Abstract
Pathogenic oral biofilms are universal, chronic, and costly. Despite advances in understanding the mechanisms of biofilm formation and persistence, novel and effective treatment options remain scarce. Nanoparticle-mediated eradication of the biofilm matrix and resident bacteria holds great potential. Particularly, nanoparticles that target specific microbial and biofilm features utilizing non-toxic materials are well-suited for clinical translation. However, much work remains to characterize the local and systemic effects of therapeutic agents topically applied to chronic biofilms, such as those that cause dental caries. This perspective summarizes the pathogenesis of oral biofilms, describes current and future nanoparticle-mediated treatment approaches, and highlights outstanding questions that are paramount to answer to effectively target and treat oral biofilms.
Introduction
Nanoparticles are a highly promising treatment modality for biofilms. Many nanoparticle strategies have aimed to inhibit biofilms within the oral cavity. Oral biofilms also serve as excellent models for other healthcare-associated and industrial biofilms that may benefit from nanoparticle approaches. Nanoparticles can be directly bactericidal or designed to enhance drug aqueous solubility, and through precise adjustments of chemical compositions, size, surface charge, and other properties, can provide unparalleled flexibility to carry, retain, and release drugs exactly when and where needed most. Additionally, nanoparticle drug delivery systems can both protect conventional drugs from pH and/or enzymatic degradation in the harsh biofilm niche, while also exploiting these unique microenvironments for stimuli-responsive drug release. While outstanding progress has been made investigating nanoparticles as anti-biofilm treatments, comprehensive evaluation of chronic exposure limits, especially for oral biofilm treatments, must be investigated to ensure safe and biocompatible delivery approaches are pursued. Alternative strategies that leverage biomimetics may also be advantageous for the prevention of chronic infections, such as caries.
This perspective provides a succinct overview of nanoparticle treatment strategies for oral biofilms with a focus on Naha et al., who reports on the robust anti-oral biofilm efficacy of iron oxide-based nanoparticles in this issue. Furthermore, we will offer perspectives on critical areas in this field that require major focus for realization of clinical translation of these therapeutic approaches. For recent, more comprehensive overviews of nanoparticle strategies to treat biofilms, the following reviews are suggested for the interested reader:1–10.
Significance of Biofilms
The majority of persistent infectious diseases in humans are caused by virulent biofilms, including those within the mouth11–12. The annual cost of treatment of oral biofilm-related infectious diseases, such as dental caries, exceeds $81 billion in the US12–13, motivating the development of new, more effective treatment modalities. Due to its ease of access and high bacterial species diversity, the oral cavity is an opportune setting to study new approaches for biofilm treatments, which can then be translated to other biofilm-associated conditions affecting human health or for use in industrial settings. The assembly of tooth-decay causing (cariogenic) biofilms is a prime example of how bacteria accumulate on surfaces and form structured communities within an extracellular matrix comprised of polymeric substances such as exopolysaccharide (EPS)11,14. Advantageously, oral biofilms can be treated topically, obviating systemic delivery hurdles of other healthcare-associated biofilms. Similar to other biofilms, the EPS-rich matrix of cariogenic biofilms creates spatial and microenvironmental heterogeneities, which modulate growth and provide protection for pathogens against intrinsic and applied antimicrobials11,14. Within the oral microbiome, Streptococcus mutans (S. mutans) adheres to the tooth pellicle then rapidly orchestrates the formation of cariogenic biofilms on teeth in the presence of dietary sucrose (Figure 1). S. mutans-released exoenzymes (e.g., glucosyltransferases) produce glucans-rich EPS from sucrose, thus promoting local colonization and accumulation of microbes as well as formation of the protective multifunctional scaffold and diffusion-limiting matrix15–17. In parallel, sugars are fermented by bacteria within this matrix, creating a highly acidic microenvironments (pH 4.5–5.5)18–19. The low pH niches induce further EPS synthesis while cariogenic (acid-tolerant and acidogenic) flora prosper17. Consequently, local acidity ensures continuous biofilm build up and demineralization of adjacent tooth enamel, leading to the onset of dental caries. The continually evolving knowledge of this intricate pathogenic process provides new opportunities for unique and effective treatment strategies (Figure 1).
Figure 1.
Bacterial biofilm developmental stages highlighting various opportunities for therapeutic interventions (reproduced with permission from6). EPS-exopolysaccharide.
Current oral biofilm treatment options
Preventing or treating pathogenic oral biofilms is challenging. Topically applied drugs suffer from rapid salivary clearance, poor penetration of the EPS matrix, and a lack of substantivity (i.e., retention on tooth surfaces) to address continual biofilm formation. The presence of EPS with its altered microenvironment reduces drug access and triggers bacterial tolerance to antibiotics10–11,14, making bacteria difficult to treat without disturbing normal flora. Furthermore, the acidic pH indicative of oral biofilms reduces efficacy of many antibiotics20–21. Importantly, the ubiquitous and chronic nature of oral biofilms requires that any therapeutic be tolerated for continual use over an extended time period with minimal toxicity and off-target effects.
Current agents for controlling oral biofilms are restricted to broad-spectrum antimicrobial drugs, such as chlorhexidine, which is limited by adverse effects (calculus formation and tooth staining) and therefore, is not suitable for daily, long-term use. Alternative anti-biofilm agents include naturally occurring drugs such as terpenoids, essential oils, and flavonoids that disrupt assembly of cariogenic biofilms and/or reduce EPS synthesis22–28. These drugs impact S. mutans viability, acid production, acid tolerance, and EPS synthesis at acidic pH24,29. However, their anti-biofilm efficacy is still hampered by poor drug solubility, EPS diffusion, and substantivity22–24.
Nanoparticle-based oral biofilm treatments
Nanoparticles hold significant promise for addressing the challenges of oral biofilm drug delivery. The chemical flexibility and relative ease of nanoparticle preparation allow for the development of unique biofilm treatments30. Nanoparticles can be directly bactericidal or designed to enhance drug aqueous solubility and transport into bacterial cells. Anti-biofilm nanoparticles can be developed from metals or metal oxides, synthetic or natural polymers, or hybrids therein. Furthermore, through precise adjustments of chemical compositions, size, surface charge and other properties, nanoparticles provide unparalleled flexibility to ensure robust biofilm targeting and retention through biofilm matrix interactions, thereby enhancing substantivity and anti-biofilm efficacy. Nanoparticles’ high surface area to volume ratios enable robust drug or drug combination loading that may result in synergistic anti-biofilm efficacy. Furthermore, the resulting highly complex antimicrobial mechanism of action may overcome common bacterial resistance mechanisms, including permeability regulation, multidrug efflux pumps, and target binding affinity site mutations5,30–31. Data suggest nanoparticles can also lower the potential for bacterial resistance and protect conventional drugs from pH and/or enzymatic degradation in the harsh biofilm microenvironments5,30. Critically, nanoparticle design can be tuned to become activated in response to unique biofilm pathologic microenvironmental triggers, such as pH or hypoxia.
Nanoparticle design properties for anti-biofilm treatments
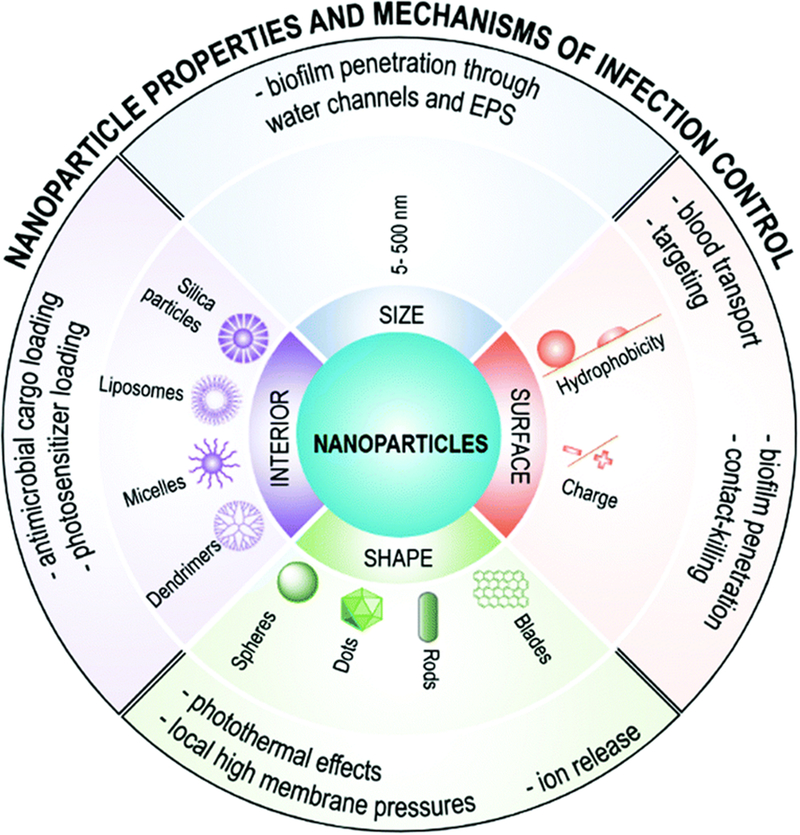
Chemistry and material architecture (solid nanoparticles, such as silica or metals, liposomes, micelles, dendrimers, etc.) define overall nanoparticle properties including size, shape, surface functionalization, and core properties that impact anti-biofilm efficacy, as recently reviewed5,8 and depicted in Figure 2. Nanoparticle size impacts diffusion into the EPS biofilm matrix after topical delivery, with diameters up to 130 nm showing robust biofilm penetration8,32. The effect of surface charge on biofilm penetration shows that positively charged nanoparticles possess excellent biofilm penetration versus anionic or uncharged counterparts, potentially due to a catch-and-release phenomenon within the anionic EPS matrix33. Additionally, hydrophobic cationic nanoparticles are taken up by bacteria while hydrophilic cationic particles remain bound to the EPS33. Nanoparticle core properties (e.g., solid or hydrophobic/hydrophilic depots) can enable loading of a variety of anti-biofilm drugs or sensitization agents for delivery. For example, cationic and hydrophobic core-shell nanoparticles capable of loading antibacterial oils showed robust anti-biofilm efficacy and selective cytotoxicity to bacteria versus fibroblast cells25. Thus, size and charge as well as nanoparticle core properties can be exploited to ensure appropriate nanoparticle localization to maximize anti-oral biofilm efficacy. The interplay between nanoparticle size and shape can also be harnessed to increase the efficacy of biofilm targeting nanoparticles34, though it has yet to be established as a critical design parameter for oral biofilm treatments.
Figure 2.
Nanoparticle properties are important for their use in anti-biofilm strategies (reproduced with permission from8)
Selectivity of therapeutics is critical when designing nanoparticle drug delivery systems, especially for the complex microenvironment of the oral cavity. In particular, S. mutans biofilm pH has been exploited to stimulate selective anti-biofilm efficacy using nanoparticles that exhibit inherent pH-responsive anti-biofilm activities or release anti-biofilm drugs via pH changes26–28,35. pH-responsive functionalities include imidazoles, amines, amides, amino acids or acid-sensitive degradable linkages, such as esters, ketals, acetals, and anhydrides. For example, we have pioneered the use of multi-surface binding and pH-responsive nanoparticles for anti-biofilm applications26–28. These polymeric diblock co-polymer nanoparticles have high affinity to tooth, pellicle, and glucose-coated biofilm surfaces due to tertiary cationic surface residues and may distribute uniformly throughout the biofilm matrix when used with saturated drug solutions27. Drug is retained within nanoparticles bound to biofilms until the pH becomes acidic. Then, the nanoparticles exhibit drug release via protonation-mediated destabilization, resulting in substantial enhancement of drug efficacy (≥ 3 log CFU) in situ and in vivo26. Importantly, due to flexibility and ease of preparation, polymeric nanoparticles can entrap other topical anti-biofilm drugs that otherwise suffer similar solubility and retention issues26–28,36–38.
Catalytic nanoparticles that target oral biofilms
In the publication by Naha et al., the acidic pH of the biofilm matrix was exploited to activate catalytic iron oxide nanoparticles, termed CAT-NP, mediating anti-biofilm activity39. Metal and metal oxide-based nanoparticles have longstanding use for their native antibacterial properties, with copper, titanium, gold, silver, and iron oxide-based nanoparticles having shown bactericidal effects40–42. Metal or metal-oxide-based nanoparticles exert antibacterial effects in a variety of ways. Mechanisms can include direct interaction with the bacterial cell wall, inhibition of biofilm formation by affecting glucans production or quorum sensing, recruiting innate and/or adaptive host immune cells, generation of reactive oxygen species (ROS), or via deleterious interactions with bacterial DNA and/or proteins41–48. All of these mechanisms align with excellent bactericidal activity, even against persister cells that are dormant and thereby resistant to traditional antibiotics49.
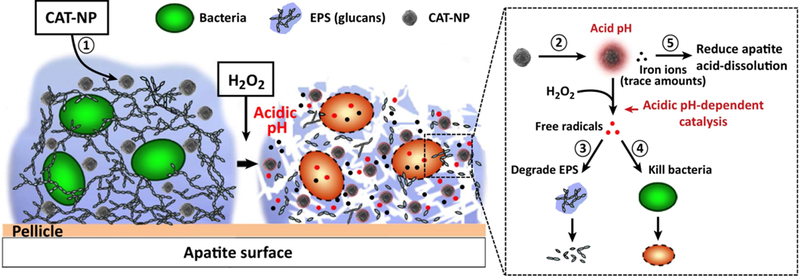
Earlier studies by Gao et al. demonstrated initial anti-biofilm efficacy of CAT-NP50. When treated in combination with hydrogen peroxide, these nanoparticles produced reactive oxygen species (Figure 3). ROS-mediated oxidative stress typically leads to oxidation of biomolecules and cell components resulting in severe cellular damage1,51–52. In this case, ROS directly contributed to biofilm exopolysaccharide matrix degradation and killing of S. mutans. The iron oxide particles, in particular, exhibited robust peroxidase-like activity only at an acidic pH characteristic of those produced by S. mutans. Importantly, and owing to the pH-responsive behavior of the nanoparticles, which limited free radical production at physiological conditions, normal tissues were protected from off-target effects. Though topical treatment once daily of a rat biofilm model was effective in reducing tooth decay, the iron oxide particles suffered from poor colloidal stability and indiscriminate tissue binding, limiting overall clinical translation50. Thus, dextran coatings were developed for CAT-NP, referred to as Dex-NZM in this issue, that maintained underlying iron oxide catalytic behaviors and enhanced the selectivity of nanoparticle binding to biofilm versus gingival tissue. Similar to bare iron oxide particles, treatments in vivo reduced the occurrence and severity of caries39.
Figure 3.
Catalytic nanoparticles (CAT-NP), comprised of iron oxide nanoparticles coated with dextran, known in this issue as Dex-NZM, result in biofilm disruption via local pH-dependent free radical production, resulting in degraded EPS and bacteria cell killing (reproduced with permission from4).
Naha et al. developed their anti-biofilm treatments strategy using a platform with long-standing clinical use, suggesting excellent systemic biocompatibility39. Feridex is a systemically delivered iron oxide-based magnetic resonance imaging (MRI) contrast agent which was approved by the Food and Drug Administration (FDA) more than 20 years ago. Oral mucosa is protected, as the iron oxide particles exhibit little enzymatic activity at physiological pH. Additionally, the native microbiota composition and diversity was largely intact post-treatment, indicating that even in close proximity, ROS-mediated damage was limited to the cariogenic S. mutans. No systemic toxicity-related adverse side effects were observed in this study (e.g., no rat weight reduction), when the iron oxide/H2O2 treatment was applied in a once daily regimen for 21 days.
The iron oxide particle-based system has many inherent advantages over other nanoparticle-based systems. It is a drug-free approach, thus overcoming limitations of drug dosing, requirement of drug loading compatibilities, and risks associated with drug resistance. However, there are still outstanding questions regarding the clinical translation of the approach including aesthetics associated with black tooth staining from nanoparticles during treatment and potential off-target effects within the oral cavity and systemically for the CAP-NP/Dex-NZM system and other nanoparticle approaches. To routinely manage oral biofilms, which are pervasive and have genetic and dietary underpinnings, chronic treatment extending for months or years will likely be necessary. Thus, the local and systemic effects of chronic exposure, whereby ingestion is the likely path, remain critical to characterize.
Perspectives and Future Outlook
Ensuring safety of nanoparticle anti-biofilm treatments
Non-specific off-target effects of oral anti-biofilm treatments can occur both immediately to local tissues and after clearance of nanoparticles. The predominant clearance route of topical treatments in the mouth is via ingestion, which may result in systemic circulation and tissue distribution. Therefore, it is critical to evaluate potential off-target biodistribution and effects prior to translation of new anti-biofilm nanotechnologies. This point is especially true for anti-oral biofilm therapies. Caries affects all ages and treatment is a persistent challenge. Thus, chronic off-target accumulation of nanoparticles may result in long-lasting effects. Metal or metal oxide-based nanoparticles can be absorbed within the gastrointestinal tract. Though bioavailability may be low (e.g., <5% of ingested dose), off-target systemic effects of nanoparticles have been reported3. Metal nanoparticles with larger doses have resulted in weight loss and increases in oxidative stress in blood and liver, brain, kidney, and spleen53 with long-term residence in brain54. Additionally, tissue fibrosis55–56 and DNA damage have been reported57. Though it is unclear if iron oxide particles will have similar toxicity profiles, their likely transport through the acidic stomach milieu, which will itself result in robust radical production, motivates careful evaluation of systemic effects of this powerful anti-oral biofilm treatment strategy as well as other nanoparticle-based approaches.
Alternative approaches should be considered that abrogate systemic exposure. For example, nanoparticle systems that dissociate into non-toxic and easily cleared components, such as those formed via self-assembly25–28 or using degradable biomaterials, should be considered. Highly ubiquitous, degradable poly(lactide-co-glycolide)-based nanoparticles with cationic functionalities have been exploited to enhance the bactericidal activity of vancomycin58. Additionally, polymers have been developed that release nitric oxide free radicals that may enable similar proton-catalyzed anti-biofilm effects seen with CAT-NPs59–63. Such NO-releasing systems can be combined with degradable polymers from polyesters64 or polyphosphazenes, which are designed to degrade as a result of nitric oxide release65, to prevent chronic nanoparticle exposure.
Potential to augment efficacy of nanoparticle delivery
Ensuring the optimal efficacy of developed nanoparticle strategies is paramount to limit potential chronic effects due to repeated treatments. While Naha et al. showed significant anti-biofilm efficacy with minimal off-target effects in short duration treatments, two approaches should be considered to further improve this and related strategies. First, introduction of targeting groups may increase selectivity towards virulent versus commensal bacteria. For example, immunoliposomes conjugated with anti-Streptococcus oralis strongly adsorb to S. oralis biofilms while showing decreased affinity to other oral bacteria biofilms66. Moreover, these immunoliposomes successfully encapsulated bactericidal agents and inhibited S. oralis growth more than that of other bacteria tested67. For S. mutans, lectins, including concanavalin-A (Con A) and wheat germ agglutinin (WGA), have been used68. A potential drawback to matrix-targeting therapies is that enhanced binding to superficial regions of the biofilm may also retard biofilm penetration, resulting in poor distribution to interior biofilm regions. As the deeper regions of biofilms harbor resistant cell types (e.g., persisters), this hurdle is significant69. Alternatively, co-delivery of agents that synergistically disrupt biofilms or activate typically dormant and highly resistant persister cells together with bactericidal agents may further increase nanoparticle therapeutic efficacy. Persister cells can be activated by introducing sugar and glycolysis intermediates, such as mannitol, glucose, fructose, and pyruvate70, DNA crosslinkers (e.g., cisplatin71), or cis‐2‐decenoic acid, which results in upregulation of protein synthesis72.
While improvements to nanoparticle-based treatment strategies will augment current anti-biofilm efficacy, it is unclear if the cost-benefit ratio will overcome standard treatment regimens of mechanical clearance (e.g., tooth brushing), antiseptic use (e.g., essential oils, cetylpyridinium chloride, chlorhexidine), and/or topical fluoride applications (e.g., toothpaste, varnish). Furthermore, the paucity of comparisons in the literature to these gold standard treatments during in vivo testing is striking. Regardless, nanoparticle-based anti-biofilm designs developed for treating dental caries will undoubtedly translate to improved treatment strategies for other healthcare-associated biofilms, such as those known to cause orthopaedic and catheter-associated infections.
Alternative biomimetic approaches
The oral microbiome is highly regulated and balanced to ensure homeostasis. The key host factor that modulates the oral microbiome is saliva, which contains a variety of mucin molecules. In particular, the mucin MUC5B plays a critical role in preventing S. mutans surface attachment and biofilm formation by maintaining planktonic growth73. Importantly MUC5B protects by reducing microbial virulence through disrupted quorum sensing rather than binding directly to the microbes73–75. These findings suggest that MUC5B biomimetics may enable balancing of the oral microbiome thereby preventing S. mutans virulence, obviating the need for chronic nanoparticle-based treatments. However, mucin structures are complex and poorly-understood, presenting challenges in the development of engineered mimetics. Alternatively, modulating salivary gland mucous acinar cell synthesis of MUC5B may also be a promising strategy for caries prevention.
Conclusions
Altogether, nanoparticle strategies have promise for anti-oral biofilm treatments, but they have yet to overcome translational hurdles for successful clinical adoption. Naha et al. present compelling data in this issue to support further translational efforts in this field. However, off-target, chronic effects due to routine therapeutic treatment regimens, opportunities for improved efficacy, and novel biomimetic strategies should be carefully considered to ensure continued forward progress for oral and other healthcare-associated biofilm nanoparticle therapeutic technologies.
Acknowledgements
The authors gratefully acknowledge the National Institutes of Health (R01 DE018023 to DB, and F31 DE026944 to KS) and the National Science Foundation (DMR 1206219 to DB) for funding and Marian Ackun-Farmmer for helpful discussions in the development and editing of this manuscript
References
- 1.Baptista PV; McCusker MP; Carvalho A; Ferreira DA; Mohan NM; Martins M; Fernandes AR, Nano-Strategies to Fight Multidrug Resistant Bacteria-”a Battle of the Titans”. Front Microbiol 2018, 9, 1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benoit DS; Koo H, Targeted, Triggered Drug Delivery to Tumor and Biofilm Microenvironments. Nanomedicine (Lond) 2016, 11, 873–879. [DOI] [PubMed] [Google Scholar]
- 3.Besinis A; De Peralta T; Tredwin CJ; Handy RD, Review of Nanomaterials in Dentistry: Interactions with the Oral Microenvironment, Clinical Applications, Hazards, and Benefits. ACS Nano 2015, 9, 2255–2289. [DOI] [PubMed] [Google Scholar]
- 4.Cormode DP; Gao L; Koo H, Emerging Biomedical Applications of Enzyme-Like Catalytic Nanomaterials. Trends Biotechnol 2018, 36, 15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta A; Landis RF; Rotello VM, Nanoparticle-Based Antimicrobials: Surface Functionality Is Critical. F1000Res 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo H; Allan RN; Howlin RP; Stoodley P; Hall-Stoodley L, Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nature reviews. Microbiology 2017, 15, 740–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krzysciak W; Jurczak A; Koscielniak D; Bystrowska B; Skalniak A, The Virulence of Streptococcus Mutans and the Ability to Form Biofilms. Eur J Clin Microbiol Infect Dis 2014, 33, 499–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y; Shi L; Su L; van der Mei HC; Jutte PC; Ren Y; Busscher HJ, Nanotechnology-Based Antimicrobials and Delivery Systems for Biofilm-Infection Control. Chem Soc Rev 2019, 48, 428–446. [DOI] [PubMed] [Google Scholar]
- 9.Marsh PD, Controlling the Oral Biofilm with Antimicrobials. J Dent 2010, 38 Suppl 1, S11–15. [DOI] [PubMed] [Google Scholar]
- 10.Lebeaux D; Ghigo JM; Beloin C, Biofilm-Related Infections: Bridging the Gap between Clinical Management and Fundamental Aspects of Recalcitrance toward Antibiotics. Microbiol Mol Biol Rev 2014, 78, 510–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall-Stoodley L; Costerton JW; Stoodley P, Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nature reviews. Microbiology 2004, 2, 95–108. [DOI] [PubMed] [Google Scholar]
- 12.Marsh PD; Moter A; Devine DA, Dental Plaque Biofilms: Communities, Conflict and Control. Periodontology 2000 2011, 55, 16–35. [DOI] [PubMed] [Google Scholar]
- 13.Flemmig TF; Beikler T, Control of Oral Biofilms. Periodontology 2000 2011, 55, 9–15. [DOI] [PubMed] [Google Scholar]
- 14.Flemming HC; Wingender J, The Biofilm Matrix. Nature reviews. Microbiology 2010, 8, 623–633. [DOI] [PubMed] [Google Scholar]
- 15.Stewart PS; Franklin MJ, Physiological Heterogeneity in Biofilms. Nature reviews. Microbiology 2008, 6, 199–210. [DOI] [PubMed] [Google Scholar]
- 16.Koo H; Falsetta ML; Klein MI, The Exopolysaccharide Matrix: A Virulence Determinant of Cariogenic Biofilm. Journal of dental research 2013, 92, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowen WH; Koo H, Biology of Streptococcus Mutans-Derived Glucosyltransferases: Role in Extracellular Matrix Formation of Cariogenic Biofilms. Caries research 2011, 45, 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo L; Hu W; He X; Lux R; McLean J; Shi W, Investigating Acid Production by Streptococcus Mutans with a Surface-Displayed Ph-Sensitive Green Fluorescent Protein. PloS one 2013, 8, e57182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao J; Klein MI; Falsetta ML; Lu B; Delahunty C; Yates JR; Heydorn A; Koo H, The Exopolysaccharide Matrix Modulates the Interaction between 3d Architecture and Virulence of a Mixed-Species Oral Biofilm. PloS Pathogens 2012, DOI: 10.1371/journal.ppat.1002623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercier C; Durrieu C; Briandet R; Domakova E; Tremblay J; Buist G; Kulakauskas S, Positive Role of Peptidoglycan Breaks in Lactococcal Biofilm Formation. Mol Microbiol 2002, 46, 235–243. [DOI] [PubMed] [Google Scholar]
- 21.Donlan RM; Costerton JW, Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin Microbiol Rev 2002, 15, 167–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koo H; Hayacibara MF; Schobel BD; Cury JA; Rosalen PL; Park YK; Vacca-Smith AM; Bowen WH, Inhibition of Streptococcus Mutans Biofilm Accumulation and Polysaccharide Production by Apigenin and Tt-Farnesol. The Journal of antimicrobial chemotherapy 2003, 52, 782–789. [DOI] [PubMed] [Google Scholar]
- 23.Koo H; Pearson SK; Scott-Anne K; Abranches J; Cury JA; Rosalen PL; Park YK; Marquis RE; Bowen WH, Effects of Apigenin and Tt-Farnesol on Glucosyltransferase Activity, Biofilm Viability and Caries Development in Rats. Oral microbiology and immunology 2002, 17, 337–343. [DOI] [PubMed] [Google Scholar]
- 24.Koo H; Schobel B; Scott-Anne K; Watson G; Bowen WH; Cury JA; Rosalen PL; Park YK, Apigenin and Tt-Farnesol with Fluoride Effects on S. Mutans Biofilms and Dental Caries. Journal of dental research 2005, 84, 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duncan B; Li X; Landis RF; Kim ST; Gupta A; Wang LS; Ramanathan R; Tang R; Boerth JA; Rotello VM, Nanoparticle-Stabilized Capsules for the Treatment of Bacterial Biofilms. ACS Nano 2015, 9, 7775–7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horev B; Klein MI; Hwang G; Li Y; Kim D; Koo H; Benoit DS, Ph-Activated Nanoparticles for Controlled Topical Delivery of Farnesol to Disrupt Oral Biofilm Virulence. ACS Nano 2015, 9, 2390–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sims KR; Liu Y; Hwang G; Jung HI; Koo H; Benoit DSW, Enhanced Design and Formulation of Nanoparticles for Anti-Biofilm Drug Delivery. Nanoscale 2018, 11, 219–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J; Horev B; Hwang G; Klein MI; Koo H; Benoit DS, Characterization and Optimization of Ph-Responsive Polymer Nanoparticles for Drug Delivery to Oral Biofilms. J Mater Chem B 2016, 4, 3075–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeon JG; Pandit S; Xiao J; Gregoire S; Falsetta ML; Klein MI; Koo H, Influences of Trans-Trans Farnesol, a Membrane-Targeting Sesquiterpenoid, on Streptococcus Mutans Physiology and Survival within Mixed-Species Oral Biofilms. International journal of oral science 2011, 3, 98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L-S; Gupta A; Rotello VM, Nanomaterials for the Treatment of Bacterial Biofilms. ACS Infectious Diseases 2015, Article ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huh AJ; Kwon YJ, “Nanoantibiotics”: A New Paradigm for Treating Infectious Diseases Using Nanomaterials in the Antibiotics Resistant Era. J Control Release 2011, 156, 128–145. [DOI] [PubMed] [Google Scholar]
- 32.Peulen TO; Wilkinson KJ, Diffusion of Nanoparticles in a Biofilm. Environ Sci Technol 2011, 45, 3367–3373. [DOI] [PubMed] [Google Scholar]
- 33.Li XN; Yeh YC; Giri K; Mout R; Landis RF; Prakash YS; Rotello VM, Control of Nanoparticle Penetration into Biofilms through Surface Design. Chem Commun 2015, 51, 282–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slomberg DL; Lu Y; Broadnax AD; Hunter RA; Carpenter AW; Schoenfisch MH, Role of Size and Shape on Biofilm Eradication for Nitric Oxide-Releasing Silica Nanoparticles. ACS Appl Mater Interfaces 2013, 5, 9322–9329. [DOI] [PubMed] [Google Scholar]
- 35.Petros RA; DeSimone JM, Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat Rev Drug Discov 2010, 9, 615–627. [DOI] [PubMed] [Google Scholar]
- 36.Mogen AB; Chen F; Ahn SJ; Burne RA; Wang D; Rice KC, Pluronics-Formulated Farnesol Promotes Efficient Killing and Demonstrates Novel Interactions with Streptococcus Mutans Biofilms. PloS one 2015, 10, e0133886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen F; Rice KC; Liu XM; Reinhardt RA; Bayles KW; Wang D, Triclosan-Loaded Tooth-Binding Micelles for Prevention and Treatment of Dental Biofilm. Pharmaceutical research 2010, 27, 2356–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen F; Liu XM; Rice KC; Li X; Yu F; Reinhardt RA; Bayles KW; Wang D, Tooth-Binding Micelles for Dental Caries Prevention. Antimicrob Agents Chemother 2009, 53, 4898–4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naha PC; Liu Y; Hwang G; Huang Y; Gubara S; Jonnakuti V; Simon-Soro A; Kim D; Gao L; Koo H; Cormode DP, Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysis for Localized and Ph-Activated Biofilm Distruption. ACS Nano 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dakal TC; Kumar A; Majumdar RS; Yadav V, Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front Microbiol 2016, 7, 1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemeg HA, Nanomaterials for Alternative Antibacterial Therapy. Int J Nanomedicine 2017, 12, 8211–8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slavin YN; Asnis J; Hafeli UO; Bach H, Metal Nanoparticles: Understanding the Mechanisms Behind Antibacterial Activity. J Nanobiotechnology 2017, 15, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aderibigbe BA, Metal-Based Nanoparticles for the Treatment of Infectious Diseases. Molecules 2017, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.AlMatar M; Makky EA; Var I; Koksal F, The Role of Nanoparticles in the Inhibition of Multidrug-Resistant Bacteria and Biofilms. Curr Drug Deliv 2018, 15, 470–484. [DOI] [PubMed] [Google Scholar]
- 45.Natan M; Banin E, From Nano to Micro: Using Nanotechnology to Combat Microorganisms and Their Multidrug Resistance. FEMS Microbiol Rev 2017, 41, 302–322. [DOI] [PubMed] [Google Scholar]
- 46.Bassegoda A; Ivanova K; Ramon E; Tzanov T, Strategies to Prevent the Occurrence of Resistance against Antibiotics by Using Advanced Materials. Appl Microbiol Biotechnol 2018, 102, 2075–2089. [DOI] [PubMed] [Google Scholar]
- 47.Katva S; Das S; Moti HS; Jyoti A; Kaushik S, Antibacterial Synergy of Silver Nanoparticles with Gentamicin and Chloramphenicol against Enterococcus Faecalis. Pharmacogn Mag 2018, 13, S828–S833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siddiqi KS; Husen A; Rao RAK, A Review on Biosynthesis of Silver Nanoparticles and Their Biocidal Properties. J Nanobiotechnology 2018, 16, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wood TK, Strategies for Combating Persister Cell and Biofilm Infections. Microb Biotechnol 2017, 10, 1054–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao L; Liu Y; Kim D; Li Y; Hwang G; Naha PC; Cormode DP; Koo H, Nanocatalysts Promote Streptococcus Mutans Biofilm Matrix Degradation and Enhance Bacterial Killing to Suppress Dental Caries in Vivo. Biomaterials 2016, 101, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dwivedi S; Wahab R; Khan F; Mishra YK; Musarrat J; Al-Khedhairy AA, Reactive Oxygen Species Mediated Bacterial Biofilm Inhibition Via Zinc Oxide Nanoparticles and Their Statistical Determination. PloS one 2014, 9, e111289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y; Zhang W; Niu J; Chen Y, Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [DOI] [PubMed] [Google Scholar]
- 53.Shrivastava R; Kushwaha P; Bhutia YC; Flora S, Oxidative Stress Following Exposure to Silver and Gold Nanoparticles in Mice. Toxicol Ind Health 2016, 32, 1391–1404. [DOI] [PubMed] [Google Scholar]
- 54.van der Zande M; Vandebriel RJ; Van Doren E; Kramer E; Herrera Rivera Z; Serrano-Rojero CS; Gremmer ER; Mast J; Peters RJ; Hollman PC; Hendriksen PJ; Marvin HJ; Peijnenburg AA; Bouwmeester H, Distribution, Elimination, and Toxicity of Silver Nanoparticles and Silver Ions in Rats after 28-Day Oral Exposure. ACS Nano 2012, 6, 7427–7442. [DOI] [PubMed] [Google Scholar]
- 55.Wang J; Zhou G; Chen C; Yu H; Wang T; Ma Y; Jia G; Gao Y; Li B; Sun J; Li Y; Jiao F; Zhao Y; Chai Z, Acute Toxicity and Biodistribution of Different Sized Titanium Dioxide Particles in Mice after Oral Administration. Toxicol Lett 2007, 168, 176–185. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z; Meng H; Xing G; Chen C; Zhao Y; Jia G; Wang T; Yuan H; Ye C; Zhao F; Chai Z; Zhu C; Fang X; Ma B; Wan L, Acute Toxicological Effects of Copper Nanoparticles in Vivo. Toxicol Lett 2006, 163, 109–120. [DOI] [PubMed] [Google Scholar]
- 57.Trouiller B; Reliene R; Westbrook A; Solaimani P; Schiestl RH, Titanium Dioxide Nanoparticles Induce DNA Damage and Genetic Instability in Vivo in Mice. Cancer Res 2009, 69, 8784–8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radovic-Moreno AF; Lu TK; Puscasu VA; Yoon CJ; Langer R; Farokhzad OC, Surface Charge-Switching Polymeric Nanoparticles for Bacterial Cell Wall-Targeted Delivery of Antibiotics. ACS Nano 2012, 6, 4279–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Namivandi-Zangeneh R; Sadrearhami Z; Bagheri A; Sauvage-Nguyen M; Ho KKK; Kumar N; Wong EHH; Boyer C, Nitric Oxide-Loaded Antimicrobial Polymer for the Synergistic Eradication of Bacterial Biofilm. Acs Macro Lett 2018, 7, 592–597. [DOI] [PubMed] [Google Scholar]
- 60.Yang L; Schoenfisch MH, Nitric Oxide-Releasing Hyperbranched Polyaminoglycosides for Antibacterial Therapy. ACS Applied Bio Materials 2018, 1, 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duong HTT; Kamarudin ZM; Erlich RB; Li Y; Jones MW; Kavallaris M; Boyer C; Davis TP, Intracellular Nitric Oxide Delivery from Stable No-Polymeric Nanoparticle Carriers. Chem Commun 2013, 49, 4190–4192. [DOI] [PubMed] [Google Scholar]
- 62.Kanayama N; Yamaguchi K; Nagasaki Y, Pegylated Polymer Micelle-Based Nitric Oxide (No) Photodonor with No-Mediated Antitumor Activity. Chem Lett 2010, 39, 1008–1009. [Google Scholar]
- 63.Sorragi CD; Shishido SM; Lemos ME; Marcondes S; Antunes E; Krieger MH, In Vitro Evaluation of the Safe Margin, Antithrombotic and Antiproliferative Actions for the Treatment of Restenosis: Nitric Oxide Donor and Polymers. Cell Biochem Funct 2011, 29, 207–214. [DOI] [PubMed] [Google Scholar]
- 64.Coneski PN; Rao KS; Schoenfisch MH, Degradable Nitric Oxide-Releasing Biomaterials Via Post-Polymerization Functionalization of Cross-Linked Polyesters. Biomacromolecules 2010, 11, 3208–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lutzke A; Neufeld BH; Neufeld MJ; Reynolds MM, Nitric Oxide Release from a Biodegradable Cysteine-Based Polyphosphazene. Journal of Materials Chemistry B 2016, 4, 1987–1998. [DOI] [PubMed] [Google Scholar]
- 66.M Robinson A; Creeth J; N Jones M, The Specificity and Affinity of Immunoliposome Targeting to Oral Bacteria. 1998; Vol. 1369, p 278–286. [DOI] [PubMed] [Google Scholar]
- 67.Robinson AM; Creeth JE; Jones MN, The Use of Immunoliposomes for Specific Delivery of Antimicrobial Agents to Oral Bacteria Immobilized on Polystyrene. Journal of biomaterials science. Polymer edition 2000, 11, 1381–1393. [DOI] [PubMed] [Google Scholar]
- 68.Forier K; Raemdonck K; De Smedt SC; Demeester J; Coenye T; Braeckmans K, Lipid and Polymer Nanoparticles for Drug Delivery to Bacterial Biofilms. J Control Release 2014, 190, 607–623. [DOI] [PubMed] [Google Scholar]
- 69.Lebeaux D; Chauhan A; Letoffe S; Fischer F; de Reuse H; Beloin C; Ghigo JM, Ph-Mediated Potentiation of Aminoglycosides Kills Bacterial Persisters and Eradicates in Vivo Biofilms. J Infect Dis 2014, 210, 1357–1366. [DOI] [PubMed] [Google Scholar]
- 70.Allison KR; Brynildsen MP; Collins JJ, Metabolite-Enabled Eradication of Bacterial Persisters by Aminoglycosides. Nature 2011, 473, 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chowdhury N; Wood TL; Martinez-Vazquez M; Garcia-Contreras R; Wood TK, DNA-Crosslinker Cisplatin Eradicates Bacterial Persister Cells. Biotechnol Bioeng 2016, 113, 1984–1992. [DOI] [PubMed] [Google Scholar]
- 72.Marques CNH; Morozov A; Planzos P; Zelaya HM, The Fatty Acid Signaling Molecule Cis-2-Decenoic Acid Increases Metabolic Activity and Reverts Persister Cells to an Antimicrobial-Susceptible State. Appl Environ Microb 2014, 80, 6976–6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frenkel ES; Ribbeck K, Salivary Mucins Protect Surfaces from Colonization by Cariogenic Bacteria. Appl Environ Microbiol 2015, 81, 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frenkel ES; Ribbeck K, Salivary Mucins in Host Defense and Disease Prevention. J Oral Microbiol 2015, 7, 29759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frenkel ES; Ribbeck K, Salivary Mucins Promote the Coexistence of Competing Oral Bacterial Species. ISME J 2017, 11, 1286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]