Abstract
Objective
To assess whether choline decreases effects of maternal infections on fetal brain circuit development and on expression of infant behavior at 3 months of age.
Study Design
A case-control study was conducted in a public hospital obstetrics and midwifery service, with prenatal assessments of maternal infection, C-Reactive Protein (CRP), and choline levels and postnatal assessments of cerebral neuronal inhibition in 162 newborns. At 3 months, 136 parents completed reports of their child’s behavior.
Results
Maternal infection at 16 weeks gestation, experienced by 41% of mothers, raised mean maternal CRP (d’ = 0.47, P = 0.002) and decreased the development of cerebral inhibition of auditory response at 1 month of age (d’ = 0.39, P <0.001). Decreased newborn cerebral inhibition manifest as decreased behavioral self-regulation at 3 months. Higher choline levels in mothers with infections were associated with better newborn inhibition of auditory cerebral response, mitigating the infection effect (β = −0.34 [95% CI, −5.35 to −0.14], P = 0.002). At 3 months of age, children of mothers with infection and higher gestational choline levels had improved development of self-regulation, approaching the level of children of mothers without infection (β = 0.29 [95% CI, 0.05 to 0.54], P = 0.03).
Conclusion
Higher maternal choline levels, now recommended by the American Medical Association, decrease adverse effects of common maternal infections during gestation on children’s early development of behavioral problems. These behavioral problems often lead to referrals to pediatricians and are associated with later serious mental illness.
Keywords: pregnancy exposure delayed effects, fetal development, choline, receptors nicotinic, sensory gating, child behavior
Maternal respiratory and genito-urinary infections generally do not infect the fetus. Nonetheless these seemingly benign infections significantly increase the risk that the child will develop mental illnesses including schizophrenia, autism, and ADHD.1–7 Early second trimester is a particularly vulnerable time, when cerebro-cortical laminae form. 1,8 The mother’s immune response activates macrophages that damage the placental chorionic villi and compromise fetal support.9–11 Maternal C-Reactive Protein (CRP) levels are related to offsprings’ subsequent autism or schizophrenia.12–14 Puberty unmasks latent effects of such prenatal insults.15 Although risk to the offspring from infection is less than risk from having a mentally ill parent, infection is more common and adds significantly to the familial genetic risk.1,16
In animal models of maternal immune activation, supplementing maternal dietary choline reduced interleukin-6 (IL-6) in the fetal brain and decreased offspring anxiety behaviors.10 Choline’s roles in fetal development include membrane synthesis, one carbon metabolism and DNA methylation, and, at higher concentrations, activation of α7-nicotinic cholinergic receptors,17–18 which promote maturation of excitatory and inhibitory neuro-circuits.19–21 Maturation of these neuro-circuits is not complete in schizophrenia.22–23 Elimination of α7-nicotinic receptors by CHRNA7 null mutation increases effects of immune activation and blocks effects of maternal choline supplementation on fetal brain development.10,19
Maternal phosphatidylcholine or choline supplementation and diets higher in choline improve childhood cognition and behavior.24–30 However, no study has examined the relationship of maternal choline levels to the effects of infection in human pregnancy. We planned to observe their interaction on newborn cerebral auditory-evoked response inhibition, a biomarker of the prenatal development of inhibitory neuro-circuits that showed negative effects of familial risk and positive effects of choline in previous studies.27,31
Methods
Mothers and infants
Pregnant women were identified from admissions to Denver Health Medical Center, a public hospital prenatal clinic, before the 16th week of gestation, timed from the last menstrual period and verified by ultrasound (Figure 1). Exclusions were fetal anomaly, severe intrauterine growth restriction, and corticosteroid use. Women with asthma or allergies were otherwise included; no women had autoimmune disorders. After informed consent approved by the Colorado Multi-Institutional Review Board, diagnoses were made using the Structured Clinical Interview for DSM-IV Axis I Disorders, converted to DSM-5 criteria. Self-ratings on Center for Epidemiological Studies of Depression-R (CESD-R), State-Trait Anxiety Inventory-State Version (STAI-S), and the Perceived Stress Scale (PSS) were performed whenever maternal infection status was assessed, and acetaminophen, antibiotic, antidepressant and other psychotropics, and nicotine, alcohol, marijuana, and other substance use were recorded. Maternal status during pregnancy, including BMI, blood pressure, and pre-eclampsia were recorded.
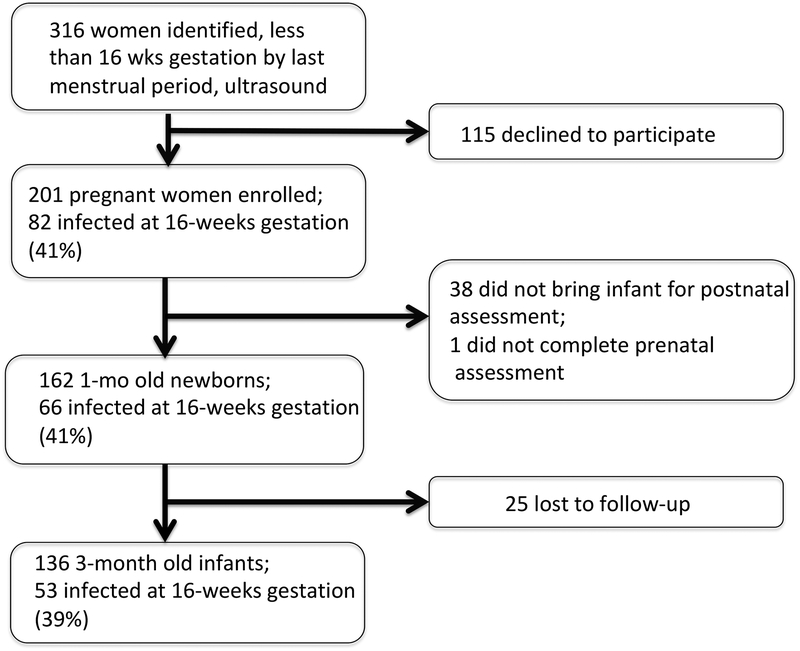
Figure 1.
Subject participation from 16 weeks gestation to 3 months post partum
Assessment of maternal infection
The medical record for all prenatal care was reviewed. A mother’s report of infection was considered significant if it was entered as a problem in the medical record. Treatment was provided for all reported genito-urinary infections. Most respiratory infections were viral and were therefore not treated. In addition, mothers had an in person review of systems for symptoms of infection at 16, 22, 28, and 34 weeks by research personnel. The correlation between symptoms rated by the mother as moderate to severe in the interview and problems in the medical record is rs = 0.96, P < 0.001.
Maternal choline levels
Maternal serum choline and its metabolite betaine at 16 weeks gestation were assayed by the Colorado Translational Research Center Metabolomics Core Laboratory, University of Colorado Denver. Serum was quickly separated by refrigerated centrifugation and stored at −80°C. Samples were extracted in methanol, acetonitrile, and water (5:3:2) and agitated for 30 minutes at 4°C. After centrifugation at 10,000g the supernatant was collected and stored at −80°C until analysis with an Ultra performance liquid chromatography-tandem mass spectrometer. Metabolites were assigned using Maven Metabolomic Analysis and Visualization Engine (Princeton, NJ).
Maternal C-Reactive Protein
Serum CRP at 16 weeks gestation was assayed by the Beckman-Coulter high sensitivity assay at the Colorado Translational Research Center, Colorado Children’s Hospital.
Physiological recording of newborn cerebral inhibition
Newborns were studied at 1 month (44 weeks) after birth adjusted for gestational age.32 Vertex electroencephalogram, electro-oculogram, submental electromyogram, and respiration were continuously recorded while infants napped. Recording of the cerebral auditory evoked potential P50 occurred in the second active sleep episode, the precursor of REM sleep, identified by low voltage desynchronized vertex activity with the absence of K-complexes, change in respirations, and large eye movements with submental atonia.33 The second active sleep episode was reached 45 minutes after sleep onset. In adults, P50 inhibition in REM and waking are equivalent.34
The P50 sensory gating paradigm assesses inhibition. The initial stimulus activates a P50S1 response, which also activates collateral inhibitory interneurons. Strength of the inhibition is tested by the decrease in P50S2 after a second stimulus.35 For comparisons between individuals, control for variance in P50S1 is desirable, which can reflect differences in excitability, but also technical factors like electrode impedance. Therefore, P50 inhibition is often assessed as amplitude ratios P50S2/P50S1 or (P50S1-P50S2)/P50S1.35 However, the skew inherent in ratios limits their power for correlation with risk factors and outcomes. P50S2 amplitude, covaried for P50S1, which is normally distributed, has been previously proposed and was used here.36 Lower P50S2 amplitudes indicate increased inhibition. The assumption is that P50S1 variance is small, compared to P50S2 variance. In 151 newborns, effect sizes for P50S1 differences between newborns whose mothers had no known risk versus women with depression or schizophrenia ranged from 0–0.16. Effect sizes for decrease in P50S2 amplitude were 0.21–0.50.31 The effect of maternal schizotypy on newborn P50 inhibition has been replicated by another group, who also found increased P50S2 amplitudes.37 Table 2 reports P50S1, P50S2, and (P50S1-P50S2)/P50S1. Technical aspects and reliability of recordings have been published.32,38–39
Table 2.
Maternal mental symptoms, choline levels, and inflammatory status at 16 weeks gestation
| Maternal Symptoms | No infection N = 96 | Infection N = 66 | Significance |
|---|---|---|---|
| Center for Epidemiological Studies of Depression Scale-Revised | 12.0 (SD 8.3) | 17.0 (SD 10.2) | 0.001 |
| State-Trait Anxiety Inventory-State Version | 33.7 (SD 9.5) | 38.7 (SD 11.9) | 0.004 |
| Perceived Stress Scale | 22.8 (SD 7.2) | 24.8 (SD 9.0) | 0.10 |
| Maternal choline and metabolite | N = 96 | N = 66 | |
| Choline 16 weeks | 6.50 (1.89) | 6.20 (1.75) | 0.3 |
| Betaine 16 weeks | 11.88 (SD .53) | 10.99 (SD 3.62) | 0.12 |
| Maternal Cytokines | N = 90 | N = 61 | |
| C-reactive protein (CRP) mg/L | 7.30 (SD 6.27) | 10.90 (SD 8.77) | 0.004 |
| Newborn electrophysiology (1 month) | N = 96 | N = 66 | |
| P50 S1 amplitude (μV) | 1.73 (SE 0.09) | 1.67 (SE 0.11) | 0.6 |
| P50 S2 amplitude (μV) | 0.75 (SE 0.05) | 0.94 (SE 0.06) | < 0.001 |
| P50 inhibition (S1–S2)/S1 | 0.56 (SE 0.03) | 0.45 (SE 0.04) | 0.003 |
| Childhood IBQ-R rated behavior (3 months) | N = 84 | N = 52 | |
| Regulation | 5.23 (SE 0.07) | 3.79 (SE 0.09) | 0.0061 |
| Surgency | 4.14 (SE 0.12) | 3.25 (SE 0.16) | 0.2 |
| Negativity | 3.05 (SE 0.10) | 4.14 (SE 0.13) | 0.5 |
Bonferroni correction for 3 IBQ-R indices
Behavioral assessment of the child
Parents completed the Infant Behavior Questionnaire-Revised Short Form (IBQ-R) when the infant was 52 weeks post gestation, generally 3 months of age.40 Parents who were primarily Spanish-speaking completed the Questionnaire in Spanish. The 91-item IBQ-R Short Form has 3 standard indices developed by the scale originators using factor analysis to summarize its 14 components: Surgency (approach, vocal reactivity, pleasure in high stimulus intensity play like rough-housing, smiling/laughter, soothability, activity level, sensory sensitivity), Negativity (sadness, distress to limitation, fear, falling distress), and Regulation (pleasure in low stimulus intensity play like toys, cuddliness/affiliation, duration of orienting, smiling/laughter, soothability). Values in a reference sample of 12 month olds are: Surgency 5.08 (SD 0.78), Negativity 3.46 (SD 0.91), Regulation 5.47 (SD 0.63).40
Statistical analyses
Choline’s effect size on P50 inhibition in a previous study was d’ = 0.7.27 We expected 20% of the women would have optimal choline levels (> 7 μM) and 33% attrition. 25,27 Therefore, we planned a sample of 200 women to have power 1-β = 0.95, α = 0.05, 1-tail.
Differences between mothers with and without infection were compared by Fisher’s exact test or t-test. The Generalized Linear Model with a linear link analyzed effects of maternal infection and choline level on P50S2, the primary physiological outcome. Multivariate General Linear Models were used for analysis of effects of maternal infection on IBQ-R indices. Infection was a categorical fixed effect, choline level was a random continuous variable, and infant sex and maternal age were covariates. Obesity (BMI > 30) and depression (CESD-R ≥ 16) were covariates because they have been associated with inflammation. The effect on CRP from infection is d’ = 0.47, P = 0.004; from obesity, d’ = 0.06 and from depression, d ‘ = 0.19, both not significant. Maternal age also reflected years of education (r = 0.32, P < 0.001, and Duncan Socioeconomic Index (r = 0.24, P = 0.002). Maternal smoking was a covariate for P50 analyses.36 Other differences between mothers with and without infection were analyzed as possible covariates (Table 1). None significantly affected the interaction between infection and choline levels. Analyses were performed using the Statistical Package for the Social Sciences version 24 (IBM, Amonk, NY). Significance levels are two-tailed and Bonferroni-corrected for multivariate analyses, except for exploratory analysis of 14 individual IBQ-R components.
Table 1.
Demographic, pregnancy, labor, and delivery differences between mother and newborn pairs by infection status at 16 weeks gestation
| Mothers 16 wk gestation | Un-infected N = 96 | Infected N = 66 | Significance |
|---|---|---|---|
| Caucasian | 80 (83%) | 51 (77%) | 0.4 |
| African-American | 6 (6%) | 4 (6%) | 0.9 |
| Native American | 4 (4%) | 6 (9%) | 0.3 |
| Biracial | 6 (6%) | 5 (8%) | 0.8 |
| Hispanic | 47 (49%) | 31 (47%) | 0.9 |
| Married | 49 (51%) | 30 (45%) | 0.5 |
| Maternal age | 30.6 (SD 6.0) | 28.6 (SD 5.8) | 0.03 |
| Education years | 14.0 (SD 3.2) | 13.0 (SD 2.9) | 0.03 |
| Duncan Socio-Economic Index | 49.3 (SD 21.5) | 41.7 (SD 18.2) | 0.02 |
| Pre-pregnancy BMI | 26.6 (5.9) | 28.2 (7.6) | 0.15 |
| Obesity BMI ≥ 30 | 16 (24%) | 31 (32%) | 0.3 |
| Bipolar disorder (DSM-5 296.5) | 1 (1.0%) | 6 (9.1%)5 | 0.02 |
| Schizophrenia (295.9, 295.7) | 2 (2.0%) | 0 | 0.5 |
| Major depressive disorder (296.21, 296.31) | 10 (10.4%) | 14 (18.2%) | 0.07 |
| Panic disorder, generalized anxiety disorder (300.01, 300,02) | 4 (4.2%) | 3 (4.5%) | 0.9 |
| Antidepressant | 9 (9%) | 13 (20%) | 0.07 |
| Acetaminophen | 77 (80%) | 56 (85%) | 0.5 |
| Cigarette smoking | 7 (7.3%) | 4 (6.1%) | 0.9 |
| Cannabis use | 9 (9.4%) | 16 (24.2%) | 0.01 |
| Alcohol use (>1 drink/wk) | 0 | 3 (4.5%) | 0.07 |
| Labor and Delivery | |||
| Diabetes | 2 (2%) | 7 (10%) | 0.03 |
| Hypertension | 6 (6%) | 4 (6%) | 0.9 |
| Preeclampsia | 9 (9%) | 5 (8%) | 0.8 |
| Proteinuria | 7 (7%) | 0 | 0.04 |
| Edema | 12 (12%) | 14 (22%) | 0.2 |
| Chorioamniotis | 8 (8%) | 3 (5%) | 0.5 |
| Premature<37 weeks | 5 (5%) | 2 (3%) | 0.7 |
| Cesarean delivery | 23 (24%) | 19 (29%) | 0.6 |
| APGAR 5min | 8.73 (1.17) | 8.85 (0.41) | 0.4 |
| Newborn | |||
| Sex male | 46 (48%) | 36 (54%) | 0.4 |
| Birth weight g | 3116 (SD 663) | 3229 (SD 514) | 0.2 |
| Birth weight %ile | 60.9 (SD 24.6) | 58.5 (SD 24.4) | 0.5 |
| Head circumference %ile | 65.9 (SD 25.4) | 70.1 (SD 21.9) | 0.3 |
| Birth length %ile | 67.3 (SD 23.6) | 68.7 (SD 22.9) | 0.7 |
| Gestational age birth | 272 (SD 20) | 274 (SD 13) | 0.5 |
| Large >90%ile for gestational age | 11 (11%) | 9 (13%) | 0.8 |
| Small <10%ile for gestational age | 6 (6%) | 1 (2%) | 0.2 |
| NICU admission>1 day | 4 (4%) | 7 (10%) | 0.12 |
| Formula fed only | 12 (12%) | 3 (5%) | 0.10 |
Results
The study enrolled 201 women before 16 weeks gestation. Of 162 who came with their infants for the 1 month post-gestation visit, 66 (41%) had reported an infection by 16 weeks gestation. Mothers who reported infection at 16 weeks gestation were younger and had less education and lower status occupations than those who did not report infection (Table 1). Eleven women had vaginal infections, 5 had urinary tract infections, 37 had viral respiratory infections, 11 had pharyngitis, 3 had influenza, and 4 had gastroenteritis. Five women had two infections. Type or number of infections had no effect on infant outcome; nor did infections later in gestation. Infection was accompanied by increases in maternal depression and anxiety symptoms and increased levels of CRP (d’ = 0.47, P < 0.004; Table 2). Choline levels were not affected by maternal infection, depression, age, or socio-economic status.41 Mothers lost to participation during their 18 months of study had no differences in infection rate or other variables (Figure 1). The principal reason for attrition was moving from Denver.
Effects of maternal infection and choline on newborn cerebral inhibition
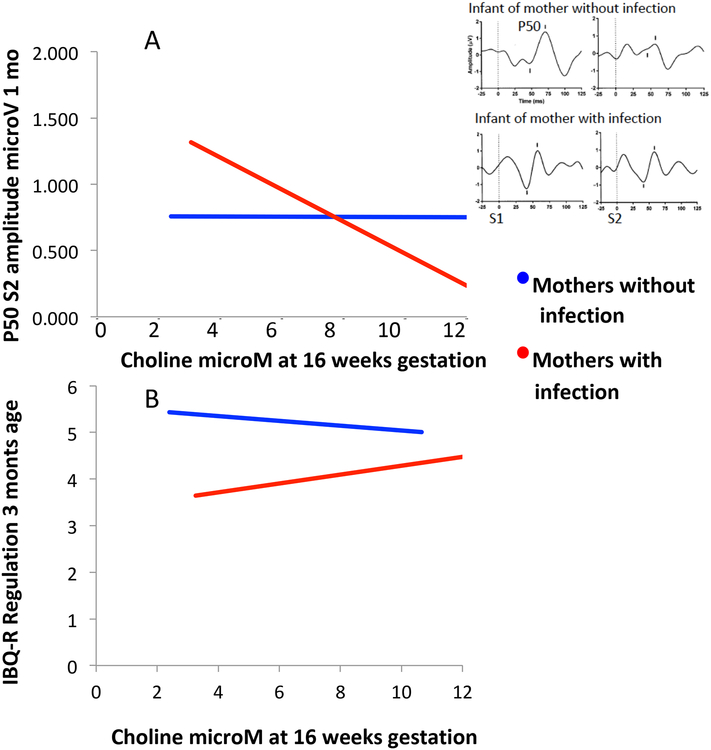
Maternal infection at 16 weeks gestation was associated with significantly decreased inhibition of the cerebral auditory-evoked potential P50 in the 1-month-old newborn. P50S2 increased by 27% in newborns of infected mothers, compared to newborns of uninfected mothers, indicative of less inhibition (d’ = 0.39, P <0.001; Table 2). Effects of choline and infection on cerebral inhibition had a significant interaction (Wald χ2 = 9.10, df 1, P = 0.003, Table 3; online). Maternal choline levels at 16 weeks gestation were associated with significantly lower P50S2 amplitudes in infants of mothers with infection (β = −0.34 [95% CI, −5.35 to −0.14], P = 0.002); there was no association in infants whose mothers were not infected (Figure 2). There were no effects of infection or choline on P50S1 amplitude.
Table 3.
Effects of maternal infection and choline level at 16 weeks gestation on newborn P50 inhibition, measured as P50S2 amplitude at 1 month post gestation
| Source | Wald Chi-Square | Sig. |
|---|---|---|
| (Intercept) | 5.685 | .017 |
| Maternal infection 16 wks | 13.045 | <.001 |
| Child sex | .357 | .500 |
| Maternal age | .008 | .930 |
| Maternal smoking | .061 | .805 |
| Maternal obesity | 2.044 | .153 |
| Maternal depression | .107 | .744 |
| P50S1 amplitude | 111.760 | <.001 |
| Choline 16wks | 6.789 | .009 |
| Infection*choline | 9.100 | .003 |
| Effect of infection 16 weeks gestation on P50S2 amplitude | Marginal Mean μV | 95% CI |
| No infection | .75 (SE .05) | .66 – .84 |
| Infection | .94 (SE .06) | .83 – 1.05 |
Figure 2.
Maternal infection and choline levels and development of newborn physiological inhibition and early childhood self-regulation. A. Maternal choline levels significantly improved cerebral inhibition, indicated by lower P50S2 amplitude in newborns of mothers who reported infection. There was no significant effect in infants whose mothers were not infected.
B. Maternal choline levels significantly improved development of Regulation, measured on the Infant Behavior Questionnaire-Revised (IBQ-R) at 3 months of age in children of mothers who reported infection; effects for children of women with no infection were not significant.
Inset: P50 averaged evoked responses to paired auditory stimuli S1 and S2, delivered 500 ms apart. P50 amplitude is measured from the positive peak voltage to the preceding negative trough. For the infant of the uninfected mother, maternal choline level was 12.9 μM; P50S2 is over 90% inhibited. For the infant of the infected mother, maternal choline level was 5.4 μM; there was no P50S2 inhibition. Horizontal scale msec, vertical μV.
Effects of maternal infection and choline on infant behavior at 3 months of age
Infection decreased Regulation rating by 28%: −1.44 (SE 0.45), P = 0.003, d’ = 0.28 (Table 2). There were no significant effects on Surgency or Negativity. Maternal infection and choline levels at 16 weeks had a significant interacting effect in a multivariate analysis of the 3 IBQ-R indices, specifically on Regulation (F3,124 = 10.71, P = 0.003, Table 4; online). The children of mothers with infections had increased Regulation associated with higher maternal choline levels; there was no such relationship for children whose mothers were not infected (infection β = 0.29 [95% CI, 0.05 to 0.54], P = 0.03; no infection β = −0.14 [95% CI, −0.31 to 0.04]). The IBQ-R component that showed most significant effects of choline in children of mothers with infection was in the Regulation index: pleasure in low stimulus intensity play (β = 3.07 [95% CI, 0.05 to 0.56], P = 0.02). IBQ-R Regulation in the children of mothers with infections was significantly associated with P50S2 at 1 month of age (β = −0.37 [95% CI, −.014 to −0.86], P = 0.04).
Table 4.
Effects of maternal infection and choline level at 16 weeks gestation on child’s IBQ-R indices at 3 months of age
| Source | Wilk’s λ | IBQ-R Indices | F (df 1,124) | Sig. |
|---|---|---|---|---|
| Child sex | λ = .997 | SURGENCY | .067 | .797 |
| Fdf3,124= .139 | NEGATIVITY | .114 | .736 | |
| P= .937 | REGULATION | .350 | .555 | |
| Maternal age | λ = .975 | SURGENCY | 1.560 | .214 |
| Fdf3,124= 1.079 | NEGATIVITY | .279 | .598 | |
| P= .361 | REGULATION | .246 | .621 | |
| Maternal obesity | λ = .939 | SURGENCY | 1.422 | .235 |
| Fdf3,124= 2.694 | NEGATIVITY | 7.620 | .0071 | |
| P= .049 | REGULATION | .151 | .699 | |
| Maternal depression | λ = .991 | SURGENCY | .221 | .639 |
| Fdf3,124= .778 | NEGATIVITY | .952 | .331 | |
| P= .937 | REGULATION | .082 | .776 | |
| Maternal infection 16 wks | λ = .924 | SURGENCY | 1.411 | .237 |
| Fdf3,124= 3.422 | NEGATIVITY | .551 | .459 | |
| P= .019 | REGULATION | 10.184 | .0022 | |
| Maternal choline 16 wks | λ = .997 | SURGENCY | .059 | .809 |
| Fdf3,124= .116 | NEGATIVITY | .003 | .959 | |
| P= .940 | REGULATION | .103 | .749 | |
| Infection* choline | λ = .922 | SURGENCY | 2.629 | .107 |
| Fdf3,124= 3.515 | NEGATIVITY | .255 | .614 | |
| P= .017 | REGULATION | 10.709 | .0013 | |
| Effect of maternal infection 16 wks, compared to uninfected: Difference (SE) | SURGENCY | −.887 (SE .747) | .237 | |
| NEGATIVITY | .456 (SE .626) | .359 | ||
| REGULATION | −1.442 (SE .452) | .0022 | ||
Bonferroni correction
P = .021;
P = .006;
P = .003
Maternal CRP levels at 16 weeks gestation decreased the child’s IBQ-R Regulation index (β = −0.64 [95% CI, −1.25 to −0.034], P = 0.04). Maternal choline and CRP levels effects on Regulation had a significant interaction (Wald χ2 = 4.79, df 1, P = 0.03). The adverse effect of higher CRP levels was negated in women with choline levels > 7μM (β= 0.28 [95% CI, −0.01 to 0.59], P = 0.06).
IBQ-R Regulation does not have a minimum threshold considered abnormal, but children below the 5th percentile on early behavior and temperament ratings are often referred for clinical intervention. Five children of 53 mothers with infection (9.4%) had Regulation levels lower than the 95th percentile of the reference sample,40 compared to 1 of 83 children of mothers without infection (1.2%, PFET = 0.03). Four of these infected mothers with infection who had children with poor Regulation also had choline levels < 7μM.
Discussion
Higher maternal choline levels were associated with increased development of cerebral inhibition and newborns and behavioral regulation in 3-month-old infants, especially in mothers who experienced common infections early in pregnancy. The timing at 16 weeks gestation is consonant with the finding that choline levels are lowest in second trimester and with the epidemiological evidence that identifies 16 weeks gestation as a vulnerable period.1,41 We did not find that the mother’s infection, socioeconomic, or mental status influenced choline levels.42
Finding of 41% of mothers infected is consistent with 37% infection found in the second trimester for 4967 control mothers in the National Birth Defects Prevention Study.43 The high frequency and unpredictability of many infections, notably respiratory infection, puts every pregnancy at potential risk of acquiring this complication. Lower socioeconomic status of women with infection has also been found in general hospital samples and attributed to stress and overcrowding, but the mechanism remains unclear.44 The age disparity in pregnancy among women of different socioeconomic status observed in this study is nationwide, according to a New York Times-commissioned study with the National Center for Health Statistics.45
The mechanisms of choline’s effects include direct activation of α7-nicotinic cholinergic receptors responsible for maturation of inhibitory and excitatory neurotransmission, as suggested by both animal models and CHRNA7 pharmacogenomic effects in studies of phosphatidylcholine supplements. 10,19–21,27–28 Newborn P50 auditory evoked potential inhibition is a putative biomarker of this effect, because of its genetic relationship to CHRNA7.46 Both P50 inhibition and CHRNA7 are involved in the pathology of major mental illness. In schizophrenia, decreased P50 inhibition is associated with poor attention and executive function in schizophrenia,47 and CHRNA7 copy number variations and polymorphisms are associated with schizophrenia, autism, and ADHD.48–50 In newborns, lower P50 inhibition predicts childhood behavior problems in attention and social withdrawal associated with ADHD and other mental illnesses.51 Lower infant P50 inhibition is also associated with family history of psychotic disorder,.31,37 In this study, decreased development of P50 inhibition presaged poorer self-regulation at 3 months of age.
The P50 response is present in newborns at nearly adult amplitudes after 30 weeks gestation.52–53 Inhibition of the P50S2 is closely related to the development of theta activity, the hallmark of infant active sleep.32 The 1 month of recording in the present study, mean gestational age 44.0 (SD 1.4) weeks. was chosen because infant active sleep patterns stabilize at this age (Harper 1981).54 Inhibition of newborn P50S2 has excellent test-retest reliability over 1.5 weeks (ricc = 0.71, P<0.01) and is also closely correlated with recordings during REM sleep when the child is 4 years of age (ricc = 0.42, P = 0.06).38–39 In the present study, a second recording was performed at 3 month of age. P50S2 amplitude at 1 month weeks predicted P50S2 amplitude at 3 months, with covariance for P50S1 at both ages (β = 2.43, 95%CI 0.74–4.25, P = 0.005).
α7-nicotinic cholinergic receptors are expressed in fetal cerebrum in large numbers early in gestation, but they do not receive acetylcholine synapses until just before birth.55–57 In the absence of acetylcholine synapses, choline is a likely initial agonist.18 Levels in the amniotic fluid are just sufficient to activate α7-nicotinic receptors.58 The 50% effective concentration in vitro is 120μM.59 However, many women are deficient in choline during pregnancy, in part because of the fetus’s need for large amounts of choline for the synthesis of cell membranes.17,60–61 In one study 56% of pregnant women and 54% in the present sample had plasma levels below 7μM at 16 weeks gestation, a level associated with liver damage from choline deprivation.25,62
Maternal plasma choline levels only indirectly reflect concentration at fetal α7-nicotinic receptors. Transport of choline is controlled by the placental choline transporter CLT1, which produces amniotic fluid levels approximately twice maternal plasma levels.58,63 Uptake is proportional to plasma concentration, which suggests that higher peak levels may be important determinants of amniotic fluid levels.64 Maternal levels obtained in non-fasting conditions, as in the present study, may be elevated after meals.65–66 No women had choline levels outside the 2 SD of the mean, which might have indicated significant genetic effects.67 Dietary history was not collected because of the low relationship of self-reported intake to maternal choline levels, r = 0.2.25,68
Higher levels of choline did not decrease CRP levels, which indicates that choline did not diminish maternal inflammation directly, although α7-receptors are involved in vagal regulation of immune response.69 The significant interaction of inflammation and choline could have occurred in the fetal cerebrum or on the effect of the inflammatory response on the placenta, where α7-receptors are also expressed.70
In a human observational study, the effects of choline cannot be rigorously isolated from the multiple environmental and genetic influences that converge in fetal development. Vitamin D levels and folic acid levels were not obtained.71 All women were in prenatal care where these supplements were strongly advised. In another study, choline and methionine levels were positively correlated, but folate and Vitamin B12 levels were not. Only choline levels affected infant outcome.25 Obesity, acetaminophen, antidepressants, and marijuana use were common and had effects on the development of P50 inhibition and childhood behavior, but these effects were independent of the interaction between infection and choline levels.72–75
Lower IBQ-R Regulation is associated with decreased reading readiness at age 4 years and decreased conscientiousness, organization, and increased distractibility at age 9 years.76–77 As the child develops, Regulation moderates the child’s Surgency and Negativity to meet cultural expectations.78 Continuity between abnormalities appearing in the first year of life and the emergence of mental disorders such as schizophrenia in adulthood is also well established.79–83 Childhood behavior does not fully predict later mental illness in any individual, but neither do interventions later in life restore function in individuals with early deficits from fetal brain development. Higher maternal choline positively affects child behavior for as long as 7 years, providing a potentially helpful continuity.24
Positive effects of higher choline levels in this study raise the issue of whether supplementation of choline in pregnancy is desirable. There have been 4 small randomized, placebo-controlled trials of choline or phosphatidylcholine supplementation.26–30 Phosphatidylcholine is more resistant to bacterial degradation than choline.65 The two forms are intraconvertable, and both have been used in the trials without significant adverse effects. We conducted a trial beginning at 17 weeks gestation of phosphatidylcholine 7300 mg (equivalent to 900 mg choline) versus placebo in which maternal infection was assessed.27 The recommended dietary intake of 550 mg choline plus supplementation equivalent to 900 mg is less than half the 3500 mg maximum choline advised for pregnant women over 18 years of age (3000mg < 18 years of age).84 The children were evaluated at 40 months of age using the Child Behavior Checklist.28 Nine of 49 mothers had experienced infections during pregnancy; the lower prevalence of infection reflects FDA mandates that excluded some higher risk mothers. Phosphatidylcholine decreased the mean number of problems in attention and aggression in children of mothers who had infection (Table 5; online). Based on these trials, which also showed positive effects on behavior and cognition in children of mothers without specific risk factors, the AMA has recommended that mothers receive “evidence-based amounts of choline in all prenatal vitamins.”85 Prenatal vitamins currently contain as little as 10 mg, and therefore additional supplementation using phosphatidylcholine or choline might be required.
Table 5.
Effects of maternal infection and phosphatidylcholine supplementation on Child Behavior Checklist Attention and Aggression Problems at 40 months of age
| No maternal infection | Maternal infection | |||
|---|---|---|---|---|
| Infection* Phosphatidylcholine | Placebo N = 22 | Phosphatidylcholine N = 18 | Placebo N=4 | Phosphatidylcholine N=5 |
| Attention F1,45 = 7.79 P = 0.008 | 2.41 (SD 1.33) | 2.06 (SD 1.73) | 4.75 (SD 3.10) | 0.83 (SD 1.33)1 |
| Aggression F1,45 = 14.07, P < 0.001 |
5.91 (SD 4.39) | 6.78 (SD 1.41) | 19.5 (SD 7.33) | 6.00 (SD 5.24)1 |
Tukey’s HSD P < 0.01 for comparison with placebo in mothers with infection
Acknowledgement:
This study was conceived and initiated by the late Randal G. Ross.
Supported by the National Institutes of Health NICHD K12HD001271–11 to MCH and NCATS UL1 TR001082 to all investigators and by the Institute for Children’s Mental Disorders and the Anschutz Foundation to RF.
Abbreviations:
- P50
Positive cerebral evoked potential, nominally 50 msec after auditory stimuli
- S1,S2
Auditory Stimulus1 followed at 500 msec by Stimulus2
- IBQ-R
Infant Behavior Questionnaire-Revised
- CRP
C-Reactive Protein
- IL-6
Interleukin-6
- CHRNA7
Gene for the α7-nicotinic cholinergic receptor peptide
- CLT1
Choline transporter 1
- DSM
American Psychiatric Association Diagnostic and Statistical Manual
- REM
Rapid eye movement sleep
- ADHD
Attention Deficit Disorder
- CESD-R
Center for Epidemiological Studies Depression Scale Revised
- STAI-S
State-Trait Anxiety Index-State
- PSS
Perceived Stress Scale
- FET
Fisher’s Exact Test
Footnotes
A correction was made to this article on July 1, 2019. This version is the correction version.
In the article, “Higher Gestational Choline Levels in Maternal Infection Are Protective for Infant Brain Developlment,” by Freedman et al, J Pediatr 2019;208:198–206e2, the authors incorrectly described the Infant Behavioral Questionnaire Revised-Short Form as completed at 1 year of age. The IBQ-R was completed at 52 weeks gestational age, generally 3 months post birth.
Data will be shared upon request.
Conflict of interest: The funders had no role in (1) study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; and (4) the decision to submit the paper for publication. The authors have no conflicts of interest with commercial or other interests.
References
- 1.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 2010; 167:261–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry 1988; 45:189–192. [DOI] [PubMed] [Google Scholar]
- 3.O’Callaghan E, Sham P, Takei N, Glover G, Murray RM. Schizophrenia after prenatal exposure to 1957 A2 influenza epidemic. Lancet 1991; 337:1248–1250. [DOI] [PubMed] [Google Scholar]
- 4.Hornig M, Bresnahan MA Che X, Schultz AF, Ukaigwe JE, Eddy ML, et al. Prenatal fever and autism risk. Mol Psychiatry 2018;23:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann JR, McDermott D. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J Atten Disord 2011;15:667–73. [DOI] [PubMed] [Google Scholar]
- 6.Werenberg DJ, Nybo-Andersen AM, Hvolby A, Garne E, Kragh-Andersen P, Berg-Beckhoff G. Fever and infections in pregnancy and risk of attention deficit/hyperactivity disorder in the offspring. J Child Psychol Psychiatry 2016;57:540–8. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg Y, D’Onofrio BM, Rickert ME, Class QA, Rosenqvist MA, Almqvist C, et al. Maternal infection requiring hospitalization during pregnancy and attention-deficit hyperactivity disorder in offspring: a quasi-experimental family-based study. J Child Psychol Psychiatry 2018. doi: 10.1111/jcpp.12959. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Xue R, Zhang J, Ren T, Richards LJ, Yarowsky P, et al. Anatomical characterization of human fetal brain development with diffusion tensor magnetic resonance imaging. J Neurosci 2009;29:4263–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson PH. Maternal effects on schizophrenia risk. Science 2007;318:576–577. [DOI] [PubMed] [Google Scholar]
- 10.Wu WL, Adams CE, Stevens KE, Chow KH, Freedman R, Patterson PH. The interaction between maternal immune activation and alpha7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain Behav Immun 2015;46:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal-inflammation? Pediatric Res 2011;69, 26R–33R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomaki S, Levisika J, Kellendonk C, McKeague IW, et al. Maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry 2014;171:960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones KL, Croen LA, Yoshida CK, Heuer L, Hansen R, Zerbe O, et al. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatry 2017;22:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koks N, Ghassabian A, Greaves-Lord K, Hofman A, Jaddoe VWV, Verhulst FC, et al. Maternal C-Reactive Protein concentration in early pregnancy and child autistic traits in the general population. Paediatric Perinatal Epidemiol 2016;30:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovanoli S, Engler H, Engler A et al. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 2013; 339:1095–1099. [DOI] [PubMed] [Google Scholar]
- 16.Clarke MC, Tanskanen A, Huttunen, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry 2009;166:1025–30. [DOI] [PubMed] [Google Scholar]
- 17.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr 2006;26:229–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TR. Acetylcholine activates an alpha-bungarotoxin sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci 1997;18:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens KE, Choo KS, Stitzel JA, Marks MJ, Adams CE. Long term improvements in sensory inhibition with gestational choline supplementation linked to alpha7 nicotinic receptors through studies in Chrna7 null mutation mice. Brain Res 2014;1552:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science 2006; 314:1610–1613. [DOI] [PubMed] [Google Scholar]
- 21.Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, et al. Glutamatergic synapse formation is promoted by α7-containing nicotinic acetylcholine receptors. J Neurosci 2012;32:7651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitri OE, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci 2011;31:11088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerwin R, Patel S, Meldrim N. Quantitative audioradiographic analysis of glutmate binding sites in the hippocampal formation in normal and schizophrenic brain. Neurosci 1990; 39:25–32. [DOI] [PubMed] [Google Scholar]
- 24.Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol 2013;177:1338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu BTF, Dyer RA, King DJ, Richardson KJ, Innis SM. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PloS One 2012; 7(8): e43448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheatham CL, Goldman BD, Fischer LM, da Costa K-A, Resnick JS, Zeisel SH. Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, placebo-controlled trial: Am J Clin Nutr 2012; 96:1465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross RG, Hunter SK, McCarthy L, Beuler J, Hutchison AK, Wagner BD, et al. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am J Psychiatry 2013;170:290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross R, Hunter SK, Hoffman MC, McCarthy L, Chambers B, Law A, et al. Perinatal phosphatidylcholine supplementation and early childhood behavior problems: Evidence for CHRNA7 moderation. Am J Psychiatry 2016;173:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caudill MA, Strupp BJ, Muscalu L, Nevins JEH, Canfield RL. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: a randomized, double-blind, controlled feeding study. FASEB J 2018;34:2712–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson SW. Carter RC. Molteno CD. Stanton ME. Herbert JS, Lindinger NM, et al. Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: a randomized, double-blind, placebo-controlled clinical trial. Alcohol Clin Exp Res 2018;42:1327–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: parental psychosis, maternal depression, and nicotine use. Schizophr Bull 2010;119:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kisley MA, Polk SD, Ross RG, Levisohn PM, Freedman R. Early postnatal development of sensory gating. NeuroReport 2003;14:693–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anders T, Emde R, Parmelee A. A manual of standardized terminology, techniques and criteria for scoring of states of sleep and wakefulness in newborn infants. Los Angeles: UCLA Brain Information Service, NINDS Neurological Information Network;1971. [Google Scholar]
- 34.Griffith JM, Freedman R. Normalization of schizophrenics’ auditory P50 gating deficit after NREM, but not REM sleep. Psychiat Res 1995;56:271–8. [DOI] [PubMed] [Google Scholar]
- 35.Adler LE, Pachtman E, Franks RD. Pecevich M Waldo MC, Freedman R Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 1982;17:639–54. [PubMed] [Google Scholar]
- 36.Smith DA, Boutros NN, Schwarzkopf SB. Reliability of P50 auditory event-related potential indices of sensory gating. Psychophysiol 1994;31:495–502. [DOI] [PubMed] [Google Scholar]
- 37.Smith E, Crawford T, Thomas M, Reid V. Schizotypy and sensory gating: a 6-month-old EEG study. Schizophr Bull 2018;44:S301–S302. [Google Scholar]
- 38.Hunter SK, Corral N, Poniscan H, Ross RG. Reliability of P50 auditory sensory gating measures in infants during active sleep. NeuroReport 2008;19:79–82. [DOI] [PubMed] [Google Scholar]
- 39.Hunter SK, Gillow SJ, Ross RG. Stability of P50 auditory sensory gating during sleep from infancy to 4 years of age. Brain Cognition 2015;94:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Putnam SP, Helbig AL, Gartstein MA, Rothbart MK, Leerkes E. Development and assessment of Short and Very Short Forms of the Infant Behavior Questionnaire–Revised. J Personal Assess 2014;96:445–458. [DOI] [PubMed] [Google Scholar]
- 41.Orczyk-Pawilowicz M, Jawien E, Deja S, Hirie L, Zabek A, Mlynarz P. Metabolomics of human amniotic fluid and maternal plasma during normal pregnancy. PLoS ONE 2016;11:e0152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Lee L, Quah PL, Saw SM et al. Maternal choline status during pregnancy, but not that of betaine, is related to antenatal mental well-being: the growing up in Singapore toward healthy outcomes cohort. Depress Anxiety 2017; 34:877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collier SA, Rasmussen SA, Feldkamp ML, Honein MA. National Birth Defects Prevention S. Prevalence of self-reported infection during pregnancy among control mothers in the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol 2009;85:193–201. [DOI] [PubMed] [Google Scholar]
- 44.Jeon CY, Muennig P, Furuya EY, Cohen B, Nash D, Larson EL. Burden of present-on-admission infections and health care-associated infections, by race and ethnicity. Am J Infect Cont 2014;42:1296–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bui Q, Miller CC. The age that women have babies: how a gap divides America. NY Times; Available from: https://www.nytimes.com/interactive/2018/08/04/upshot/up-birth-age-gap.html [Accessed 6th Nov 2018]. [Google Scholar]
- 46.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. PNAS 1997;94:587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamilton HK, Williams TJ, Ventura J, Jasperse LJ, Owens EM, et al. Clinical and cognitive significance of auditory sensory processing deficits in schizophrenia. Am J Psychiatry 2018;175:275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stefansson H, Rujescu D, Cichon S, Pietilinen P, Olli PH, Ingason A, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen-Brady K, Robison R, Cannon D, Varvil T, Villalobos M, Pingree C, et al. Genome-wide linkage in Utah autism pedigrees. Mol Psychiatry 2010;15:1006–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stergiakouli E, Hamshere M, Holmans P, Langley K, Zaharieva I, et al. Investigating the contribution of common genetic variants to the risk and pathogenesis of ADHD. Am J Psychiatry 2012;169:186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hutchison AK, Hunter SK, Wagner BD, Calvin EA, Zerbe GO, Ross RG. Diminished infant P50 sensory gating predicts increased 40-month-old attention, anxiety/depression, and externalizing symptoms. J Atten Disord 2007;21:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wunderlich JL, Cone-Wesson BK, Shepherd R. Maturation of the cortical auditory evoked potential in infants and young children Hearing Res 2006;212:185–202. [DOI] [PubMed] [Google Scholar]
- 53.Bisiacchi PS, Mento G, Suppiej A. Cortical auditory processing in preterm newborns: an ERP study. Biol Psychol 2009;82:176–85. [DOI] [PubMed] [Google Scholar]
- 54.Harper RM, Leake B, Miyahara L, Mason J, Hoppenbrouwers T, Sterman MB, Hodgman J. Temporal sequencing in sleep and waking states during the first 6 months of life. Exp Neurol 1981;72:294–307. [DOI] [PubMed] [Google Scholar]
- 55.Birnbaum R, Jaffe AE, Hyde TM, Kleinman JE, Weinberger DR. Prenatal expression patterns of genes associated with neuropsychiatric disorders. Am J Psychiatry 2014;171:758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Court JA, Lloyd S, Johnson M, Griffiths M, Birdsall NJ, Piggott MA, et al. Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Brain Res Dev Brain Res 1997;101:93–105. [DOI] [PubMed] [Google Scholar]
- 57.Descarries L, Aznavour N, Hamel E. The acetylcholine innervation of cerebral cortex: new data on its normal development and its fate in the hAPP (SW,IND) mouse model of Alzheimer’s disease. J Neural Transm 2005;112:149–62. [DOI] [PubMed] [Google Scholar]
- 58.Ilcol YO, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in plasma during pregnancy, after delivery, and in newborns. Arch Physiol Biochem 2002;110:393–9. [DOI] [PubMed] [Google Scholar]
- 59.Horenstein NA, Leonik FM, and Roger L Papke RL Multiple pharmacophores for the selective activation of nicotinic α7-type acetylcholine receptors. Mol Pharmacol 2008;74: 1496–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen HH, Batres-Marquez SP, Carriquiry A, Schalinske KL. Choline in the diets of the U.S. population: NHANES, 2003–2004. FASEB J. 2007;21:lb219 [Google Scholar]
- 61.Masih S, Plumptre L, Ly A, Berger H, Lausman AY, Croxford R, et al. Pregnant Canadian women achieve recommended intakes of one-carbon nutrients through prenatal supplementation but the supplement composition, including choline, requires reconsideration. J Nutr 2015;145:1824–34. [DOI] [PubMed] [Google Scholar]
- 62.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography–tandem mass spectrometry. Clin Chem 2033;49:286–94. [DOI] [PubMed] [Google Scholar]
- 63.Baumgartner HK, Trinder KM, Galimanis CE, Post A, Phang T, Ross RG, Winn VD. Characterization of choline transporters in the human placenta over gestation. Placenta 2015;36:1362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwao B, Yara M, Hara N, Kawai Y, Yamanaka T, Nishihara H, et al. Functional expression of choline transporter like-protein 1 (CTL1) and CTL2 in human brain microvascular endothelial cells. Neurochem Int 2016;93:40–50. [DOI] [PubMed] [Google Scholar]
- 65.Zeisel SH, Growdon JH, Wurtman RJ, Magil SG, Logue M. Normal plasma choline response to ingested lecithin. Neurology 1980;30:1226–9. [DOI] [PubMed] [Google Scholar]
- 66.Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high-throughput method based on normal-phase chromatography–tandem mass spectrometry. Clin Chem 2033;49:286–94. [DOI] [PubMed] [Google Scholar]
- 67.Fischer LM, da Costa KA, Galanko J, Sha W, Stephenson B, Vick J et al. Choline intake and genetic polymorphisms influence choline metabolite concentrations in human breast milk and plasma. Am J Clin Nutr 2010;92:336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abratte CM, Wang W, Li R, JAxume J, Moriarty DJ, Caudill MA. Choline status is not a reliable indicator of moderate changes in dietary choline consumption in premenopausal women. J Nutr Biochem 2009;20:62–9. [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Yu M, Ochani M, Amelia CA, Tanovic M, Susaria S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003;421:384–8. [DOI] [PubMed] [Google Scholar]
- 70.Lips KS, Bruggmann D, Pfeil U, Brugmann D, Pfeil U, Vollerthun R, et al. Nicotinic acetylcholine receptors in rat and human placenta. Placenta 2005;26:735–46. [DOI] [PubMed] [Google Scholar]
- 71.McGrath JJ, Eyles DW, Pedersen CB, Anderson C, Ko P, et al. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch Gen Psychiatry 2010;67:889–94. [DOI] [PubMed] [Google Scholar]
- 72.Brion MJ, Zeegers M, Jaddoe V, Verhulst F, Tiemeier H, Lawlor DA, et al. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics 2011;127:e202–e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stergiakouli E, Thapar A, Davey Smith G. Association of acetaminophen use during pregnancy with behavioral problems in childhood: evidence against confounding. JAMA Pediatrics 2016;170:964–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sujan AC, Rickert ME, Oberg AS, Quinn PD, Hernandez-Diaz S et al. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA 2017;317:1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunter SK, Mendoza JH, D’Anna K, McCarthy L, Freedman R et al. Antidepressants may mitigate the effects of prenatal maternal anxiety on infant auditory sensory gating. Am J Psychiatry 2012;169:616–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gartstein MA, Putnam SP, Kliewer R. Do infant temperament characteristics predict core academic abilities in preschool-aged children? Learn Individ Diff 2016; 45:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Slobodskaya HR, Kozlova EA. Early temperament as a predictor of later personality. Personality Individ Diff 2016; 99:127–32. [Google Scholar]
- 78.Ahadi SA, Rothbart MK. Children’s temperament in the US and China: similarities and differences. Eur J Personality 1993;7:359–77. [Google Scholar]
- 79.Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schiz Bull 1994;20:441–51. [DOI] [PubMed] [Google Scholar]
- 80.Fish B Longitudinal observations of biological deviations in a schizophrenic infant. Am J Psychiatry 1959;116:25–31. [DOI] [PubMed] [Google Scholar]
- 81.Mednick SA, Schulsinger F. Some premorbid characteristics related to breakdown in children with schizophrenic mothers. J Psychiatric Res 1968;6:Supp 1:267–91. [Google Scholar]
- 82.Erlenmeyer-Kimling L, Cornblatt B. The New York High-Risk Project: a followup report. Schizophr Bull 1987;13:451–61. [DOI] [PubMed] [Google Scholar]
- 83.Serdarevic F, Ghassabian A, van Batenburg-Eddes T, van Battenburg-Eddes T, Tahirovic E et al. Infant neuromotor development and childhood problem behavior. Pediatrics 2017;140:e20170884. [DOI] [PubMed] [Google Scholar]
- 84.Food and Drug Administration. Food labeling: revision of the nutrition and supplement facts labels. Federal Register 2016;May 27:903–4. [PubMed] [Google Scholar]
- 85.AMA. Proceedings of the 2017 Annual Meeting House of Delegates. https://www.ama-assn.org/about/proceedings-2017-annual-meeting-house-delegates. [Accessed 27th Nov 27, 2017].