanahan M, Feilding A, Tagliazucchi E, Chialvo DR, & Nutt DJ (2014). The entropic brain: a theory of conscious states informed by neuroimaging research with psychedelic drugs. Front Hum Neurosci, 8, 20. doi: 10.3389/fnhum.2014.00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Hood RWJ, Qi W, & Watson PJ (2011a). Common Core Thesis and Qualitative and Quantitative Analysis of Mysticism in Chinese Buddhist Monks and Nuns. Journal for the Scientific Study of Religion, 50(4), 654–670. [Google Scholar]
- Chen Z, Hood RWJ, Yang L, & Watson PJ (2011b). Mystical Experience Among Tibetan Buddhists: The Common Core Thesis Revisited. Journal for the Scientific Study of Religion, 50(2), 328–338. [Google Scholar]
- Chen Z, Zhang Y, Hood RWJ, & Watson PJ (2012). Mysticism in Chinese Christians and Non-Christians: Measurement Invariance of the Mysticism Scale and Implications for the Mean Differences. International Journal for the Psychology of Religion, 2(2), 155–168. [Google Scholar]
- d’Aquili EG & Newberg AB (2000). The Neuropsychology of Aesthetic, Spiritual, and Mystical States. Zygon, 35(1), 39–51. [Google Scholar]
- Denny BT, Kober H, Wager TD, & Ochsner KN (2012). A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci, 24(8), 1742–1752. doi: 10.1162/jocn_a_00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich A (1998). The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry, 31, 80–84. Doi: 10.1055/s-2007-979351 [DOI] [PubMed] [Google Scholar]
- Dittrich A, Lamparter D, & Maurer M (2010) 5D-ASC: Questionnaire for the assessment of altered states of consciousness. A short introduction. Zurich, Switzerland: PSIN PLUS. [Google Scholar]
- Dobkin de Rios M (1984). Visionary Vine: Hallucinogenic Healing in the Peruvian Amazon. Prospet Heights, Illinois: Waveland Press. [Google Scholar]
- Dobkin de Rios M (1996). On “Human Pharmacology of Hoasca”: A Medical Anthropology Perspective. The Journal of Nervous and Mental Disease, 184(2), 95–98. [DOI] [PubMed] [Google Scholar]
- Doblin R (1991). Pahnke’s “Good Friday Experiment”: A Long-Term Follow-up and Methodological Critique. The Journal of Transpersonal Psychology, 23(1), 1–28. [Google Scholar]
- Doucet G, Naveau M, Petit L, Delcroix N, Zago L, Crivello F, Jobard G, Tzourio-Mazoyer N, Mazoyer B, Mellet E, & Joliot M (2011). Brain activity at rest: a multiscale hierarchical functional organization. J Neurophysiol, 105(6), 2753–2763. doi: 10.1152/jn.00895.2010 [DOI] [PubMed] [Google Scholar]
- El-Seedi HR, De Smet PAGM, Beck O, Possnert G, & Bruhn JG (2005). Prehistoric peyote use: Alkaloid analysis and radiocarbon dating of archaeological specimens of Lophophora from Texas. Journal of Enthnopharmacology, 101, 238–242. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A, 102(27), 9673–9678. doi: 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsson A (2010). Strong experiences with music Handbook of Music and Emotion. Oxford University Press. [Google Scholar]
- Garcia-Romeu A, Griffiths RR, & Johnson MW (2015). Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr Drug Abuse Rev, 7(3), 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Santoyo JF, Davis JH, Thornhill T. A. t., Kerr CE, & Brewer JA (2013). Effortless awareness: using real time neurofeedback to investigate correlates of posterior cingulate cortex activity in meditators’ self-report. Front Hum Neurosci, 7, 440. doi: 10.3389/fnhum.2013.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Scheinost D, Worhunsky PD, Elwafi HM, Thornhill T. A. t., Thompson E, Saron C, Desbordes G, Kober H, Hampson M, Gray JR, Constable RT, Papademetris X, & Brewer JA (2013). Real-time fMRI links subjective experience with brain activity during focused attention. Neuroimage, 81, 110–118. doi: 10.1016/j.neuroimage.2013.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Steele JD, Lawrie SM, Frith CD, & Burgess PW (2006). Functional specialization within rostral prefrontal cortex (area 10): a meta-analysis. J Cogn Neurosci, 18(6), 932–948. doi: 10.1162/jocn.2006.18.6.932 [DOI] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Schreckenberger M, Sabri O, Arning C, Thelen B, Spitzer M, Kovar KA, Hermle L, Büll U, & Sass H (1999). Neurometabolic effects of psilocybin, 3,4-methylenedioxyethylamphetamine (MDE) and d-methamphetamine in healthy volunteers. A double-blind, placebo-controlled PET study with [18F]FDG. Neuropsychopharmacology, 20(6), 565–581. doi: 10.1016/S0893-133X(98)00089-X [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, & Jesse R (2006). Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology (Berl), 187(3), 268–283; discussion 284–292. doi: 10.1007/s00213-006-0457-5 [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Richards W, Johnson M, McCann U, & Jesse R (2008). Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol, 22(6), 621–632. doi: 10.1177/0269881108094300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, & Jesse R (2011). Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl), 218(4), 649–665. doi: 10.1007/s00213-011-2358-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, Umbricht A, Richards WA, Richards BD, Cosimano MP, Klinedinst MA (2016). Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharm, 30(12): 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob CS, McKenna DJ, Callaway JC, Brito GS, Neves ES, Oberlaender G, Saede OL, Labigalini E, Tacla C, Miranda CT, Strassman RJ, & Boone KB (1996). Human Psychopharmacology of Hoasca, A Plant Hallucinogen Used in Ritual Context in Brazil. The Journal of Nervous and Mental Disease, 184(2), 86–94. [DOI] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, Hagerty M, McKay CR, Halberstadt AL, & Greer GR (2011). Pilot Study of Psilocybin Treatment of Anxiety in Patients with Advanced-Stage Cancer. Arch Gen Psychiatry, 68(1), 71–78. [DOI] [PubMed] [Google Scholar]
- Guzmán G (1983). The Genus Psilocybe: A Systematic Revision of the Known Species Including the History, Distribution and Chemistry of the Hallucinogenic Species. Cramer, Vaduz, Germany: Nova Hedwigia Heft 74. [Google Scholar]
- Guzmán G (2008). Hallucinogenic Mushrooms in Mexico: An Overview. Economic Botany, 62(3), 404–412. [Google Scholar]
- Halpern JH, Sherwood AR, Hudson JI, Yurgelun-Todd D, & Pope HG Jr. (2005). Psychological and cognitive effects of long-term peyote use among Native Americans. Biol Psychiatry, 58(8), 624–631. doi: 10.1016/j.biopsych.2005.06.038 [DOI] [PubMed] [Google Scholar]
- Hermle L, Fünfgeld M, Oepen G, Botsch H, Borchardt D, Gouzoulis E, Fehrenbach RA, & Spitzer M (1992). Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biol Psychiatry, 32(11), 976–991. [DOI] [PubMed] [Google Scholar]
- Hood RWJ (1975). The construction and preliminary validation of a measure of reported mystical experience. Journal for the Scientific Study of Religion(14), 29–41. [Google Scholar]
- Hood RWJ, Morris RJ, & Watson PJ (1990). Quasi-experimental elicitation of differential report of religious experience among intrinsic and indiscriminately pro-religious types. Journal for the Scientific Study of Religion, 29(2), 164–172. [Google Scholar]
- Hood RW, & Williamson WP (2000). An Empirical Test of the Unity Thesis: The Structure of Mystical Descriptors in Various Faith Samples. Journal of Psychology and Christianity, 19(3), 232–244. [Google Scholar]
- Hood RWJ, Ghorbani N, Watson PJ, Ghramaleki AF, Bing MN, Davidson HK, Morris RJ, & Williamson WP (2001). Dimensions of the Mysticism Scale: Confirming the Three-Factor Structure in the United States and Iran. Journal for the Scientific Study of Religion, 40(4), 691–705. [Google Scholar]
- Hood RWJ (2003). Conceptual and Empirical Consequences of the Unity Thesis In Belzen JA and Geels A (Eds), Mysticism: A variety of psychological perspectives. New York: Rodopi. [Google Scholar]
- Hood RWJ (2006). The Common Core Thesis in the Study of Mysticism Where God and Science Meet (Vol. 3, pp. 119–138). Westport: Praeger. [Google Scholar]
- Hood RWJ (2009). Mysticism In Hood RWJ, Hill PC & Spilka B (Eds.), The Psychology of Religion (4th Edition ed.). New York: The Guilford Press. [Google Scholar]
- Humphreys GF, & Lambon Ralph MA (2014). Fusion and Fission of Cognitive Functions in the Human Parietal Cortex. Cereb Cortex. doi: 10.1093/cercor/bhu198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A (1947). The Perennial Philosophy. London: Chatto & Windus. [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, & Griffiths RR (2014). Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. J Psychopharmacol, 28(11), 983–992. doi: 10.1177/0269881114548296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josipovic Z (2010). Duality and nonduality in meditation research. Consciousness and Cognition, 19, 1119–1121. [DOI] [PubMed] [Google Scholar]
- Josipovic Z, Dinstein I, Weber J, & Heeger DJ (2011). Influence of meditation on anti-correlated networks in the brain. Front Hum Neurosci, 5, 183. doi: 10.3389/fnhum.2011.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josipovic Z (2014). Neural correlates of nondual awareness in meditation. Ann N Y Acad Sci, 1307, 9–18. doi: 10.1111/nyas.12261 [DOI] [PubMed] [Google Scholar]
- Katz ST (Ed.) (1978). Mysticism and philosophical analysis. New York: Oxford University Press. [Google Scholar]
- Katz ST (Ed.) (1983). Mysticism and religious traditions. New York: Oxford University Press. [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, & Heatherton TF (2002). Finding the self? An event-related fMRI study. J Cogn Neurosci, 14(5), 785–794. doi: 10.1162/08989290260138672 [DOI] [PubMed] [Google Scholar]
- Kometer M, Schmidt A, Jancke L, & Vollenweider FX (2013). Activation of serotonin 2A receptors underlies the psilocybin-induced effects on alpha oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci, 33(25), 10544–10551. doi: 10.1523/JNEUROSCI.3007-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubit B, & Jack AI (2013). Rethinking the role of the rTPJ in attention and social cognition in light of the opposing domains hypothesis: findings from an ALE-based meta-analysis and resting-state functional connectivity. Front Hum Neurosci, 7, 323. doi: 10.3389/fnhum.2013.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, & Sharp DJ (2014). The role of the posterior cingulate cortex in cognition and disease. Brain, 137(Pt 1), 12–32. doi: 10.1093/brain/awt162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, & Davidson RJ (2008). Attention regulation and monitoring in meditation. Trends in Cognitive Sciences, 12(4), 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Leoutsakos JM, Johnson MW, & Griffiths RR (2012). Factor Analysis of the Mystical Experience Questionnaire: A Study of Experiences Occasioned by the Hallucinogen Psilocybin. J Sci Study Relig, 51(4), 721–737. doi: 10.1111/j.1468-5906.2012.01685.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majic T, Schmidt TT, & Gallinat J (2015). Peak experiences and the afterglow phenomenon: When and how do therapeutic effects of hallucinogens depend on psychedelic experiences? Journal of Psychopharmacology. doi: 10.1177/0269881114568040 [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, & Macrae CN (2007). Wandering minds: the default network and stimulus-independent thought. Science, 315(5810), 393–395. doi: 10.1126/science.1131295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, & C’de Baca J (2001). Quantum Change. New York: Guilford Press. [Google Scholar]
- Muthukumaraswamy SD, Carhart-Harris RL, Moran RJ, Brookes MJ, Williams TM, Errtizoe D, Sessa B, Papadopoulos A, Bolstridge M, Singh KD, Feilding A, Friston KJ, & Nutt DJ (2013). Broadband cortical desynchronization underlies the human psychedelic state. J Neurosci, 33(38), 15171–15183. doi: 10.1523/JNEUROSCI.2063-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JD & Newberg A (2013). Toward a unifying taxonomy and definition for meditation. Frontiers in Psychology, 4(806), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberg A, Alavi A, Baime M, Pourdehnad M, Santanna J, & d’Aquili E (2001). The measurement of regional cerebral blood flow during the complex cognitive task of meditation: a preliminary SPECT study. Psychiatry Res, 106(2), 113–122. [DOI] [PubMed] [Google Scholar]
- Newberg AB & Iversen J (2003). The neural basis of the complex mental task of meditation: neurotransmitter and neurochemical considerations. [Review]. Med Hypotheses, 61(2), 282–291. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Wintering NA, Morgan D, & Waldman MR (2006). The measurement of regional cerebral blood flow during glossolalia: a preliminary SPECT study. Psychiatry Res, 148(1), 67–71. doi: 10.1016/j.pscychresns.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Newberg AB, Wintering N, Waldman MR, Amen D, Khalsa DS, & Alavi A (2010). Cerebral blood flow differences between long-term meditators and non-meditators. Conscious Cogn, 19(4), 899–905. doi: 10.1016/j.concog.2010.05.003 [DOI] [PubMed] [Google Scholar]
- Nichols DE (2004). Hallucinogens. Pharmacol Ther, 101(2), 131–181. doi: 10.1016/j.pharmthera.2003.11.002 [DOI] [PubMed] [Google Scholar]
- Nichols DE (2016). Psychedelics. Pharmacol Rev, 68, 264–355. doi: 10.1124/pr.115.011478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, & Panksepp J (2006). Self-referential processing in our brain--a meta-analysis of imaging studies on the self. Neuroimage, 31(1), 440–457. doi: 10.1016/j.neuroimage.2005.12.002 [DOI] [PubMed] [Google Scholar]
- Osmond H (1970). Peyote Night In Aaronson B & Osmond H (Eds.), Psychedelics: The Uses and Implications of Hallucinogenic Drugs (pp. 67–85). Garden City: Anchor Books. [Google Scholar]
- Pahnke WN (1963). Drugs and mysticism: An analysis of the relationship between psychedelic drugs and the mystical consciousness. Cambridge, MA: Harvard University Press. [Google Scholar]
- Pahnke WN (1967). LSD and religious experience In DeBold RC & Leaf RC (Eds.), LSD man & society. Middletown, CT: Wesleyan University Press. [Google Scholar]
- Pahnke WN (1969). Psychedelic drugs and mystical experience. Int Psychiatry Clin, 5, 149–162. [PubMed] [Google Scholar]
- Penman J & Becker J (2009). Religious Ecstatics, “Deep Listeners,” and Musical Emotion. Empirical Musicology Review, 4(2), 49–70. [Google Scholar]
- Petri G, Expert P, Turkheimer F, Carhart-Harris R, Nutt D, Hellyer PJ, & Vaccarino F (2014). Homological scaffolds of brain functional networks. J R Soc Interface, 11(101), 20140873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AG, Laumann TO, Miezin FM, Schlaggar BL, & Petersen SE (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. doi: 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot W (1985). Religious experience. Berkeley, CA: University of California Press. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME & Snyder AZ (2007). A default mode of brain function: A brief history of an evolving idea. NeuroImage, 37, 1083–1090. [DOI] [PubMed] [Google Scholar]
- Renes RA, van Haren NE, Aarts H, & Vink M (2014). An exploratory fMRI study into inferences of self-agency. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nsu106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Anderer P, Morte A, Urbano G, Jane F, Saletu B, & Barbanoj MJ (2002). Topographic pharmaco-EEG mapping of the effects of the South American psychoactive beverage ayahuasca in healthy volunteers. Br J Clin Pharmacol, 53(6), 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riba J, Anderer P, Jane F, Saletu B, & Barbanoj MJ (2004). Effects of the South American psychoactive beverage ayahuasca on regional brain electrical activity in humans: a functional neuroimaging study using low-resolution electromagnetic tomography. Neuropsychobiology, 50(1), 89–101. doi: 10.1159/000077946 [DOI] [PubMed] [Google Scholar]
- Riba J, Romero S, Grasa E, Mena E, Carrio I, & Barbanoj MJ (2006). Increased frontal and paralimbic activation following ayahuasca, the pan-Amazonian inebriant. Psychopharmacology (Berl), 186(1), 93–98. doi: 10.1007/s00213-006-0358-7 [DOI] [PubMed] [Google Scholar]
- Richards WA, Rhead JC, DiLeo FB, Yensen R, & Kurland AA (1977). The peak experience variable in DPT-assisted psychotherapy with cancer patients. J Psychedelic Drugs, 9, 1–10. [Google Scholar]
- Richards WA (2014). The Rebirth of Research with Entheogens: Lessons from the Past and Hypotheses for the Future In Ellens JH (Ed.), Seeking the Sacred with Psychoactive Substances: Chemical Paths to Spirituality and to God (Vol. 2, pp. 1–14). Santa Barbara: Praeger. [Google Scholar]
- Roberts TB (Ed.). (2001). Psychoactive Sacramentals: Essays on Entheogens and Religion. San Francisco: Council on Spiritual Practices. [Google Scholar]
- Roseman L, Leech R, Feilding A, Nutt DJ, & Carhart-Harris RL (2014). The effects of psilocybin and MDMA on between-network resting state functional connectivity in healthy volunteers. Front Hum Neurosci, 8, 204. doi: 10.3389/fnhum.2014.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, Agin-Liebes G, Malone T, Cohen B, Mennenga SE, Belser A, Kalliontzi K, Babb J, Su., Zhe., Corby P, Schmidt BL (2016). Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. J Psychopharm, 30(12): 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Triantafyllou C, Whitfield-Gabrieli S, Brown EN, & Saxe R (2009). Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PLoS One, 4(3), e4869. doi: 10.1371/journal.pone.0004869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultes RE (1969). Hallucinogens of plant origin. Science, 163, 245–254. [DOI] [PubMed] [Google Scholar]
- Schultes RE, Hofmann A, & Rätsch C (2001). Plants of the Gods: Their Sacred, Healing, and Hallucinogenic Powers: Healing Arts Press. [Google Scholar]
- Seghier ML (2013). The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist, 19(1), 43–61. doi: 10.1177/1073858412440596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf RH (1988). Experience In Taylor M (Ed), Critical Terms for Religious Studies (pp. 94–116). Chicago: University of Chicago Press. [Google Scholar]
- Shermer M (2015). The Moral Arc: How science and reason lead humanity toward truth, justice, and freedom. New York: Henry Holt and Co. [Google Scholar]
- Smith H (2000). Cleansing the doors of perception: the religious significance of entheogenic plants and chemicals. New York: Tarcher/Putnam. [Google Scholar]
- Stace WT (1960a). Mysticism and philosophy. New York: MacMillan Press. [Google Scholar]
- Stace WT (1960b). The teachings of the mystics: New American Library. [Google Scholar]
- Studerus E, Gamma A, & Vollenweider FX (2010). Psychometric Evaluation of the Altered States of Consciousness Rating Scale (OAV). PLoS One 5(8):e12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, & Levine B (2006). The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia, 44(12), 2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, Carhart-Harris R, Leech R, Nutt D, & Chialvo DR (2014). Enhanced repertoire of brain dynamical states during the psychedelic experience. Hum Brain Mapp, 35(11), 5442–5456. doi: 10.1002/hbm.22562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupper KW (2008). The globalization of ayahuasca: Harm reduction or benefit maximization? International Journal of Drug Policy, 19, 297–303. [DOI] [PubMed] [Google Scholar]
- Underhill E (1911 [2009]). Mysticism: Evinity Publishing. [Google Scholar]
- Urgesi C, Aglioti SM, Skrap M, & Fabbro F (2010). The spiritual brain: selective cortical lesions modulate human self-transcendence. Neuron, 65(3), 309–319. doi: 10.1016/j.neuron.2010.01.026 [DOI] [PubMed] [Google Scholar]
- van Veluw SJ & Chance SA (2014). Differentiating between self and others: an ALE meta-analysis of fMRI studies of self-recognition and theory of mind. Brain Imaging Behav, 8(1), 24–38. doi: 10.1007/s11682-013-9266-8 [DOI] [PubMed] [Google Scholar]
- Vollenweider FX & Kometer M (2010). The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nature Reviews Neuroscience, 11, 642–651. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, & Angst J (1997). Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology, 16(5), 357–372. doi: 10.1016/S0893-133X(96)00246-1 [DOI] [PubMed] [Google Scholar]
- von Stein A & Sarnthein J (2000). Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol, 38(3), 301–313. [DOI] [PubMed] [Google Scholar]
- Wasson RG, Hofman A, & Ruck CAP (1998). The Road to Eleusis: Unveiling the Secret of the Mysteries. Los Angeles: Hermes Press. [Google Scholar]
- Watts A (1970). Psychedelics and Religious Experience In Aaronson B & Osmond H (Eds.), Psychedelics: The Uses and Implications of Hallucinogenic Drugss (pp. 131–144). New York: Anchor Books. [Google Scholar]
- Westermeyer J (1988). The Pursuit of Intoxication: Our 100 Century-Old Romance with Psychoactive Substances. Am J. Drug Alcohol Abuse, 14(2), 175–187. [DOI] [PubMed] [Google Scholar]
- Wulff DM (1991). Psychology of religion; classic and contemporary views. New York: Wiley. [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, & Buckner RL (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol, 106(3), 1125–1165. doi: 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]