Malassezia are a monophyletic phylum of 18 species of commensal/ pathogenic yeasts that are prominent in the mammalian mycobiome, and recently linked to risk of Crohn’s Disease. Malassezia are challenging to study...
Keywords: Malassezia furfur, insertional mutagenesis, CRISPR/Cas9, protein phosphatase 2A, pleiotropic drug resistance
Abstract
Malassezia encompasses a monophyletic group of basidiomycetous yeasts naturally found on the skin of humans and other animals. Malassezia species have lost genes for lipid biosynthesis, and are therefore lipid-dependent and difficult to manipulate under laboratory conditions. In this study, we applied a recently-developed Agrobacterium tumefaciens-mediated transformation protocol to perform transfer (T)-DNA random insertional mutagenesis in Malassezia furfur. A total of 767 transformants were screened for sensitivity to 10 different stresses, and 19 mutants that exhibited a phenotype different from the wild type were further characterized. The majority of these strains had single T-DNA insertions, which were identified within open reading frames of genes, untranslated regions, and intergenic regions. Some T-DNA insertions generated chromosomal rearrangements while others could not be characterized. To validate the findings of our forward genetic screen, a novel clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system was developed to generate targeted deletion mutants for two genes identified in the screen: CDC55 and PDR10. This system is based on cotransformation of M. furfur mediated by A. tumefaciens, to deliver both a CAS9-gRNA construct that induces double-strand DNA breaks and a gene replacement allele that serves as a homology-directed repair template. Targeted deletion mutants for both CDC55 and PDR10 were readily generated with this method. This study demonstrates the feasibility and reliability of A. tumefaciens-mediated transformation to aid in the identification of gene functions in M. furfur, through both insertional mutagenesis and CRISPR/Cas9-mediated targeted gene deletion.
THE genus Malassezia is a lipophilic, monophyletic group of basidiomycetous yeasts that colonize sebaceous skin sites and represents > 90% of the skin mycobiome (Findley et al. 2013; Wu et al. 2015; Byrd et al. 2018). In addition to their ubiquitous presence on the skin of humans and animals, recent data support the hypothesis that Malassezia fungi are much more widespread than previously thought. Metagenomic studies have revealed the presence of Malassezia DNA in a number of unexpected areas, such as in association with corals and sea sponges in the ocean (Amend et al. 2019).
Malassezia are highly divergent from other fungi that are commonly found on the skin, such as Candida species and the dermatophytes, and there are currently 18 species within the Malassezia genus. Malassezia species belong to the Ustilaginomycotina subphylum, which includes the plant pathogens Ustilago, Sporisorium, and Tilletia, and are highly divergent from other basidiomycetous fungi that infect humans, such as Cryptococcus neoformans. A defining characteristic of the Malassezia genus is the lack of a fatty acid synthase, a Δ9 desaturase, and a Δ2,3 enoyl-CoA isomerase, making them lipid-dependent, and difficult to study and manipulate under laboratory conditions. Recent classifications have revealed that Malassezia represents a sister group to the blast yeast-like fungi Moniliella (Wang et al. 2014, 2015), which also includes species reported to be pathogenic on human and animal skin (McKenzie et al. 1984; Pawar et al. 2002).
In the last decade, there has been increasing scientific interest in Malassezia, with several sequencing projects aimed at defining genomic features and gene content for 15 broadly recognized Malassezia species. All haploid Malassezia species have small genomes compared to other phylogenetically related fungi (7–9 Mb compared to ∼20 Mb), with the exception of Malassezia furfur hybrid species whose genomes are twice the size of other Malassezia species. Genome analyses have revealed intriguing features, such as (1) loss of the RNA interference pathway components; (2) evidence of horizontal gene transfer events from bacteria; (3) the presence of genes unique to Malassezia; and (4) expansion of secreted protein, lipase, and protease gene families that encode products predicted to breakdown lipids and proteins important for growth, and host–microbe interactions (Xu et al. 2007; Gioti et al. 2013; Triana et al. 2015; Wu et al. 2015; Park et al. 2017; Zhu et al. 2017; Kim et al. 2018; Lorch et al. 2018; Cho et al. 2019; Morand et al. 2019). It is likely that the genomes of Malassezia species have reduced over time concomitantly with their evolution as a commensal organism and adaptation to the skin (Wu et al. 2015). There are other cases in which fungal genome reduction correlates with niche specialization, with the most remarkable examples being the obligate Pneumocystis species, with genomes of ∼7–8 Mb (Ma et al. 2016), and Microsporidia species, with genomes as small as 2.9 Mb (Cuomo et al. 2012).
Aside from their commensal lifestyle, Malassezia fungi have been associated with several skin disorders, including pityriasis versicolor, dandruff, severe atopic dermatitis in humans, and otitis in dogs (Gaitanis et al. 2012; Wu et al. 2015). However, the exact role of Malassezia in these clinical conditions has been controversial, with recent studies even hypothesizing a protective role of M. globosa against Staphylococcus aureus, a bacterium that is associated with severe atopic dermatitis (Ianiri et al. 2018; Li et al. 2018). The lack of knowledge regarding Malassezia functions within the skin mycobiome is due, in part, to the dearth of experimental systems for studying Malassezia–host interactions; current knowledge is based solely on in vitro experiments with isolated host cells (Watanabe et al. 2001; Ishibashi et al. 2006; Donnarumma et al. 2014; Glatz et al. 2015; Sparber and LeibundGut-Landmann 2017). Recently, two groundbreaking studies reported novel experimental murine models for studying Malassezia interactions with the skin and intestinal mucosa (Limon et al. 2019; Sparber et al. 2019), and their importance has been highlighted in two commentaries (Dawson 2019; Wrighton 2019).
Although these models represent an important advancement of our understanding of the mechanisms of host responses to Malassezia, a lack of technologies for functional genetic studies has hampered the identification and characterization of the fungal components that promote inflammation, and induce host responses. We developed a transformation system based on Agrobacterium tumefaciens-mediated transformation (AtMT) that is effective for both insertional and targeted mutagenesis, and enabled the first genetic manipulation of M. furfur and M. sympodialis (Ianiri et al. 2016, 2017a). Subsequently, M. pachydermatis has also been transformed (Celis et al. 2017).
CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 was originally discovered as a mechanism of adaptive bacterial immunity (Jinek et al. 2012). The CRISPR/Cas9 system has been modified for use in other organisms and, at present, represents a revolutionary technology that has allowed gene editing in a number of cell types, including fungi (Shi et al. 2017; Adli 2018). The system consists of two elements, a specific endonuclease (Cas9) and a guide RNA (gRNA), that form a complex that catalyzes double-strand breaks (DSBs) at a specific DNA site flanking a protospacer adjacent motif (PAM) sequence of the host genome. After the DSB is generated, the DNA is repaired either through nonhomologous end joining or through homology-directed repair (HDR) when donor DNA is provided (Shi et al. 2017).
The present study is divided into two sections. In the first, we built upon the previously developed AtMT technology to perform the first transfer (T)-DNA-mediated genetic screen in M. furfur. The aim was to generate a library of random insertional mutants, select for mutants with a phenotype of interest, and characterize insertion sites within the M. furfur genome to infer the function of genes involved in physiological and clinical processes of interest. In the second part of this study, we developed the first efficient, transient CRISPR/Cas9 mutagenesis system for Malassezia, and successfully generated targeted deletion mutants of two genes identified in the forward screen: CDC55, which encodes a subunit of protein phosphatase 2A (PP2A), and PDR10, which encodes an ATP-binding cassette (ABC) transporter predicted to be involved in pleiotropic drug resistance. While validating the effectiveness of the T-DNA insertional mutagenesis for gene function studies, this novel CRISPR/Cas9 method enabled us to overcome issues related to the reduced rate of homologous recombination (HR) observed in M. furfur, and we expect that this technology will facilitate future molecular research on Malassezia fungi.
Materials and Methods
Strains and culture conditions
The haploid M. furfur strain CBS14141 (previously known as JPLK23) was used as the wild-type (WT) strain for transformation experiments. This strain was maintained on modified Dixon’s media (mDixon) [mycological peptone (10 g/liter), malt extract (36 g/liter), glycerol (2 ml/liter), tween 60 (10 ml/liter), desiccated ox-bile (10 g/liter), and agar (20 g/liter) for solid media]. Transformants were maintained on mDixon supplemented with the antifungal agents nourseothricin (NAT) or G418 (NEO).
Forward genetics screen in M. furfur
Insertional mutagenesis was performed through AtMT using A. tumefaciens strain EHA105 engineered with the binary vectors pAIM2 or pAIM6, which contain NAT and NEO resistance markers under the control of the M. sympodialis ACT1 promoter and terminator, respectively (Ianiri et al. 2016). Initially, transformations were performed using previously developed methods (Ianiri et al. 2016; Celis et al. 2017). Selected transformants were colony purified on selective media and arrayed in 96-well plates containing 100 µl of mDixon + NAT or mDixon + NEO for in vitro assays and long-term storage.
For the primary screen, a 1.5-µl aliquot of cellular suspension of transformants was spotted on mDixon agar containing the following chemicals: Congo red (0.5%), sodium chloride (NaCl, 1 M), sodium dodecyl sulfate (SDS, 0.3%), or fluconazole (FLC, 150 µg/ml) for cell wall and plasma membrane stress; NaNO2 (100 mM) for nitrosative stress; or cadmium sulfate (CdSO4, 30 µM) for protein-folding defects and heavy-metal stress. Transformants were also exposed to UV light (250–450 µJ/cm2 × 100), elevated temperature (37°), pH (pH 7.5), and nutrient-limiting conditions [yeast nitrogen base media (YNB)]. When used, arginine and tyrosine were added at 30 mg/liter. Transformants selected in the primary screen as having a phenotype different from the WT were confirmed through a standard 1:10 serial dilution method by spotting 1.5 µl of cellular suspension on mDixon agar in the conditions that allowed their selection.
Molecular characterization of the T-DNA insertional mutants of M. furfur
Insertional mutants with a phenotype of interest were single-colony purified and grown overnight (ON) in 25 ml of liquid mDixon for genomic DNA extraction using a CTAB extraction buffer (Pitkin et al. 1996). To identify the insertion sites of the T-DNA in the M. furfur genome, inverse PCR (iPCR) was performed (Idnurm et al. 2004; Ianiri and Idnurm 2015). Briefly, ∼2 µg of DNA were digested with the restriction enzymes PvuII, XhoI, SacII, ApaI, EcoRI (6-bp recognition site), or TaqI (4-bp recognition site), column purified, and eluted in 30 µl of elution buffer. Then, 8.5 µl of digested DNA were self-ligated with T4 DNA ligase (New England Biolabs, Beverly, MA) ON at 4°, and 1 µl was used as template for iPCR using primers ai76-ai77 for DNA digested with restriction enzymes that cut outside the T-DNA region, or ai076-M13F and ai077-M13R for DNA digested with restriction enzymes that cut inside the T-DNA (Idnurm et al. 2004). iPCR conditions were: initial denaturation at 94° for 2 min, denaturation at 94° for 30 sec, annealing at 55° for 30 sec, and extension at 72° for 2.5 min. PCR reactions were performed using ExTaq polymerase (Taqara Bio, Shiga Japan). When ExTaq was unsuccessful, we used LaTaq polymerase (Taqara Bio) suitable for highly G+C-rich regions with an annealing temperature of 55 or 60°. Amplicons were either PCR- or gel-purified, and Sanger sequenced. Sequences were subjected to basic local alignment search tool nucleotide (BLASTn) analysis against the M. furfur CBS14141 genome assembly available on the National Center for Biotechnology Information (NCBI) website (reported with the previous designation as JPLK23) (Wu et al. 2015) and against an unpublished PacBio (Pacific Biosciences) assembly. Gene boundaries and regulatory regions were determined using unpublished RNA sequencing (RNAseq) data, which allowed us to define the accurate locations of T-DNA insertions. Retrieved M. furfur sequences were subjected first to BLASTx analysis against the latest genome assembly of M. sympodialis (Zhu et al. 2017) and subsequently on the Saccharomyces Genome Database (SGD), to identify orthologs and infer gene function. Genes were named based on orthologous genes in Saccharomyces cerevisiae. Gene annotation was carried out manually based on BLAST searches and with Augustus (http://bioinf.uni-greifswald.de/augustus/submission.php), using RNAseq for untranslated regions (UTRs) and introns.
For Southern blot analysis, ∼2 µg genomic DNA were digested with SacII (no cut sites are within the NAT or NEO cassette, thus allowing us to determine the number of T-DNA insertions), resolved on a 0.8% agarose gel in 1× Tris-acetate EDTA (TAE) buffer, transferred to a Zeta-Probe membrane, and probed with NAT or NEO cassettes labeled with [32P]dCTP. NAT and NEO cassettes were amplified from plasmids pAIM2 and pAIM6, respectively, with universal M13F and M13R primers.
RNA extraction was performed using the standard TRIzol method (Rio et al. 2010). RNA was treated with the TURBO DNAse enzyme (Thermo Fisher Scientific) according to the manufacturer’s instructions, and quality was assessed using a NanoDrop spectrophotometer. Then, 3 µg of purified RNA were converted into cDNA via the Affinity Script QPCR cDNA synthesis kit (Agilent Technologies). cDNA synthesized without the RT/RNAse block enzyme mixture was used as a control for genomic DNA contamination. Approximately 500 pg of cDNA were used to measure the relative expression level of target genes through quantitative real-time PCR (RT-qPCR) using the Brilliant III ultrafast SYBR green QPCR mix (Agilent Technologies) in an Applied Biosystems (Foster City, CA) 7500 Real-Time PCR System. A “no-template control” was included for each target. Technical and biological triplicates were performed for each sample. Gene expression levels were normalized using the endogenous reference gene TUB2 and determined using the comparative ΔΔCt method.
Generation of plasmids for CRIPSR/Cas9 targeted mutagenesis in M. furfur
Plasmids for targeted mutagenesis of M. furfur CDC55 and PDR10 through A. tumefaciens-mediated transformation were assembled in S. cerevisiae using the binary vector pGI3, as previously reported (Ianiri et al. 2016, 2017a,b). The NAT cassette was amplified from plasmid pAIM1 using primers JOHE43277 and JOHE43278. The 5ʹ and 3ʹ flanking regions for HR were amplified from the genomic DNA of M. furfur CBS14141, using primer pairs JOHE45209-JOHE45210 and JOHE45211-JOHE45212 for CDC55, and JOHE45201-JOHE45212 and JOHE45203-JOHE45204 for PDR10, respectively. The PCR products and the double-digested (KpnI and BamHI) binary vector pGI3 were transformed into S. cerevisiae using lithium acetate and PEG 3750, as previously reported (Ianiri et al. 2016). To assess correct recombination of the newly generated plasmids, single colonies of S. cerevisiae transformants were screened by PCR using primers specific for the NAT marker (JOHE43281-JOHE43282) in combination with primers homologous to outside of the region of the plasmid pGI3 involved in the recombination event (JOHE43279-JOHE43280). Positive clones of S. cerevisiae were grown ON in YPD and subjected to phenol–chloroform–isoamyl alcohol (25:24:1) plasmid extraction using a previously reported protocol (Hoffman 2001). The plasmid DNA was then introduced into the A. tumefaciens EHA105 strain by electroporation and transformants were selected on LB + 50 µg/ml kanamycin. PCRs were performed using ExTaq and/or LATaq polymerase as described previously, except with a 1.5-min extension time.
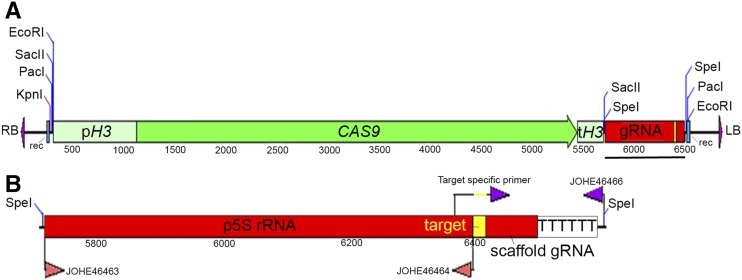
To generate the components of the CRISPR/Cas9 system in Malassezia, histone H3 was identified in the M. sympodialis ATCC42132 genome assembly (Zhu et al. 2017) through BLASTp analysis using S. cerevisiae H3 as the query. The 813-bp upstream and 257-bp downstream regions, including the M. sympodialis H3 promoter and terminator (pH3 and tH3), respectively, were PCR-amplified using JOHE46457-JOHE46458 and JOHE46461-JOHE46462, respectively. High-Fidelity (HF) Phusion Taq polymerase (New England Biolabs) was used with an annealing temperature of 55° for 30 sec and extension for 1 min at 72°. Primer JOHE46457 includes a chimeric region for recombination in pPZP-201BK and a multicloning site. Primers JOHE46458 and JOHE46461 include chimeric regions for recombination with JOHE46459 and JOHE46460, which were used to amplify the CAS9 open reading frame (ORF) from plasmid pXL1-Cas9 (Fan and Lin 2018). JOHE46462 has SacII and SpeI restriction sites, and a region for recombination with the promoter of the 5S rRNA of M. sympodialis used to drive expression of the single gRNA. CAS9 amplification did not work well with HF Phusion Taq, so we used ExTaq polymerase as described above, but with fewer cycles (20 cycles) and a 4-min extension. The M. sympodialis ATCC42132 ribosomal cluster was identified in the latest genome assembly and annotation (Zhu et al. 2017) through BLASTn analysis using internal transcribed spacer (ITS) sequences from M. sympodialis CBS7222 (GenBank accession number NR_103583). We PCR-amplified a 674-bp region from the end of the rRNA-eukaryotic large subunit ribosomal RNA (position 612351 on chromosome 5), including the rRNA-5S ribosomal RNA gene (position 613025 on chromosome 5), using primers JOHE46463 and JOHE46464. Primer JOHE46463 has a chimeric region complementary to JOHE46462. This PCR was performed using the touchdown protocol with an initial denaturation of 94° for 5 min, followed by 24 cycles of denaturation at 94° for 30 sec, annealing at a gradient temperature of 62° for 30 sec minus 1° per cycle, and extension at 72° for 1 min. This was followed by 16 cycles of denaturation at 94° for 30 sec, annealing at 50° for 30 sec, extension at 72° for 1 min, and final extension of 72° for 5 min. The gRNA scaffold was amplified from plasmid pSDMA64 (Arras et al. 2016) using primers JOHE46465 and JOHE46466. JOHE46466 includes SpeI and SacII restriction sites, six thymine residues (6T terminator), and a chimeric region for recombination in pPZP-201BK.
The specific target sequence for CDC55 was identified using EuPaGDT (http://grna.ctegd.uga.edu/), available on FungiDB (https://fungidb.org/fungidb/). A specific target sequence for CDC55 was added by PCR with primers JOHE46468 and JOHE46466 using the gRNA scaffold as template. Primer JOHE46468 has a chimeric region for recombination with both the 5S rRNA sequence and the gRNA scaffold, with an intervening target sequence specific for CDC55. We used HF Phusion Taq as reported above. All components were gel purified, and equimolar amounts of the purified amplicons were used for overlap PCR to generate the Cas9 expression cassette (pH3- CAS9, tH3) and the complete gRNA (5S rRNA promoter fused with the gene-specific gRNA scaffold). PCRs were carried out using HF Phusion Taq and the touchdown protocol as above, with the only difference being extension times of 5 and 1 min, respectively. The two resulting amplicons were cloned into the T-DNA of pPZP201BK digested with KpnI and BamHI through HiFi (New England Biolabs) assembly and recovered in Escherichia coli DH5α. We screened E. coli clones for recombinant plasmids by PCR using primers specific for the plasmid backbone (JOHE43279 and JOHE43280) with JOHE46458 and JOHE46463. The plasmid sequence for CRISPR/Cas9 deletion of CDC55 (named pGI40) was confirmed by Sanger sequencing.
To generate the CRISPR/Cas9 plasmid for targeted mutagenesis of PDR10, the binary vector pGI40 was digested with SpeI to remove the CDC55-specific gRNA, recovered from the gel, and purified. A PDR10-specific target sequence designed using EuPaGDT was added to the gRNA scaffold by PCR using primers JOHE46466 and JOHE46467, which have chimeric regions for recombination with both the 5S rRNA sequence and the gRNA scaffold, with the specific target sequence for PDR10 in between.
This amplicon and the 5S rRNA previously generated were recombined through HiFi assembly within the T-DNA of the SpeI-digested pGI40 plasmid, and the novel recombinant plasmids with the PDR10-specific gRNA (named pGI48) were identified by Sanger sequencing. This procedure is reported in Figure 4B. Recombinant plasmids were introduced into A. tumefaciens through electroporation.
Figure 4.
Development of a CRISPR/Cas9 system in M. furfur. (A) Complete T-DNA necessary for CAS9 expression and gRNA excision in M. furfur. The promoter and terminator of the histone H3 gene of M. sympodialis ATCC42132 (pH3 and tH3, respectively) and the CAS9 gene are shown in green. The gRNA is shown in red, and the gene-specific target region is shown in yellow. Sites for recombination are shown in blue, and the RB and LB of the T-DNA are shown in purple. Restriction sites were added to facilitate further molecular manipulation. The black bar indicates the full-length gRNA that is shown in greater resolution in (B). (B) The gRNA includes the 5S rRNA promoter region (p5S rRNA) obtained with primers JOHE46463 and JOHE46464 through touchdown PCR with M. sympodialis ATCC42132 genomic DNA. The gene-specific gRNA was obtained by PCR using a target-specific primer, which overlaps with the p5S rRNA and the gRNA scaffold, and includes a 20-nt target sequence (represented in yellow). This was used in combination with JOHE46466, which includes the 6T terminator (and a region for recombination in pPZP201BK, which is not shown). A SpeI restriction site was added to facilitate further use of the CRISPR/Cas9 system in Malassezia. To perform targeted mutagenesis of another gene, we recommend using SpeI digestion of the plasmid in (A) (pGI40), and cloning the two PCR products (p5S rRNA and gene-specific gRNA) through Gibson or HiFi assembly. We used this strategy to generate binary vector pGI48 for CRISPR/Cas9-mediated mutagenesis of the PDR10 gene. CRISPR, clustered regularly interspaced short palindromic repeats; HiFi, plasmid recombination kit; gRNA, guide RNA; LB, left border; RB, right border; T-DNA, transfer DNA.
AtMT was performed with modifications that increase transformation efficiency compared to our previous protocol used to generate insertional mutants. Briefly, M. furfur was grown for 2 days at 30° and the culture was diluted to OD600 ∼1.0. The engineered A. tumefaciens strains with the gene deletion cassettes and the CRISPR/Cas9 expression system were grown ON, diluted to an OD600 ∼0.1, and incubated for 4–6 hr in shaking cultures (30°) in liquid induction medium (IM) until OD600 reached a value of 0.6–0.8. These bacterial cellular suspensions were mixed in 1:1, 1:2, and 2:1 ratios, respectively, and added to M. furfur cellular suspensions at 1:2 and 1:5 ratios, respectively. These cellular suspensions were centrifuged at 5200 × g for 15 min, the supernatants were discarded, and ∼500 µl to 1 ml of these fungal and bacterial mixes were spotted directly onto nylon membranes placed on modified IM (mIM) agar containing 200 µM acetosyringone. These were co-incubated for 5 days at room temperature (plates maintained without Parafilm), prior to transferring the dual cultures to mDixon supplemented with NAT (100 µg/ml) to select for fungal transformants and cefotaxime (350 µg/ml) to inhibit Agrobacterium growth.
M. furfur transformants resistant to NAT were colony purified, and subjected to phenotypic and molecular characterization. Putative mutants for the CDC55 gene were exposed to UV light (250–300 µJ/cm2 × 100) to identify those with impaired growth according to the results of the forward genetic screen. For molecular analysis, 23 representative NAT-resistant, UV light-sensitive transformants were subjected to phenol–chloroform–isoamyl alcohol (25:24:1) DNA extraction, and the correct replacement of the target loci was assessed by PCR. Diagnostic PCRs to identify HR events for the CDC55 gene were carried out with primers JOHE45213 or JOHE45874 in combination with specific primers for the NAT gene (JOHE43281 and JOHE43282, respectively), and with primers JOHE45215 and JOHE45216 specific for the internal region of CDC55. To evaluate the overall rate of HR of the CRISPR/Cas9 system, a larger number of cdc55Δ candidate mutants were tested for sensitivity to hydroxyurea, which was found to be the stressor with the strongest phenotype. Similarly, putative pdr10∆ mutants were exposed to FLC (150 µg/ml) for phenotypic characterization, and transformants displaying impaired growth were subjected to DNA extraction for molecular characterization. Diagnostic PCRs were carried out using primers JOHE45205 and JOHE45206, alone and in combination with specific primers for the NAT gene (JOHE43281 and JOHE43282, respectively), and with primers JOHE45207 and JOHE45208 specific for the internal region of PDR10. PCR analyses consisted of 34 cycles of denaturation at 94° for 30 sec, annealing at 55° for 30 sec, extension at 72° of 1 min/kb with an initial denaturation at 94° for 2 min, and a final extension at 72° for 5 min. PCR analyses were performed using ExTaq (Takara) according the manufacturer’s instructions. To detect HR events at the 3ʹ region of CDC55 and to amplify the full-length PDR10 gene, LATaq polymerase (Takara) with Buffer I was used. PCR for CAS9 was carried out with ExTaq, and the touchdown protocol with primers JOHE46459–JOHE46461. PCR for the gRNA was carried out with JOHE46465 and JOHE46466 using ExTaq as reported above. All the primers used are listed in Supplemental Material, Table S1.
Phenotypic analysis of the target mutants was performed on mDixon agar by spotting 1.5 µl of 1:10 dilutions of each cellular suspension under the following conditions: UV (250–450 µJ × 100), hydroxyurea (50 mM), benomyl (50 µM), FLC (150 µg/ml), amphotericin B (AmB, 50 µg/ml), 5-flucytosine (1 mg/ml), caspofungin (100 µg/ml), cyclosporin A (CsA) at 100 µg/ml both alone and in combination with 10 mM of lithium chloride, tacrolimus (FK506) at 100 µg/ml both alone and in combination with 10 mM of lithium chloride, and dimethyl sulfoxide (40 µl), which was used to dissolve benomyl (10 mM).
Genomic comparison and phylogeny of the ABC transporter of M. furfur and M. sympodialis
The predicted amino acid sequences of S. cerevisiae Pdr10, Pdr5, Pdr15, Pdr12, Snq2, Pdr18, Aus1, and Pdr11 were used as queries for tBLASTn and BLASTp searches against the genomes of M. furfur CBS14141 and M. sympodialis ATCC 42132, available on GenBank (Wu et al. 2015; Zhu et al. 2017). The Malassezia best hits were retrieved, and the encoded proteins for M. furfur were predicted using Augustus (http://bioinf.uni-greifswald.de/augustus/submission.php) based on RNAseq evidence for UTRs and introns. The web portal of ACT Artemis (https://www.webact.org/WebACT/home) was used for synteny analysis of an ∼15,000-bp region of M. sympodialis and M. furfur, containing orthologs of the Pdr10-encoding genes. tBLASTx analysis with a E-value of 0.1 was performed. For phylogeny, the aforementioned predicted ABC transporter proteins were aligned using MUSCLE and the phylogenetic tree was generated with MEGA 7 (http://www.megasoftware.net/) (Kumar et al. 2016), using the maximum likelihood method (LG model, five discrete γ categories) and 100 bootstrap replications.
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8309342.
Results
Molecular and phenotypic characterization of M. furfur insertional mutants
Insertional mutants of M. furfur were generated through AtMT using both NAT and NEO dominant drug-resistance markers. A total of 767 insertional mutants were isolated and their growth was assayed under several stress conditions (see Materials and Methods, and Table 1). A total of 19 mutants (∼2.5%) displayed phenotypes different from the WT and were selected for further characterization.
Table 1. Insertional mutants for M. furfur isolated in the forward genetic screen.
| Strains | Phenotype | Hit gene | Position | Comments |
|---|---|---|---|---|
| 1A7 | UV | CDC55 | ORF | Fourth exon |
| 1F12 | CdSO4 | NA | NA | NA |
| 2A8 | YNB | ARG1 | ORF | Exon |
| 2F4 | CdSO4 | SEC13-PRP43 | SEC13-5′ region PRP43-5′ region | Intergenic |
| 2G9 | FLC | NA | NA | Tandem insertion |
| 2H11 | FLC, NaCl | ERG5/PDA1 | ERG5-ORF/PDA1-5′ region | Chromosomal rearrangement |
| 3A1 | CdSO4 | JLP2/TCP1 | JLP2-ORF/TCP1-5′ region | Chromosomal rearrangement |
| 4A10 | NaNO2, SDS | Uncharacterized enoyl-CoA hydratase | ORF | 2 T-DNA insertions |
| 4B1 | CdSO4 | MAE1 | 3′-UTR | 2 T-DNA insertions |
| 5D11 | FLC | NA | NA | Tandem insertion |
| 5F1 | 37°C | GPD1/INO80 | GPD1-5′ region/INO80-5′ region | Chromosomal rearrangement |
| 5F10 | NaCl | DUG1/RPC10 | DUG1-3′UTR/RPC10-5′-UTR | Chromosomal rearrangement |
| 6A10 | FLC | SIP5 | ORF | Altered stop codon |
| 6B2 | 37°C | Uncharacterized Rho GTPase | ORF | 2 T-DNA insertions |
| 6C8 | YNB | TYR1 uncharacterized gene | ORF | Nonstandard T-DNA insertion |
| 7D5 | 37°C | NA | NA | NA |
| 7D9 | FLC | PDR10- UCB6 | PDR10-5′ region UBC6-3′ region | Intergenic |
| 7F8 | FLC | ADY2 | 3′-UTR | 260 bp after the predicted stop codon |
| 7H6 | 37°C | JEN1 uncharacterized protein with RNA-binding domain | JEN1-5′ region uncharacterized 3′ region | Intergenic |
FLC, fluconazole; NA: inverse PCR failed to identify T-DNA insertions; T-DNA, transfer DNA; YNB, yeast nitrogen base; (/), chromosomal rearrangements.
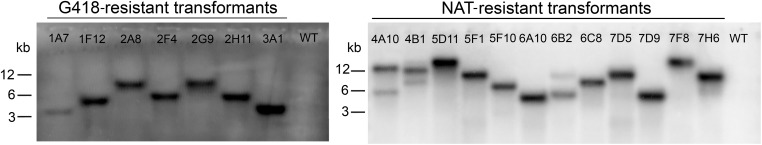
iPCR was utilized to identify the genes inactivated by the T-DNA insertions. The sequenced amplicons were compared to the unannotated M. furfur CBS14141 genome assemblies (one reported in NCBI, and another unpublished based on PacBio sequencing) coupled with RNAseq data, which facilitated the identification of the coding and regulatory sequences. This allowed an accurate determination of T-DNA insertion sites. Southern blot analysis was performed to determine the number of T-DNA insertions, revealing that 16 transformants harbored single T-DNA insertions and three transformants had two T-DNA insertions (strains 4A10, 4B1, and 6B2) (Figure 1). In parallel, iPCR allowed the characterization of 15 T-DNA insertions (Figure 2). Six strains had T-DNA insertions within predicted ORFs, two strains had insertions within UTRs, and three strains had T-DNA insertions in intergenic regions. Of the remaining eight strains, four were suspected to have chromosomal rearrangements because the T-DNA borders were found in different locations in the M. furfur CBS14141 genome, and the junctions between the T-DNA and the M. furfur genome could not be identified in four other strains. Table 1 summarizes the results of the forward genetics approach performed in this study, and properties of the T-DNA insertions are reported in Figure 2.
Figure 1.
Southern blot analysis of M. furfur insertional mutants selected in the insertional genetic screen as having a phenotype different from WT. Genomic DNA was digested with SacII, which does not cut within the NAT or NEO cassette, and hybridized with the ORFs of the NEO (left) and NAT (right) genes. Each hybridization band corresponds to a single T-DNA insertion. The names of the transformants and of the M. furfur WT are indicated. NAT, nourseothricin; NEO, G418; T-DNA, transfer DNA; WT, wild-type.
Figure 2.
T-DNA insertion sites in 15 transformants of M. furfur as determined by iPCR. For each transformant, the mutated region and its corresponding region in the WT are shown. The region altered by the T-DNA is indicated above the sequence. When the sequences of two regions are shown above the sequence, it indicates that the T-DNA insertion involved different locations of the M. furfur genome. The borders of the T-DNA are depicted in bold. Uppercase letters represent nucleotides corresponding to regions with RNAseq coverage (and hence representing either 5ʹ- or 3ʹ-UTRs as indicated, or ORFs), while lowercase letters represent intergenic regions with no RNAseq coverage. The red nucleotides in strain 6A10 indicate a TAG stop codon. The symbol “−/−” indicates chromosomal rearrangement, with genomic locations shown in parentheses for the two transformants (2H11 and 5F10) having rearrangements involving the same chromosome. iPCR, inverse PCR; RNAseq, RNA sequencing; T-DNA, transfer DNA; WT, wild-type.
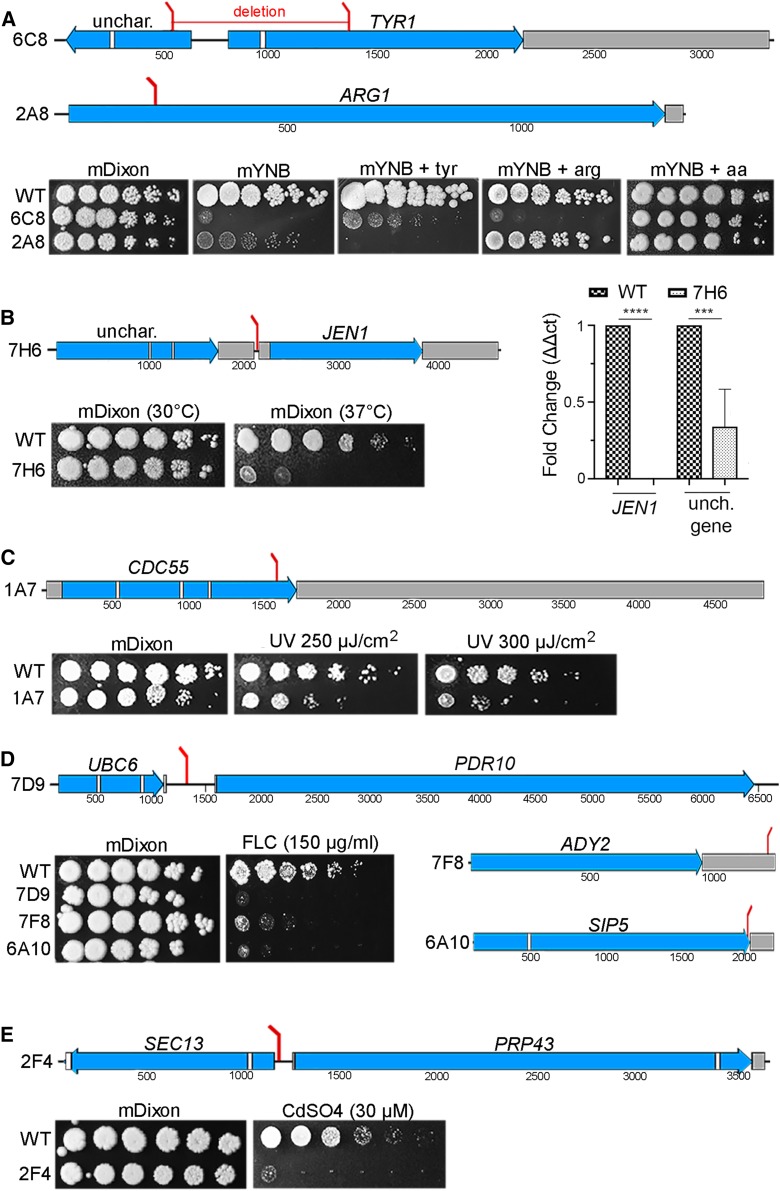
Two mutants with reduced growth on YNB were identified, and analysis of the genome sequence flanking the T-DNA revealed insertions in genes involved in amino acid biosynthesis (Figure 3A). Strain 6C8 had a nonstandard T-DNA insertion that generated a deletion of ∼800 bp in the M. furfur genome. Moreover, we were unable to identify the left border sequence from iPCR and the first nucleotides obtained mapped within the ORF of TYR1, which encodes a prephenate dehydrogenase involved in tyrosine biosynthesis (Mannhaupt et al. 1989). Conversely, the right border was within the ORF of the adjacent gene encoding an uncharacterized protein with no conserved domains that shares similarity with several other Malassezia species and basidiomycetes. Although addition of tyrosine did not restore the growth of strain 6C8 to the WT level (Figure 3A), YNB media supplemented with all amino acids did restore growth, suggesting that Tyr1 is involved in the biosynthesis of other amino acids. In strain 2A8, T-DNA inserted within the ORF of ARG1, which encodes the enzyme arginosuccinate synthetase in the arginine biosynthesis pathway (Jauniaux et al. 1978). Addition of l-arginine to YNB was sufficient to restore a WT phenotype, confirming that M. furfur ARG1 is involved in arginine biosynthesis (Figure 3A).
Figure 3.
Position of T-DNA insertions in M. furfur mutants and their associated phenotypes. Each section shows mutants sensitive to the same stress or condition, such as reduced growth on mYNB medium (A), reduced growth at 37° (B), and sensitivity to UV light (C), FLC (D), or CdSO4 (E). The positions of the T-DNA insertions are indicated by red bars, and in the same panel the phenotypes of the mutants are also shown. Exons are represented in blue, introns in white, and UTRs in gray. In the qPCR of (B) **** P < 0.0001 and *** P < 0.001 for each pairwise comparison. FLC, fluconazole; mDixon; modified Dixon’s media; qPCR, quantitative PCR; T-DNA, transfer DNA; unch./unchar., uncharacterized; WT, wild-type; mYNB, minimal yeast nitrogen base.
Four insertional mutants with decreased growth at an elevated temperature (37°) were identified. Of these, only strain 7H6 had a standard T-DNA insertion, which integrated between two genes: downstream of an RNA-binding domain-containing protein and upstream of JEN1 (Figure 3B). While the RNA-binding domain-containing protein is uncharacterized in S. cerevisiae, Jen1 is a plasma membrane monocarboxylate/proton symporter that transports pyruvate, acetate, lactate, and other substrates (Casal et al. 1999). To assess which gene was affected by the T-DNA insertion and therefore responsible for the phenotype of interest, RT-qPCR was performed. Expression levels were normalized to the TUB2 gene of M. furfur WT grown at 30°. Expression of the uncharacterized gene encoding the RNA-binding domain-containing protein in strain 7H6 was ∼60% lower compared to the WT, whereas expression of JEN1 was undetectable, indicating that either or both genes could be responsible for the temperature-sensitive phenotype (Figure 3B). Other transformants that displayed a temperature-sensitive phenotype had a nonstandard T-DNA insertion pattern, and they included strains 5F1, 6B2, and 7D5 (Table 1).
Strain 1A7 showed increased sensitivity to UV light (250 and 350 µJ/cm2 × 100) compared to WT M. furfur CBS14141 (Figure 3C). The T-DNA inserted into the fourth exon of the CDC55 gene in this strain. In S. cerevisiae, CDC55 encodes a regulatory subunit of PP2A. CDC55 is involved in cell cycle control, and is required for successful chromosome segregation and nuclear division (Healy et al. 1991; Bizzari and Marston 2011).
Six strains showed increased sensitivity to the antifungal FLC (150 µg/ml) compared to the WT strain (Figure 3D). Strain 6A10 had a T-DNA insertion in the predicted stop codon of the S. cerevisiae ortholog SIP5. The function of this protein is unknown and it has no known domains. However, it has been reported to interact with both the Reg1/Glc7 phosphatase and the Snf1 kinase in response to glucose starvation (Sanz et al. 2000). In strain 7D9, the T-DNA was found in the intergenic region between the 5ʹ end of an ABC multidrug transporter gene and the 3ʹ end of the UBC6 gene. As shown in Figure 6E, the closest S. cerevisiae homolog is the ABC transporter PDR10, which is the designation that we adopted. ABC multidrug transporters are involved in pleiotropic drug responses that mediate resistance to xenobiotic compounds including mutagens, fungicides, steroids, and anticancer drugs (Sipos and Kuchler 2006). UBC6 encodes a ubiquitin-conjugating enzyme involved in ER-associated protein degradation (Walter et al. 2001). As confirmed by targeted mutagenesis (discussed below), the FLC-sensitive phenotype of strain 7D9 is due to T-DNA insertion in the promoter region of PDR10. In strain 7F8, the T-DNA inserted within the 3′-UTR of ADY2 (Figure 3D), which encodes an ammonium and acetate transmembrane transporter involved in nitrogen utilization (Rabitsch et al. 2001; Paiva et al. 2004). For the remaining three FLC-sensitive isolates, strain 2H11 had a chromosomal rearrangement involving the ERG5 gene and the region close to the 5ʹ end of the PDA1 gene, and in strains 2G9 and 5D11, the T-DNA likely integrated in tandem repeats because iPCR amplicons consisted of both the left and right borders of the T-DNA fused together, and this prevented retrieval of the junctions between the T-DNA and the genome.
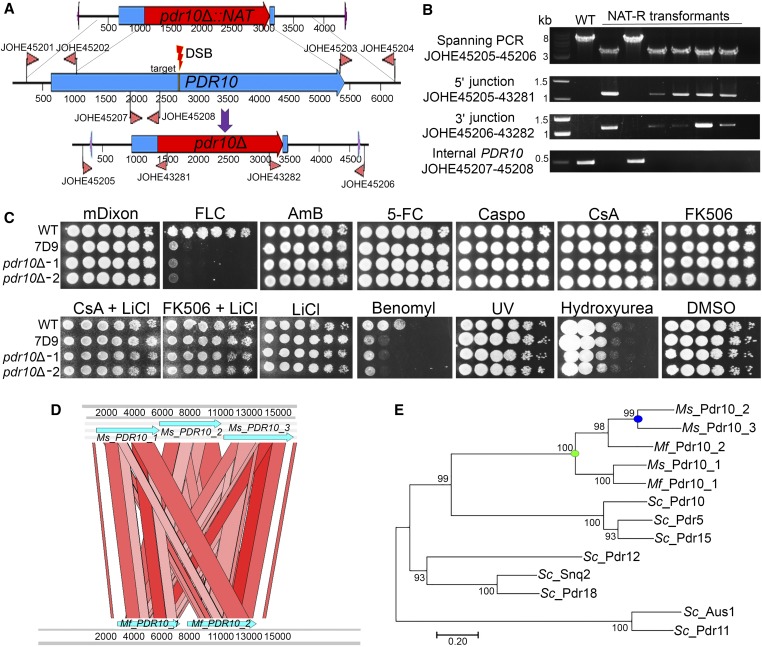
Figure 6.
Targeted CRISPR/Cas9-mediated gene replacement of the Mf PDR10 gene. (A) The T-DNA including the pdr10Δ::NAT HDR template is shown at the top. The PDR10 gene, the primers used to amplify the 5ʹ and 3ʹ regions for HR, the internal primer for the PDR10 gene, and the 20-nt target sequence (yellow) for the DSB are shown in the middle. The bottom part shows the pdr10Δ mutant allele and the primers used for PCR. (B) Diagnostic PCR analyses of Mf WT, and five FLC-sensitive and one FLC-resistant, transformants for the identification of pdr10Δ mutants. Each panel used a combination of primers that are represented in (A). (C) Cellular suspension (1.5 µl) of the WT strain CBS14141, insertional mutant 7D9, and two independent pdr10Δ mutants were spotted on mDixon (control), FLC (150 µg/ml), AmB (50 µg/ml), 5-FC (1 mg/ml), Caspo (100 µg/ml), CsA alone or with 10 mM of LiCl (100 µg/ml), FK506 (100 µg/ml) alone or with 10 mM of LiCl, benomyl (50 µM), UV (300 µJ/cm2 × 100), hydroxyurea (50 mM), or DMSO [800 µl/liter (solvent used to resuspend benomyl)]. (D) ACT Artemis synteny comparison of a 15-kb region including the three copies of the PDR10 gene of Ms and the two copies of the PDR10 gene of Mf. (E) The predicted proteins of the Sc ATP-binding cassette transporters Pdr10, Pdr5, Pdr15, Pdr12, Snq2, Pdr18, Aus1, and Pdr11; Ms Pdr10_1, Pdr10_2, and Pdr10_3; and Mf Pdr10_1 and Pdr10_2 were used to generate a maximum likelihood phylogenetic tree with the LG + G method (100 bootstrap replications). AmB, amphotericin B; Caspo, caspofungin; CRISPR, clustered regularly interspaced short palindromic repeats; CsA, cyclosporine A; DSB, double-strand break; 5-FC, 5-fluorocytosine; FLC, fluconazole; G, γ category; gRNA, guide RNA; HDR, homology-directed repair; HR, homologous recombination; mDixon; modified Dixon’s media; Mf, M. furfur; Ms, M. sympodialis; NAT-R, nourseothricin-resistant; Sc, S. cerevisiae; T-DNA, transfer DNA; WT, wild-type.
Four strains showed sensitivity to CdSO4 (30 µM), and only one (strain 2F4) showed a standard T-DNA insertion in the 5ʹ regions of both SEC13 and PRP43 (Figure 3E). SEC13 in S. cerevisiae encodes an essential protein that is a structural component of the coat protein complex II, of the nuclear pore outer ring, and of the Seh-1-associated complex. S. cerevisiae PRP43 encodes an RNA helicase protein that is also essential for viability and contributes to the biogenesis of ribosomal RNA, and it is also involved in spliceosomal complex disassembly (Arenas and Abelson 1997; Giaever et al. 2002). RT-qPCR did not show clear downregulation of either gene (data not shown), and whether either or both genes are responsible for the cadmium sulfate-sensitive growth defect remains to be established. Because the T-DNA inserted in the 5ʹ region of SEC13 and PRP43, whose orthologs are essential in S. cerevisiae, we speculate that the functions of both genes are affected or that the phenotype observed is unlinked to the T-DNA insertion. In strain 3A1, we identified a rearrangement involving the JLP2 and TCP1 genes, and for strain 4B1, Southern blotting indicated two T-DNA insertions, one of which was identified within the 3′-UTR of the MAE1 gene. For strain 1F12, iPCR using different restriction enzymes was unsuccessful. We also identified a strain (4A10) that was sensitive to sodium nitrite (NaNO2) and SDS. According to Southern blot analysis, strain 4A10 has two T-DNA insertions, one of which was identified in an uncharacterized enoyl-CoA hydratase gene. Another strain (5F10) was sensitive to NaCl and iPCR, and revealed the presence of a chromosomal rearrangement involving the 3ʹ region of the DUG1 gene and the 5ʹ-UTR of the RPC10 gene.
Development of a CRISPR/Cas9 gene deletion system to generate cdc55Δ M. furfur mutants
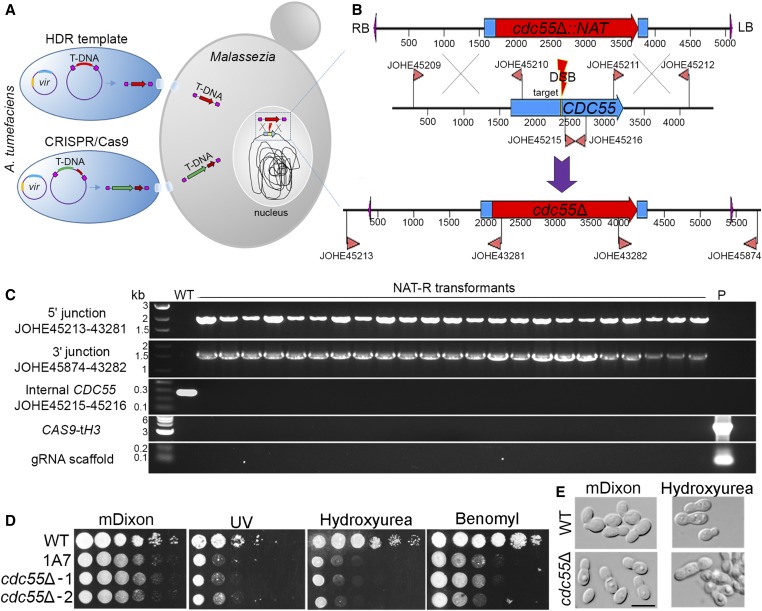
To validate the results of the insertional mutagenesis screen, the insertional mutants 1A7 and 7D9, and their associated mutated genes, were chosen for further analysis. First, we focused on the UV-sensitive strain 1A7 with a T-DNA insertion in the CDC55 gene. We were intrigued by this strain because CDC55 mutation is not known to be responsible for UV sensitivity in other fungi.
To determine if the UV phenotype of the original insertional mutant in 1A7 is attributable to CDC55 mutation, we first generated a cdc55Δ-targeted mutant. For targeted mutagenesis, molecular biology techniques were performed following our previously published methods (Ianiri et al. 2016). Regions of 1500 and 1000 bp flanking the 5ʹ and 3ʹ ends of the CDC55 target gene, respectively, were amplified from M. furfur genomic DNA and fused with the NAT marker within the T-DNA borders of plasmid pGI3. The recombinant plasmid (pGI41) bearing the cdc55Δ::NAT allele was identified in S. cerevisiae by colony PCR and transformed in A. tumefaciens EHA105 via electroporation. Several rounds of AtMT were performed, and NAT-resistant transformants of M. furfur were single-colony purified and subjected to diagnostic PCR to confirm CDC55-targeted mutagenesis. None of the transformants tested (0 out of > 100) showed full replacement of CDC55.
Next, we developed a CRISPR/Cas9 system for M. furfur to increase HR efficiency. Because the plasmid for targeted gene replacement of CDC55 was already available, we generated an additional plasmid to make a DNA DSB in CDC55, and then used the available cdc55Δ::NAT allele as the HDR template to repair the break. For expression of Cas9, the ORF of the CAS9 endonuclease was cloned under the control of the strong promoter and terminator of the histone H3 gene of M. sympodialis ATCC42132. To drive expression of gRNA specific for the target CDC55 gene, the promoter of the 5S rRNA was chosen. Because the ribosomal cluster is well annotated in the newly released genome of M. sympodialis (Zhu et al. 2017), and we have evidence that M. sympodialis promoters and terminators are functional in M. furfur (Ianiri et al. 2016), a 689-bp region including the 5S rRNA and its upstream region was amplified from M. sympodialis ATCC42132. The forward primer for the p5S rRNA contained restriction sites for SacII and SpeI to facilitate genetic manipulations. The scaffold gDNA was also obtained by PCR, and 6 thymine residues were included as a terminator. A 20-nt oligonucleotide target of gRNA was designed to match a region of the CDC55 gene adjacent to a PAM site, and it included the 5ʹ and 3ʹ regions that overlapped with the 5S rRNA promoter and the gRNA scaffold, respectively. This target oligonucleotide was added to the gRNA scaffold through PCR as reported in Figure 4B. The five PCR fragments (pH3, CAS9, tH3, p5S rRNA, and gRNA) were used as templates for overlap PCRs, and two final amplicons (pH3-CAS9-tH3 and p5SrRNA-gRNA) were cloned in pPZP201BK to generate plasmid pGI40 (Table 2) (Figure 4, A and B).
Table 2. Plasmids used in the present study.
| Name | Background | Relevant features | Purpose | References |
|---|---|---|---|---|
| pAIM2 | pPZP-201BK | pACT1-NAT-tACT1 | Random insertional mutagenesis | Ianiri et al. (2016) |
| pAIM6 | pPZP-201BK | pACT1-NEO-tACT1 | Random insertional mutagenesis | Ianiri et al. (2016) |
| pPZP-201BK | NA | KAN-R | Binary vector that replicates in E. coli and A. tumefaciens | Covert et al. (2001) |
| pGI3 | pPZP-201BK | ScURA3 + 2µ; KAN-R | Binary vector that replicates in E. coli, A. tumefaciens, and S. cerevisiae | Ianiri et al. (2017b) |
| pGI40 | pPZP-201BK | pH3-CAS9-tH3 + p5S rRNA-gRNA CDC55 | Cas9 endonuclease and CDC55 target gRNA | This study |
| pGI41 | pGI3 | cdc55::NAT | HDR template to generate cdc55Δ | This study |
| pGI42 | pGI3 | pdr10::NAT | HDR template to generate pdr10Δ | This study |
| pGI48 | pGI40 | pH3-CAS9-tH3 + p5S rRNA-gRNA PDR10 | Cas9 endonuclease and PDR10_1 target gRNA | This study |
HDR, homology-directed repair; KAN-R, kanamycin-resistant; gRNA, guide RNA.
AtMT of M. furfur CBS14141 was conducted to test the developed CRISPR/Cas9 system to generate targeted gene replacement of the CDC55 gene. Since our previous reports of AtMT of Malassezia (Ianiri et al. 2016, 2017a), we have optimized the protocol to achieve greater transformation efficiency. The main change involves the use of a 2:1 to 5:1 Malassezia:A. tumefaciens mixture that is concentrated through centrifugation before the co-incubation step on mIM (Ianiri et al. 2016); the detailed procedure is reported in the Materials and Methods. A representative subset of 23 M. furfur NAT-resistant transformants were single-colony purified and subjected to molecular characterization through PCR. Genotyping was performed using: (1) primers designed beyond the regions of DNA used in the generation of the deletion allele in combination with specific NAT primers; (2) primers internal to the gene CDC55; (3) primers specific for the CAS9 genes, and (4) primers specific for the gRNA (Figure 5B). Specific amplicons of ∼1.6 and ∼1.4 kb for the 5ʹ (left) and 3ʹ (right) T-DNA-genomic DNA junctions, respectively, were obtained for all of the 23 randomly selected transformants. Accordingly, the internal region of CDC55 was amplified only from the WT strain. No amplicons for CAS9 or the gRNA were obtained. These results indicate that all transformants tested had full replacement of the CDC55 gene, and an absence of CAS9 and gRNA integration in the genome (Figure 5C). Furthermore, 64 additional random cdc55Δ mutant candidates were tested for sensitivity to hydroxyurea, which we found to be the most effective stressor for the cdc55Δ phenotype, and we found that 62 displayed impaired growth compared to WT (Figure S1). Therefore, molecular and phenotypic analyses revealed that out of 87 transformants analyzed, 85 were cdc55Δ mutants, resulting in a rate of successful HR of 97.7%.
Figure 5.
CRISPR/Cas9-mediated target mutagenesis of M. furfur CDC55. (A) Cotransformation of M. furfur mediated by two A. tumefaciens strains, one that delivers a T-DNA including the HDR template (i.e., cdc55Δ::NAT deletion construct in red), and another that includes the CAS9 cassette (in green) and the gene-specific gRNA (dark red). Also depicted are the vir plasmids present in A. tumefaciens cells that are necessary for T-DNA excision and transfer to the M. furfur nucleus where HR occurs. (B) Magnification of the HR event that occurs in the M. furfur nucleus. The top construct represents the T-DNA of the plasmid pGI41 bearing the cdc55Δ::NAT HDR template. The middle panel represents the native M. furfur CDC55 locus, the primers used to amplify the 5ʹ and 3ʹ regions for HR, the internal primer for the CDC55 gene, and the 20-nt target sequence (yellow). The gRNA guides Cas9 to the target site to generate a DSB that is repaired using the deletion allele as template, resulting in the targeted replacement of CDC55 with a NAT dominant marker (lower panel). Primers outside the region in which HR events occur are used in combination with primers for the NAT marker to identify cdc55Δ mutants. (C) Diagnostic PCR analyses of M. furfur WT and NAT-resistant transformants for the identification of cdc55Δ mutants. Each panel used the indicated combination of primers, whose position can be found in (B). PCR primers for CAS9 and gRNA are reported in Table S1. (D) Phenotypic analysis of M. furfur WT, insertional mutant 1A7, and two independent cdc55Δ mutants on mDixon (control), UV (300 µJ/cm2 × 100), hydroxyurea (50 mM), and benomyl (50 µM); 1.5 µl of 10-fold serial dilutions were spotted on the agar plates, incubated at 30° for 3–7 days, and then photographed. (E) Microscopic analysis of cell morphology of WT and a representative cdc55Δ mutant, after growth on mDixon and mDixon supplemented with hydroxyurea (50 mM). The black bar indicates 5 µm. CRISPR, clustered regularly interspaced short palindromic repeats; DSB, double-strand break; gRNA, guide RNA; HDR, homology-directed repair; HR, homologous recombination; mDixon; modified Dixon’s media; NAT, nourseothricin; T-DNA, transfer DNA; WT, wild-type.
In S. cerevisiae, CDC55 positively regulates mitotic entry at the G2/M phase transition, mitotic spindle assembly, and the morphogenesis checkpoint, and negatively regulates mitotic exit (Wang and Burke 1997; Bizzari and Marston 2011). Null cdc55Δ mutants display abnormally elongated buds, decreased growth rate, and increased sensitivity to γ rays and hydroxyurea (DNA-damaging agents that interfere with DNA replication), to benomyl and nocodazole (which interfere with microtubule polymerization), and cold-induced stress. Phenotypic characterization of two representative independent cdc55Δ mutants confirmed that they were sensitive to UV light (Figure 5D), corroborating the phenotype of the insertional mutant 1A7. Moreover, the cdc55Δ mutants had slower growth rates compared to the WT strain, and increased sensitivity to hydroxyurea and benomyl (Figure 5D). Due to the inability of M. furfur WT to grow at low temperatures, cold sensitivity could not be determined for M. furfur cdc55Δ. The cdc55Δ mutants were subjected to microscopy analysis both under normal and stress conditions, and when exposed to hydroxyurea, the cells displayed abnormal morphology and elongated buds, similar to S. cerevisiae cdc55Δ mutants (Figure 5E).
Generation of a pdr10Δ M. furfur mutant with CRISPR/Cas9
The other insertional mutant of interest was strain 7D9, which has a T-DNA insertion between the PDR10 and UBC6 genes, and exhibited FLC sensitivity. It was hypothesized that the phenotype of strain 7D9 was due to the T-DNA interfering with the function of PDR10, which is well known to mediate an antifungal drug response and therefore was chosen for targeted mutagenesis. The pdr10Δ::NAT gene disruption cassette was generated as previously described for CDC55, and the vector was named pGI42. For the gRNA, a primer with a specific PDR10 target between regions that overlap with the 5S rRNA promoter and the gRNA scaffold was added to the gRNA scaffold by PCR. The resulting amplicon was then cloned together with the p5S rRNA in the T-DNA of pGI40 digested with SpeI, as reported in Figure 4B; this vector was named pGI48.
Cotransformation of M. furfur CBS14141 was performed using A. tumefaciens strains bearing plasmids pGI42 and pGI48, as reported in Figure 5A. Sixty M. furfur NAT-resistant transformants were single-colony purified and streaked onto mDixon + FLC. Five (8.3%) transformants that displayed FLC sensitivity plus a randomly selected FLC-resistant control strain were subjected to molecular characterization (Figure 6B). PCR analysis using external screening primers designed beyond the region of DNA utilized to generate the pdr10Δ::NAT deletion allele produced two amplicons: a 6183-bp amplicon for the WT and the FLC-resistant strain, and a 3933-bp amplicon for the five transformants that displayed FLC sensitivity. For these five transformants, PCR carried out using the external primers with specific NAT primers generated amplicons of ∼1.1 and ∼1.3 kb on the 5ʹ and 3ʹ regions, respectively. PCR using primers internal to the PDR10 gene generated an amplicon of 486 bp only in the WT and the randomly selected NAT-R strain. These PCR results indicate full replacement of the PDR10 gene in the five transformants that displayed FLC sensitivity.
Mutants of pdr10Δ showed hypersensitivity to FLC, indicating that the phenotype of strain 7D9 was due to T-DNA interfering with the function of PDR10. Because ABC transporters are involved in pleiotropic drug resistance and cellular detoxification, the phenotypic response of pdr10Δ mutants was tested against other antifungal drugs of clinical relevance. Surprisingly, M. furfur pdr10Δ mutants showed only sensitivity to FLC and grew at the WT level on AmB, 5-flucytosine, caspofungin, FK506, and CsA both alone and in combination with the plasma membrane stressor lithium chloride (Figure 6C and data not shown), which we previously showed enhances antifungal activity of tacrolimus against M. furfur (Ianiri et al. 2017a). M. furfur pdr10Δ mutants did not display sensitivity to the DNA-damaging agents UV or hydroxyurea, and only displayed sensitivity to the fungicide benomyl (Figure 6C).
During BLAST searches, we noted that M. furfur has two adjacent ABC transporter-encoding genes that are orthologs of three adjacent ABC transporter-encoding genes in M. sympodialis (Figure 6D), a Malassezia species that is a model for genomic comparison within the genus because of the high quality of its genome assembly (Zhu et al. 2017). Interestingly, BLASTp of these ABC transporters against S. cerevisiae revealed a high level of similarity (i.e., E-value 0.0) with several ABC transporters, such as Pdr18, Pdr12, Pdr5, Pdr10, Pdr15, Aus1, and Pdr11. Reciprocal BLAST (BLASTp and tBLASTn) of these proteins against M. furfur and M. sympodialis identified only the aforementioned adjacent ABC transporters, which we named Mf (M. furfur) and Ms (M. sympodialis) PDR10_1, PDR10_2, and PDR10_3. The mutated gene in M. furfur corresponds to PDR10_1. Phylogenetic analysis revealed that ABC transporters of M. furfur and M. sympodialis cluster together in a maximum likelihood tree, and are related to the S. cerevisiae Pdr10 ABC transporter, which is the gene designation that we selected. This analysis suggests a common duplication event of the Malassezia PDR10 (green dot on Figure 6E), followed by another more recent duplication in M. sympodialis (blue dot on Figure 6E).
Discussion
AtMT is considered a “silver bullet” in functional genomics of fungi, and its main applications as well as the major discoveries that it has allowed have been recently reviewed (Idnurm et al. 2017a). Because A. tumefaciens can grow under a variety of conditions, the transformation method is versatile and has been successfully applied in a number of fungi, including those with particular nutrient requirements and that are recalcitrant to other transformation approaches, such as Malassezia (Ianiri et al. 2016; Celis et al. 2017).
In this report, we present the first application of forward genetics in M. furfur, a representative species of the fungemia-causing Malassezia group. The goal was to generate random insertional mutants, expose them to stress to isolate strains displaying sensitivity compared to the WT, and identify the corresponding T-DNA insertion sites to determine the function of the genes causing the phenotypes. Given the lack of knowledge on gene function in Malassezia, insertional mutants were assayed on a variety of conditions that are known to interfere with: (1) important cellular processes, such as those involved in plasma membrane and cell wall maintenance, growth under nutrient-limiting conditions, and protein folding; (2) response to environmental stresses, such as osmotic and nitrosative stresses, UV light, elevated temperature and pH, and heavy metals; and (3) response to the antifungal FLC, which is of clinical relevance.
This loss-of-function screen allowed the characterization of eight M. furfur insertional mutants (1A7, 2A8, 2F4, 6A10, 6C8, 7D9, 7F8, and 7H6) that had single T-DNA insertions as determined by Southern blot analysis (Figure 1), and that displayed sensitivity to one or more stress conditions (Figure 3 and Table 1). In four strains, the T-DNA inserted within the ORFs of genes, and in one strain it was found to lie within a 3ʹ-UTR (Figure 2, Figure 3, and Table 1), thus allowing us to define a direct link between genotype and phenotype with high confidence. Clear examples of this were M. furfur transformants 2A8 and 1A7. Strain 2A8 was selected because of its reduced growth on minimal medium (YNB), and it was found to have a T-DNA insertion in the ARG1 gene. Strain 1A7 was selected for its increased sensitivity to UV light, and was found to have a T-DNA insertion in the CDC55 gene. Two approaches were employed to validate the findings of the insertional mutagenesis screen. For strain 2A8, the addition of arginine was sufficient to rescue growth to a WT level (Figure 3A), while targeted M. furfur cdc55Δ mutants (Figure 5) confirmed the role of CDC55 in UV sensitivity for strain 1A7.
In three other mutants of interest, the T-DNA inserted between two adjacent genes and further experiments were conducted to identify the gene(s) responsible for the observed phenotype. A successful approach for strain 7H6 was gene expression analysis through RT-qPCR, which revealed downregulation of both genes flanking the T-DNA insertion (Figure 3B). Strain 7D9 was sensitive to FLC, and had a T-DNA insertion between the 3ʹ region of UBC6 and the 5ʹ region of PDR10; targeted mutagenesis confirmed that the observed phenotype was due to T-DNA insertion in the promoter region of PDR10 (Figure 6). Lastly, the RT-qPCR approach did not allow us to define which genes were responsible for the cadmium sulfate-sensitive phenotype of strain 2F4 (data not shown).
Despite the benefits of an AtMT random mutagenesis approach, analysis of the T-DNA insertion events also revealed limitations. For example, 11 of 19 M. furfur insertional mutants selected (∼58%) were not useful for gene function analysis due to either multiple or tandem T-DNA insertions, difficulties in identifying T-DNA insertion sites, or T-DNA insertion mediating chromosomal rearrangements (Figure 1 and Table 1). Although AtMT represents a powerful method for random mutagenesis, we and other authors have commonly found nonstandard T-DNA insertion events in the genome of both ascomycetous and basidiomycetous fungi [for more details see the following reviews and references within them (Michielse et al. 2005; Bourras et al. 2015; Idnurm et al. 2017a; Hooykaas et al. 2018)]. For example, in a recent study on systematic T-DNA insertion events in the red yeast Rhodosporidium toruloides, Coradetti et al. (2018) found that only 13% of mutants had regular T-DNA insertions and 21% of total insertions were useful to identify the mutated genes (Coradetti et al. 2018). Moreover, in classical forward genetic screens in which loss-of-function events are selected, it is common to isolate strains with chromosomal rearrangements that originated following the insertion of the T-DNA because these strains are generally less fit and display increased sensitivity to stress (Ianiri and Idnurm 2015). These undesirable events have been also described following AtMT of plants (Clark and Krysan 2010).
In forward genetics, the link between T-DNA insertion and phenotype is typically confirmed through: (1) sexual crosses and analysis of the phenotype in the recombinant progeny, (2) functional complementation, or (3) generation of an independent targeted mutation for the identified gene (Idnurm et al. 2017a). Because of the lack of a known sexual cycle in Malassezia and the difficulty of genetic manipulation for complementation studies, we aimed to generate M. furfur mutants specifically for the genes CDC55 and PDR10, to validate their involvement in UV and FLC resistance, respectively. Despite several rounds of transformation, we did not obtain any CDC55 or PDR10 mutants. Therefore, we developed a system based on CRISPR/Cas9 to increase HR and facilitate the generation of targeted mutants in M. furfur. Because AtMT is the only effective transformation technique for Malassezia, both a functional CAS9 cassette and a gRNA needed to be cloned within the T-DNA of a binary vector, together with a marker for selection. We also needed a specific gene replacement construct to serve as a template to repair the DSB for HR-mediated targeted mutagenesis. In fungi, CRISPR/Cas9 has been used with AtMT in two studies (Idnurm et al. 2017b; Kujoth et al. 2018). For CRISPR/Cas9 in Malassezia, we opted for a system that would be suitable for targeted gene replacement through HR based on cotransformation of M. furfur with two A. tumefaciens strains, one bearing the binary vector with the HDR gene deletion allele and the other with a binary vector engineered for the CRISPR/Cas9 system without a gene marker. The rationale for generating a marker-free binary vector was to: (1) have a CRISPR/Cas9 transient expression system with a reduced rate of CAS9 and/or gRNA ectopic integration in the host genome, similar to a system developed for C. albicans and C. neoformans (Min et al. 2016; Fan and Lin 2018); (2) allow further genetic manipulation of the NAT-generated mutant using the other available Malassezia-specific gene marker encoding resistance to NEO; and (3) reduce recombination within the actin promoter, and terminator regions of the NAT and NEO gene markers.
The choice of an appropriate promoter for gRNA expression has been a major challenge for the application of CRISPR/Cas9 technology in fungi. Common approaches include the use of a strong promoter recognized by RNA polymerase II, such as that of the ACT1 or GDP1 genes, coupled with a hammerhead ribozyme and/or hepatitis δ virus ribozyme for gRNA excision (Idnurm et al. 2017b; Kujoth et al. 2018); the use of RNA polymerase III promoters, such as the U6 promoters of small nuclear RNA used for C. neoformans (Wang et al. 2016; Fan and Lin 2018); or the promoters of the tRNA or rRNA, with or without ribozymes (Shi et al. 2017). In this study, we first tested a strategy based on the use of the 5S rRNA promoter of M. sympodialis (Figure 4B). While we were working on developing this system, Zheng and colleagues reported a similar approach in Aspergillus niger, demonstrating high efficiency of gene editing using both the 5S rRNA promoter alone or in combination with the hepatitis δ virus ribozyme (Zheng et al. 2018). In this study, we utilized the regulatory regions of the histone H3 gene of M. sympodialis to drive Cas9 expression (Figure 4A). During our first CRISPR/Cas9 attempt, we generated M. furfur cdc55Δ mutants with a HR rate of ∼98% (Figure 4C and Figure S1), similar to the high rates observed in other studies (Fan and Lin 2018; Zheng et al. 2018). Given these positive results, we did not test the use of ribozymes flanking the 5S rRNA promoter. Phenotypic analysis confirmed the involvement of CDC55 in UV resistance, and further assays revealed sensitivity of the M. furfur cdc55Δ mutant to benomyl and hydroxyurea (Figure 5D), which also induced abnormal bud morphology (Figure 5E). This indicates a conserved function of the cell division cycle protein Cdc55 in M. furfur and S. cerevisiae.
The CRISPR/Cas9 technology was then successfully employed for targeted mutagenesis of PDR10, although the rate of HR was lower for this gene, relative to CDC55. This could be due to several factors, such as shorter flanking regions of ∼800 bp used in the HDR pdr10Δ::NAT template, the length of the PDR10 gene (> 4 kb), the genomic location, or lower activity of the PDR10-specific gRNA. Analysis of M. furfur pdr10Δ mutants revealed an unexpected specificity of M. furfur PDR10 for resistance to the clinically relevant drug FLC and to the antifungal agent benomyl (Figure 6C). While there are multiple studies on the pleiotropic drug-resistance function of ABC transporters in nonpathogenic (S. cerevisiae) and pathogenic (C. albicans) yeasts (Sipos and Kuchler 2006; Coste et al. 2008; Paul and Moye-Rowley 2014), specific analysis of Pdr10 mutation in response to several drugs has yet to be performed, and therefore it is not possible to provide a detailed comparison analysis that supports conserved or divergent functions of PDR10 in M. furfur. Further bioinformatics analyses revealed that Pdr10 is the only ABC transporter present in the genome of two Malassezia species, and that it underwent ancestral and more recent gene duplication events (Figure 6, D and E). This suggests profound differences with other fungi, and further studies are needed to elucidate the evolution and specific roles of these Malassezia ABC transporters in resistance to chemicals, and their network of interactions.
Historically Malassezia research has been hampered by the fastidious nature, and growth and temperature requirements of species within this genus, and by their difficult identification and classification. Although understanding of Malassezia genetics is still limited, the T-DNA-mediated random insertional mutagenesis applied in this study coupled with a novel and efficient CRISPR/Cas9 system represent straightforward approaches to advance molecular genetics in this understudied organism. Furthermore, the available genome sequence and annotation of most Malassezia species, the recent introduction of animal models to study Malassezia interactions with the skin and the gastrointestinal tract (Limon et al. 2019; Sparber et al. 2019), and the development of molecular technologies (Ianiri et al. 2016, 2017a; Celis et al. 2017) represent key scientific advances to study the biology and pathophysiology of Malassezia.
Acknowledgments
We thank Tom Dawson for sharing unpublished genome and RNAseq information prior to publication, and Shelby Priest, Ci Fu, and Cecelia Wall for critical comments on the manuscript. This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) grant R01 AI50113-15 and NIH/NIAID R37 MERIT award AI39115-21 (to J.H.).
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8309342.
Communicating editor: A. Mitchell
Literature Cited
- Adli M., 2018. The CRISPR tool kit for genome editing and beyond. Nat. Commun. 9: 1911 10.1038/s41467-018-04252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amend A., Burgaud G., Cunliffe M., Edgcomb V. P., Ettinger C. L., et al. , 2019. Fungi in the marine environment: open questions and unsolved problems. mBio 10: e01189-18. 10.1128/mBio.01189-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas J. E., Abelson J. N., 1997. Prp43: an RNA helicase-like factor involved in spliceosome disassembly. Proc. Natl. Acad. Sci. USA 94: 11798–11802. 10.1073/pnas.94.22.11798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arras S. D., Chua S. M., Wizrah M. S., Faint J. A., Yap A. S., et al. , 2016. Targeted genome editing via CRISPR in the pathogen Cryptococcus neoformans. PLoS One 11: e0164322 10.1371/journal.pone.0164322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizzari F., Marston A. L., 2011. Cdc55 coordinates spindle assembly and chromosome disjunction during meiosis. J. Cell Biol. 193: 1213–1228. 10.1083/jcb.201103076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourras S., Rouxel T., Meyer M., 2015. Agrobacterium tumefaciens gene transfer: how a plant pathogen hacks the nuclei of plant and nonplant organisms. Phytopathology 105: 1288–1301. 10.1094/PHYTO-12-14-0380-RVW [DOI] [PubMed] [Google Scholar]
- Byrd A. L., Belkaid Y., Segre J. A., 2018. The human skin microbiome. Nat. Rev. Microbiol. 16: 143–155. 10.1038/nrmicro.2017.157 [DOI] [PubMed] [Google Scholar]
- Casal M., Paiva S., Andrade R. P., Gancedo C., Leão C., 1999. The lactate-proton symport of Saccharomyces cerevisiae is encoded by JEN1. J. Bacteriol. 181: 2620–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis A. M., Vos A. M., Triana S., Medina C. A., Escobar N., et al. , 2017. Highly efficient transformation system for Malassezia furfur and Malassezia pachydermatis using Agrobacterium tumefaciens-mediated transformation. J. Microbiol. Methods 134: 1–6. 10.1016/j.mimet.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Cho Y.-J., Park M., Jung W. H., 2019. Resequencing the genome of Malassezia restricta strain KCTC 27527. Microbiol. Resour. Announc. 8: e00213-19. 10.1128/MRA.00213-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K. A., Krysan P. J., 2010. Chromosomal translocations are a common phenomenon in Arabidopsis thaliana T-DNA insertion lines. Plant J. 64: 990–1001. 10.1111/j.1365-313X.2010.04386.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coradetti S. T., Pinel D., Geiselman G. M., Ito M., Mondo S. J., et al. , 2018. Functional genomics of lipid metabolism in the oleaginous yeast Rhodosporidium toruloides. eLife 7: e32110. 10.7554/eLife.32110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste A. T., Ramsdale M., Ischer F., Sanglard D., 2008. Divergent functions of three Candida albicans zinc-cluster transcription factors (CTA4, ASG1 and CTF1) complementing pleiotropic drug resistance in Saccharomyces cerevisiae. Microbiology 154: 1491–1501. 10.1099/mic.0.2007/016063-0 [DOI] [PubMed] [Google Scholar]
- Covert S. F., Kapoor P., Lee M.-H., Briley A., Nairn C. J., 2001. Agrobacterium tumefaciens-mediated transformation of Fusarium circinatum. Mycol. Res. 105: 259–264. 10.1017/S0953756201003872 [DOI] [Google Scholar]
- Cuomo C. A., Desjardins C. A., Bakowski M. A., Goldberg J., Ma A. T., et al. , 2012. Microsporidian genome analysis reveals evolutionary strategies for obligate intracellular growth. Genome Res. 22: 2478–2488. 10.1101/gr.142802.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. L., 2019. Malassezia: the forbidden kingdom opens. Cell Host Microbe 25: 345–347. 10.1016/j.chom.2019.02.010 [DOI] [PubMed] [Google Scholar]
- Donnarumma G., Perfetto B., Paoletti I., Oliviero G., Clavaud C., et al. , 2014. Analysis of the response of human keratinocytes to Malassezia globosa and restricta strains. Arch. Dermatol. Res. 306: 763–768. 10.1007/s00403-014-1479-1 [DOI] [PubMed] [Google Scholar]
- Fan Y., Lin X., 2018. Multiple applications of a transient CRISPR-Cas9 coupled with electroporation (TRACE) system in the Cryptococcus neoformans species complex. Genetics 208: 1357–1372. 10.1534/genetics.117.300656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley K., Oh J., Yang J., Conlan S., Deming C., et al. , 2013. Topographic diversity of fungal and bacterial communities in human skin. Nature 498: 367–370. 10.1038/nature12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitanis G., Magiatis P., Hantschke M., Bassukas I. D., Velegraki A., 2012. The Malassezia genus in skin and systemic diseases. Clin. Microbiol. Rev. 25: 106–141. 10.1128/CMR.00021-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G., Chu A. M., Ni L., Connelly C., Riles L., et al. , 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. 10.1038/nature00935 [DOI] [PubMed] [Google Scholar]
- Gioti A., Nystedt B., Li W., Xu J., Andersson A., et al. , 2013. Genomic insights into the atopic eczema-associated skin commensal yeast Malassezia sympodialis. mBio 4: e00572–e00512. 10.1128/mBio.00572-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz M., Bosshard P. P., Hoetzenecker W., Schmid-Grendelmeier P., 2015. The role of Malassezia spp. in atopic dermatitis. J. Clin. Med. 4: 1217–1228. 10.3390/jcm4061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy A. M., Zolnierowicz S., Stapleton A. E., Goebl M., Depaoli-Roach A. A., et al. , 1991. CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization, and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell. Biol. 11: 5767–5780. 10.1128/MCB.11.11.5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, C. S., 2001 Preparation of yeast DNA, pp. 13.11.1–13.11.14 in Current Protocols in Molecular Biology, edited by F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman et al. John Wiley & Sons, New York. [DOI] [PubMed] [Google Scholar]
- Hooykaas P. J. J., van Heusden G. P. H., Niu X., Reza Roushan M., Soltani J., et al. , 2018. Agrobacterium-mediated transformation of yeast and fungi, pp. 349–374 in Agrobacterium Biology: From Basic Science to Biotechnology, edited by Gelvin S. B. Springer International Publishing, Cham: 10.1007/82_2018_90 [DOI] [PubMed] [Google Scholar]
- Ianiri G., Idnurm A., 2015. Essential gene discovery in the basidiomycete Cryptococcus neoformans for antifungal drug target prioritization. mBio 6: e02334-14. 10.1128/mBio.02334-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiri G., Averette A. F., Kingsbury J. M., Heitman J., Idnurm A., 2016. Gene function analysis in the ubiquitous human commensal and pathogen Malassezia genus. mBio 7: e01853-16. 10.1128/mBio.01853-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiri G., Applen Clancey S., Lee S. C., Heitman J., 2017a. FKBP12-dependent inhibition of calcineurin mediates immunosuppressive antifungal drug action in Malassezia. MBio 8: e01752-17 (erratum: MBio 8: e02055-17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiri G., Boyce K. J., Idnurm A., 2017b. Isolation of conditional mutations in genes essential for viability of Cryptococcus neoformans. Curr. Genet. 63: 519–530. 10.1007/s00294-016-0659-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianiri G., Heitman J., Scheynius A., 2018. The skin commensal yeast Malassezia globosa thwarts bacterial biofilms to benefit the host. J. Invest. Dermatol. 138: 1026–1029. 10.1016/j.jid.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A., Reedy J. L., Nussbaum J. C., Heitman J., 2004. Cryptococcus neoformans virulence gene discovery through insertional mutagenesis. Eukaryot. Cell 3: 420–429. 10.1128/EC.3.2.420-429.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A., Bailey A. M., Cairns T. C., Elliott C. E., Foster G. D., et al. , 2017a. A silver bullet in a golden age of functional genomics: the impact of Agrobacterium-mediated transformation of fungi. Fungal Biol. Biotechnol. 4: 6 10.1186/s40694-017-0035-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A., Urquhart A. S., Vummadi D. R., Chang S., Van de Wouw A. P., et al. , 2017b. Spontaneous and CRISPR/Cas9-induced mutation of the osmosensor histidine kinase of the canola pathogen Leptosphaeria maculans. Fungal Biol. Biotechnol. 4: 12 10.1186/s40694-017-0043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi Y., Sugita T., Nishikawa A., 2006. Cytokine secretion profile of human keratinocytes exposed to Malassezia yeasts. FEMS Immunol. Med. Microbiol. 48: 400–409. 10.1111/j.1574-695X.2006.00163.x [DOI] [PubMed] [Google Scholar]
- Jauniaux J.-C., Urrestarazu L. A., Wiame J.-M., 1978. Arginine metabolism in Saccharomyces cerevisiae: subcellular localization of the enzymes. J. Bacteriol. 133: 1096–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., et al. , 2012. A Programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821. 10.1126/science.1225829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Cho Y. J., Park M., Choi Y., Hwang S. Y., et al. , 2018. Genomic tandem quadruplication is associated with ketoconazole resistance in Malassezia pachydermatis. J. Microbiol. Biotechnol. 28: 1937–1945. [DOI] [PubMed] [Google Scholar]
- Kujoth G. C., Sullivan T. D., Merkhofer R., Lee T.-J., Wang H., et al. , 2018. CRISPR/Cas9-mediated gene disruption reveals the importance of zinc metabolism for fitness of the dimorphic fungal pathogen Blastomyces dermatitidis. mBio 9: e00412-18. 10.1128/mBio.00412-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K., 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33: 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Goh B., Teh W. K., Jiang Z., Goh J. P. Z., et al. , 2018. Skin commensal Malassezia globosa secreted protease attenuates Staphylococcus aureus biofilm formation. J. Invest. Dermatol. 138: 1137–1145. 10.1016/j.jid.2017.11.034 [DOI] [PubMed] [Google Scholar]
- Limon J. J., Tang J., Li D., Wolf A. J., Michelsen K. S., et al. , 2019. Malassezia is associated with Crohn’s disease and exacerbates colitis in mouse models. Cell Host Microbe 25: 377–388.e6. 10.1016/j.chom.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch J. M., Palmer J. M., Vanderwolf K. J., Schmidt K. Z., Verant M. L., et al. , 2018. Malassezia vespertilionis sp. nov.: a new cold-tolerant species of yeast isolated from bats. Persoonia 41: 56–70. 10.3767/persoonia.2018.41.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Chen Z., Huang da W., Kutty G., Ishihara M., et al. , 2016. Genome analysis of three Pneumocystis species reveals adaptation mechanisms to life exclusively in mammalian hosts. Nat. Commun. 7: 10740 10.1038/ncomms10740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhaupt G., Stucka R., Pilz U., Schwarzlose C., Feldmann H., 1989. Characterization of the prephenate dehydrogenase-encoding gene, TYR1, from Saccharomyces cerevisiae. Gene 85: 303–311. 10.1016/0378-1119(89)90422-8 [DOI] [PubMed] [Google Scholar]
- McKenzie R. A., Connole M. D., McGinnis M. R., Lepelaar R., 1984. Subcutaneous phaeohyphomycosis caused by Moniliella suaveolens in two cats. Vet. Pathol. 21: 582–586. 10.1177/030098588402100606 [DOI] [PubMed] [Google Scholar]
- Michielse C. B., Hooykaas P. J., van den Hondel C. A., Ram A. F., 2005. Agrobacterium-mediated transformation as a tool for functional genomics in fungi. Curr. Genet. 48: 1–17. 10.1007/s00294-005-0578-0 [DOI] [PubMed] [Google Scholar]
- Min K., Ichikawa Y., Woolford C. A., Mitchell A. P., 2016. Candida albicans gene deletion with a transient CRISPR-Cas9 system. mSphere 1: e00130-16. 10.1128/mSphere.00130-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand S. C., Bertignac M., Iltis A., Kolder I. C. R. M., Pirovano W., et al. , 2019. Complete genome sequence of Malassezia restricta CBS 7877, an opportunist pathogen involved in dandruff and seborrheic dermatitis. Microbiol. Resour. Announc. 8: e01543-18. 10.1128/MRA.01543-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva S., Devaux F., Barbosa S., Jacq C., Casal M., 2004. Ady2p is essential for the acetate permease activity in the yeast Saccharomyces cerevisiae. Yeast 21: 201–210. 10.1002/yea.1056 [DOI] [PubMed] [Google Scholar]
- Park M., Cho Y. J., Lee Y. W., Jung W. H., 2017. Whole genome sequencing analysis of the cutaneous pathogenic yeast Malassezia restricta and identification of the major lipase expressed on the scalp of patients with dandruff. Mycoses 60: 188–197. 10.1111/myc.12586 [DOI] [PubMed] [Google Scholar]
- Paul S., Moye-Rowley W. S., 2014. Multidrug resistance in fungi: regulation of transporter-encoding gene expression. Front. Physiol. 5: 143 10.3389/fphys.2014.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar S., Murray D., Khalife W., Robinson-Dunn B., McGinnis M., 2002. Human infection caused by Moniliella suaveolens. Clin. Microbiol. Newsl. 24: 53–55. 10.1016/S0196-4399(02)80011-3 [DOI] [Google Scholar]
- Pitkin J. W., Panaccione D. G., Walton J. D., 1996. A putative cyclic peptide efflux pump encoded by the TOXA gene of the plant-pathogenic fungus Cochliobolus carbonum. Microbiology 142: 1557–1565. 10.1099/13500872-142-6-1557 [DOI] [PubMed] [Google Scholar]
- Rabitsch K. P., Tóth A., Gálová M., Schleiffer A., Schaffner G., et al. , 2001. A screen for genes required for meiosis and spore formation based on whole-genome expression. Curr. Biol. 11: 1001–1009. 10.1016/S0960-9822(01)00274-3 [DOI] [PubMed] [Google Scholar]
- Rio D. C., Ares M., Jr., Hannon G. J., Nilsen T. W., 2010. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010: pdb.prot5439 10.1101/pdb.prot5439 [DOI] [PubMed] [Google Scholar]
- Sanz P., Ludin K., Carlson M., 2000. Sip5 interacts with both the Reg1/Glc7 protein phosphatase and the Snf1 protein kinase of Saccharomyces cerevisiae. Genetics 154: 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T.-Q., Liu G.-N., Ji R.-Y., Shi K., Song P., et al. , 2017. CRISPR/Cas9-based genome editing of the filamentous fungi: the state of the art. Appl. Microbiol. Biotechnol. 101: 7435–7443. 10.1007/s00253-017-8497-9 [DOI] [PubMed] [Google Scholar]
- Sipos G., Kuchler K., 2006. Fungal ATP-binding cassette (ABC) transporters in drug resistance & detoxification. Curr. Drug Targets 7: 471–481. 10.2174/138945006776359403 [DOI] [PubMed] [Google Scholar]
- Sparber, F., and S. LeibundGut-Landmann, 2017 Host responses to Malassezia spp. in the mammalian skin. Front. Immunol. 8: 1614 10.3389/fimmu.2017.01614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparber F., De Gregorio C., Steckholzer S., Ferreira F. M., Dolowschiak T., et al. , 2019. The skin commensal yeast Malassezia triggers a type 17 response that coordinates anti-fungal immunity and exacerbates skin inflammation. Cell Host Microbe 25: 389–403.e6. 10.1016/j.chom.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Triana S., González A., Ohm R. A., Wösten H. A. B., de Cock H., et al. , 2015. Draft genome sequence of the animal and human pathogen Malassezia pachydermatis strain CBS 1879. Genome Announc. 3: e01197-15. 10.1128/genomeA.01197-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J., Urban J., Volkwein C., Sommer T., 2001. Sec61p-independent degradation of the tail-anchored ER membrane protein Ubc6p. EMBO J. 20: 3124–3131. 10.1093/emboj/20.12.3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. M., Theelen B., Groenewald M., Bai F. Y., Boekhout T., 2014. Moniliellomycetes and Malasseziomycetes, two new classes in Ustilaginomycotina. Persoonia 33: 41–47. 10.3767/003158514X682313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q. M., Begerow D., Groenewald M., Liu X. Z., Theelen B., et al. , 2015. Multigene phylogeny and taxonomic revision of yeasts and related fungi in the Ustilaginomycotina. Stud. Mycol. 81: 55–83. 10.1016/j.simyco.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Burke D. J., 1997. Cdc55p, the B-type regulatory subunit of protein phosphatase 2A, has multiple functions in mitosis and is required for the kinetochore/spindle checkpoint in Saccharomyces cerevisiae. Mol. Cell. Biol. 17: 620–626. 10.1128/MCB.17.2.620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wei D., Zhu X., Pan J., Zhang P., et al. , 2016. A ‘suicide’ CRISPR-Cas9 system to promote gene deletion and restoration by electroporation in Cryptococcus neoformans. Sci. Rep. 6: 31145 10.1038/srep31145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Kano R., Sato H., Nakamura Y., Hasegawa A., 2001. The effects of Malassezia yeasts on cytokine production by human keratinocytes. J. Invest. Dermatol. 116: 769–773. 10.1046/j.1523-1747.2001.01321.x [DOI] [PubMed] [Google Scholar]
- Wrighton K. H., 2019. Malassezia restricta plays CARDs in the gut. Nat. Rev. Microbiol. 17: 266–267. 10.1038/s41579-019-0188-3 [DOI] [PubMed] [Google Scholar]
- Wu G., Zhao H., Li C., Rajapakse M. P., Wong W. C., et al. , 2015. Genus-wide comparative genomics of Malassezia delineates its phylogeny, physiology, and niche adaptation on human skin. PLoS Genet. 11: e1005614 10.1371/journal.pgen.1005614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Saunders C. W., Hu P., Grant R. A., Boekhout T., et al. , 2007. Dandruff-associated Malassezia genomes reveal convergent and divergent virulence traits shared with plant and human fungal pathogens. Proc. Natl. Acad. Sci. USA 104: 18730–18735. 10.1073/pnas.0706756104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Zheng P., Zhang K., Cairns T. C., Meyer V., et al. , 2018. 5S rRNA promoter for guide RNA expression enabled highly efficient CRISPR/Cas9 genome editing in Aspergillus niger. ACS Synth. Biol. DOI: 10.1021/acssynbio.7b00456. 10.1021/acssynbio.7b00456 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Engstrom P. G., Tellgren-Roth C., Baudo C. D., Kennell J. C., et al. , 2017. Proteogenomics produces comprehensive and highly accurate protein-coding gene annotation in a complete genome assembly of Malassezia sympodialis. Nucleic Acids Res. 45: 2629–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8309342.