Abstract
Recombination between divergent DNA sequences is actively prevented by heteroduplex rejection mechanisms. In baker’s yeast, such antirecombination mechanisms can be initiated by the recognition of DNA mismatches in heteroduplex DNA by MSH proteins, followed by recruitment of the Sgs1-Top3-Rmi1 helicase–topoisomerase complex to unwind the recombination intermediate. We previously showed that the repair/rejection decision during single-strand annealing recombination is temporally regulated by MSH (MutS homolog) protein levels and by factors that excise nonhomologous single-stranded tails. These observations, coupled with recent studies indicating that mismatch repair (MMR) factors interact with components of the histone chaperone machinery, encouraged us to explore roles for epigenetic factors and chromatin conformation in regulating the decision to reject vs. repair recombination between divergent DNA substrates. This work involved the use of an inverted repeat recombination assay thought to measure sister chromatid repair during DNA replication. Our observations are consistent with the histone chaperones CAF-1 and Rtt106, and the histone deacetylase Sir2, acting to suppress heteroduplex rejection and the Rpd3, Hst3, and Hst4 deacetylases acting to promote heteroduplex rejection. These observations, and double-mutant analysis, have led to a model in which nucleosomes located at DNA lesions stabilize recombination intermediates and compete with MMR factors that mediate heteroduplex rejection.
Keywords: heteroduplex rejection, homologous recombination, mismatch repair, histone chaperones, chromatin modifiers
HOMOLOGOUS recombination (HR) is a conservative DNA repair pathway that is critical for repairing DNA double-strand breaks (DSBs). This process is regulated to prevent recombination between divergent DNA sequences [reviewed in George and Alani (2012)]. Such regulation, which can prevent deleterious chromosomal rearrangements, is initiated through the recognition of mismatches in heteroduplex DNA that form during strand invasion steps involving single-stranded DNA (ssDNA) from a broken chromosome and a divergent duplex donor. In the yeast Saccharomyces cerevisiae, MutS homolog (MSH) Msh2-Msh6 mismatch repair (MMR) complexes recognize mismatches in heteroduplex DNA and recruit the RecQ family helicase–topoisomerase complex Sgs1-Top3-Rmi1 to unwind the recombination intermediate in a process known as heteroduplex rejection (Datta et al. 1996; Chen and Jinks-Robertson 1999; Nicholson et al. 2000; Myung et al. 2001; Spell and Jinks-Robertson 2004; Sugawara et al. 2004; Goldfarb and Alani 2005; Chakraborty et al. 2016; Hum and Jinks-Robertson 2019). If rejection does not occur, MSH complexes initiate repair of the mismatches in heteroduplex DNA after/while the break is repaired. We refer to this regulation as the repair/rejection decision.
An important aspect of the repair/rejection decision is that tolerating multiple mismatches in heteroduplex DNA formed from divergent substrates can lead to chromosomal rearrangements, but a highly efficient rejection system can result in DSBs that are not repaired. Various factors are likely to influence this decision [reviewed in Chakraborty and Alani (2016)]. For example, we found that MSH protein levels influence the repair/rejection decision during single-strand annealing (SSA) (Chakraborty et al. 2016). During SSA, HR is initiated by a DSB located between two closely spaced repeat sequences. Resection of the DSB promotes annealing of homologous sequences, followed by the clipping of 3′ nonhomologous tails that must be excised before repair steps are completed. During SSA involving divergent repeat sequences, modest overexpression of Msh6 results in a significant increase in heteroduplex rejection due to a decrease in the availability of Msh2-Msh3 to clip the 3′ tails. Thus 3′ tail clipping during SSA serves as a regulatory step, with rejection favored prior to 3′ tail removal. Consistent with these findings, Anand et al. (2017) showed in a break-induced repair recombination system that 3′ nonhomologous tails promote heteroduplex rejection, and that the absence of such tails prevents it. These observations indicate cross talk between the rejection machinery and the local environment that likely occurs prior to steps in HR that involve repair DNA synthesis.
Recent studies have indicated that chromatin structure can influence HR outcomes. Several nucleosome remodeling complexes have been shown to be recruited to DSBs in steps thought to increase chromatin accessibility, and allow strand resection and presynaptic filament formation [reviewed in Hauer and Gasser (2017)]. Such chromatin remodelers were shown to promote chromatin mobility during DSB formation and the increased mobility correlated to more efficient repair by HR (Dion et al. 2012; Miné-Hattab and Rothstein 2012; Neumann et al. 2012; Hauer et al. 2017). Also, histone chaperones, which act in DNA replication-dependent nucleosome assembly, have been implicated in DNA repair. These chaperones include CAF-1, Asf1, and Rtt106, which are all involved in DNA replication-dependent nucleosome assembly (Tyler et al. 1999; Tagami et al. 2004; Huang et al. 2005). For example, nucleosome assembly mediated by CAF-1 during DNA repair is coupled with DNA synthesis and requires an interaction with PCNA (Gaillard et al. 1996; Tyler et al. 1999; Linger and Tyler 2005; Polo et al. 2006; Pietrobon et al. 2014), and CAF-1 and Asf1 play roles in restoring chromatin after DNA repair in budding yeast at repair sites by turning off the DNA damage checkpoint (Chen et al. 2008; Kim and Haber 2009; Diao et al. 2017).
Histone modifications have also been shown to affect genome stability. For example, deacetylation of an acetylated lysine residue at amino acid 56 in histone 3 (H3K56) by Hst3 and Hst4 is required for the suppression of mutations and gross chromosomal rearrangements, and acetylation of H3K56 by Rtt109 is also required for the suppression of mutations (Kadyrova et al. 2013). In contrast, histone deacetylases such as Rpd3L and Hda1 promote trinucleotide repeat expansions associated with various neurodegenerative diseases (Debacker et al. 2012). Thus, histone acetylation and deacetylation are likely to play important roles in various stages of HR. In support of roles for acetylases and deacetylases in HR, Tamburini and Tyler (2005) showed that histone acetyltransferases such as Gcn5 and Esa1 are recruited to a HO (homothallism) endonuclease-induced DSB in S. cerevisiae, followed at a later stage by recruitment of histone deacetylases such as Sir2, Hst1, and Rpd3. Furthermore, they showed that mutating acetylable lysine residues in histone subunit 4, or deleting GCN5 or RPD3, caused inviability in response to HO endonuclease-induced lesions repaired primarily by HR. These observations suggest that, after DSB formation, histone acetylases participate in nucleosome removal in the vicinity of the break and “open up” the chromatin structure, increasing the accessibility of the underlying DNA to repair factors. Once DNA repair is underway, histone deacetylases likely modify the chromatin to a “closed” conformation that serves as a commitment step to complete the repair process and restore chromatin to its original state.
The NAD-dependent histone deacetylase Sir2, known for its role in transcriptional silencing and heterochromatin formation, also plays direct roles in forming a repressive local chromatin environment around most euchromatic replication origins (Gartenberg and Smith 2016; Hoggard et al. 2018). Sir2 is also involved in DNA repair pathways such as nonhomologous end joining and nucleotide excision repair (Tsukamoto et al. 1997; Boulton and Jackson 1998; Guintini et al. 2017). sir2 mutants are hypersensitive to DNA-damaging agents, and a number of studies have reported that in response to DNA DSBs, a significant fraction of the histone-bound SIR complex was displaced from subtelomeric regions and relocated to sites of DSBs in a DNA checkpoint-dependent manner, suggesting that the recruitment of SIR complexes reflects the assembly of a repressed chromatin state following DNA repair (Martin et al. 1999; McAinsh et al. 1999; Mills et al. 1999).
A number of studies have shown that nucleosome assembly on newly synthesized DNA during replication and MMR are mutually inhibitory processes. MMR during DNA replication is thought to be restricted to the short time window between the formation of the mismatch and the chaperone-assisted assembly of nucleosomes on the newly replicated DNA (Li et al. 2009; Kadyrova et al. 2011; Schöpf et al. 2012; Rodriges Blanko et al. 2016). The human MSH2-MSH6 complex has been shown to interact with CAF-1 in vitro (Schöpf et al. 2012). Additionally, human MSH2-MSH6 inhibits CAF-1 and ASF1A-dependent packaging of a DNA mismatch into a nucleosome, and deposition of the (H3-H4)2 tetramers on DNA protects the discontinuous daughter strand from unnecessary degradation during MMR (Rodriges Blanko et al. 2016). Moreover, Pietrobon et al. (2014) have shown in fission yeast that CAF-1 stabilizes D-loops in an HR pathway, by counteracting their disassembly mediated by the RecQ family helicase Rqh1 when cells replicate a damaged template.
How is the balance between heteroduplex rejection and repair through HR maintained? We tested roles for histone acetylases, deacetylases, and chaperones in regulating the repair/rejection decision in baker’s yeast. To our knowledge, this is the first study to examine roles for epigenetic factors and chromatin modifiers in modulating heteroduplex rejection. We show that histone chaperones CAF-1 and Rtt106 suppress heteroduplex rejection in steps dependent on mismatch recognition. Additionally, the histone deacetylase Sir2 appears to act in a common pathway with CAF-1 and/or Rtt106 to suppress rejection. However, other factors involved in nucleosome assembly during DNA replication, such as Asf1 and Rtt109, do not affect rejection efficiency. Similarly, histone acetylases such as Gcn5, which assemble early at recombination sites, and other histone deacetylases such as Hst1 do not affect the efficiency of rejection. However, mutants lacking the Rpd3, or Hst3 and Hst4 histone deacetylases show defects in rejection. Taken together, these results are consistent with the idea that nucleosomes at DNA lesions, which are likely to be localized independently of DNA synthesis, stabilize recombination intermediates and thus prevent access/unwinding by antirecombination factors.
Materials and Methods
Yeast strains and plasmids
Yeast strains used in this study are listed in Table 1, and were constructed and grown using standard techniques (Rose et al. 1990). Gene disruptions used to make the strains are described in Table 1 and Table 2 (geneXΔ::KANMX), and were obtained by PCR amplification (details provided upon request) of chromosomal DNA derived from the yeast knockout collection (Brachmann et al. 1998). PCR products, linear DNA fragments obtained from pEAI98 (msh2Δ::hisG-URA3-hisG) and pEAA633 (pol30-8::KANMX, see below), and 2μ vectors (see below) were introduced into yeast strains using standard transformation procedures (Gietz and Schiestl 1991). The presence of mutant alleles was confirmed by PCR analysis of chromosomal DNA, and in some cases DNA sequencing of the amplified DNA fragments.
Table 1. Strains used in this study.
| Strain | Description |
|---|---|
| SJR328 | MATα, ade2-101, his3Δ200, ura3-Nhe, lys2ΔRV::hisG, leu2-R |
| SJR769 | SJR328, cβ2/cβ2:LEU2, homologous 350-bp substrates |
| GCY615 | SJR328, cβ2/cβ2-ns:LEU2, predicted to form four, base–base mismatches in the 350-bp cβ2 substrates |
| GCY559 | SJR328, cβ2/cβ2-4L:LEU2, predicted to form four 4-nt loops in the 350-bp cβ2 substrates |
| EAY1605,1606 | SJR769, msh2Δ::hisG-URA3-hisG |
| EAY1609,1610 | GCY615, msh2Δ::hisG-URA3-hisG |
| EAY3853-3856 | SJR769, hst3Δ::KANMX |
| EAY3857-3860 | GCY615, hst3Δ::KANMX |
| EAY3865-3868 | SJR769, hst4Δ::KANMX |
| EAY3869-3872 | GCY615, hst4Δ::KANMX |
| EAY3877-3880 | SJR769, hst3Δ::KANMX hst4Δ::NATMX |
| EAY3881-3884 | GCY615, hst3Δ::KANMX hst4Δ::NATMX |
| EAY3964-3967 | SJR769, hst1Δ::KANMX |
| EAY3968-3971 | GCY615, hst1Δ::KANMX |
| EAY3841-3844 | SJR769, sir2Δ::KANMX |
| EAY3845-3848 | GCY615, sir2Δ::KANMX |
| EAY3849-3852 | GCY559, sir2Δ::KANMX |
| EAY4002-4005 | SJR769, rpd3Δ::KANMX |
| EAY4006-4009 | GCY615, rpd3Δ::KANMX |
| EAY4047-4050 | SJR769, gcn5Δ::KANMX |
| EAY4051-4054 | GCY615, gcn5Δ::KANMX |
| EAY3889-3892 | SJR769, cac1Δ::KANMX |
| EAY3893-3896 | GCY615, cac1Δ::KANMX |
| EAY3897-3900 | GCY559, cac1Δ::KANMX |
| EAY3972,3974,3975 | SJR769, rtt106Δ::KANMX |
| EAY3976-3978 | GCY615, rtt106Δ::KANMX |
| EAY3901-3904 | SJR769, asf1Δ::KANMX |
| EAY3905-3908 | GCY615, asf1Δ::KANMX |
| EAY3909-3912 | GCY559, asf1Δ::KANMX |
| EAY4127-4129 | SJR769, pol30-8::KANMX |
| EAY4130-4132 | GCY615, pol30-8::KANMX |
| EAY3925, 3926 | SJR769, cac1Δ::KANMX asf1Δ::NATMX |
| EAY3929, 3930 | GCY615, cac1Δ::KANMX asf1Δ::NATMX |
| EAY3933, 3934 | GCY559, cac1Δ::KANMX asf1Δ::NATMX |
| EAY4018-4021 | SJR769, rtt106Δ::KANMX asf1Δ::NATMX |
| EAY4022-4025 | GCY615, rtt106Δ::KANMX asf1Δ::NATMX |
| EAY3913-3916 | SJR769, rtt109Δ::KANMX |
| EAY3917-3920 | GCY615, rtt109Δ::KANMX |
| EAY4010-4013 | SJR769, rtt106Δ::KANMX cac1Δ::NATMX |
| EAY4014-4017 | GCY615, rtt106Δ::KANMX cac1Δ::NATMX |
| EAY4027-4029 | SJR769, sir2Δ::KANMX cac1Δ::NATMX |
| EAY4030-4033 | GCY615, sir2Δ::KANMX cac1Δ::NATMX |
| EAY4034-4037 | SJR769, rtt106Δ::KANMX sir2Δ::NATMX |
| EAY4038-4041 | GCY615, rtt106Δ::KANMX sir2Δ::NATMX |
| EAY4042-4044 | SJR769, rtt106Δ::KANMX cac1Δ::NATMX sir2Δ::HYGMX |
| EAY4045,4046 | GCY615, rtt106Δ::KANMX cac1Δ::NATMX sir2Δ::HYGMX |
| EAY4069-4072 | SJR769, cac1Δ::KANMX msh2Δ::hisG-URA3-hisG |
| EAY4073-4076 | GCY615, cac1Δ::KANMX msh2Δ::hisG-URA3-hisG |
| EAY4077-4080 | SJR769, rtt106Δ::KANMX msh2Δ::hisG-URA3-hisG |
| EAY4081-4084 | GCY615, rtt106Δ::KANMX msh2Δ::hisG-URA3-hisG |
| EAY4216-4218 | SJR769, sir2Δ::KANMX msh2Δ::hisG-URA3-hisG |
| EAY4220,4222,4223 | GCY615, sir2Δ::KANMX msh2Δ::hisG-URA3-hisG |
SJR328, SJR769, GCY615, and GCY559 were obtained from Sue Jinks-Robertson and are described in Nicholson et al. (2000).
Table 2. Recombination rates as measured in the inverted repeat reporter assay.
| Genotype | Cβ2/Cβ2 His+ homologous recombination rate (×10−6) | Cβ2/Cβ2-ns His+ divergent recombination rate (×10−6) | Cβ2/Cβ2 rate/Cβ2/Cβ2-ns ratea |
|---|---|---|---|
| Wild-type | 0.52 (0.48–0.56)b | 0.027 (0.024–0.029)b | 20 (17–22)b |
| msh2Δ | 0.92 (0.70–1.15) | 2.55 (2.31–2.80) | 0.36 (0.28–0.47) |
| cac1Δ | 1.19 (1.00–1.38) | 0.030 (0.021–0.040) | 40 (28–57) |
| rtt106Δ | 1.87 (1.55–2.20) | 0.028 (0.018–0.038) | 68 (46–101) |
| asf1Δ | 3.96 (3.30–4.61) | 0.27 (0.22–0.32) | 15 (11–19) |
| rtt109Δ | 3.59 (2.93–4.24) | 0.22 (0.16–0.27) | 16 (12–23) |
| cac1Δ asf1Δ | 0.60 (0.32–0.89) | 0.093 (0.059–0.13) | 6.4 (3.5–12) |
| rtt106Δ asf1Δ | 1.98 (1.65–2.31) | 0.14 (0.097–0.18) | 14 (10–20) |
| cac1Δ rtt106Δ | 2.33 (1.85–2.80) | 0.018 (0.0095–0.027) | 134 (78–229) |
| pol30-8 | 0.74 (0.61–0.87) | 0.026 (0.020–0.033) | 28 (21–39) |
| hst3Δ | 1.67 (1.44–1.89) | 0.090 (0.066–0.11) | 19 (14–25) |
| hst4Δ | 5.31 (4.29–6.34) | 0.36 (0.29–0.43) | 15 (11–19) |
| hst3Δ hst4Δ | 11.3 (8.66–14.8) | 4.53 (3.79–5.42) | 2.5 (1.8–3.5) |
| hst1Δ | 1.34 (1.13–1.55) | 0.047 (0.033–0.063) | 29 (20–41) |
| sir2Δ | 2.12 (1.93–2.31) | 0.050 (0.040–0.059) | 43 (35–53) |
| rpd3Δ | 0.25 (0.22–0.29) | 0.026 (0.020–0.033) | 9.7 (7.4–13) |
| gcn5Δ | 0.98 (0.86–1.10) | 0.068 (0.054–0.082) | 15 (11–19) |
| sir2Δ cac1Δ | 4.44 (3.77–5.11) | 0.030 (0.015–0.046) | 151 (87–264) |
| sir2Δ rtt106Δ | 5.40 (4.36–6.43) | 0.12 (0.089–0.16) | 44 (31–62) |
| sir2Δ cac1Δ rtt106Δ | 6.32 (5.26–7.37) | 0.18 (0.13–0.24) | 35 (25–50) |
| msh2Δ cac1Δ | 3.18 (2.58–3.78) | 5.61 (5.01–6.20) | 0.57 (0.45–0.70) |
| msh2Δ rtt106Δ | 4.07 (3.49–4.65) | 3.35 (2.98–3.67) | 1.2 (1.0–1.4) |
| msh2Δ sir2Δ | 4.87 (4.23–5.50) | 3.58 (3.05–4.11) | 1.4 (1.1–1.7) |
| Wild-type-2μ empty vectorc | 0.74 (0.63–0.85) | 0.034 (0.022–0.048) | 22 (15–33) |
| Wild-type-2μ MSH2-MSH6c | 5.69 (4.82–6.54) | 0.061 (0.043–0.080) | 94 (67–132) |
| sir2Δ-2μ empty vector | 4.35 (3.58–5.13) | 0.067 (0.045–0.089) | 66 (45–96) |
| sir2Δ-2μ MSH2-MSH6 | 4.55 (3.52–5.59) | 0.040 (0.027–0.053) | 116 (77–173) |
| rtt106Δ-2μ empty vector | 2.01 (1.59–2.43) | 0.044 (0.028–0.061) | 46 (30–71) |
| rtt106Δ-2μ MSH2-MSH6 | 2.49 (2.01–2.97) | 0.15 (0.10–0.20) | 17 (12–25) |
| Cβ2/Cβ2-4L rate (×10−6) | Cβ2/Cβ2 rate/Cβ2/Cβ2-4L rate | ||
| Wild-type | 0.057 (0.042–0.072) | 9.2 (7.0–12.1) | |
| cac1Δ | 0.070 (0.048–0.093) | 17 (12–25) | |
| asf1Δ | 0.61 (0.48–0.74) | 6.5 (4.9–8.6) | |
| cac1Δ asf1Δ | 0.25 (0.17–0.32) | 2.4 (1.4–4.3) | |
| sir2Δ | 0.15 (0.12–0.18) | 14 (11–17) |
Recombination rates were calculated as described in the Materials and Methods. The genotypes of the parental strains are shown in Table 1. Cβ2/Cβ2, homologous substrate; Cβ2/Cβ2-4L = 4-nt loop mismatch substrate; Cβ2/Cβ2-ns, base–base mismatch substrate.
Homologous rate (Cβ2-Cβ2)/divergent rate for strains with the same genotype or overexpression plasmid.
Numbers in parentheses indicate 95% credible intervals.
Data obtained from Chakraborty et al. (2018).
The mutations comprising the pol30-8 allele (R61A, D63A) were introduced into pEAA578 (POL30::KANMX) by Q5 site-directed mutagenesis (New England Biolabs, Beverly, MA) to create the single-step integrating vector pEAA633 (pol30-8::KANMX). Plasmids pRS426 (2μ, URA3) (Christianson et al. 1992) and pEAM272 (MSH2, MSH6, 2μ, URA3) ( Chakraborty et al. 2018) were used in the MSH overexpression experiments presented in Table 2.
Inverted repeat recombination assay
Strains used to measure HR and divergent recombination are listed in Table 1. Strains lacking plasmids were initially struck onto synthetic complete plates and those containing 2μ plasmids were struck onto minimal dropout media plates (Rose et al. 1990). A total of 10–117 single colonies per strain were then inoculated into 5 ml of synthetic complete or minimal dropout medium containing 4% galactose and 2% glycerol, and grown to saturation for ∼2 days at 30°. Appropriate dilutions of cells were plated onto minimal media (2% galactose and 2% glycerol) plates lacking histidine and the amino acid required to maintain the 2μ plasmid (selective), and onto minimal media (2% glucose) plates lacking the amino acid required to maintain the 2μ plasmid (permissive). Plates were incubated for 4 days at 30° (5 days for experiments involving rpd3Δ) and then scored for frequency of His+ colonies. Rates of HR and divergent recombination were calculated as described below.
Analysis of mutation rates
Asteris and Sarkar (1996) showed that Bayesian estimators of mutation rates from fluctuation experiments are more accurate than even the maximum likelihood estimator. We used an extension of their approach to calculate the posterior distribution of the mutation rate per cell division, µ = m/Nt, where m is the estimated mean number of mutations and Nt is the total number of cells in the culture. This permitted credible intervals of the mutation rate ratios to be accurately calculated. Posterior distributions were calculated using the Ma–Sandri–Sarkar likelihood function (Ma et al. 1992) for the Lea–Coulson model (Lea and Coulson 1949) with a noninformative (constant) prior over log µ. The results were insensitive to the choice of prior: Changing to a constant prior over µ or to a 1/µ2 prior changed the estimated mutation rate ratios by < 10% of the distance to the credible interval boundaries (Root-Mean-Square (RMS) deviation = 4%).
Depending on the mutation rates estimated in pilot experiments, fractions f, ranging between 0.005 and 0.4 of the cultures, were plated on nonpermissive medium and counted. The total number of cells in the culture, Nt, was measured in parallel in each case by plating a small aliquot on permissive medium. The likelihood function was calculated independently for each culture using the number of His+ colonies, Nt, and f. The statistical error introduced by the partial plating with fraction f was included using the method of Zheng (2008); the statistical error in determining each Nt was included assuming Poisson sampling.
The variation of mutation rates between transformants of the same genotype, except for hst3Δ hst4Δ, were modest (Supplemental Material, Table S1). Therefore, the data for these genotypes were pooled, and the mutation rates (Figure 3 and Table 2) were computed from the overall posterior distributions and using a quadratic loss function. The 95% credible intervals were computed using the highest posterior density. Figure 2 displays the geometric means of the HR rate (H)/divergent recombination rate (D) ratios computed using the marginal posterior distribution of , which was computed by integration over the joint distribution (Gelman et al. 2014). The corresponding 95% credible intervals for were computed from the highest posterior density over The posterior distributions were very close to normal; the Hellinger distances between them and the best-fit normal distributions were 0.04; therefore, integrals were approximated using this assumption.
Figure 3.
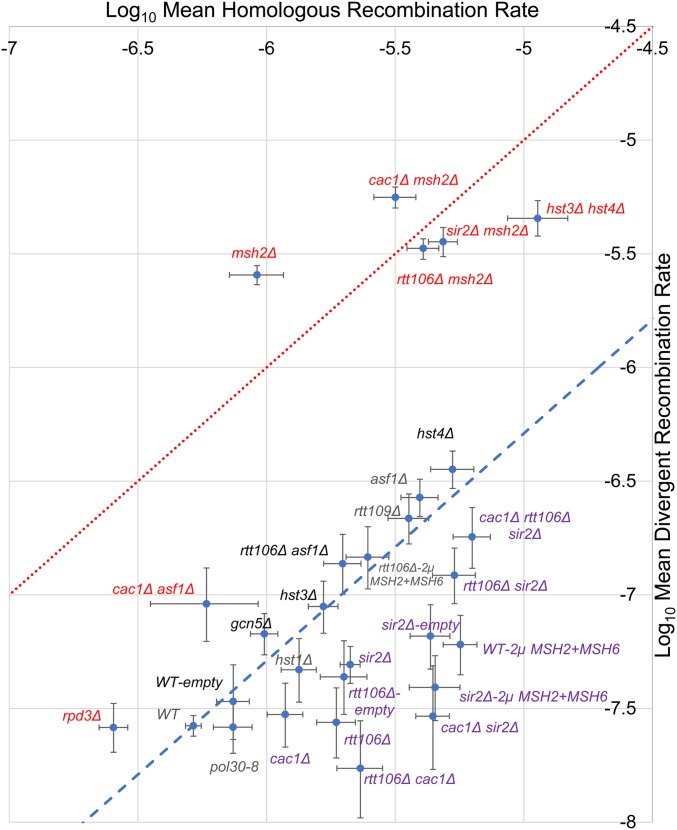
Roles for histone chaperones, acetylases, and deacetylases in regulating heteroduplex rejection efficiency. Homologous and divergent recombination rates (calculated as described in the Materials and Methods) are shown for base–base mismatches, for wild-type and mutant strains presented in this study. The data in Table 2 were plotted to represent the least squared mean of the log10-transformed recombination rates (Materials and Methods). Error bars represent the 95% credible interval. The red dashed line represents a hypothetical homologous/divergent recombination ratio of 1, as seen in mutants defective in heteroduplex rejection. The blue dashed line represents a homologous/divergent recombination rate of 20, as seen in wild-type. Mutants that deviate from wild-type are shown with red fonts indicating a reduced heteroduplex rejection ratio and purple fonts indicating an increased ratio.
Figure 2.
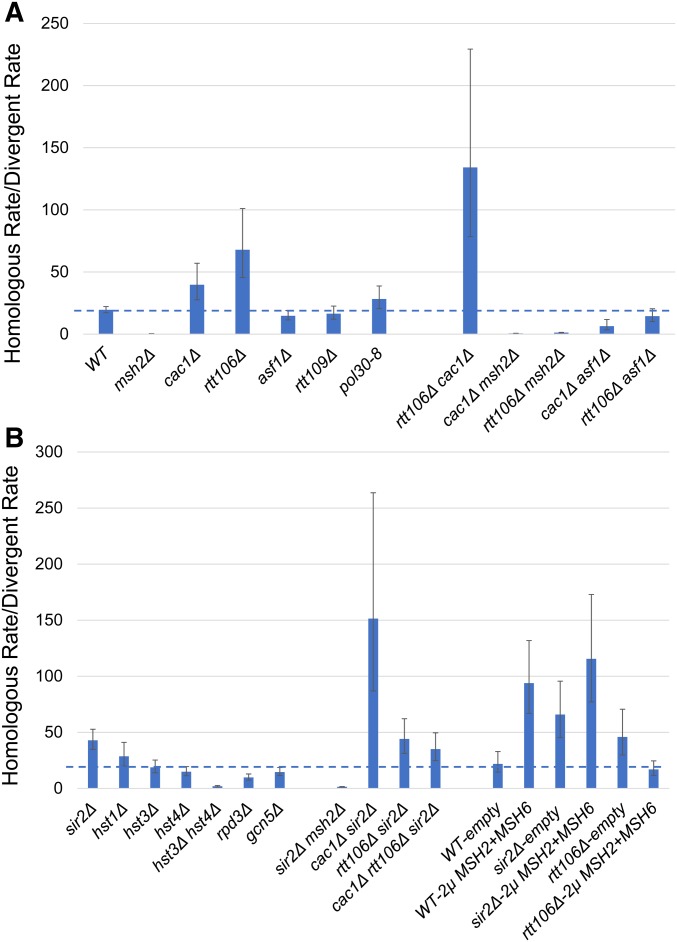
Recombination rates as measured in the inverted repeat reporter assay. Homologous and divergent recombination rates (calculated as described in the Materials and Methods, with 95% credible intervals, see Table 2) are shown in a bar graph for base–base mismatches for wild-type (WT) and the indicated mutant strains. (A) Analysis of the effect of mutations in chromatin remodelers on the homologous rate/divergent rate ratio. (B) Analysis of the effect of mutations in histone acetylases and deacetylases on the homologous rate/divergent rate ratio.
The hst3Δ hst4Δ data displayed anomalies that required special treatment. About 17% of the histidine dropout plates in which independent cultures of hst3Δ hst4Δ mutants were plated (both homologous and divergent strains) had zero colonies, or one colony in one case, that were extremely inconsistent (P < 10−6) with the Lea–Coulson model (Lea and Coulson 1949) that provides the basis for the analysis (the median number of colonies in the other cultures were > 100.) They were omitted since these outliers (eight homologous and seven divergent independent cultures) would distort the analysis and yield extreme recombination rate outliers. This strain displays extremely high levels of genetic instability (Kadyrova et al. 2013), and thus the lack of His+ colonies on these plates likely resulted from this phenotype. Even when these values were included, the ratio of HR /divergent recombination rates increased by less than twofold, presumably because the events that caused these unusual values were similar in the two strains.
Because of the above issue and possibly related twofold variation in mutation rates of the different homologous transformants, we computed the mutation rates for each hst3Δ hst4Δ transformant separately. The weighted (inverse-variance) means and SD of the transformant log μ values were computed. The ratio and 95% credible intervals were computed using the normal approximation to the posterior distribution.
Repetition of experiments
The inverted repeat recombination assays were repeated on a minimum of two separate days, with roughly an equal number of repetitions per day, and two to four independent transformants were analyzed for each genotype.
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Table S1, variation in recombination rate between transformants, can be found at the GSA FigShare portal. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8292140.
Results
Rationale for the experiments performed in this study
CAF-1 was shown to stabilize D-loops in Schizosaccharomyces pombe that occur as the result of template switching at replication forks (Pietrobon et al. 2014). This observation encouraged us to test whether the histone chaperones CAF-1 and Rtt106 play roles in stabilizing recombination intermediates during HR, and in turn in suppressing rejection. If these factors play such a role, heteroduplex rejection would occur more frequently in cac1Δ (deletion of the large subunit of CAF-1) and rtt106Δ mutants; however, heteroduplex rejection functions in these mutant backgrounds should remain dependent on mismatch recognition functions if CAF-1 and Rtt106 affect the stabilization of recombination intermediates, but not the mechanisms of antirecombination mediated by heteroduplex rejection factors through mismatch recognition.
We also tested roles for other histone chaperones and chromatin-modifying enzymes associated with DNA replication. These included Asf1, which binds to newly synthesized H3/H4 dimers that are then acetylated at H3K56 by Rtt109 (Tyler et al. 1999; Driscoll et al. 2007; Han et al. 2007), and Rtt109, which mediates histone modification that promotes binding of H3 to the histone chaperones CAF-1 and Rtt106, and, subsequently, the binding of CAF-1 to PCNA, facilitating histone deposition near the replication fork (Li et al. 2008). We were encouraged to study the above factors because much is known about the interactions, and cross talk, between the replication apparatus and nucleosome assembly machinery. For example, CAF-1 interacts with the replication processivity clamp PCNA and Asf1 interacts with RFC, which loads PCNA, and the (Minichromosome Maintenance) helicase (Franco et al. 2005; Groth et al. 2007). In addition to their roles in nucleosome assembly, Asf1 and Rtt109 indirectly promote nucleosome disassembly through H3K56 acetylation (Adkins et al. 2004; Adkins and Tyler 2004; Korber et al. 2006; Schwabish and Struhl 2006). The multifunctional roles of Asf1 and Rtt109 (promoting nucleosome disassembly as well as binding of H3 to CAF-1 and Rtt106) thus make it difficult to predict what roles these factors might play in modulating heteroduplex rejection.
Finally, we were encouraged to test roles for the silencing factor Sir2 and related deacetylases (additional rationale provided below) based on work showing that Sir2 is recruited by CAF-1 and Rtt106 to sites of chromatin formation (Huang et al. 2007). In the model outlined above, histone chaperones would act in steps that involve the stabilization of recombination intermediates; Sir2 would act in conjunction with CAF-1 and Rtt106 to stabilize recombination intermediates. Thus, Sir2 would be predicted to provide additional steps to stabilize recombination intermediates, and sir2Δ mutants would be predicted to increase the frequency of heteroduplex rejection, perhaps to levels similar to that seen in cac1Δ and rtt106Δ mutants.
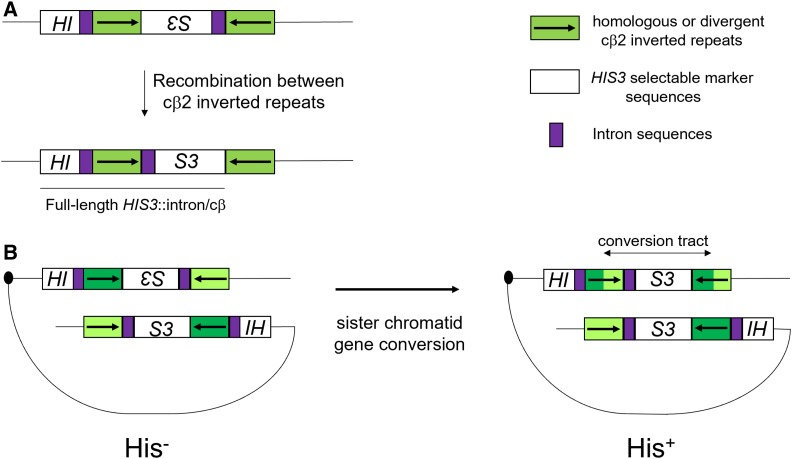
We used an inverted repeat recombination assay in baker’s yeast to study roles for chromatin modifiers in regulating the repair/rejection decision (Nicholson et al. 2000; Figure 1). Specifically, this assay measures spontaneous recombination events between homologous or divergent repeat sequences, which reorient HIS3 and intron sequences to yield a functional HIS3 gene. Such events, thought to be initiated by DNA lesions that occur during or shortly after the replication of the recombination substrates, are consistent with repair through sister chromatid gene conversion (Chen and Jinks-Robertson 1998). For each genotype, we calculated the ratio of recombination rates (± 95% credible intervals) in a strain containing identical inverted repeat DNA substrates to those seen in a strain containing divergent inverted repeats (Materials and Methods). In the divergent strains, heteroduplex recombination intermediates would form four single-nucleotide mismatches or four 4-nt loop mismatches in a 350-bp substrate (Figure 1). In wild-type cells, this ratio is ∼20 for single-nucleotide mismatches; in strains containing deletions in MSH2 or SGS1 the ratio ∼1, indicating that HR and divergent recombination rates are similar, whereas deletions in PMS1 and MLH1 confer more modest effects (Figure 2 and Table 2; Nicholson et al. 2000; Myung et al. 2001; Spell and Jinks-Robertson 2004). Similar genetic dependencies have been seen in other assays that measured recombination between divergent DNA substrates (Selva et al. 1995; Sugawara et al. 2004). These observations have led to heteroduplex rejection models in which MSH proteins recognize mismatches in heteroduplex DNA and recruit the Sgs1-Top3-Rmi1 complex to unwind the recombination intermediate (Myung et al. 2001; Spell and Jinks-Robertson 2004; Sugawara et al. 2004).
Figure 1.
Schematic of an intron-based recombination assay involving inverted repeat sequences that form a functional HIS3 reporter following homologous recombination [adapted from Nicholson et al. (2000)]. (A) Repeat sequences depicted by the green boxes are either identical in sequence (homologous substrate), or differ (divergent) by four SNPs (cβ2/cβ2-ns) or four 4-nt insertions (cβ2/cβ2-4L). The cβ2/cβ2-ns and cβ2/cβ2-4L substrates are predicted to form base–base and 4-nt loop mismatches in heteroduplex DNA, respectively. In this assay, spontaneous His+ colonies result from recombination between the 350-bp repeat sequences that reorient HIS3 (white boxes) and intron (purple boxes) sequences to yield a full-length HIS3::intron gene. (B) A model of how His+ recombinants arise in this system (Chen and Jinks-Robertson 1998). His+ recombinants are thought to result from gene conversion events between inverted repeat sequences present on sister chromatids that reorient HIS3 and intron sequences. In this example, divergent sequences are represented by different shades of green and the length of the gene conversion tract is shown. Homologous and divergent recombination rates for base–base and 4-nt loop substrates were calculated as described in the Materials and Methods, and the ratio of homologous to divergent recombination rates is presented as a measure of heteroduplex rejection efficiency.
The absence of histone chaperones CAF-1 and Rtt106, but not Asf1 and Rtt109, improves antirecombination
As shown in Figure 2 and Table 2, the cac1Δ mutation (deletion of the large subunit of CAF-1) increased the rejection ratio by twofold, compared to wild-type, in recombination assays involving base–base or 4-nt loop mismatches. The rtt106Δ mutation conferred a three-to-fourfold increase in this ratio, as measured in the base–base mismatch recombination assay. msh2Δ cac1Δ and msh2Δ rtt106Δ mutants showed rejection ratios similar to msh2Δ strains, indicating that the increases in the rejection ratios in cac1Δ and rtt106Δ strains were dependent on the rejection machinery. Interestingly, the cac1Δ rtt106Δ double mutant showed a rejection ratio significantly higher (sevenfold increase compared to wild-type) than the single mutants, suggesting a redundant function for these two factors analogous to redundant roles for these proteins in DNA replication (Li et al. 2008). Together, these observations suggested that deposition of histones by CAF-1 and Rtt106 during DSB repair stabilized recombination intermediates, making them less accessible to heteroduplex rejection factors.
In contrast, deleting Asf1 or Rtt109, factors thought to act upstream of CAF-1 and Rtt106, did not confer an effect on heteroduplex rejection (Table 2). These factors are required to acetylate histone H3 at K56, and the resulting H3K56ac-H4 dimers are then transferred to histone chaperones such as CAF-1 and Rtt106 (Schneider et al. 2006; Driscoll et al. 2007; Han et al. 2007; Tsubota et al. 2007). Interestingly, the asf1Δ mutation suppressed the increase in rejection seen in cac1Δ or rtt106Δ mutants, and for cac1Δ asf1Δ mutants the rejection ratio was lower than in wild-type cells. Additionally, we performed recombination assays in 4-nt loop mismatch strains where heteroduplex rejection depends primarily on the Msh2-Msh3-complex (Nicholson et al. 2000). We found that the asf1Δ mutation had a minimal, if any, effect on rejection and that it suppressed the increased rejection seen in cac1Δ strains (to lower than wild-type), in agreement with observations from base–base mismatch strains (Figure 2 and Table 2). This observation also indicates that the effects on heteroduplex rejection seen in chromatin modifier mutants in the base pair mismatch strains (where heteroduplex rejection is primarily dependent on Msh2-Msh6) were not specific to a particular MSH complex.
Previous studies have shown that mutants defective in the CAF-1 complex are sensitive to DNA-damaging agents, and have suggested that during DNA repair, CAF-1 is recruited by the replication processivity clamp PCNA to facilitate DNA synthesis repair steps (Moggs et al. 2000; Linger and Tyler 2005). The Stillman group identified an allele of POL30 [gene encoding PCNA; pol30-8 (R61A, D63A)] in baker’s yeast that displayed reduced binding to CAF-1 and compromised the recruitment of CAF-1 to replicating DNA (Shibahara and Stillman 1999; Zhang et al. 2000), and Linger and Tyler (2005) showed that pol30-8 mutant yeasts were similarly sensitive to DNA-damaging agents as cac2Δ (subunit of the heterotrimeric CAF-1 complex) and cac2Δ pol30-8 mutants. As shown in Figure 2 and Table 2, the pol30-8 allele, which conferred sensitivity to MMS, increased heteroduplex rejection by slightly (1.4-fold), although the 95% credible interval of the ratio was large (0.8–2.7), indicating a significant overlap between the rejection ratios for wild-type and pol30-8.
Deletion of the Sir2 silencing factor increases antirecombination
The silencing factors Sir2 and Sir3 are recruited by the histone chaperones CAF-1 and Rtt106 to sites of heterochromatin formation. In the absence of CAF-1 and Rtt106, Sir proteins are mislocalized (Huang et al. 2007). These observations encouraged us to test whether the histone deacetylases Hst1, Hst3, Hst4, or Sir2 are involved in the regulation of rejection efficiency. We were further encouraged to test these factors because Tamburini and Tyler (2005) showed in S. cerevisiae that the histone acetylases Gcn5 and Esa1 (essential for viability), and the histone deacetylases Rpd3, Sir2, and Hst1, were recruited to an HO endonuclease-induced lesion during HR. We were also interested in Hst3 and Hst4, which act to remove H3K56ac marks from newly generated chromatin in G2/M, because studies from Muñoz-Galván et al. (2013) have suggested that acetylation and deacetylation of H3K56 are important for selecting the sister chromatid as a template for the repair of DSBs that occur during DNA replication. As shown in Figure 2 and Table 2, the sir2Δ mutation increased the efficiency of heteroduplex rejection by 2.2- (1.7–2.8, 95% credible intervals) and 1.5-fold (1.1–2.1, 95% credible intervals) in the nucleotide substitution and 4-nt loop strains, respectively. The sir2Δ msh2Δ mutant displayed a homoduplex/divergent recombination ratio similar to msh2Δ, indicating that the increased rejection ratios seen in sir2Δ strains required the heteroduplex rejection machinery. Compared to wild-type cells, deletion of HST3 or HST4 conferred no significant effect on the rejection ratio. However, the hst3Δ hst4Δ double mutant showed an eightfold reduction in antirecombination of base–base mismatches compared to wild-type strains, but the strain also showed variation in recombination rates between transformants (unrelated to whether the strain contains the HR or divergent recombination substrate; see Materials and Methods) that is likely due to the high genomic instability (Kadyrova et al. 2013) seen in this mutant. Thus, there is a need for caution in interpreting the hst3Δ hst4Δ results.
The increased heteroduplex rejection phenotype observed in sir2Δ strains and the opposite phenotype seen in hst3Δ hst4Δ strains encouraged us to test if deletion mutations in the histone deacetylase Hst1, the acetylase Gcn5, and Rpd3 (a modifier of Sir2 function) affected rejection. As shown in Figure 2 and Table 2, hst1Δ and gcn5Δ mutations did not confer an effect. We then tested a strain deleted for Rpd3, which encodes a subunit of both the Rdp3S and Rpd3L histone deacetylase complexes that act in chromatin remodeling. Several studies have reported that Rpd3 and Sir2 have antagonistic effects on silent chromatin propagation and replication timing (Zhou et al. 2009; Ehrentraut et al. 2010; Yoshida et al. 2014; Thurtle-Schmidt et al. 2016). The rpd3Δ mutation decreased rejection twofold in the nucleotide substitution strain. Together, these observations provide evidence that chromatin modifiers can suppress and enhance heteroduplex rejection (see Discussion).
SIR2, CAC1, and RTT106 may act in similar steps
To determine if CAF-1 and Rtt106 function in similar steps as Sir2 to prevent heteroduplex rejection, we examine the phenotypes of double mutants. rtt106Δ sir2Δ mutant strains exhibited rejection ratios similar to those of rtt106Δ or sir2Δ single mutants, whereas cac1Δ sir2Δ showed a higher ratio compared to cac1Δ or sir2Δ (Figure 2 and Table 2). Curiously, the rejection ratio was lower or similar for sir2Δ cac1Δ rtt106Δ mutants compared to any of the single mutants, indicating a more complex genetic interaction (see below).
Previously, we showed that cooverexpression of the Msh2 and Msh6 MMR proteins in wild-type strains increased the frequency of heteroduplex rejection in the inverted repeat assay by 3.5-fold, and hypothesized that this was due to the increased availability of functional MSH complexes acting in heteroduplex rejection (Chakraborty et al. 2016, 2018). Because both the sir2Δ mutation, and Msh2 and Msh6 cooverexpression increased rejection, we asked if the increased rejection seen in sir2Δ strains would rise to an even higher level in the presence of pEAM272 (2μ, MSH2, MSH6 plasmid). Such an experiment tests if the increases in rejection seen in the two conditions reflect common or distinct regulatory steps. In wild-type strains bearing pEAM272, the Msh2-Msh6 complex is overexpressed by around eightfold (Chakraborty et al. 2018). Wild-type strains containing pEAM272 display a 4.3-fold increase, compared to those containing the empty vector pRS426, in the rejection ratio, consistent with an increased concentration of Msh2-Msh6 resulting in improved rejection by increasing the likelihood of mismatch recognition in heteroduplex DNA (Table 2). sir2Δ strains containing pEAM272 display a higher rejection ratio [1.8-fold (1.1–3.1, 95% credible intervals) higher than sir2Δ with empty vector, but with some overlap in 95% credible intervals], suggesting that the SIR2 effect on antirecombination may not be similar to that seen in the wild-type strain containing pEAM272. One interpretation of this observation is that the Sir2 effect on rejection occurs in steps that compete with mismatch recognition. Curiously, rtt106Δ strains containing pEAM272 showed a rejection ratio that was similar to the wild-type lacking pEAM272 (Table 2), indicating a more complex phenotype reminiscent of the decreased rejection ratio seen in sir2Δ cac1Δ rtt106Δ triple mutants.
Altered recombination rates in chromatin remodeling mutants do not correlate with their heteroduplex rejection phenotypes
The inverted repeat assay detects spontaneous recombination events. msh2Δ mutants, which are defective in heteroduplex rejection, display elevated levels of HR (Table 2; Datta et al. 1997; Nicholson et al. 2000). Previously the Jinks-Robertson group hypothesized that the increased HR seen in msh2Δ reflects the fact that the length of perfect homology required to avoid heteroduplex rejection (610 bp) is larger than the length of the 350-bp repeats present in the inverted repeat substrate (Datta et al. 1997). However, for other mutants, increased HR could be due to increases in the formation of DNA lesions that increase the initiation of such events. As shown in Figure 3 and Table 2, the rates of recombination between homologous sequences in the mutants analyzed in this study vary, but do not appear to correlate to changes in their repair/rejection ratios. For example, asf1Δ and rtt109Δ mutants show recombination levels higher than that of msh2Δ, but an HR/divergent recombination ratio similar to wild-type cells. In contrast, cac1Δ and rtt106Δ, which show HR levels between wild-type and msh2Δ, and sir2Δ, which shows recombination levels similar to msh2Δ, displayed HR/divergent recombination ratios higher than wild-type. A similar lack of correlation was seen for the double-mutant combinations presented. This information suggests that overall levels of HR do not impact the rejection ratio.
Discussion
This study focused on understanding roles for chromatin structure and modifications in regulating the heteroduplex rejection/DNA repair decision. Improving repair at the cost of reduced fidelity can lead to gene conversion, chromosomal rearrangement, and loss of heterozygosity, whereas high fidelity can compromise repair efficiency [reviewed in Chakraborty and Alani (2016)]. We found that the histone chaperones CAF-1 and Rtt106, and the deacetylase Sir2, act to suppress heteroduplex rejection. In contrast, a large set of factors involved in nucleosome assembly during DNA replication or in modifying histones (Rtt109, Hst1, and Gcn5) do not affect rejection efficiency. These results are consistent with repair pathways in which the presence of nucleosomes at DNA lesions acts to stabilize recombination intermediates and inhibit antirecombination (Figure 4).
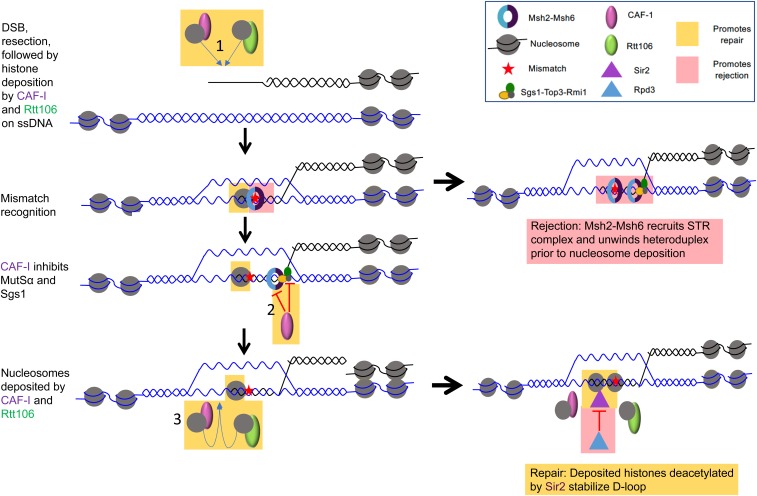
Figure 4.
A model outlining possible steps during homologous recombination where chromatin modifiers regulate the repair/rejection decision. We hypothesize that factors promoting nucleosome assembly/maintenance, such as CAF-1 and Rtt106, deposit nucleosomes at DNA lesions independent of DNA synthesis on ssDNA prior to strand invasion (1). Such deposition/maintenance, coupled with the formation of Sir2-dependent chromatin marks that promote a closed chromatin state, stabilizes D-loops to suppress rejection. Additionally, we hypothesize that CAF-1 physically interacts with Msh6, and possibly Sgs1, to inhibit the rejection factors from unwinding heteroduplex substrates (2). Finally, DNA synthesis-coupled nucleosome deposition by CAF-1 and Rtt106, combined with Sir2-dependent modifications of these nucleosomes (negatively regulated by Rpd3), are likely to stabilize heteroduplex D-loop intermediates, making it more difficult for rejection factors to unwind the intermediates (3). In this model, MSH proteins are recruited to a DSB either directly or through specific histone marks, and nucleosome maintenance limits the time frame in which antirecombination can be performed, and promotes repair of the broken chromosome and ultimately mismatch repair steps (see text for additional details). Factors/steps that promote rejection are highlighted in pink boxes and those that promote repair are highlighted in yellow boxes. DSB, double-strand break; ssDNA, single-stranded DNA; MSH, MutS homolog; STR, Sgs1-Top3-Rmi1.
The model presented in Figure 4 outlines a recombination event involving divergent sequences initiated by a DSB. DNA mismatches in heteroduplex DNA that form during strand invasion are recognized by MMR proteins, which in turn recruit the Sgs1-Top3-Rmi1 helicase–topoisomerase complex to unwind and reject the recombination intermediate. In this model, heteroduplex rejection is repressed by the presence of nucleosomes in a process regulated by CAF-1 and Rtt106, representing a regulatory step in the repair/rejection decision. The maintenance of nucleosomes by the histone chaperones CAF-1 and Rtt106 acts to stabilize heteroduplex DNA. This is followed by Sir2 deacetylating newly deposited histones, creating a closed chromatin structure that prevents rejection but promotes the completion of repair through DNA synthesis steps [see the access-repair-restore model of Tamburini and Tyler (2005)]. In other words, a closed chromatin structure, particularly in later stages in repair, would promote the completion of HR and prevent access to MMR factors that can act in heteroduplex rejection. Thus, the window for heteroduplex rejection would occur prior to the formation of closed chromatin.
The model presented in Figure 4 is supported by the following observations:
Deleting CAC1 or RTT106 resulted in increased heteroduplex rejection. Furthermore, cac1Δ rtt106Δ strains showed an even greater increase in heteroduplex rejection, consistent with CAF-1 and Rtt106 acting redundantly in nucleosome assembly (Table 2; Li et al. 2008).
A number of studies have shown that MMR and nucleosome assembly during replication are mutually inhibitory processes, and that human MutSα interacts physically with CAF-1 (Li et al. 2009; Kadyrova et al. 2011; Schöpf et al. 2012; Rodriges Blanko et al. 2016). Thus, in a manner analogous to their interaction during postreplicative MMR, nucleosome deposition could suppress MMR-mediated heteroduplex rejection, forcing the latter process to occur in the time window after strand invasion and before nucleosome deposition.
Various studies have shown in fission yeast and humans that RecQ helicases (Rqh1 in S. pombe, and BLM and WRN in humans) physically interact with the large subunit of CAF-1 (Jiao et al. 2004, 2007; Pietrobon et al. 2014). Additionally, Pietrobon et al. (2014) showed that CAF-1 suppresses D-loop disassembly by Rqh1 during template switching. Based on these observations, it is possible that CAF-1 physically interacts with Sgs1 in budding yeast and counteracts its unwinding activity during heteroduplex rejection.
The removal of 3′ nonhomologous tails during SSA acts as a temporal switch; rejection is favored before tail removal, prior to DNA synthesis steps, and repair is favored after (Chakraborty et al. 2016). Thus, analogous to 3′ nonhomologous tail removal during SSA, nucleosome maintenance by CAF-1 and Rtt106, followed by nucleosome deacetylation by Sir2 during HR, could provide another type of temporal commitment step that regulates the rejection vs. repair decision. In support of this idea, Tamburini and Tyler (2005) showed that Sir2 localizes to sites of DSBs after histone acetylases such as Gcn5 and Esa1, suggesting that Sir2 is likely to localize to sites of HR at later stages of repair, likely after the deposition of nucleosomes, to modify histones by deacetylating them and further compacting the repair substrates, making it harder for the rejection machinery to act.
Together, these observations support the idea that CAF-1 and Rtt106 function redundantly to deposit nucleosomes on recombination intermediates that stabilize the DNA heteroduplex and suppress rejection.
asf1Δ mutation suppresses the hyper-rejection phenotype seen in cac1Δ and rtt106Δ strains
Curiously, the asf1Δ and rtt109Δ mutations did not alter heteroduplex rejection ratios, and asf1Δ suppressed the increased ratio seen in cac1Δ and rtt106Δ strains (both base–base and 4-nt loop mismatch substrates). This was surprising, given that Asf1 and Rtt109 act upstream of CAF-1 and Rtt106 in the nucleosome deposition pathway. Asf1 and Rtt109 have been implicated in nucleosome removal during DNA replication and transcriptional activation (Adkins et al. 2004; Adkins and Tyler 2004; Korber et al. 2006; Schwabish and Struhl 2006; Groth et al. 2007; Ransom et al. 2010). Such a nucleosome removal activity could also act during DNA recombination and, in its absence, lead to chromatin acting to stabilize strand invasion intermediates that are refractory to heteroduplex rejection. In this model, a lack of, or delay in, nucleosome deposition in cac1Δ or rtt106Δ mutants resulting in increased rejection could be compensated for by the lack of nucleosome removal in asf1Δ mutants. Such a scenario is supported by recent in vivo and in vitro findings indicating that histones are present at ssDNA (Adkins et al. 2017; Huang et al. 2018). Adkins et al. (2017) also showed in vitro that histones remain bound to ssDNA as resection proceeds for longer distances.
We recognize that testing the effect of H3K56Q and H3K56R mutations, which mimic histone H3 acetylation and a lack of acetylation at lysine 56, respectively [summarized in Kadyrova et al. (2013)], in the inverted repeat assay could provide additional insights into the unexpected finding that asf1Δ suppresses the elevated rejection phenotype seen in cac1Δ and rtt106Δ mutants. However, H3K56 acetylation is required for both histone disassembly by Asf1, and for histone assembly by CAF-1 and Rtt106 (Tyler et al. 1999; Driscoll et al. 2007; Han et al. 2007; Li et al. 2008; Williams et al. 2008). Thus, similar to what was seen in an asf1 mutant, histone modifications would likely inhibit or promote (depending on the mutant) both histone disassembly and assembly, and thus a test of histone mutant alleles in heteroduplex rejection assays may not provide additional insights. More detailed analyses are planned to understand the asf1Δ suppression phenotype, as well as to determine if any of the phenotypes that we observed are the results of indirect effects of mutations in chromatin-modifying factors on recombination between divergent DNA sequences (e.g., genome-wide effects on transcription).
Does CAF-1 regulation of heteroduplex rejection depend on its interaction with PCNA?
CAF-1 has been implicated in DNA synthesis-coupled nucleosome deposition via its interaction with PCNA (Shibahara and Stillman 1999; Zhang et al. 2000; Krawitz et al. 2002). We found that strains bearing the pol30-8 allele, which significantly weakens the PCNA-CAF-1 interaction, showed heteroduplex rejection ratios only slightly higher than wild-type (Figure 2 and Table 2). One explanation for this phenotype is that CAF-1 regulation of heteroduplex rejection does not depend, or only partially depends, on its interaction with PCNA. In support of the former possibility, Hoek et al. (2011) found that a mutation in human CAF-1 that causes a defect in PCNA interactions (N-terminal truncation of the p150 subunit of CAF-1) does not affect the recruitment of CAF-1 to sites of DNA damage or confer sensitivity to DNA-damaging agents. They also showed direct interactions between CAF-1, and the KU complex and 14-3-3 proteins, both of which are involved in DNA damage responses. In addition, during baker’s yeast meiosis, CAF-1 is recruited independently of its interaction with PCNA to meiotic DSBs at a step prior to strand invasion, though deletion of the large subunit of CAF-1 did not affect meiotic DSB repair or crossover formation (Brachet et al. 2015). Finally, Huang et al. (2018) have suggested that CAF-1 and ASF1 are involved in chromatin assembly on ssDNA prior to Rad51 nucleoprotein filament formation, implying that it occurs in a PCNA-independent manner, as PCNA might be expected to localize much later during DNA synthesis steps.
MMR factors are likely to associate with sites of recombination early in the process
Our data suggest that the deposition of nucleosomes and their subsequent modification are likely to suppress rejection, and limit the time window during which the rejection machinery can act to unwind recombination intermediates involving divergent substrates. Previously, we showed that during SSA, removal of 3′ nonhomologous tails serves a similar role with respect to providing a limited time frame for rejection to occur (Chakraborty et al. 2016). Several studies also support this idea. For example, Li et al. (2013) showed in mammalian cells that MSH2-MSH6 is recruited via a PWWP motif in MSH6 to H3K36me3 histones before or during early S phase. In the context of rejection, this would ensure that mismatch recognition proteins are localized to chromatin before replication fork stalling-related recombination events occur. Although yeast Msh6 and human MSH3 proteins do not have PWWP motifs, one can imagine that these proteins may be recruited by other histone marks to chromatin. Additionally, MSH proteins have been shown to localize rapidly to DSBs, even in the absence of a donor template, and act in the DNA damage response (Evans et al. 2000; Hong et al. 2008; Lyndaker et al. 2008; Burdova et al. 2015). Interestingly, overexpression of Msh2 and Msh6 increased, albeit weakly, the rejection ratio further in sir2Δ strains, suggesting that mismatch recognition effects on rejection are distinct and are likely to precede changes in chromatin structure (Table 2). Taken together, these data suggest that mismatch recognition proteins localize to sites of recombination either before DSBs are formed or soon afterward, and that the chromatin environment could alter this localization and thus impact heteroduplex rejection.
Why do rpd3Δ strains display defects in heteroduplex rejection?
A large-scale screen for mutants defective in trinucleotide repeat instability identified a mutation in Sin3, which is a subunit of the histone deacetylases Rpd3L and Rpd3S (Debacker et al. 2012). In the analysis, sin3 mutants displayed significant reductions (9–18-fold) in expansion rates for a trinucleotide repeat reporter. Trinucleotide repeat expansions are also suppressed by the disruption of factors involved in heteroduplex rejection such as Msh2 and Msh3; such MSH factors are hypothesized to promote trinucleotide repeat expansions by stabilizing slipped-strand intermediates or through altered MMR (McMurray 2010). Based on these observations, the defect in heteroduplex rejection observed in rpd3Δ strains could reflect the downregulation of MSH factors that act in mismatch recognition. Alternatively, the rpd3Δ phenotype results from improved localization of Sir2 to an initiating DSB site, thus lowering rejection. The latter explanation fits with observations obtained from Zhou et al. (2009), who showed that, in the absence of Rpd3, increased localization was seen for Sir2 at telomeres and homologous mating type loci, leading to an extension of silent chromatin in these areas.
Can the heteroduplex rejection machinery be saturated?
As shown in Figure 2 and Table 2, the hst3Δ hst4Δ double mutant appears strongly compromised for antirecombination and displays high rates of HR. Kadyrova et al. (2013) showed that hst3Δ hst4Δ mutants display very high mutation rates that result from base substitutions, 1-bp insertions/deletions, and spontaneous gross chromosomal rearrangements. The rate of mutations in hst3Δ hst4Δ, as measured in forward mutation and reversion assays, was similar to that seen in MMR-defective strains, and msh2Δ hst3Δ hst4Δ triple mutants displayed greater than additive mutation rates compared to msh2Δ and hst3Δ hst4Δ. Based on these and other observations, they proposed that Hst3 and Hst4 participate in genetic stability mechanisms, which work with MMR and replicative polymerase proofreading mechanisms to suppress spontaneous mutagenesis. In this framework, the decreased rejection seen in hst3Δ hst4Δ strains could result from high rates of mutagenesis saturating the MMR machinery and thus reducing the pool of MSH proteins available to participate in heteroduplex rejection. Another possibility is that the lack of Hst3 and Hst4 leads to higher levels of H3K56 acetylation, which in turn favors nucleosome deposition by CAF-1 and Rtt106 factors that selectively bind to H3K56-acetylated histones. Such a situation could stabilize the strand invasion intermediate and suppress antirecombination. In contrast, the presence of Hst3 and Hst4 would promote rejection by deacetylating H3K56, and thus suppress nucleosome deposition by CAF-1 and Rtt106. In support of this idea, Celic et al. (2006) showed that H3K56 sites are hyperacetylated in yeast lacking Hst3 and Hst4. Additional studies will be required to distinguish between these models.
Do chromatin modification factors have indirect effects on heteroduplex rejection?
Studies in yeast showed that the Fun30, RSC, and INO80 chromatin remodelers promote resection of DNA DSB ends (Chen et al. 2012; Daley et al. 2015; Lademann et al. 2017), and studies in human cells showed that ASF1 protects such ends from excessive resection (Huang et al. 2018). Could changes in resection rates impact heteroduplex rejection? Resection of DNA DSB ends is a critical initiating step in HR because it generates 3′ single-strand tails that participate in the formation of heteroduplex DNA. Thus, changes in resection rates could impact the stability of recombination intermediates by altering heteroduplex tract lengths. Consistent with this idea, a recent study in yeast showed that defects in resection, as well as increased amounts of the single-strand-binding protein RPA, enhanced the efficiency of repair through an ectopic donor locus (Lee et al. 2016). We were unable to test effects on resection because His+ recombinants result from the repair of spontaneous DNA lesions. However, Datta et al. (1997) estimated, in the system that we used, the length of perfect homology required to initiate stable heteroduplex formation (20 bp) and avoid heteroduplex rejection (610 bp, which is larger than the 350-bp repeat). These estimates, the work of Lee et al. (2016), and our finding that HR is not decreased in the chromatin remodeling mutants analyzed suggest to us that the increased rejection seen in cac1Δ, rtt106Δ, and sir2Δ mutants is not likely due to alterations in resection rates, though we cannot exclude the possibility that greater resection could lead to longer heteroduplex tract intermediates with more mismatches that are substrates for rejection. Also, we cannot exclude the possibility that the decreased rejection seen in hst3Δ hst4Δ and rpd3Δ mutants resulted from severely limited resection if only short heteroduplex tracts form that contain perfect homology tracts, without branch migration steps that would lead to the formation of mismatches.
We also recognize that the HR/divergent recombination ratio cannot tell us if chromatin-modifying mutations affect the utilization of specific recombination pathways that have different sensitivities to heteroduplex rejection. For example, Spell and Jinks-Robertson (2003, 2004) showed that Rad51-dependent recombination had more stringent requirements for homology between recombination substrates than Rad51-independent pathways. Using the same assay that we used here, they found that a null mutation in the Srs2 helicase, which acts to prevent Rad51-dependent recombination, conferred an increase in the HR/divergent recombination ratio, a phenotype similar to what we observed in cac1Δ and rtt106Δ mutants (Krejci et al. 2003; Spell and Jinks-Robertson 2003, 2004; Veaute et al. 2003). Based on these observations, Spell and Jinks-Robertson (2004) suggested that increased rejection occurred in srs2Δ becauseRad51-dependent recombination is favored in such an environment, leading to more stringent homology requirements. Interestingly, they found that the increased rejection seen in srs2Δ mutants was suppressed by the msh2Δ mutation, suggesting that “much of the prevention of hyperrecombination between divergent recombination substrates is dependent on mismatch recognition, which also would be expected for RAD51-dependent recombination.” We observed similar suppression in cac1Δ msh2Δ and rtt106Δ msh2Δ mutants (Table 2), suggesting that even if different recombination pathways are utilized in rtt106Δ and cac1Δ strains, the heteroduplex rejection machinery in these strains responds to mismatch recognition. The above concerns will need to be further explored in recombination systems where heteroduplex rejection can be monitored through DSB events induced at specific sites (e.g., HO and I-SceI).
Acknowledgments
We thank members of the Alani laboratory, Jessica Tyler, Mike Fasullo, Michael Lichten, and Scott Keeney for helpful comments and advice; Sue Jinks-Robertson for providing us with the inverted repeat recombination strains SJR769, GCY615, and GCY559; and Deniz Akdemir (Cornell Statistical Consulting Unit) for his advice on the statistical analysis. U.C., B.M., and E.A. were supported by National Institutes of Health (NIH) grant GM-53085. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the NIH.
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8292140.
Communicating editor: J. Nickoloff
Literature Cited
- Adkins M. W., Tyler J. K., 2004. The histone chaperone Asf1p mediates global chromatin disassembly in vivo. J. Biol. Chem. 279: 52069–52074. 10.1074/jbc.M406113200 [DOI] [PubMed] [Google Scholar]
- Adkins M. W., Howar S. R., Tyler J. K., 2004. Chromatin disassembly mediated by the histone chaperone Asf1 is essential for transcriptional activation of the yeast PHO5 and PHO8 genes. Mol. Cell 14: 657–666. 10.1016/j.molcel.2004.05.016 [DOI] [PubMed] [Google Scholar]
- Adkins N. L., Swygert S. G., Kaur P., Niu H., Grigoryev S. A., et al. , 2017. Nucleosome-like, single-stranded DNA (ssDNA)-histone octamer complexes and the implication for DNA double-strand break repair. J. Biol. Chem. 292: 5271–5281. 10.1074/jbc.M117.776369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand R., Beach A., Li K., Haber J., 2017. Rad51-mediated double-strand break repair and mismatch correction of divergent substrates. Nature 544: 377–380. 10.1038/nature22046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asteris G., Sarkar S., 1996. Bayesian procedures for the estimation of mutation rates from fluctuation experiments. Genetics 142: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton S. J., Jackson S. P., 1998. Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing. EMBO J. 17: 1819–1828. 10.1093/emboj/17.6.1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachet E., Béneut C., Serrentino M.-E., Borde V., 2015. The CAF-1 and Hir histone chaperones associate with sites of meiotic double-strand breaks in budding yeast. PLoS One 10: e0125965 10.1371/journal.pone.0125965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., et al. , 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132. [DOI] [PubMed] [Google Scholar]
- Burdova K., Mihaljevic B., Sturzenegger A., Chappidi N., Janscak P., 2015. The mismatch-binding factor MutSβ can mediate ATR activation in response to DNA double-strand breaks. Mol. Cell 59: 603–614. 10.1016/j.molcel.2015.06.026 [DOI] [PubMed] [Google Scholar]
- Celic I., Masumoto H., Griffith W. P., Meluh P., Cotter R. J., et al. , 2006. The sirtuins hst3 and Hst4p preserve genome integrity by controlling histone h3 lysine 56 deacetylation. Curr. Biol. 16: 1280–1289. 10.1016/j.cub.2006.06.023 [DOI] [PubMed] [Google Scholar]
- Chakraborty U., Alani E., 2016. Understanding how mismatch repair proteins participate in the repair/anti-recombination decision. FEMS Yeast Res. 16: fow071 10.1093/femsyr/fow071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty U., George C. M., Lyndaker A. M., Alani E., 2016. A delicate balance between repair and replication factors regulates recombination between divergent DNA sequences in Saccharomyces cerevisiae. Genetics 202: 525–540. 10.1534/genetics.115.184093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty U., Dinh T. A., Alani E., 2018. Genomic instability promoted by overexpression of mismatch repair factors in yeast: a model for understanding cancer progression. Genetics 209: 439–456. 10.1534/genetics.118.300923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Carson J. J., Feser J., Tamburini B., Zabaronick S., et al. , 2008. Acetylated lysine 56 on histone H3 drives chromatin assembly after repair and signals for the completion of repair. Cell 134: 231–243. 10.1016/j.cell.2008.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Jinks-Robertson S., 1998. Mismatch repair proteins regulate heteroduplex formation during mitotic recombination in yeast. Mol. Cell. Biol. 18: 6525–6537. 10.1128/MCB.18.11.6525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Jinks-Robertson S., 1999. The role of the mismatch repair machinery in regulating mitotic and meiotic recombination between diverged sequences in yeast. Genetics 151: 1299–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Cui D., Papusha A., Zhang X., Chu C. D., et al. , 2012. The Fun30 nucleosome remodeller promotes resection of DNA double-strand break ends. Nature 489: 576–580. 10.1038/nature11355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P., 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110: 119–122. 10.1016/0378-1119(92)90454-W [DOI] [PubMed] [Google Scholar]
- Daley J. M., Niu H., Miller A. S., Sung P., 2015. Biochemical mechanism of DSB end resection and its regulation. DNA Repair (Amst.) 32: 66–74. 10.1016/j.dnarep.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A., Adjiri A., New L., Crouse G. F., Jinks-Robertson S., 1996. Mitotic crossovers between diverged sequences are regulated by mismatch repair proteins in Saccharomyces cerevisiae. Mol. Cell. Biol. 16: 1085–1093. 10.1128/MCB.16.3.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A., Hendrix M., Lipsitch M., Jinks-Robertson S., 1997. Dual roles for DNA sequence identity and the mismatch repair system in the regulation of mitotic crossing-over in yeast. Proc. Natl. Acad. Sci. USA 94: 9757–9762. 10.1073/pnas.94.18.9757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debacker K., Frizzell A., Gleeson O., Kirkham-McCarthy L., Mertz T., et al. , 2012. Histone deacetylase complexes promote trinucleotide repeat expansions. PLoS Biol. 10: e1001257 10.1371/journal.pbio.1001257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao L. T., Chen C. C., Dennehey B., Pal S., Wang P., et al. , 2017. Delineation of the role of chromatin assembly and the Rtt101Mms1 E3 ubiquitin ligase in DNA damage checkpoint recovery in budding yeast. PLoS One 12: e0180556 10.1371/journal.pone.0180556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion V., Kalck V., Horigome C., Towbin B. D., Gasser S. M., 2012. Increased mobility of double-strand breaks requires Mec1, Rad9 and the homologous recombination machinery. Nat. Cell Biol. 14: 502–509. 10.1038/ncb2465 [DOI] [PubMed] [Google Scholar]
- Driscoll R., Hudson A., Jackson S. P., 2007. Yeast Rtt109 promotes genome stability by acetylating histone H3 K56 on lysine 56. Science 315: 649–652. 10.1126/science.1135862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrentraut S., Weber J. M., Dybowski J. N., Hoffmann D., Ehrenhofer-Murray A. E., 2010. Rpd3-dependent boundary formation at telomeres by removal of Sir2 substrate. Proc. Natl. Acad. Sci. USA 107: 5522–5527. 10.1073/pnas.0909169107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E., Sugawara N., Haber J. E., Alani E., 2000. The Saccharomyces cerevisiae Msh2 mismatch repair protein localizes to recombination intermediates in vivo. Mol. Cell 5: 789–799. 10.1016/S1097-2765(00)80319-6 [DOI] [PubMed] [Google Scholar]
- Franco A. A., Lam W. M., Burgers P. M., Kaufman P. D., 2005. Histone deposition protein Asf1 maintains DNA replisome integrity and interacts with replication factor C. Genes Dev. 19: 1365–1375. 10.1101/gad.1305005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard P. H., Martini E. M., Kaufman P. D., Stillman B., Moustacchi E., et al. , 1996. Chromatin assembly coupled to DNA repair: a new role for chromatin assembly factor I. Cell 86: 887–896. 10.1016/S0092-8674(00)80164-6 [DOI] [PubMed] [Google Scholar]
- Gartenberg M. R., Smith J. S., 2016. The nuts and bolts of transcriptionally silent chromatin in Saccharomyces cerevisiae. Genetics 203: 1563–1599. 10.1534/genetics.112.145243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A., Carlin J. B., Stern H. S., Dunson D. B., Vehtari A., et al. , 2014. Bayesian Data Analysis, Ed. 3 CRC Press, Boca Raton, FL. [Google Scholar]
- George C. M., Alani E., 2012. Multiple cellular mechanisms prevent chromosomal rearrangements involving repetitive DNA. Crit. Rev. Biochem. Mol. Biol. 47: 297–313. 10.3109/10409238.2012.675644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz R. D., Schiestl R. H., 1991. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast 7: 253–263. 10.1002/yea.320070307 [DOI] [PubMed] [Google Scholar]
- Goldfarb T., Alani E., 2005. Distinct roles for the Saccharomyces cerevisiae mismatch repair proteins in heteroduplex rejection, mismatch repair and nonhomologous tail removal. Genetics 169: 563–574. 10.1534/genetics.104.035204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth A., Rocha W., Verreault A., Almouzni G., 2007. Chromatin challenges during DNA replication and repair. Cell 128: 721–733. 10.1016/j.cell.2007.01.030 [DOI] [PubMed] [Google Scholar]
- Guintini L., Tremblay M., Toussaint M., D’Amours A., Wellinger R. E., et al. , 2017. Repair of UV-induced DNA lesions in natural Saccharomyces cerevisiae telomeres is moderated by Sir2 and Sir3, and inhibited by yKu-Sir4 interaction. Nucleic Acids Res. 45: 4577–4589. 10.1093/nar/gkx123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Zhou H., Horazdovsky B., Zhang K., Xu R. M., et al. , 2007. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science 315: 653–655. 10.1126/science.1133234 [DOI] [PubMed] [Google Scholar]
- Hauer M. H., Gasser S. M., 2017. Chromatin and nucleosome dynamics in DNA damage and repair. Genes Dev. 31: 2204–2221. 10.1101/gad.307702.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer M. H., Seeber A., Singh V., Thierry R., Sack R., et al. , 2017. Histone degradation in response to DNA damage enhances chromatin dynamics and recombination rates. Nat. Struct. Mol. Biol. 24: 99–107. 10.1038/nsmb.3347 [DOI] [PubMed] [Google Scholar]
- Hoek M., Myers M. P., Stillman B., 2011. An analysis of CAF-1-interacting proteins reveals dynamic and direct interactions with the KU complex and 14–3-3 proteins. J. Biol. Chem. 286: 10876–10887. 10.1074/jbc.M110.217075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard T. A., Chang F., Perry K. R., Subramanian S., Kenworthy J., et al. , 2018. Yeast heterochromatin regulators Sir2 and Sir3 act directly at euchromatic DNA replication origins. PLoS Genet. 14: e1007418 10.1371/journal.pgen.1007418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z., Jiang J., Hashiguchi K., Hoshi M., Lan L., et al. , 2008. Recruitment of mismatch repair proteins to the site of DNA damage in human cells. J. Cell Sci. 121: 3146–3154. 10.1242/jcs.026393 [DOI] [PubMed] [Google Scholar]
- Huang S., Zhou H., Katzmann D., Hochstrasser M., Atanasova E., et al. , 2005. Rtt106p is a histone chaperone involved in heterochromatin-mediated silencing. Proc. Natl. Acad. Sci. USA 102: 13410–13415. 10.1073/pnas.0506176102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Zhou H., Tarara J., Zhang Z., 2007. A novel role for histone chaperones CAF-1 and Rtt106p in heterochromatin silencing. EMBO J. 26: 2274–2283. 10.1038/sj.emboj.7601670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. H., Fowler F., Chen C. C., Shen Z. J., Sleckman B., et al. , 2018. The histone chaperones ASF1 and CAF-1 promote MMS22L-TONSL-mediated Rad51 loading onto ssDNA during homologous recombination in human cells. Mol. Cell 69: 879–892.e5. 10.1016/j.molcel.2018.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hum Y. F., Jinks-Robertson S., 2019. Mismatch recognition and subsequent processing have distinct effects on mitotic recombination intermediates and outcomes in yeast. Nucleic Acids Res. 47: 4554–4568. 10.1093/nar/gkz126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao R., Bachrati C. Z., Pedrazzi G., Kuster P., Petkovic M., et al. , 2004. Physical and functional interaction between the Bloom’s syndrome gene product and the largest subunit of chromatin assembly factor 1. Mol. Cell. Biol. 24: 4710–4719. 10.1128/MCB.24.11.4710-4719.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao R., Harrigan J. A., Shevelev I., Dietschy T., Selak N., et al. , 2007. The Werner syndrome protein is required for recruitment of chromatin assembly factor 1 following DNA damage. Oncogene 26: 3811–3822. 10.1038/sj.onc.1210150 [DOI] [PubMed] [Google Scholar]
- Kadyrova L. Y., Blanko E. R., Kadyrov F. A., 2011. CAF-I-dependent control of degradation of the discontinuous strands during mismatch repair. Proc. Natl. Acad. Sci. USA 108: 2753–2758. 10.1073/pnas.1015914108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadyrova L. Y., Mertz T. M., Zhang Y., Northam M. R., Sheng Z., et al. , 2013. A reversible histone H3 acetylation cooperates with mismatch repair and replicative polymerases in maintaining genome stability. PLoS Genet. 9: e1003899 10.1371/journal.pgen.1003899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. A., Haber J. E., 2009. Chromatin assembly factors Asf1 and CAF-1 have overlapping roles in deactivating the DNA damage checkpoint when DNA repair is complete. Proc. Natl. Acad. Sci. USA 106: 1151–1156. 10.1073/pnas.0812578106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber P., Barbaric S., Luckenback T., Schmid A., Schermer U. J., et al. , 2006. The histone chaperone Asf1 increases the rate of histone eviction at the yeast PH05 and PH08 promoters. J. Biol. Chem. 281: 5539–5545. 10.1074/jbc.M513340200 [DOI] [PubMed] [Google Scholar]
- Krawitz D. C., Kama T., Kaufman P. D., 2002. Chromatin assembly factor I mutants defective for PCNA binding require Asf1/Hir proteins for silencing. Mol. Cell. Biol. 22: 614–625. 10.1128/MCB.22.2.614-625.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci L., Van Komen S., Li Y., Villemain J., Reddy M. S., et al. , 2003. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature 423: 305–309. 10.1038/nature01577 [DOI] [PubMed] [Google Scholar]
- Lademann C. A., Renkawitz J., Pfander B., Jentsch S., 2017. The INO80 complex removes H2A.Z to promote presynaptic filament formation during homologous recombination. Cell Rep. 19: 1294–1303. 10.1016/j.celrep.2017.04.051 [DOI] [PubMed] [Google Scholar]
- Lea D. E., Coulson C. A., 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49: 264–285. 10.1007/BF02986080 [DOI] [PubMed] [Google Scholar]
- Lee C.-S., Wang R. W., Chang H.-H., Capurso D., Segal M. R., et al. , 2016. Chromosome position determines the success of double-strand break repair. Proc. Natl. Acad. Sci. USA 113: E146–E154. 10.1073/pnas.1523660113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Tian L., Gu L., Li G. M., 2009. Evidence that nucleosomes inhibit mismatch repair in eukaryotic cells. J. Biol. Chem. 284: 33056–33061. 10.1074/jbc.M109.049874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Mao G., Tong D., Huang J., Gu L., et al. , 2013. The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSα. Cell 153: 590–600. 10.1016/j.cell.2013.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Zhou H., Wurtele H., Davies B., Horazdovsky B., et al. , 2008. Acetylation of histone H3 lysine 56 regulates replication-coupled nucleosome assembly. Cell 134: 244–255. 10.1016/j.cell.2008.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linger J., Tyler J. K., 2005. The yeast histone chaperone chromatin assembly factor 1 protects against double-strand DNA-damaging agents. Genetics 171: 1513–1522. 10.1534/genetics.105.043000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyndaker A. M., Goldfarb T., Alani E., 2008. Mutants defective in Rad1-Rad10-Slx4 exhibit a unique pattern of viability during mating-type switching in Saccharomyces cerevisiae. Genetics 179: 1807–1821. 10.1534/genetics.108.090654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W. T., Sandri G. H., Sarkar S., 1992. Analysis of the Luria-Delbrück distribution using discrete convolution powers. J. Appl. Probab. 29: 255–267. 10.2307/3214564 [DOI] [Google Scholar]
- Martin S. G., Laroche T., Suka N., Grunstein M., Gasser S. M., 1999. Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97: 621–633. 10.1016/S0092-8674(00)80773-4 [DOI] [PubMed] [Google Scholar]
- McAinsh A. D., Scott-Drew S., Murray J. A., Jackson S. P., 1999. DNA damage triggers disruption of telomeric silencing and Mec1p-dependent relocation of Sir3p. Curr. Biol. 9: 963–966. 10.1016/S0960-9822(99)80424-2 [DOI] [PubMed] [Google Scholar]
- McMurray C. T., 2010. Mechanisms of trinucleotide repeat instability during human development. Nat. Rev. Genet. 11: 786–799 (erratum: Nat. Rev. Genet. 11: 886). 10.1038/nrg2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K. D., Sinclair D. A., Guarente L., 1999. MEC1-dependent redistribution of the Sir3 silencing protein from telomeres to DNA double-strand breaks. Cell 97: 609–620. 10.1016/S0092-8674(00)80772-2 [DOI] [PubMed] [Google Scholar]
- Miné-Hattab J., Rothstein R., 2012. Increased chromosome mobility facilitates homology search during recombination. Nat. Cell Biol. 14: 510–517. 10.1038/ncb2472 [DOI] [PubMed] [Google Scholar]
- Moggs J. G., Grandi P., Quivy J. P., Jónsson Z. O., Hübscher U., et al. , 2000. A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol. Cell. Biol. 20: 1206–1218. 10.1128/MCB.20.4.1206-1218.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Galván S., Jimeno S., Rothstein R., Aguilera A., 2013. Histone H3K56 acetylation, Rad52, and non-DNA repair factors control double-strand break repair choice with the sister chromatid. PLoS Genet. 9: e1003237 10.1371/journal.pgen.1003237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung K., Datta A., Chen C., Kolodner R. D., 2001. SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat. Genet. 27: 113–116. 10.1038/83673 [DOI] [PubMed] [Google Scholar]
- Neumann F. R., Dion V., Gehlen L. R., Tsai-Pflugfelder M., Schmid R., et al. , 2012. Targeted INO80 enhances subnuclear chromatin movement and ectopic homologous recombination. Genes Dev. 26: 369–383. 10.1101/gad.176156.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A., Hendrix M., Jinks-Robertson S., Crouse G. F., 2000. Regulation of mitotic homeologous recombination in yeast: functions of mismatch repair and nucleotide excision repair genes. Genetics 154: 133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon V., Fréon K., Hardy J., Costes A., Iraqui I., et al. , 2014. The chromatin assembly factor 1 promotes Rad51-dependent template switches at replication forks by counteracting D-loop disassembly by the RecQ-type helicase Rqh1. PLoS Biol. 12: e1001968 10.1371/journal.pbio.1001968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S. E., Roche D., Almouzni G., 2006. New histone incorporation marks sites of UV repair in human cells. Cell 127: 481–493. 10.1016/j.cell.2006.08.049 [DOI] [PubMed] [Google Scholar]
- Ransom M., Dennehey B. K., Tyler J. K., 2010. Chaperoning histones during DNA replication and repair. Cell 140: 183–195. 10.1016/j.cell.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriges Blanko E., Kadyrova L. Y., Kadyrov F. A., 2016. DNA mismatch repair interacts with CAF-1- and ASF1A-H3-H4-dependent histone (H3-H4)2 tetramer deposition. J. Biol. Chem. 291: 9203–9217. 10.1074/jbc.M115.713271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Winston F., Hieter P., 1990. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Schneider J., Bajwa P., Johnson F. C., Bhaumik S. R., Shilatifard A., 2006. Rtt109 is required for proper H3K56 acetylation: a chromatin mark associated with the elongating RNA polymerase II. J. Biol. Chem. 281: 37270–37274. 10.1074/jbc.C600265200 [DOI] [PubMed] [Google Scholar]
- Schöpf B., Bregenhorn S., Quivy J.-P., Kadyrov F. A., Almouzni G., et al. , 2012. Interplay between mismatch repair and chromatin assembly. Proc. Natl. Acad. Sci. USA 109: 1895–1900. 10.1073/pnas.1106696109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabish M. A., Struhl K., 2006. Asf1 mediates histone eviction and deposition during elongation by RNA polymerase II. Mol. Cell 22: 415–422. 10.1016/j.molcel.2006.03.014 [DOI] [PubMed] [Google Scholar]
- Selva E. M., New L., Crouse G. F., Lahue R. S., 1995. Mismatch correction acts as a barrier to homeologous recombination in Saccharomyces cerevisiae. Genetics 139: 1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara K., Stillman B., 1999. Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell 96: 575–585. 10.1016/S0092-8674(00)80661-3 [DOI] [PubMed] [Google Scholar]
- Spell R. M., Jinks-Robertson S., 2003. Role of mismatch repair in the fidelity of RAD51- and RAD59-dependent recombination in Saccharomyces cerevisiae. Genetics 165: 1733–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spell R. M., Jinks-Robertson S., 2004. Examination of the roles of Sgs1 and Srs2 helicases in the enforcement of recombination fidelity in Saccharomyces cerevisiae. Genetics 168: 1855–1865. 10.1534/genetics.104.032771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N., Goldfarb T., Studamire B., Alani E., Haber J. E., 2004. Heteroduplex rejection during single-strand annealing requires Sgs1 helicase and mismatch repair proteins Msh2 and Msh6 but not Pms1. Proc. Natl. Acad. Sci. USA 101: 9315–9320. 10.1073/pnas.0305749101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami H., Ray-Gallet D., Almouzni G., Nakatani Y., 2004. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116: 51–61. 10.1016/S0092-8674(03)01064-X [DOI] [PubMed] [Google Scholar]
- Tamburini B. A., Tyler J. K., 2005. Localized histone acetylation and deacetylation triggered by the homologous recombination pathway of double-strand DNA repair. Mol. Cell. Biol. 25: 4903–4913. 10.1128/MCB.25.12.4903-4913.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurtle-Schmidt D. M., Dodson A. E., Rine J., 2016. Histone deacetylases with antagonistic roles in Saccharomyces cerevisiae heterochromatin formation. Genetics 204: 177–190. 10.1534/genetics.116.190835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubota T., Berndsen C. E., Erkmann J. A., Smith C. L., Yang L., et al. , 2007. Histone H3–K56 acetylation is catalyzed by histone chaperone-dependent complexes. Mol. Cell 25: 703–712. 10.1016/j.molcel.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto Y., Kato J., Ikeda H., 1997. Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature 388: 900–903. 10.1038/42288 [DOI] [PubMed] [Google Scholar]
- Tyler J. K., Adams C. R., Chen S. R., Kobayashi R., Kamakaka R. T., et al. , 1999. The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature 402: 555–560. 10.1038/990147 [DOI] [PubMed] [Google Scholar]
- Veaute X., Jeusset J., Soustelle C., Kowalczykowski S. C., Le Cam E., et al. , 2003. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 423: 309–312. 10.1038/nature01585 [DOI] [PubMed] [Google Scholar]
- Williams S. K., Truong D., Tyler J. K., 2008. Acetylation in the globular core of histone H3 on lysine-56 promotes chromatin disassembly during transcriptional activation. Proc. Natl. Acad. Sci. USA 105: 9000–9005. 10.1073/pnas.0800057105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Bacal J., Desmarais D., Padioleau I., Tsaponina O., et al. , 2014. The histone deacetylases Sir2 and Rpd3 act on ribosomal DNA to control the replication program in budding yeast. Mol. Cell 54: 691–697. 10.1016/j.molcel.2014.04.032 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Shibahara K., Stillman B., 2000. PCNA connects DNA replication to epigenetic inheritance in yeast. Nature 408: 221–225. 10.1038/35041601 [DOI] [PubMed] [Google Scholar]
- Zheng Q., 2008. A note on plating efficiency in fluctuation experiments. Math. Biosci. 216: 150–153. 10.1016/j.mbs.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Zhou J., Zhou B. O., Lenzmeier B. A., Zhou J. Q., 2009. Histone deacetylase Rpd3 antagonizes Sir2-dependent silent chromatin propagation. Nucleic Acids Res. 37: 3699–3713. 10.1093/nar/gkp233 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Table S1, variation in recombination rate between transformants, can be found at the GSA FigShare portal. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8292140.