Abstract
The target of rapamycin (TOR) pathway is an evolutionarily conserved signal transduction system that governs a plethora of eukaryotic biological processes, but its role in Cryptococcus neoformans remains elusive. In this study, we investigated the TOR pathway by functionally characterizing two Tor-like kinases, Tor1 and Tlk1, in C. neoformans. We successfully deleted TLK1, but not TOR1. TLK1 deletion did not result in any evident in vitro phenotypes, suggesting that Tlk1 is dispensable for the growth of C. neoformans. We demonstrated that Tor1, but not Tlk1, is essential and the target of rapamycin by constructing and analyzing conditionally regulated strains and sporulation analysis of heterozygous mutants in the diploid strain background. To further analyze the Tor1 function, we constructed constitutive TOR1 overexpression strains. Tor1 negatively regulated thermotolerance and the DNA damage response, which are two important virulence factors of C. neoformans. TOR1 overexpression reduced Mpk1 phosphorylation, which is required for cell wall integrity and thermoresistance, and Rad53 phosphorylation, which governs the DNA damage response pathway. Tor1 is localized to the cytoplasm, but enriched in the vacuole membrane. Phosphoproteomics and transcriptomics revealed that Tor1 regulates a variety of biological processes, including metabolic processes, cytoskeleton organization, ribosome biogenesis, and stress response. TOR inhibition by rapamycin caused actin depolarization in a Tor1-dependent manner. Finally, screening rapamycin-sensitive and -resistant kinase and transcription factor mutants revealed that the TOR pathway may crosstalk with a number of stress signaling pathways. In conclusion, our study demonstrates that a single Tor1 kinase plays pleiotropic roles in C. neoformans.
Keywords: rapamycin, nutrient sensing, DNA damage response, thermotolerance
THE target of rapamycin (TOR) pathway is an evolutionarily conserved signal transduction system that regulates a plethora of eukaryotic cellular processes. These include: (1) ribosome biosynthesis, (2) the initiation and elongation of protein translation, (3) amino acid import, (4) transcriptional regulation of diverse metabolic pathways, (5) autophagy, and (6) endocytosis (Raught et al. 2001). Rapamycin is a lipophilic macrolide isolated from a strain of Streptomyces hygroscopicus (Vézina et al. 1975), which has an inhibitory effect on the growth of fungi, and an immunosuppressive effect in humans, by inhibiting T-cell activation. Rapamycin binds to the intracellular rapamycin receptor, the peptidyl-prolyl isomerase FKBP12 (an FK506-binding protein having a molecular weight of 12 kDa), and the rapamycin-FKBP12 complex directly binds and inhibits TOR kinases and its downstream signaling pathways. The TOR kinases belong to the phosphatidylinositol kinase-related kinases (PIKKs) protein family, and function as Ser/Thr protein kinases (Hoekstra 1997).
Saccharomyces cerevisiae and Schizosaccharomyces pombe—budding and fission yeast models, respectively—each have two TOR orthologs (Weisman and Choder 2001). However, mammals contain a single TOR protein, named mTOR (mammalian TOR). TOR proteins contain multiple functional domains, including an N-terminal region with multiple HEAT repeats (Huntington, elongation factor 3, the A subunit of type 2A protein phosphatase, and Tor), which are involved in protein–protein interactions, a central FAT (focal adhesion kinase, targeting) domain regulating cell growth and TOR kinase activity, the FRB (FKBP-rapamycin-binding) domain that binds to the rapamycin-FKBP12 complex, and the kinase domain (Figure 1A). The mTOR protein contains a unique repressor domain at the C-terminus (Rohde et al. 2001). In S. cerevisiae, TOR proteins form two multi-protein complexes. TOR complex 1 (TORC1) is composed of Kog1, Lst8, and Tco89, in association with either Tor1 or Tor2, and regulates ribosome biogenesis, protein synthesis, transcription, and actin polarization (Wedaman et al. 2003; Reinke et al. 2004). In contrast, TOR complex 2 (TORC2) is comprised of Tor2, Lst8, Avo1, Avo2, Avo3, Bit2, and Bit61, and controls cell integrity, actin polarization, and stress responses. Even eukaryotic organisms with only a single TOR protein appear to have two TOR complexes (Rohde et al. 2001).
Figure 1.
TOR1 is required for viability in Cryptococcus neoformans. (A) Maximum likelihood tree of the identified Tor1 and Tlk1 proteins in S. cerevisiae (Sc), C. albicans (Ca), A. fumigatus (Af), C. neoformans (Cn), M. sympodialis (Ms), and U. maydis (Um). (B) The domain structure of C. neoformans TOR1 and TLK1. (C) Alignment of FKBP12-rapamycin binding domains of S. cerevisiae TOR1 (ScTOR1), Tor2 (ScTOR2), C. neoformans TOR1 (CnTOR1), and TLK1 (CnTLK1). Multiple sequence alignment is by ClustalW alignment using MacVector software. The position of the serine residue required for inhibition by rapamycin is in the box. (D) WT, PCTR4:TOR1 (YSB3176 and YSB3178), and PCTR4:TLK1 (YSB3043 and YSB3044) strains were spotted on YPD or YNB medium containing 200 μM BCS or 25 μM CuSO4. The plates were incubated at 30° and photographed daily for 5 days. This spot assay was repeated more than three times, and one representative image was shown here. (E) Schematic representation of the TOR1 gene before and after the targeted replacement. Position of primers used for confirmation are marked by arrows. (F) Δ indicates the NAT resistant heterozygote tor1Δ/TOR1 mutant generated. Random basidiospores (150) were dissected from TOR1/tor1Δ, and 14 that germinated were grown on four medium types for genetic analysis; 12 strains showed a NAT-sensitive phenotype and independent segregation of the other genetic markers. Conversely, spores #7 and #8 yielded colonies with irregular segregation, and hence were subjected to further molecular characterization reported in (G). (G) Molecular characterization of the four markers that segregate during meiosis (NAT, URA5, ADE2, MAT) in NATR progeny #7 and #8 obtained from the sporulation of the heterozygous TOR1/tor1Δ. The parental TOR1/tor1Δ and the diploid AI187 were included as control. The asterisk (*) indicates the mutated tor1Δ and ura5 alleles.
Regardless of the potential antifungal activity of rapamycin, there is a paucity of information on the function and regulation of the TOR pathway in fungal pathogens. Studies in Candida albicans and Cryptococcus neoformans provide some evidence that the TOR pathway plays an important role in virulence and may be a novel antifungal drug target. Rapamycin toxicity to C. neoformans depends on Tor1 and Frr1 (FKBP12 homolog), and spontaneous mutations in the FRB domain of Tor1 (S1862L) confer resistance to the drug (Cruz et al. 1999). Tor1 physically interacts with Frr1 only in the presence of rapamycin in C. neoformans (Cruz et al. 1999). At a sublethal concentration, rapamycin inhibits the filamentous growth of C. neoformans, C. albicans, and Candida lusitaniae under nitrogen-starved conditions (Cutler et al. 2001), indicating that the TOR pathway is involved in fungal morphogenic transitions. H. Lee et al. (2012) demonstrated that Sin1, which potentially forms a complex with Tor1, and its downstream target kinase Ypk1 are required for both fluconazole resistance and for the virulence of C. neoformans. TOR also regulates phosphorylation of mRNA decapping enzyme Dcp2, which subsequently modulates the RCK family protein Vad1 to control autophagy-related transcript degradation in C. neoformans (Hu et al. 2015). Notably, overexpression of VAD1 not only suppresses autophagy and also attenuates the virulence of C. neoformans, strongly suggesting that TOR-mediated autophagy regulation is critical for cryptococcal pathogenesis (Hu et al. 2015).
Despite the importance of the TOR pathway in fungal pathogens, the C. neoformans Tor1 kinase itself has not been directly investigated in detail because it is hypothesized to be an essential protein. In Cryptococcus deneoformans (JEC21 strain background), Tor1 was predicted to be essential because TOR1 could be disrupted in the diploid strain, but not in the haploid strain (Davidson et al. 2002). Tor1 was also predicted to be essential in C. neoformans (H99 strain background) because it could not be deleted (H. Lee et al. 2012). However, the essentiality of Tor1 has not been confirmed experimentally. Notably, C. neoformans contains another TOR-like kinase Tlk1, which shares 57% identity with S. cerevisiae Tor1 but has a less well conserved FRB domain (H. Lee et al. 2012). Deletion of TLK1 is possible, but does not affect fluconazole and rapamycin resistance (H. Lee et al. 2012). Currently, the physiological function of Tlk1 in C. neoformans is not known.
In this study, we investigated the function and regulatory mechanisms of Tor1 and Tlk1 kinases in C. neoformans through gene manipulation approaches. We experimentally verified that Tor1, but not Tlk1, is essential for growth, and is indeed a target of rapamycin. Using constitutive TOR1 overexpression strains, we demonstrate that Tor1 is required for thermotolerance by regulating phosphorylation of the mitogen-activated protein kinase (MAPK) Mpk1, and also regulates the DNA damage response and adaptation by affecting Rad53 phosphorylation. Furthermore, we found that Tor1 is involved in actin cytoskeleton organization. Finally, using phosphoproteomics and transcriptomics approaches, we define the Tor1-signaling networks in C. neoformans.
Materials and Methods
Strain and media
Supplemental Material, Tables S1 and S2 list the strains and primers used in this study. C. neoformans strains were cultured in yeast extract-peptone-dextrose (YPD) medium, yeast nitrogen base (YNB) medium containing 25 μM CuSO4 [for repressing gene expression in the CTR4 (copper transporter 4) promoter replacement strain] or 200 μM bathocuproinedisulfonic acid (BCS; for inducing gene expression in the CTR4 promoter replacement strain). The diploid C. neoformans strain AI187 (ade2/ADE2 ura5/URA5 MATa/MATα) was used as a parental strain for constructing the heterozygous TOR1/tor1Δ mutant.
Identification of Tor1 and Tor1-like kinase in representative ascomycetes and basidiomycetes fungi
The Tor1 and Tor2 proteins of S. cerevisiae were retrieved from Saccharomyces Genome Database (SGD) and used for BLASTp and tBLASTn to identify orthologs in representative ascomycetes and basidiomycetes fungi, such as C. albicans, Aspergillus fumigatus, C. neoformans, Malassezia sympodialis, and Ustilago maydis. Bidirectional BLAST against S. cerevisiae was used to confirm the identity of the proteins. The retrieved proteins were aligned used the software MUSCLE and the phylogenetic tree generated with MEGA 7 (http://www.megasoftware.net/) using the maximum likelihood method (LG, five discrete gamma categories) and 100 bootstrap replications.
Construction of the promoter replacement, gene deletion, and GFP tagging strains
To construct promoter replacement strains, the native promoter of each gene was replaced with the CTR4 promoter or the histone H3 promoter of C. deneoformans strain JEC21. The promoter replacement cassettes were generated by two rounds of PCR with the primers listed in Table S2 using a split marker/double-joint PCR strategy that has been reported previously (Kim et al. 2009). The 5′-flanking and a portion of the 5′ exon regions of the TOR1, TLK1, and HSF1 gene, the NAT (nourseothricin acetyltransferase)-CTR4 promoter, and the NEO (neomycin phosphotransferase), or NAT-H3 promoter region were amplified by PCR with the primers listed in Table S2. PCR amplifications were performed using the Ex-Taq polymerase (TAKARA). The generated cassettes were purified using the Gel SV kit (Geneall) and coated on to gold microcarrier beads [0.6-μm (Bio-Rad)] and introduced into the wild-type (WT) strain (H99S) using a Bio-Rad particle delivery system (Toffaletti et al. 1993). The mutant strains were confirmed by diagnostic PCR and Southern blot analysis (Figure S1). The expression levels of TOR1, TLK1, and HSF1 in PH3:TOR1, PH3:TLK1, and PH3:HSF1 strains were measured by Northern blot analysis (Figure S1). To construct the PH3:TOR1 sch9Δ strain, the SCH9 disruption cassette was generated by the split marker/double-joint PCR strategy with the primers listed in Table S2, introduced in the PH3:TOR1 (YSB3144) strain through biolistic transformation, and confirmed by diagnostic PCR and Southern blot analysis (Figure S1).
To generate the heterozygous TOR1/tor1Δ strain in the diploid C. neoformans WT strain AI187 (Idnurm 2010), the tor1Δ::NAT construct was generated by in vivo recombination in S. cerevisiae using the strain FY834 and the plasmid pRS426 as previously described (Colot et al. 2006). The construct consisted of 1.5 kb regions for homologous recombination fused to the NAT cassette; primers were designed to replace a 6989 bp region of the TOR1 gene. The constructs were amplified from S. cerevisiae transformants using the primers ALID1229 and ALID1230. The replacement allele was introduced into AI187 using biolistic transformation. Nourseothricin resistant (NATR) transformants were screened for homologous recombination events by PCR using primers specific to the NAT gene (ai006, ai37, ai270, or ai290) in combination with screening primers (ALID2265–ALID2266) located outside the regions targeted. The TOR1/tor1Δ strain was streaked onto Murashige-Skoong (MS) agar medium and kept in the dark for 4–6 weeks to induce meiosis and sporulation. Haploid basidiospores from mixed populations were dissected on YPD agar + adenine (20 mg/liter) and incubated for 3 days at 30°. Spores were transferred to a 96-well plate containing 100 μl of YPD, and tested for the four genetic markers that segregate during meiosis (NATR, ura5/URA5, ade2/ADE2, and MATa/MATα) by spotting 3 μl of the cellular suspensions onto YPD + nourseothricin (100 μg/ml), YNB + adenine (20 mg/liter) or YNB + uracil (40 mg/liter) plates. The mating type marker was scored by crossing the haploid progeny with C. deneoformans strains JEC20 and JEC21 on MS media supplemented with adenine and uracil, and evaluating the formation of sexual structures under a microscope. Molecular characterization of the heterozygote TOR1/tor1Δ strains and selected progeny was performed as previously described (Ianiri and Idnurm 2015), with some modifications. Briefly, the presence of the WT and mutated TOR1 gene was assessed by PCR based on the different lengths of the amplicons produced using screening primers (ALID2265–ALID2266). Mating type was assessed by PCR using primers specific for each MAT locus, with ai144–ai145 used for the α allele and ai150–ai151 for the a allele. The complete URA5 gene was amplified by PCR with primers ALID0375–ALID0376 and analyzed by restriction fragment length polymorphism (RFLP) through digestion with ClaI, since a base substitution altered the restriction site in the ura5 gene. A fragment of the gene ADE2 was amplified with primers ALID0380–ALID0381 by PCR and sequenced, confirming the replacement of a C with a T. The sequences of the primers used are listed in Table S2.
The TOR1/TOR1-3xGFP strain was constructed as follows. To generate the pNAT_PGAL7_Cre construct, the Cre recombinase of pJL519 (Patel et al. 2010) and the HOG1 terminator (HOG1t) region of H99 strain were amplified, and CRE-HOG1t was generated using overlap PCR. The GAL7 promoter (PGAL7) in H99 strain was also amplified with the primers listed in Table S2. CRE-HOG1t and PGAL7 fragments were cloned into pTOP vectors (Enzynomics) and sequenced. The PGAL7 region was then subcloned into pTOP_Cre-HOG1t generating the plasmid pTOP_PGAL7_Cre-HOG1t. The plasmid pNAT was digested with HindIII and subcloned into pTOP_PGAL7_Cre-HOG1t to generate pNAT_PGAL7_Cre-HOG1t. The plasmid pNAT_PGAL7_Cre-HOG1t was introduced biolistically into the diploid strain AI187 and its ectopic integration was confirmed by diagnostic PCR and quantitative reverse transcription-PCR (qRT-PCR). To construct the internal 3xGFP tagged Tor1 strain, 5′ and 3′- regions flanking residue N321 of TOR1 (5′-FR and 3′-FR), the loxP-NEO-loxP region of pJK517 (Patel et al. 2010), and 3xGFP were amplified using PCR, cloned into pTOP vectors, and sequenced. The plasmid pTOP_3′-FR was subcloned into pTOP_3xGFP to generate pTOP_3xGPF_3′-FR. The plasmids pTOP_loxP_NEO_loxP and pTOP_5′-FR were subcloned into pTOP_3xGFP_3′-FR. The plasmid pTOP_5′-FR_loxP_NEO_loxP_3xGFP_3′-FR was then digested with HincII and XmaI and introduced into the diploid AI187 strain harboring Cre recombinase (YSB4868). The TOR1/TOR1-loxP-NEO-loxP-3xGFP strains were confirmed by diagnostic PCR and Southern blot analysis (Figure S1).
Chemical and stress sensitivity tests
Cells were incubated in YPD medium overnight at 30°, serially diluted (1–104 dilutions) in distilled water, and spotted (3 μl) onto solid YPD medium containing the indicated concentrations of stress-inducing reagents. Each plate was incubated for 2–5 days and photographed during the incubation period. To test temperature sensitivity, plates were incubated at 30, 37, and 39°.
Total RNA isolation and quantitative RT-PCR
WT and TOR1 overexpression (TOR1oe) strains were grown in YPD medium at 30° for 16 hr, subcultured into fresh YPD medium, and incubated for ∼4 hr at 30° until the culture reached an optical density at 600 nm (OD600) of 0.6. The cells were harvested by centrifugation and the supernatant was discarded. Prewarmed (30, 37, or 39°) fresh YPD medium was added to the remaining pellet and further incubated for 30 min at 30, 37, or 39°. For the rapamycin-treated sample, a half of the culture was treated with rapamycin and incubated for an additional 30 min at 30, 37, or 39°. A portion of the liquid culture (50 ml) was taken at each time point, frozen in liquid nitrogen, and lyophilized. Total RNAs were isolated by the EasyBlue (Intron) as previously described (Ko et al. 2009), and purified using RNeasy spin columns (Qiagen). The cDNA was synthesized using a PrimeScript 1ST strand cDNA synthesis kit (TAKARA). Relative gene expression levels were monitored using the gene-specific primers listed in Table S2.
Phosphorylation and λ-phosphatase assays
To monitor Rad53 phosphorylation, the Rad53-4xFLAG (Jung et al. 2019) and Rad53-4xFLAG TOR1oe strains were grown in YPD medium overnight at 30°. The overnight culture was inoculated into fresh YPD medium and further incubated for 5 hr at 30° until the OD600 was ∼0.6. The subcultured cells were divided in half. One-half was treated with methyl methanesulfonate (MMS; 0.02%) and rapamycin (3 ng/ml), while the other was only treated with 0.02% MMS. The strains were further incubated for 90 min and harvested with centrifugation. The cell pellet was resuspended in a lysis buffer [50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.5 mM EDTA, and 0.5% Triton X-100 supplemented with a protease inhibitor cocktail (Invitrogen) and phenylmethylsulfonyl fluoride (PMSF)] with or without a phosphatase inhibitor cocktail (Sigma). The cells were disrupted with 0.5-mm zirconia/silica beads (BioSpec Products). The cell lysates were collected and incubated with PMP buffer [50 mM HEPES pH 7.5, 100 mM NaCl, 2 mM dithiothreitol (DTT), 0.01% Brij 35, and 1 mM MnCl2] and 800 units of λ-phosphatase (New England BioLabs) for 1 hr at 30°. The protein concentration of each sample was determined using a Pierce BCA Protein Assay Kit (Thermo Scientific), and an equal amount of protein was run on a 10% SDS-PAGE gel and transferred to an immunoblot PVDF membrane (Bio-Rad). The phosphorylated forms of Rad53-4xFLAG were detected using anti-FLAG antibody (Santa Cruz Biotechnology) and a secondary anti-mouse IgG horseradish peroxidase-conjugated antibody (SC-2013; Santa Cruz Biotechnology) in an ECL solution.
Subcellular localization of Tor1
The TOR1/TOR1-loxP-NEO-loxP-3xGFP strain was incubated overnight at 30° in YPD medium and subcultured in fresh YPD medium until OD600 reached ∼0.6. The cells were harvested by centrifugation, resuspended in fresh YPG (yeast extract-peptone-galactose) medium to activate the Cre-Lox system, and further incubated for 3 hr at 30°. FM4-64 dye (Life Technologies) was added to the cells to a final concentration of 10 μM and incubated at 30° for an additional 30 min. The cells were pelleted by centrifugation, resuspended with fresh liquid YPD medium, and then incubated for 30 min at 30°. The cells were pelleted again, washed three times with phosphate-buffered saline (PBS), and resuspended in PBS. The cell suspension (10 μl) was mixed with mounting solution (10 μl; Biomeda), spotted on glass slides, and observed with Nikon Eclipse Ti and Olympus BX51 microscopes.
TiO2 enrichment-based phosphoproteomics
To identify Tor1 phosphorylated targets, the WT and TOR1oe strains were incubated overnight in YPD medium at 30°, subcultured into fresh YPD medium, and further incubated until they reached early logarithmic phase (OD600 = 0.7). For the rapamycin-treated sample, the WT cells were divided in half. One-half was treated with rapamycin (3 ng/ml) and both halves were incubated for 3 hr at 30°. Whole cell lysates were prepared from each sample with lysis buffer containing 50 mM Tris-Cl (pH 7.5), 1% sodium deoxycholate, 5 mM sodium pyrophosphate, 0.2 mM sodium orthovanadate, 50 mM sodium fluoride (NaF), 0.1% SDS, 1% Triton X-100, 0.5 mM PMSF, and 2.5× protease inhibitor cocktail solution (Merck Millipore). The protein concentration of each cell lysate was determined using a Pierce BCA protein kit (Life Technologies). Disulfide bonds between cysteine residues in the protein lysates were reduced by incubating 10 mg of total protein lysate with 10 mM DTT at room temperature (25°) for 1 hr followed by alkylation with 50 mM iodoacetamide in the dark for 1 hr at room temperature. The samples were once again treated with 40 mM DTT for 30 min at room temperature and digested with trypsin overnight at 37° (Sequencing-grade trypsin; Promega) at an enzyme:substrate ratio of 1:50 (w/w). The trypsin-digested protein lysates were purified with Sep-Pak C18 columns (Waters), lyophilized, and stored at −80°. Phosphopeptides were enriched using TiO2 Mag Sepharose beads (GE Healthcare), and lyophilized for liquid chromatography-tandem mass analysis (LC-MS/MS). Mass spectrometric analyses were performed using a Q Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Scientific), equipped with a Dionex U 3000 RSLC nano HPLC system, a nano-electrospray ionization source and fitted with a fused silica emitter tip (New Objective). All phosphopeptide samples were reconstituted in solution A [water/acetonitrile (98:2, v/v), 0.1% formic acid], and then injected into an LC- nano ESI-MS/MS system. Samples were first trapped on the Acclaim PepMap 100 trap column (100 μm i.d. × 2 cm, nanoViper C18, 5 μm particle size, 100 Å pore size, Thermo Scientific), and washed for 6 min with 98% solution A at a flow rate of 4 μl min−1, and then separated on an Acclaim PepMap 100 capillary column (75 μm i.d. × 15 cm, nanoViper C18, 3 μm particle size, 100 Å pore size; Thermo Scientific) at a flow rate of 400 nl min−1. Peptides were eluted with a gradient of 2–35% solution B [water/acetonitrile (2:98, v/v), 0.1% formic acid] over 90 min, 35–90% over 10 min, followed by 90% for 5 min, and finally 5% for 15 min. The separated peptides were electrosprayed through a coated silica tip (PicoTip emitter) at an ion spray voltage of 2000 eV. Peptides were identified by searching the C. neoformans H99S protein database (http://www.uniprot.org) for the MS/MS spectra using the Sequest TH search algorithm through the Proteome Discoverer platform (version 2.2, Thermo Scientific). The MS and MS/MS tolerances were set to 10 ppm and 0.5 Da, respectively. PhosphoRS node was used to calculate individual site probabilities for phosphorylated peptides with the following search parameters: cysteine carbamidomethylation as a fixed modification, methionine oxidation and serine/threonine/tyrosine phosphorylation as variable modifications, and allowing two missed trypsin cleavages for peptide identification. Peptide identification was filtered by a 1% false discovery rate (FDR) cut-off. A PhosphoRS probability score ≥0.75 was required to confirm the site of phosphorylation. Spectral counts were used to estimate relative phosphopeptide abundance in the WT, TOR1oe mutant strains and rapamycin-treated WT cells. The data were further normalized by dividing each ratio by the median value of the ratio of each LC-MS/MS run, resulting in normalized relative phosphopeptide abundance (fold change). If fold change value was between 0 and 1, it was calculated by a minus reciprocal (−1/fold change) to effectively represent the downregulation. Each sample (WT, TOR1oe and rapamycin-treated WT) was analyzed in three independent biological replicates. If a given phosphopeptide was identified in more than two independent biological replicates of WT, TOR1oe or rapamycin-treated WT sample and its relative abundance in comparison with WT was more than 1.5-fold change, it was considered as a putative Tor1 target phosphopeptide. If a phosphopeptide was exclusively identified in a single sample between two compared groups, in which fold change calculation is not possible, it was also considered as a putative Tor1 target phosphopeptide. Two-way ANOVA followed by Bonferroni’s multiple comparison test was further used to assess the statistical significance of the difference between the samples.
Transcriptome analysis by RNA-sequencing
WT (H99S) and TOR1oe strains were incubated in YPD liquid medium overnight at 30°, subcultured in fresh YPD liquid medium, and then further incubated at 30° until OD600 was ∼0.6–0.8. The WT cells were divided in half. One-half was used as a WT control while the other half was treated with rapamycin (3 ng/ml), and further incubated for 3 hr at 30°. The WT, TOR1oe, and rapamycin-treated WT cells were harvested and lyophilized. Three biologically independent cultures were prepared for each strain. Total RNA was isolated by Easy-BLUE (iNtRON) and purified with an RNeasy Mini Kit (Qiagen). cDNA libraries were constructed with the TruSeq RNA library kit using 1 μg of total RNA. The protocol included polyA-selected RNA extraction, RNA fragmentation, random hexamer primed reverse transcription, and 100 nt paired-end sequencing by Illumina HiSeq4000. The libraries were quantified using quantitative PCR (qPCR) according to the qPCR Quantification Protocol Guide and an Agilent Technologies 2100 Bioanalyzer. Reads were processed from the sequencer and aligned onto the C. neoformans H99 genome data using Tophat v2.0.13 (Trapnell et al. 2009). Internally Tophat uses Bowtie v2.2.3 (Langmead et al. 2009) to perform the alignment. Tophat initially removes a portion of the reads based on the quality information accompanying each read before it maps reads to the reference genome. The reference genome sequence of C. neoformans H99 and annotation data were downloaded from the Broad Institute ftp server (archive.broadinstitute.org). Tophat allows multiple alignments per read (up to 20 by default) and a maximum of two mismatches when mapping the reads to the reference. After aligning reads to the genome Cufflinks v2.2.1 (Trapnell et al. 2010) was used to assemble aligned reads into transcripts and to estimate their abundance. To correct for sequence expression count bias, the “–max-bundle-frags 50000000” option was used. The “-G” option was employed to enable use of known gene annotation information. Other parameters were left at their default values. The transcript counts for each isoform were calculated, and the relative transcript abundances were measured in FPKM (Fragments Per Kilobase of exon per Million fragments mapped) from Cufflinks. Gene expression levels were also calculated from the transcript counts. These values were used for differentially expressed gene (DEG) analysis later. Gene-level relative abundances were calculated as the sum of FPKMs of transcripts in a gene. Genes with a raw FPKM signal value of zero in any of the nine samples were excluded from the analysis. To facilitate log2 transformation, “1” was added to the FPKM value of each filtered gene. Filtered data were log2-transformed, converted to a linear scale using exponential power of 2, and subjected to quantile normalization. If fold change value was between 0 and 1, it was calculated by a minus reciprocal (−1/fold change) to effectively represent the downregulation. Statistical significance of differential expression was determined using independent t-test and fold change with the null hypothesis that no difference exists among the groups. The FDR was controlled by adjusting the P value (<0.05) using the Benjamini-Hochberg algorithm. For the DEG set, hierarchical clustering analysis was performed using complete linkage and Euclidean distance as a measure of similarity. Gene-enrichment and functional annotation analysis for the significant genes was performed using DAVID (http://david.abcc.ncifcrf.gov/).
Actin cytoskeleton staining
WT (H99S) and TOR1oe strains were treated with rapamycin (10 ng/ml) for 1 hr, and fixed for 15 min in 3.7% formaldehyde. Fixed cells were permeabilized with 0.1% Triton X-100 for 15 min and stained with rhodamine-phalloidin (Invitrogen) for 30 min at room temperature. After incubation, the cells were washed two or more times with PBS. The actin cytoskeleton organization was observed with Olympus BX51 microscope.
Data availability
Strains and plasmids are available upon request. Supplemental Figures and Tables are available at FigShare. Figure S1 contains data for construction of promoter replacement strains. Table S1 contains information for strains used in this study. Table S2 contains information for primers used in this study. Table S3 contains detailed information regarding phosphopeptides identified in wild-type, TOR1oe, or rapamycin-treated WT of C. neoformans. Table S4 contains the RNA-seq data generated in this study. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al. 2019) partner repository with the dataset identifier PXD012983 and 10.6019/PXD012983. The RNA-seq data generated by this study are available at Gene Expression Omnibus (GEO accession no. GSE129227). Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8208569.
Results
Tor1 kinase, but not Tor-like kinase 1 (Tlk1), is essential in C. neoformans
Bidirectional BLAST analysis revealed that the ascomycetes C. albicans and A. fumigatus, and the basidiomycetes M. sympodialis and U. maydis have only one copy of a TOR1-encoding gene, and all lack TOR2 (Figure 1A). Interestingly, the basidiomycetes C. neoformans has two homologs of the S. cerevisiae Tor kinase, Tor1 (CNAG_06642) and Tlk1 for TOR-like kinase 1 (CNAG_05220). Both Tor1 and Tlk1 have an FRB domain (Figure 1B). The FRB domain of TOR kinases contains an evolutionarily conserved serine residue, which plays a crucial role in binding the rapamycin-FKBP12 complex (Weisman and Choder 2001). In S. cerevisiae, mutation of this residue (S1972) leads to rapamycin-resistance (Weisman and Choder 2001). In C. neoformans, the serine residue is conserved only in the FRB domain of Tor1, but not in Tlk1 (Figure 1C). This indicates that Tlk1 is not likely to be a rapamycin-target, as strongly supported also by phylogenetic analysis that places Tlk1 of C. neoformans in a separate branch compared to the Tor1 proteins (Figure 1A).
To characterize the function of Tor1 in C. neoformans, we attempted to delete the TOR1 gene by homologous recombination in the C. neoformans H99 strain background. Repeated attempts to delete TOR1 were not successful (data not shown) as has been reported by others (J. Lee et al. 2012). In contrast, we successfully constructed two independent tlk1∆ mutants previously (Lee et al. 2016). These data indicate that Tor1, but not Tlk1, could be essential in C. neoformans. To verify the essentiality of Tor1 in C. neoformans, we replaced the native TOR1 and TLK1 promoters with the copper-regulated CTR4 promoter (Figure S1). Two independently constructed PCTR4:TOR1 strains exhibited highly reduced growth under CTR4-repressing conditions (25 μM CuSO4), but grew as well as the WT strain when CTR4 was induced (200 μM BCS) (Figure 1D). In contrast, the PCTR4:TLK1 strains did not show any growth defect under either CTR4 repressing or inducing conditions (Figure 1D).
The essentiality of Tor1 was further confirmed by constructing the heterozygous TOR1/tor1Δ mutant in a diploid C. neoformans strain background (Al187) using the NAT selectable marker (Figure 1E). NATR transformants were screened by PCR for correct homologous recombination, and one positive strain that was confirmed to bear a NAT insertion in a single TOR1 locus by Southern blot (data not shown) was chosen as the heterozygous TOR1/tor1Δ strain. The heterozygous TOR1/tor1Δ strain was streaked onto MS medium to induce sporulation and basidiospores production. Of 150 haploid basidiospores dissected, only 14 (∼9%) germinated, and hence could be used for genetic analysis. Since TOR1 was suspected to be an essential gene, no NATR progeny were expected from the heterozygous TOR1/tor1Δ strain. However, while 12 spores were NATS and showed independent segregation of the other gene markers, two (spores #7 and #8) were able to grow on medium containing nourseothricin. Both spores showed prototrophy for adenine and uracil, and progeny #7 successful crossed with MATα whereas progeny #8 produced filamentous structures when crossed with either MATa or MATα (Figure 1F). Since there is evidence that aneuploidy can cause irregular segregation during meiosis and altered phenotypes (Ni et al. 2013), spores #7 and #8 were subjected to molecular characterization of their genetic markers. The diploid AI187 and the heterozygous TOR1/tor1Δ strains were used as controls. PCR with primers for the TOR1 gene (screening primers, ALID2265–ALID2266) yielded two amplicons of ∼10 kb (TOR1) and ∼5 kb (tor1Δ) for both progenies #7 and #8, which are identical to those obtained from the heterozygote TOR1/tor1Δ (Figure 1G), indicating that both spores are likely diploid or aneuploid for chromosome 7, in which the TOR1 gene is located. Progeny #7 has the same URA5 RFLP profile as the diploid AI187 and TOR1/tor1Δ, indicating the presence of both ura5 and URA5 on chromosome 8, but has only one mating type locus (MATa) and one WT ADE2 allele (Figure 1G). Therefore, progeny #7 is likely to be aneuploid. In contrast, progeny #8 showed the same molecular profile of heterozygous TOR1/tor1Δ (Figure 1G), suggesting that this strain is either diploid or aneuploid. Collectively, these results confirmed that TOR1 is an essential gene in C. neoformans.
Tor1, but not Tlk1, is the target of rapamycin in C. neoformans
We next addressed whether Tor1 and/or Tlk1 are the target of rapamycin. We first examined the susceptibility of the PCTR4:TOR1 strain to rapamycin under TOR1-repression (CuSO4) and induction (BCS) conditions. The PCTR4:TOR1 strain showed enhanced rapamycin susceptibility when TOR1 expression was repressed compared with the WT strain, whereas the PCTR4:TOR1 strain exhibited enhanced rapamycin resistance when TOR1 expression was induced (Figure 2A). Furthermore, the heterozygous TOR1/tor1Δ strain was more susceptible to rapamycin than the TOR1/TOR1 diploid strain, and was as susceptible to rapamycin as the haploid H99 strain, indicating that haploinsufficiency contributed to rapamycin susceptibility (Figure 2B). We also constructed constitutive TOR1 and TLK1 overexpression strains by replacing each promoter with the histone 3 (H3) promoter (TOR1oe and TLK1oe) (Figure 2C and Figure S1). Through qRT-PCR, we quantified the expression levels of TOR1 in WT, TOR1oe, and rapamycin-treated (3 ng/ml) WT cells. TOR1 was overexpressed by ∼50-fold in the TOR1oe strain compared with WT cells (Figure 2C). Independently constructed TOR1oe strains all exhibited greater rapamycin-resistance than the WT (Figure 2D). In contrast, the tlk1Δ mutant and TLK1oe strains were as susceptible to rapamycin as the WT (Figure 2D). Collectively, these results confirmed that Tor1, but not Tlk1, is the target of rapamycin in C. neoformans.
Figure 2.
Tor1 is the target of rapamycin in C. neoformans. (A) WT (H99) and PCTR4:TOR1 (YSB3176) strains were cultured in liquid YPD medium overnight at 30°, 10-fold serially diluted, and spotted on YNB agar medium containing either 25 μM CuSO4 or 200 μM BCS with or without 7 ng/ml rapamycin. This spot assay was repeated more than three times and one representative image was shown here. (B and D) The following strains were used: TOR1 (haploid WT H99), TOR1/TOR1 (diploid AI187), and TOR1/tor1Δ (YSB3450) for (B); WT (H99), TOR1oe (YSB3144), TLK1oe (YSB3118), and tlk1Δ (YSB3153) for (D). Each strain was cultured in liquid YPD medium overnight at 30°, 10-fold serially diluted, and spotted on YPD medium containing 10 ng/ml rapamycin. The plates were further incubated at 30° and photographed daily for 5 days. This spot assay was repeated more than three times and one representative image is shown here. (C) Quantitative RT-PCR analysis of TOR1 expression in WT, TOR1oe (YSB3144), and rapamycin-treated (3 ng/ml) WT cells. Three independent biological experiments with three technical replicates were performed. Error bars indicate SEM. Statistical significance of the differences was determined by one-way ANOVA with Bonferroni’s multiple-comparison test (***P < 0.0001).
Tor1 negatively regulates the thermotolerance of C. neoformans
To identify the functions of Tor1, besides its essential role in growth, we analyzed different phenotypic traits of TOR1oe strains under diverse in vitro conditions (e.g., such as growth at different temperatures, mating efficiency, chemical and antifungal drug stress susceptibility, virulence factor production). One striking phenotype was the thermotolerance properties of the TOR1oe strain. The TOR1oe strain showed reduced growth at 37° and 39°, but not at 30° (Figure 3A). Rapamycin treatment abolished the increased thermosensitivity of the TOR1oe strain (Figure 3A), suggesting that Tor1 kinase activity is responsible for this phenotype. These results suggest that Tor1 negatively regulates the thermotolerance, which is one of key virulence factors for C. neoformans.
Figure 3.
Tor1 negatively regulates the thermotolerance of C. neoformans. (A) To test thermotolerance, cells were cultured at 30° for 16 hr. Each culture was serially diluted and spotted onto YPD medium with or without 10 ng/ml rapamycin. Strains were then incubated at 30, 37, and 39°. The plates were photographed daily for 4 days. This spot assay was repeated more than three times, and one representative image is shown here. (B) Analysis of HSF1, HSP90, HSP104, and ERG11 expression levels under high temperature conditions in WT (H99), TOR1oe (YSB3144), and rapamycin-treated (3 ng/ml) WT strains. The cDNA was synthesized from the total RNA extracted from cells exposed to temperature upshift (from 30 to 37° or 39°) for 30 min. The specific primers for the amplification of HSF1, HSP90, HSP104, ERG11, and ACT1 are described in Table S2. Three independent biological experiments with three technical replicates were performed. Error bars indicate SEM. Statistical significance of the differences was determined by one-way ANOVA with Bonferroni’s multiple-comparison test (* P < 0.05, ** P < 0.001, *** P < 0.0001; ns: not significant). (C) WT cells (H99) and TOR1oe strains were grown to midlogarithmic phase and exposed to 10 ng/ml rapamycin for the indicated time. Mpk1 phosphorylation levels were monitored using anti-P-p44/p42 antibody. The blot was stripped and used for detection of Hog1 with a polyclonal anti-Hog1 antibody as a loading control. This western blot analysis was repeated twice, and one representative result is shown here. (D) The proposed Tor1 regulatory mechanism for thermotolerance of C. neoformans. Tor1 negatively regulates thermotolerance in an Hsf1-independent and Sch9 and Mpk1-dependent manner.
We next addressed how Tor1 regulates thermotolerance in C. neoformans. In S. cerevisiae, Sch9 is a well-known direct phosphorylation target of Tor1 (Swinnen et al. 2014). Notably, SCH9 deletion markedly increases thermotolerance of C. neoformans (Wang et al. 2004; Yang et al. 2017), indicating that Sch9 negatively regulates thermotolerance in the pathogen. Therefore, we hypothesized that TOR1 overexpression might hyperactivate Sch9, which in turn reduce C. neoformans thermotolerance. However, while SCH9 deletion improved the thermotolerance of the WT strain at 39°, the TOR1oe sch9Δ strain did not show any markedly enhanced thermotolerance at 39° like the sch9Δ strain although it was marginally more thermotolerant than the TOR1oe strain (Figure 3A). These results indicate that Tor1 may have additional downstream targets, other than Sch9, for controlling thermotolerance.
Recently, it has been reported that the essential heat shock factor 1 (Hsf1) transcription factor is involved in C. neoformans thermotolerance (Yang et al. 2017). Although HSF1 expression is downregulated during temperature upshift, HSF1 overexpression (HSF1oe) marginally enhances C. neoformans thermotolerance (Yang et al. 2017). Here, we observed that TOR1 overexpression indeed reduced the thermotolerance of the HSF1oe strain and that the TOR1oe HSF1oe strain was as thermosensitive as the TOR1oe strain, implying that Tor1 may negatively act upstream of Hsf1, or negatively regulate the thermotolerance of C. neoformans in an Hsf1-independent manner. Supporting the latter hypothesis, we found that TOR1 overexpression did not affect downregulation of HSF1 expression upon temperature upshift, or affect induction or repression of several Hsf1-dependent temperature-regulated genes, including HSP90, HSP104, and ERG11 (Figure 3B). Tor1 inhibition by rapamycin treatment also did not significantly affect the expression patterns of HSF1, HSP90, HSP104, and ERG11. Therefore, Tor1 may negatively regulate the thermotolerance of C. neoformans in an Hsf1-independent manner.
In C. neoformans, the Mpk1 MAPK pathway is also required for maintaining cell wall integrity and thermotolerance (Kraus et al. 2003). Therefore, we monitored whether TOR1 overexpression affects Mpk1 activation at high temperatures. As shown in Figure 3C, rapamycin treatment rapidly induced Mpk1 phosphorylation followed by a reduction over time (Figure 3C). In contrast, we found that even basal Mpk1 phosphorylation was highly decreased in TOR1oe strains (Figure 3C). These results indicate that Tor1 kinase negatively regulates C. neoformans thermotolerance through the Mpk1 MAPK pathway. Collectively, Tor1 regulates thermotolerance of C. neoformans through Sch9-and Mpk1-dependent pathways (Figure 3D).
Tor1 negatively regulates the DNA damage response in C. neoformans
Another notable phenotype observed in the TOR1oe strain was an increased susceptibility to DNA damaging agents, such as hydroxyurea (HU), methyl methanesulfonate (MMS), and bleomycin (Bleo) (Figure 4A). Suppressing Tor1 activity by rapamycin treatment completely abolished increased genotoxic stress susceptibility in the TOR1oe strain (Figure 4A), indicating that the TOR pathway is indeed involved in the DNA damage response and adaptation, which is another virulence attribute of C. neoformans (Jung et al. 2019).
Figure 4.
Tor1 negatively regulates the C. neoformans DNA damage response. (A) WT (H99), TOR1oe (YSB3144), and rad53Δ (YSB3785) strains were cultured in liquid YPD medium overnight at 30°, 10-fold serially diluted, and spotted on YPD medium containing 0.01% MMS or 90 mM HU, or 3 μg/ml bleomycin with and without 5 ng/ml rapamycin. This spot assay was repeated more than three times, and one representative image is shown here. (B) Expression levels of putative Rad53-dependent genes were verified by qRT-PCR analysis using cDNA of the WT, TOR1oe, and rapamycin (3 ng/ml)-treated WT strains with and without MMS treatment. Three independent biological experiments with three technical replicates were performed. Error bars indicate SEM. Statistical significance of the differences were determined by one-way ANOVA with Bonferroni’s multiple-comparison test (* P < 0.05, ** P < 0.001, *** P < 0.0001). (C) Phosphorylation of Rad53 was monitored using western blot with anti-FLAG antibody. The strains were grown to midlogarithmic phase, and then treated with MMS (0.02%) or rapamycin for 90 min. The cell extracts were incubated at 30° for 1 hr with and without λ-phosphatase and phosphatase inhibitor. This experiment was repeated twice, and one representative result is shown here.
We next addressed how Tor1 regulates the DNA damage response and adaptation. Through a recent transcriptome and genetic analyses of radiation resistance regulatory networks in C. neoformans (Jung et al. 2016), a number of DNA damage response genes have been uncovered, including RAD51, RAD54, RDH54, RIG1, RIG2 and BDR1. All these genes are highly induced by genotoxic agents, and their deletion significantly reduces genotoxic stress resistance in C. neoformans (Jung et al. 2016). In agreement with these published results, we confirmed that expression of these genes was strongly induced by MMS in the WT strain (Figure 4B). We observed that TOR1 overexpression significantly reduced induction of RAD51, RAD54, RDH54, RIG1, RIG2, and BDR1 in response to MMS. Notably, TOR1 overexpression markedly blocked the increase in transcript levels of BDR1, which encodes a unique bZIP transcription factor that is one of master regulators controlling expression of DNA repair genes in C. neoformans (Figure 4B). In agreement with these data, rapamycin treatment increased basal expression levels of some DNA repair genes, such as RAD54 (P = 0.0434), BDR1 (P = 0.0353), RIG1 (P = 0.0310), and RIG2 (P = 0.0109). In response to MMS, rapamycin treatment also reduced the induction of several DNA repair genes, such as RAD51, RAD54, RIG1, and RIG2 (Figure 4B), suggesting that Tor1 inhibition can also affect the normal DNA repair response and adaptation.
It has been recently demonstrated that Rad53 is the central protein kinase in the regulation of genes involved in DNA damage repair in C. neoformans (Jung et al. 2019). Therefore, we questioned whether TOR1 overexpression might influence Rad53 activity. As previously reported (Jung et al. 2019), Rad53 was quickly phosphorylated in response to MMS in the WT strain (Figure 4C; left panel). However, MMS-mediated Rad53 phosphorylation did not occur in the TOR1oe strain (Figure 4C; middle panel). Supporting this, we found that rapamycin treatment itself increased Rad53 phosphorylation even in the absence of MMS treatment (Figure 4C; right panel). Collectively, these data suggest that the TOR pathway negatively regulates the Rad53-mediated DNA damage response pathway in C. neoformans.
Cellular localization of Tor1 in C. neoformans
Cellular localization of Tor1 in S. cerevisiae has been previously investigated. Li et al. (2006) used an antibody against the unique amino terminus of Tor1 in fixed cells to show that in nutrient-rich conditions Tor1 localizes to both the cytoplasm and nucleus, albeit with a higher concentration in the nucleus. In response to nutrient starvation or rapamycin treatment, Tor1 nuclear staining decreases. In contrast, Sturgill et al. (2008) demonstrated that Tor1 localizes to the vacuolar membrane by using live yeast cells with a functional Tor1-3xGFP allele, in which three copies of green fluorescent protein (GFP) are internally integrated into the variable region of Tor1. Therefore, to address the cellular localization of Tor1 in C. neoformans, we employed the latter approach. Because Tor1 tagged with GFP at either the N- or C-terminus was not functional (data not shown), which was also the case in S. cerevisiae Tor1 (Sturgill et al. 2008), we constructed an internal 3xGFP tagged Tor1 strain in the diploid AI187 strain background using the Cre-LoxP system (Baker and Lodge 2012) as modified in this study (Figure 5A). The Tor1-3xGFP allele was considered to be functional because the TOR1/TOR1-3xGFP strain was as resistant to rapamycin as the WT TOR1/TOR1 diploid strain, whereas the TOR1/tor1-3xGFP strain (before activation of the Cre recombinase) was more susceptible to rapamycin than the WT strain (Figure 5B).
Figure 5.
Tor1 localizes to the vacuolar membrane in C. neoformans. (A) Schematic diagram of construction of the Tor1-3xGFP strain. (B) Strains [WT H99 strain (haploid), TOR1oe (YSB3144), AI187 (diploid strain), TOR1/TOR1-loxP-NEOr-loxP-3xGFP (YSB4869), and TOR1/TOR1-3xGFP (YSB4870)] were spotted on YPD medium containing rapamycin. The plates were then incubated at 30° and photographed daily for 5 days. This spot assay was repeated more than three times, and one representative image was shown here. (C and D) Tor1-3xGFP cellular localization was monitored by fluorescence microscopy. (C) To activate the Cre-Lox system, the TOR1/tor1-3xGFP strain (YSB4869) was incubated in YPG (galactose) medium for 3 hr. (D) The Tor1-3xGFP strain was grown to mid-log phase and treated with MMS or further incubated at 39° for the indicated times. FM4-64 and Hoechst staining were used to stain the vacuole membrane and nucleus, respectively. Bar, 10 μm.
Using the TOR1/TOR1-3xGFP strain, cellular localization of Tor1 was monitored. Tor1-3xGFP was enriched in the vacuolar membrane in C. neoformans (Figure 5C), which is in agreement with the behavior of Tor1-3xGFP in S. cerevisiae (Sturgill et al. 2008). We next addressed whether the localization of Tor1-3xGFP was altered in response to high temperature and DNA damage, because the Tor1 pathway was found to be involved in adaptation to these stresses (Figure 5D). Tor1 was localized predominantly in the vacuolar membrane, regardless of the presence of MMS or heat shock (Figure 5D). In summary, Tor1 localizes to the vacuolar membrane in C. neoformans, and its cellular localization does not change in response to stresses such as DNA damage or heat shock.
Identification of Tor1 downstream targets in C. neoformans
To identify additional direct and indirect targets of Tor1 in C. neoformans, we compared the phosphoproteome and transcriptome profiles of the WT strain to those of rapamycin-treated WT and TOR1oe strains. A total 44 nonredundant phosphorylated proteins were reproducibly identified as described in Materials and Methods (Table S3). Of these, 12 phosphorylated peptides, which exhibited more than a 1.5-fold change in a given comparison (TOR1oe/WT and rapamycin-treated WT/WT or TOR1oe). Among these, relative phosphopeptide abundance for CNAG_00091 and CNAG_06576 was significantly increased by rapamycin treatment (P < 0.05 by two-way ANOVA), indicating that phosphorylation of these two uncharacterized proteins may be negatively regulated by Tor1. Furthermore, 32 phosphorylated peptides were identified exclusively in WT, TOR1oe or rapamycin-treated WT sample. Notably, Sch9 and Slm1, which are known downstream targets of Tor1 in S. cerevisiae, were identified in our phosphoproteomic analysis. Phosphopeptide abundance for Sch9 and Slm1 increased and decreased, respectively, upon rapamycin treatment (Table S3). In addition to these known targets, a number of as yet unknown direct and indirect Tor1 targets have also been identified (Table S3).
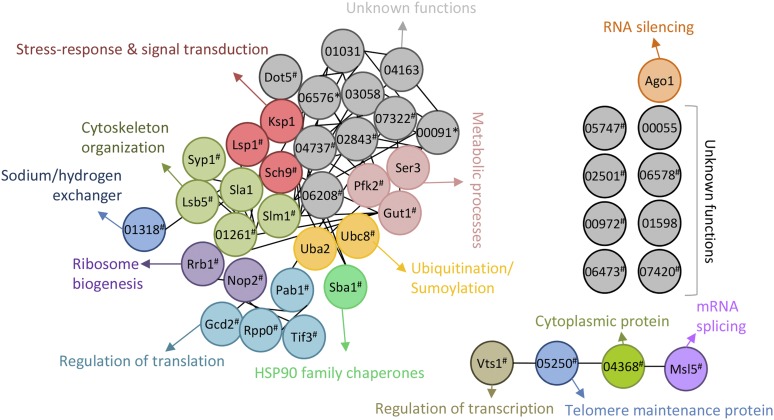
Using these potential Tor1 targets, we constructed a correlation network using CryptoNet (http://www.inetbio.org/cryptonet), which we previously published (Kim et al. 2015), to gain further insight into Tor1-dependent functional networks (Figure 6). CryptoNet is a genome-scale cofunctional network for C. neoformans and covers ∼81% of the coding genome, providing an efficient link between functional genomics data and putative phenotypic traits for given set of genes (Kim et al. 2015). This in silico analysis revealed that Tor1 plays diverse roles in a plethora of biological and physiological processes, including metabolic processes, cytoskeleton organization, ribosome biogenesis, telomere maintenance, regulation of transcription and translation, ubiquitination/sumoylation, DNA damage response, protein folding, tRNA modification, mRNA splicing, ribosome biogenesis, stress response, and signal transduction (Figure 6).
Figure 6.
Functional network relationships of the Tor1 phospho-target proteins using CryptoNet. The phosphopeptides in WT, TOR1oe, and/or rapamycin-treated WT strains reproducibly identified 44 proteins listed in Table S3. We used the search option “infer functions from network neighbors” from the CryptoNet web server (http://www.inetbio.org/cryptonet) to get a functional insight for uncharacterized targets of Tor1. These uncharacterized genes were connected by similar functions to form network. *, statistically significant difference (P < 0.05 by two-way ANOVA). #, exclusively identified in either WT, TOR1oe, or rapamycin-treated WT sample.
To further elucidate the Tor1 signaling pathway in C. neoformans, we compared the transcriptome profile of the WT strain with that of rapamycin-treated WT and TOR1oe strains using RNAseq analysis (Figure 7A). Upon rapamycin treatment, expression levels of 793 genes either increased (433 genes) or decreased (360 genes) in a statistically significant manner (P < 0.05, |Fold Change| ≧ 2) (Figure 7, B and C and Table S4). Based on gene functional annotation using DAVID (http://david.abcc.ncifcrf.gov/), the function of rapamycin-regulated genes include transmembrane transport, pseudouridine synthesis, metabolic process, rRNA processing, and ribosome biogenesis (Figure 7D), which is in agreement with the phosphoproteomics data. On the other hand, only 20 genes exhibited more than a twofold change in expression in the TOR1oe strain (Figure 7B), suggesting that simple overexpression may not be sufficient to fully maximize the level of Tor1 kinase activity.
Figure 7.
Transcriptome profiles of WT, TOR1oe, and rapamycin-treated WT strains. (A) Gene clustering of WT, TOR1oe, and rapamycin-treated WT (WT+Rapa). Yellow in the heat map denotes upregulation while blue denotes downregulation. (B) The number of genes whose expression was significantly upregulated or downregulated by more than twofold change (FC) in each comparison (WT vs. TOR1oe and WT vs. WT+Rapa) was indicated. (C) The scatter plots show the expression levels of each gene between the corresponding conditions. (D) Functional categories of genes regulated by rapamycin using the DAVID tool (http://david.abcc.ncifcrf.gov/). False discovery rate (FDR) was used to determine statistical significance (P value <0.05).
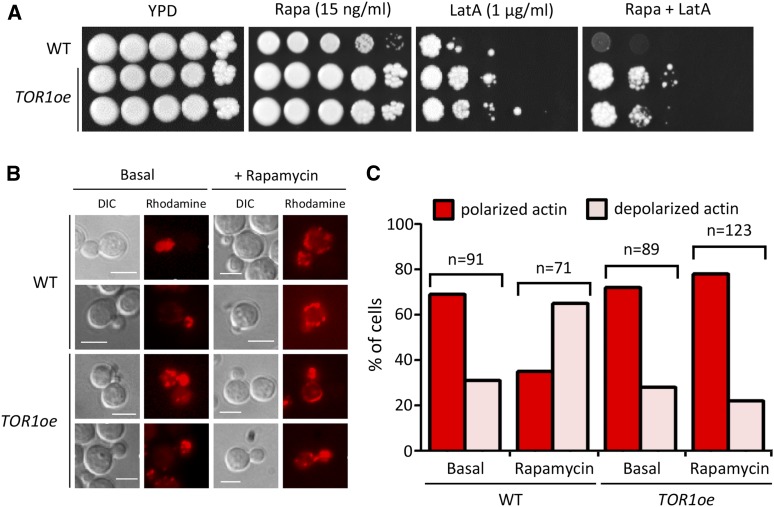
Interestingly, phosphoproteomics analysis predicted that the C. neoformans Tor1 kinase could be involved in cytoskeleton organization. In addition, transcriptomic analysis showed that the cytoskeleton organization (SLM1, LSB5, and CNAG_01261) as well as actin dynamics related genes (CNAG_04222, CNAG_01375, CNAG_01341, CNAG_04777, CNAG_03897, and CNAG_06399) were differentially expressed between WT and rapamycin-treated WT in a statistically significant manner (Table S4, P < 0.05). Supporting this, the TOR1oe strain was more resistant to latrunculin A, an inhibitor of actin polymerization, than the WT strain (Figure 8A). Furthermore, we sought to determine whether inhibition of Tor1 by rapamycin affects the organization of the actin cytoskeleton. About 70% of WT C. neoformans cells showed polarized actin distribution in small or midsize budded cells under basal conditions (Figure 8, B and C). In response to rapamycin treatment, the percentage of WT C. neoformans showing polarized actin distribution decreased (∼30%; Figure 8, B and C), which is similar to the case in S. cerevisiae (Aronova et al. 2007). In contrast, >70% of the TOR1oe cells maintained polarized actin distribution in budded cells even after rapamycin treatment (Figure 8, B and C). Therefore, we hypothesized that C. neoformans Tor1 is involved in cytoskeleton organization—a typical function of S. cerevisiae TORC2—as well as in the functions of S. cerevisiae TORC1, such as metabolic processes, ribosome biogenesis, stress response, and signal transduction.
Figure 8.
Tor1 is involved in C. neoformans actin cytoskeleton organization. (A) Strains [WT H99 strain, TOR1oe (YSB3144 and YSB3147)] were spotted on YPD medium containing rapamycin (15 ng/ml) and/or LatA (Latrunculin A; actin cytoskeleton inhibitor, 1 μg/ml). The plates were incubated at 30° and photographed daily for 7 days. This spot assay was repeated more than three times, and one representative image is shown here. (B) Distribution of polymerized actin upon rapamycin treatment in WT and TOR1oe WT and TOR1oe strains were grown to midlogarithmic phase at 30°. The cells were exposed to 10 ng/ml rapamycin for 1 hr. Then, cells were fixed, stained with rhodamine phalloidin, and visualized by fluorescence microscopy. Bar, 10 μm. (C) Rapamycin treatment significantly perturb the actin cytoskeleton. The graph shows the actin distribution in rapamycin treatment in WT and TOR1oe strains. (n; counted number of cells)
Signaling components and pathways that potentially cross-talk with the TOR pathway
To identify signaling components and pathways that potentially cross-talk with, and are functionally related to, the Tor1 pathway, we examined rapamycin-susceptibility in previously constructed transcription factor and kinase mutant libraries (Jung et al. 2015; Lee et al. 2016) (Figure 9). In the transcription factor mutant library, two mutants (crz1Δ and skn7Δ) were more susceptible to rapamycin than the WT strain, while five mutants (hlh5Δ, bzp1Δ, hob1Δ, ada2Δ, and atf1Δ) were more resistant (Figure 9A and Figure S2). In the kinase mutant library, 31 kinase mutants showed altered rapamycin-susceptibility. Deletion of BUD32, SCH9, RIM15, SSN3, IPK1, TCO1, CKA1, KSP1, FBP26, YPK1, CHK1, VPS15, MPK1, MKK2, BCK1, TCO6, CDC7, and CDC2801 increased susceptibility to rapamycin, whereas deletion of PKA1, GSK3, PIK1, CRK1, PAN3, IRK5, MAK3202, IGI1, KIN4, SSK2, TCO2, PBS2, and HOG1 decreased rapamycin susceptibility (Figure 9A and Figure S2). Notably, the fact that the sch9Δ mutant exhibited increased rapamycin susceptibility suggested that Sch9 could be positively regulated by Tor1, regardless of the proteomics data showing that Sch9 phosphorylation was increased by 3 hr-rapamycin treatment (Table S3). Among 38 transcription factor/kinase mutants exhibiting altered rapamycin-susceptibility, a majority (29 out of 38) were previously shown to exhibit altered susceptibility to thermal or DNA damage stresses (Jung et al. 2015; Lee et al. 2016), indicating that these signaling components are highly likely to crosstalk with the TOR pathway. Collectively, the TOR pathway functionally interacts with a variety of signaling pathways in C. neoformans, supporting the proteomics and transcriptomics data shown above.
Figure 9.
The TOR pathway has a functional relationship with the signaling pathway modulating thermotolerance and DNA damage response, as well as the HOG MAPK pathway in C. neoformans. (A) Phenome heat map for transcription factor/kinase mutants exhibiting altered rapamycin-susceptibility. Red and blue circles in the heat map represent susceptibility and tolerance, respectively, against rapamycin, temperature stress, and DNA damage stress. The level of susceptibility and tolerance are distinguished by the sizes of circle and gradients of red or blue color. The heat map data for the thermal stress and DNA damage response of transcription factor/kinase mutants were retrieved from previous papers (Jung et al. 2015; Lee et al. 2016). (B) C. neoformans strains were grown overnight at 30° in liquid YPD medium. The 10-fold serially diluted cells (1–104 dilutions) were spotted on YPD plates containing rapamycin (10 ng/ml), and the plates were incubated at 30° and photographed daily for 5 days. [Strains: WT H99, tco1∆ (YSB278), tco2∆ (YSB281), ssk1∆ (YSB261), ssk2∆ (YSB264), pbs2∆ (YSB123), hog1∆ (YSB64), atf1∆ (YSB676), and tco1∆ tco2∆ (YSB324)]. This spot assay was repeated more than three times, and one representative image was shown here. (C) WT cells (H99) and TOR1oe strains were grown to midlogarithmic phase, and exposed to 10 ng/ml rapamycin for the indicated time. Hog1 phosphorylation levels were monitored using anti-P-p38 antibody. The blot was stripped and used for detection of Hog1 with a polyclonal anti-Hog1 antibody as a loading control This western blot analysis was repeated twice, and one representative result is shown here. HU, hydroxylurea; MMS, methyl methanesulphonate.
Notably, two stress-sensing MAPK pathways appeared to have opposite effects on rapamycin resistance. Mutation of BCK1, MKK2, and MPK1, which belong to the cell wall integrity MAPK pathway, markedly enhanced rapamycin susceptibility, indicating that the Mpk1 pathway may control the Tor1 pathway. This is in contrast to our previous finding that Mpk1 became more phosphorylated in response to rapamycin treatment, and that TOR1 overexpression decreased basal Mpk1 phosphorylation levels (Figure 3D). Therefore, it is likely that the Tor1 and Mpk1 MAPK pathways may reciprocally regulate each other. In contrast, mutation of SSK1, SSK2, PBS2, and HOG1, which belong to the stress-sensing HOG pathway, increased rapamycin resistance (Figure 9, A and B). Interestingly, deletion of TCO1 and TCO2, which are two major hybrid histidine kinases upstream of the HOG pathway, enhances rapamycin susceptibility and resistance, respectively, and the tco1Δ tco2Δ double mutant was as resistant to rapamycin as the tco2Δ single mutant (Figure 9B). Therefore, the role of Tco1 in rapamycin susceptibility could be mediated through Tco2. In C. neoformans, Hog1 is highly phosphorylated under basal conditions, and undergoes dephosphorylation in response to environmental stresses, which is in stark contrast to S. cerevisiae Hog1 (Bahn et al. 2006). Notably, in response to rapamycin, the level of Hog1 phosphorylation increased at initial time points (15–30 min) before decreased over time (Figure 9C). However, the overexpression of TOR1 did not markedly affect the phosphorylation level of Hog1. Collectively, all these results suggest that the TOR pathway has a functional relationship with the Mpk1 and Hog1 MAPK pathways in C. neoformans.
Discussion
The target of rapamycin (TOR) proteins are essential for signal transduction in eukaryotic cells. However, this fundamental nature has made their study difficult beyond model organisms. In this study, we used molecular and genetic analyses to characterize the TOR signaling pathway in the human pathogen C. neoformans. Although this fungus contains two proteins, Tor1 and Tlk1, which are orthologous to yeast TOR kinases, only Tor1 has characteristics as the target of rapamycin. The tlk1Δ mutant does not have any evident in vitro phenotypes, and, therefore, its physiological roles in C. neoformans remain unclear. Several lines of evidences indicated that the Tor1 kinase is involved in a plethora of biological processes, including primary metabolism, cytoskeleton organization, ribosome biogenesis, telomere maintenance, regulation of transcription and translation, stress response, and signal transduction. Most notably, Tor1 modulates growth and stress responses of C. neoformans in opposite manners.
Although we found that Tor1 is essential for the growth of C. neoformans, the Tor1 pathway negatively regulates the thermotolerance and DNA damage response in the pathogen. In C. neoformans, TOR1 overexpression increased thermosensitivity at 37° and 39°. This is in stark contrast to the previous finding that Tor1 promotes S. pombe growth at high temperature (Kawai et al. 2001). In this study, we demonstrated that the Tor1 pathway may negatively regulate the thermotolerance of C. neoformans by affecting Sch9 and Mpk1 pathways, which were previously described to be involved in the thermotolerance of the fungal pathogen (Bhabhra and Askew 2005; Jung and Bahn 2009). Sch9 is a known Tor1 phosphorylation target in S. cerevisiae (Urban et al. 2007) and negatively regulates thermotolerance in C. neoformans (Yang et al. 2017). However, the fact that the thermosensitivity of the TOR1oe strain is partly rescued by SCH9 deletion, while TOR1 overexpression markedly reduced thermotolerance of the sch9Δ mutant, indicates that Tor1 could negatively regulate thermotolerance in both a Sch9-dependent and -independent manner, and that Sch9 could be phosphorylated and regulated by a kinase(s), other than Tor1, when the Tor1 pathway was inhibited. In S. cerevisiae, the stress response protein kinase C (Pkc1) modulates the Tor1 pathway in response to heat stress, which influences longevity by controlling the diffusion barrier in the nuclear envelope (Baldi et al. 2017). Furthermore, in S. cerevisiae and C. albicans, the activity of Slt2/Mpk1 and Mkc1 (ERK orthologs) cell integrity MAPK pathways is altered in response to TORC1 inhibition (Tsao et al. 2009). In C. neoformans, Pkc1 works upstream of the cell wall integrity Mpk1 MAPK pathway and is critical for controlling thermotolerance (Gerik et al. 2008). However, it still remains unclear whether the Pkc1 pathway regulates or cross-talks with the Tor1 pathway in controlling thermotolerance; this is because TOR1 overexpression did not cause cell wall integrity defects, but did increase thermosensitivity in C. neoformans. The fact that rapamycin treatment and TOR1 overexpression induced and repressed Mpk1 phosphorylation, respectively, indicates that Tor1 negatively regulates Mpk1 activation. However, the fact that all Mpk1 pathway mutants (bck1Δ, mkk2Δ, or mpk1Δ) showed markedly increased rapamycin-susceptibility suggests that the Mpk1 pathway may control the TOR pathway. Taken together, these data strongly indicated that the Tor1 and Mpk1 pathways are interconnected and may be reciprocally regulated in C. neoformans. This regulatory mechanism needs further study.
The most obvious phenotype of the C. neoformans TOR1oe strain is a marked susceptibility to various genotoxic stresses, indicating that the Tor1 pathway negatively regulates the DNA damage response and adaptation. This is in stark contrast to the previous finding that the TOR pathway promotes the survival of S. cerevisiae upon DNA damaging stress by maintaining replication fork stability (Shen et al. 2007). Cotreatment with rapamycin, which specifically targets Tor1, but not Tor2, and MMS, dramatically reduces the survival of S. cerevisiae, suggesting that the TORC1 complex promotes the DNA damage response in the budding yeast (Shen et al. 2007). In contrast, we found that rapamycin treatment did not further enhance MMS susceptibility in WT C. neoformans. Instead, TOR1 overexpression markedly enhanced MMS susceptibility in C. neoformans. Furthermore, in S. cerevisiae, rapamycin treatment alone did not phosphorylate Rad53, which is a central regulator of DNA damage response (Shen et al. 2007). In contrast, we found that, like MMS, rapamycin treatment alone could induce Rad53 phosphorylation in C. neoformans, further supporting the hypothesis that the Tor1 pathway negatively regulates the DNA damage response in the basidiomycete pathogen. In S. cerevisiae, the rapamycin-insensitive TORC2 complex is also involved in maintaining genome stability through Ypk1/2-mediated actin cytoskeleton regulation when DNA strand breaks occur (Shimada et al. 2013). Therefore, chemical or genetic inhibition of TORC2 in the presence of zeocin, a DNA-break-inducing bleomycin family antibiotic, results in yeast chromosome shattering (Shimada et al. 2013). However, we observed that TOR1 overexpression, but not Tor1 inhibition by rapamycin, dramatically increased sensitivity to bleomycin in C. neoformans. Assuming that, like mTOR, Tor1 may take part in both the TORC1 and TORC2 complexes in C. neoformans, the TOR pathway regulates the DNA damage response pathway of the pathogen in a negative manner. Supporting this, expression of RAD51, RAD54, BDR1, RDH54, RIG1, and RIG2, which are DNA repair genes, were highly induced after MMS treatment in the WT strain, but significantly less induced in the TOR1oe strain. Recently, we reported that the Rad53-and Chk1-dependent DNA damage response pathways are required for full virulence of C. neoformans (Jung et al. 2019). Therefore, Tor1 is not only essential for the growth of C. neoformans, but also plays a pivotal role in the virulence of the pathogen through regulation of thermotolerance and DNA response pathways.
Phosphoproteomics and transcriptomic analyses of rapamycin-treated cryptococcal cells and TOR1oe strains and large-scale screening mutant libraries of genes encoding transcription factors and kinases for rapamycin susceptibility revealed that a number of other signaling pathways could also be functionally associated with the Tor1 pathway. In particular, we found that there is crosstalk between the Tor1 and Hog1 MAPK pathways in C. neoformans. Deletion of the HOG pathway genes, except for TCO1, increased rapamycin resistance and rapamycin rapidly induced phosphorylation of Hog1, which was then eventually dephosphorylated. In mammalian cells, the p38 MAPK isoforms, which are orthologous to yeast Hog1 MAPKs, also affect mTORC1 activation (Durán and Hall 2012). Similarly, rapamycin lowers the phosphorylation level of C. albicans Hog1 through the two Hog1 tyrosine phosphatases Ptp2 and Ptp3. In hyphal development, reduced Tor1 signaling downregulates Hog1 basal activity (Su et al. 2013). Therefore, Hog1 functions downstream of Tor1 in C. albicans (Su et al. 2013). However, it remains unclear how Hog1 phosphorylation was regulated in C. neoformans. In our previous study, we reported that the stress-dependent induction of PTP1 and PTP2 requires Hog1 kinase activity (Lee et al. 2014). Ptp2 was especially involved in mediating vegetative growth, sexual differentiation, antifungal drug resistance, stress response, and virulence factor regulation through the negative feedback loop of the Hog1 MAPK pathway and suppressed the hyper-phosphorylation of Hog1 (Lee et al. 2014). Therefore, it remains to be determined whether the Hog1 phosphorylation is regulated in a Ptp1-and Ptp2-dependent or independent manner upon rapamycin treatment.
Interestingly, our phosphoproteomic and transcriptomics analyses predicted that C. neoformans Tor1 was involved in cytoskeleton organization. Like S. cerevisiae TORC2, we observed that the actin cytoskeleton was depolarized in response to rapamycin in C. neoformans. Several previous studies have highlighted the importance of actin cytoskeleton regulation in the pathogenicity of C. neoformans. Wsp1, which is a GBD/CRIB domain-containing WASP homolog and regulates actin assembly in C. neoformans, is required for growth at 37°, mating, production of polysaccharide capsule and melanin pigment, and secretion of urease enzyme (Shen et al. 2011). Therefore, the wsp1Δ mutant shows attenuated virulence in a murine model of cryptococcosis (Shen et al. 2011). Wsp1 controls actin cytoskeleton as an effector of GTPases Cdc42 and Rac1 in C. neoformans (Shen et al. 2012). Furthermore, Cin1, which is a multi-modular protein orthologous to human endocytic protein ITSN1, interacts with Cdc42 and Wsp1 and promotes growth and virulence factor production in C. neoformans (Shen et al. 2010). All these previous and current results indicate that actin cytoskeleton organization is closely related to the pathogenicity of Cryptococcus, and further studies will be conducted to determine how the Tor1 kinase regulates actin dynamics in association with the Cin1-Wsp1-Cdc42/Rac1 pathway in the pathogen.
In conclusion, our study demonstrates that the TOR signaling pathway can regulate a number of biological and physiological processes as a central regulator in C. neoformans. Although its pleiotropic roles appear to be evolutionarily conserved among fungi, the regulatory mechanism of the TOR pathway and its connection to other signaling pathways are highly divergent in different fungal species.
Acknowledgments
This work was supported by the National Research Foundation (NRF) grants funded by the Korea government (MSIT) (2016R1E1A1A01943365 and 2018R1A5A1025077 to Y.-S.B.), and in part by the Strategic Initiative for Microbiomes in Agriculture and Food funded by Ministry of Agriculture, Food and Rural Affairs (918012-4) (to Y.-S.B.). This work was also supported in part by the United States National Institutes of Health (AI094364) and Australian Research Council (FT130100146) (to A.I.).
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8208569.
Communicating editor: A. Mitchell
Literature Cited
- Aronova S., Wedaman K., Anderson S., Yates J., III, Powers T., 2007. Probing the membrane environment of the TOR kinases reveals functional interactions between TORC1, actin, and membrane trafficking in Saccharomyces cerevisiae. Mol. Biol. Cell 18: 2779–2794. 10.1091/mbc.e07-03-0274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn Y. S., Kojima K., Cox G. M., Heitman J., 2006. A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol. Biol. Cell 17: 3122–3135. 10.1091/mbc.e06-02-0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L. G., Lodge J. K., 2012. Multiple gene deletion in Cryptococcus neoformans using the Cre-lox system. Methods Mol. Biol. 845: 85–98. 10.1007/978-1-61779-539-8_6 [DOI] [PubMed] [Google Scholar]
- Baldi S., Bolognesi A., Meinema A. C., Barral Y., 2017. Heat stress promotes longevity in budding yeast by relaxing the confinement of age-promoting factors in the mother cell. eLife 6: e28329 10.7554/eLife.28329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhabhra R., Askew D. S., 2005. Thermotolerance and virulence of Aspergillus fumigatus: role of the fungal nucleolus. Med. Mycol. 43: S87–S93. 10.1080/13693780400029486 [DOI] [PubMed] [Google Scholar]
- Colot H. V., Park G., Turner G. E., Ringelberg C., Crew C. M., et al. , 2006. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc. Natl. Acad. Sci. USA 103: 10352–10357 (erratum: Proc. Natl. Acad. Sci. USA 103: 16614). 10.1073/pnas.0601456103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz M. C., Cavallo L. M., Gorlach J. M., Cox G., Perfect J. R., et al. , 1999. Rapamycin antifungal action is mediated via conserved complexes with FKBP12 and TOR kinase homologs in Cryptococcus neoformans. Mol. Cell. Biol. 19: 4101–4112. 10.1128/MCB.19.6.4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler N. S., Pan X., Heitman J., Cardenas M. E., 2001. The TOR signal transduction cascade controls cellular differentiation in response to nutrients. Mol. Biol. Cell 12: 4103–4113. 10.1091/mbc.12.12.4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R. C., Blankenship J. R., Kraus P. R., de Jesus Berrios M., Hull C. M., et al. , 2002. A PCR-based strategy to generate integrative targeting alleles with large regions of homology. Microbiology 148: 2607–2615. 10.1099/00221287-148-8-2607 [DOI] [PubMed] [Google Scholar]
- Durán R. V., Hall M. N., 2012. Regulation of TOR by small GTPases. EMBO Rep. 13: 121–128. 10.1038/embor.2011.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerik K. J., Bhimireddy S. R., Ryerse J. S., Specht C. A., Lodge J. K., 2008. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot. Cell 7: 1685–1698. 10.1128/EC.00146-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra M. F., 1997. Responses to DNA damage and regulation of cell cycle checkpoints by the ATM protein kinase family. Curr. Opin. Genet. Dev. 7: 170–175. 10.1016/S0959-437X(97)80125-6 [DOI] [PubMed] [Google Scholar]
- Hu G., McQuiston T., Bernard A., Park Y. D., Qiu J., et al. , 2015. A conserved mechanism of TOR-dependent RCK-mediated mRNA degradation regulates autophagy. Nat. Cell Biol. 17: 930–942. 10.1038/ncb3189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiri G., Idnurm A., 2015. Essential gene discovery in the basidiomycete Cryptococcus neoformans for antifungal drug target prioritization. MBio 6: e02334-14 10.1128/mBio.02334-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A., 2010. A tetrad analysis of the basidiomycete fungus Cryptococcus neoformans. Genetics 185: 153–163. 10.1534/genetics.109.113027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K. W., Bahn Y. S., 2009. The stress-activated signaling (SAS) pathways of a human fungal pathogen, Cryptococcus neoformans. Mycobiology 37: 161–170. 10.4489/MYCO.2009.37.3.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K. W., Yang D. H., Maeng S., Lee K. T., So Y. S., et al. , 2015. Systematic functional profiling of transcription factor networks in Cryptococcus neoformans. Nat. Commun. 6: 6757 10.1038/ncomms7757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K. W., Yang D. H., Kim M. K., Seo H. S., Lim S., et al. , 2016. Unraveling fungal radiation resistance regulatory networks through the genome-wide transcriptome and genetic analyses of Cryptococcus neoformans. MBio 7: e01483-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K. W., Lee Y., Huh E. Y., Lee S. C., Lim S., et al. , 2019. Rad53- and Chk1-dependent DNA damage response pathways cooperatively promote fungal pathogenesis and modulate antifungal drug susceptibility. MBio 10: e01726-18 10.1128/mBio.01726-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Nakashima A., Ueno M., Ushimaru T., Aiba K., et al. , 2001. Fission yeast tor1 functions in response to various stresses including nitrogen starvation, high osmolarity, and high temperature. Curr. Genet. 39: 166–174. 10.1007/s002940100198 [DOI] [PubMed] [Google Scholar]
- Kim H., Jung K. W., Maeng S., Chen Y. L., Shin J., et al. , 2015. Network-assisted genetic dissection of pathogenicity and drug resistance in the opportunistic human pathogenic fungus Cryptococcus neoformans. Sci. Rep. 5: 8767 10.1038/srep08767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. S., Kim S. Y., Yoon J. K., Lee Y. W., Bahn Y. S., 2009. An efficient gene-disruption method in Cryptococcus neoformans by double-joint PCR with NAT-split markers. Biochem. Biophys. Res. Commun. 390: 983–988. 10.1016/j.bbrc.2009.10.089 [DOI] [PubMed] [Google Scholar]
- Ko Y. J., Yu Y. M., Kim G. B., Lee G. W., Maeng P. J., et al. , 2009. Remodeling of global transcription patterns of Cryptococcus neoformans genes mediated by the stress-activated HOG signaling pathways. Eukaryot. Cell 8: 1197–1217. 10.1128/EC.00120-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus P. R., Fox D. S., Cox G. M., Heitman J., 2003. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Mol. Microbiol. 48: 1377–1387. 10.1046/j.1365-2958.2003.03508.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Trapnell C., Pop M., Salzberg S. L., 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10: R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Khanal Lamichhane A., Garraffo H. M., Kwon-Chung K. J., Chang Y. C., 2012. Involvement of PDK1, PKC and TOR signalling pathways in basal fluconazole tolerance in Cryptococcus neoformans. Mol. Microbiol. 84: 130–146. 10.1111/j.1365-2958.2012.08016.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Moir R. D., McIntosh K. B., Willis I. M., 2012. TOR signaling regulates ribosome and tRNA synthesis via LAMMER/Clk and GSK-3 family kinases. Mol. Cell 45: 836–843. 10.1016/j.molcel.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. T., Byun H. J., Jung K. W., Hong J., Cheong E., et al. , 2014. Distinct and redundant roles of protein tyrosine phosphatases Ptp1 and Ptp2 in governing the differentiation and pathogenicity of Cryptococcus neoformans. Eukaryot. Cell 13: 796–812. 10.1128/EC.00069-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. T., So Y. S., Yang D. H., Jung K. W., Choi J., et al. , 2016. Systematic functional analysis of kinases in the fungal pathogen Cryptococcus neoformans. Nat. Commun. 7: 12766 10.1038/ncomms12766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Tsang C. K., Watkins M., Bertram P. G., Zheng X. F., 2006. Nutrient regulates Tor1 nuclear localization and association with rDNA promoter. Nature 442: 1058–1061. 10.1038/nature05020 [DOI] [PubMed] [Google Scholar]
- Ni M., Feretzaki M., Li W., Floyd-Averette A., Mieczkowski P., et al. , 2013. Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans. PLoS Biol. 11: e1001653 10.1371/journal.pbio.1001653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. D., Lodge J. K., Baker L. G., 2010. Going green in Cryptococcus neoformans: the recycling of a selectable drug marker. Fungal Genet. Biol. 47: 191–198. 10.1016/j.fgb.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., et al. , 2019. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res. 47: D442–D450. 10.1093/nar/gky1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B., Gingras A. C., Sonenberg N., 2001. The target of rapamycin (TOR) proteins. Proc. Natl. Acad. Sci. USA 98: 7037–7044. 10.1073/pnas.121145898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke A., Anderson S., McCaffery J. M., Yates J., III, Aronova S., et al. , 2004. TOR complex 1 includes a novel component, Tco89p (YPL180w), and cooperates with Ssd1p to maintain cellular integrity in Saccharomyces cerevisiae. J. Biol. Chem. 279: 14752–14762. 10.1074/jbc.M313062200 [DOI] [PubMed] [Google Scholar]
- Rohde J., Heitman J., Cardenas M. E., 2001. The TOR kinases link nutrient sensing to cell growth. J. Biol. Chem. 276: 9583–9586. 10.1074/jbc.R000034200 [DOI] [PubMed] [Google Scholar]
- Shen C., Lancaster C. S., Shi B., Guo H., Thimmaiah P., et al. , 2007. TOR signaling is a determinant of cell survival in response to DNA damage. Mol. Cell. Biol. 27: 7007–7017. 10.1128/MCB.00290-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G., Whittington A., Song K., Wang P., 2010. Pleiotropic function of intersectin homologue Cin1 in Cryptococcus neoformans. Mol. Microbiol. 76: 662–676. 10.1111/j.1365-2958.2010.07121.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G., Whittington A., Wang P., 2011. Wsp1, a GBD/CRIB domain-containing WASP homolog, is required for growth, morphogenesis, and virulence of Cryptococcus neoformans. Eukaryot. Cell 10: 521–529. 10.1128/EC.00274-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G., Zhou E., Alspaugh J. A., Wang P., 2012. Wsp1 is downstream of Cin1 and regulates vesicle transport and actin cytoskeleton as an effector of Cdc42 and Rac1 in Cryptococcus neoformans. Eukaryot. Cell 11: 471–481. 10.1128/EC.00011-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K., Filipuzzi I., Stahl M., Helliwell S. B., Studer C., et al. , 2013. TORC2 signaling pathway guarantees genome stability in the face of DNA strand breaks. Mol. Cell 51: 829–839. 10.1016/j.molcel.2013.08.019 [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Cohen A., Diefenbacher M., Trautwein M., Martin D. E., et al. , 2008. TOR1 and TOR2 have distinct locations in live cells. Eukaryot. Cell 7: 1819–1830. 10.1128/EC.00088-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C., Lu Y., Liu H., 2013. Reduced TOR signaling sustains hyphal development in Candida albicans by lowering Hog1 basal activity. Mol. Biol. Cell 24: 385–397. 10.1091/mbc.e12-06-0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen E., Ghillebert R., Wilms T., Winderickx J., 2014. Molecular mechanisms linking the evolutionary conserved TORC1-Sch9 nutrient signalling branch to lifespan regulation in Saccharomyces cerevisiae. FEMS Yeast Res. 14: 17–32. 10.1111/1567-1364.12097 [DOI] [PubMed] [Google Scholar]
- Toffaletti D. L., Rude T. H., Johnston S. A., Durack D. T., Perfect J. R., 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175: 1405–1411. 10.1128/jb.175.5.1405-1411.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L., Salzberg S. L., 2009. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–1111. 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Williams B. A., Pertea G., Mortazavi A., Kwan G., et al. , 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28: 511–515. 10.1038/nbt.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C. C., Chen Y. T., Lan C. Y., 2009. A small G protein Rhb1 and a GTPase-activating protein Tsc2 involved in nitrogen starvation-induced morphogenesis and cell wall integrity of Candida albicans. Fungal Genet. Biol. 46: 126–136. 10.1016/j.fgb.2008.11.008 [DOI] [PubMed] [Google Scholar]
- Urban J., Soulard A., Huber A., Lippman S., Mukhopadhyay D., et al. , 2007. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol. Cell 26: 663–674. 10.1016/j.molcel.2007.04.020 [DOI] [PubMed] [Google Scholar]
- Vézina C., Kudelski A., Sehgal S. N., 1975. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiot. (Tokyo) 28: 721–726. 10.7164/antibiotics.28.721 [DOI] [PubMed] [Google Scholar]
- Wang P., Cox G. M., Heitman J., 2004. A Sch9 protein kinase homologue controlling virulence independently of the cAMP pathway in Cryptococcus neoformans. Curr. Genet. 46: 247–255. 10.1007/s00294-004-0529-1 [DOI] [PubMed] [Google Scholar]
- Wedaman K. P., Reinke A., Anderson S., Yates J., III, McCaffery J. M., et al. , 2003. Tor kinases are in distinct membrane-associated protein complexes in Saccharomyces cerevisiae. Mol. Biol. Cell 14: 1204–1220. 10.1091/mbc.e02-09-0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman R., Choder M., 2001. The fission yeast TOR homolog, tor1+, is required for the response to starvation and other stresses via a conserved serine. J. Biol. Chem. 276: 7027–7032. 10.1074/jbc.M010446200 [DOI] [PubMed] [Google Scholar]
- Yang D. H., Jung K. W., Bang S., Lee J. W., Song M. H., et al. , 2017. Rewiring of signaling networks modulating thermotolerance in the human pathogen Cryptococcus neoformans. Genetics 205: 201–219. 10.1534/genetics.116.190595 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. Supplemental Figures and Tables are available at FigShare. Figure S1 contains data for construction of promoter replacement strains. Table S1 contains information for strains used in this study. Table S2 contains information for primers used in this study. Table S3 contains detailed information regarding phosphopeptides identified in wild-type, TOR1oe, or rapamycin-treated WT of C. neoformans. Table S4 contains the RNA-seq data generated in this study. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al. 2019) partner repository with the dataset identifier PXD012983 and 10.6019/PXD012983. The RNA-seq data generated by this study are available at Gene Expression Omnibus (GEO accession no. GSE129227). Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8208569.