Abstract
Background:
The main purpose of the present study was to examine the startle reflex in individuals diagnosed with Generalized Anxiety Disorder (GAD) and control participants in terms of three questions. First, is the basic startle reflex modulated by autonomic nervous system (ANS) activation and/or attentional focus? Second, are induced and self-reported emotional states related to the magnitude of the startle response? And third, do individuals with GAD and their controls show differential startle responses?
Methods:
Experimental tasks designed to elicit sympathetic and parasympathetic activation and requiring internal and external attention foci were administered to nine individuals with GAP and nine controls.
Results:
Individuals with GAD showed a greater startle reflex than controls during involvement in tasks that either induced worry or relaxation but not during a baseline period. Startle responses differed in terms of intentional focus but not ANS activity. During baseline and emotional induction, self-reported negative emotionality was significantly correlated with magnitude of the startle response.
Conclusions:
These results suggest that negative emotionality at the time of the startle probe is an important determinant. Further, attentional focus plays a more important role in startle modulation than autonomic nervous system manipulation. These results are discussed in relation to negative emotion, focus of attention, and use of the startle response as a measure of change during psychotherapy.
Keywords: Generalized Anxiety Disorder, startle response, negative emotionality
INTRODUCTION
The startle reflex is a set of skeletomuscular contractions[1–3] viewed as a behavioral interrupt that prepares the organism for action.[4] It is elicited by an intense stimulus of sudden onset and generally measured by its eyeblink component in humans.[5] Animal research suggests that the neuronal pathway of the acoustic startle is primitive and consists of only three synapses that connect the cochlear root neurons, neurons in the nucleus reticularis pontis caudalis, and motorneurons in the facial motor nucleus.[2,6]
The startle response can be amplified as an expression of fear and is significantly increased in the presence of an aversive conditioned stimulus.[7] Through a series of lesion and stimulation studies, it has been determined that regions of the limbic brain, specifically the central nucleus of the amygdala, potentiate the startle response to explicit aversive cues at the level of the nucleus reticularis pontis caudalis.[8] Further, benzodiazepine anxiolytics block fear-potentiated startle in both rodents[9] and humans.[10]
Given the simple neural circuitry and ease of measurement, the startle response has proven an important tool for understanding emotional information processing in both animals[6,11] and humans [for overviews, see].[12–14] Indeed, numerous studies suggest that emotional state is an important variable in the modulation of the exaggerated startle reflex].[15] Research with non-anxious participants indicates that both anticipation of aversive situations (e.g., anticipation of shock) and processing negatively valenced information (e.g., viewing slides, imagining fearful situations) augment the startle reflex.[11,16–21] Positive states related to picture viewing diminish the reflex.[11]
This pattern of startle reflex modulation by valence is a robust finding that holds across a variety of negatively and positively valent states when arousal/activation level is controlled for as well as across methods for eliciting these states in both humans and animals with few exceptions [for reviews, see].[11, 12, 19] This work has theoretically utilized the distinction made by[22, 23] of two motivational systems of which one is appetitive reflecting approach and pleasantness and the other aversive reflecting avoidance and unpleasantness.[11] suggested that the startle response activates the aversive system and will be augmented if it is presented during a negative stimulus and reduced if presented during a positive stimulus. It should be noted, however, that modulation of startle by valence is directly related to arousal level such that at higher levels of arousal greater modulation by valence is found.[24] Moreover, some reports suggest that arousal alone may play a more important role than valence in modulation of startle activity when reflexes other than eyeblink are measured.[25,26] For instance, emotions marked by readiness to respond (activation/arousal) to both positively and negatively valenced stimuli resulted in enhanced reflexive responding in a leg muscle compared to that seen in response to neutral stimuli in response to a hammertap to the heel tendon.
Historically, an exaggerated startle reflex has been associated with a variety of psychopathological disorders including “combat neurosis” and posttraumatic stress disorder [PTSD].[3,27] In the revised third edition of the Diagnostic and Statistical Manual of Mental Disorders [DSM-III-R],[28] an exaggerated startle reflex was one possible diagnostic criterion for generalized anxiety disorder (GAD) and PTSD. DSM-IV (1994),[29] included an exaggerated startle reflex as an associated feature, rather than a diagnostic criterion, of GAD. Given that to date no published study has examined the startle reflex in individuals diagnosed with GAD, we undertook this study to elucidate the relationship between GAD and the startle response by comparing participants with GAD to a non-anxious control group during both relaxation and worry inductions.
Additional mechanisms such as attentional engagement[30,31] and motivational states reflected in the autonomic nervous system [ANS][19,32] have also been implicated in contributing to startle amplitude. Startle reflex studies involving social anxiety suggest that an attentional component associated with inward directed attention is involved in attenuating startle magnitude.[33] Furthermore,[34] reported larger startle responses during a self-focused attentional task (being monitored by a video camera) than in a non-self-focus condition with socially anxious undergraduates. Given that anxiety processes such as worry and self-monitoring are internal in nature[35] and that attentional focus has been found to be important from a psychophysiological perspective,[36–38] we sought to probe the attentional component by utilizing tasks that directly manipulate attentional focus in participants with and without GAD.
In terms of ANS activation,[32] reported that the startle response is augmented during sympathetic stimulation by epinephrine and reduced using the β-blocker esmolol. Although to our knowledge parasympathetic activation has not been examined in a startle study, Thayer et al.[39] suggested differential (reduced) parasympathetic activation in GAD individuals compared to controls. Overall, this would suggest that the startle response would be greater on tasks that are associated with sympathetic activation and lessened on those involving parasympathetic activation. In this study the influence of attentional and ANS activation on startle magnitude was studied in a 2 × 2 manner by presenting stimuli that involved either external or internal focus of attention in tasks known to increase sympathetic or parasympathetic nervous system responding.
Startle studies involving anxiety-related diagnoses have included PTSD,[40–42] panic disorder,[19,43] and specific phobias.[44,45] In response to aversive or threatening stimuli, PTSD individuals showed larger startle responses than non-anxious controls.[41,46] Studies that examined baseline startle with PTSD have reported inconsistent results (both increases and no differences) in startle responses compared to controls.[47–49] With an adolescent PTSD population,[48] even reported a reduced startle during baseline.[50] sought to test the hypothesis that PTSD combat veterans suffer from an enhanced sensitivity to stressful experimental contexts during a two-session experiment. In the first session, in which there was no experimental stress, baseline startle in the PTSD group did not differ from matched controls. In the second session in which aversive shocks were anticipated, startle magnitude was elevated throughout the session in the PTSD group. Studies with panic disorder and phobia have likewise found exaggerated startle to fear stimuli (e.g., spiders in spider phobics), but failed to find increased startle responses during baseline conditions,[43] which suggests that an exaggerated startle reflex is not specific to any one anxiety disorder and is most likely modulated by the emotional state of the individual. However, given that individuals with GAD are characterized by a high degree of internally focused worry in many situations, it remains an open question as to whether GAD individuals will show exaggerated startle in baseline as well as in anxiety-provoking situations.
We designed this study to examine GAD participants’ startle responses in comparison to matched controls in three conditions: a baseline situation; experimental tasks designed to elicit sympathetic/parasympathetic activation by internal/external attention foci; and a relaxation and a worry induction condition. Self-report data were used as a manipulation check and control for emotionality, including activation, throughout the study.
METHODS
PARTICIPANTS
Participants were nine female Caucasian treatment-seeking individuals who met DSM-IV criteria for primary GAD according to two separate diagnostic interviews by independent raters. Nine controls who matched for age, sex, ethnicity, and education composed a comparison group. All controls were free of lifetime and current axis I disorders and had never received psychotherapy or psychopharmacological treatments. No GAD or control individual was taking psychotropic medications at the time of the study. All participants were diagnosed with the Anxiety Disorders Interview Schedule for DSM-IV diagnoses.[51] The mean age of the sample was 36 for the GAD group and 35 for the control group.
PROCEDURE
A week before the recording session, participants were familiarized with the psychophysiological laboratory and recording procedures. In addition to the structured diagnostic interviews during which the Hamilton Depression and Anxiety Scales[52,53] were administered, participants had also completed in a separate session a number of self-report measures including the Penn State Worry Questionnaire,[54] the Beck Depression Inventory,[55] and the Trait version of the Spielberger State–Trait Anxiety Inventory.[56] At the beginning of the recording session, participants gave informed consent and then were seated in a recliner and the appropriate electrodes attached. To measure startle reflex amplitude, two 4-mm Ag/AgCl electrodes were filled with high-conductivity electrode gel and placed over the orbicularis oculi muscle below the left pupil. If the impedance levels were not below 10 kΩ this procedure was repeated. All contact lens wearers removed contact lenses before electrodes were affixed and wore eye glasses when needed throughout the experiment. Electrodes to measure electroencephalograph and heart rate (HR) activity were also attached. Before the startle section of the experiment, participants engaged in preliminary relaxation and worry induction procedures that lasted for 10 min each without any startle stimuli.
After all electrodes were attached, a standardized overview of the experiment was provided. Each participant was then asked to select a topic about which she was currently most worried so that the topic would be readily available when instructions were later received for the worry task. After the experimenter left the room, audiotape instructions directed the participant to familiarize herself with a modified version of Osgood’s Semantic Differential Scale.[57] Participants rated 15 differentials used to assess emotional state after each of the experimental tasks.
At the beginning of the startle portion of the experiment, all participants were informed that they may hear brief noises and to pay no attention to them. The session was composed of a baseline period (5 min), the attentional (internal and external) tasks by ANS (sympathetic and parasympathetic) tasks (each task lasted 2.5 min), and the subsequent relaxation and worry induction tasks that lasted 5 min each. The sympathetic tasks required participants to silently verbalize the colors in which various color adjectives were displayed (Stroop) and to subtract 7 from 11,023 consecutively (mental math). The parasympathetic tasks included watching a video of birds flying over the ocean with pleasant background music and imagining eating one’s favorite food. Two tasks required internal focus (mental math and imagining food) and two tasks required external focus (Stroop and video). After each task, each participant completed the modified Osgood self-report measures to indicate how she felt during that task. To wipe out the effect of each task, the participant was instructed to relax with eyes closed for 90 s after completing the self-report measure and before proceeding to the next task. The only parts of the experiment during which participants had their eyes open during assessment of electromyographic (EMG) activity were the Stroop and relaxing video tasks. After the presentation of these four attention-focusing tasks, participants engaged in a 5-min period of relaxing themselves through slowed breathing and a 5-min period of worrying about their previously chosen topic. Electrodes were removed, participants were debriefed, and the experimenter ensured that any adverse experiences were discussed and that there were no adverse effects of the experiment.
The startle stimulus consisted of a 95 dB fast rise time 50-ms white noise signal presented biaurally through headphones. In sum, a total of 28 startle probes were delivered during this experiment with six startle probes delivered during the 5-min baseline task, three during each of the four 2.5-min sympathetic/parasympathetic by internal/external tasks, and five each during the final relaxation and worry tasks. All startle probes were presented at random intervals within each task, with the constraint that (a) at least 30 s elapsed during any task before the presentation of the first probe and (b) a minimum of 30 s always elapsed between startle probes.
DATA REDUCTION
Digitized raw EMG signals from the orbicularis oculi were amplified at a constant level for all participants and sampled at a 1,000 Hz rate 200 ms before and 800 ms after the startle stimulus. A high-pass filter was used to remove slow wave activity. The EMG signal was half-wave rectified and integrated using Matlab software developed by our lab. Graphic presentations of these data (i.e., raw EMG, half-wave rectified EMG, integrated EMG) for the 1,000-ms period were visually inspected to ensure the startle response occurred within the 30–50-ms poststartle probe window.[5] Of the 504 startle probes analyzed in this study, 28 could not be used because of technical difficulties or because the response occurred before 30 or after 50 ms. The maximum of the integrated signal was used as the startle measure for this study.
To measure cardiovascular activity, a Matlab program was used to identify R-spikes within the sampled data to measure HR and also calculate the time between adjacent R-spikes (heart period). Heart period data were analyzed separately from HR and used to conduct a power spectral analysis of HR variability using the Porges–Bohrer filter. Spectral power in the 0.12–0.40 Hz band has been shown to be a good measure of parasympathetic activity and often referred to as vagal tone.
RESULTS
DATA ANALYSES
Startle reflex magnitude.
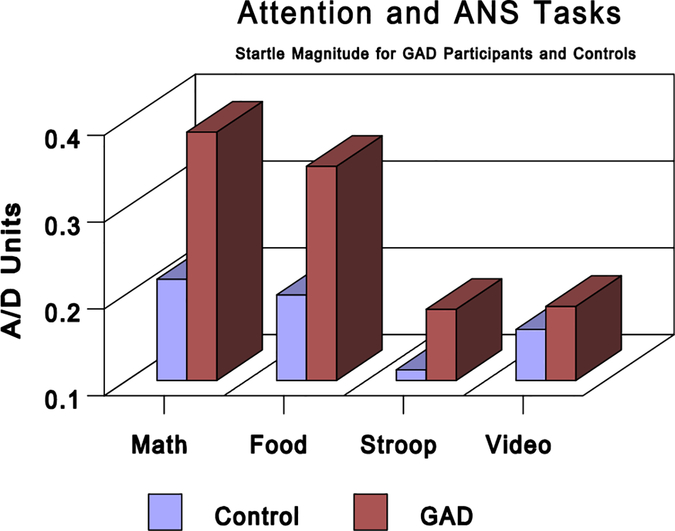
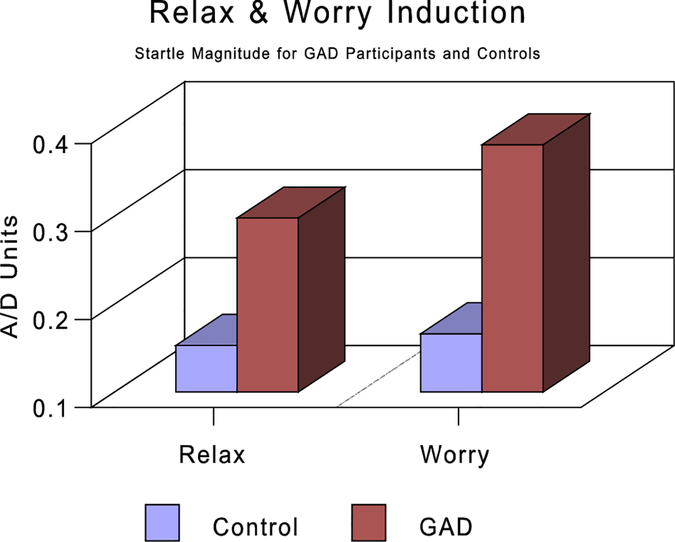
Startle reflex responses were averaged over each of the following periods: (a) startle baseline, (b) Stroop task, (c) food imagery, (d) mental math, (e) video, (f) relaxation, and (g) worry. To determine the effects of attentional focus and ANS activation, a 2 (internal versus external focus-of-attention tasks) × 2 (sympathetic versus parasympathetic tasks) analysis of variance (ANOVA) was performed with GAD/control status as a between-group factor. A main effect for group was found in which GAD participants had a significantly larger startle magnitude (.28 versus .17) than controls, F(1, 15) = 6.36, P<.023. There was also a significant main effect for type of attention tasks, F(1, 16) = 22.04, P<.001, but not for type of ANS tasks, F(1, 16) = 0.01, ns. Larger startle responses were seen during the internal as opposed to external focus-of-attention task (.29 versus .16). The group by attention interaction approached significance, F(1, 16) = 4.15, P<.059, with the GAD participants showing greater differentiation between the attentional task than the controls (see Fig. 1). A separate group (GAD versus control) by task (relaxation and worry) ANOVA showed significantly larger startle responses for participants with GAD as compared to controls, F(1, 14) = 5.22, P<.037. Neither the task, nor the task by group interaction, was statistically significant (see Fig. 2). Further, a between-groups ANOVA with startle response at baseline as the dependent measure showed no significant differences between the startle magnitude of the controls (m = .29, SD = .22) compared to those with GAD (m = .39, SD = .18; F(1, 16) = 1.248, P<.28).
Figure 1.
Startle magnitude in A/D units for GAD participants and controls during attentional and autonomic nervous system (ANS) tasks. The tasks are mental math (internal, sympathetic), imagining favorite food (internal, parasympathetic), Stroop (external, sympathetic), and watching a video (external, parasympathetic). GAD, generalized anxiety disorder.
Figure 2.
Startle magnitude in A/D units for GAD participants and controls during relaxation and worry induction. See text for complete description. GAD, generalized anxiety disorder.
Cardiovascular measures.
The effects of attentional focus and ANS activation were analyzed with a 2 (internal versus external focus-of-attention tasks) × 2 (sympathetic versus parasympathetic tasks) ANOVA for average HR and vagal tone between the GAD and control groups. As expected, parasympathetic tasks (food imagery and video) as compared to sympathetic tasks showed significantly lower HR (70.05 versus 73.34 BPM, F(1, 15) = 16.59, P<.001) and higher vagal tone (6.12 versus 5.86, F(1, 14) = 5.97, P<.028). There was also a significant attentional effect with internal tasks showing higher HRs than external tasks (72.85 versus 70.53, F(1, 15) = 17.37, P<.001) and lower vagal tone (5.88 versus 6.10, F(1, 14) = 4.84, P<.05). The interaction of attention focus and group was not significant for either HR or vagal tone. The interaction of ANS task type with group was not significant for HR but was for vagal tone (F(1, 14) = 8.57, P<.01) with the control group showing greater vagal tone during the parasympathetic than the sympathetic tasks (6.3 versus 5.7, respectively) than that shown by the group with GAD (5.9 versus 6.0, respectively). An overall interaction between group, attentional focus, and ANS tasks was not significant.
Self-report measures.
Factor analytic studies have shown that items such as those found on our semantic differential form two factors, one for activation or arousal and one for emotional valence.[57] In this study cluster analyses of the 15 semantic differentials for each condition were used to derive two summary measures, that of activation and valence. Every item in the activation composite measure and every item in the valence composite measure except for one loaded only on its expected cluster for all seven conditions (baseline and tasks); the exceptional item was consistent for six of seven conditions. The arousal/activation measure consisted of the anxiety/relaxed, angry/glad, agitated/peace, tension/calm, fear/courage, displeasure/pleasure differentials. The valence/power measure consisted of the happy/sad, kind/cruel, powerful/weak, active/passive, and positive/negative differentials. A group by activation/valence multivariate analysis of variance was significant for baseline (F(4, 10) = 8.37, P<.001), attention by sympathetic/parasympathetic tasks (F(4, 10) = 6.23, P<.001), and relaxation/worry inductions (F(4, 10) = 7.43, P<.001). Univariate tests showed significant group differences for both measures on all conditions except the activation ratings on the Stroop and mental math task and the worry induction. Specifically, the group with GAD reported significantly greater levels of activation than the controls during all tasks except for the Stroop, mental math, and worry tasks. The group with GAD reported a higher level of negative emotion than the controls during all tasks. Table 1 shows the rating on these two measures for all conditions of the experiment by group. The two composite self-report measures were also correlated with the startle magnitude during the seven conditions of the experiment. These data are shown in Table 2 and reveal that startle magnitude increases were significantly associated with reports of greater activation during the relax and worry periods and with negative valence during the baseline, relax, worry, and Stroop periods.
TABLE 1.
Self-report rating for the activation and valence differentials during the experimental conditions
| Period | |||||||
|---|---|---|---|---|---|---|---|
| Item rated | Baseline | Stroop | Imagery | Math | Video | Relax | Worry |
| Activation | |||||||
| Control | 4.05 (1.22) | 5.35 (2.11) | 6.97 (1.88) | 3.77 (1.23) | 7.38 (1.62) | 7.16 (1.90) | 4.24 (2.26) |
| GAD | 1.95 (1.33) | 3.60 (2.12) | 3.14 (1.34) | 2.54 (1.61) | 3.87 (2.10) | 4.16 (1.34) | 3.44 (2.39) |
| Valence | |||||||
| Control | 4.38 (0.97) | 2.34 (1.22) | 3.28 (1.27) | 3.34 (1.87) | 4.09 (1.11) | 4.70 (1.08) | 4.55 (1.20) |
| GAD | 5.79 (0.81) | 4.67 (2.02) | 5.56 (1.32) | 5.95 (2.27) | 6.30 (1.17) | 6.10 (1.17) | 6.04 (1.20) |
Note: Larger numbers reflect less activation (e.g., more relaxation) and more negative valence. GAD, generalized anxiety disorder.
TABLE 2.
Correlations between the self-report composite measures and startle magnitude for all participants
| Period | |||||||
|---|---|---|---|---|---|---|---|
| Item rated | Baseline | Relax | Worry | Stroop | Imagery | Math | Video |
| Activation | −0.35 | −0.56* | −0.61* | −0.47 | −0.31 | −0.22 | 0.10 |
| Valence | 0.59* | 0.66** | 0.75** | 0.54* | 0.34 | 0.30 | −0.19 |
Note: Larger numbers reflect less activation (e.g., more relaxation) and more negative valence.
P<.05;
P<.01.
DISCUSSION
To better understand the startle response in anxiety, we examined ANS and attentional factors previously associated with differential startle responses. This can be conceptualized in terms of three questions. First, is the basic startle reflex modulated by ANS activation and or attentional focus? Second, are induced and self-reported emotional states related to the magnitude of the startle response? In addition, third, do individuals with GAD and their controls show differential startle responses?
ANS functioning has been implicated in contributing to startle amplitude with[28] showing augmented startle with sympathetic stimulation and reduced startle with β-blockers, a sympathetic antagonist. Also, both GAD and worry have been associated with less parasympathetic nervous system activity and thus differential activation of the ANS compared to controls by.[39] This led us to directly test the manner in which startle responses might vary in relation to differential ANS activity in general and whether there would be a differential effect in those with GAD compared to controls. In our study, tasks designed to elicit differential ANS functioning had no influence on the startle response of either the group with GAD or the controls. Unlike,[32] we found ANS influence to be unrelated to startle response. One difference in our work and theirs is that we did not directly manipulate physiological state through pharmacological agents. Further,[58] studied young boys and reported that the startle response appeared to be independent of the immediate ANS and central nervous system conditions within the person. Overall, our data suggest that the tasks we used to differentially activate the two divisions of the ANS were successful in producing the expected cardiovascular responses and that differential ANS activation was not associated with modulation of the startle reflex.
In terms of attentional focus, our work showed that greater startle magnitude occurred during internal tasks (mental math and imagining favorite food) as compared to external tasks (Stroop and relaxing video). Further, the GAD individuals showed greater startle magnitudes as compared to the controls on the internal tasks, whereas both groups showed similar responses on the external tasks. These results are consistent with[34] who found larger startle responses in socially anxious individuals during a self-focused attention condition. Given that our GAD participants showed larger startle response on both a traditionally stressful task (mental math) and a traditionally non-stressful task (imagining one’s favorite food), it is difficult to argue that the exaggerated startle on internal tasks was modulated by a negative emotional state resulting solely from the task itself. Overall, our second conclusion is that attentional focus plays a larger role in determining startle magnitude than ANS activity or self-reported anxiety levels in those with GAD compared to controls.
The second condition of our study examined emotional state differences as reflected in a relaxation and worry task. We found a significantly larger startle response in participants with GAD compared to controls during both conditions. As can be seen in Figure 2, the control participants displayed similar startle levels irrespective of conditions. The lack of differentiation on the part of controls is somewhat surprising but given the self-reported valence measures it appears that the worry condition for the controls did not generate a significant level of distress. Further, as seen in Table 1 the self-reported arousal and valence results suggest that the relaxation and worry inductions influenced arousal/activation more than valence by both groups. Additionally, the controls reported more differentiation in terms of self-reported arousal and valence between the relaxation and worry conditions than did the GAD participants. This is consistent with thought sampling research during self-relaxation in which non-anxious individuals report mostly positive imagery and few thoughts, whereas participants with GAD report equal amounts of negatively toned thoughts and images.[59] On an individual differences level, startle response magnitude was associated with both increased arousal and negatively valenced self-reports across all participants. Our third conclusion is that GAD participants show exaggerated startle responses during relaxation and worry tasks both of which appear to be characterized by a negative emotional state for them.
The third purpose of this study was to examine the startle reflex in individuals diagnosed with GAD. In an examination of the startle response, individuals with GAD, in comparison to controls, did not display an exaggerated startle response in the baseline condition. This is consistent with previous research using other clinical anxiety populations (e.g., PTSD) that suggest that it is only in response to specific situations (e.g., under threat or stress) that one sees potentiated startle reflective of diagnostic status[41, 50] and emotional state at the time of startle probe delivery. We put considerable effort to reduce the stressfulness of the laboratory situation by introducing the GAD individuals to the lab before the psychophysiological session. Thus, we conclude that GAD individuals, like other clinical anxiety populations, do not display exaggerated startle responses in a baseline lab condition with which they have become familiar. However, it should be noted that during the baseline condition self-report ratings of negative valence were significantly correlated with startle amplitude across all participants with more negatively valenced self-reports being associated with larger startle responses. Our data are also consistent with Lang’s observation that although GAD individuals report apprehension and distress, they paradoxically appear from a psychophysiological perspective less fearful.[60] Thus we found increased startle associated with self-reported negative emotional state, but not with a diagnosis of GAD per se. We thus conclude that the state of the individual at the time of startle probe delivery is an important predictor of enhanced startle reflex responding.
Whereas an internal focus of attention increased startle magnitude in the group with GAD, but not in the control group, it is possible that exaggerated startle during the emotional tasks was indeed related to differences in attention focus between the GAD participants and controls. This is intriguing because GAD individuals are characterized by the very nature of their chronic worrying to have a strong degree of internal focus of attention. Given that the GAD individuals also showed larger startle responses on the two internal focus-of-attention tasks (mental math and food imagery), it may be the case that any time attention is inwardly directed for individuals with GAD, it is associated with a negative emotional state. Thus, in the GAD individuals, the attentional effect could have been supplemented with a valence effect. This suggests that treatment interventions that teach clients with GAD to focus their attention on the external environment will be associated with fewer behavioral indicators of anxiety such as exaggerated startle responses and reported negative emotional states. Indeed, training GAD clients to focus their attention on actual environmental events is one of the interventions used in our therapy research to decrease worry.[61]
As previously mentioned, the amygdala central nucleus has been associated with expressions of fear paired with explicit conditioned stimuli.[62] have additionally demonstrated that the bed nucleus of the stria terminalis, a structure adjacent to the amygdala, is crucial for potentiated startle responses to contextual cues. Indeed, the authors go so far as to conceptualize fear startle responses as those exhibited in the presence of an immediate threat stimulus and anxiety startle responses as those in which the threat cues are more distal. Further, distinguishing between these two types of startle responses is the finding that the peptide called corticotrophin releasing factor, associated with the release of the stress hormone cortisol, has been linked with the modulation of potentiated startle to contexutal cues while leaving potentiated startle responses to explicit cues unaffected.[63] This supports previous clinical models of GAD individuals who worry as a cognitive mode of processing threat cues that are more removed from the individual or never actually materialize.[64, 65] Integrating the clinical conceptualization of worry in GAD with these psychophysiological findings suggests that individuals with GAD may process affective states (both positive and negative) with increased levels of stress hormone. Thus, the potentiated startle response can be conceptualized as a behavioral index of a neuroendocrinary system of anxiety. The degree to which changes in cognition (e.g., via cognitive behavioral therapy) impact these multiple levels of neurophysiology remains to be tested.
Research involving both animals and humans see[66] has shown that the startle probe magnitude decreases with repeated presentations. Given the sensitivity of psychophysiological responses to biologically significant stimuli that have been shown to habituate more slowly than motor responses in animals cf.[67] and that startle responses to relevant stimuli have been shown to habituate after therapy,[68–70] then the startle probe may be an important and sensitive tool in evaluating changes in emotional information processing after therapy.
Acknowledgments.
Research was supported in part by NIMH grant 58593.
Contract grant sponsor: National Insitute of Mental Health; Contract grant number: 58593.
Footnotes
This article is a US Government work and, as such, is in the public domain in the United States of America.
REFERENCES
- 1.Davis M The mammalian startle response In: Eaton RC, ed. Neural mechanisms of startle behavior. New York: Plenum Publishing Corporation; 1984. [Google Scholar]
- 2.Davis M Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol 2006;61:741–756. [DOI] [PubMed] [Google Scholar]
- 3.Landis C, Hunt W. The startle pattern. New York: Farrar & Rinehart; 1939. [Google Scholar]
- 4.Graham F Distinguishing among orienting, defense, and startle reflexes In: Kimmel H, van Olst E, Orlebeke J, eds. The orienting reflex in humans. Hillsdale, NJ: Erlbaum; 1979: 137–167. [Google Scholar]
- 5.Berg K, Balaban M. Startle elicitation: stimulus parameters, recording techniques, and quantification In: Dawson M, Schell A, Böhmelt A, eds. Startle modulation. New York: Cambridge University Press; 1999. [Google Scholar]
- 6.Davis M Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry 1998;44:1239–1247. [DOI] [PubMed] [Google Scholar]
- 7.Brown JS, Kalish HI, Farber IE. Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. J Exp Psychol 1951;41:317–328. [DOI] [PubMed] [Google Scholar]
- 8.Rosen JB, Hitchcock JM, Sananes CB, Miserendino MJD, Davis M. A direct projection from the central nucleus of the amygdala to the acoustic startle pathway: anterograde and retrograde tracing studies. Behav Neurosci 1991;105:817–825. [DOI] [PubMed] [Google Scholar]
- 9.Diazepam Davis M. and flurazepam: effects on conditioned fear as measured with the potentiated startle paradigm. Psychopharmacology 1979;62:1–7. [DOI] [PubMed] [Google Scholar]
- 10.Patrick CJ, Berthot B, Moore JD. Diazepam blocks fear-potentiated startle in humans. J Abnorm Psychol 1996;105: 89–96. [DOI] [PubMed] [Google Scholar]
- 11.Lang PJ. The emotion probe: studies of motivation and attention. Am Psychol 1995;50:372–385. [DOI] [PubMed] [Google Scholar]
- 12.Dawson M, Schell A, Böhmelt A. Startle modulation. New York: Cambridge University Press; 1999. [Google Scholar]
- 13.Filion DL, Dawson ME, Schell AM. The psychological significance of human startle eyeblink modification: a review. Biol Psychol 1998;47:1–43. [DOI] [PubMed] [Google Scholar]
- 14.Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol 2003;114:1557–1579. [DOI] [PubMed] [Google Scholar]
- 15.Lang P, Bradley M, Cuthbert B. Emotion, attention, and the startle reflex. Psychol Rev 1990;97:377–395. [PubMed] [Google Scholar]
- 16.Davis M. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behav Neurosci 1986;100:814–824. [DOI] [PubMed] [Google Scholar]
- 17.Grillon C, Ameli R, Woods S, Merikangas K, Davis M. Fear-potentiated startle in humans: effects for anticipatory anxiety on the acoustic blink reflex. Psychophysiology 1991;28:588–595. [DOI] [PubMed] [Google Scholar]
- 18.Grillon C, Ameli R, Woods S, Merikangas K, Davis M. Measuring the time-course of anxiety using the fear-potentiated startle reflex. Psychophysiology 1993;30:340–346. [DOI] [PubMed] [Google Scholar]
- 19.Lang PJ, Cuthbert BN, Bradley MM. Measuring emotion in therapy: imagery, activation, and feeling. Behav Ther 1998;29:655–674. [Google Scholar]
- 20.Smith J, Bradley M, Lang P. State anxiety and affective physiology: effects of sustained exposure to affective pictures. Biol Psychol 2005;69:247–260. [DOI] [PubMed] [Google Scholar]
- 21.Vrana S, Lang P. Fear imagery and the startle probe reflex. J Abnorm Psychol 1990;99:189–197. [DOI] [PubMed] [Google Scholar]
- 22.Konorski J Conditioned reflexes and neuron organization. Cambridge: Cambridge University Press; 1948. [Google Scholar]
- 23.Konorski J Integrative activity of the brain. Chicago, IL: University of Chicago Press; 1967. [Google Scholar]
- 24.Cuthbert BN, Bradley MM, Lang PJ. Probing picture perception: activation and emotion. Psychophysiology 1996;33:103–111. [DOI] [PubMed] [Google Scholar]
- 25.Bonnet M, Bradley MM, Lang PJ, Requin J. Modulation of spinal relexex: arousal, pleasure, action. Psychophysiology 1995;32:367–372. [DOI] [PubMed] [Google Scholar]
- 26.Both S, Everaerd W, Laan E. Modulation of spinal reflexes by aversive and sexually appetitive stimuli. Psychophysiology; 40:174–183. [DOI] [PubMed] [Google Scholar]
- 27.Cook E Affective individual differences, psychopathology, and startle reflex modulation In: Dawson M, Schell A, Böhmelt A, eds. Startle modulation. New York: Cambridge University Press; 1999. [Google Scholar]
- 28.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 3rd revised ed Washington, DC: Author; 1987. [Google Scholar]
- 29.American Psychiatric Association; Diagnostic and statistical manual of mental disorders, 4th ed Washington, DC: Author; 1994. [Google Scholar]
- 30.Graham F Attention: the heartbeat, the blink, and the brain In: Campbell B, Hayne H, Richardson R, eds. Attention and information processing in infants and adults: perspectives from human and animal research. Hillsdale, NJ: Erlbaum; 1992:3–29. [Google Scholar]
- 31.Hackley S Blink reflex studies of attention and consciousness In: Dawson M, Schell A, Böhmelt A, eds. Startle modulation. New York: Cambridge University Press; 1999. [Google Scholar]
- 32.Lüthy M, Schächinger H. Impact of sympathetic activation on the acoustic startle response. Psychophysiology 1998;35:s54. [Google Scholar]
- 33.Blumenthal T, Chapman J, Muse K. Effects of social anxiety, attention, and extraversion on the acoustic startle eyeblink response. Pers Individ Differences 1995;19:797–807. [Google Scholar]
- 34.Panayiotou G, Vrana S. Effect of self-focused attention on the startle reflex, heart rate, and memory performance among socially anxious and nonanxious individuals. Psychophysiology 1998;35:328–336. [DOI] [PubMed] [Google Scholar]
- 35.Carver C A cybernetic model of self-attention processes. J Pers Soc Psychol 1979;37:1251–1281. [Google Scholar]
- 36.Darrow C Differences in the physiological reactions to sensory and ideational stimuli. Psychol Bull 1929;26:185–201. [Google Scholar]
- 37.Lacey J, Lacey B. Two-way communication between the heart and the brain. Am Psychol 1978;33:99–113. [DOI] [PubMed] [Google Scholar]
- 38.Ray WJ, Cole H. EEG alpha reflects attentional demands, beta reflects emotional and cognitive processes. Science 1985;228: 750–752. [DOI] [PubMed] [Google Scholar]
- 39.Thayer J, Friedman B, Borkovec T. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry 1996;39: 255–266. [DOI] [PubMed] [Google Scholar]
- 40.Grillon C Startle reactivity and anxiety disorders: aversive condition, context, and neurobiology. Biol Psychiatry 2002;52: 958–975. [DOI] [PubMed] [Google Scholar]
- 41.Grillon C, Morgan CA. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol 1999;108: 134–142. [DOI] [PubMed] [Google Scholar]
- 42.Miller M, Litz B. Emotional-processing in posttraumatic stress disorder II: startle reflex modulation during picture processing. J Abnorm Psychol 2004;113:451–463. [DOI] [PubMed] [Google Scholar]
- 43.Grillon C, Ameli R, Goddard A, Woods SW, Davis M. Baseline and fear-potentiated startle in panic disorder patients. Biol Psychiatry 1994;35:431–439. [DOI] [PubMed] [Google Scholar]
- 44.Hamm A, Cuthbert B, Globisch J, Vaitl D. Fear and the startle reflex: blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology 1997; 34:97–107. [DOI] [PubMed] [Google Scholar]
- 45.Merckelback H, deJong PJ, Leeuw I, VanDenHout MA. Startle responses of spider phobics to masked stimuli: a pilot study. Int J Neurosci 1995;81:169–175. [DOI] [PubMed] [Google Scholar]
- 46.Morgan CA, Grillon C, Southwick SM, Davis M, Charney D. Exaggerated acoustic startle reflex in Gulf War veterans with posttraumatic stress disorder. Am J Psychiatry 1996;153: 64–68. [DOI] [PubMed] [Google Scholar]
- 47.Grillon C, Morgan C, Southwick S, Davis M, Charneuy D. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res 1996;64:169–178. [DOI] [PubMed] [Google Scholar]
- 48.Ornitz EM, Pynoos RS. Startle modulation in children with posttraumatic stress disorder. Am J Psychiatry 1989;146: 866–870. [DOI] [PubMed] [Google Scholar]
- 49.Orr SP, Lasko N, Shalev A, Pitman RK. Physiologic responses to loud tones in Vietnam veterans with posttraumatic stress disorder. J Abnorm Psychol 1995;104:75–82. [DOI] [PubMed] [Google Scholar]
- 50.Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with PTSD. Biol Psychiatry 1998;44: 1027–1036. [DOI] [PubMed] [Google Scholar]
- 51.Brown T, DiNardo P, Barlow D. Anxiety disorders interview schedule for DSM-IV. Albany, NY: Graywood Publications; 1994. [Google Scholar]
- 52.Hamilton M The measurement of anxiety states by rating. Br J Med Psychol 1959;32:50–55. [DOI] [PubMed] [Google Scholar]
- 53.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther 1990;28:487–495. [DOI] [PubMed] [Google Scholar]
- 55.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;41:561–571. [DOI] [PubMed] [Google Scholar]
- 56.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State–Trait Anxiety Inventory (Form Y). Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 57.Osgood CE, Suci GS, Tannenbaum PH. The measurement of meaning. Urbana: University of Illinois Press; 1957. [Google Scholar]
- 58.Ornitz EM, Russell AT, Yuan H, Liu M. Autonomic, electroencephalographic, and myogenic activity accompanying startle and its habituation during mid-childhood. Psychophysiology 1996;33:507–513. [DOI] [PubMed] [Google Scholar]
- 59.Borkovec T, Inz J. The nature of worry in generalized anxiety disorder: a predominance of thought activity. Behav Res Ther 1990;28:153–158. [DOI] [PubMed] [Google Scholar]
- 60.Lang PJ, McTeague L, Cuthbert B. Fear, anxiety, depression, and the anxiety disorder spectrum: a psychophysiological analysis In: Treat T, Bootzin R, Baker T, eds. Psychological clinical science. New York: Psychology Press; 2007. [Google Scholar]
- 61.Borkovec TD, Sharpless B. Generalized anxiety disorder: bringing cognitive behavioral therapy into the valued present In: Hayes S, Follette V, Linehan M. eds. New directions in behavior therapy. New York: Guilford Press; 2004;209–242. [Google Scholar]
- 62.Davis M, Walker DL, Lee Y. Neurophysiology and neruopharmacology of startle and its affective modulation In: Dawson ME, Schell A, Böhmelt A, eds. Startle modulation. New York: Cambridge University Press; 1999:95–113. [Google Scholar]
- 63.Lee Y, Davis M. The role of bed nucleus of the stria terminalis in CRH-enhanced startle: an animal model of anxiety. Soc Neurosci Abstr 1996;22:465. [Google Scholar]
- 64.Borkovec TD. The nature, functions, and origins of worry In: Davey GCL, Tallis F, eds. Worrying: perspectives on theory, assessment, and treatment. New York: Wiley; 1994. [Google Scholar]
- 65.Craske MG. Anxiety disorders: psychological approaches to theory and treatment. Boulder: Westview; 1999. [Google Scholar]
- 66.Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: habituation in humans. Behav Neurosci 1993;107: 970–980. [DOI] [PubMed] [Google Scholar]
- 67.Hinde RA. Behavioral habituation In: Horn G, Hinde RA, eds. Short term changes in neural activity and behavior. Cambridge, England: Cambridge University Press; 1970:3–40. [Google Scholar]
- 68.deJong PJ, Merckelbach H, Arntz A. Eye blink startle responses in spider phobics before and after treatment: a pilot study. J Psychopathol Behav Assess 1991;13:213–223. [Google Scholar]
- 69.deJong P, Visser S, Merckelbach H. Startle and spider phobia: unilateral probes and the prediction of treat effects. J Psychophysiol 1996;10:150–160. [Google Scholar]
- 70.Vrana SR, Constantine JA, Westman JS. Startle reflex modification as an outcome measure in the treatment of phobia: two case studies. Behav Assess 1992;14:279–291. [Google Scholar]