Abstract
The nucleolytic ribozymes carry out site-specific RNA cleavage reactions by nucleophilic attack of the 2′-oxygen atom on the adjacent phosphorus with an acceleration of a million-fold or greater. A major part of this arises from concerted general acid–base catalysis. Recent identification of new ribozymes has expanded the group to a total of nine and this provides a new opportunity to identify sub-groupings according to the nature of the general base and acid. These include nucleobases, hydrated metal ions, and 2′-hydroxyl groups. Evolution has selected a number of different combinations of these elements that lead to efficient catalysis. These differences provide a new mechanistic basis for classifying these ribozymes.
Keywords: Nucleolytic ribozymes, general acid-base catalysis, metal ions
Ribozymes are enzymes that are built from RNA rather than from protein. Although they plausibly played a key role in the emergence of primitive life early in the history of the planet, some are still with us, catalyzing some extremely important cellular reactions such as the condensation of amino acids to form polypeptides in the core of the ribosome. Therefore, understanding how RNA could catalyze cellular reactions is important and recently this field has seen considerable progress that is reviewed here. This review focuses on a sub-group of the ribozymes where some general principles can be discerned.
The nucleolytic ribozymes
The nucleolytic ribozymes are relatively small RNAs (all but one have fewer than 100 nucleotides) that bring about site-specific cleavage of the backbone 1, 2. In some ribozymes, the reverse (ligation) reaction can occur 3– 8. In the protein enzymes, such phosphoryl transfer reactions are catalyzed in one of two ways, either by the use of metal ions to organize and activate the active center or by general acid–base catalysis using histidine. RNA has a small fraction of the chemical resources of a protein. It has only four similar heterocyclic nucleobases, the 2′-hydroxyl group, and bound hydrated metal ions. Although some nucleolytic ribozymes certainly employ hydrated metal ions in their catalysis, the common theme among these species is the use of concerted general acid–base catalysis. Evolution has found a variety of ways of employing the limited resources of RNA in different combinations to accelerate the reactions by around one million-fold or more and it is this feat of chemical catalysis that we wish to understand.
There are now nine members within this group, and they have distinct folds that are generally based on helical junctions or pseudoknot structures or both. The hammerhead 9, 10, hairpin 3, 11, 12, Varkud satellite (VS) 13, and hepatitis delta virus (HDV) 14– 16 ribozymes have been known for several decades, but more recently the group has expanded significantly thanks to the work of the Breaker lab, starting with the glmS ribozyme 17. More recently, their bioinformatic pipeline 18 has added four more distinct species: the twister, TS, pistol, and hatchet ribozymes 19. This complete group ( Table 1) now provides a new opportunity to compare structures and mechanisms and to contrast and perceive sub-groupings.
Table 1. General bases and acids participating in the cleavage reactions of the nucleolytic ribozymes.
| Group | Ribozyme | Base | Acid |
|---|---|---|---|
| G + A | |||
| Hairpin | G N1 | A N1 | |
| VS | G N1 | A N1 | |
| G + A(N3) | |||
| Twister | G N1 | A N3 | |
| M + C | |||
| HDV | M 2+ OH − | C N3 | |
| TS | M 2+ OH − | C N3? | |
| G O2′ | |||
| Hammerhead | G N1 | O2′ | |
| G + M | |||
| Pistol | G N1 | M 2+ H 2O | |
| G + coenzyme | |||
| glmS | G N1 | Glc6P amine |
The groups are named by the general base followed by the general acid in the cleavage reaction. M designates a hydrated divalent metal ion directly participating in catalysis. HDV, hepatitis delta virus; VS, Varkud satellite; Glc6P, glucosamine-6-phosphate
Mechanism and catalysis of phosphoryl transfer
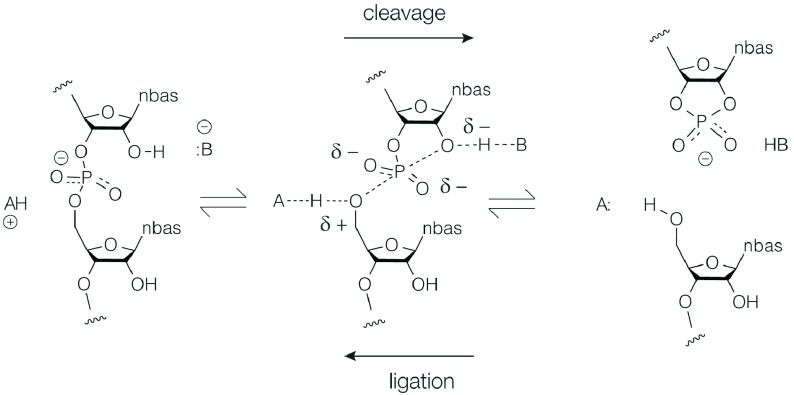
The standard reaction carried out by the nucleolytic ribozymes is shown in Figure 1. The cleavage reaction (left to right) is brought about by nucleophilic attack of the O2′ on its adjacent phosphate group. The S N2 reaction passes through a trigonal bipyramidal phosphorane intermediate 20 that is probably close to the transition state that decomposes by departure of the O5′to leave the cyclic 2′3′phosphate 21. The reaction is typically accelerated by at least a million-fold by the ribozymes, although the estimation requires knowledge of the baseline rate of the uncatalyzed reaction which is not easy to estimate. If the products are held in place by the RNA structure, then the reverse ligation reaction (right to left) can also occur, as it does, for example, with the hammerhead and hairpin ribozymes.
Figure 1. The chemical mechanism of the nucleolytic ribozymes, showing catalysis by general acid–base catalysis.
The cleavage reaction is left to right and the ligation reaction right to left. The central phosphorane structure will be close to the transition state in the reaction trajectory. In the cleavage reaction, B − acts as a general base to deprotonate the O2′ nucleophile, and the general acid AH + protonates the 5′-oxyanion leaving group.
There are four potential elements to the catalysis 22– 24. First, if the RNA can facilitate a near in-line geometry of attack, this will provide a small contribution to rate enhancement. Second, the phosphorane could be stabilized by a combination of hydrogen bonding and electrostatics. Third, conversion of the 2′OH to an alkoxide by a general base will generate a much stronger nucleophile. Lastly, protonation of the O5′ by a general acid will create a better leaving group. The last two jointly constitute concerted general acid–base catalysis and undoubtedly make the largest contribution to the catalytic rate enhancement 25. Of course, ultimately, all catalytic strategy can be reduced to transition state stabilization, and general acid–base catalysis also reduces charge separation in the transition state.
The nucleolytic ribozymes use a variety of functionalities in different combinations to perform general acid–base catalysis. Nucleobases are used most frequently, and G, A, and C are all employed in different ribozymes. They suffer from the disadvantage that their natural p K a values are not close to neutrality. The observed rate of cleavage ( k obs) will be related to the intrinsic rate ( k cat) by:
k obs = k cat . f A . f B
f A and f B are the fractions of protonated acid and deprotonated base, respectively, at any given pH. For example, if a ribozyme uses a general base and acid of p K a = 9 and 5, respectively (values close to those of a typical G+A ribozyme), then f A . f B will be 10 −4 at neutrality. Application of this to the maximum rates observable for the VS ribozyme gives a k cat that is not greatly slower than that of ribonuclease A 26. By shifting p K a values closer to neutrality and thus raising f A. f B, faster rates can be obtained. In an electronegative environment of RNA, it is easy to see how the p K a of adenine or cytosine can be raised and an extreme case of this is observed in the twister ribozyme (see the “G + A(N3): the twister ribozyme” section below). Lowering a higher p K a value will likely require juxtaposition of a metal ion. It should be noted that although the fraction of catalyst that is in the correct state of protonation is important, there is a degree of compensation between this and the reactivity of the groups undergoing proton transfer 27.
General base catalysis by guanine nucleobases
The most common element in the mechanisms of the nucleolytic ribozymes is a guanine nucleobase acting as a general base, which is true for the all the ribozymes except the group containing HDV and TS. The guanine nucleobase acts rather like the analog of the imidazole group of histidine in protein enzymes and accepts the proton from the O2′ nucleophile at its N1 position. In order to do this, it must be in its deprotonated form, and in each case this has been demonstrated by the pH dependence of the cleavage reaction, generally combined with atomic mutagenesis. The normal p K a of guanine is around 9.5; but for maximum catalytic efficiency, this would ideally be lowered. In the VS ribozyme, this has been measured at 8.4 28, where proximity of a bound metal ion likely reduces the p K a.
Grouping the nucleolytic ribozymes
While guanine acting as general base is very common, the general acid is rather more variable. This is a nucleobase in (probably) five cases, in three of which it is adenine. But it is also frequently not a nucleobase. Consideration of the general base and acid provides a way to group the nucleolytic ribozymes mechanistically ( Table 1 and Figure 2).
Figure 2. The six mechanisms observed in the nucleolytic ribozymes, classified according to the general base and acid used.
For clarity, charges are not shown in these depictions. The groupings correspond to those listed in Table 1. CoE refers to the use of an exogenous coenzyme; glmS binds glucosamine-6-phosphate to act as a general acid.
G + A: the hairpin and VS ribozymes
These ribozymes use the N1 of a guanine and an adenine in proton transfer. The role of an adenine nucleobase as a general acid has been proven by phosphorothiolate substitution in the hairpin 29 and VS 26 ribozymes, and despite having very different overall folds, the active centers of the hairpin and VS ribozymes are closely similar. Both are generated by interaction of two internal loops, and the disposition of the guanine general base, adenine general acid, and scissile phosphate is the same for both 26, 30, 31. Both ribozymes exhibit activity in high concentrations of monovalent ions as the sole ion 32 and thus there is no evidence for a direct role for metal ions in these ribozymes, although some electrostatic stabilization of the transition state is possible. In order for adenine to act as a general acid, it must be protonated at N1. Thus, the concerted general acid–base catalysis requires an unprotonated guanine and a protonated adenine ( Figure 2A). Although the p K a of unperturbed adenine is 4 or lower, these values are typically raised in the electronegative context of the folded RNA. For example, that measured in the VS ribozyme is 5.2 28. The pH profile for the VS ribozyme is bell-shaped, reflecting the requirement for an unprotonated guanine acting as a general base and a protonated adenine acting as a general acid. By the principle of microscopic reversibility, the ligation reaction will proceed via the same chemical mechanism in reverse, so that neutral adenine will act as the general base deprotonating the O5′ nucleophile, and neutral guanine will act as the general acid protonating the O2′ leaving group.
G + A(N3): the twister ribozyme
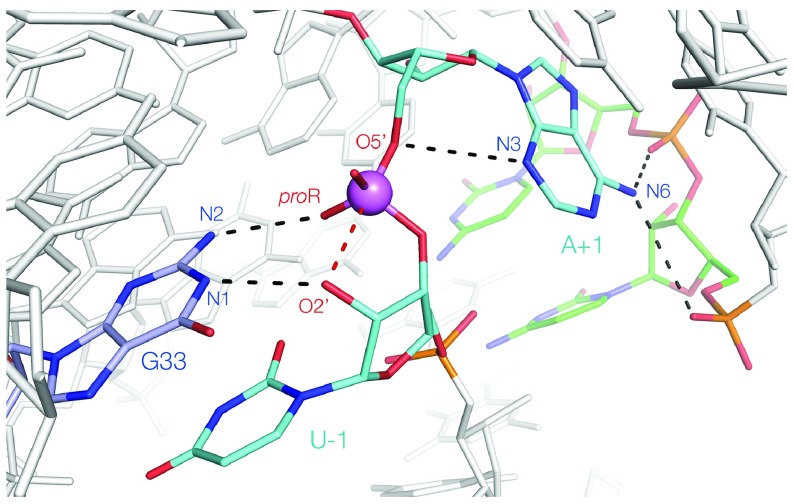
The twister ribozyme 33 is a third G+A ribozyme but with two significant differences. It was the first of the group of new ribozymes, and three crystal structures have been determined, showing that the fold is a unique inverted double pseudoknot 34– 36. As a result of extensive mechanistic studies 24 in addition to the structural analysis, it is arguably the best understood of the nucleolytic ribozymes. Although none of the structures is well aligned for nucleophilic attack (and the one that was closest has other significant disruption of the structure 36), it was easy to see how to rotate the 5′ nucleotide in the active center to bring it into an in-line geometry 34, 37. Moreover, this had the effect of bringing the guanine G33 that was known to act as a general base into position in addition to stabilizing the transition state by bonding its N2 to the scissile phosphate ( Figures 2B and Figure 3). So with three catalytic strategies identified, what is the general acid? Surprisingly, it turned out to be the adenine immediately 3′ to the scissile phosphate (A+1). Moreover, it was not the usual N1 that acts in proton transfer (in fact, geometrically, this is not possible given its position) but rather the much more acidic N3. This was confirmed by deaza substitution 24. Microscopic p K a values of the N1 and N3 atoms indicate that a proton will be 100-fold more likely to reside on N1 rather than N3 38 yet this is partially compensated by the unusual environment in the active center. The N6 of A+1 donates both its protons to successive phosphate groups. The net effect of this is to raise the p K a of A+1 to neutrality, thus offsetting its low probability of protonation at N3. The twister is thus another G+A ribozyme but with a difference. The active site shows how evolution has generated a perfect environment for catalysis of the transesterification, comprising in-line attack, transition state stabilization, and concerted general acid–base catalysis ( Figure 3). An alternative mechanism has been proposed for the twister ribozyme; for a neutral perspective on this, the reader is directed to an excellent critical evaluation by Breaker 39 and a very recent computational study 40.
Figure 3. The proposed structure of the active site of the twister ribozyme.
This is derived from the crystal structure, with a rotation of U-1 that brings the O2′ (modeled) into an in-line position 34. G33 both acts as a general base (accepting a proton at N1) and stabilizes the transition state (N2 donates a proton to the proR non-bridging O atom of the scissile phosphate). A+1 acts as a general acid, donating a proton from N3. Its N6 donates protons to successive phosphates in the backbone, thereby raising its p K a.
M 2+ + C: metal ions as the general base and cytosine as the general acid: the HDV and TS ribozymes
Although the hairpin and VS ribozymes have been shown to retain some activity at pH = 8 in the presence of 4 M Li + as the only metal ion 32 and the hairpin ribozyme is fully active in Co(III) hexammine ions 41– 43, the HDV ribozyme is essentially inactive in either condition 44. This indicates a direct role for a metal in the catalytic mechanism. Nevertheless, the rate of cleavage by the HDV ribozyme depends upon pH 44 in a manner consistent with general acid–base catalysis. The genomic and antigenomic forms of the ribozyme adopt a double pseudoknot fold 45, 46. In these structures, there are no guanine nucleobases closer than 9 Å from the scissile phosphate. However, there is a cytosine nucleobase oriented so that its N3 points toward the scissile phosphate at a distance of 3.7 Å. Doudna et al. 45 suggested that C75 could act as a general base to catalyze cleavage. Bevilacqua 44 showed that the rate of cleavage of the HDV ribozyme increased log-linearly with pH up to 6.5 and remained at a plateau at higher pH values. The data fitted a p K a of 6, potentially corresponding to a cytosine in an electronegative environment. However, in the absence of divalent cations, the pH dependence inverted 44. Since the rate fell with increasing pH, this indicates that C75 acts as the general acid. This was proven by Das and Piccirilli 47, who obtained almost full restoration of activity of a C-to-U variant when coupled with 5′-phosphorothiolate substitution. The bridging sulfur substitution creates a facile leaving group that no longer requires acid catalysis, consistent with cytosine acting as the general acid in the cleavage reaction. Taken together, the data suggest a model for catalysis in which a metal hydroxide acts as the general base and cytosine N3 acts as the general acid ( Figure 2C). The positions of these functionalities in available crystal structures 45, 46 are not fully consistent with their mechanistic roles, suggesting that the structures may not fully reflect the active conformation.
The TS ribozyme was identified by bioinformatic analysis and called twister-sister 19, and two structures have been solved in different forms 48, 49. The rate of cleavage of the ribozyme increases with pH up to neutrality, remaining at a plateau at higher pH, and increased log-linearly with Mg 2+ ion concentration 19. However, the activity was extremely low in either 1 M Li + or 1 mM Co(III) hexammine ions 48. Withdrawal of divalent cations had an effect similar to that in HDV, suggesting direct participation of a metal ion in the reaction. Importantly, the rate of cleavage was log-linearly dependent on the p K a of the divalent metal ion present (that is, the ease of deprotonation of an inner-sphere water molecule) 48. This showed that a hydrated metal ion is involved in proton transfer. A metal ion directly bound to the cytosine immediately 5′ to the scissile phosphate was observed in the crystal 48, placing an inner-sphere water molecule close to the O2′nucleophile. However, the geometry around the scissile phosphate was far from in-line and it is likely that this structure would require significant reorganization to move into the active conformation. If the hydrated metal ion acts as a general base in cleavage, that leaves the question of what acts as a general acid. Mutagenesis suggests that modification of a cytosine (C7) results in the largest reduction of cleavage activity. In our crystal structure, C7 is 12 Å from the scissile phosphate and is involved in an H-bonded network. But remodeling the active center to create the required in-line geometry might also reposition C7, allowing it to participate in catalysis. Computational modeling has indicated that the geometry around the active center can be readily altered and that C7 can move closer to the scissile phosphate, whereupon it could either act as a general acid or bind a metal ion that acts as a general acid 50. In a more recent crystal structure of the TS ribozyme 49, the conformation about the scissile phosphate is quite different (though still far from in-line), consistent with the TS active center being quite malleable. Interestingly, in that structure, C7 is rather closer to the O5′ atom. Although TS remains one of the most incompletely understood ribozymes, the key catalytic participants are most probably a bound hydrated metal ion and a cytosine nucleobase. Thus, TS is provisionally placed with the HDV ribozyme as a discrete sub-group of the nucleolytic ribozymes ( Table 1). For both ribozymes, it is likely that the crystal structures are not fully reflective of the active conformations.
G + O2′ and G + M 2+: the hammerhead and pistol ribozymes
It might not be immediately obvious that the hammerhead and pistol ribozymes should be discussed together, but there are striking structural and mechanistic similarities. Even comparison of the secondary structures and location of conserved nucleotides suggests a strong similarity between the two ribozymes 51. A new class of hammerhead ribozymes in which stems I and II interact through a pseudoknot has been described 19. A pseudoknot is also a key feature in the pistol ribozyme structure 19, 52, and if the two ribozyme secondary structures are rotated into the same frame, the strong similarity is apparent, and the positions of the conserved nucleotides and the scissile phosphate are very similar 51. Comparison of the three-dimensional structures in crystals of the hammerhead 53 and pistol 51, 54, 55 ribozymes also reveals a close similarity. If the pseudoknot interactions are stripped away, what remains is a three-way helical junction in both cases and the scissile phosphate is at the center. In both cases, the nucleotide 3′ to the scissile phosphate is base-paired to splay apart the 5′ and 3′ nucleotides, thereby generating the local geometry required for in-line attack. Furthermore, in both cases, there is a guanine nucleobase poised to remove the proton from the O2′ nucleophile. These (G12 in the hammerhead 56, 57 and G40 in the pistol 51) have been demonstrated to act as the general bases in the cleavage reactions.
This leaves the question of what serves as a general acid in these ribozymes. In neither structure is there a nucleobase close to the O5′ leaving group. However, in the hammerhead ribozyme, the O2′ of G8 is 3.2 Å from the O5′ atom and therefore could serve as the general acid. The p K a of a 2′-OH group is very high, but computations of York et al. 58 suggested that a metal ion could move close and thus lower the p K a. Thomas and Perrin 57 showed that the reduced cleavage activity of a G8 O2′H variant could be restored by 5′-phosphorothiolate substitution at the scissile phosphate. This demonstrated the importance of the G8 O2′, but the effect could be indirect. The direct role of the G8 O2′ was demonstrated by substitution by 2′-amino ribose, whereupon the pH profile changed completely to a flattened bell shape 51. The modified ribozyme, rather like the hairpin or VS ribozymes, operates with one participant of high p K a (G12 N1) and one of low p K a (G8, 2′NH 2).
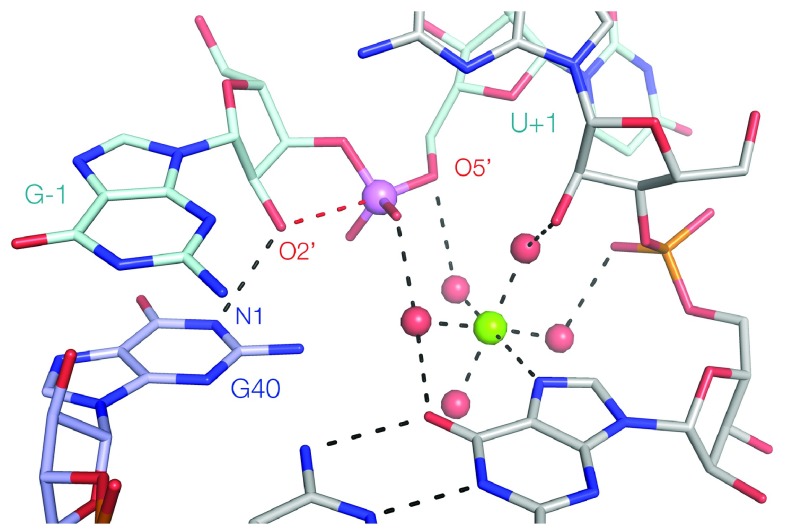
When the same 2′NH 2 substitution was performed at A32 in the pistol ribozyme (this lies in an analogous position to G8 in the hammerhead), a very different response was found. While absolute rates of cleavage were reduced, the shape of the pH profile was essentially unchanged from the unmodified ribozyme 51. Thus, unlike the hammerhead ribozyme, the corresponding 2′OH clearly does not act directly as the general acid. Instead, the available evidence indicates that pistol is the first ribozyme known to employ a hydrated metal ion as the general acid. The rate of cleavage increases in a log-linear manner with the p K a of the metal ion used, consistent with an increase in reactivity of inner-sphere water molecules acting as a general acid 51. A metal ion is observed in the crystal structures of the pistol ribozyme 51, 54, directly bound to N7 of G33. Inner-sphere water molecules can be modeled so as to be bound to a number of active center functionalities, including the G32 O2′. The reaction rate is lowered if this O2′ is modified. The water molecule that is in the axial position with respect to G33 N7 is just 3.1 Å from the O5′ and thus is well positioned to act as the general acid ( Figure 4).
Figure 4. The proposed structure of the active site of the pistol ribozyme.
This is derived from the crystal structure of the ribozyme 51. G40 acts as a general base, accepting a proton at N1 from the O2′ nucleophile. The green sphere is a Mg 2+ ion that is directly bound to N7 of the guanine nucleobase. The red spheres are inner-sphere water molecules, the positions of which have been modeled to maximize interactions with the RNA. The water molecule apical with N7 is well positioned to act as a general acid, protonating the O5′ leaving group.
In many ways, the hammerhead and pistol ribozymes are very similar but ultimately they are mechanistically distinct. In terms of the general acid, they are in sense mirror images. The hammerhead uses an O2′ that is probably activated by a metal ion ( Figure 2D), whereas the pistol ribozyme uses a hydrated metal ion that is held in part by an O2′ ( Figure 2E).
Some final perspectives
Two of the nucleolytic ribozymes have not been discussed. The structure of the glmS ribozyme 17 is known 59, 60 and it is another ribozyme that uses guanine N1 as the general base in cleavage 61. However, uniquely, the general acid is the amine of bound glucosamine-6-phosphate ( Figure 2F); that is, this ribozyme uses a coenzyme. At this time, the structure of the hatchet ribozyme is not known 19. However, biochemical data 62 are consistent with another metalloenzyme ribozyme of the HDV type.
Overall, we see that the use of nucleobases in proton transfers occurs frequently, and guanine is used as a general base in 6/8 ribozymes. However, the general acid is much more variable, and every possibility has been exploited in the group overall. Despite the limited catalytic resources of RNA, evolution has refined the use of various combinations of functional groups, hydrated metal ions, and even a coenzyme. But the expanding number of these ribozymes now allows these to be rationalized into a number of distinct mechanistic classes.
Acknowledgments
The author thanks Tim Wilson and Yijin Liu (University of Dundee) for their many contributions to the structure and mechanisms of ribozymes in the Dundee laboratory, Tim Wilson for valuable discussion, and Xiamen University for conferring a visiting professorship.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Ronald Breaker, Molecular, Cellular and Developmental Biology, Yale University, New Haven, USA
Yoshiya Ikawa, Department of Chemistry, Graduate School of Science and Engineering, University of Toyama, Toyama, Japan
Darrin M York, Center for Integrative Proteomics Research and Department of Chemistry and Chemical Biology, Rutgers University, New Jersey, USA
Funding Statement
The author was supported by a Cancer Research UK grant (A18604) for financial support in Dundee.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. van Tol H, Buzayan JM, Feldstein PA, et al. : Two autolytic processing reactions of a satellite RNA proceed with inversion of configuration. Nucleic Acids Res. 1990;18(8):1971–5. 10.1093/nar/18.8.1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slim G, Gait MJ: Configurationally defined phosphorothioate-containing oligoribonucleotides in the study of the mechanism of cleavage of hammerhead ribozymes. Nucleic Acids Res. 1991;19(5):1183–8. 10.1093/nar/19.6.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buzayan JM, Gerlach WL, Bruening G: Non-enzymatic cleavage and ligation of RNAs complementary to a plant virus satellite RNA. Nature. 1986;323:349–53. 10.1038/323349a0 [DOI] [Google Scholar]
- 4. Saville BJ, Collins RA: RNA-mediated ligation of self-cleavage products of a Neurospora mitochondrial plasmid transcript. Proc Natl Acad Sci U S A. 1991;88(19):8826–30. 10.1073/pnas.88.19.8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Komatsu Y, Koizumi M, Sekiguchi A, et al. : Cross-ligation and exchange reactions catalyzed by hairpin ribozymes. Nucleic Acids Res. 1993;21(2):185–90. 10.1093/nar/21.2.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones FD, Ryder SP, Strobel SA: An efficient ligation reaction promoted by a Varkud Satellite ribozyme with extended 5'- and 3'-termini. Nucleic Acids Res. 2001;29(24):5115–20. 10.1093/nar/29.24.5115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLeod AC, Lilley DM: Efficient, pH-dependent RNA ligation by the VS ribozyme in trans. Biochemistry. 2004;43(4):1118–25. 10.1021/bi035790e [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Canny MD, Jucker FM, Pardi A: Efficient ligation of the Schistosoma hammerhead ribozyme. Biochemistry. 2007;46(12):3826–34. 10.1021/bi062077r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hutchins CJ, Rathjen PD, Forster AC, et al. : Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986;14(9):3627–40. 10.1093/nar/14.9.3627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forster AC, Symons RH: Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987;49(2):211–20. 10.1016/0092-8674(87)90562-9 [DOI] [PubMed] [Google Scholar]
- 11. Haseloff J, Gerlach WL: Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988;334(6183):585–91. 10.1038/334585a0 [DOI] [PubMed] [Google Scholar]
- 12. Hampel A, Tritz R: RNA catalytic properties of the minimum (-)sTRSV sequence. Biochemistry. 1989;28(12):4929–33. 10.1021/bi00438a002 [DOI] [PubMed] [Google Scholar]
- 13. Saville BJ, Collins RA: A site-specific self-cleavage reaction performed by a novel RNA in Neurospora mitochondria. Cell. 1990;61(4):685–96. 10.1016/0092-8674(90)90480-3 [DOI] [PubMed] [Google Scholar]
- 14. Kuo MY, Sharmeen L, Dinter-Gottlieb G, et al. : Characterization of self-cleaving RNA sequences on the genome and antigenome of human hepatitis delta virus. J Virol. 1988;62(12):4439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perrotta AT, Been MD: The self-cleaving domain from the genomic RNA of hepatitis delta virus: sequence requirements and the effects of denaturant. Nucleic Acids Res. 1990;18(23):6821–7. 10.1093/nar/18.23.6821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Perrotta AT, Been MD: A pseudoknot-like structure required for efficient self-cleavage of hepatitis delta virus RNA. Nature. 1991;350(6317):434–6. 10.1038/350434a0 [DOI] [PubMed] [Google Scholar]
- 17. Winkler WC, Nahvi A, Roth A, et al. : Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428(6980):281–6. 10.1038/nature02362 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Weinberg Z, Wang JX, Bogue J, et al. : Comparative genomics reveals 104 candidate structured RNAs from bacteria, archaea, and their metagenomes. Genome Biol. 2010;11(3):R31. 10.1186/gb-2010-11-3-r31 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Weinberg Z, Kim PB, Chen TH, et al. : New classes of self-cleaving ribozymes revealed by comparative genomics analysis. Nat Chem Biol. 2015;11(8):606–10. 10.1038/nchembio.1846 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Westheimer FH: Pseudo-rotation in the hydrolysis of phosphate esters. Acc Chem Res. 2002;1(3):70–8. 10.1021/ar50003a002 [DOI] [Google Scholar]
- 21. Oivanen M, Kuusela S, Lönnberg H: Kinetics and Mechanisms for the Cleavage and Isomerization of the Phosphodiester Bonds of RNA by Brønsted Acids and Bases. Chem Rev. 1998;98(3):961–90. 10.1021/cr960425x [DOI] [PubMed] [Google Scholar]
- 22. Soukup GA, Breaker RR: Relationship between internucleotide linkage geometry and the stability of RNA. RNA. 1999;5(10):1308–25. 10.1017/s1355838299990891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koo S, Novak T, Piccirilli JA: Catalytic Mechanism of the HDV Ribozyme. In Lilley, D. M. J. and Eckstein, F. (eds.), Ribozymes and RNA catalysis.Roy. Soc. Chemistry, Cambridge.2008. 10.1039/9781847557988-00092 [DOI] [Google Scholar]
- 24. Wilson TJ, Liu Y, Domnick C, et al. : The Novel Chemical Mechanism of the Twister Ribozyme. J Am Chem Soc. 2016;138(19):6151–62. 10.1021/jacs.5b11791 [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Breaker RR: Kinetics of RNA Degradation by Specific Base Catalysis of Transesterification Involving the 2‘-Hydroxyl Group. J Am Chem Soc. 1999;121(23):5364–72. 10.1021/ja990592p [DOI] [Google Scholar]
- 26. Wilson TJ, Li NS, Lu J, et al. : Nucleobase-mediated general acid-base catalysis in the Varkud satellite ribozyme. Proc Natl Acad Sci U S A. 2010;107(26):11751–6. 10.1073/pnas.1004255107 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Bevilacqua PC: Mechanistic considerations for general acid-base catalysis by RNA: revisiting the mechanism of the hairpin ribozyme. Biochemistry. 2003;42(8):2259–65. 10.1021/bi027273m [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Wilson TJ, McLeod AC, Lilley DM: A guanine nucleobase important for catalysis by the VS ribozyme. EMBO J. 2007;26(10):2489–500. 10.1038/sj.emboj.7601698 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Kath-Schorr S, Wilson TJ, Li NS, et al. : General acid-base catalysis mediated by nucleobases in the hairpin ribozyme. J Am Chem Soc. 2012;134(40):16717–24. 10.1021/ja3067429 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Rupert PB, Ferré-D'Amaré AR: Crystal structure of a hairpin ribozyme-inhibitor complex with implications for catalysis. Nature. 2001;410(6830):780–6. 10.1038/35071009 [DOI] [PubMed] [Google Scholar]
- 31. Suslov NB, DasGupta S, Huang H, et al. : Crystal structure of the Varkud satellite ribozyme. Nat Chem Biol. 2015;11(11):840–6. 10.1038/nchembio.1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murray JB, Seyhan AA, Walter NG, et al. : The hammerhead, hairpin and VS ribozymes are catalytically proficient in monovalent cations alone. Chem Biol. 1998;5(10):587–95. 10.1016/S1074-5521(98)90116-8 [DOI] [PubMed] [Google Scholar]
- 33. Roth A, Weinberg Z, Chen AG, et al. : A widespread self-cleaving ribozyme class is revealed by bioinformatics. Nat Chem Biol. 2014;10(1):56–60. 10.1038/nchembio.1386 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Liu Y, Wilson TJ, McPhee SA, et al. : Crystal structure and mechanistic investigation of the twister ribozyme. Nat Chem Biol. 2014;10(9):739–44. 10.1038/nchembio.1587 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Eiler D, Wang J, Steitz TA: Structural basis for the fast self-cleavage reaction catalyzed by the twister ribozyme. Proc Natl Acad Sci U S A. 2014;111(36):13028–33. 10.1073/pnas.1414571111 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Ren A, Košutić M, Rajashankar KR, et al. : In-line alignment and Mg²⁺ coordination at the cleavage site of the env22 twister ribozyme. Nat Commun. 2014;5:5534. 10.1038/ncomms6534 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Gaines CS, York DM: Ribozyme Catalysis with a Twist: Active State of the Twister Ribozyme in Solution Predicted from Molecular Simulation. J Am Chem Soc. 2016;138(9):3058–65. 10.1021/jacs.5b12061 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Kapinos LE, Operschall BP, Larsen E, et al. : Understanding the acid-base properties of adenosine: the intrinsic basicities of N1, N3 and N7. Chemistry. 2011;17(29):8156–64. 10.1002/chem.201003544 [DOI] [PubMed] [Google Scholar]
- 39. Breaker RR: Mechanistic Debris Generated by Twister Ribozymes. ACS Chem Biol. 2017;12(4):886–91. 10.1021/acschembio.7b00010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Gaines CS, Giese TJ, York DM: Cleaning Up Mechanistic Debris Generated by Twister Ribozymes Using Computational RNA Enzymology. ACS Catal. 2019;9(7):5803–15. 10.1021/acscatal.9b01155 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Hampel A, Cowan JA: A unique mechanism for RNA catalysis: the role of metal cofactors in hairpin ribozyme cleavage. Chem Biol. 1997;4(7):513–7. 10.1016/S1074-5521(97)90323-9 [DOI] [PubMed] [Google Scholar]
- 42. Hegg LA, Fedor MJ: Kinetics and thermodynamics of intermolecular catalysis by hairpin ribozymes. Biochemistry. 1995;34(48):15813–28. 10.1021/bi00048a027 [DOI] [PubMed] [Google Scholar]
- 43. Young KJ, Gill F, Grasby JA: Metal ions play a passive role in the hairpin ribozyme catalysed reaction. Nucleic Acids Res. 1997;25(19):3760–6. 10.1093/nar/25.19.3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nakano S, Chadalavada DM, Bevilacqua PC: General acid-base catalysis in the mechanism of a hepatitis delta virus ribozyme. Science. 2000;287(5457):1493–7. 10.1126/science.287.5457.1493 [DOI] [PubMed] [Google Scholar]
- 45. Ferré-D'Amaré AR, Zhou K, Doudna JA: Crystal structure of a hepatitis delta virus ribozyme. Nature. 1998;395(6702):567–74. 10.1038/26912 [DOI] [PubMed] [Google Scholar]
- 46. Ke A, Zhou K, Ding F, et al. : A conformational switch controls hepatitis delta virus ribozyme catalysis. Nature. 2004;429(6988):201–5. 10.1038/nature02522 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Das SR, Piccirilli JA: General acid catalysis by the hepatitis delta virus ribozyme. Nat Chem Biol. 2005;1(1):45–52. 10.1038/nchembio703 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Liu Y, Wilson TJ, Lilley DMJ: The structure of a nucleolytic ribozyme that employs a catalytic metal ion. Nat Chem Biol. 2017;13(5):508–13. 10.1038/nchembio.2333 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Zheng L, Mairhofer E, Teplova M, et al. : Structure-based insights into self-cleavage by a four-way junctional twister-sister ribozyme. Nat Commun. 2017;8(1):1180. 10.1038/s41467-017-01276-y [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Gaines CS, York DM: Model for the Functional Active State of the TS Ribozyme from Molecular Simulation. Angew Chem Int Ed Engl. 2017;56(43):13392–5. 10.1002/anie.201705608 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Wilson TJ, Liu Y, Li NS, et al. : Comparison of the Structures and Mechanisms of the Pistol and Hammerhead Ribozymes. J Am Chem Soc. 2019;141(19):7865–7875. 10.1021/jacs.9b02141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Harris KA, Lünse CE, Li S, et al. : Biochemical analysis of pistol self-cleaving ribozymes. RNA. 2015;21(11):1852–8. 10.1261/rna.052514.115 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Martick M, Scott WG: Tertiary contacts distant from the active site prime a ribozyme for catalysis. Cell. 2006;126(2):309–20. 10.1016/j.cell.2006.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Ren A, Vušurović N, Gebetsberger J, et al. : Pistol ribozyme adopts a pseudoknot fold facilitating site-specific in-line cleavage. Nat Chem Biol. 2016;12(9):702–8. 10.1038/nchembio.2125 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Nguyen LA, Wang J, Steitz TA: Crystal structure of Pistol, a class of self-cleaving ribozyme. Proc Natl Acad Sci U S A. 2017;114(5):1021–6. 10.1073/pnas.1611191114 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Han J, Burke JM: Model for general acid-base catalysis by the hammerhead ribozyme: pH-activity relationships of G8 and G12 variants at the putative active site. Biochemistry. 2005;44(21):7864–70. 10.1021/bi047941z [DOI] [PubMed] [Google Scholar]
- 57. Thomas JM, Perrin DM: Probing general acid catalysis in the hammerhead ribozyme. J Am Chem Soc. 2009;131(3):1135–43. 10.1021/ja807790e [DOI] [PubMed] [Google Scholar]
- 58. Lee TS, Silva López C, Giambaşu GM, et al. : Role of Mg 2+ in hammerhead ribozyme catalysis from molecular simulation. J Am Chem Soc. 2008;130(10):3053–64. 10.1021/ja076529e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Klein DJ, Ferré-D'Amaré AR: Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science. 2006;313(5794):1752–6. 10.1126/science.1129666 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Cochrane JC, Lipchock SV, Strobel SA: Structural investigation of the GlmS ribozyme bound to Its catalytic cofactor. Chem Biol. 2007;14(1):97–105. 10.1016/j.chembiol.2006.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Klein DJ, Been MD, Ferré-D'Amaré AR: Essential role of an active-site guanine in glmS ribozyme catalysis. J Am Chem Soc. 2007;129(48):14858–9. 10.1021/ja0768441 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Li S, Lünse CE, Harris KA, et al. : Biochemical analysis of hatchet self-cleaving ribozymes. RNA. 2015;21(11):1845–51. 10.1261/rna.052522.115 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation