Abstract
Purpose
Pulmonary fibrosis (PF) is a common clinical disease, which results in serious respiratory impairment. Xin Jia Xuan Bai Cheng Qi Decoction (XJXBCQ) is a traditional prescription commonly used in treating lung diseases. We investigate the effect of XJXBCQ against PF and its mechanism via the regulation of TGF-β1/Smad in vitro and in vivo.
Materials and methods
XJXBCQ was first extracted and probed for chemical characterization. An PF model in vitro and in vivo was established in rats and in MRC-5 cells. In bleomycin (BLM)-induced rats model, lung function such as peak expiratory flow (PEF), minute ventilation (MV) and hydroxyproline (HYP) were measured; histopathological changes of lung tissue and TGF-β1 in peripheral blood of rats were detected. TGF-β receptor, Smad2 and its phosphorylation expression were tested by Western blot assay in rats model. Then the effects of XJXBCQ on TGF-β1/Smad signal pathway were assessed by Western blot analysis in vitro, and IL-17A and IL-25 levels were evaluated by ELISA in vivo.
Results
Our results showed that XJXBCQ significantly enhanced the lung functions, such as PEF, MV and HYP, by reducing the expression level of lung inflammatory cytokine and the content and fibrosis of lung collagen. Moreover, XJXBCQ effectively inhibited TGF-β1, Smad2 and its phosphorylation expression, and the activation of Smad7 in vitro and in vivo. Furthermore, XJXBCQ had an inhibitory effect on the α-smooth muscle actin (α-SMA) and fibronectin (Fn) in vitro and downregulated IL-17A and IL-25 by inhibiting the activation of TGF-β1/Smad signaling pathway in vitro and in vivo. Further, XJXBCQ effectively inhibitied ventilation volume and peak expiratory content remodeling and hydroxyproline content through inhibition of TGF-βRⅡ, Smad2 and its phosphorylation expression, and activation of Smad7 in vivo.
Conclusion
XJXBCQ extract had an anti-PF effect in vitro and in vivo, which could be attributed to the inhibition of the expression of p-Smad2 and increase in the expression of Smad7 by regulating the TGF-β1/Smad activity.
Keywords: pulmonary fibrosis, lung inflammatory cytokines, transforming growth factor beta family, Smads family, Xin Jia Xuan Bai Cheng Qi decoction
Introduction
Pulmonary fibrosis (PF) is a chronic, progressive and life-threatening fibrotic disease of the lungs, which results in serious respiratory impairment.1 The prevalence of PF is 2–29 per 100,000 individuals, and its incidence is approximately 10 per 100,000 individuals per year with an upward trend.2 Unfortunately, no therapies have been proven effective for treating PF aside from lung transplantation; however, pirfenidone has been approved as a novel antifibrotic agent in some countries.3 Nevertheless, these drugs have unavoidable side effects such as gastrointestinal disorders, skin-related abnormalities, abnormal increases in alanine and aspartate aminotransferase, and they can only delay pulmonary failure, but not reverse the process of PF. Traditional therapy for PF includes warfarin, prednisone, azathioprine and N-acetylcysteine.4,5 The pathogenesis and drug therapy of PF remain poorly elucidated and controversial.
In recent years, Chinese herbal prescriptions have attracted increasing attention due to their complementary therapeutic effects to Western drugs but with minimum side effects.6 Chinese herbal medicines have been used for many years by Chinese investigators in treating PF due to their benefits.7 Herbal medicines have significant effects against pulmonary injury and fibrosis by blocking cell signal pathway, which is associated with their anti-inflammatory and fibrogenic-inhibiting molecules.8,9
TGF-β1 is a member of the TGF-β superfamily, which is considered as a hallmark in PF. As multifunctional cytokine, TGF-β1 regulates the growth, differentiation and function of various cell types.10 The principal effect of TGF-β1 on mesenchymal cells is the stimulation of extracellular matrix (ECM) deposition. Moreover, TGF-β1 increases the expression levels of collagen types I, III, VI, VII and X, as well as the expression levels of fibronectin and proteoglycans.11–13
Smads are intracellular signal-transduction molecules of the TGF-β super family.14 According to differences in structure and function, nine Smads have been reported and classified into three groups. Smad2 and Smad3 are called R-Smads in the pathway and Smad4 (Co-Smads) for all the pathways. Smad6, Smad7 and Smad8 are inhibitory factors of these Smads. When TGF-β1 binds to its receptor, Smad2/3 phosphorylates and binds with Smad4; together, they move into the nucleus for translation and expression of the target gene.15,16
The disease characterized of PF by progressive and diffuse PF and restrictive ventilation dysfunction that leads to declines in pulmonary function, and, eventually, torespiratory failure.17,18 Many studies have demonstrated that Smad2 is one of the two major downstream regulators that promotes TGF-β-mediated tissue fibrosis.19-22 Furthermore, blocking Smad7 with a specific antisense oligonucleotide restores TGF-β signaling and allows TGF-β to inhibit proinflammatory cytokine production.23,24 This result indicates the importance of Smad7 in the pathogenesis of inflammatory disorders and suggests that Smad7 can be a target for the treatment of disorders with disturbed TGF-β signaling.
Xin Jia Xuan Bai Cheng Qi decoction (XJXBCQ) is a Chinese herbal formula composed of six herbs, namely, Gypsum fibrosum (Shi Gao), Rhei radix et rhizome (Da Huang), Semen Armeniacae amarum (Ku Xing Ren), Trichosanthis pericarpium (Gua Lou Pi), Persicae semen (Tao Ren) and Eupolyphaga steleophaga (Tu Bie Chong), in 10:3:2:5:3:3 ratio. The component of the herbal formula is mostly derived from the classic formula Xia Yu Xue Tang and Xuan Bai Cheng Qi Tang in traditional Chinese medicine (TCM), which have a long history of use for the treatment of lung diseases, such as phlegm and lung deflation.25
Previous clinical studies suggest that the decoction of Gualou Xiebai herbal formula plays an effective role in ameliorating myocardial fibrosis induced by cardiac infarction with ligated left anterior descending coronary artery by inhibiting the TGF-β1 signaling pathway.26 In addition, some herbal contents in XJXBCQ include Gua Lou Pi (Trichosanthis pericarpium), Da Huang (Rhei radix et rhizome) and Ku Xing Ren (Semen Armeniacae amarum), which are commonly used drugs for lung-intestine-related diseases, with differences for different types of syndrome. Two additional drugs, that is, amygdalin and emodin, exhibit anti-fibroblast activation effect and have a potential in treating PF.27,28
Although the XJXBCQ herbal formula has been previously reported to possess anticancer effect, its underlying mechanisms in improving pulmonary function and PF remain unknown. Thus, the present study aims to elucidate the effect and the molecular mechanism of Chinese herbal formula XJXBCQ in PF and fibrotic diseases of the lung.
Materials and methods
Cell culture and reagents
Human embryonic lung fibroblast MRC-5 cells were obtained from the cell bank of Chinese Academy of Sciences and maintained in a MEM medium supplemented with 10% (v/v) heat-inactivated fetal calf serum, 2 mM glutamine, 100 units/mL of penicillin and 100 μg/mL of streptomycin (Invitrogen, Carlsbad, CA) at 37°C in a 5% CO2 humidified atmosphere.
Animal model
A total of 60 6-week-old SPF male Wistar rats weighing 190–210 g and have never mated were used in the study. All animals were obtained from Shanghai Super-B&K Animal Laboratory Co., Ltd. (Shanghai, China). The Wistar rats were housed in Shanghai University of Traditional Chinese Medicine’s animal facility under controlled temperature, humidity and dark/light cycle conditions. The animal protocol used in this study was reviewed and approved by the Ethics Committees of Shanghai University of Traditional Chinese Medicine (2013001). Animals were treated according to the guiding principles of the Guide for the Care and Use of Laboratory Animals of Shanghai University of Traditional Chinese Medicine (Shanghai, China). The protocol approval number is SCXK (Shanghai) 2013–0016.
The 6-week-old SPF male Wistar rats were randomly divided into 6 treatment groups with 10 rats each as follows: control group; PF model group [bleomycin (BLM)], positive control group (BLM+prednisone, 0.167 mg/kg), low-dose XJXBCQ (BLM+XJXBCQ, 3.60 g/kg), medium-dose XJXBCQ group (BLM+XJXBCQ 7.19 g/kg) and high-dose XJXBCQ group (BLM+XJXBCQ, 14.38 g/kg). According to the improved method,29 rats from the PF model group underwent intranasal instillation of a single dose of 7 μg/g BLM in saline (150 μL), and rats from the control group underwent intranasal instillation of normal saline (NS) of equal volume (150 μL). At 8 hrs after the model establishment, rats from the XJXBCQ groups were intragastrically administrated with different XJXBCQ doses, and rats from the positive control group were treated with prednisone acetate once a day. Meanwhile, rats from the control and PF model groups were given NS of equal volume. All groups were sacrificed at the 28th day.
Preparation of Chinese XJXBCQ herb formula
The formula for creating one dose of XJXBCQ is presented in Table 1. Chinese medicines were purchased from Shanghai Hua Yu Chinese Herbs Co., Ltd. (Shanghai, China). The Chinese medicines included Shi Gao (lot# 130125), Da Huang (lot# 130129), Ku Xing Ren (lot# 130220), Gua Lou Pi (lot# 130115), Tao Ren (lot# 130326) and Tu Bie Chong (lot# 130419). All herbs were authenticated by Associate Researcher Tao Yang according to the Pharmacopoeia of the People’s Republic of China (2015). The vouchers of all components were deposited at the herbarium located in the College of Pharmacy, Shanghai University of TCM (Shanghai, China).
Table 1.
Formula of XJXBCQ (one dose)
| Chinese medicine | Sources | Medicinal parts | Origin (Province) | Voucher number | Weight (g) |
|---|---|---|---|---|---|
| Shi Gao | Gypsum fibrosum | - | Hubei | 211803 | 30 |
| Da Huang | Rheum palmatum L. | Root and rhizome | Qinghai | 221304 | 9 |
| Ku Xing Ren | Prunus armeniaca L. | Seed | Hebei | 201508 | 6 |
| Gua Lou Pi | Trichosanthes kirikrwii Maxim. | Fruit | Xinjiang | 226115 | 15 |
| Tao Ren | Prunus persica L. Batsch (Rosaceae) | Seed | Neimenggu | 217036 | 9 |
| Tu Bie Chong | Eupolyphaga sinensis Walker | Female | Jiangxi | 223810 | 9 |
XJXBCQ extracts were prepared according to a validated method.30 All herbs were added with appropriate amounts of water (except Shi Gao and Da Huang), soaked for 60 mins, and decocted twice for 3 hrs for the first time and 2 hrs for the second time. Afterward, we combined the decoctions. Shi Gao was first decocted for 0.5 hrs, and Da Huang was decocted in the last 5 mins, according to the approaches of decocted earlier and decocted late. The decoction was dried by decompressing at 60±2°C (1 g extract contained 15 g of herbal mixtures) as the dry extract. This decoction was stored at −20°C, dissolved in distilled water, and diluted with physiologic saline for animal tests.
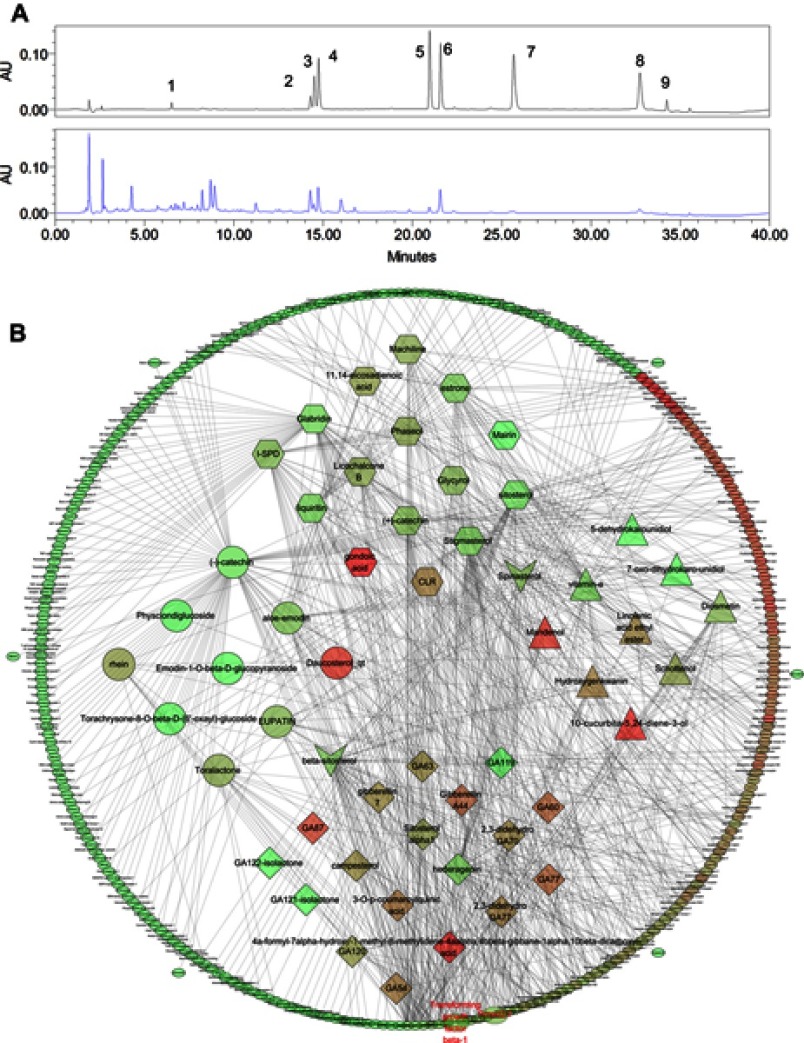
For quality control, the fingerprint spectrum for XJXBCQ was performed by Waters 2695 system (Waters, Milford, Massachusetts, USA) equipped with a quaternary gradient pump, an autosampler and a photodiode array detector. The components were eluted with a gradient system consisting of graded water (mobile phase A) and LC-MS grade acetonitrile (mobile phase B; time, min/B%: 0/95, 5/75, 10/70, 15/60, 20/40, 30/40, 32/5, 36/5 and 40/95). The chromatographic column used was the CNW Athena C18-WP (4.6 mm×150 mm, 3 µm). The mobile phase flow rate was 1 mL/min, and the column temperature was maintained at 30°C. The contents of amygdalin, aloe emodin, rhein, chrysophanol and emodin detected using the HPLC method were 18.1, 0.77, 5.10, 0.52 and 1.16 mg/g in the extracts, respectively (Figure 1A).
Figure 1.
HPLC fingerprint of mix standards and XJXBCQ. (A)The chromatographic profile of XJXBCQ extracts (stationary phase: CNW Athena C18-WP [(4.6 mm×150 mm, 3 µm]; mobile phase: 0.1% formic acid in LC-MS grade water [mobile phase A] and LC-MS grade acetonitrile [mobile phase B; time, min/B%: 0/95, 5/75, 10/70, 15/60, 20/40, 30/40, 32/5, 36/5 and 40/95; flow rate: 1 mL/min]). Peak nos.: 1) amygdalin, 2) barbaloin, 3) emodin-8-O-β-D-glucopyranoside, 4) chrysopha, 5) Aloe emodin, 6) rhein, 7) emodin, 8) chrysophanol and 9) physcion. (B) Interaction network diagram between the active ingredients of XJXBCQ and their targets using prediction software of Cytoscape 3.6.1. Active compound-active compound target network of XJXBCQ consists of 53 active compounds and 290 active compound targets (circle, diamond, hexagon, triangle stand for active compounds of Shengdahuang, Taoren, Kuxingren, Gualoupi; vee of “beta-sitosterol” stands for common active compound of Shengdahuang and Taoren, vee of “Spinasterol” stands for common active compound of Kuxingren and Gualoupi; ellipse stands for compound targets). All active compounds and target proteins change from red to green according to the number of edges. In this figure, 12 compounds were associated with TGF-β1, and 8 compounds were associated with Smads, indicating that XJXBCQ is closely related to TGF-β1 and Smads.
Network construction
The potential targets for the components of XJXBCQ were retrieved from Therapeutic Targets Database including TCMSP (http://lsp.nwu.edu.cn/tcmsp.php), TCM database @Taiwan (http://tcm.cmu.edu.tw) and Drugbank (http://www.drugbank.ca). In these networks (Figure 1B), XJXBCQ and its targets are represented as nodes, while the edges indicate interaction or relatedness.31
Lung function tests
Rats were anesthetized with 3% pentobarbital sodium by intraperitoneal injection and transferred into a plethysmographic chamber (4665 mL) to determine the pulmonary function by using the PowerLab and respiratory amplifier. The recorded variables included minute ventilation (MV) and peak expiratory flow (PEF).32
Assessment of hydroxyproline (HYP) content in lung tissue
Approximately 100 mg of lung tissue samples (stored at −70°C) were hydrolyzed with 1 mL of hydrolysate and boiled for 15 mins. The HYP contents in lung tissue were determined using an HYP kit (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions.33 HYP content was expressed as μg/mg.
Lung of histopathological changes
H&E stain and Sirius red staining were used to assess the histopathological changes and the severity of alveolitis and fibrosis. Histopathological changes in lung tissues were observed under a light microscope. The degree of fibrosis was shown through the areas of brownish yellow-stained collagen on the sections stained with immunohistochemistry. The red collagen fibers were positively stained with Sirius red, and the images of Sirius red were collected with Image-Pro Plus 6.0 professional image analysis software system. A total of 9–10 sections were taken from each group. Six high-power fields (×200) were randomly selected in the positive staining area, and each field of view (μm2) was measured.
Immunohistochemical analysis
Hydrated paraffin sections were incubated in a blocking solution (10% goat serum+5% nonfat dry milk+4% BSA+0.1% Triton X-100) for 10 mins and then incubated at 4°C overnight with anti-Smad2 and Smad7. The analysis was conducted as previously described.28 Cell labeling was scored as follows: 0 (negative), 1 (positive cells≤10%), 2 (positive cells between 11% and 50%), 3 (positive cells between 51% and 75%) and 4 (positive cells>75%). Discrepancies (<5%) were resolved by simultaneous re-evaluation. The significance of the correlation was determined using Pearson’s χ2 test.
Western blot analysis
Whole cell lysates for SDS-PAGE and Western blot analysis for the expression levels of α-SMA, fibronectin (Fn), Smad2, Smad7 and TGF-βRⅡ were prepared as previously reported.26 To prepare lysate from dissected in vivo lung tissues, samples were snap-frozen in liquid nitrogen immediately after sacrificing the animals and were stored at −80°C. The lysate was incubated on ice in an immunoprecipitation assay buffer for 2 hrs before being homogenized using a mortar and pestle. The homogenized sample was centrifuged, and the supernatant was collected and stored at −80°C. Equal loading was confirmed with β-actin/GAPDH. Densitometric analysis was conducted using Scion Imaging Software (Scion Co.).
ELISA
Cells were seeded in a 24-well plate at a density of 1×105 cells per well. After attachment, the medium was replaced with fresh medium supplemented with 1% FBS. After treatment, cell supernatants were collected, and the amounts of IL-17A, TGF-β and IL-25 were determined with a human ELISA kit (Multi Sciences, Shanghai, China) according to the manufacturer’s instructions.34 Cells were harvested by trypsinization and were counted afterward.
Statistical analysis
All values were expressed as the mean±SD and analyzed by one-way analysis of variance, followed by Duncan’s multiple range test by using SPSS version 21.0 software. A P-value of less than 0.05 was considered significant.
Results
Effect of XJXBCQ on PF in vivo
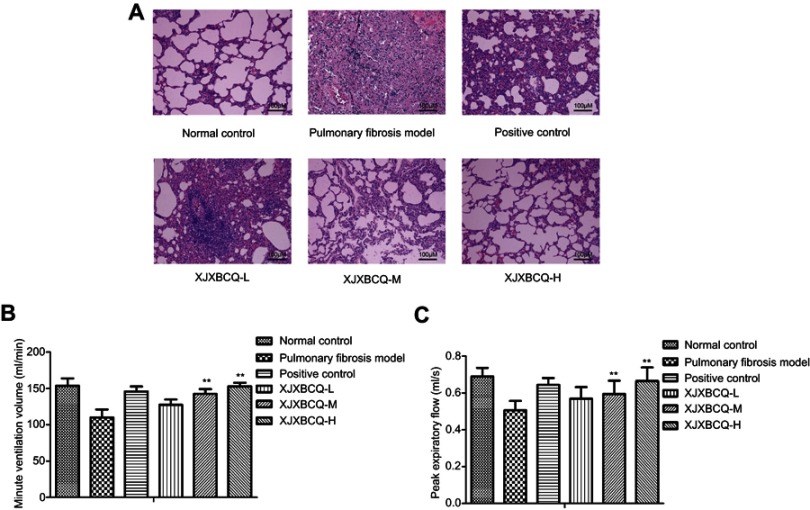
Previous studies have demonstrated that BLM can induce PF.29 In the present study, we used a concentration of 7 mg/kg bleomycin (volume of 200 μL each) to establish a PF model by nasal instillation into each rat, and the PF model can be successfully established by only one administration (Supplementary Fig. 1 & 2). We used prednisone in the positive control group. Histologically, in comparison with the control group, an increase in collagen deposition in the interstitium was observed in approximately 28 days after nasal BLM instillation (Figure 2A). In addition, the collagen deposition appeared diffusively in the lung parenchyma. The bronchial epithelium was papillary hyperplasia protruding into the cavity primarily within the alveolar walls in the lung and was accompanied by severe damages of lung architectures and papillary hyperplasia protruding in the bronchial epithelium and cellular infiltrates (Figure 2A). Moreover, in the groups treated with XJXBCQ, namely, XJXBCQ-L, XJXBCQ-M and XJXBCQ-H at concentrations of 3.60, 7.19 and 14.38 g/kg/day, all of the aforementioned alveolitis and fibrosis changes were significantly ameliorated compared with those of the PF model group. To determine the amount of pulmonary function of rats, we analyzed the dose-course changes in the content of MV and PEF in the rat models. As shown in Figure 2B and C, treatment with XJXBCQ significantly improved lung function in the BLM treatment group (PF Model).
Figure 2.
Effect of XJXBCQ on PF in BLM-induced rat. (A) Representative images of lung pathologic abnormalities with H&E staining: the inflammatory cells and collagen deposition in rats with PF. Scale bar, 100 μm. The lungs of rats were performed from rats treated with saline; BLM (7 μg/g), positive control and BLM+XJXBCQ on the 28th day. (B and C) Effects of MV and PEF in rats with PF from different groups. The values are shown as mean±SEM of the three experiments. **P<0.01 versus the model group.
Effect of XJXBCQ on PF and its mesenchymal marker
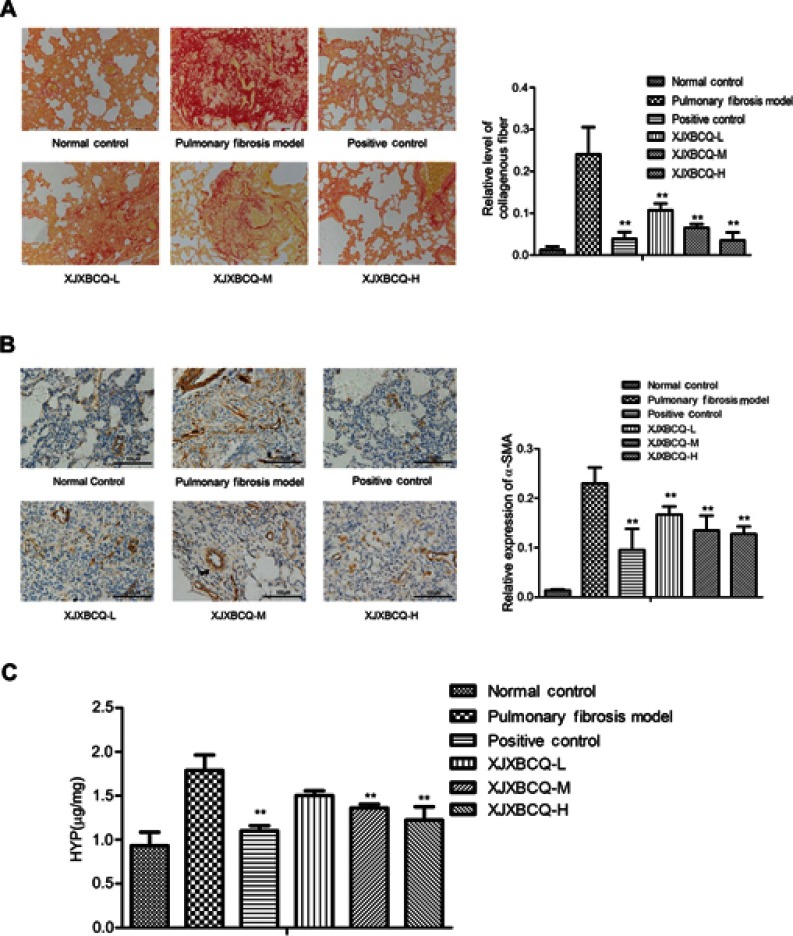
Lung fibrosis was further scored by histopathological observations of lung sections on the 28th day stained with Sirius red staining from normal control, PF model, positive control and XJXBCQ-treated groups (ie, XJXBCQ-L, XJXBCQ-M and XJXBCQ-H groups). As shown in Figure 2A, the lung section of rats receiving saline showed almost normal alveolar structure with a small amount of red collagen fibers in the alveolar septum (Figure 3A). The PF model produced characteristic histological changes, including infiltration areas of inflammatory cells, different grades of bronchial walls and alveolar spaces. Similar to the results of H&E, severe collagen deposition and fibrotic lesions were also improved by XJXBCQ in a dose-dependent manner.
Figure 3.
Effect of XJXBCQ on PF in vivo. (A) Representative images of lung collagen deposition by picro-sirius red staining from rat treated with saline; BLM (7 μg/g), positive control and BLM+XJXBCQ on the 28th day. (n=5); scale bar, 200 μm. (B) Protein expression levels of α-SMA in lung tissues. Scale bar, 100 μm. (C) HYP content in lung tissues of BLM-induced rat. The values are shown as mean±SEM of the three experiments. **P<0.01 versus the model group.
α-SMA is mesenchymal marker involved in inflammation and fibrosis. Thus, to determine whether it was involved in the inhibitory effect on PF of XJXBCQ, we assessed its expressions via immunohistochemical analysis. As shown in Figure 3B, the expression level of α-SMA in the lungs decreased in a dose-dependent manner after the XJXBCQ treatment. We used the image pro plus (ipp) as the quantitative image analysis to quantify the level of α-SMA expression in different groups. The acquisition of α-SMA dramatically decreased via the XJXBCQ treatment (Figure 3B). These results revealed an antifibrotic effect of XJXBCQ against BLM-induced PF in vivo.
To validate the effect of XJXBCQ, we evaluated lung fibrosis by measuring the HYP content in lungs and serum as an index of collagen accumulation. As expected, the XJXBCQ-treated groups (ie, XJXBCQ-L, XJXBCQ-M and XJXBCQ-H groups) significantly inhibited the increased HYP levels compared with the PF model group (Figure 3C).
Effect of XJXBCQ on TGF-β1 and p-Smad2 phosphorylation
rNumerous studies have recognized that the expression of TGF-β1 is increased predominantly in pulmonary endothelial cells and fibroblasts,35,36 and its downstream mediators, that is, Smads, are also involved in the fibrotic response. The Smad complex translocates into the nucleus, where it binds to a specific cis-acting element in the regulatory region of the TGF-β1 target genes (eg, α-SMA).37 In the present study, the protein expression levels of Smad2, p-Smad2 and Smad7 in the lungs were also assessed by Western blot analyses. Similar to the immunohistochemical results of the expression levels of Smad2 and Smad7 (Figure 4A), the protein levels of Smad2, p-Smad2 and TGF-βRⅡ decreased. Moreover, the levels of Smad7 increased via the XJXBCQ treatment (Figure 4B–D). As determined by ELISA, XJXBCQ induced a dramatic decrease in the expression of lung TGF-β1 protein compared with the PF model group (Figure 4E).
Figure 4.
Effect of XJXBCQ on TGF-β1 and p-Smad2 phosphorylation. (A) Immunohistochemical and Western blotting assays were conducted to detect the expression levels of Smad2, p-Smad2, Smad7 and TGF-βRII in different lung tissue groups. Scale bar, 100 μm. (B–D) Western blot with an antibody to GAPDH/β-actin was used to ensure equal loading of proteins in each lane. The blots were photographed and quantified for each sample. The data shown were obtained from three independent experiments. (E) The expression levels of TGF-β1 in the serum of rats were detected by ELISA. Each value is presented as the mean±SD of the three independent experiments. **P<0.01 ##P<0.01 versus the model group.
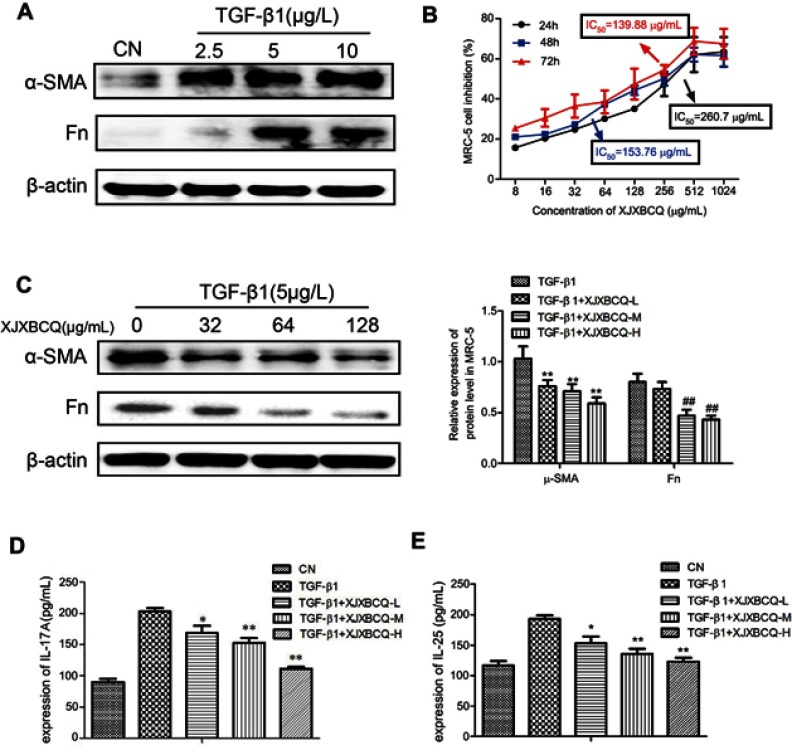
Effect of XJXBCQ on TGF-β1-mediated PF in MRC-5 cells
Fibroblasts (MRC-5 cells) were used to access the effect of XJXBCQ in vitro as previous research.38 Thus, we examined whether the activation of Smad2 via TGF-β1 contributed to the abrogated fibrotic response. The results showed that after treatment with different TGF-β1 concentrations for 48 hrs, the expression levels of α-SMA and Fn significantly increased (Figure 5A). Then, we tested their effects on the growth of the MRC-5 cells in vitro. As shown in Figure 5B, after treatment for 48 hrs and 72 hrs, XJXBCQ showed insignificant changes in the cell viability of MRC-5 cells, as analyzed via CCK-8 assay. However, in comparison with the control of MRC-5 cells without XJXBCQ treatment, the expression levels of α-SMA and Fn decreased significantly in TGF-β1-treated MRC-5 cells (Figure 5C). Simultaneously, we also used ELISA techniques to investigate the expression levels of IL-17A and IL-25 in the cellular supernatant of MRC-5 cells. The results were consistent with the findings of Western blot. After the XJXBCQ treatment in MRC-5 cells, the expression levels of IL-17A and IL-25 decreased remarkably compared with those of the TGF-β1-treated MRC-5 cells (Figure 5D and E).
Figure 5.
Effect of XJXBCQ on TGF-β1-mediated PF in MRC-5 cells. (A and C) Western blot assay was conducted to detect the levels of α-SMA and Fn in MRC-5 cells. The MRC-5 cells were treated with TGF-β1 for 48 hrs. Western blot with an antibody to GAPDH was used to ensure equal loading of proteins in each lane. Blots were photographed and quantified for each sample. The data presented were obtained from three independent experiments. (B) Cells were treated with various concentrations of XJXBCQ and analyzed by CCK-8 analyses. (D and E) The expression levels of IL-17A and IL-25 in the serum of rats were detected by ELISA. Each value is presented as the mean±SD determined from three independent experiments. *P<0.05 versus the TGF-β1 group; **P<0.01, ##P<0.01 versus the TGF-β1 group.
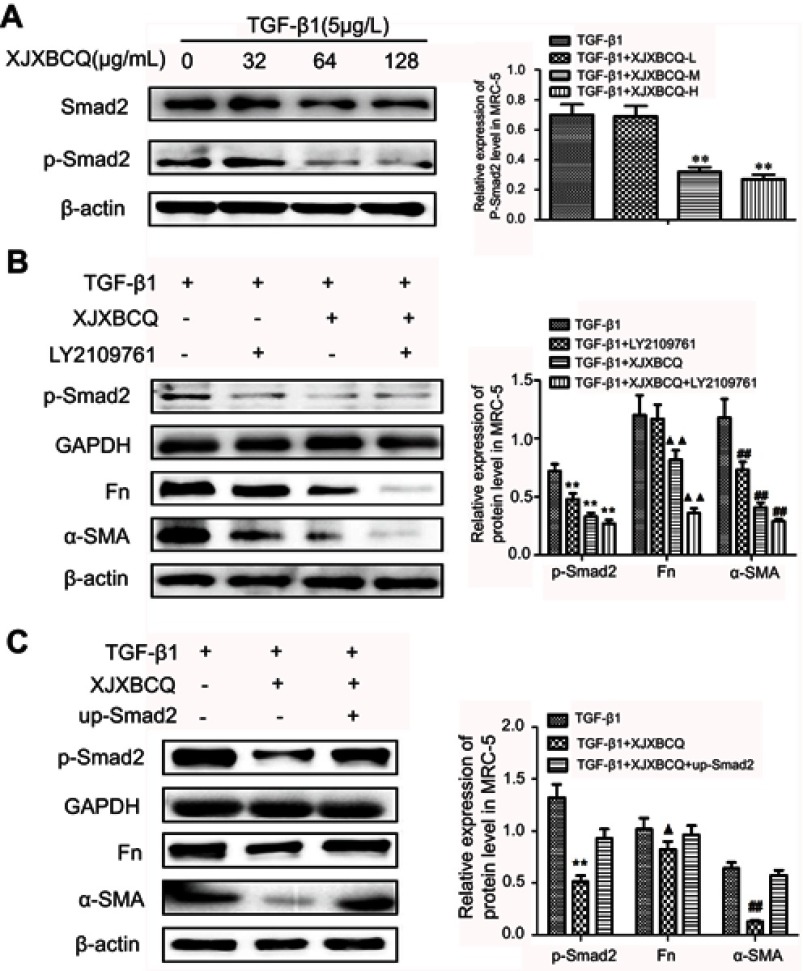
Effect of XJXBCQ on PF via TGF-β1/Smad2 pathway in MRC-5 cells
A previous study demonstrated that the TGF-β1 inhibitor (LY2109761) could inhibit the phosphorylation of TGF-β1-induced Smad2/3 in PF cells.39 In the present study, we initially examined the inhibitory activity of XJXBCQ (64 μg/mL) against TGF-β1-induced Smad phosphorylation after 48 hrs of TGF-β1 stimulation. The data showed that XJXBCQ could downregulate the levels of p-Smad2 in TGF-β1-stimulated MRC-5 cells in a dose-dependent manner, whereas the expression of Smad2 showed minimal change (Figure 6A). XJXBCQ can regulate Smad signaling pathway (Figure 6A and Supplementary Figure 3), decreasing Smad2 expression and increasing Smad7 expression in mRNA and protein level (Supplementary Fig. 4). Thus, investigating whether XJXBCQ could inhibit the PF through the TGF-β1-mediated Smad signaling pathway is necessary. To verify this hypothesis, we used TGF-β1 inhibitor LY2109761 to block the TGF-β1/Smad signaling pathway; afterward, XJXBCQ was added. The results showed that under the condition, XJXBCQ had a more significant inhibiting effect on the expression levels of α-SMA and Fn (Figure 6B). In addition, to enhance the integrity of the aforementioned results, we used the previously constructed the overexpressing vector Smad2 and test the effect of cell proliferation with vector and up-Smad2 vector (Supplementary Fig. 5). We found that the re-expression of Smad2 could partially recover the expression of α-SMA and Fn that had been inhibited by XJXBCQ (Figure 6C). All these data suggested that XJXBCQ may regulate PF through TGF-β1/Smad signaling pathway, thereby inhibiting TGF-β1-induced PF, which may be one of the mechanisms by which XJXBCQ inhibited PF.
Figure 6.
Effect of XJXBCQ on PF via TGF-β1/p-Smad2 pathway in MRC-5 cells. (A–C) Western blot assay was conducted to detect the levels of Smad2, p-Smad2, α-SMA and Fn in MRC-5 cells. MRC-5 cells were treated with TGF-β1 for 48 hrs. Western blot with an antibody to β-actin was used to ensure equal loading of proteins in each lane. Blots were photographed and quantified for each sample. The data presented were obtained from three independent experiments. Each value is presented as the mean±SD of three independent experiments. ▲P<0.05 versus the TGF-β1 group; **P<0.01, ##P<0.01, ▲▲P<0.01 versus the TGF-β1 group.
Discussion
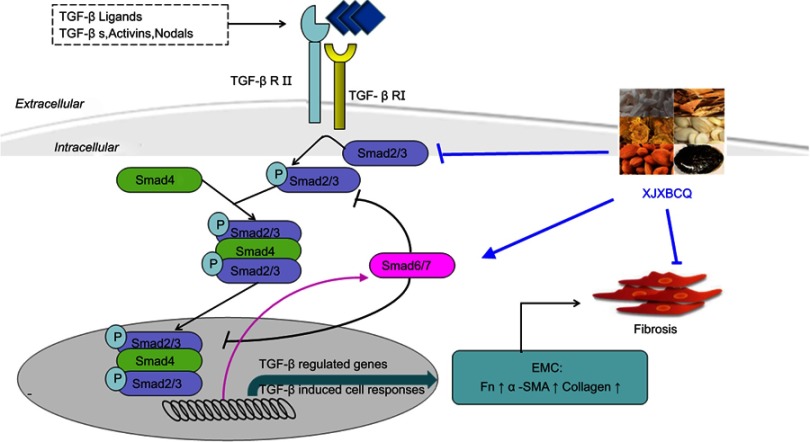
In this study, we used a simple and accurate HPLC method for the simultaneous separation and identification of six components to evaluate the quality of XJXBCQ. The interaction network diagram among all the active ingredients of XJXBCQ is shown in Figure 1A, and their targets were determined by using the prediction software of TCMSP, TCM database @Taiwan and Drugbank (Supplementary Table 1 & 2 and Figure 1B). Our results indicated that XJXBCQ had protective effects against BLM-associated lung inflammation in rat by reducing the expression levels of lung inflammatory cytokines; decreasing the lung collagen content and fibrosis; lowering the HYP content and impeding the pulmonary function, including MV, PEF and HYP contents. Furthermore, we demonstrated that XJXBCQ significantly downregulated the mesenchymal marker, which could be mediated by decreasing the expression of p-Smad2 via the TGF-β1/Smad signaling pathway. The results of the MRC-5 cell showed that TGF-β1/Smad2 activation was partially altered by treatment with XJXBCQ through the inhibition of Smad2 phosphorylation. To our knowledge, it is the first work to demonstrate that Chinese Herbal medicine XJXBCQ could inhibit lung inflammation in vivo and in vitro, at least in part, via the inhibition of Smad2 phosphorylation in the TGF-β1 signaling pathway (Figure 7).
Figure 7.
Proposed working model of XJXBCQ on the TGF-β1/Smad2 signaling pathway-mediated PF.
It is well known that TGF-β exerts its biological effects by activating downstream mediators including Smad2, Smad3 and Smad4, while is negatively regulated by Smad7 expression.40–42 XJXBCQ can effectively act on regulating the expression of Smad2, p-Smad2 and Smad7 in vivo; meanwhile, it promoted Smad7 expression and downregulated p-Smad2 expression, which could attribute to TGF-β1/p-Smad2 signaling pathway. Our data demonstrated that XJXBCQ significantly lowered the HYP contents and impeded pulmonary function in BLM-induced PF rat model. According to the histological findings of the rat lung, XJXBCQ markedly ameliorated the scores of alveolitis and fibrosis of the lung and suppressed lung inflammation. The aforementioned results clearly revealed that XJXBCQ could not only inhibit PF, which was confirmed by the decrease in acute inflammatory injury, collagen deposition and fibroblast, but also ameliorate the pulmonary function in the XJXBCQ treatment group. Moreover, the levels of HYP in medium doses of XJXBCQ treatment group were significantly lower than those in high and low doses of XJXBCQ treatment groups. This result may suggest that the medium dose of XJXBCQ is the best treatment dose.
Meanwhile, in the XJXBCQ-treated rat, a decreased expression of α-SMA-positive was observed, despite the comparable expression of α-SMA cell in BLM rat among groups by immunohistochemistry. This result indicated that XJXBCQ may inhibit the formation of fibrosis by inhibiting the expression of α-SMA and relieving PF. In addition, we determined the collagen content in the lung tissue by detecting HYP. Our results demonstrated that XJXBCQ treatment groups could markedly decrease the levels of HYP and suppress collagen deposition. XJXBCQ treatment not only distinctly inhibited the overexpression of collagen deposition and α-SMA but also attenuated the pulmonary function in rat after the BLM challenge. This result indicated that XJXBCQ may be an effective therapeutic drug against fibrogenesis in response to BLM. Next, we further studied and explored the mechanism and therapeutic targets of XJXBCQ.
TGF-β1 is a pleiotropic cytokine that has a crucial role in many aspects of lung fibrotic response and has long been believed to be a central mediator of this response.43,44 Smad proteins are thought to play an important role in regulating intracellular responses to TGF-β1. Smad proteins, such as Smad2 and Smad3, are activated by TGF-β1 receptors and are then translocated to the nucleus, where they regulate transcription, thereby further modifying multiple CF functions, including proliferation, differentiation and secretion.35 Our study demonstrated that TGF-β1-induced Smad2 and Smad phosphorylation in vivo and in vitro. Notably, the protein levels of α-SMA and Fn were significantly suppressed when Smad2 was knocked down or when TGF-β1 was inhibited. However, they were increased when Smad2 was overexpressed following TGF-β1 stimulation. However, for Smad7, an induction of inhibitory Smad protein, which represented a negative feedback loop, inhibited the TGF-β1/Smad signaling pathway in the activation progress of TGF-β1/Smad signaling pathway.
In the long-term course of treatment, traditional Chinese prescriptions and formulae, which are based on TCM principles, have been identified to be effective anti-pulmonary fibrotic drugs in patients with PF. Our previous clinical studies demonstrated that XJXBCQ improves the symptom of patients with PF.45 Adding herb extracts to cancer cells may improve the therapeutic outcomes among patients with cancer. To examine the potential mechanisms responsible for the therapeutic effects of XJXBCQ, we performed a series of experiments.
The discovery that XJXBCQ restores lung injury under pathological conditions suggests that XJXBCQ exerts its anti-pulmonary fibrotic effects, at least in part, through its regulation of a key pathway. Pulmonary protection induced by XJXBCQ is indicated by a number of studies related to its compounds extracted from natural plants. For example, emodin significantly relieves lung edema and fibrotic changes, decreases collagen deposition and suppresses myofibroblast differentiation via the suppression of TGF-β1 signaling pathway and inhibition of inflammation and oxidative activities in rats with BLM-induced PF.46 Resveratrol also interrupts TGF-β signaling and attenuates the expression of fibrogenic protein in rat with renal interstitial fibrosis.47 Moreover, rhein displays broad pharmacological activities against various cellular pathological processes, including oxidative stress, inflammation, apoptosis and carcinogenesis.48,49 However, the intracellular targets of XJXBCQ are not firmly established, and its inhibition mode of action remains poorly understood. The findings in the present study indicated that XJXBCQ treatment significantly suppressed BML-induced PF by inhibiting TGF-β1-induced Smad2, p-Smad2 activity, promoting Smad7 expression and decreasing α-SMA and Fn activity.
In conclusion, the present study confirmed the anti-PF effect of XJXBCQ in vitro and in vivo. The therapeutic effect of XJXBCQ may be mainly derived from its inhibition on the TGF-β1/Smad-mediated PF. Clarification regarding the underlying mechanism of XJXBCQ will provide new therapeutic targets for PF.
Acknowledgment
The authors thank Dr Xiao-Ling Wang for assisting in the copyediting of article. This work was funded by the National Natural Science Foundation of China (No. 81001491), the Science Foundation for Shanghai Committee of Science Project (No. 15ZR1441100) and Program for Application and Research of the Classical Prescription Theory (No. A1-Z183020110).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gonzalez-Gonzalez FJ, Chandel NS, Jain M, Budinger GRS. Reactive oxygen species as signaling molecules in the development of lung fibrosis. Transl Res 2017;190:61-68. doi: 10.1016/j.trsl.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao J, Ren Y, Qu Y, Jiang W, Lv C. Pharmacodynamic and pharmacokinetic assessment of pulmonary rehabilitation mixture for the treatment of pulmonary fibrosis. Sci Rep. 2017;7(1):3458. doi: 10.1038/s41598-017-02774-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staitieh BS, Renzoni EA, Veeraraghavan S. Pharmacologic therapies for idiopathic pulmonary fibrosis, past and future. Ann Med. 2015;47(2):100–105. doi: 10.3109/07853890.2014.991751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim HJ, Perlman D, Tomic R. Natural history of idiopathic pulmonary fibrosis. Respir Med. 2015;109(6):661–670. doi: 10.1016/j.rmed.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Tian SL, Yang Y, Liu XL, Xu QB. Emodin attenuates bleomycin-Induced pulmonary fibrosis via anti-Inflam -matory and anti-oxidative activities in rats. Med Sci Monit. 2018;1(24):1–10. doi: 10.12659/MSM.905496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu X, Sun S, Guo Y, et al. Citri reticulatae pericarpium (Chenpi): botany, ethnopharmacology, phytochemistry, and pharmacology of a frequently used traditional Chinese medicine. J Ethnopharmacol. 2018;220:265–282. doi: 10.1016/j.jep.2018.03.031 [DOI] [PubMed] [Google Scholar]
- 7.Guo J, Li B, Li W, et al. Chinese herbal medicines compared with N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: protocol for a systematic review. Medicine. 2018;97(44):e13077. doi: 10.1097/MD.0000000000013077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong SH, Liu YW, Wei F, Tan HZ, Han ZD. Asiatic acid ameliorates pulmonary fibrosis induced by bleomycin (BLM) via suppressing pro-fibrotic and inflammatory signaling pathways. Biomed Pharmacother. 2017;89:1297–1309. doi: 10.1016/j.biopha.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 9.Zhou XM, Wen GY, Zhao Y, Liu YM, Li JX. Inhibitory effects of alkaline extract of citrus reticulata on pulmonary fibrosis. J Ethnopharmacol. 2013;146(1):372–378. doi: 10.1016/j.jep.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 10.Gendron DR, Lemay AM, Lecours PB, et al. FTY720 promotes pulmonary fibrosis when administered during the remodelling phase following a bleomycin-induced lung injury. Pulm Pharmacol Ther. 2017;44:50–56. doi: 10.1016/j.pupt.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 11.Mamuya FA, Xie D, Lei L, et al. Deletion of β1-integrin in collecting duct principal cells leads to tubular injury and renal medullary fibrosis. Am J Physiol Renal Physiol. 2017;313(4):F1026–F1037. doi: 10.1152/ajprenal.00038.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griggs LA, Hassan NT, Malik RS, et al. Fibronectin fibrils regulate TGF-β1-induced epithelial-mesenchymal transition. Matrix Biol. 2017;60–61:157–175. doi: 10.1016/j.matbio.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins LM, Horst B, Lancaster CL, Mythreye K. Dually modified transmembrane proteoglycans in development and disease. Cytokine Growth Factor Rev. 2018;39:124–136. doi: 10.1016/j.cytogfr.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.David CJ, Massagué J. Contextual determinants of TGFβ action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19(7):419–435. doi: 10.1038/s41580-018-0007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y, Katuri V, Srinivasan R, et al. Transforming growth factor-beta suppresses nonmetastatic colon cancer through smad4 and adaptor protein elf at an early stage of tumorigenesis. Cancer Res. 2005;65(10):4228–4237. doi: 10.1158/0008-5472.CAN-04-4585 [DOI] [PubMed] [Google Scholar]
- 16.Hariharan R, Pillai MR. Structure-function relationship of inhibitory Smads: structural flexibility contributes to functional divergence. Proteins. 2008;71(4):1853–1862. doi: 10.1002/prot.21869 [DOI] [PubMed] [Google Scholar]
- 17.Behr J. The diagnosis and treatment of idiopathic pulmonary fibrosis. Dtsch Arztebl Int. 2013;110(51–52):875–881. doi: 10.3238/arztebl.2013.0875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou XM, Wang GL, Wang XB, et al. GHK peptide inhibits bleomycin-induced pulmonary fibrosis in mice by suppressing TGFβ1/smad-mediated epithelial-to-mesenchymal transition. Front Pharmacol. 2017;8:904. doi: 10.3389/fphar.2017.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Tu W, Tian K, et al. Sirtuin 6 inhibits myofibroblast differentiation via inactivating transforming growth factor-β1/smad2 and nuclear factor-κB signaling pathways in human fetal lung fibroblasts. J Cell Biochem. 2019;120(1):93–104. doi: 10.1002/jcb.27128 [DOI] [PubMed] [Google Scholar]
- 20.Jiang H, Chen Y, Yu T, et al. Inhibition of lncRNA PFRL prevents pulmonary fibrosis by disrupting the miR-26a/Smad2 loop. Am J Physiol Lung Cell Mol Physiol. 2018;315(4):L563–L575. doi: 10.1152/ajplung.00434.2017 [DOI] [PubMed] [Google Scholar]
- 21.Kawami M, Harabayashi R, Miyamoto M, Harada R, Yumoto R, Takano M. Methotrexate-induced epithelial-mesenchymal transition in the alveolar epithelial cell line A549. Lung. 2016;194(6):923–930. doi: 10.1007/s00408-016-9935-7 [DOI] [PubMed] [Google Scholar]
- 22.Liu B, Rong Y, Sun D, et al. Costunolide inhibits pulmonary fibrosis via regulating NF-kB and TGF-β1/Smad2/Nrf2-NOX4 signaling pathways. Biochem Biophys Res Commun. 2019;510(2):329–333. doi:10.1016.j.bbrc.2019.01.104 [DOI] [PubMed] [Google Scholar]
- 23.Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-β1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108(4):601–609. doi: 10.1172/JCI12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y, Gu S, Li W, et al. Smad7 enables STAT3 activation and promotes pluripotency independent of TGF-β signaling. Proc Natl Acad Sci U S A. 2017;114(38):10113–10118. doi: 10.1073/pnas.1705755114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu M, Zhong X, Li Y, et al. Xuan Bai Cheng Qi formula as an adjuvant treatment of acute exacerbation of chronic obstructive pulmonary disease of the syndrome type phlegm-heat obstructing the lungs: a multicenter, randomized, double-blind, placebo-controlled clinical trial. BMC Complement Altern Med. 2014;14:239. doi: 10.1186/1472-6882-14-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding YF, Peng YR, Li J, Shen H, Shen MQ, Fang TH. Gualou Xiebai Decoction prevents myocardial fibrosis by blocking TGF-beta/Smad signalling. J Pharm Pharmacol. 2013;65(9):1373–1381. doi: 10.1111/jphp.12102 [DOI] [PubMed] [Google Scholar]
- 27.Du HK, Song FC, Zhou X, Li H, Zhang JP. Effect of amygdalin on serum proteinic biomarker in pulmonary fibrosis of bleomycin-induced rat. Chin J Ind Hyg Occup Dis. 2010;28(4):260–263. [PubMed] [Google Scholar]
- 28.Guan R, Wang X, Zhao X, et al. Emodin ameliorates bleomycin-induced pulmonary fibrosis in rats by suppressing epithelial-mesenchymal transition and fibroblast activation. Sci Rep. 2016;6:35696. doi: 10.1038/srep35696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin C, von der Thüsen J, Daalhuisen J, et al. Pharmacological targeting of protease-activated receptor 2 affords protection from bleomycin-induced pulmonary fibrosis. Mol Med. 2015;21:576–583. doi: 10.2119/molmed.2015.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng W, Sui H, Wang Q, et al. A Chinese herbal formula, Yi-Qi-Fu-Sheng, inhibits migration/invasion of colorectal cancer by down-regulating MMP-2/9 via inhibiting the activation of ERK/MAPK signaling pathways. BMC Complement Altern Med. 2013;13:65. doi: 10.1186/1472-6882-13-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sui H, Zhao J, Zhou L, et al. Tanshinone IIA inhibits β-catenin/VEGF-mediated angiogenesis by targeting TGF-β1 in normoxic and HIF-1α in hypoxic microenvironments in human colorectal cancer. Cancer Lett. 2017;403:86–97. doi: 10.1016/j.canlet.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 32.Delaunois A, Dedoncker P, Hanon E, Guyaux M. Repeated assessment of cardiovascular and respiratory functions using combined telemetry and whole-body plethysmography in the rat. J Pharmacol Toxicol Methods. 2009;60(2):117–129. doi: 10.1016/j.vascn.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 33.You XY, Xue Q, Fang Y, et al. Preventive effects of ecliptae herba extract and its component, ecliptasaponin A, on bleomycin-induced pulmonary fibrosis in mice. J Ethnopharmacol. 2015;175:172–180. doi: 10.1016/j.jep.2015.08.034 [DOI] [PubMed] [Google Scholar]
- 34.Asano K, Shikama Y, Shoji N, Hirano K, Suzaki H, Nakajima H. Tiotropium bromide inhibits TGF-β-induced MMP production from lung fibroblasts by interfering with Smad and MAPK pathways in vitro. Int J Chron Obstruct Pulmon Dis. 2010;5:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimbori C, Bellaye PS, Xia J, et al. Fibroblast growth factor-1 attenuates TGF-β1-induced lung fibrosis. J Pathol. 2016;240(2):197–210. doi: 10.1002/path.4768 [DOI] [PubMed] [Google Scholar]
- 36.Bellaye PS, Shimbori C, Upagupta C, et al. Lysyl oxidase-like 1 protein deficiency protects mice from adenoviral transforming growth factor-β1-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2018;58(4):461–470. doi: 10.1165/rcmb.2017-0252OC [DOI] [PubMed] [Google Scholar]
- 37.Bagnato GL, Irrera N, Pizzino G, et al. Dual αvβ3 and αvβ5 blockade attenuates fibrotic and vascular alterations in a murine model of systemic sclerosis. Clin Sci. 2018;132(2):231–242. doi: 10.1042/CS20171426 [DOI] [PubMed] [Google Scholar]
- 38.Li XF, Liao J, Xin ZQ, Lu WQ, Liu AL. Relaxin attenuates silica-induced pulmonary fibrosis by regulating collagen type I and MMP-2. Int Immunopharmacol. 2013;17(3):537–542. doi: 10.1016/j.intimp.2013.07.020 [DOI] [PubMed] [Google Scholar]
- 39.Higgins SP, Tang Y, Higgins CE, et al. TGF-β1/p53 signaling in renal fibrogenesis. Cell Signal. 2018;43:1–10. doi: 10.1016/j.cellsig.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen L, Yang T, Lu DW, et al. Central role of dysregulation of TGF-β/Smad in CKD progression and potential targets of its treatment. Biomed Pharmacother. 2018;101:670–681. doi: 10.1016/j.biopha.2018.02.090 [DOI] [PubMed] [Google Scholar]
- 41.Walton KL, Johnson KE, Harrison CA. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Front Pharmacol. 2017;8:461. doi: 10.3389/fphar.2017.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu HH, Chen DQ, Wang YN, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 43.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–338. doi: 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, Zhang Q, Gao Y, et al. Induced pluripotent stem cell-conditioned medium suppresses pulmonary fibroblast-to-myofibroblast differentiation via the inhibition of TGF-β1/smad pathway. Int J Mol Med. 2018;41(1):473–484. doi: 10.3892/ijmm.2017.3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu J, Guo YJ. Therapeutic effect of xuanbai chengqi decoction on the acute phase of pulmonary diseases. J Emergency Traditional Chin Med. 2009;18(8):1286–1287. [Google Scholar]
- 46.Guan R, Zhao X, Wang X, et al. Emodin alleviates bleomycin-induced pulmonary fibrosis in rats. Toxicol Let. 2016;262:161–172. doi: 10.1016/j.toxlet.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 47.Zhang YQ, Liu YJ, Mao YF, Dong WW, Zhu XY, Jiang L. Resveratrol ameliorates lipopolysaccharide-induced epithelial mesenchymal transition and pulmonary fibrosis through suppression of oxidative stress and transforming growth factor-β1 signaling. Clin Nutr. 2015;34(4):752–760. doi: 10.1016/j.clnu.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 48.Ge H, Tang H, Liang Y, et al. Rhein attenuates inflammation through inhibition of NF-κB and NALP3 inflammasome in vivo and in vitro. Drug Des Devel Ther. 2017;11:1663–1671. doi: 10.2147/DDDT.S133069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C, Cao H, Zhou H, et al. Research progress on the antitumor effects of rhein: literature review. Anticancer Agents Med Chem. 2017;17(12):1624–1632. doi: 10.2174/1871520615666150930112631 [DOI] [PubMed] [Google Scholar]