Abstract
Background
Lyme disease is an infection caused by the spirochete Borrelia burgdorferi and, in most of North America, is transmitted by the blacklegged tick Ixodes scapularis. Climate change has contributed to the expansion of the geographic range of blacklegged ticks in Ontario, increasing the risk of Lyme disease for Ontarians.
Objective
To identify the number of cases and incidence rates, as well as the geographic, seasonal and demographic distribution of Lyme disease cases reported in Ontario in 2017, with comparisons to historical trends.
Methods
Data for confirmed and probable Lyme disease cases with episode dates from January 1, 2012, through December 31, 2017, were extracted from the integrated Public Health Information System (iPHIS). Data included public health unit (PHU) of residence, episode date, age and sex. Population data from Statistics Canada were used to calculate provincial and PHU-specific incidence rates per 100,000 population. The number of cases reported in 2017 by PHU of residence, month of occurrence, age and sex was compared to the 5-year averages for the period 2012–2016.
Results
There were 959 probable and confirmed cases of Lyme disease reported in Ontario in 2017. This was three times higher than the 5-year (2012–2016) average of 313. The provincial incidence rate for 2017 was 6.7 cases per 100,000 population, although this varied markedly by PHU. The highest incidence rates were found in Leeds-Grenville and Lanark District (128.8 cases per 100,000), Kingston-Frontenac, Lennox and Addington (87.2 cases per 100,000), Hastings and Prince Edward Counties (28.6 cases per 100,000), Ottawa (18.1 cases per 100,000) and Eastern Ontario (13.5 cases per 100,000). Cases occurred mostly from June through September, were most common among males, and those aged 5–14 and 50–69 years.
Conclusion
In 2017, Lyme disease incidence showed a marked increase in Ontario, especially in the eastern part of the province. If current weather and climate trends continue, blacklegged ticks carrying tick-borne pathogens, such as those causing Lyme disease, will continue to spread into suitable habitat. Monitoring the extent of this geographic spread will inform future clinical and public health actions to detect and mitigate the impact of Lyme disease in Ontario.
Keywords: Borrelia, epidemiology, expansion, Ixodes ticks, public health, range, risk, surveillance
Introduction
Lyme disease is a spirochete infection caused by Borrelia burgdorferi and, in much of North America, is transmitted to humans through the bite of an infectious blacklegged tick Ixodes scapularis. The B. burgdorferi infection typically begins with a rash and influenza-like symptoms (1–6). In the majority of cases, treatment with antibiotics results in full recovery. However, if unrecognized and left untreated, infection can progress to disseminated disease with an increased probability of morbidity, long-term sequelae and post-treatment Lyme disease syndrome (7-9).
Lyme disease case counts in Canada increased by a factor of six from 2009 through 2015, with the majority of cases reported in Ontario (10). This has been associated with an expansion in the geographic range of blacklegged ticks in Canada, including northern regions of Ontario. A driving force behind this expansion is climate change, i.e. an increase in annual cumulative degree days above 0 °C (11-13). Public health officials in Ontario monitor Lyme disease risks by conducting blacklegged tick surveillance and reporting human cases of Lyme disease.
An understanding of Lyme disease epidemiology is essential to inform clinical and public health efforts to increase awareness, prevention, early detection and mitigation efforts. The objective of this study was to identify the number of cases and incidence rates as well as the geographic, seasonal and demographic distribution of Lyme disease cases reported in Ontario in 2017, and compare this to historical trends over the previous five years.
Methods
Study location and population
Ontario has a population of approximately 14.2 million that is largely concentrated in the south of the province (14). Southern Ontario has a moderate, humid, continental climate and mixtures of agricultural, deciduous/mixed forests and built environments (15). In 2017, 36 public health units (PHUs) administered public health services in Ontario, including human Lyme disease case follow-up and blacklegged tick surveillance.
Data collection and analysis
When notified of a new case of Lyme disease, public health professionals conduct follow-up of cases and collect information pertaining to demographics, exposures, symptoms, hospitalizations and deaths. If the case meets the provincial surveillance case definition (see text box), then all the data are reported to the provincial integrated Public Health Information System (iPHIS).
Ontario’s surveillance case definitions for confirmed and probable cases of Lyme disease (2012–2017): Ontario, Canada (16)
CONFIRMED CASE
clinician-confirmed erythema migrans greater than 5 cm in diameter in a person with a history of residence in, or visit to, a Lyme disease endemic area or risk area OR
clinical evidence of Lyme disease with laboratory confirmation by polymerase chain reaction (PCR) or culture OR
clinical evidence of Lyme disease with laboratory support by serological methods, and a history of residence in, or visit to, an endemic area or risk area
PROBABLE CASE
clinical evidence of Lyme disease with laboratory support by serological methods, but with no history of residence in, or visit to an endemic area or risk area OR
clinician-confirmed erythema migrans greater than 5 cm in diameter but with no history of residence in, or visit to an endemic area or risk area
For this study, we extracted data from the iPHIS for confirmed and probable Lyme disease cases with episode dates from January 1, 2012, through December 31, 2017, and calculated the number and proportion of cases by PHU of residence, month of occurrence, age and sex.
We used an individual’s PHU of residence and earliest episode date in calculating case counts and incidence rates. Episode dates also enabled us to determine seasonality. Episode dates were defined as the date of earliest symptom onset, specimen collection or date reported. Due to the incompleteness of data and the possibility of multiple exposure opportunities, we did not attempt to determine if a case was locally acquired (exposure within PHU of residence) or travel related (travel outside of PHU of residence). Population estimates (2012–2016) and projections (2017), obtained from Statistics Canada via IntelliHEALTH Ontario, were used to calculate provincial and PHU-specific incidence rates per 100,000 population. We aggregated Lyme disease incidence rates by PHUs for mapping using a geographic information system, ESRI ArcGIS v10.3 (Incidence rates by PHU were then manually organized into incidence rate classes: 0, 0.1–5.0, 5.1–10.0, 10.1–30.0, >30.0). The 2017 data were compared to the 5-year averages for the period 2012–2016. Descriptive analyses were conducted using Microsoft Excel 2010 (Microsoft, Redmond, Washington, US) and SAS 9.3 (Statistical Analysis System, Cary, North Carolina, US).
This manuscript reports on routine surveillance activities and not research; therefore, research ethics committee approval was not required. Data are available upon request via Public Health Ontario (PHO) at http://www.publichealthontario.ca/en/About/Pages/privacy.aspx.
Results
Case counts, incidence rates and geographic distribution
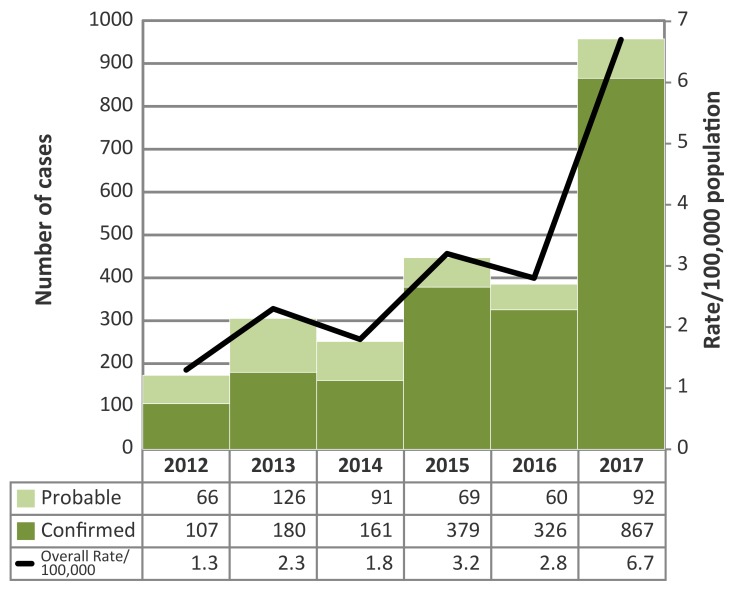
In 2017, there were 959 confirmed and probable cases of Lyme disease reported in Ontario (Figure 1).
Figure 1. Number of probable and confirmed Lyme disease cases and incidence rate per 100,000 population by year: Ontario, Canada (2012–2017).
The 2017 total was 3.1 times higher than the 5-year (2012–2016) average of 313 (Table 1). The majority of cases in 2017 were reported by Leeds-Grenville and Lanark District (LGL) (n=219, 22.8% of the provincial total), Kingston-Frontenac, Lennox and Addington (KFL) (n=180, 18.8%) and Ottawa (n=180, 18.8%). These three PHUs made up 9.7% of the Ontario population, yet reported 60.4% of the province’s Lyme disease cases. In 2017, the LGL case count was 4.6 times higher than the 5-year average; KFL and Ottawa counts were 3.6 and 3.8 times higher than their 5-year averages, respectively.
Table 1. Number of probable and confirmed Lyme disease cases and incidence rates by public health unit: Ontario, Canada (2017).
| Public health unita | Number of cases | % | 5-year average | Incidence per 100,000 population |
|---|---|---|---|---|
| Algoma District (ALG) | 2 | 0.2 | 2.5 | 1.7 |
| Brant County (BRN) | 1 | 0.1 | 2.0 | 0.7 |
| Chatham-Kent (CHK) | 3 | 0.3 | 2.2 | 2.9 |
| City of Hamilton (HAM) | 5 | 0.5 | 6.2 | 0.9 |
| Ottawa (OTT) | 180 | 18.8 | 47.4 | 18.1 |
| Durham Regional (DUR) | 46 | 4.8 | 14.2 | 6.7 |
| Eastern Ontario (EOH) | 28 | 2.9 | 17.0 | 13.5 |
| Elgin-St. Thomas (ELG) | 0 | 0.0 | 1.3 | 0.0 |
| Grey Bruce (GBO) | 3 | 0.3 | 2.0 | 1.8 |
| Haldimand-Norfolk (HDN) | 7 | 0.7 | 2.2 | 6.3 |
| Haliburton-Kawartha-Pine Ridge District (HKP) | 17 | 1.8 | 5.2 | 9.3 |
| Halton Regional (HAL) | 13 | 1.4 | 7.4 | 2.2 |
| Hastings and Prince Edward Counties (HPE) | 47 | 4.9 | 18.2 | 28.6 |
| Huron County (HUR) | 1 | 0.1 | 1.7 | 1.7 |
| Kingston-Frontenac and Lennox & Addington (KFL) | 180 | 18.8 | 49.6 | 87.2 |
| Lambton County (LAM) | 1 | 0.1 | 3.0 | 0.8 |
| Leeds-Grenville and Lanark District (LGL) | 219 | 22.8 | 47.8 | 128.8 |
| Middlesex-London (MSL) | 15 | 1.6 | 5.2 | 3.1 |
| Niagara Regional (NIA) | 19 | 2.0 | 9.2 | 4.1 |
| North Bay Parry Sound District (NPS) | 1 | 0.1 | 1.3 | 0.8 |
| Northwestern (NWR) | 2 | 0.2 | 3.2 | 2.5 |
| Oxford County (OXF) | 2 | 0.2 | 1.8 | 1.8 |
| Peel Regional (PEE) | 12 | 1.3 | 5.8 | 0.8 |
| Perth District (PDH) | 1 | 0.1 | 1.3 | 1.3 |
| Peterborough County-City (PTC) | 12 | 1.3 | 2.8 | 8.4 |
| Porcupine (PQP) | 0 | 0.0 | 1.0 | 0.0 |
| Renfrew County and District (REN) | 7 | 0.7 | 3.2 | 6.5 |
| Simcoe Muskoka District (SMD) | 12 | 1.3 | 3.8 | 2.1 |
| Sudbury and District (SUD) | 3 | 0.3 | 1.5 | 1.5 |
| Thunder Bay District (THB) | 0 | 0.0 | 1.0 | 0.0 |
| Timiskaming (TSK) | 0 | 0.0 | 0.0 | 0.0 |
| Toronto (TOR) | 76 | 8.0 | 31.0 | 2.6 |
| Waterloo (WAT) | 7 | 0.7 | 4.2 | 1.3 |
| Wellington-Dufferin-Guelph (WDG) | 9 | 0.9 | 2.4 | 3.1 |
| Windsor-Essex County (WEC) | 7 | 0.7 | 4.0 | 1.7 |
| York Regional (YRK) | 21 | 2.2 | 9.8 | 1.8 |
| Total | 959 | 100.0b | 313 (average) | 6.7 (average) |
a 3-letter abbreviation
b The percentage does not add to 100 percent due to rounding
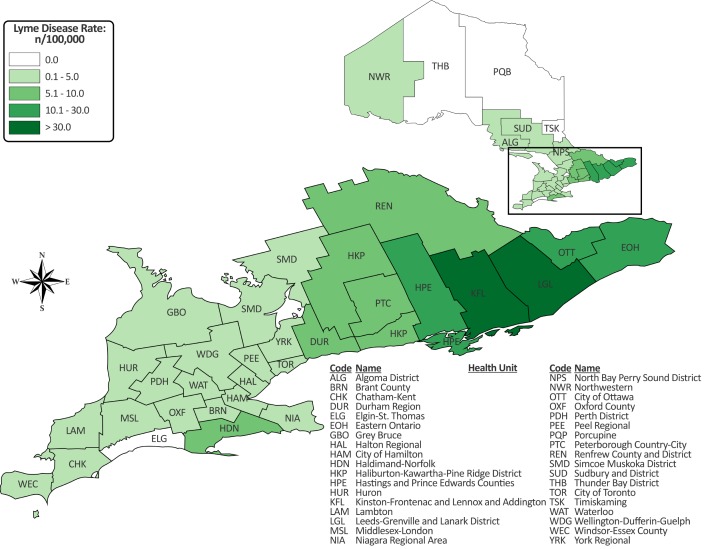
The highest incidence rates in 2017 occurred in LGL (128.8 cases per 100,000), KFL (87.2 cases per 100,000), Hastings and Prince Edward Counties (HPE) (28.6 cases per 100,000), Ottawa (18.1 cases per 100,000) and Eastern Ontario (EOH) (13.5 cases per 100,000) (Figure 2).
Figure 2. Incidence rate of Lyme disease (per 100,000 population) by public health unit: Ontario, Canada (2017).
Abbreviations: n, number, > superior to
Seasonality
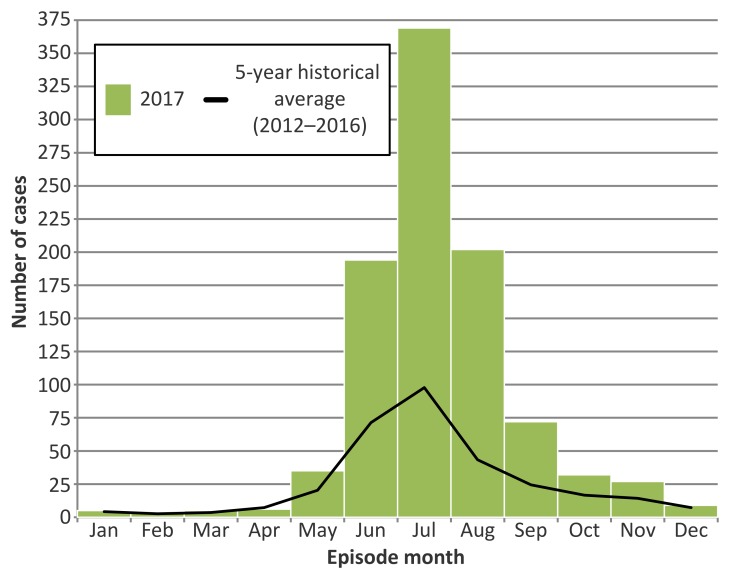
In 2017, the majority of cases occurred from June through September, with July having the highest number (n=369) (Figure 3). Monthly case counts were above 5-year averages for June (2.7 times higher), July (3.8), August (4.7) and September (3.0).
Figure 3. Number of probable and confirmed Lyme disease cases by episode month in 2017, compared to 5-year averages (2012–2016): Ontario, Canada.
Age and sex
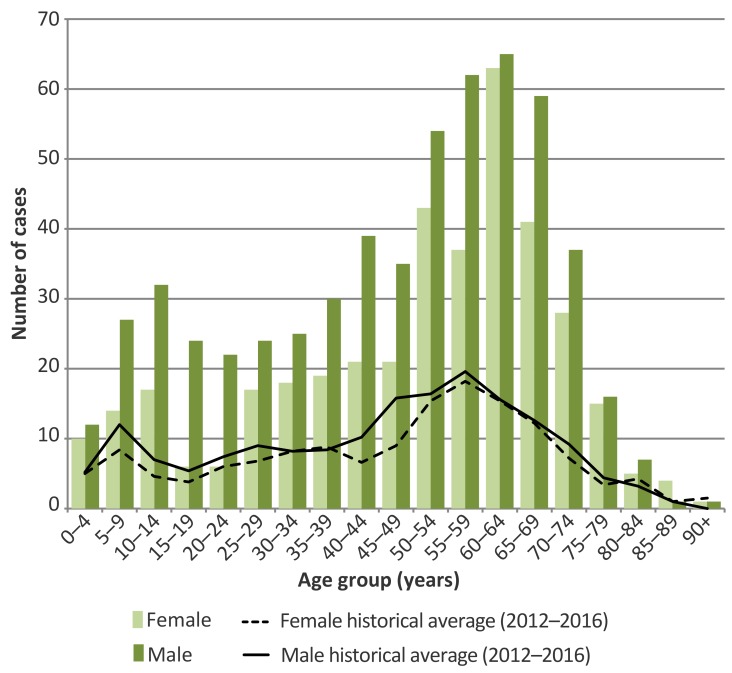
Lyme disease case counts in Ontario displayed a bimodal pattern in 2017, with relatively higher counts in those aged 5–14 and 50–69 years and relatively fewer in those aged 15–24 years (Figure 4). Over half – 59.8% – of cases were male. The ratio of male to female cases was higher than the 5-year average in most age groups. The male to female ratio was almost two times higher in the 5–14, 35–39, 40–49 and 55–59 year old age groups and was three to four times higher in the 15–19 and 20–24 year age groups.
Figure 4. Number of confirmed and probable Lyme disease cases by age group and sex in 2017, compared to 5-year averages (2012–2016): Ontario, Canadaa.
a Cases with unknown age (n=3) or sex (n=3) were excluded. Age group refers to the age group of the individual at the time of illness
Discussion
Ontario Lyme disease incidence in 2017 was at its highest recorded level since it became a reportable disease in 1988. The increase in Lyme disease incidence in Ontario is not uniform, but is concentrated in the eastern part of the province, which contains more blacklegged ticks and blacklegged ticks with relatively higher B. burgdorferi percent positivity (17,18). Consistent with the rest of the Canada and elsewhere, Lyme disease cases were more frequent between June and September, and were more common among those aged 5–14 and 50–69 years and among males.
The strength of our study is that it provides the most up-to-date data available on Lyme disease incidence in Ontario as well as an analysis of the geographic, seasonal and demographic trends in Lyme disease infection.
There are several limitations to consider. First, the true incidence of an infection such as Lyme disease is subject to varying degrees of underreporting due to a variety of factors, such as variable disease awareness, health care–seeking behaviours, clinical diagnoses, reporting behaviours and treatment of clinical early-localized Lyme disease without reporting to the PHU (19,20). Although the degree of provincial underreporting has not been determined, we assume cases reported through passive surveillance skew towards cases confirmed by serology.
Second, the iPHIS is a disease reporting system that allows for ongoing updates to data previously entered. As a result, data extractions from iPHIS represent a snapshot of the database at the time of extraction and may differ from previous or subsequent reports. Third, it was not possible to assess whether B. burgdorferi infections were acquired locally or when travelling elsewhere, so the PHU of residence is not necessarily the location of exposure. Nonetheless, the most likely exposure location for a case is near their home or, more broadly, in the PHU of residence (21,22).
Next steps
There are both research and public health implications to these data. Reportable disease databases such as iPHIS, offer an opportunity to explore the epidemiology of Lyme disease in Ontario. For example, research using iPHIS and other health care databases could examine spatiotemporal trends in reporting and treatment of cases with and without laboratory confirmation.
These data have important public health implications in that the identified trends offer an opportunity for better targeting of Lyme disease prevention awareness, such as educational programs for children and parents and the need for protection during summer activities. Summer is associated with the nymph stage of the tick, so increased awareness of the smaller size of the tick at this time may also be useful.
Conclusion
Lyme disease incidence is increasing in Ontario. This trend is likely to continue as climate change progresses and enables blacklegged ticks to survive and propagate in new areas. Ongoing surveillance of both human cases and tick distribution can continue to inform clinical and public health actions to prevent, detect and mitigate the impact of Lyme disease in Ontario.
Acknowledgements
We would like to thank our public health colleagues throughout Ontario for their continued commitment to Lyme disease case management, surveillance and reporting. We thank Lisa Fortuna (PHO), Shelley Deeks (PHO), Patricia Huston (Public Health Agency of Canada) and an anonymous reviewer for reviewing earlier drafts of the manuscript and for providing valuable input on its improvement.
Conflict of interest: None.
Funding: Public Health Ontario supported this work as part of its core mandate.
References
- 1.Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am 2015. Jun;29(2):269–80. 10.1016/j.idc.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith RP, Schoen RT, Rahn DW, Sikand VK, Nowakowski J, Parenti DL, Holman MS, Persing DH, Steere AC. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med 2002. Mar;136(6):421–8. 10.7326/0003-4819-136-6-200203190-00005 [DOI] [PubMed] [Google Scholar]
- 3.Halperin JJ. Nervous system Lyme disease. Clin Lab Med 2015. Dec;35(4):779–95. 10.1016/j.cll.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 4.Robinson ML, Kobayashi T, Higgins Y, Calkins H, Melia MT. Lyme carditis. Infect Dis Clin North Am 2015. Jun;29(2):255–68. 10.1016/j.idc.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 5.Nadelman RB. Erythema migrans. Infect Dis Clin North Am 2015. Jun;29(2):211–39. 10.1016/j.idc.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Steere AC, Bartenhagen NH, Craft JE, Hutchinson GJ, Newman JH, Rahn DW, Sigal LH, Spieler PN, Stenn KS, Malawista SE. The early clinical manifestations of Lyme disease. Ann Intern Med 1983. Jul;99(1):76–82. 10.7326/0003-4819-99-1-76 [DOI] [PubMed] [Google Scholar]
- 7.Aucott JN. Posttreatment Lyme disease syndrome. Infect Dis Clin North Am 2015. Jun;29(2):309–23. 10.1016/j.idc.2015.02.012 [DOI] [PubMed] [Google Scholar]
- 8.Kalish RA, Kaplan RF, Taylor E, Jones-Woodward L, Workman K, Steere AC. Evaluation of study patients with Lyme disease, 10-20-year follow-up. J Infect Dis 2001. Feb;183(3):453–60. 10.1086/318082 [DOI] [PubMed] [Google Scholar]
- 9.Shadick NA, Phillips CB, Logigian EL, Steere AC, Kaplan RF, Berardi VP, Duray PH, Larson MG, Wright EA, Ginsburg KS, Katz JN, Liang MH. The long-term clinical outcomes of Lyme disease. A population-based retrospective cohort study. Ann Intern Med 1994. Oct;121(8):560–7. 10.7326/0003-4819-121-8-199410150-00002 [DOI] [PubMed] [Google Scholar]
- 10.Gasmi S, Ogden NH, Lindsay LR, Burns S, Fleming S, Badcock J, Hanan S, Gaulin C, Leblanc MA, Russell C, Nelder M, Hobbs L, Graham-Derham S, Lachance L, Scott AN, Galanis E, Koffi JK. Surveillance for Lyme disease in Canada: 2009-2015. Can Commun Dis Rep 2017. Oct;43(10):194–9. 10.14745/ccdr.v43i10a01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clow KM, Leighton PA, Ogden NH, Lindsay LR, Michel P, Pearl DL, Jardine CM. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario, Canada. PLoS One 2017. Dec;12(12):e0189393. 10.1371/journal.pone.0189393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden NH, Maarouf A, Barker IK, Bigras-Poulin M, Lindsay LR, Morshed MG, O’callaghan CJ, Ramay F, Waltner-Toews D, Charron DF. Climate change and the potential for range expansion of the Lyme disease vector Ixodes scapularis in Canada. Int J Parasitol 2006. Jan;36(1):63–70. 10.1016/j.ijpara.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 13.Leighton PA, Koffi JK, Pelcat Y, Lindsay LR, Ogden NH. Predicting the speed of tick invasion: an empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J Appl Ecol 2012;49(2):457–64. 10.1111/j.1365-2664.2012.02112.x [DOI] [Google Scholar]
- 14.Statistics Canada. Table 17-10-0009-01. Population estimates, quarterly. Ottawa (ON): Government of Canada; 2018. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901
- 15.Wadkins L. The forest resources of Ontario 2011. Sault Ste. Marie (ON): Ontario Ministry of Natural Resources, Forest Evaluation and Standards Section, Forests Branch; 2011. [Google Scholar]
- 16.Ministry of Health and Long-Term Care. Appendix B: Provincial case definitions for reportable diseases: Lyme disease. Toronto (ON): Government of Ontario; 2017. http://www.health.gov.on.ca/en/pro/programs/publichealth/oph_standards/docs/lyme_disease_cd.pdf
- 17.Ontario Agency for Health Protection and Promotion (Public Health Ontario). Ontario Lyme disease map 2017: estimated risk areas map. Toronto (ON): Queen's Printer for Ontario; 2017 Jun. https://www.publichealthontario.ca//eRepository/Lyme_disease_risk_areas_map.pdf
- 18.Nelder MP, Russell C, Lindsay LR, Dhar B, Patel SN, Johnson S, Moore S, Kristjanson E, Li Y, Ralevski F. Population-based passive tick surveillance and detection of expanding foci of blacklegged ticks Ixodes scapularis and the Lyme disease agent Borrelia burgdorferi in Ontario, Canada. PLoS One 2014. Aug;9(8):e105358. 10.1371/journal.pone.0105358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS. Incidence of clinician-diagnosed Lyme disease, United States, 2005-2010. Emerg Infect Dis 2015. Sep;21(9):1625–31. 10.3201/eid2109.150417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 2014. Sep;59(5):676–81. 10.1093/cid/ciu397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falco RC, Fish D. Ticks parasitizing humans in a Lyme disease endemic area of southern New York State. Am J Epidemiol 1988. Nov;128(5):1146–52. 10.1093/oxfordjournals.aje.a115057 [DOI] [PubMed] [Google Scholar]
- 22.Stafford KC. Tick management handbook: an integrated guide for homeowners, pest control operators, and public health officials for the prevention of tick-associated disease, 2cd edition. The Connecticut Agricultural Experiment Station, Bulletin No. 1010, New Haven, CT (USA), 78 pp. https://stacks.cdc.gov/view/cdc/11444