Abstract
Background
Human papillomavirus (HPV), the major cause of cervical cancer worldwide, is associated with infection of HPV (Oncogenic HPV). Cancer patients who develop drug resistance are resulted in failure of chemotherapy.
Objective
We investigated the mechanisms for the HPV E6/E7 oncoprotein-mediated 5-fluorouracil (5-Fu) sensitivity.
Methods
HPV-16 E6/E7 was transfected into human cervical cancer cell lines. Glycolysis rate was assessed. Xenograft model was established to examine the in vivo therapeutic effects of E6/E7 inhibition and 5-Fu treatments.
Results
The HPV-16 E6/E7 oncoprotein induces 5-Fu resistance in cervical cancer cells. Overexpression of E6/E7 renders CaSki and SiHa cells resistant to 5-Fu treatments. We found E6/E7 expressions were significantly upregulated in 5-Fu-resistant cells compared with parental cells. Moreover, the cellular glycolysis rate was significantly increased in 5-Fu-resistant cells. The glucose uptake, lactate production, and expressions of glycolysis enzymes were upregulated in 5-Fu-resistant cells. We report the E6/E7-mediated 5-Fu resistance was through upregulation of glycolysis pathway. Importantly, inhibition of E6/E7 by shRNA effectively decreased cellular glycolysis and overcame 5-Fu resistance using in vitro and in vivo xenograft model.
Conclusion
Our study contributed to understanding the molecular mechanisms for HPV E6/E7-mediated 5-Fu resistance and development of new therapeutic strategies against cervical cancer.
Keywords: human papillomavirus, Warburg effect, chemoresistance, 5-Fu, cervical cancer, HPV E6/E7
Introduction
The human papillomavirus (HPV) is the major cause of cervical cancer worldwide.1 Currently, the association between cervical cancer and high-risk strains of HPV (Oncogenic HPV) is widely studied.2 A bulk of human cervical dysplasia and cancers result from persistent infection with oncogenic HPVs.3 In addition to the infection of HPV to cervical epithelial cells, a variety of cofactors and cellular events determine the development of cervical cancer.4 HPVs encode two oncoproteins, E6 and E7.5 It is known that continuous expression of the E6 and E7 oncoproteins contributes to the development of HPV-induced cervical carcinogenesis.6 However, the detailed molecular basis for HPV E6/E7 carcinogenesis is still under investigation.
5-Fu, a pyrimidine analog modified by fluorination of uracil on position 5 of the pyrimidine ring, is currently being administrated as a widely used anticancer chemotherapy to treat cervical cancer patients.7 The primary mechanisms of 5-Fu have been well studied: a) direct incorporation of fluorouridine triphosphate into RNA to disrupt RNA synthesis;8–10 b) incorporation of fluorodeoxyuridine triphosphate and deoxyuridine triphosphate into DNA;8–10 and c) inhibition of thymidylate synthase (TS).8–10 However, a part of patients with advanced cervical cancer eventually developed 5-Fu resistance despite some patients responded initially to 5-Fu therapy.11 Thus, identifying molecular mechanisms of 5-Fu resistance in cervical cancer patient contributes to develop novel therapeutic strategies.
Recently, dysregulated cellular metabolism has been intensively studied as a new “Hallmarker” that tumors exhibit metabolic alterations which support their malignant growth.12 Cancer cells, which prefer anaerobic glycolysis as a source of energy rather than the more efficient mitochondrial pathway of oxidative phosphorylation even in the presence of oxygen.13 This is called “Warburg effect”.14 In addition, studies reported that cancer cells were more sensitive to glucose deprivation or glucose metabolism inhibitor due to the dependence on glucose as major nutrition and energy supply.15 Therefore, targeting dysregulated cellular glucose metabolism has emerged as a new therapeutic strategy against cancer.
In the present study, we sought to assess the effects of human HPV-16 E6/E7 oncoprotein on the 5-Fu sensitivity of cervical cancer cells. CaSki 5-Fu-resistant cell line was established to investigate the molecular mechanisms of 5-Fu resistance and the correlation between HPV-16 E6/E7, cellular glycolysis, and 5-Fu sensitivity using in vitro and in vivo xenograft model. Our study will contribute to understand the molecular mechanisms for HPV E6/E7-mediated 5-Fu resistance and development of new therapeutic strategies against cervical cancer.
Materials and methods
Cell culture
Human HPV16-positive (SiHa, CaSki) cervical cancer cells were obtained from the American Tissue Culture Collection (ATCC). Cells were cultured separately in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) with 10% fetal bovine serum and 100 IU/mL penicillin G and 100 μg/mL streptomycin (Sigma-Aldrich, Saint Louis, MO, USA) at 37°C in a humidified atmosphere with 5% CO2.
Antibodies and reagents
Goat polyclonal anti-HPV16 E6 (sc-1584) and mouse monoclonal anti-HPV16 E7 (sc-6981) were purchased from Santa Cruz (Dallas, TXs, USA). Mouse monoclonal anti-β-actin (A2228) was purchased from Sigma Aldrich (St. Louis, MO, USA). Rabbit monoclonal anti-LDHA (#3582), anti-HK2 (#2867), anti-Glut1 (#12,939), anti-Akt (#9272), and anti-p-Akt S473 (#4060) were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Akt inhibitor, LY294002 (#9901) was purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). 5-FU was purchased from Sigma Aldrich (St. Louis, MO, USA). Transfection reagents were purchased from Invitrogen (Carlsbad, CA, USA).
Plasmid and siRNA transfection
Plasmid transfection was performed using the Lipofectamine 3000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. The HPV16 E6, E7 were cloned into pEF1a lentiviral vector and packaged in 293 LTV cells according to previous report (16). siRNA transfection was performed using the Lipofectamine™ RNAiMAX (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Three E6/E7 si/shRNA target sequences were pooled for transfection. Individual sequences were as follows: HPV16 E6/E7 #1, 5ʹ-CCGGACAGAGCCCAUUACA-3ʹ; #2, 5ʹ-CACCUACAUUGCAUGAAUA-3ʹ; #3, 5ʹ-CAACUGAUCUCUACUGUUA-3ʹ. Seventy-two hours post-transfection, cells were collected for downstream experiments.
Total RNA isolation and qRT-PCR
RNA was isolated using TRIZOL methods according to the manufacturer’s protocols. For cDNA synthesis, reverse transcription of 1 μg RNA was carried out by the PrimeScript RT Reagent Kit (TaKaRa Biotechnology, Dalian, China). qRT-PCR reactions were performed using the SYBR Green PCR Master Mix (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocols. The thermal cycling conditions were as follows: 95°C for 30 s; 40 cycles at 95°C for 5 s, 60°C for 31 s; and a final dissociation stage. All of the reactions were performed in triplicate in an ABI PRISM 7300 real-time PCR system. Primers used were as follows: Glut1: forward: 5ʹ-AACTCTTCAGCCAGGGTCCAC-3ʹ and reverse: 5ʹ-CACAGTGAAGATGATGAAGAC-3ʹ; HK2: forward: 5ʹ-ATTGTCCAGTGCATCGCGGA-3ʹ and reverse: 5ʹ-AGGTCAAACTCCTCTCGCCG-3ʹ; LDHA: forward primer, 5ʹ-AGCCCGATTCCGTTACCT-3ʹ and reverse primer: 5ʹ-CACCAGCAACATTCATTCCA-3ʹ; β-actin: forward: 5ʹ-CCAAAGCAACCGTGAGGA-3ʹ and reverse 5ʹ-CCAGAGGCGTACAGGACA-3ʹ. β-actin was used as an internal standard. Relative mRNA expressions were calculated by 2−∆∆Ct method. All experiments were performed in triplicate and repeated three times.
Cell viability
MTT assay representing the percentage of cell viability induced by increasing drug concentrations was performed to assess cell viability according to the previous report.16 SiHa and CaSki cells were seeded into 96-well plates (1000 cells/well) for 24 hrs before MTT assay. The 3-(4,5-dimethylthiazol-2-yl)−2, 5-diphenyltetrazolium bromide reagent (Sigma, St. Louis, MO) (20 mg/mL) was added into cell culture medium, which was removed 4 hrs later and 100 µL DMSO was added. After incubating at room temperature with light prevention for 2 hrs, the absorbance values were measured at 570 nm. Experiments were performed in triplicate and repeated three times.
Measurements of glycolysis rate
The glucose uptake and lactate production were determined using the Glucose Uptake Colorimetric Assay kit (cat. no. K676-100; BioVision, Inc., Milpitas, CA, USA) and the Lactate Colorimetric/Fluorometric Assay kit (cat. no. K607-100; BioVision, Inc.) following the manufacturer’s protocols. The relative of glucose and lactate were normalized by cell numbers of each well. The Extracellular Acidification Rate (ECAR) was measured using the XF24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA, USA). Cells (3.5×104/well) were plated in XF24 cell-culture microplate and incubated 24 hrs. Culture medium was replaced with XF assay medium containing 2 mM L-glutamine. ECAR was measured by sequential addition of glucose, oligomycin (2.5 μM) and 2-deoxyglucose (100 mM) in an XF24 flux analyzer. Reading from each well was normalized by protein concentrations. Each experiment was performed in triplicates and repeated three times.
Western blot
Cells were collected and directly lysed with RIPA buffer containing protease inhibitors cocktail from Sigma Aldrich (St. Louis, MO, USA). After 30-min incubation on ice, cell lysates were centrifuged at 12,000 g for 15 mins at 4°C and the supernatants were collected. Protein concentration was determined by Bradford assay. Equal amounts of protein samples were separated by SDS-PAGE (15%) and then transferred onto the nitrocellulose membrane (BioRad). Following blocking with 5% BSA in TBS-T (TBS containing 0.1% Tween 20) at room temperature for 1 hr, membranes were incubated with primary antibodies at 1:1000 dilutions at 4°C for overnight. Membranes were washed three times and incubated with secondary antibody (IRDye conjugated IgG, LI-COR) with 1:5000 dilutions in TBS-T containing 5% BSA for 1 hr. The signals were then detected with Odyssey Imaging System (LI-COR).
Tumor xenograft experiment
The BALB/c-nude mice (6–7 weeks old) were purchased from Beijing Huafukang Bioscience Co. Inc. (Beijing, China). Mice were housed in a barrier facility on a 12-hr light-dark cycle with a constant temperature (23–25°C) and humidity (40–50%). Animal protocols were approved by the Institutional Animal Care and Use Committee animal facility at the Tianjin Third Central Hospital and were performed in accordance with the guidelines of the “UK Animals (Scientific Procedures) Act 1986”. Mice were injected subcutaneously into a mouse mammary fat pad with 0.2-mL, 0.5×107 CaSki 5-Fu-resistant cells. When the tumor reached a size of greater than 150 mm3, mice were randomly divided into four groups (each group, n=9) as follows: PBS control; 5-Fu alone [10 mg/kg intraperitoneal (i.p.), 2 times/week], E6/E7 shRNA [intratumoral, 2 times/week] and 5-Fu plus E6/E7 shRNA. Tumors developed in mice were measured 2 times a week with calipers. The volume was calculated with the following formula: V=(length×width2)/2.
Statistical analysis
Statistical analysis was performed by GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). The Student’s t-test was used to analyze the statistical significance between two groups. The comparison of variance in multiple groups was analyzed by one-way ANOVA. p-values of p<0.05 (*), p<0.01 (**), and p<0.001 (***) were considered statistically significant.
Results
HPV16 E6/E7 oncoproteins contribute to 5-Fu resistance in cervical cancer cells
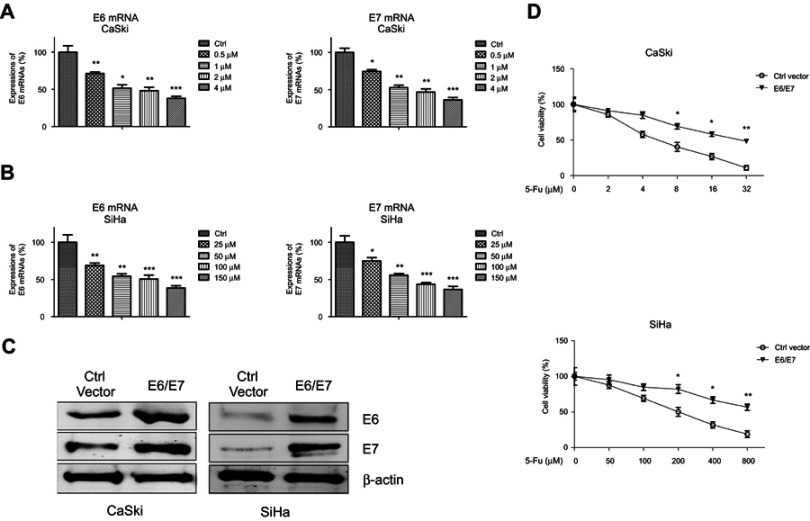
We started to exam the potential roles of HPV E6/E7 in chemosensitivity. Two HPV16-positive human cervical cancer cell lines, SiHa cells were treated with 5-Fu at 0, 25, 50, 100, and 150 µM and CaSki cells were treated with 5-Fu at 0, 0.5, 1, 2, and 4 µM for 24 hrs, respectively. Notably, viral E6/E7 expressions detected by qPCR were efficiently suppressed by 5-Fu treatments in both cells (Figure 1A, B), suggesting the HPV E6/E7 might be targets of anti-cancer agents. To investigate the effects of E6/E7 oncoprotein on 5-Fu sensitivity, we induced the E6/E7 expressions in CaSki and SiHa cervical cancer cell lines by transient transfection of E6/E7 overexpression plasmid (Figure 1C). Cells with or without E6/E7 oncoprotein overexpression were treated with elevated 5-Fu concentrations for 48 hrs. As we expected, ectopic overexpression of E6/E7 significantly decreased 5-Fu sensitivity of cervical cancer cells (Figure 1D). Taken together, the above results demonstrated overexpression of oncoproteins E6/E7 contributed to 5-Fu resistance of cervical cancer cells.
Figure 1.
Correlation between E6/E7 oncoprotein and 5-Fu in HPV16-positive cervical cancer cells. (A) E6 (left) and E7 (right) mRNAs were measured in SiHa cells with 5-Fu at 0, 25, 50, 100, or 150 µM for 48 hrs. (B) E6 (left) and E7 (right) mRNAs were measured in SiHa cells with 5-Fu at 0, 25, 50, 100, or 150 µM for 48 hrs. (C) CaSki and SiHa cells were transfected with control plasmid or E6/E7 overexpression plasmid for 48 hrs; protein expressions of E6/E7 were detected by Western blot. β-actin was a loading control. (D) CaSki (upper) and SiHa (lower) cells without or with E6/E7 overexpression were treated with the indicated concentrated 5-Fu for 48 hrs, followed by cell viability analysis by MTT. Results are shown as mean±SEM. *p<0.05; **p<0.01; ***p<0.001.
5-Fu-resistant cervical cancer cells display upregulated E6/E7 expressions and glycolysis rate
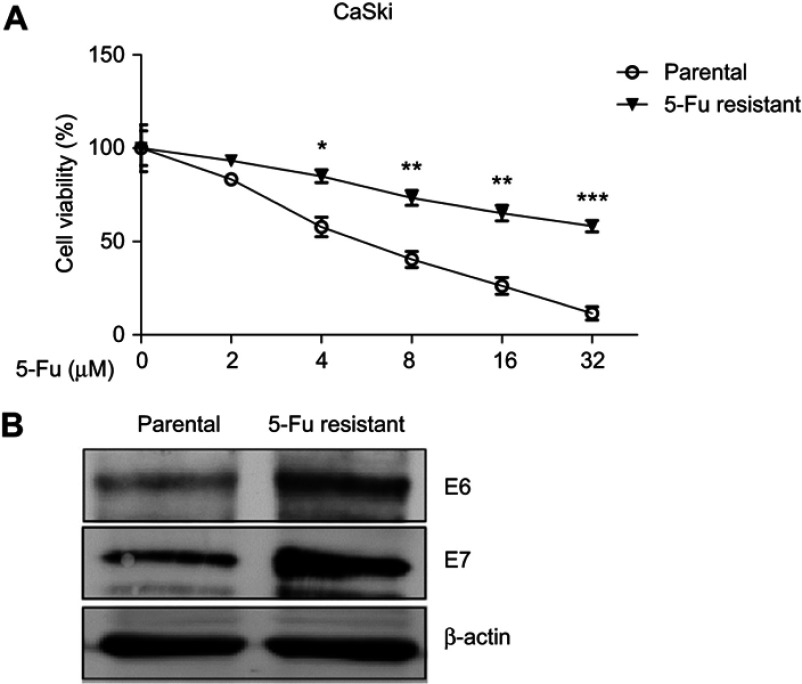
In line with the above findings that ectopic E6/E7 promoted 5-Fu resistance, we asked questions that whether endogenous E6/E7 expressions were correlated with 5-Fu resistance. To test this, CaSki cells were treated with gradually elevated concentrations of 5-Fu to select cervical cells which developed 5-Fu resistance.17 Results in Figure 2A showed CaSki 5-Fu-resistant cells were less sensitive to 5-Fu treatments at 4, 8, 16, and 32 µM, compared with their parental cells. The 5-Fu-resistant cells obtained a higher IC50 (52.05 µM) than parental cells (5.21 µM). As we expected, the E6/E7 protein expressions were significantly upregulated in 5-Fu-resistant cells compared with their parental cells (Figure 2B), suggesting E6/E7 might be targets of 5-Fu against 5-Fu resistance. It was known that dysregulated cellular glucose metabolism was a hallmarker of cancer cells and correlated to chemosensitivity (12–15).12–15 To further investigate the underline mechanisms of the E6/E7-mediated 5-Fu sensitivity, we assessed the cellular glucose metabolism of 5-Fu-resistant cells. Compared with their parental cells, 5-Fu-resistant cells showed increased extracellular glycolysis rates: the glucose uptake and lactate production were significantly higher in 5-Fu-resistant cells (Figure 3A). To further document the dynamic glycolysis capacity of CaSki parental and 5-Fu-resistant cells, we measured extracellular acidification rate (ECAR) using Seahorse XF24 Extracellular Flux analyzer. As shown in Figure 3B, the ECAR was significantly higher in 5-Fu-resistant cells compared to parental cells, consistent with the glucose uptake and lactate production results. Moreover, we observed that the 5-Fu-resistant cells had upregulated glycolytic speed-limit enzymes (Glut1, HK2, and LDHA) (Figure 3C, D). Taken together, the above results demonstrated a positive correlation between 5-Fu-resistant, E6/E7 expressions, and cellular glycolysis rate.
Figure 2.
5-Fu-resistant cervical cancer cells show increased E6/E6 expressions. (A) CaSki parental cells and 5-Fu-resistant cells were treated with 5-Fu at 0, 2, 4, 8, 16, or 32 µM for 48 hrs. The cell viabilities were assessed by MTT assay. (B) Protein expressions of E6/E7 from CaSki parental- and 5-Fu-resistant cells were measured by Western blot. β-actin was a loading control. Results are shown as mean±SEM. *p<0.05; **p<0.01; ***p<0.001.
Figure 3.
Elevated cellular glycolysis in 5-Fu-resistant cervical cancer cells. (A) Glucose uptake and lactate production were compared in CaSki parental- and 5-Fu-resistant cells. (B) Real-time comparison of extracellular acidification rate (ECAR) between CaSki parental- and 5-Fu-resistant cells. (C) Protein or (D) mRNA expressions of glycolysis enzymes, Glut1, HK2, and LDHA from CaSki parental- and 5-Fu-resistant cells were measured by Western blot or qRT-PCR. β-actin was a loading control. Results are shown as mean±SEM. *p<0.05; **p<0.01; ***p<0.001.
Inhibition of the E6/E7-mediated glycolysis sensitizes 5-Fu-resistant cells
To assess the potential synergistic effects of glycolysis and 5-Fu in cervical cancer cells, we asked whether inhibiting the E6/E7-regulated glycolysis could counteract 5-Fu resistance of cervical cancer cells. CaSki 5-Fu-resistant cells were transfected with E6/E7 shRNA or glycolysis inhibitor, 2-deoxy-D-glucose (2-DG) at 1 mM and 5-Fu at 0, 2, 4, 8, 16, or 32 µM for 48 hrs. Resistant cells with E6/E7 shRNA or 2-DG treatments showed significantly increased 5-Fu sensitivity (Figure 4A, B). Obviously, we detected glucose uptake and lactate production of 5-Fu-resistant cells were significantly attenuated by E6/E7 knockdown or glycolysis inhibitor treatment (Figure 4C, D). Furthermore, expressions of glycolysis enzymes were significantly decreased in CaSki 5-Fu-resistant cells with E6/E7 shRNA or 2-DG treatments compared with control cells (Figure 4E, F). Taken together, these results directly support the oncoprotein E6/E7-promoted cellular glycolysis is a therapeutic target against 5-Fu resistance in cervical cancer.
Figure 4.
Knockdown E6/E7 sensitizes cervical cancer cells to 5-Fu. (A) CaSki was transfected with E6/E7 shRNA for 48 hrs, followed by 5-Fu treatments at the indicated concentrations for 48 hrs. Cell viability was measured by MTT assay. (B) CaSki was co-treated with 2-DG at 1 mM and 5-Fu at the indicated concentrations for 48 hrs. Cell viability was measured by MTT assay. (C) The glucose uptake and lactate production were detected in CaSki cells without or with E6/E7 knockdown and (D) CaSki cells without or with 2-DG treatments. (E) The mRNA expressions of glycolysis key enzymes, Glut1, HK2, and LDHA were measured in CaSki cells without or with E6/E7 knockdown and (F) CaSki cells without or with 2-DG treatments. Results are shown as mean±SEM. *p<0.05; **p<0.01; ***p<0.001.
E6/E7 promotes 5-Fu resistance through upregulation of Akt-glycolysis axis
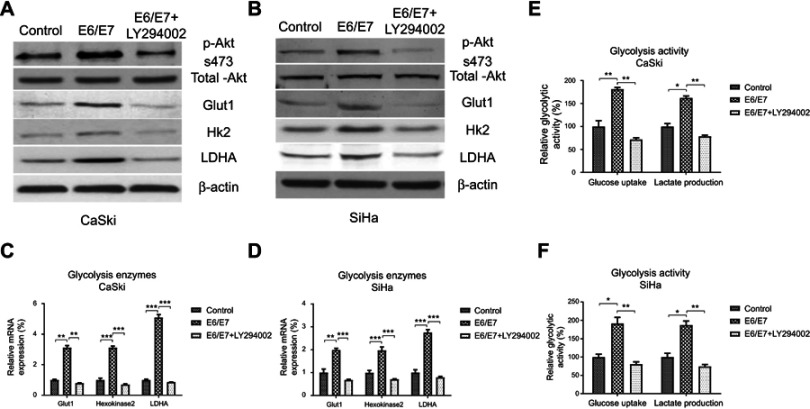
To investigate the molecular mechanisms for the E6/E7-promoted cellular glycolysis, we assessed the Akt pathway of cervical cancer cells with or without E6/E7 overexpression. Results from Figure 5A–D demonstrated significantly increased phosphorylation of Akt and upregulation of Glut-1, HK2, and LDHA by E6/E7 overexpression in CaSki and SiHa cells. Moreover, this stimulation by E6/E7 was blocked by specific Akt inhibitor-LY294002 (Figure 5A and B). These results suggested E6/E6 upregulated Akt pathway, led to increased glycolysis rate of cervical cancer cells. We next addressed the question whether the E6/E7-mediated 5-Fu resistant is through the upregulation of cellular glycolysis. In this case, we analyzed the glycolysis rate between control and E6/E7 overexpressed cervical cancer cells. CaSki and SiHa cells with E6/E7 overexpression displayed increased glucose uptake and lactate production rate (Figure 5E, F). To investigate whether the E6/E7-mediated Akt-glycolysis upregulation results in 5-Fu resistance, we assessed the 5-Fu sensitivity of CaSki and SiHa cells with or without E6/E7 overexpression co-treated with Akt inhibitor or glycolysis inhibitor, 2DG. As we expected, inhibition of Akt or glycolysis apparently sensitized CaSki and SiHa cells to 5-Fu treatments (Figure 6A–D). Importantly, although E6/E7 overexpression rendered cervical cancer cells resistant to 5-Fu, Akt or glycolysis inhibitor treatment overcame 5-Fu resistance of E6/E7 overexpressing cells (Figure 6A–D). The 5-Fu IC50 of E6/E7 overexpressed cells with Akt inhibitor or 2-DG treatment was lower than that of parental cells with 5-Fu treatments alone. These results strongly demonstrated that the E6/E7-promoted 5-Fu resistance was through upregulation of Akt-glycolysis axis.
Figure 5.
5-Fu sensitivity of cervical cancer cells is mediated by the E6/E7-promoted glycolysis. (A) CaSki and (B) SiHa cells were transfected with control or E6/E7 overexpression vector for 48 hrs, cells were treated with or without Akt inhibitor for 16 hrs. Western blot was performed to check the phosphorylation of Akt and Glut-1, HK2, and LDHA protein expressions. (C) CaSki and (D) SiHa cells were treated with the same protocol and the mRNAs of Glut-1, HK2, and LDHA were measured by qRT-PCR. (E) CaSki and (F) SiHa cells were treated with the same protocol and the glucose uptake and lactate production were detected. *p<0.05; **p<0.01; ***p<0.001.
Figure 6.
Sensitization of cervical cancer cells to 5-Fu through Akt-glycolysis pathway. (A) CaSki and (B) SiHa cells were transfected with control, E6/E7 overexpression vector for 48 hrs, with or without Akt inhibitor treatment, followed by 5-Fu treatments at the indicated concentrations. Cell viability was measured by MTT assay. (A) CaSki and (B) SiHa cells were transfected with control, E6/E7 overexpression vector for 48 hrs, with or without glycolysis inhibitor treatment, followed by 5-Fu treatments at the indicated concentrations. Cell viability was measured by MTT assay. Results are shown as mean±SEM. *p<0.05; **p<0.01; ***p<0.001.
Efficacy of combined E6/E7 inhibition and 5-Fu treatment on subcutaneous cervical tumors
Given that E6/E7-mediated cellular glucose metabolism is crucial in cervical cancer 5-Fu resistance, we explored the therapeutic efficacy of the combination of E6/E7 inhibition and 5-Fu using xenograft mouse models. E6/E7 shRNA from retrovirus alone, 5-Fu alone, or the combination of E6/E7 shRNA and 5-Fu were intravenously injected into nude mice bearing established subcutaneous tumors from CaSki 5-Fu-resistant cells. The effects on tumor growth were monitored. Results showed that systemic delivery of E6/E7 shRNA alone or 5-Fu alone resulted in a moderate decrease in tumor burden compared with treatment with control vehicle (Figure 7A). As we expected, the anti-tumor effect of treatments with E6/E7 shRNA plus 5-Fu was more pronounced than other therapeutic options (Figure 7B). The tumor volumes were smaller with the combined treatments than E6/E7 shRNA or 5-Fu alone, consistent with our in vitro results (Figure 7C). In addition, significant reduction in glycolysis enzymes was observed in the tumor xenografts of E6/E7 shRNA plus 5-Fu treatment mice (Figure 7D, E, F). Taken together, these in vivo results strongly supported that inhibiting the E6/E7-mediated glycolysis contributes anti-5-Fu resistance in cervical cancers.
Figure 7.
The combination of E6/E7 shRNA and 5-Fu suppresses growth of 5-Fu-resistant cervical cancer xenografts in vivo. (A) Mice tumor growth in CsSki 5-Fu-resistant cells xenografts after intravenous administration of 5-Fu and intratumor administration of E6/E7 shRNA. Mice were grouped as: control treatment, E6/E7 shRNA injection alone, 5-Fu alone, or the combination of E6/E7 shRNA and 5-Fu. Tumor volume was measured every 3 days until day 21 when mice with tumors were euthanized. (B, C) The representative mice tumor from the above xenograft experiments. The relative mRNA expressions of (D) Glut1, (E) HK2, and (F) LDHA from tumors of the above mice were measured by qRT-PCR. Results are shown as mean±SEM.
Discussion
Cervical cancer, one of the most common women cancers in the world, is tightly associated with infection by human papillomavirus.1–5 The continuous expression of the viral E6/E7 oncogenes is essential for HPV-positive cervical cancer cells growth.6 Thus, identification of the cellular targets attacked by the viral oncogenes is of high interest to explore new molecular pathways for therapeutic intervention. This study investigated the roles of HPV E6/E7 in regulating cellular metabolism and 5-Fu sensitivity of cervical cancer cell.
Strong evidence suggests that cancer cells depend on anaerobic glycolysis for energy source as well as metabolic intermediates to develop tumors.12–14 Studies have reported association of cellular glucose metabolism alterations and cervical carcinoma with an increase in lactate dehydrogenase activity.18 It was known that HPV E6/E7 activated cancer oncogenic signals, such as the AKT-mTOR-associated pathways,16 while inactivated the p53 and pRb tumor-suppressive pathways,19 suggesting HPV E6/E7 might regulate cellular glucose metabolism of cervical cancers. Recent studies revealed cervical cancer cells with HPV infection were more glycolytic with elevated HK2, LDH, and PKM2.20 We found overexpression of HPV-16 E6/E7 promoted glycolysis and knockdown E6/E7 significantly suppressed glycolysis rate of cervical cancer cells, those results were consistent with previous report.
5-Fu, a pyrimidine analog modified by fluorination of uracil on position 5 of the pyrimidine ring, is currently being administrated as a widely used anti-cervical cancer chemotherapy to achieve optimal response and postoperative survival.8–10 However, in many cases, the HPV carrying cervical cancer cells generated 5-Fu resistance and the molecular mechanism of 5-Fu resistance remains unclear.11 Multiple groups reported elevated glycolysis of cancer cells contributes to the acquired 5-Fu resistance,21–23 indicating targeting glycolysis pathway might be an effective strategy against chemo-resistance. We demonstrated an HPV E6/E7-glycolysis-5-Fu resistance pathway, presenting a new molecular mechanism responsible for the chemosensitivity of cervical cancer. From the established 5-Fu-resistant cervical cancer cell line, we showed 5-Fu-resistant cells displayed upregulated E6/E7 expressions and increased glycolysis rate. In addition, inhibiting glycolysis overcame the obtained 5-Fu resistance in E6/E7 ectopic overexpressing cervical cancer cells, suggesting that dysregulated glycolysis acts as a downstream cellular process of E6/E7 oncoprotein, leading to develop E6/E7 inhibition as a new strategy against elevated glycolytic pathway for sensitization of cervical cancer cells to 5-Fu.
In summary, this study revealed the elevated glycolysis in HPV E6/E7 overexpressed cervical cancer cell, leading to 5-Fu resistance. From in vitro and in vivo experiments, we demonstrated inhibition of the E6/E7-mediated glycolysis facilitated overcoming 5-Fu resistance. Thus, targeting cellular glucose metabolism may be a valid therapeutic strategy to reduce or eliminate chemoresistant tumors.
Acknowledgments
This work was supported by the Department of Obstetrics and Gynecology, Tianjin Third Central Hospital. We thank all the medical doctors, faculties, and staffs in the Department of Obstetrics and Gynecology, Tianjin Third Central Hospital for academic and technical assistance.
Author contributions
DM, YH, and SS designed the study; DM performed research; DM, YH, and SS analyzed data; and DM, YH, and SS wrote the paper. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798 [DOI] [PubMed] [Google Scholar]
- 2.Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. 2015;25 Suppl 1:2–23. doi: 10.1002/rmv.1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBride AA, Warburton A. The role of integration in oncogenic progression of HPV-associated cancers. PLoS Pathog. 2017;13:e1006211. doi: 10.1371/journal.ppat.1006211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer. 2017;141:664–670. doi: 10.1002/ijc.30716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoppe-Seyler K, Bossler F, Braun JA, Herrmann AL, Hoppe-Seyler F. The HPV E6/E7 oncogenes: key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. 2018;26:158–168. doi: 10.1016/j.tim.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 6.Yeo-Teh NSL, Ito Y, Jha S. High-risk human papillomaviral oncogenes E6 and E7 target key cellular pathways to achieve oncogenesis. Int J Mol Sci. 2018;19:1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folprecht G, Köhne CH. Drug insight: metastatic colorectal cancer–oral fluoropyrimidines and new perspectives in the adjuvant setting. Nat Clin Pract Oncol. 2005;2:578–587. doi: 10.1038/ncponc0353 [DOI] [PubMed] [Google Scholar]
- 8.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074 [DOI] [PubMed] [Google Scholar]
- 9.Ceilley RI. Mechanisms of action of topical 5-fluorouracil: review and implications for the treatment of dermatological disorders. J Dermatolog Treat. 2012;23:83–89. doi: 10.3109/09546634.2010.507704 [DOI] [PubMed] [Google Scholar]
- 10.Ordikhani F, Erdem Arslan M, Marcelo R, et al. Drug delivery approaches for the treatment of cervical cancer. Pharmaceutics. 2016;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang N, Yin Y, Xu SJ, Chen WS. 5-Fluorouracil: mechanisms of resistance and reversal strategies. Molecules. 2008;13:1551–1569. doi: 10.3390/molecules13081551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyanaraman B. Teaching the basics of cancer metabolism: developing antitumor strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017;12:833–842. doi: 10.1016/j.redox.2017.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otto AM. Warburg effect(s)-a biographical sketch of Otto Warburg and his impacts on tumor metabolism. Cancer Metab. 2016;4:5. doi: 10.1186/s40170-016-0145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luengo A, Gui DY, Vander Heiden MG. Targeting metabolism for cancer therapy. Cell Chem Biol. 2017;24:1161–1180. doi: 10.1016/j.chembiol.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng Q, Chen J, Li Y, et al. LKB1 inhibits HPV-associated cancer progression by targeting cellular metabolism. Oncogene. 2017;36:1245–1255. doi: 10.1038/onc.2016.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vishnoi K, Mahata S, Tyagi A, et al. Human papillomavirus oncoproteins differentially modulate epithelial-mesenchymal transition in 5-FU-resistant cervical cancer cells. Tumour Biol. 2016;37:13137–13154. doi: 10.1007/s13277-016-5143-6 [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, Su J, Xue SL, et al. HPV E6/p53 mediated down-regulation of miR-34a inhibits Warburg effect through targeting LDHA in cervical cancer. Am J Cancer Res. 2016;6:312–320. [PMC free article] [PubMed] [Google Scholar]
- 19.Pastrez PRA, Mariano VS, Da Costa AM, et al. The relation of HPV infection and expression of p53 and p16 proteins in esophageal squamous cells carcinoma. J Cancer. 2017;8:1062–1070. doi: 10.7150/jca.17080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoppe-Seyler K, Honegger A, Bossler F, et al. Viral E6/E7 oncogene and cellular hexokinase 2 expression in HPV-positive cancer cell lines. Oncotarget. 2017;8:106342–106351. doi: 10.18632/oncotarget.22463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T, Ning K, Sun X, Zhang C, Jin LF, Hua D. Glycolysis is essential for chemoresistance induced by transient receptor potential channel C5 in colorectal cancer. BMC Cancer. 2018;18:207. doi: 10.1186/s12885-018-4242-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grasso C, Jansen G, Giovannetti E. Drug resistance in pancreatic cancer: impact of altered energy metabolism. Crit Rev Oncol Hematol. 2017;114:139–152. doi: 10.1016/j.critrevonc.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 23.He J, Xie G, Tong J, et al. Overexpression of microRNA-122 re-sensitizes 5-FU-resistant colon cancer cells to 5-FU through the inhibition of PKM2 in vitro and in vivo. Cell Biochem Biophys. 2014;70:1343–1350. doi: 10.1007/s12013-014-0062-x [DOI] [PubMed] [Google Scholar]