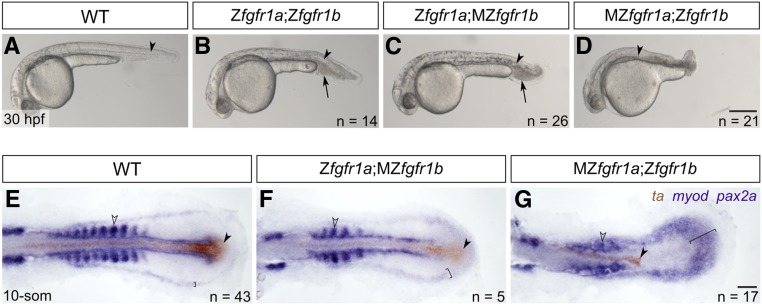
Figure 3.
fgfr1a and fgfr1b function redundantly to regulate posterior mesoderm development. (A–D) Lateral view of 30 hpf wild-type (WT; A), and Zfgfr1a;Zfgfr1b (B), Zfgfr1a;MZfgfr1b (C), and MZfgfr1a;Zfgfr1b (D) mutant embryos. Arrowheads denote the notochord; arrows mark pooled blood cells in (B and C). Anterior is to the left, dorsal is up. (E–G) Mesodermal derivative marker analysis of Zfgfr1a;MZfgfr1b and MZfgfr1a;Zfgfr1b double mutant embryos at the 10-somite stage. The notochord (labeled with ta, brown; filled arrowhead) extends down the length of the trunk and tail in wild-type (E) and Zfgfr1a;MZfgfr1b double mutant embryos (F), but is truncated in MZfgfr1a;Zfgfr1b double mutant embryos (G). Defined somites (labeled with myod, purple; open arrowhead) are present in wild-type embryos (E) and Zfgfr1a;MZfgfr1b embryos (F); however, the latter have distinctly fewer somites (5 compared to 10). Although MZfgfr1a;Zfgfr1b mutants retain some myod-positive cells, there are no definitive somites (G). Pronephric precursors (labeled with pax2a, purple; brackets) are restricted to a defined band around the trunk and tail of wild-type embryos (E), a region that is expanded in both Zfgfr1a;MZfgfr1b (F) and MZfgfr1a;Zfgfr1b double mutant embryos (G). Bars: in (D), 200 μm for (A–D); in (G), 50 μm for (E–G).