Version Changes
Revised. Amendments from Version 1
In this updated version an additional table (Table 9) has been added with an overview of the different administration routes of DMSO used in the included studies.
Abstract
Background: Dimethyl sulfoxide (DMSO) has been used for medical treatment and as a pharmacological agent in humans since the 1960s. Today, DMSO is used mostly for cryopreservation of stem cells, treatment of interstitial cystitis, and as a penetrating vehicle for various drugs. Many adverse reactions have been described in relation to the use of DMSO, but to our knowledge, no overview of the existing literature has been made. Our aim was to conduct a systematic review describing the adverse reactions observed in humans in relation to the use of DMSO.
Methods: This systematic review was reported according to the PRISMA-harms (Preferred Reporting Items for Systematic reviews and Meta-Analysis) guidelines. The primary outcome was any adverse reactions occurring in humans in relation to the use of DMSO. We included all original studies that reported adverse events due to the administration of DMSO, and that had a population of five or more.
Results: We included a total of 109 studies. Gastrointestinal and skin reactions were the commonest reported adverse reactions to DMSO. Most reactions were transient without need for intervention. A relationship between the dose of DMSO given and the occurrence of adverse reactions was seen.
Conclusions: DMSO may cause a variety of adverse reactions that are mostly transient and mild. The dose of DMSO plays an important role in the occurrence of adverse reactions. DMSO seems to be safe to use in small doses.
Registration: PROSPERO CRD42018096117.
Keywords: Dimethyl Sulfoxide, DMSO, Adverse reactions, Toxicology
Introduction
The first medical report on the use of dimethyl sulfoxide (DMSO) as a pharmacological agent was published in 1964 1. A year later, the use of DMSO in humans was terminated because experimental studies had shown refractive index changes to the lens of the eye 1, 2. Years later, DMSO was again approved for use in humans since this side effect was only proven in animal studies 2. DMSO has since been used for a variety of purposes, such as treatment of musculoskeletal and dermatological diseases, cryopreservation of stem cells, treatment of interstitial cystitis, treatment of increased intracranial pressure, and many more 3– 9.
DMSO is a colourless liquid, which is rapidly absorbed when administered dermally or orally 10, 11. DMSO is used as a cryoprotectant because it decreases osmotic stress and cellular dehydration, and thereby enables stem cells to be stored for several years 12. DMSO is mostly excreted through the kidneys, but a small part is excreted through the lungs and liver 10. Part of the DMSO is transformed to the volatile metabolite dimethyl sulfide, which gives a characteristic garlic- or oyster-like smell when excreted through the lungs 10. DMSO may induce histamine release, which can be the reason for adverse reactions such as flushing, dyspnoea, abdominal cramps, and cardiovascular reactions 11.
To our knowledge, no systematic reviews have been performed on the adverse reactions of DMSO. Our aim was therefore to provide an extensive overview of the suspected adverse reactions to DMSO in humans.
Methods
Protocol and eligibility criteria
Our study-protocol is registered at PROSPERO (Registration number: CRD42018096117). The systematic review was performed according to PRISMA-harms (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines 13.
No limitations were set on the date of publication. The language was restricted to English, Danish, Swedish, Norwegian, and Russian. We included all original studies that administered DMSO to humans and included five or more participants. There was no gender or age restriction. For a study to be included, the authors had to suspect that an observed adverse reaction could be caused by DMSO.
Primary outcome
The primary outcome was any adverse reaction seen in relation to the use of DMSO in humans.
Literature search
The search was performed in PubMed (1966-present), EMBASE (1980-present), and the Cochrane Library. The databases were last searched on February 23, 2018. Our search strategy was formulated with the help of a medical research librarian.
The search string used in PubMed was: ((dimethyl sulfoxide) OR DMSO) AND ((((((administration and dosage) OR adverse reactions) OR alternate effects) OR secondary response) OR toxicology) OR side effects)). The search was restricted to humans. The search string was adapted to EMBASE and Cochrane Library using the same search-words as abovementioned.
The search string used in EMBASE was: ((dmso or dimethyl sulfoxide) and ((side effect or toxicology or secondary response or alternate effects or alternate reactions or (administration and dosage)) and (dmso or dimethyl sulfoxide))).mp. The search was restricted to humans, articles and Medline journals were excluded.
The search string used in Cochrane was: (adverse drug events and dimethyl sulfoxide). The search was restricted to trials.
Study selection and data extraction
Two authors (B.K.M. and D.Z.) independently screened title and abstract according to the eligibility criteria using www.covidence.org. Discrepancies were resolved by discussion. One author screened the full-text articles (B.K.M.). Russian articles were screened by an author fluent in Russian (M.H.). If M.H. was in doubt regarding inclusion of a study the results were presented to B.K.M. and then discussed until a mutual decision was made.
After the screening process was finished, all included studies were imported to an Excel sheet (Microsoft Excel 2016). Data extraction was performed by two authors (M.H. extracted from the Russian articles and B.K.M. extracted from the rest). Data extracted were: author, publication year, country, study characteristics (study design, sample size, size of comparison group if present, time to follow-up), use of DMSO (reason for use, treatment duration, administration route, dose of DMSO), and adverse reactions observed (number of persons experiencing an adverse reaction, method of assessing, and duration of adverse reaction).
Analysis
The Newcastle-Ottawa-Scale was used to assess the risk of bias in non-randomized observational studies 14. Risk of bias in randomized controlled trials was assessed using the Cochrane Handbook “Risk of Bias” assessment tool 15. Risk of bias was assessed at the outcome level.
The primary summary measure was percentage of persons experiencing an adverse reaction, as well as the range in which a reaction occurred in the studies included. No meta-analysis and further summery measures were planned due to the expected large heterogeneity of the studies.
Results
Study selection
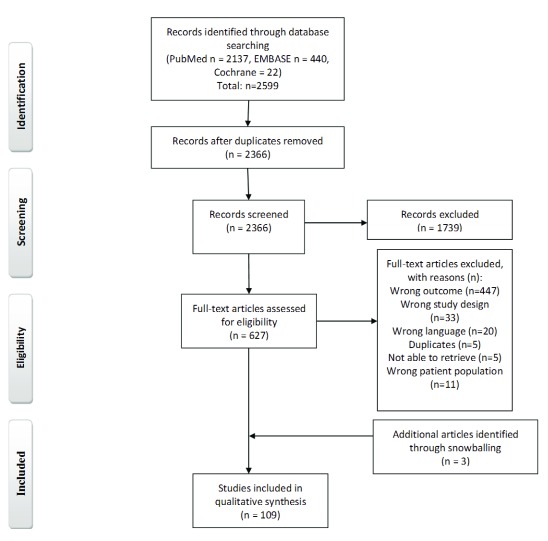
Our primary search identified 2599 studies ( Figure 1). After the evaluation process, 109 studies were included in the final review 2, 4, 6– 9, 16– 118.
Figure 1. PRISMA flow diagram.
Gastrointestinal reactions
Gastrointestinal adverse reactions were reported in 61 studies. Of these, 10 studies were randomized controlled trials 16, 30, 33, 55, 57, 59, 67, 79, 93, 95, 49 were cohort studies 2, 4, 7, 9, 18, 19, 23, 25– 27, 29, 35, 38– 43, 45, 46, 48, 50– 54, 58, 60, 66, 68, 69, 71, 73, 83, 85– 88, 90, 94, 97, 98, 101, 104, 105, 112, 113, 115, 118, and 2 were case series 84, 109. Most studies reported the number of patients experiencing an adverse reaction ( Table 1). Other studies reported adverse reactions observed in relation to the number of treatments given ( Table 2).
Table 1. Gastrointestinal adverse reactions observed per number of patients.
| Adverse reaction | Studies | Total
patients, n |
Patients
with adverse reaction, n (%) |
(%, min-max) † |
|---|---|---|---|---|
| Nausea (overall incidence) | [ 2, 18, 27, 33, 45, 46, 48, 53, 55, 57, 59, 60, 67, 84, 90, 93, 109, 118] | 2214 | 257 (12) | (2–41) [ 55] - [ 48] |
| Intravenous administration | [ 18, 27, 33, 46, 48, 53, 59, 90, 118] | 1154 | 199 (17) | (2–41) [ 59] - [ 48] |
| Transdermal application | [ 2, 45, 55, 57, 67, 93, 109] | 1039 | 51 (5) | (2–32) [ 55] - [ 2] |
| >1 administration route | [ 60, 84] | 21 | 7 (33) | (29–36) [ 84] - [ 60] |
| Vomiting (overall incidence) | [ 2, 18, 27, 33, 46, 48, 55, 57, 59, 118] | 1611 | 115 (7) | (0–64) [ 55] - [ 48] |
| Intravenous administration | [ 18, 27, 33, 46, 48, 59, 118] | 972 | 108 (11) | (2–64) [ 59] - [ 48] |
| Transdermal application | [ 2, 55, 57] | 639 | 7 (1) | (0–6) [ 55] - [ 2] |
| Nausea and vomiting ‡ | [ 7, 38, 41, 54, 66, 69, 73, 85, 87, 115] | 4529 | 591 (13) | (0–46) [ 66] - [ 73] |
|
Abdominal cramps/stomach
ache (overall incidence) |
[ 18, 26, 27, 39, 41, 54, 55, 59, 73, 85, 87, 93, 115] | 1629 | 88 (5) | (1–52) [ 117] - [ 116] |
| Intravenous administration | [ 18, 26, 27, 39, 41, 54, 59, 73, 85, 87, 115] | 1253 | 72 (6) | (1–52) [ 18] - [ 26] |
| Transdermal application | [ 55, 93] | 376 | 16 (4) | (2–16) [ 55] - [ 93] |
|
Halitosis/garlic-like breath
(overall incidence) |
[
4,
9,
16,
19,
29,
30,
35,
42,
43,
45,
50,
52,
55,
57,
58,
66–
68,
79,
83,
85, 88, 94, 95, 97, 98, 109, 112, 113] |
5782 | 607 (11) | (0–100) [
30] -
[ 19, 43, 45, 83, 98] |
| Intravenous administration | [ 16, 85, 94, 98] | 239 | 14 (6) | (1–100) [ 85] - [ 98] |
| Transdermal application | [
4,
19,
29,
30,
42,
45,
50,
52,
55,
57,
58,
66,
67,
79,
83,
88,
95,
109,
112, 113] |
5333 | 556 (10) | (0–100) [
30]
- [ 19, 45, 83] |
| Intravesical administration | [ 35, 43, 97] | 165 | 33 (20) | (1–100) [ 35] - [ 43] |
| Oral administration | [ 9] | 15 | 4 (27) | |
| Diarrhea (overall incidence) | [ 2, 18, 41, 54, 57, 85, 93] | 1107 | 27 (2) | (1–6) [ 85] - [ 93] |
| Intravenous administration | [ 18, 41, 54, 85] | 744 | 15 (2) | (1–6) [ 85] - [ 41] |
| Transdermal application | [ 2, 57, 93] | 363 | 12 (3) | (2–6) [ 57] - [ 93] |
†Incidences of the adverse reactions have been calculated for all the individual studies. (min%–max%) are the lowest and highest observed incidence of an adverse reaction observed in the group of studies included.
‡ Nausea and vomiting are reported as one combined adverse reaction in some studies.
Table 2. Gastrointestinal adverse reactions observed per number of treatments.
| Adverse reaction | Studies | Total
treatments, n |
Adverse reactions
observed, n (%) |
(min%–max%) † |
|---|---|---|---|---|
| Nausea (overall incidence) | [ 40, 51, 68, 84, 105] | 474 | 161 (34) | (16–57) [ 105] - [ 40] |
| Intravenous administration | [ 40, 51, 68] | 323 | 137 (42) | (41–57) [ 68] - [ 40] |
| Intravesical administration | [ 105] | 151 | 24 (16) | |
| Vomiting ‡ | [ 51, 68] | 316 | 112 (35) | (29 - 71) [ 68] - [ 51] |
| Nausea and/or vomiting ‡ | [ 25, 74, 101] | 1557 | 220 (14) | (8–17) [ 25] - [ 101] |
| Abdominal cramps/stomach ache ‡ | [ 51, 68, 101] | 495 | 16 (5) | (1–19) [ 68] - [ 51] |
| Halitosis ‡ | [ 68] | 262 | 4 (2) | |
| Diarrhea ‡ | [ 51, 101] | 233 | 2 (1) | (1–2) [ 101] - [ 51] |
† Incidences of the adverse reactions have been calculated for all the individual studies. (min%–max%) are the lowest and highest observed incidence of an adverse reaction.
‡ Intravenous administration.
The most commonly reported gastrointestinal adverse reactions were nausea and vomiting. The incidence of nausea seems to be less common with the transdermal administration of DMSO compared with intravenous administration. The majority of studies reported an incidence of nausea between 2–14%, with the exception of one study, reporting an incidence of 32% 2. In one study that failed to specify the dose, 8 of 42 patients reported nausea and anorexia, but the symptoms disappeared in five of the eight patients when the dose of DMSO was reduced 45.
Often the studies had short follow-up periods (less than 24 hours), especially when DMSO was used as a cryoprotectant. The study reporting the highest incidence of nausea had a follow-up period of 5 days 48, and the authors concluded that the high incidence of nausea observed might be due to the long follow-up period 48. In another article using the same data 119, it was suggested that the delayed nausea was due to gastrointestinal mucosal damage, and only the initial nausea could be related to DMSO, and therefore we decided only to include the data from the first 2 days after infusion 48.
Halitosis was reported in 29 studies 4, 9, 16, 19, 29, 30, 35, 42, 43, 45, 50, 52, 55, 57, 58, 66– 68, 79, 83, 85, 88, 94, 95, 97, 98, 109, 112, 113. In five studies, patients discontinued treatment due to halitosis 9, 45, 83, 94. In five studies, all patients experienced halitosis 9, 45, 83, 94. Unlike halitosis, other gastrointestinal side effects were reported more often when DMSO was administered intravenously, than transdermally or intravesically.
One study reported a severe case of nausea, vomiting, and abdominal cramps in one patient with an acute allergic reaction 59. However, in most studies the reported gastrointestinal reactions were transient and mild, often lasting only minutes to a couple of hours 16, 38, 41, 68, 85, 87, 90. Several studies reported a relationship between the dose of DMSO and the occurrence of gastrointestinal adverse reactions 26, 33, 53, 73, 83, 85.
Cardiovascular and respiratory reactions
Cardiovascular and respiratory adverse reactions were reported in 33 studies. Of these, two were randomized controlled trials 33, 59, 30 were cohort studies 7, 18, 23, 25– 27, 36, 39– 41, 51, 54, 61, 65, 66, 68, 73, 74, 80, 85– 87, 90, 100– 102, 104, 115, 117, and one was a preliminary report 91. Except for one study 66, all studies reporting cardiovascular and respiratory reactions administered DMSO intravenously ( Table 3 and Table 4).
Table 3. Cardiovascular and respiratory adverse reactions observed per number of patients.
| Adverse reaction | Studies | Total
patients, n |
Patients with adverse
reactions, n (%) |
(min%–max%) † |
|---|---|---|---|---|
| Cardiac | ||||
| Hypotension ‡ | [ 7, 18, 23, 33, 71, 73, 87, 104, 115] | 2752 | 115 (4) | (1–14) [ 18, 71] - [ 87] |
| Hypertension § | [ 7, 18, 23, 33, 41, 54, 61, 73, 85, 87, 102] | 2998 | 385 (13) | (2–95) [ 85] - [ 61] |
| Bradycardia (mild and severe) ‡ | [ 23, 36, 54, 61, 65, 85, 90, 91, 115, 117] | 882 | 94 (11) | (0–49) [ 36] - [ 61] |
| Decrease in heart rate ‡ | [ 41, 54, 61, 80] | 193 | 152 (79) | (11–94) [ 80] - [ 41] |
| Tachycardia ‡ | [ 23, 27, 36] | 565 | 13 (2) | (0–6) [ 36] - [ 23] |
| Ventricular extrasystoles ‡ | [ 73] | 22 | 11 (50) | |
| Cardiac event, unspecified ‡ | [ 26, 86] | 165 | 18 (11) | (5–12) [ 26] - [ 86] |
| Asystole ¶ | [ 91, 100] | 45 | 3 (7) | (3–20) [ 100] - [ 91] |
| Left cardiac insufficiency | [ 85] | 194 | 1 (1) | |
| Chest discomfort/tightness ‡ | [ 18, 27, 54, 73, 87, 91, 115] | 901 | 22 (2) | (1–10) [ 27] - [ 54] |
| Respiratory | ||||
|
Unspecified respiratory
symptoms ‡ |
[ 26, 86] | 165 | 43 (26) | (21–62) [ 86] - [ 26] |
| Dyspnea d | [ 18, 27, 54, 66, 85] | 2748 | 26 (1) | (0–10) [ 66] - [ 54] |
| Cough | [ 85, 101] | 373 | 52 (14) | (5–22) [ 101] [ 85] |
| Lung edema ‡ | [ 59, 85] | 241 | 3 (1) | (1–2) [ 85] - [ 59] |
†Incidences of the adverse reactions have been calculated for all the individual studies. (min%–max%) are the lowest and highest observed incidence of an adverse reaction.
‡ DMSO was administered intravenously in all studies.
§ DMSO was administered intravenously in all studies. Horacek et al. [ 102] measured 42 patients with an increase in systolic blood pressure, and 31 patients with an increase in diastolic blood pressure. This was counted as 73 cases of hypertension.
¶ In both studies, asystole occurred because of DMSO effect on the trigeminal nerve and activation of the trigeminal cardiac reflex. d) in one study DMSO was administered transdermally
Table 4. Cardiovascular and respiratory adverse reactions observed per number of treatments.
| Adverse reaction | Studies | Total number of
treatments |
Adverse reactions
observed, n (%) |
(min%–max%) † |
|---|---|---|---|---|
| Cardiac | ||||
| Hypotension ‡ | [ 40, 51, 68] | 323 | 10 (3) | (2–14) [ 68] - [ 40] |
| Hypertension ‡ | [ 25, 51, 68] | 425 | 60 (14) | (3–21) [ 25] - [ 68] |
| Bradycardia (mild and severe) ‡ | [ 51] | 54 | 4 (7) | |
| Decrease in heartrate ‡ | [ 39] | 32 | 30 (94) | |
| Tachycardia ‡ | [ 51] | 54 | 4 (7) | |
| Cardiac event, unspecified ‡ | [ 74] | 1269 | 35 (3) | |
| Chest discomfort/tightness ‡ | [ 25, 68, 74] | 1640 | 83 (5) | (0–6) [ 68] - [ 74] |
| Respiratory | ||||
| Dyspnea | [ 25, 68] | 371 | 3 (1) | (0–2) [ 68] - [ 25] |
| Shortness of breath ‡ | [ 74] | 1269 | 40 (3) |
†Incidences of the adverse reactions have been calculated for all the individual studies. (%, min-max) are the lowest and highest observed incidence of an adverse reaction observed in the group of studies included.
‡ DMSO was administered intravenously.
Bradycardia was defined as a heart rate less than 60 beats per minute 41, 61 and was often transient 23, 61, 90, 115, but cases where atropine was needed are described 49, 96. A lowered heart rate not enough to be considered bradycardia was observed in four studies 39, 41, 54, 61.
In some studies, hypertension did not require intervention 61, 102, but cases where medication was needed to control the hypertension, or where treatment was stopped due to hypertension, are described 41, 54, 85. Hypotension was also described as transient most of the time 18, 23, 68, 87, 104, with some cases needing intervention 40, 51, 54.
One study reported 11 cases of transient extrasystoles in 22 patients receiving cryopreserved autologous blood stem cells, monitored with Holter during infusion 73. There were two studies reporting cases of asystole during embolization of dural arteriovenous fistulas with a substance called Onyx, a non-adhesive liquid embolic agent dissolved in DMSO 91, 100.
Dyspnea was reported in seven studies 18, 25, 27, 54, 66, 68, 85. A single study reported eight patients with transient shock after stem cell transfusion 51. Some of these patients developed loss of consciousness and cyanosis but recovered promptly and had no need for additional therapy, whereas the rest of the patients developed severe hypotension or transient dyspnea, which was described as the reason for the transient shock. Further description of the condition was not provided.
Several of the studies found a correlation between the dose of DMSO used and the incidence of cardiovascular adverse reactions 41, 67, 71, 75, 78, 85, 86, 93, 101, 115.
Dermatological reactions
Dermatological side effects are common when DMSO is administered transdermally. Skin reactions or allergic reactions were reported in 58 studies. DMSO was applied transdermally in 43 studies 2, 4, 6, 17, 19– 22, 24, 28– 32, 37, 44, 45, 52, 55, 57, 63, 64, 66, 67, 69, 72, 75, 76, 78, 79, 82, 83, 88, 89, 93, 95, 96, 106, 108, 109, 111– 113, intravenously in 14 studies 25, 40, 41, 51, 59, 73, 74, 77, 85, 86, 92, 98, 101, 110 and intraarticular in one 103 ( Table 5).
Table 5. Dermatological and allergic adverse reactions observed per number of patients.
| Adverse reactions | Studies | Total
patients, n |
Patients
with adverse reactions, n (%) |
(%, min-max) † |
|---|---|---|---|---|
| Skin reactions | ||||
| Erythema ‡ | [ 19, 32, 64, 66, 82, 95] | 2352 | 201 (9) | (3–95) [ 95] - [ 82] |
| Itching/Pruritus ‡ | [ 6, 55, 57, 64, 66, 72, 82, 93] | 3421 | 215 (6) | (0–70) [ 55] - [ 82] |
| Urticaria ‡ | [ 24, 31, 83] | 58 | 9 (16) | (4–59) [ 24] - [ 83] |
| Rash | [ 29, 30, 55, 57, 64, 93, 101, 111] | 2682 | 121 (5) | (1–40) [ 30] - [ 93] |
|
Paresthesia/burning or
stinging sensation § ‡ |
[ 17, 21, 24, 28, 30, 44, 45, 55, 57, 67, 69, 79, 91, 93, 106] | 2141 | 335 (16) | (0–100) [ 30] - [ 45] |
|
Scaling of skin/desquamation/
dry skin/local irritant ‡ |
[ 22, 29, 30, 37, 52, 55, 57, 64, 66, 69, 75, 82, 88, 89, 106] | 4739 | 731 (15) | (1–96) [ 66] - [ 52] |
| Blistering ‡ | [ 31, 32, 66, 69, 93, 112] | 2038 | 79 (4) | (3–20) [ 66] - [ 112] |
|
Roughness and/or thickening
of skin ‡ |
[ 66, 82, 93] | 1986 | 191 (10) | (6–10) [ 93] - [ 82] |
|
Bullous dermatitis/dermatitis
with vesicles ‡ |
[ 20, 29, 64] | 1116 | 79 (7) | (1–9) [ 64] - [ 29] |
| Contact dermatitis ‡ | [ 6, 20, 28– 30, 64, 111] | 2587 | 161 (6) | (1–13) [ 28] - [ 29] |
| Skin reaction, unspecified ‡ | [ 2, 78, 96, 113] | 457 | 159 (35) | (4–48) [ 96] - [ 113] |
| Increase in skin pigmentation ‡ | [ 6] | 548 | 28 (5) | |
| Peripheral edema ‡ | [ 45, 55, 66, 109] | 2291 | 22 (0) | (1–14) [ 66] - [ 109] |
| Allergic reactions | [ 37, 44, 59, 86, 98, 110] | 309 | 75 (24) | (3–55) [ 44, 110] - [ 86] |
| Intravenous administration | [ 59, 86, 98, 110] | 229 | 66 (29) | (2–55) [ 59] - [ 86] |
| Transdermal application | [ 37, 44] | 86 | 9 (10) | (3–19) [ 44] - [ 37] |
| Flushing ¶ | [ 41, 54, 73] | 292 | 34 (12) | (2–9) [ 54] - [ 73] |
†Incidences of the adverse reactions have been calculated for all the individual studies. (min%–max%) are the lowest and highest observed incidence of an adverse reaction observed in the group of studies included.
‡ Transdermal application only.
§ One study administered DMSO through intraarticular injection [ 38].
¶ DMSO was administered intravenously in all studies.
The most common skin reaction was a local burning sensation reported in 13 studies 17, 21, 24, 28, 30, 45, 55, 57, 67, 69, 79, 93, 106. In one study, all participants experienced this burning sensation 45. In the same study, four participants experienced a transient peripheral edema associated with itching and erythema 45. A single study described a burning sensation in four of 669 patients when DMSO was given as a local injection 92; another study described burning in two out of 17 patients when DMSO was injected intraarticularly 103.
Most skin reactions were transient, only lasting minutes 17, 24, 32, 67, 72, but some studies reported cases described as serious, causing discontinuation of treatment 2, 6, 52, 63, 78, 96. There were two studies describing that skin reactions to DMSO would disappear after days of continuous treatment 45, 83. Another study reported that 1 of 18 patients treated for psoriasis with DMSO was hospitalized due to exfoliative erythroderma 63. In another study, two patients, diagnosed with dermographia developed prominent areas of weals after DMSO application 95.
Acute allergic reactions due to use of DMSO were reported in six studies 37, 44, 59, 86, 98, 110. One study reported that 63 of 144 patients experienced allergic reactions, which was not described as serious adverse events (bronchospasms, facial flushing, rash) 86. In two other studies, acute allergic reactions were characterized as serious adverse events 59, 110.
Flushing was regarded as an allergic reaction in this review and was only reported when DMSO was administered intravenously 25, 40, 41, 51, 54, 73, 74. A total of four studies, not depicted in Table 5, reported 204 cases of flushing during 1439 stem cell infusions 25, 40, 51, 74. Several studies observed a relationship between the dose of DMSO and the occurrence of adverse reactions 67, 75, 78, 83, 88, 93.
Neurological reactions
Headache is the most common neurological adverse reaction reported. In one study, headache was the reason for withdrawal of 2 out of 21 patients being treated with DMSO 116.
Three studies using DMSO as a cryoprotectant in stem cell transfusions described seizures after administration 18, 36, 47. Severe encephalopathy was observed in one patient 99, and transient cranial nerve III and IV palsy was observed in one patient after Onyx embolization 34. One study described neurological symptoms occurring during and after transfusion, but they did not define neurological symptoms in detail 86.
Urogenital reactions
Few urogenital reactions were described ( Table 6 and Table 7). Hemoglobinuria was described as an adverse reaction seen after transfusion of stem cell products 39, 51, 56, 73. However, hemoglobinuria is often attributed to erythrocyte debris in the transplant material and has thus not been interpreted as being caused by DMSO 39, 73. The other urogenital reactions ( Table 6 and Table 7) all occurred after DMSO instillation in the bladder 38, 49, 97.
Table 6. Neurological and urogenital adverse reactions observed per number of patients.
| Adverse reaction | Studies | Total
patients, n |
Patients
with adverse reactions, n (%) |
(min%–max%) † |
|---|---|---|---|---|
| Neurological | ||||
| Headache | [ 2, 18, 29, 33, 38, 41, 55, 59, 70, 71, 81, 84, 85, 98, 101, 104, 116] | 2516 | 150 (6) | (1–50) [ 101] - [ 70] |
| Intravenous administration | [ 18, 33, 41, 59, 70, 71, 81, 85, 98, 101, 104] | 1271 | 42 (3) | (1–50) [ 101] - [ 70] |
| Transdermal application | [ 2, 29, 55] | 1197 | 102 (8) | (5–35) [ 55] - [ 2] |
| Intravesical administration | [ 38] | 20 | 1 (5) | |
| Rectal administration | [ 116] | 21 | 3 (14) | |
| >1 administration route | [ 84] | 7 | 2 (29) | |
| Seizures | [ 18, 36, 47] | 301 | 2 (1) | (0–2) [ 18] - [ 47] |
|
Neurological symptoms,
unspecified |
[ 86] | 144 | 5 (3) | |
| Transient CN III and IV palsy | [ 34] | 12 | 1 (8) | |
| Severe encephalopathy | [ 99] | 124 | 1 (1) | |
| Urogenital | ||||
|
Pelvic discomfort/pain/
irritation |
[ 38, 49, 97] | 107 | 10 (9) | (6–30) [ 49] - [ 38] |
| Dysuria/strangury | [ 49] | 36 | 6 (17) | |
| Renal and urinary disorder | [ 49] | 36 | 8 (22) |
†Incidences of the adverse reactions have been calculated for all the individual studies. (min%–max%) are the lowest and highest observed incidence of an adverse reaction observed in the group of studies included.
Table 7. Neurological and urogenital adverse reactions observed per number of treatments.
| Adverse reaction | Studies | Total
treatments, n |
Adverse reactions
observed, n (%) |
(min%–max%) † |
|---|---|---|---|---|
| Neurological | ||||
| Headache | [ 39, 51] | 86 | 40 (47) | (6 - 73) [ 39] - [ 51] |
| Urogenital | ||||
| Urethral irritation | [ 73] | 151 | 110 (73) |
†Incidences of the adverse reactions have been calculated for all the individual studies. (%, min-max) are the lowest and highest observed incidence of an adverse reaction observed in the group of studies included.
Other reactions
Only one study in this review administered DMSO as eye-drops 114. In this study, two patients experienced severe conjunctival hyperemia due to allergic reactions, and 25% of patients experienced a stinging sensation when eye-drops were applied 114. Other studies performed eye examinations to determine whether DMSO caused changes in the lens; however, no such cases were observed 2, 45.
Hyponatremia occurred in six patients after they received large doses of DMSO as treatment for cranial hypertension 62. This adverse reaction was not reported in other studies ( Table 8).
Table 8. Other adverse reactions observed per number of patients.
| Adverse
reaction |
Studies | Total
patients, n |
Patients with
reaction, n (%) |
(min%–max%) † |
|---|---|---|---|---|
| Fever | [ 27, 71, 73, 77, 101] | 547 | 44 (8) | (2–19) [ 27] - [ 77] |
| Chills | [ 27, 33, 70, 71, 81, 85, 101] | 852 | 60 (7) | (1–31) [ 101] - [ 71] |
| Dizziness | [ 2, 46, 55, 85, 101] | 885 | 18 (2) | (1–15) [ 55] - [ 2] |
| Weakness | [ 33, 45, 46] | 293 | 19 (6) | (1–29) [ 46] - [ 45] |
| Sedation | [ 2] | 78 | 34 (44) | |
| Hyponatremia | [ 62] | 6 | 6 (100) |
†Incidences of the adverse reactions have been calculated for all the individual studies. (%, min-max) are the lowest and highest observed incidence of an adverse reaction observed in the group of studies included.
Very few cases of serious adverse reactions associated with DMSO have been described 18, 36, 51, 59.
Overall, most studies administered DMSO intravenously or transdermally ( Table 9)
Table 9. Way of administration of DMSO in included studies.
| Administration | Number
of studies |
References |
|---|---|---|
| Intravenous | 49 | [
7,
16,
18,
23,
25,
26,
33,
34,
36,
39–
41,
46–
48,
51,
53,
54,
56,
59,
61,
62,
65,
68,
70–
74,
77, 80, 81, 84– 87, 90, 91, 94, 98– 102, 104, 110, 115, 117, 118] |
| Transdermal | 48 | [
2,
4,
6,
17,
19–
22,
24,
28–
32,
37,
42,
44,
45,
50,
52,
55,
57,
58,
63,
64,
66,
67,
69,
72,
75,
76,
78,
79,
82– 84, 88, 89, 93, 95, 96, 106– 109, 111– 113] |
| Intravesical | 7 | [ 8, 35, 38, 43, 49, 97, 105] |
| Oral | 2 | [ 9, 60] |
| Eye-drops | 1 | [ 114] |
| Local injection | 1 | [ 92] |
| Intra-articular | 1 | [ 103] |
| Rectal | 1 | [ 116] |
Risk of bias within studies
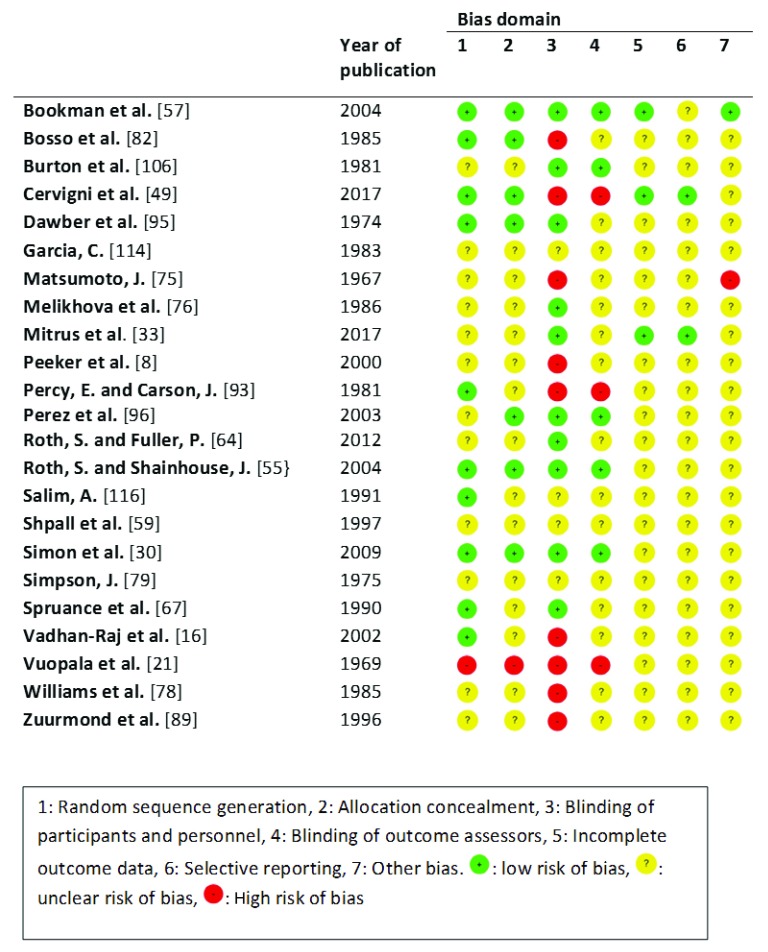
In this review, we included 76 cohort studies, of which 64 were prospective 2, 4, 6, 7, 20, 22, 24– 27, 29, 31, 32, 34– 38, 40– 45, 48, 50– 54, 56, 58, 60, 63, 65, 66, 68– 70, 72, 73, 77, 80, 81, 83, 85, 88, 90, 92, 94, 97, 98, 101– 104, 107, 108, 110, 112, 115, 117, 118 and 13 were retrospective 9, 18, 23, 39, 46, 47, 61, 71, 74, 86, 87, 100, 105. Bias was assessed using The Newcastle-Ottawa-Scale 14. Using this scale, studies were given zero to nine stars. A high number of stars equals low risk of bias and vice versa. The studies in this review had a median value of 5 stars, with a range of 2–8. No studies received the highest possible value of nine stars. Very few studies had a comparison group that did not receive DMSO, and often the occurrence of adverse reactions was poorly described. There were 24 randomized controlled trials ( Figure 2). Many studies received an unclear risk of bias because often it was vaguely described how adverse reactions were reported.
Figure 2. Risk of bias in randomized controlled trials.
Overall, there was a high risk of bias when assessing the description of adverse reactions. Some studies were not assessed for bias due to being case-reports, preliminary trials, or because they included more than one study design 17, 19, 28, 62, 84, 91, 99, 109, 111, 113.
Discussion
Gastrointestinal and dermatological adverse reactions were the most commonly reported in the included studies. Cardiac adverse reactions only occurred when DMSO was administered intravenously, whereas dermatological reactions mostly occurred when DMSO was administered on the skin. Serious neurological and cardiac reactions were rare and only described in few studies. There seems to be a dose-response relationship between DMSO and adverse reactions with no or mild reactions in low doses.
Many studies on the use of DMSO have been performed in Russia. These studies have not been readily accessible to the global community due to the language barrier. In this review, we have included not only studies dating back almost 50 years, but also articles written in Russian, which is an important strength of the review. This study has several limitations: 1) Some studies used the NCI-CTC (National Cancer Institute’s Common Terminology Criteria for adverse events), but often no scale was used, and the occurrence of adverse reactions were poorly reported. 2) It was difficult to make conclusions on the frequency of a specific adverse reaction, because the exact number of patients experiencing a reaction was often not stated. 3) Several studies using DMSO as a cryoprotectant concluded that other factors affected the occurrence of adverse reactions 7, 85, 86. One study prospectively looked at the adverse reactions observed in relation to autologous transplantation in 64 European Blood and Marrow Transplant Group centers 7. They had difficulties isolating the effects of DMSO from confounding factors such as cell breakdown products and conditioning chemotherapy. Factors such as age, gender, volume transfused, granulocyte concentration, clumping of transplant material, and amount of red blood cells played a role in the occurrence of adverse reactions 61, 86, 120– 122. Another study believed that acute volume expansion, electrolyte imbalance and vagal responses to the coldness of the freshly thawed infusate were more likely reasons for cardiac arrhythmias during stem cell transfusions than the DMSO infused 123. This differs from other studies, which found a clear connection between dose of DMSO and occurrence of cardiac adverse reactions 41, 67, 71, 75, 78, 85, 86, 93, 101, 115. Therefore, it is possible that some adverse reactions are more or less common than found in this review. The rarer side effects are often reported in case reports, which often did not meet the eligibility criteria in this review. However, we have included several larger studies in this review, and they found a very small occurrence of serious adverse events 7, 55, 66, 74.
In conclusion, adverse reactions due to DMSO are often mild and transient and do not qualify as serious adverse events. Cardiovascular and respiratory adverse reactions occur mostly when DMSO is administered intravenously, whereas dermatological reactions have a higher incidence when DMSO is administered transdermally. An important finding is that the occurrence of adverse reactions seems to be related to the dose of DMSO, and it therefore seems safe to continue the use of DMSO in small doses.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved]
Supplementary material
Supplementary File 1. Completed PRISMA harms checklist.
References
- 1. Brown JH: Dimethyl sulfoxide (DMSO)--a unique therapeutic entity. Aviat Space Environ Med. 1982;53(1):82–8. [PubMed] [Google Scholar]
- 2. Brobyn RD: The human toxicology of dimethyl sulfoxide. Ann N Y Acad Sci. 1975;243:497–506. 10.1111/j.1749-6632.1975.tb25394.x [DOI] [PubMed] [Google Scholar]
- 3. Swanson BN: Medical use of dimethyl sulfoxide (DMSO). Rev Clin Basic Pharm. 1985;5(1–2):1–33. [PubMed] [Google Scholar]
- 4. Paul MM: Interval therapy with dimethyl sulfoxide. Ann N Y Acad Sci. 1967;141(1):586–98. 10.1111/j.1749-6632.1967.tb34928.x [DOI] [PubMed] [Google Scholar]
- 5. Kulah A, Akar M, Baykut L: Dimethyl sulfoxide in the management of patient with brain swelling and increased intracranial pressure after severe closed head injury. Neurochirurgia (Stuttg). 1990;33(6):177–80. 10.1055/s-2008-1053579 [DOI] [PubMed] [Google Scholar]
- 6. Rosenbaum EE, Herschler RJ, Jacob SW: Dimethyl Sulfoxide in Musculoskeletal Disorders. JAMA. 1965;192:309–13. 10.1001/jama.1965.03080170037009 [DOI] [PubMed] [Google Scholar]
- 7. Morris C, de Wreede L, Scholten M, et al. : Should the standard dimethyl sulfoxide concentration be reduced? Results of a European Group for Blood and Marrow Transplantation prospective noninterventional study on usage and side effects of dimethyl sulfoxide. Transfusion. 2014;54(10):2514–22. 10.1111/trf.12759 [DOI] [PubMed] [Google Scholar]
- 8. Peeker R, Haghsheno MA, Holmäng S, et al. : Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis: a prospective, randomized double-blind study. J Urol. 2000;164(6):1912–5, discussion 1915–6. 10.1016/S0022-5347(05)66916-9 [DOI] [PubMed] [Google Scholar]
- 9. Amemori S, Iwakiri R, Endo H, et al. : Oral dimethyl sulfoxide for systemic amyloid A amyloidosis complication in chronic inflammatory disease: a retrospective patient chart review. J Gastroenterol. 2006;41(5):444–9. 10.1007/s00535-006-1792-3 [DOI] [PubMed] [Google Scholar]
- 10. Hucker HB, Miller JK, Hochberg A, et al. : Studies on the absorption, excretion and metabolism of dimethylsulfoxide (DMSO) in man. J Pharmacol Exp Ther. 1967;155(2):309–17. [PubMed] [Google Scholar]
- 11. Kligman AM: Topical Pharmacology and Toxicology of Dimethyl Sulfoxide. 1. JAMA. 1965;193:796–804. 10.1001/jama.1965.03090100042010 [DOI] [PubMed] [Google Scholar]
- 12. Lovelock JE, Bishop MW: Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature. 1959;183(4672):1394–5. 10.1038/1831394a0 [DOI] [PubMed] [Google Scholar]
- 13. Zorzela L, Loke YK, Ioannidis JP, et al. : PRISMA harms checklist: improving harms reporting in systematic reviews. BMJ. 2016;352:i157. 10.1136/bmj.i157 [DOI] [PubMed] [Google Scholar]
- 14. Wells G, Shea B, O’Connell D, et al. : The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Reference Source [Google Scholar]
- 15. Higgins JPT, Altman DG, Sterne JAC: Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT GS. Cochrane Handbook for Systematic Reviews of InterventionsVersion 5.1.0 (updated March 2011). The Cochrane Collaboration.2008. 10.1002/9780470712184.ch8 [DOI] [Google Scholar]
- 16. Vadhan-Raj S, Kavanagh JJ, Freedman RS, et al. : Safety and efficacy of transfusions of autologous cryopreserved platelets derived from recombinant human thrombopoietin to support chemotherapy-associated severe thrombocytopenia: a randomised cross-over study. Lancet. 2002;359(9324):2145–52. 10.1016/S0140-6736(02)09090-6 [DOI] [PubMed] [Google Scholar]
- 17. Ogden HD: Experiences with DMSO in treatment of headache. Ann N Y Acad Sci. 1967;141(1):646–8. 10.1111/j.1749-6632.1967.tb34934.x [DOI] [PubMed] [Google Scholar]
- 18. Martin-Henao GA, Resano PM, Villegas JM, et al. : Adverse reactions during transfusion of thawed haematopoietic progenitor cells from apheresis are closely related to the number of granulocyte cells in the leukapheresis product. Vox Sang. 2010;99(3):267–73. 10.1111/j.1423-0410.2010.01341.x [DOI] [PubMed] [Google Scholar]
- 19. Savastano AA: Clinical experiences with dimethyl sulfoxide (DMSO) in human subjects. Approval must be withheld until safety in extended use is established. R I Med J. 1984;67(3):119–21. [PubMed] [Google Scholar]
- 20. Ivanov OL, Potekaev NS, Aliab’eva AP: [Dimethyl sulfoxide applications in the treatment of erythema nodosum]. Ter Arkh. 1983;55(9):104–7. [PubMed] [Google Scholar]
- 21. Vuopala U, Isomäki H, Kaipainen WJ: Dimethyl sulfoxide (DMSO) ointment in the treatment of rheumatoid arthritis. A double blind study. Acta Rheumatol Scand. 1969;15(2):139–44. 10.3109/rhe1.1969.15.issue-1-4.21 [DOI] [PubMed] [Google Scholar]
- 22. Menne T: Nickel allergy--reliability of patch test. Evaluated in female twins. Derm Beruf Umwelt. 1981;29(6):156–60. [PubMed] [Google Scholar]
- 23. Castillo N, Garcia-Cadenas I, Garcia O, et al. : Few and nonsevere adverse infusion events using an automated method for diluting and washing before unrelated single cord blood transplantation. Biol Blood Marrow Transplant. 2015;21(4):682–7. 10.1016/j.bbmt.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 24. Pandhi R, Kaur I, Kumar B: Lack of effect of dimethylsulphoxide in cutaneous amyloidosis. J Dermatolog Treat. 2002;13(1):11–4. 10.1080/09546630252775180 [DOI] [PubMed] [Google Scholar]
- 25. Halle P, Tournilhac O, Knopinska-Posluszny W, et al. : Uncontrolled-rate freezing and storage at -80 degrees C, with only 3.5-percent DMSO in cryoprotective solution for 109 autologous peripheral blood progenitor cell transplantations. Transfusion. 2001;41(5):667–73. 10.1046/j.1537-2995.2001.41050667.x [DOI] [PubMed] [Google Scholar]
- 26. Syme R, Bewick M, Stewart D, et al. : The role of depletion of dimethyl sulfoxide before autografting: on hematologic recovery, side effects, and toxicity. Biol Blood Marrow Transplant. 2004;10(2):135–41. 10.1016/j.bbmt.2003.09.016 [DOI] [PubMed] [Google Scholar]
- 27. Ozdemir E, Akgedik K, Akdogan S, et al. : The lollipop with strawberry aroma may be promising in reduction of infusion-related nausea and vomiting during the infusion of cryopreserved peripheral blood stem cells. Biol Blood Marrow Transplant. 2008;14(12):1425–8. 10.1016/j.bbmt.2008.09.010 [DOI] [PubMed] [Google Scholar]
- 28. Silvestri DL, Corey L, Holmes KK: Ineffectiveness of topical idoxuridine in dimethyl sulfoxide for therapy for genital herpes. JAMA. 1982;248(8):953–9. 10.1001/jama.1982.03330080035025 [DOI] [PubMed] [Google Scholar]
- 29. Shainhouse JZ, Grierson LM, Naseer Z: A long-term, open-label study to confirm the safety of topical diclofenac solution containing dimethyl sulfoxide in the treatment of the osteoarthritic knee. Am J Ther. 2010;17(6):566–76. 10.1097/MJT.0b013e3181d169b5 [DOI] [PubMed] [Google Scholar]
- 30. Simon LS, Grierson LM, Naseer Z, et al. : Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain. 2009;143(3):238–45. 10.1016/j.pain.2009.03.008 [DOI] [PubMed] [Google Scholar]
- 31. Engel MF: Dimethyl sulfoxide in the treatment of scleroderma. South Med J. 1972;65(1):71–3. [DOI] [PubMed] [Google Scholar]
- 32. Ludwig CU, Stoll HR, Obrist R, et al. : Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23(3):327–9. 10.1016/0277-5379(87)90077-0 [DOI] [PubMed] [Google Scholar]
- 33. Mitrus I, Smagur A, Fidyk W, et al. : Reduction of DMSO concentration in cryopreservation mixture from 10% to 7.5% and 5% has no impact on engraftment after autologous peripheral blood stem cell transplantation: results of a prospective, randomized study. Bone Marrow Transplant. 2018;53(3):274–280. 10.1038/s41409-017-0056-6 [DOI] [PubMed] [Google Scholar]
- 34. Elhammady MS, Wolfe SQ, Farhat H, et al. : Onyx embolization of carotid-cavernous fistulas. J Neurosurg. 2010;112(3):589–94. 10.3171/2009.6.JNS09132 [DOI] [PubMed] [Google Scholar]
- 35. Hung MJ, Chen YT, Shen PS, et al. : Risk factors that affect the treatment of interstitial cystitis using intravesical therapy with a dimethyl sulfoxide cocktail. Int Urogynecol J. 2012;23(11):1533–9. 10.1007/s00192-012-1699-x [DOI] [PubMed] [Google Scholar]
- 36. Lemarie C, Camels B, Malenfant C, et al. : Clinical experience with the delivery of thawed and washed autologous blood cells, with an automated closed fluid management device: CytoMate. Transfusion. 2005;45(5):737–42. 10.1111/j.1537-2995.2005.04126.x [DOI] [PubMed] [Google Scholar]
- 37. Mendelow AY, Forsyth A, Florence AT, et al. : Patch testing for nickel allergy. The influence of the vehicle on the response rate to topical nickel sulphate. Contact Dermatitis. 1985;13(1):29–33. 10.1111/j.1600-0536.1985.tb02488.x [DOI] [PubMed] [Google Scholar]
- 38. Fowler JE, Jr: Prospective study of intravesical dimethyl sulfoxide in treatment of suspected early interstitial cystitis. Urology. 1981;18(1):21–6. 10.1016/0090-4295(81)90489-1 [DOI] [PubMed] [Google Scholar]
- 39. Perseghin P, Balduzzi A, Bonanomi S, et al. : Infusion-related side-effects in children undergoing autologous hematopoietic stem cell transplantation for acute leukemia. Bone Marrow Transplant. England; 2000;26(1):116–8. 10.1038/sj.bmt.1702462 [DOI] [PubMed] [Google Scholar]
- 40. Rowley SD, Feng Z, Yadock D, et al. : Post-thaw removal of DMSO does not completely abrogate infusional toxicity or the need for pre-infusion histamine blockade. Cytotherapy. 1999;1(6):439–46. 10.1080/0032472031000141303 [DOI] [PubMed] [Google Scholar]
- 41. Davis J, Rowley SD, Santos GW: Toxicity of autologous bone marrow graft infusion. Prog Clin Biol Res. 1990;333:531–40. [PubMed] [Google Scholar]
- 42. Bertelli G, Gozza A, Forno GB, et al. : Topical dimethylsulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13(11):2851–5. 10.1200/JCO.1995.13.11.2851 [DOI] [PubMed] [Google Scholar]
- 43. Barker SB, Matthews PN, Philip PF, et al. : Prospective study of intravesical dimethyl sulphoxide in the treatment of chronic inflammatory bladder disease. Br J Urol. 1987;59(2):142–4. 10.1111/j.1464-410X.1987.tb04805.x [DOI] [PubMed] [Google Scholar]
- 44. Tseraidis GS, Popova SS, Zykova NIa: [Use of a progesterone ointment in hirsutism]. Vestn Dermatol Venerol. 1986; (7):14–7. [PubMed] [Google Scholar]
- 45. Scherbel AL, McCormack LJ, Layle JK: Further observations on the effect of dimethyl sulfoxide in patients with generalized scleroderma. (Progressive systemic sclerosis). Ann N Y Acad Sci. 1967;141(1):613–29. 10.1111/j.1749-6632.1967.tb34931.x [DOI] [PubMed] [Google Scholar]
- 46. Mitrus I, Smagur A, Giebel S, et al. : A faster reconstitution of hematopoiesis after autologous transplantation of hematopoietic cells cryopreserved in 7.5% dimethyl sulfoxide if compared to 10% dimethyl sulfoxide containing medium. Cryobiology. 2013;67(3):327–31. 10.1016/j.cryobiol.2013.09.167 [DOI] [PubMed] [Google Scholar]
- 47. Mueller LP, Theurich S, Christopeit M, et al. : Neurotoxicity upon infusion of dimethylsulfoxide-cryopreserved peripheral blood stem cells in patients with and without pre-existing cerebral disease. Eur J Haematol. 2007;78(6):527–31. 10.1111/j.1600-0609.2007.00851.x [DOI] [PubMed] [Google Scholar]
- 48. Gonella S, Di Giulio P: Delayed chemotherapy-induced nausea and vomiting in autologous hematopoietic cell transplant patients: an exploratory analysis. Tumori. 2015;101(6):e154–9. 10.5301/tj.5000296 [DOI] [PubMed] [Google Scholar]
- 49. Cervigni M, Sommariva M, Tenaglia R, et al. : A randomized, open-label, multicenter study of the efficacy and safety of intravesical hyaluronic acid and chondroitin sulfate versus dimethyl sulfoxide in women with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2017;36(4):1178–86. 10.1002/nau.23091 [DOI] [PubMed] [Google Scholar]
- 50. Vuopala U, Kaipainen WJ: DMOS in the treatment of Dupuytren’s contracture. A therapeutic experiment. Acta Rheumatol Scand. 1971;17(1):61–2. 10.3109/rhe1.1971.17.issue-1-4.09 [DOI] [PubMed] [Google Scholar]
- 51. Okamoto Y, Takaue Y, Saito S, et al. : Toxicities associated with cryopreserved and thawed peripheral blood stem cell autografts in children with active cancer. Transfusion. 1993;33(7):578–81. 10.1046/j.1537-2995.1993.33793325053.x [DOI] [PubMed] [Google Scholar]
- 52. Zuckner J, Uddin J, Gantner GE, Jr: Local application of dimethyl sulfoxide and DMSO combined with triamcinolone acetonide in rheumatoid arthritis. Ann N Y Acad Sci. 1967;141(1):555–9. 10.1111/j.1749-6632.1967.tb34924.x [DOI] [PubMed] [Google Scholar]
- 53. Akkök CA, Liseth K, Nesthus I, et al. : Autologous peripheral blood progenitor cells cryopreserved with 5 and 10 percent dimethyl sulfoxide alone give comparable hematopoietic reconstitution after transplantation. Transfusion. 2008;48(5):877–83. 10.1111/j.1537-2995.2008.01648.x [DOI] [PubMed] [Google Scholar]
- 54. Davis JM, Rowley SD, Braine HG, et al. : Clinical toxicity of cryopreserved bone marrow graft infusion. Blood. 1990;75(3):781–6. [PubMed] [Google Scholar]
- 55. Roth SH, Shainhouse JZ: Efficacy and safety of a topical diclofenac solution (pennsaid) in the treatment of primary osteoarthritis of the knee: a randomized, double-blind, vehicle-controlled clinical trial. Arch Intern Med. 2004;164(18):2017–23. 10.1001/archinte.164.18.2017 [DOI] [PubMed] [Google Scholar]
- 56. Galmés A, Besalduch J, Bargay J, et al. : A simplified method for cryopreservation of hematopoietic stem cells with -80 degrees C mechanical freezer with dimethyl sulfoxide as the sole cryoprotectant. Leuk Lymphoma. 1995;17(1–2):181–4. 10.3109/10428199509051720 [DOI] [PubMed] [Google Scholar]
- 57. Bookman AA, Williams KS, Shainhouse JZ: Effect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: a randomized controlled trial. CMAJ. 2004;171(4):333–8. 10.1503/cmaj.1031793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Aliab’eva AP, Melikhova NI, Murav’ev IuV: [Use of heparin applications in a dimethyl sulfoxide medium in the overall treatment of rheumatoid arthritis in children]. Pediatriia. 1980; (9):50–1. [PubMed] [Google Scholar]
- 59. Shpall EJ, LeMaistre CF, Holland K, et al. : A prospective randomized trial of buffy coat versus CD34-selected autologous bone marrow support in high-risk breast cancer patients receiving high-dose chemotherapy. Blood. 1997;90(11):4313–20. [PubMed] [Google Scholar]
- 60. Fuks JZ, Egorin MJ, Aisner J, et al. : Cyclophosphamide and dimethylsulfoxide in the treatment of squamous carcinoma of the lung. Therapeutic efficacy, toxicity, and pharmacokinetics. Cancer Chemother Pharmacol. 1981;6(2):117–20. 10.1007/BF00262327 [DOI] [PubMed] [Google Scholar]
- 61. Styler MJ, Topolsky DL, Crilley PA, et al. : Transient high grade heart block following autologous bone marrow infusion. Bone Marrow Transplant. 1992;10(5):435–8. [PubMed] [Google Scholar]
- 62. Marshall LF, Camp PE, Bowers SA: Dimethyl sulfoxide for the treatment of intracranial hypertension: a preliminary trial. Neurosurgery. 1984;14(6):659–63. 10.1227/00006123-198406000-00002 [DOI] [PubMed] [Google Scholar]
- 63. Engel MF: Dimethyl sulfoxide (DMSO) in clinical dermatology. South Med J. 1966;59(11):1318–9. [DOI] [PubMed] [Google Scholar]
- 64. Roth SH, Fuller P: Pooled safety analysis of diclofenac sodium topical solution 1.5% (w/w) in the treatment of osteoarthritis in patients aged 75 years or older. Clin Interv Aging. 2012;7:127–37. 10.2147/CIA.S30884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lv X, Jiang C, Li Y, et al. : Percutaneous transvenous packing of cavernous sinus with Onyx for cavernous dural arteriovenous fistula. Eur J Radiol. 2009;71(2):356–62. 10.1016/j.ejrad.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 66. Demos CH, Beckloff GL, Donin MN, et al. : Dimethyl sulfoxide in musculoskeletal disorders. Ann N Y Acad Sci. 1967;141(1):517–23. 10.1111/j.1749-6632.1967.tb34920.x [DOI] [PubMed] [Google Scholar]
- 67. Spruance SL, Stewart JC, Freeman DJ, et al. : Early application of topical 15% idoxuridine in dimethyl sulfoxide shortens the course of herpes simplex labialis: a multicenter placebo-controlled trial. J Infect Dis. 1990;161(2):191–7. 10.1093/infdis/161.2.191 [DOI] [PubMed] [Google Scholar]
- 68. Bojanic I, Cepulic BG, Mazic S, et al. : Toxicity related to autologous peripheral blood haematopoietic progenitor cell infusion is associated with number of granulocytes in graft, gender and diagnosis of multiple myeloma. Vox Sang. 2008;95(1):70–5. 10.1111/j.1423-0410.2008.01060.x [DOI] [PubMed] [Google Scholar]
- 69. Blumenthal LS, Fuchs M: The clinical use of dimethyl sulfoxide on various headaches, musculoskeletal, and other general medical disorders. Ann N Y Acad Sci. 1967;141(1):572–85. 10.1111/j.1749-6632.1967.tb34927.x [DOI] [PubMed] [Google Scholar]
- 70. Hoang BX, Le BT, Tran HD, et al. : Dimethyl sulfoxide-sodium bicarbonate infusion for palliative care and pain relief in patients with metastatic prostate cancer. J Pain Palliat Care Pharmacother. 2011;25(4):350–5. 10.3109/15360288.2011.606294 [DOI] [PubMed] [Google Scholar]
- 71. Stroncek DF, Fautsch SK, Lasky LC, et al. : Adverse reactions in patients transfused with cryopreserved marrow. Transfusion. 1991;31(6):521–6. 10.1046/j.1537-2995.1991.31691306250.x [DOI] [PubMed] [Google Scholar]
- 72. Kaidbey KH: Therapy of resistant psoriasis with topical corticosteroids and dimethylsulfoxide. Dermatologica. 1976;152(5):316–20. 10.1159/000251276 [DOI] [PubMed] [Google Scholar]
- 73. Zambelli A, Poggi G, Da Prada G, et al. : Clinical toxicity of cryopreserved circulating progenitor cells infusion. Anticancer Res. 1998;18(6B):4705–8. [PubMed] [Google Scholar]
- 74. Otrock ZK, Sempek DS, Carey S, et al. : Adverse events of cryopreserved hematopoietic stem cell infusions in adults: a single-center observational study. Transfusion. 2017;57(6):1522–6. 10.1111/trf.14072 [DOI] [PubMed] [Google Scholar]
- 75. Matsumoto J: Clinical trials of dimethyl sulfoxide in rheumatoid arthritis patients in Japan. Ann N Y Acad Sci. 1967;141(1):560–8. 10.1111/j.1749-6632.1967.tb34925.x [DOI] [PubMed] [Google Scholar]
- 76. Melikhova NI, Murav’ev IuV, Sigidin IaA, et al. : [Effectiveness of the treatment of juvenile rheumatoid arthritis with dimethyl sulfoxide gel]. Pediatriia. 1986; (6):53–4. [PubMed] [Google Scholar]
- 77. Holbro A, Graf L, Topalidou M, et al. : Cryopreserved stem cell products containing dimethyl sulfoxide lead to activation of the coagulation system without any impact on engraftment. Transfusion. 2014;54(6):1508–14. 10.1111/trf.12511 [DOI] [PubMed] [Google Scholar]
- 78. Williams HJ, Furst DE, Dahl SL, et al. : Double-blind, multicenter controlled trial comparing topical dimethyl sulfoxide and normal saline for treatment of hand ulcers in patients with systemic sclerosis. Arthritis Rheum. 1985;28(3):308–14. 10.1002/art.1780280311 [DOI] [PubMed] [Google Scholar]
- 79. Simpson JR: Idoxuridine in the treatment of herpes zoster. Practitioner. 1975;215(1286):226–9. [PubMed] [Google Scholar]
- 80. Lv X, Li Y, Jiang C, et al. : The incidence of trigeminocardiac reflex in endovascular treatment of dural arteriovenous fistula with onyx. Interv Neuroradiol. 2010;16(1):59–63. 10.1177/159101991001600107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hoang BX, Tran DM, Tran HQ, et al. : Dimethyl sulfoxide and sodium bicarbonate in the treatment of refractory cancer pain. J Pain Palliat Care Pharmacother. 2011;25(1):19–24. 10.3109/15360288.2010.536306 [DOI] [PubMed] [Google Scholar]
- 82. Bosso JA, Spruance SL, Wenerstrom G: Tolerance and percutaneous absorption of topically applied arildone. J Clin Pharmacol. 1985;25(2):95–9. 10.1002/j.1552-4604.1985.tb02808.x [DOI] [PubMed] [Google Scholar]
- 83. Ozkaya-Bayazit E, Kavak A, Güngör H, et al. : Intermittent use of topical dimethyl sulfoxide in macular and papular amyloidosis. Int J Dermatol. 1998;37(12):949–54. 10.1046/j.1365-4362.1998.00600.x [DOI] [PubMed] [Google Scholar]
- 84. Vinnik CA, Jacob SW: Dimethylsulfoxide (DMSO) for human single-stage intraoperative tissue expansion and circulatory enhancement. Aesthetic Plast Surg. 1991;15(4):327–37. 10.1007/BF02273881 [DOI] [PubMed] [Google Scholar]
- 85. Donmez A, Tombuloglu M, Gungor A, et al. : Clinical side effects during peripheral blood progenitor cell infusion. Transfus Apher Sci. 2007;36(1):95–101. 10.1016/j.transci.2006.05.019 [DOI] [PubMed] [Google Scholar]
- 86. Cordoba R, Arrieta R, Kerguelen A, et al. : The occurrence of adverse events during the infusion of autologous peripheral blood stem cells is related to the number of granulocytes in the leukapheresis product. Bone Marrow Transplant. 2007;40(11):1063–7. 10.1038/sj.bmt.1705861 [DOI] [PubMed] [Google Scholar]
- 87. Alessandrino P, Bernasconi P, Caldera D, et al. : Adverse events occurring during bone marrow or peripheral blood progenitor cell infusion: analysis of 126 cases. Bone Marrow Transplant. 1999;23(6):533–7. 10.1038/sj.bmt.1701609 [DOI] [PubMed] [Google Scholar]
- 88. Binnick SA, Shore SS, Corman A, et al. : Failure of dimethyl sulfoxide in the treatment of scleroderma. Arch Dermatol. 1977;113(10):1398–402. 10.1001/archderm.1977.01640100076013 [DOI] [PubMed] [Google Scholar]
- 89. Zuurmond WW, Langendijk PN, Bezemer PD, et al. : Treatment of acute reflex sympathetic dystrophy with DMSO 50% in a fatty cream. Acta Anaesthesiol Scand. 1996;40(3):364–7. 10.1111/j.1399-6576.1996.tb04446.x [DOI] [PubMed] [Google Scholar]
- 90. Galmés A, Besalduch J, Bargay J, et al. : Cryopreservation of hematopoietic progenitor cells with 5-percent dimethyl sulfoxide at -80 degrees C without rate-controlled freezing. Transfusion. 1996;36(9):794–7. 10.1046/j.1537-2995.1996.36996420755.x [DOI] [PubMed] [Google Scholar]
- 91. Amiridze N, Darwish R: Hemodynamic instability during treatment of intracranial dural arteriovenous fistula and carotid cavernous fistula with Onyx: preliminary results and anesthesia considerations. J Neurointerv Surg. 2009;1(2):146–50. 10.1136/jnis.2009.000042 [DOI] [PubMed] [Google Scholar]
- 92. Vinokurov VL, Zharinov GM, Val’kovich AA, et al. : [The prevention of radiation injuries to the rectum and bladder in cervical cancer patients]. Vopr Onkol. 1990;36(9):1119–20. [PubMed] [Google Scholar]
- 93. Percy EC, Carson JD: The use of DMSO in tennis elbow and rotator cuff tendonitis: a double-blind study. Med Sci Sports Exerc. 1981;13(4):215–9. [DOI] [PubMed] [Google Scholar]
- 94. Weigel BJ, Blaney SM, Reid JM, et al. : A phase I study of 17-allylaminogeldanamycin in relapsed/refractory pediatric patients with solid tumors: a Children’s Oncology Group study. Clin Cancer Res. 2007;13(6):1789–93. 10.1158/1078-0432.CCR-06-2270 [DOI] [PubMed] [Google Scholar]
- 95. Dawber R: Idoxuridine in herpes zoster: further evaluation of intermittent topical therapy. Br Med J. 1974;2(5918):526–7. 10.1136/bmj.2.5918.526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Perez RS, Zuurmond WW, Bezemer PD, et al. : The treatment of complex regional pain syndrome type I with free radical scavengers: a randomized controlled study. Pain. 2003;102(3):297–307. 10.1016/S0304-3959(02)00414-1 [DOI] [PubMed] [Google Scholar]
- 97. Stav K, Beberashvili I, Lindner A, et al. : Predictors of response to intravesical dimethyl-sulfoxide cocktail in patients with interstitial cystitis. Urology. 2012;80(1):61–5. 10.1016/j.urology.2012.03.030 [DOI] [PubMed] [Google Scholar]
- 98. Duijvestein M, Vos AC, Roelofs H, et al. : Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59(12):1662–9. 10.1136/gut.2010.215152 [DOI] [PubMed] [Google Scholar]
- 99. Marcacci G, Corazzelli G, Becchimanzi C, et al. : DMSO-associated encephalopathy during autologous peripheral stem cell infusion: a predisposing role of preconditioning exposure to CNS-penetrating agents? Bone Marrow Transplant.England,2009;44(2):133–5. 10.1038/bmt.2008.442 [DOI] [PubMed] [Google Scholar]
- 100. Rabinov JD, Yoo AJ, Ogilvy CS, et al. : ONYX versus n-BCA for embolization of cranial dural arteriovenous fistulas. J Neurointerv Surg. 2013;5(4):306–10. 10.1136/neurintsurg-2011-010237 [DOI] [PubMed] [Google Scholar]
- 101. Milone G, Mercurio S, Strano A, et al. : Adverse events after infusions of cryopreserved hematopoietic stem cells depend on non-mononuclear cells in the infused suspension and patient age. Cytotherapy. 2007;9(4):348–55. 10.1080/14653240701326756 [DOI] [PubMed] [Google Scholar]
- 102. Horacek JM, Jebavy L, Jakl M, et al. : Cardiovascular changes associated with infusion of hematopoietic cell grafts in oncohematological patients -- impact of cryopreservation with dimethylsulfoxide. Exp Oncol. 2009;31(2):121–2. [PubMed] [Google Scholar]
- 103. Murav’ev IuV: [Treatment of rheumatoid synovitis by intra-articular administration of dimethyl sulfoxide and corticosteroids]. Ter Arkh. 1986;58(7):104–5. [PubMed] [Google Scholar]
- 104. Akkok CA, Holte MR, Tangen JM, et al. : Hematopoietic engraftment of dimethyl sulfoxide-depleted autologous peripheral blood progenitor cells. Transfusion. 2009;49(2):354–61. 10.1111/j.1537-2995.2008.01949.x [DOI] [PubMed] [Google Scholar]
- 105. Rössberger J, Fall M, Peeker R: Critical appraisal of dimethyl sulfoxide treatment for interstitial cystitis: discomfort, side-effects and treatment outcome. Scand J Urol Nephrol. 2005;39(1):73–7. 10.1080/00365590410018738 [DOI] [PubMed] [Google Scholar]
- 106. Burton WJ, Gould PW, Hursthouse MW, et al. : A multicentre trial of Zostrum (5 percent idoxuridine in dimethyl sulphoxide) in herpes zoster. N Z Med J. 1981;94(696):384–6. [PubMed] [Google Scholar]
- 107. Lockie LM, Norcross BM: A clinical study on the effects of dimethyl sulfoxide in 103 patients with acute and chronic musculoskeletal injuries and inflammations. Ann N Y Acad Sci. 1967;141(1):599–602. 10.1111/j.1749-6632.1967.tb34929.x [DOI] [PubMed] [Google Scholar]
- 108. Evstaf’ev VV: [The use of a heparin ointment in combination with dimexide in treating psoriasis]. Vestn Dermatol Venerol. 1989; (9):71–2. [PubMed] [Google Scholar]
- 109. Parsons JL, Shepard WL, Fosdick WM: DMSO an adjutant to physical therapy in the chronic frozen shoulder. Ann N Y Acad Sci. 1967;141(1):569–71. 10.1111/j.1749-6632.1967.tb34926.x [DOI] [PubMed] [Google Scholar]
- 110. Delaney C, Milano F, Cicconi L, et al. : Infusion of a non-HLA-matched ex-vivo expanded cord blood progenitor cell product after intensive acute myeloid leukaemia chemotherapy: a phase 1 trial. Lancet Haematol. 2016;3(7):e330–9. 10.1016/S2352-3026(16)30023-0 [DOI] [PubMed] [Google Scholar]
- 111. Stewart BH: Dimethyl sulfoxide (DMSO) in the treatment of troublesome genitourinary disorders: a preliminary report. Cleve Clin Q. 1966;33(2):81–4. [DOI] [PubMed] [Google Scholar]
- 112. Olver IN, Aisner J, Hament A, et al. : A prospective study of topical dimethyl sulfoxide for treating anthracycline extravasation. J Clin Oncol. 1988;6(11):1732–5. 10.1200/JCO.1988.6.11.1732 [DOI] [PubMed] [Google Scholar]
- 113. Brown JH: Clinical experience with DMSO in acute musculoskeletal conditions comparing a noncontrolled series with a controlled double blind study. Ann N Y Acad Sci. 1967;141(1):496–505. 10.1111/j.1749-6632.1967.tb34918.x [DOI] [PubMed] [Google Scholar]
- 114. Garcia CA: Ocular toxicology of dimethyl sulfoxide and effects on retinitis pigmentosa. Ann N Y Acad Sci. 1983;411:48–51. 10.1111/j.1749-6632.1983.tb47285.x [DOI] [PubMed] [Google Scholar]
- 115. Kim DH, Jamal N, Saragosa R, et al. : Similar outcomes of cryopreserved allogeneic peripheral stem cell transplants (PBSCT) compared to fresh allografts. Biol Blood Marrow Transplant. 2007;13(10):1233–43. 10.1016/j.bbmt.2007.07.003 [DOI] [PubMed] [Google Scholar]
- 116. Salim AS: Role of oxygen-derived free radical scavengers in the treatment of recurrent pain produced by chronic pancreatitis. A new approach. Arch Surg. 1991;126(9):1109–14. 10.1001/archsurg.1991.01410330067010 [DOI] [PubMed] [Google Scholar]
- 117. Martino M, Morabito F, Messina G, et al. : Fractionated infusions of cryopreserved stem cells may prevent DMSO-induced major cardiac complications in graft recipients. Haematologica. 1996;81(1):59–61. [PubMed] [Google Scholar]
- 118. Eisenberg S, Wickline M, Linenberger M, et al. : Prevention of dimethylsulfoxide-related nausea and vomiting by prophylactic administration of ondansetron for patients receiving autologous cryopreserved peripheral blood stem cells. Oncol Nurs Forum. 2013;40(3):285–92. 10.1188/13.ONF.285-292 [DOI] [PubMed] [Google Scholar]
- 119. Gonella S, Berchialla P, Bruno B, et al. : Are orange lollies effective in preventing nausea and vomiting related to dimethyl sulfoxide? A multicenter randomized trial. Support Care Cancer. 2014;22(9):2417–24. 10.1007/s00520-014-2227-y [DOI] [PubMed] [Google Scholar]
- 120. Kessinger A, Schmit-Pokorny K, Smith D, et al. : Cryopreservation and infusion of autologous peripheral blood stem cells. Bone Marrow Transplant. 1990;5 Suppl 1:25–7. [PubMed] [Google Scholar]
- 121. Foïs E, Desmartin M, Benhamida S, et al. : Recovery, viability and clinical toxicity of thawed and washed haematopoietic progenitor cells: analysis of 952 autologous peripheral blood stem cell transplantations. Bone Marrow Transplant. 2007;40(9):831–5. 10.1038/sj.bmt.1705830 [DOI] [PubMed] [Google Scholar]
- 122. Calmels B, Lemarié C, Esterni B, et al. : Occurrence and severity of adverse events after autologous hematopoietic progenitor cell infusion are related to the amount of granulocytes in the apheresis product. Transfusion. 2007;47(7):1268–75. 10.1111/j.1537-2995.2007.01267.x [DOI] [PubMed] [Google Scholar]
- 123. Keung YK, Lau S, Elkayam U, et al. : Cardiac arrhythmia after infusion of cryopreserved stem cells. Bone Marrow Transplant. 1994;14(3):363–7. [PubMed] [Google Scholar]