Abstract
Background:
Treatment with opioid agonists is effective for opioid use disorder, but early discontinuation of treatment is a major obstacle to success. Intensive longitudinal methods — which take many repeated measurements over time, usually in the field— have provided unique insight into the effects of stress, mood and craving on drug use while people are being treated; these methods might also be useful for studying the processes that lead people to drop out of treatment.
Methods:
Ecological momentary assessment (EMA) was conducted for up to 17 weeks by obtaining multiple electronic diary entries per day from 238 participants being treated with methadone or buprenorphine-naloxone. Survival analysis was used to study two outcomes: dropping out of treatment and noncompliance with EMA self-report requirements. Self-reports of stress, craving, and mood were used as time-varying predictors. Demographic and psychosocial variables measured with the Addiction Severity Index at the start of treatment were used as time-invariant predictors.
Results:
Dropping out of treatment was more likely in participants with more reported hassles (a measure of stress), higher levels of cocaine craving, lower levels of positive mood, a recent history of emotional abuse, a recent history of being bothered frequently by psychological problems, and with buprenorphine rather than methadone as their medication. In contrast, study noncompliance was not significantly associated with any of the variables analyzed.
Conclusions:
Assessment of stress, craving and mood during treatment might identify people who are at greater risk of dropping out, and therapeutic interventions targeting these processes might increase retention.
Keywords: Ecological Momentary Assessment, Opioid Use Disorder, Dropout, Noncompliance, Buprenorphine, Methadone
1. Introduction
Opioid use, addiction, and overdose are epidemic in the United States, and science-based treatments for addiction offer essential tools for combatting this crisis (Epstein et al., 2018). Available treatments for opioid use disorder include counseling, intensive treatment in a residential setting, twelve-step programs, contingency management, opioid agonist or antagonist medication, and combinations of these treatments (Ainscough et al., 2017; Galanter, 2018; Mumba et al., 2018). Among these, the opioid agonist medications methadone and buprenorphine are considered to be the best available treatments (Mattick et al., 2014; Volkow et al., 2014); they can decrease mortality, drug use, drug craving, and criminal activity (Bart, 2012; Salsitz and Wiegand, 2016) and increase quality of life (Mitchell et al., 2015). However, early dropout from treatment is a major obstacle to obtaining such benefits (Hser et al., 2014). We know that stress, mood and craving are influential factors that determine whether people will use drugs such as opioids and cocaine while they are in methadone or buprenorphine treatment (Furnari et al., 2015; Marhe et al., 2013; Preston et al., 2018a), but it is unclear whether these same factors are important in determining who will drop out of treatment.
Dropout from treatment has sometimes been found to be more prevalent for buprenorphine than methadone (Hser et al., 2014; Mattick et al., 2014), especially early in treatment. For example, previous studies have found lower retention for buprenorphine than methadone over the first 4 weeks (but not 12 weeks) (Gerra et al., 2004), or the first 24 weeks (Hser et al., 2014) when treatments were randomly assigned, or over the first 50 days when compared across cohorts without random assignment (Gryczynski et al., 2013).
Basic demographic variables such as race and sex have been studied extensively but do not reliably predict dropout from addiction treatment (Brorson et al., 2013; Damian et al., 2017), with the possible exception that younger people tend to be at higher risk of dropping out. However, there is evidence that dropping out is affected by psychosocial variables such as mental distress (Andersson et al., 2018; Dobkin et al., 2002), anxiety sensitivity (Lejuez et al., 2008), and co-occurring psychiatric diagnoses (Amodeo et al., 2008; Brorson et al., 2013). For example, Daughters et al. (2009) found that people who were more reactive to a standardized stressor prior to starting treatment for substance use disorder were more likely to drop out of treatment early. If craving and stress increase the risk of dropping out, it could be beneficial to identify and target these symptoms early and throughout treatment to increase retention.
The relationship between psychosocial variables and behavior can be studied effectively using ecological momentary assessment (EMA), in which people make electronic diary entries over an extended length of time (Bolger and Laurenceau, 2013; Lukasiewicz et al., 2007; Shiffman et al., 2008). In our research, EMA has been critical for establishing the effects of mental states on drug use during treatment with opioid agonist medications (Epstein et al., 2009; Furnari et al., 2015; Kowalczyk et al., 2018; Preston et al., 2018a, b). However, to our knowledge, intensive longitudinal methods such as EMA have not been used to study retention in treatment for substance use disorder. Based on the rationale that the richly-detailed information obtained with EMA can provide unique insight into the effects of fluctuating psychological variables, our aim was to determine whether stress, craving, and mood contribute to early dropout from methadone and buprenorphine treatment. We also consider the characteristics of another group of participants who did not choose to drop out of treatment but whose study participation was terminated because they were unable to comply with the demands of our EMA protocol.
2. Methods
2.1. Participants
The participants (N=238) were part of an intensive longitudinal study (Bolger and Laurenceau, 2013; Ginexi et al., 2014) of stress, drug craving, and environment-related influences on drug use during treatment for opioid use disorder in Baltimore, Maryland (NTC-00787423). The study used a natural-history design and had a primary objective of using smartphones as electronic diaries to obtain measures of stress and drug use in an outpatient treatment population. The present paper describes a secondary analysis; other analyses have been published previously (Epstein et al., 2009; Furnari et al., 2015; Moran et al., 2018; Preston et al., 2017, 2018a, b; Preston et al., 2018c); the present analysis includes all participants who were included in these previous reports. The study had two arms (“Office-Based Opioid Treatment (OBOT)” and “Methadone-Buprenorphine”) that used similar procedures; the substantive difference between the arms was the number of days participants were expected to visit the clinic (2 days/week vs. 5-7 days/week, respectively; this change was driven by budgetary/staffing concerns). Data from both arms were combined for analysis, with 81.5% of participants in the methadone-buprenorphine arm. All participants received either methadone (47%) or buprenorphine-naloxone (53%) at our research/treatment clinic. Buprenorphine-naloxone has been the standard of care for buprenorphine treatment for over a decade; the naloxone component is intended to deter misuse of the medicine by intravenous injection, but the naloxone dose has no appreciable effect when the medicine is used as directed (i.e., orally). For brevity, we simply refer to “buprenorphine” treatment when discussing our study or other studies that involved buprenorphine-naloxone or buprenorphine without naloxone. Participants in the Methadone-Buprenorphine arm visited the clinic to receive medication 5-7 days per week and provided urine samples for drug screening three times per week (Moran et al., 2018; Preston et al., 2017, 2018b). Participants in the OBOT arm visited the clinic twice a week to receive medication and provide urine samples. In both arms, administration of medication was observed when the participant was at the clinic, and take-home doses were provided for non-clinic days. Enrollment ran from July 2009 through September 2017. Individual participants received only methadone or only buprenorphine throughout the study, but which one they received was determined by 1) what we were offering at the time they were recruited; 2) their personal preference; and 3) the clinical judgement of the study physician. Due mostly to changes in budget and staffing, we offered different treatments during the years of the study: daily methadone when the study started, daily methadone and buprenorphine between August 2011 and August 2015, and only buprenorphine (in a twice-weekly OBOT model with take-home doses) after September 2015. Individuals who requested a medication that was not available at the time of recruitment were offered referral to a community treatment program. Medication doses were optimized for each participant to minimize withdrawal symptoms and prevent illicit opioid use without causing intolerable side effects. Data were analyzed from the maintenance phase of treatment (i.e., the period after the induction phase and before the optional the taper phase), which lasted 16-18 weeks depending on the duration of their induction and the arm of the study. At the end of the study, participants were assisted in transitioning to a community program or given an 8-week taper. During the study, some participants also participated in brief substudies that involved automated sensing devices, but these substudies did not disrupt treatment or the ongoing collection of EMA data.
The general demographics were: 66% African American and 78% male, with a mean (± SEM; i.e., standard error of the mean) of 43 (± 0.7) years of age, 14.3 (± 0.7) years of heroin use, and 6.4 (± 0.6) years of polydrug use (i.e., using more than one substance per day, including alcohol). Data from all participants who did not self-identify as African American were combined for analysis of race, with two self-identifying as Asian American and the rest as white. The study was reviewed and approved by the Addictions Institutional Review Board of the National Institutes of Health. Participants gave written informed consent before the study and were paid for completing the research components of the study. Small incentives were used to encourage compliance with EMA and discourage the use of opioids and cocaine. Specifically, participants received $10 per visit if their urine samples were negative and consistent with their EMA-reported drug use or $6 per visit if their samples were positive and consistent with their EMA-reported drug use. They could earn an additional $10 per week (methadone-buprenorphine arm) or $30 per week (OBOT arm) for completing at least 82% of randomly prompted EMA entries.
2.2. Data Collection
During a screening session, each participant completed the Addiction Severity Index (ASI) (McLellan et al., 1985), a semi-structured interview covering a wide range of psychosocial variables. We focus here on ASI items that pertain to the person’s history of drug use or to psychological/emotional problems such as depression and anxiety (see Table 1).
Table 1.
Variables used in modeling process.
| Demographics and Drug History | Units or Levels |
|---|---|
| Medication | buprenorphine, methadone |
| Race | African American, other |
| Sex | female, male |
| Age | years |
| Children | number |
| History of heroin use | years |
| History of >1 substance per day (including alcohol) | years |
| ASI Psychiatric Status and Family/ Social Relationships | Units or Levels |
| Abused by anyone emotionally in the past 30 days | yes, no |
| Days (in the past 30) experiencing psychological or emotional problems* | days |
| Participant rating of how troubled or bothered by psychological or emotional problems* | rating (1-5) |
| Participant rating of importance of treatment now for psychological problems* | rating (1-5) |
| Interviewer rating of need for treatment for these problems* | rating (0-6) |
| Stress EMA | Units |
| How much stress (random prompt) | rating (1-5) |
| Perceived Stress (end of day) | rating (0-20) |
| Hassles (end of day) | items endorsed (0-32) |
| Craving and Mood EMA | Units |
| Cocaine craving | rating (1-5) |
| Heroin craving | rating (1-5) |
| Positive mood | composite rating (1-5) |
| Negative mood | composite rating (1-5) |
refers to specific serious problems not directly resulting from drug use: depression, anxiety, hallucinations, trouble understanding, concentrating or remembering, trouble controlling violent behavior, thoughts of suicide, attempted suicide
ASI = Addiction Severity Index; EMA = ecological momentary assessment
One week after beginning methadone or buprenorphine, each participant was issued a smartphone or personal digital assistant and trained to use it as an electronic diary for collection of EMA. There were four kinds of EMA reports: 1) randomly-prompted reports on current mood, environment, activities, and levels of drug craving and stress; 2) participant-initiated, event-contingent reports of occasions involving more-than-usual stress (Preston et al., 2017); 3) participant-initiated, event-contingent reports of drug use (Furnari et al., 2015), which are not a focus of the analyses presented here; and 4) scheduled end-of-day reports (Preston et al., 2018c). During randomly prompted reports, self-ratings of 24 mood adjectives were taken and used to produce two composite scores (positive mood and negative mood) that were previously shown to be orthogonal (Epstein et al., 2014). Randomly-prompted reports also included Likert-scale ratings of heroin craving, cocaine craving, and how much stress the participant was feeling at the time of the prompt. In end-of-day reports, stress on that day was measured with five items from the Perceived Stress Scale (Cohen et al., 1983; Preston et al., 2018c); each item was measured on a Likert scale from 0-4, and the five scores were summed within each report to produce a composite measure. Participants also indicated whether they had experienced each of 32 potential hassles (e.g., money, housing, transportation, family, work) during the day (Preston et al., 2018c).
2.3. Outcomes
Participants were classified as reaching one of four outcomes: Completed, Dropped Out (of treatment), Noncompliant (with study requirements), or Other. Participants were considered to have Completed the study if they remained in the study for the designated length of their participation (16-18 weeks). The criteria for being classed as Dropped Out or Noncompliant were specified a priori in the study protocol and made known to the participants through the consent form they signed and the consent quiz they passed before joining the study. According to these criteria, participants were considered Dropped Out if they stopped coming to the clinic without arranging a taper or transferring to another agonist-treatment program; specifically, these participants missed 3 consecutive visits or any 12 visits at which urine samples or other data-collection procedures were scheduled (Methadone-Buprenorphine arm), or missed 4 visits to pick up their medication (OBOT arm). We have no further information about these participants after they left the study. Although the specific criteria (i.e., number of missed visits) for becoming Dropped Out differed between the two arms, in practice they were functionally equivalent because participants were repeatedly notified when they came close to meeting the criteria. Participants were deemed Noncompliant and removed from the study if they did not comply with EMA requirements; specifically, if they failed to make at least 82% of scheduled EMA reports (i.e., randomly-prompted and end-of-day reports) within a week for two weeks (not necessarily consecutive) or damaged or lost two of the electronic diaries that we provided. Participants who became Noncompliant were offered options of continuing to receive their medication (with the dose tapered over 21 days) or being transferred with our assistance to a community treatment program. Participants who Dropped Out are the main focus of this analysis, since they chose to discontinue treatment. Participants who were Noncompliant are also relevant to EMA research in general because they could shed light on why certain people have difficulty meeting the demands of an intensive longitudinal study. All participants who left the study early but could not be considered Dropped Out or Noncompliant made up the Other category; this included those who voluntarily transferred to a different treatment program or who reported specific life events that precluded their participation in the study, such as extended hospitalization, incarceration, or moving out of the Baltimore area. Since participants in the Other category left the study for a variety of reasons, they cannot be considered as a coherent group. However, they were still at risk for becoming Dropped Out up until the time that they left. Thus, as described below, data from participants in the Other category provide a necessary context for the survival analyses used to identify differences between participants who Dropped Out or became Noncompliant versus those who did not.
2.4. Statistical Analysis
Two kinds of analysis were performed. First, EMA, demographic and ASI variables were used to describe the general characteristics of participants in the Completed, Dropped Out and Noncompliant outcome classes. For this purpose, EMA variables were averaged for each participant, collapsed across the participant’s whole time in the study. Numeric variables were compared between outcome classes using ANOVA followed by paired comparisons, with familywise error controlled using the Holm procedure. Contingency tables of non-numeric variables were analyzed with χ2.
Second, survival analysis with Cox proportional hazards regression was used to identify characteristics that were associated with Dropping Out or becoming Noncompliant, focusing specifically on the time period just before they left the study. At each time point, survival analysis compares the participants who left the study at that point to all participants who were still in the study (i.e., still at risk of leaving). Dropping Out and becoming Noncompliant were treated as competing events (Noordzij et al., 2013), which involves performing the analysis once with the Dropped Out participants compared to all other participants and once with the Noncompliant participants compared to all others.
Demographic and ASI variables were treated as time-invariant predictors, and EMA variables were treated as time-dependent predictors (Therneau et al., 2014). Although EMA data were collected daily, the criteria for becoming Dropped Out or Noncompliant were based on failures that occurred over several days, so survival analysis was based on each participant’s EMA data averaged by week. Survival analysis was applied to a 17-week period. Participants who were enlisted for 16 weeks and completed the study were right-censored at that point in the survival analysis. No participants dropped out or became noncompliant after week 17.
All of the variables that we analyzed were selected because they are relevant to opioid use disorder and its treatment and because we judged them to be potentially relevant to dropout. Some of these variables overlap conceptually with each other (e.g., measuring related aspects of stress, craving or mood), which could be problematic if they were combined in a single model of survival. Therefore, we used a two-stage process to select a parsimonious set of predictors that work well together. In the first stage, four sets of plausible variables (see Table 1) were each processed with stepwise survival analysis (Heinze et al., 2018) to select a small set of candidate variables. In the second stage, all variables retained from the first stage were combined and processed with stepwise analysis to select the final model. Forward-backward stepwise analysis was performed using the MASS package (Modern Applied Statistics with S; R Core Team, 2018; Venables and Ripley, 2002) based on Akaike’s information criterion, which rewards goodness of fit (sensitivity) and penalizes model complexity (overfitting) (Dziak et al., 2012). Some variables retained in the final model are not individually significant, but controlling for their effects improves the precision of the estimates for the other predictors and contributes to interpretation of the model. The global statistical significance of the final model was tested with a likelihood ratio test. Validity of the proportional hazards assumption was confirmed by assessing the slopes of Schoenfeld residuals over time. Lack of multicollinearity was confirmed by assessing variance inflation factors. The discriminative validity of the model when applied to individual cases was measured as concordance (Harrell et al., 1996); considering all possible pairs of individuals, concordance is the proportion of pairs where the individual with the longer survival time is correctly identified by the model, with 0.5 indicating chance level and 1.0 indicating perfect discrimination. Results are reported as hazard ratios with 95% confidence intervals. Because hazard ratios are affected by the measurement units of the predictor, we also report standardized effect-size values (reffect) (Rosnow et al., 2000); this measure is useful for comparing effects within our results but has not been formally tested in the context of intensive longitudinal data and should be interpreted with caution in terms of cross-study comparisons.
3. Results
3.1. Overview of Outcomes
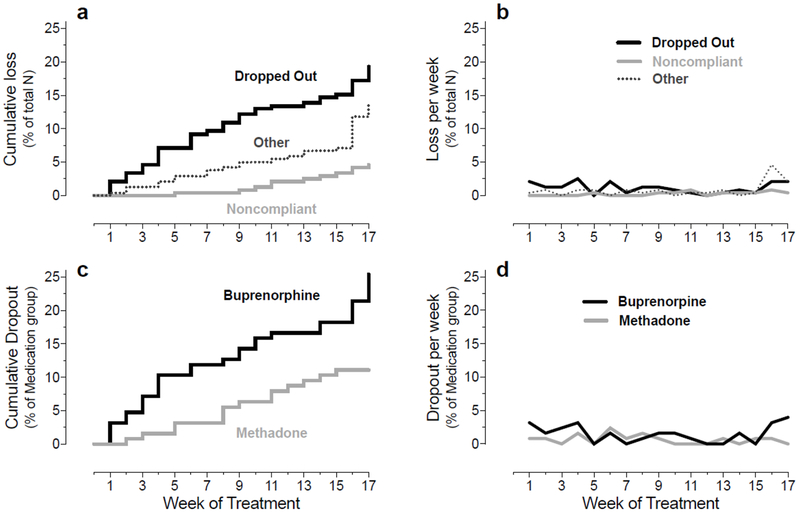
Most participants Completed the entire study (n=148; 62%), but substantial numbers Dropped Out (n=46; 19%) or left the study for reasons that placed them into the Other category (n=33; 14%). Fewer participants became Noncompliant with the study’s EMA requirements (n=11; 5%). Dropping Out occurred throughout the study (Figure 1a) but was slightly higher during the first six weeks and the last two weeks (Figure 1b). The percentage of participants who Dropped Out was higher within the buprenorphine group than within the methadone group (Figure 1c); this difference was observed not only during the earliest part of treatment but also during the last two weeks (Figure 1d).
Figure 1.
a: Cumulative loss of participants for each of the three outcome classes, expressed as a percentage of all participants who started the study.
b: Loss per week for each outcome class, expressed as a percentage of all participants who started the study.
c: Cumulative dropout within the buprenorphine group and within the methadone group, expressed as a percentage of participants who started the study in each medication group.
d. Dropout per week within the buprenorphine group and within the methadone group, expressed as a percentage of participants who started the study in each medication group.
3.2. Stress, Craving and Mood in EMA Reports
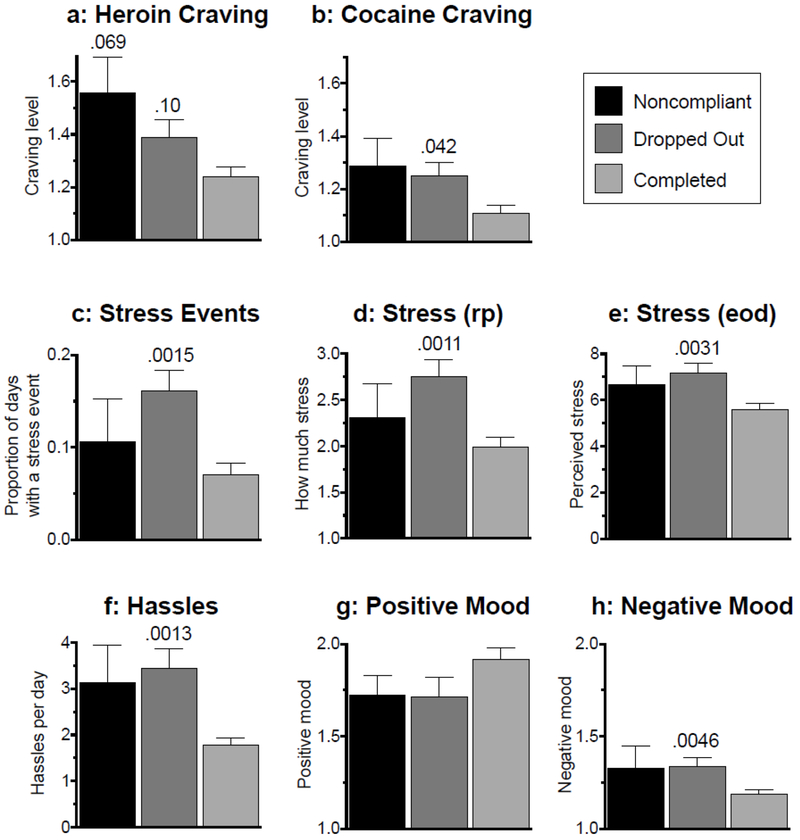
Averaged over time, levels of stress and craving were higher in participants who Dropped Out than in participants who Completed the study (Figure 2). The following F-tests refer to ANOVA results for each panel of Figure 2 or 3, and unless otherwise indicated, effect sizes refer to comparisons between Dropped Out and Completed participants; Holm-adjusted p-values for these comparisons are indicated in the figure. Dropped Out participants reported higher levels of heroin craving (Figure 2a; F2,202 = 4.2, p = .016; reffect = .25), cocaine craving (Figure 2b; F2,202 =4.0, p = .02; reffect = .17) and stress (Figure 2d; F2,200 = 6.59, p = .0017; reffect = .25) in randomly-prompted EMA entries. Dropped Out participants also initiated event-contingent stress reports more frequently (Figure 2c; F2.202 = 6.45, p = .002; reffect = .08) and reported more perceived stress (Figure 2e; F2,197 = 5.85, p = .0034; reffect = .25) and hassles (Figure 2f; F2,97 = 5.48, p = .001; reffect = .25) in end-of-day EMA entries. Positive mood did not differ significantly across outcome classes (Figure 2g), but negative mood was higher in Dropped Out participants compared to Completed participants (Figure 2h; F2,200 = 5.92, p = .0033; reffect = .22). In contrast to these differences between Dropped Out and Completed participants, differences between Noncompliant and Completed participants only approached significance for one EMA variable: heroin craving (Figure 2A; reffect = .16 for Noncompliant vs. Completed).
Figure 2.
Mean (+ SEM, i.e., standard error of the mean) values of ecological momentary assessment (EMA) variables within each outcome class, collapsed across weeks.
a: Level of heroin craving reported in randomly-prompted EMA.
b: Level of cocaine craving reported in randomly-prompted EMA.
c: Within-participant proportion of days that included a participant-initiated report of greater-than-usual stress.
d: Level of stress reported in randomly-prompted (“rp”) EMA, responding to the question, “How much stress are you feeling right now?”
e: Level of perceived stress reported in end-of-day (“eod”) EMA.
f. Number of hassles endorsed in end-of-day EMA, from a list of 32 items.
g: Level of positive mood reported in randomly-prompted EMA.
h: Level of negative mood reported in randomly-prompted EMA. Numeric values over bars represent Holm-adjusted p values for Noncompliant or Dropped Out compared to Completed.
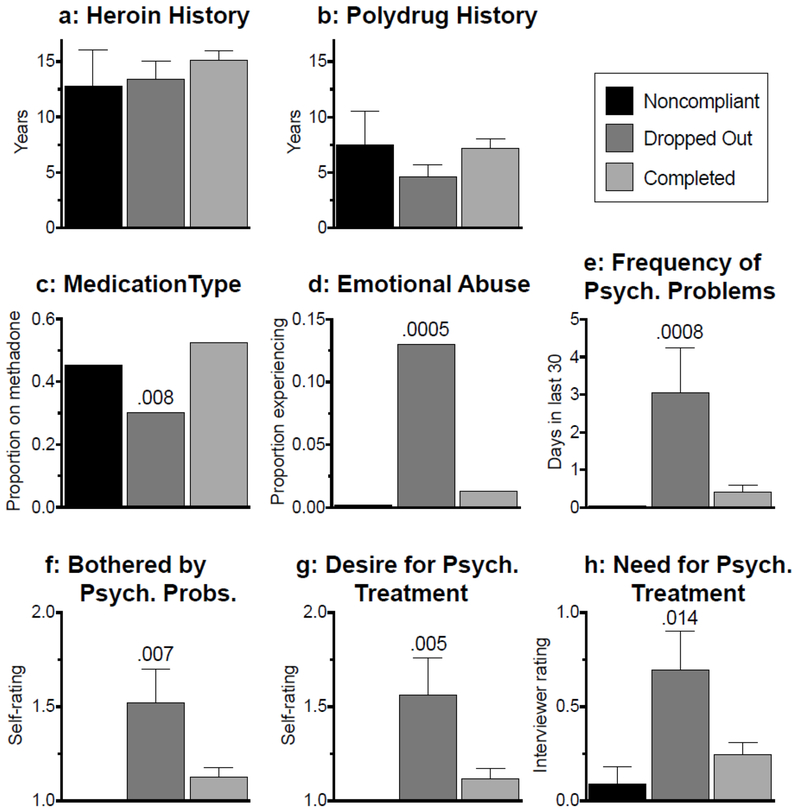
Figure 3.
Mean (± SEM) values of drug-history and psychosocial variables within each outcome class, as assessed at the beginning of the study.
a: Years of heroin use.
b: Years of polydrug use.
c: Proportion of participants who received methadone as opposed to buprenorphine.
d: Proportion of participants who responded “yes” to having been abused emotionally in the past 30 days.
e: Frequency of experiencing serious, non-drug-related psychological (“Psych.”) problems in the past 30 days.
f: Self-rating of how bothered the participant was by these psychological problems.
g: Self-rating of desire to be treated for these psychological problems.
h: Interviewer rating of the participant’s need to be treated for these psychological problems. Numeric values over bars represent Holm-adjusted p values for Noncompliant or Dropped Out compared to Completed.
3.3. Non-EMA Subject Variables
Participants’ histories of heroin use (Figure 3a) and poly drug use (Figure 3b) did not differ significantly across outcome classes, although Dropped Out participants tended to have fewer years of polydrug use. Unlike the other outcome classes, the Dropped Out participants had a preponderance of participants who were receiving buprenorphine rather than methadone (Figure 3c; χ22 = 7.0, p = .031). About 13% of Dropped Out participants reported being emotionally abused by someone during the 30 days before starting treatment, and this was 10-fold more prevalent than in Completed participants (Figure 3d; χ22 = 13.3, p = .0014). Dropped Out participants had a higher frequency of experiencing serious psychological problems during the 30 days before starting treatment (Figure 3e; F2,202 = 7.16, p = .001; reffect = .26). They were also more bothered by these problems (Figure 3f; F2,202 = 5.26, p = .006; reffect = .22), expressed a stronger desire to receive treatment for them (Figure 3g; F2.202 = 5.48, p = .005; reffect = .22), and were rated by interviewers as having a higher need for such treatment (Figure 3h; F2,202 = 4.51, p = .012; reffect = .2).
3.4. Survival Analysis: Variables Affecting Dropping Out
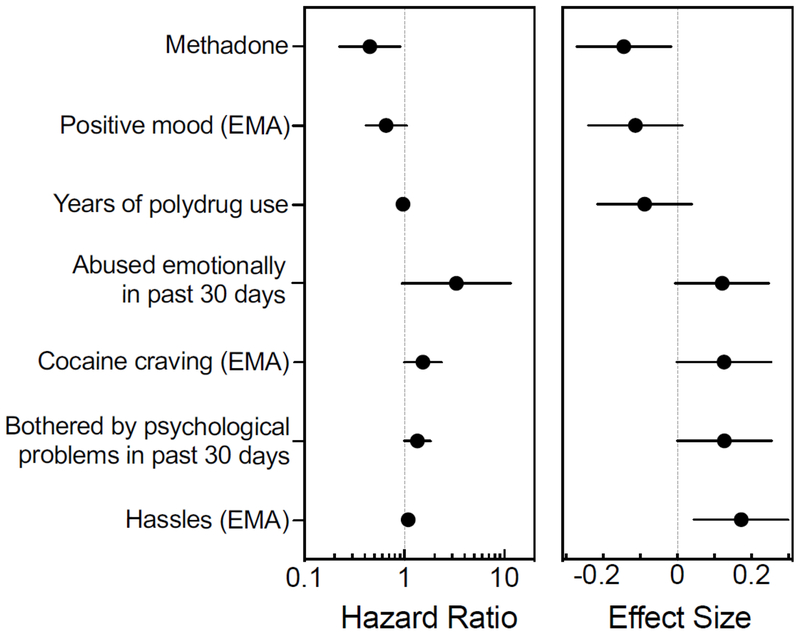
The first stage of model building was used to identify variables that distinguished between participants with different outcomes within each of the four sets of predictors. The first-stage variables that were retained as being relevant to Dropping Out were four demographic variables (medication type, race, years of heroin use, and number of children), two ASI psychological variables (being abused emotionally during the 30 days before treatment and frequency of being bothered by non-drug-related psychological problems during the 30 days before treatment), two EMA stress variables (number of hassles and level of perceived stress), and two mood and craving EMA variables (cocaine craving and positive mood). It should be noted that unlike the results depicted in Figures 2 and 3, where for example negative mood but not positive mood was found to differ between outcomes when averaged over timepoints, the survival analysis is based only on comparisons between those who left the study at a given timepoint and those who were at risk of leaving at that same timepoint.
The second stage of model building was used to develop a coherent and parsimonious picture of how variables from the four sets influence the outcome. The final model for Dropping Out included seven variables (Figure 4) and was statistically significant (likelihood ratio test χ27 =32.27, p<.0001) with a concordance of 0.72 (within a range typically seen in published survival studies). Four of these variables increased the hazard of Dropping Out: more hassles reported in end-of-day EMA, more cocaine craving in randomly-prompted EMA, experience of emotional abuse during the 30 days prior to starting treatment, and being bothered frequently by personal psychological problems during the 30 days prior to starting treatment. The other three variables had protective effects, decreasing the likelihood of Dropping Out: treatment with methadone as opposed to buprenorphine, higher self-ratings of positive mood in random prompts, and more years of polydrug use. Hassles and medication type had the largest effect sizes. A lower level of positive mood (Figure 4) distinguished people who Dropped Out in a given week from those who remained (including participants who would later Drop Out, become Noncompliant, or leave the study for Other reasons), consistent with the trend in the same direction when positive mood was averaged over time (Figure 2g). It should be noted that, although 14.7% of participants endorsed “0 years” of polydrug use (defined as using more than one drug per day), this does not imply that they did not have experience with cocaine; in fact, all participants endorsed craving cocaine in at least one EMA report. The estimated effect sizes and 95% confidence intervals for the variables in the final model were: methadone (−0.14; −0.27 to −0.02), positive mood (−0.11; −0.24 to 0.01), years of polydrug use (−0.09; −0.22 to 0.04), abused emotionally in past 30 days (0.12; −0.01 to 0.25), cocaine craving (0.13; 0.00 to .25), bothered by psychological problems (0.13; 0.00 to 0.25) and hassles (0.17; 0.04 to 0.30).
Figure 4.
Hazard ratios and effect sizes from the final model describing how dropping out of treatment is affected by demographics and recent psychological problems and by stress, craving and mood assessed through ecological momentary assessment (EMA) reports. Left panel shows the hazard ratio (with 95% confidence interval) for each variable in the final model. Right panel shows the effect size (reffect with 95% confidence interval) for each variable in the final model. Values to the left of the dashed vertical lines represent decreased hazard of dropping out (i.e., a protective influence), and values to the right represent increased hazard of dropping out.
3.5. Survival Analysis: Variables Affecting Noncompliance
The first-stage models for the Noncompliant outcome retained four demographic variables (race, years of heroin use, years of polydrug use, and number of children), three ASI psychological variables (being abused emotionally during the 30 days prior to starting treatment, number of days experiencing psychological problems during the 30 days prior to starting treatment, and interviewer determination that the participant needed treatment for these psychological problems), two EMA stress variables (number of hassles and level of perceived stress), and two EMA mood variables (positive mood and negative mood).
The final model for the Noncompliant outcome retained only one variable (the number of hassles reported in end-of-day EMA reports) and was not significant (likelihood ratio test χ21 =2.61, p = .11), with a concordance (0.551) near chance level. The hassles variable in the Noncompliant participants had a hazard ratio of 1.12 (95% confidence interval: 0.99 to 1.25) and an effect size of 0.12 (95% confidence interval: −0.04 to 1.84).
4. Discussion
Electronic diary entries related to stress and mood during the previous week distinguished participants who dropped out of treatment. Specifically, survival analysis indicated that these participants reported more hassles (an indicator of stress), less experience of positive mood, and more cocaine craving than participants who did not drop out. In contrast, survival analysis did not identify any significant predictors that distinguished participants who were unable to comply with the demands of intensive longitudinal research, although the number of hassles had a marginal effect.
These results suggest that greater attention to stress and craving (either through counseling or adjunct medications) might improve treatment retention, particularly for people receiving buprenorphine. Alpha-2 adrenergic agonists such as clonidine and lofexidine represent one type of medication that has been used to treat stress-related disorders and that should be considered for use as an adjunct medication in treating opioid use disorder. Lofexidine has been found to increase retention or increase time to dropout in studies of opioid taper (Gorodetzky et al., 2017; Yu et al., 2008). We have shown that clonidine, given during opioid maintenance rather than taper, decreased stress-induced craving and delayed relapse to drug use, but it did not reduce the level of dropout (Kowalczyk et al., 2015; see also Weinstein et al., 2017). However, since the clonidine/placebo intervention in that study was being tested for relapse prevention, it was not initiated until after at least six weeks of treatment and two weeks of abstinence, possibly missing the most vulnerable period for dropout. Thus, there is a clear need for research specifically designed to test the effects of clonidine and other medications on stress-induced dropout starting from the outset of opioid agonist therapy.
In agreement with a meta-analysis of studies that examined dropout from treatment for addiction (Brorson et al., 2013), basic demographic variables such as race and sex did not have reliable effects in the present study. One exception might be years of polydrug use, which had a mild protective effect against dropping out and might be a partial proxy for age. Our final model for dropping out of treatment did include two person-level variables related to problems that existed prior to starting treatment. One of these, recent experience of emotional abuse, has previously been associated with suicide attempts (Hakansson et al., 2010) and was identified by White et al. (1998) as a predictor of dropout from an intensive outpatient program for addiction treatment. The other person-level variable— a history of emotional, physical or sexual abuse— has previously been associated with dual diagnosis of substance use and other psychiatric conditions (Palacios et al., 1999). Notably, the emotional-abuse item on the ASI is part of the family/social relations section of the inventory and is distinct from the other ASI item that predicted dropping out (i.e., being bothered by depression, anxiety and other psychological problems; see Table 1), which is part of the psychiatric status section. The fact that recent emotional abuse and recent psychological problems are both retained in the final model also suggests that each contributes independently to the risk of dropout.
As in many earlier studies (Gerra et al., 2004; Gryczynski et al., 2013; Hser et al., 2014; Mattick et al., 2014), the dropout rate was higher for participants treated with buprenorphine (a partial μ-opioid agonist) than for those treated with methadone (a full μ-opioid agonist). Since dosage is an important consideration, and doses that are too low will be ineffective at decreasing craving and opioid use, it is notable that dosage was individually tailored in this study, limited only by adverse effects rather than an arbitrary maximum; thus, methadone and buprenorphine were each given at an optimal level. Noncompliance with EMA was not affected by medication type. Unlike some earlier studies (Gerra et al., 2004; Gryczynski et al., 2013), the higher dropout rate we observed in buprenorphine-treated participants was not restricted to the earliest part of treatment. However, it might be reasonable to consider our entire 17-week study as representing an early stage of treatment, since it is recommended that agonist treatment be continued for years (National Institute on Drug Abuse, 2018). The effects of medication type on dropout in studies of treatment might be influenced by whether participants are randomly assigned to a medication or allowed to choose between methadone and buprenorphine. In the present study, the medication for each individual was determined in a naturalistic way based on what our clinic was offering at the time of recruitment, whether the participant expressed a preference, and a safety assessment that considered comorbidities, concurrent medications, and the participant’s past experience with medication side effects and tolerability.
In a recent review of behavioral interventions that have been tested in combination with buprenorphine treatment, contingency management emerged as the one type of intervention that was effective at improving outcome (Carroll and Weiss, 2017). Our study employed contingency management in two ways, to enhance compliance with study procedures and to reward abstinence from opioids and cocaine (as described in section 2.1). Thus, one way to look at the present findings is that stress, mood, and psychological problems predicted a poorer outcome with buprenorphine treatment, even in the presence of a generally effective behavioral intervention. This suggests that future research should examine contingency management (promoting decreased drug use) in combination with interventions that target stress and other psychological problems.
Several studies have examined antidepressant medications for treatment of depression among patients maintained on methadone (for a review see Nunes et al., 2004). Studies with low rates of placebo response showed beneficial effects of antidepressant treatment (Nunes et al., 1998; Titievsky et al., 1982; Woody et al., 1975). Many other studies showed high rates of placebo response, which might stem from improvements in stress and mood upon entering an effective treatment for addiction (Nunes et al., 2004). Obtaining EMA data from patients starting buprenorphine or methadone treatment could be a way to track and identify those patients whose stress and mood are not improving and who may benefit most from interventions such as antidepressant medication or behavioral therapy for psychological problems.
In addition to monetary incentives given for complying with study requirements, the constant presence of a study-provided electronic device and frequent encounters with recovery-related EMA questions might have increased retention and medication adherence for some participants, similar to the effects of text-message reminders (Bauer et al., 2010; Krishna et al., 2009; Milward et al., 2014) or interventions that provide feedback based on EMA (Aharonovich et al., 2017a; Aharonovich et al., 2017b). Although maintaining a device and complying with EMA can be burdensome to some, the fact that few participants became noncompliant suggests that this burden was not a major factor contributing to nonretention. Future research should examine technology-based interventions as adjuncts to medication-based treatment for opioid use disorder.
There are several limitations of the present study. The distinction between participants who dropped out of treatment and those who were unwilling or unable to comply with the demands of EMA is not perfect, since the reasons that led some people to miss clinic visits (i.e., to meet the criteria for being considered Dropped Out) might overlap with the reasons that led other people to miss EMA reports (i.e., to meet the criteria for being Noncompliant). The fact that the Other category was heterogeneous— comprising people who switched to a different treatment program and/or left our study for reasons beyond their control— means that we cannot draw conclusions about them; we can only use their data to provide part of the context for identifying salient characteristics of people in the other categories. EMA responses from participants who had difficulty complying with EMA requirements might have been distorted in some unknown way. The fact that noncompliance was rare in our study limited our statistical power for detecting its predictors. People can drop out of treatment for a variety of reasons (Nordheim et al., 2018), sometimes with the intention of returning later or trying a different kind of treatment; identifying such cases would require measures that are not available in our data. Finally, remaining in treatment is only a step toward recovery and does not guarantee success (Walker, 2009). Nonetheless, it is known that dropping out of opioid treatment puts people at increased risk of early death by overdose and other causes (Bauer et al., 2008; Pierce et al., 2016), and our findings regarding mood, stress and psychiatric problems suggest that those who dropped out were not merely people who were doing so well that they could afford to leave.
Highlights.
Electronic diary data effectively identified mental states that predict dropout.
Dropouts reported more hassles, cocaine craving than those who stayed in treatment.
People who dropped out had lower levels of positive mood at the time of dropout.
Recent emotional abuse, psychiatric problems increased the likelihood of dropout.
Dropout rates were higher with buprenorphine than with methadone.
Acknowledgments
Role of Funding Source
This study was supported by the Intramural Research Program of the National Institute on Drug Abuse, National Institutes of Health. The funding source had no further role in the writing of this report.
Conflict of Interest
E.V.N. served as an investigator on a study funded by Braeburn, Inc., has served as an investigator on studies where study medication was supplied, in kind, by Alkermes, Inc., or Reckitt (Indivior), and has served as a consultant without compensation to Braeburn-Camurus and Alkermes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharonovich E, Sarvet A, Stohl M, DesJarlais D, Tross S, Hurst T, Urbina A, Hasin D, 2017a. Reducing non-injection drug use in HIV primary care: A randomized trial of brief motivational interviewing, with and without HealthCall, a technology-based enhancement. J. Subst. Abuse Treat. 74, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Stohl M, Cannizzaro D, Hasin D, 2017b. HealthCall delivered via smartphone to reduce co-occurring drug and alcohol use in HIV-infected adults: A randomized pilot trial. J. Subst. Abuse Treat. 83, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainscough TS, McNeill A, Strang J, Calder R, Brose LS, 2017. Contingency Management interventions for non-prescribed drug use during treatment for opiate addiction: A systematic review and meta-analysis. Drug Alcohol Depend. 178, 318–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodeo M, Chassler D, Oettinger C, Labiosa W, Lundgren LM, 2008. Client retention in residential drug treatment for Latinos. Eval. Program Plann. 31, 102–112. [DOI] [PubMed] [Google Scholar]
- Andersson HW, Steinsbekk A, Walderhaug E, Otterholt E, Nordfjaern T, 2018. Predictors of dropout from inpatient substance use treatment: A prospective cohort study. Subst. Abuse Res. Treat 12, 1178221818760551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart G, 2012. Maintenance medication for opiate addiction: The foundation of recovery. J. Addict. Dis 31, 207–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, de Niet J, Timman R, Kordy H, 2010. Enhancement of care through self-monitoring and tailored feedback via text messaging and their use in the treatment of childhood overweight. Patient Educ. Couns. 79, 315–319. [DOI] [PubMed] [Google Scholar]
- Bauer SM, Loipl R, Jagsch R, Gruber D, Risser D, Thau K, Fischer G, 2008. Mortality in opioid-maintained patients after release from an addiction clinic. Eur. Addict. Res 14, 82–91. [DOI] [PubMed] [Google Scholar]
- Bolger N, Laurenceau JP, 2013. Intensive longitudinal methods: An introduction to diary and experience sampling research. Guilford Press, New York. [Google Scholar]
- Brorson HH, Ajo Arnevik E, Rand-Hendriksen K, Duckert F, 2013. Drop-out from addiction treatment: A systematic review of risk factors. Clin. Psychol. Rev 33, 1010–1024. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Weiss RD, 2017. The role of behavioral interventions in buprenorphine maintenance treatment: A review. Am. J. Psychiatry 174, 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R, 1983. A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396. [PubMed] [Google Scholar]
- Damian AJ, Mendelson T, Agus D, 2017. Predictors of buprenorphine treatment success of opioid dependence in two Baltimore City grassroots recovery programs. Addict. Behav 73, 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughters SB, Richards JM, Gorka SM, Sinha R, 2009. HPA axis response to psychological stress and treatment retention in residential substance abuse treatment: A prospective study. Drug Alcohol Depend. 105, 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin PL, De CM, Paraherakis A, Gill K, 2002. The role of functional social support in treatment retention and outcomes among outpatient adult substance abusers. Addiction 97, 347–356. [DOI] [PubMed] [Google Scholar]
- Dziak JJ, Coffman DL, Lanza ST, Li R, 2012. Sensitivity and specificity of information criteria, technical report series (#12-119). The Methodology Center, Pennsylvania State University, University Park, PA: pp. 1–30. [Google Scholar]
- Epstein DH, Heilig M, Shaham Y, 2018. Science-based actions can help address the opioid crisis. Trends Pharmacol. Sci. 39, 911–916. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Tyburski M, Craig IM, Phillips KA, Jobes ML, Vahabzadeh M, Mezghanni M, Lin JL, Furr-Holden CDM, Preston KL, 2014. Real-time tracking of neighborhood surroundings and mood in urban drug misusers: Application of a new method to study behavior in its geographical context. Drug Alcohol Depend. 134, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL, 2009. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch. Gen. Psychiatry 66, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari M, Epstein DH, Phillips KA, Jobes ML, Kowalczyk WJ, Vahabzadeh M, Lin JL, Preston KL, 2015. Some of the people, some of the time: Field evidence for associations and dissociations between stress and drug use. Psychopharmacology 232, 3529–3537. [DOI] [PubMed] [Google Scholar]
- Galanter M, 2018. Combining medically assisted treatment and Twelve-Step programming: A perspective and review. Am. J. Drug Alcohol Abuse 44, 151–159. [DOI] [PubMed] [Google Scholar]
- Gerra G, Borella F, Zaimovic A, Moi G, Bussandri M, Bubici C, Bertacca S, 2004. Buprenorphine versus methadone for opioid dependence: Predictor variables for treatment outcome. Drug Alcohol Depend. 75, 37–45. [DOI] [PubMed] [Google Scholar]
- Ginexi EM, Riley W, Atienza AA, Mabry PL, 2014. The promise of intensive longitudinal data capture for behavioral health research. Nicotine Tob. Res. 16, S73–S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodetzky CW, Walsh SL, Martin PR, Saxon AJ, Gullo KL, Biswas K, 2017. A phase III, randomized, multi-center, double blind, placebo controlled study of safety and efficacy of lofexidine for relief of symptoms in individuals undergoing inpatient opioid withdrawal. Drug Alcohol Depend. 176, 79–88. [DOI] [PubMed] [Google Scholar]
- Gryczynski J, Mitchell SG, Jaffe JH, Kelly SM, Myers CP, O’Grady KE, Olsen YK, Schwartz RP, 2013. Retention in methadone and buprenorphine treatment among African Americans. J. Subst. Abuse Treat. 45, 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakansson A, Bradvik L, Schlyter F, Berglund M, 2010. Factors associated with the history of attempted suicide. Crisis 31, 12–21. [DOI] [PubMed] [Google Scholar]
- Harrell FE Jr., Lee KL, Mark DB, 1996. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med 15, 361–387. [DOI] [PubMed] [Google Scholar]
- Heinze G, Wallisch C, Dunkler D, 2018. Variable selection-- A review and recommendations for the practicing statistician. Biom. J 60, 431–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Jacobs P, Teruya C, McLaughlin P, Wiest K, Cohen A, Ling W, 2014. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction 109, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk WJ, Moran LM, Bertz JW, Phillips KA, Ghitza UE, Vahabzadeh M, Lin JL, Epstein DH, Preston KL, 2018. Using ecological momentary assessment to examine the relationship between craving and affect with opioid use in a clinical trial of clonidine as an adjunct medication to buprenorphine treatment. Am. J. Drug Alcohol Abuse 44, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk WJ, Phillips KA, Jobes ML, Kennedy AP, Ghitza UE, Agage DA, Schmittner JP, Epstein DH, Preston KL, 2015. Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: A randomized controlled trial with ecological momentary assessment. Am. J. Psychiatry 172, 760–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S, Boren SA, Balas EA, 2009. Healthcare via cell phones: A systematic review. Telemed. J. E. Health 15, 231–240. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Zvolensky MJ, Daughters SB, Bornovalova MA, Paulson A, Tull MT, Ettinger K, Otto MW, 2008. Anxiety sensitivity: A unique predictor of dropout among inner-city heroin and crack/cocaine users in residential substance use treatment. Behav. Res. Ther 46, 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasiewicz M, Fareng M, Benyamina A, Blecha L, Reynaud M, Falissard B, 2007. Ecological momentary assessment in addiction. Expert Rev. Neurother. 7, 939–950. [DOI] [PubMed] [Google Scholar]
- Marhe R, Waters AJ, van de Wetering BJ, Franken IH, 2013. Implicit and explicit drug-related cognitions during detoxification treatment are associated with drug relapse: An ecological momentary assessment study. J. Consult. Clin. Psychol 81, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M, 2014. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. Cd002207. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP, 1985. New data from the Addiction Severity Index. Reliability and validity in three centers. J. Nerv. Ment. Dis 173, 412–423. [DOI] [PubMed] [Google Scholar]
- Milward J, Lynskey M, Strang J, 2014. Solving the problem of non-attendance in substance abuse services. Drug Alchol Rev. 33, 625–636. [DOI] [PubMed] [Google Scholar]
- Mitchell SG, Gryczynski J, Schwartz RP, Myers CP, O’Grady KE, Olsen YK, Jaffe JH, 2015. Changes in quality of life following buprenorphine treatment: Relationship with treatment retention and illicit opioid use. J. Psychoactive Drugs 47, 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Kowalczyk WJ, Phillips KA, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, Preston KL, 2018. Sex differences in daily life stress and craving in opioid-dependent patients. Am. J. Drug Alcohol Abuse 44, 512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumba MJ, Findlay L,E, . Snow D., 2018. Treatment options for opioid use disorders: A review of the relevant literature. J. Addict. Nurs 29, 221–225. [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse, 2018. Principles of drug addiction treatment: A research-based guide, 3rd ed. U.S. Department of Health and Human Services, Washington, D.C. [Google Scholar]
- Noordzij M, Leffondre K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ, 2013. When do we need competing risks methods for survival analysis in nephrology? Nephrol. Dial. Transplant 28, 2670–2677. [DOI] [PubMed] [Google Scholar]
- Nordheim K, Walderhaug E, Alstadius S, Kern-Godal A, Arnevik E, Duckert F, 2018. Young adults’ reasons for dropout from residential substance use disorder treatment. Qual. Soc. Work 17, 24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EV, Quitkin FM, Donovan SJ, Deliyannides D, Ocepek-Welikson K, Koenig T, Brady R, McGrath PJ, Woody G, 1998. Imipramine treatment of opiate-dependent patients with depressive disorders. A placebo-controlled trial. Arch. Gen. Psychiatry 55, 153–160. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Sullivan MA, Levin FR, 2004. Treatment of depression in patients with opiate dependence. Biol. Psychiatry 56, 793–802. [DOI] [PubMed] [Google Scholar]
- Palacios WR, Urmann CF, Newel R, Hamilton N, 1999. Developing a sociological framework for dually diagnosed women. J. Subst. Abuse Treat. 17, 91–102. [DOI] [PubMed] [Google Scholar]
- Pierce M, Bird SM, Hickman M, Marsden J, Dunn G, Jones A, Millar T, 2016. Impact of treatment for opioid dependence on fatal drug-related poisoning: A national cohort study in England. Addiction 111, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, 2017. Context and craving during stressful events in the daily lives of drug-dependent patients. Psychopharmacology 234, 2631–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, 2018a. Before and after: Craving, mood, and background stress in the hours surrounding drug use and stressful events in patients with opioid-use disorder. Psychopharmacology 235, 2713–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, 2018b. Exacerbated craving in the presence of stress and drug cues in drug-dependent patients. Neuropsychopharmacology 43, 859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Schroeder JR, Kowalczyk WJ, Phillips KA, Jobes ML, Vahabzadeh M, Lin JL, Mezghanni M, Epstein DH, 2018c. End-of-day reports of daily hassles and stress in men and women with opioid-use disorder: Relationship to momentary reports of opioid and cocaine use and stress. Drug Alcohol Depend. 193, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2018. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Rosnow RL, Rosenthal R, Rubin DB, 2000. Contrasts and correlations in effect-size estimation. Psychol. Sci 11, 446–453. [DOI] [PubMed] [Google Scholar]
- Salsitz E, Wiegand T, 2016. Pharmacotherapy of opioid addiction: “Putting a real face on a false demon”. J. Med. Toxicol 12, 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR, 2008. Ecological momentary assessment. Ann. Rev. Clin. Psychol 4, 1–32. [DOI] [PubMed] [Google Scholar]
- Therneau T, Crowson C, Clinic M, 2014. Using time dependent covariates and time dependent coefficients in the Cox model. R Foundation for Statistical Computing, Vienna. [Google Scholar]
- Titievsky J, Seco G, Barranco M, Kyle EM, 1982. Doxepin as adjunctive therapy for depressed methadone maintenance patients: A double-blind study. J. Clin. Psychiatry 43, 454–456. [PubMed] [Google Scholar]
- Venables WN, Ripley BD, 2002. Modern applied statistics with S. Springer, New York. [Google Scholar]
- Volkow ND, Frieden TR, Hyde PS, Cha SS, 2014. Medication-assisted therapies-- Tackling the opioid-overdose epidemic. N. Engl. J. Med 370, 2063–2066. [DOI] [PubMed] [Google Scholar]
- Walker R, 2009. Retention in treatment-- Indicator or illusion: An essay. Subst. Use Misuse 44, 18–27. [DOI] [PubMed] [Google Scholar]
- Weinstein ZM, Cheng DM, Quinn E, Hui D, Kim H, Gryczynski G, Samet JH, 2017. Psychoactive medications and disengagement from office based opioid treatment (obot) with buprenorphine. Drug Alcohol Depend. 170, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Winn KI, Young W, 1998. Predictors of attrition from an outpatient chemical dependency program. Subst. Abuse 19, 49–59. [DOI] [PubMed] [Google Scholar]
- Woody GE, O’Brien CP, Rickels K, 1975. Depression and anxiety in heroin addicts: A placebo-controlled study of doxepin in combination with methadone. Am. J. Psychiatry 132, 447–450. [DOI] [PubMed] [Google Scholar]
- Yu E, Miotto K, Akerele E, Montgomery A, Elkashef A, Walsh R, Montoya I, Fischman MW, Collins J, McSherry F, Boardman K, Davies DK, O’Brien CP, Ling W, Kleber H, Herman BH, 2008. A Phase 3 placebo-controlled, double-blind, multi-site trial of the alpha-2-adrenergic agonist, lofexidine, for opioid withdrawal. Drug Alcohol Depend. 97, 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]