Abstract
The pituitary adenylate cyclase-activating polypeptide (PACAP) type 1 (PAC1) receptor is a G protein-coupled receptor and class II receptor member. The receptor domains critical for signaling are unknown. To explore the role of the C terminus, truncations of 63 residues (Tr406), 53 residues (Tr416), 49 residues (Tr420), 44 residues (Tr424), and 37 residues (Tr433) were constructed and expressed in NIH/3T3 cells, and immunofluorescence, radioligand binding, adenylyl cyclase (AC) and phospholipase C (PLC) assays were performed. 125I-PACAP-27 binding (Kd = 0.6–1.5 nM) for the Tr406 and Tr433 were similar to wild type Hop and Null splice variants (Kd = ~1.1 nM). Although internalization of ligand for both the Tr406 and Tr433 mutants was reduced to 50–60% at 60 min compared with 76–87% for WT, loss of G protein coupling did not account for differences in internalization. Despite similar binding properties Tr406 and Tr416 mutants showed no AC or PLC response. Addition of 14 amino acids distal to HopTr406 resulted in normal AC and PLC responses. Site-directed mutagenesis indicated that Arg416 and Ser417 are essential for G protein activation. The proximal C terminus mediates signal transduction, and the distal is involved with internalization. Two residues within the C terminus, Arg416 and Ser417 conserved among class II receptors are the likely sites for G protein coupling.
It has been previously demonstrated for receptors belonging to the class I heptahelical receptor family that specific intracellular amino acid residues are critical for internalization and coupling to guanine nucleotide-binding proteins (G proteins). These cytoplasmic domains have been partially characterized for the α- and β-adrenergic receptors (1, 2), the muscarinic receptors (3), and for rhodopsin (4). Studies of the β2-adrenergic (2) and muscarinic receptors (3) have suggested that substitution, deletion, and/or insertion of residues in the third intracellular loop affect the ability of these receptors to couple to G proteins.
At present, the role of the cytoplasmic domains for members of the class II family for receptor coupling to intracellular G proteins has not been fully explored. Based on studies with receptors belonging to the class I receptor family, we speculated that amino acids in either the third intracellular loop or the C terminus of the pituitary adenylate cyclase-activating polypeptide receptor (PAC1),1 may be important for coupling to G proteins (5). Furthermore, the C terminus has been proposed to be necessary for the formation of a functional receptor-G protein complex and to result in heterodimerization for some GPCRs such as type B receptor for GABA and GABAB-like orphan receptor (6).
Pituitary adenylate cyclase-activating polypeptide (PACAP) hormone was originally isolated from bovine hypothalamus and is one of the most recently identified peptides in the VIP/secretin/glucagon family (7). The cloning of the rat and human PAC1 cDNA and genes identified a receptor protein composed of ~468 amino acids containing seven transmembrane domains that are characteristic of G protein-coupled receptors (5, 8). The PAC1 belongs to the class II GPCR superfamily that includes VPAC1 (the classical VIP1 receptor), VPAC2 (VIP2 receptor), secretin, glucagon, and calcitonin receptors (9); these molecules can be distinguished on the basis of their relative affinities for the agonists PACAP-27, PACAP-38, VIP, and helodermin (10). The cloning of the rat and human PAC1 gene have identified two unique exons that can be alternatively spliced yielding different third intracellular loops. The four major splice variants are named Null, Hip, Hop, and Hip-Hop (5, 8), and they have been shown to be differentially expressed in different tissues and are able to couple with different efficacies and potencies to phospholipase C (PLC) and adenylyl cyclase (AC) (5, 8). Therefore, the expression of splice variants in the third intracellular loop and the close similarity of the general structure of PAC1 compared with the members of the class II family make it a good candidate for developing a receptor model to understand the role of the C terminus for the activation of signal transduction cascades.
Previously, the major efforts that have been made have focused on the receptor domains involved in receptor internalization, desensitization, and recycling, but these studies have mainly focused on members of the class I receptor family, such as the cholecystokinin type A, gastrin-releasing peptide, and β-adrenergic receptors (11–14). The specific amino acid residues involved in these processes have been identified for only a few receptors such as the angiotensin II (15), parathyroid hormone (16), interleukin 8 (17), and the cholecystokinin-B receptor (18). Alternatively, the third intracellular loop has been indicated to be important for the muscarinic receptor (19). In contrast to these class I receptor family studies, only a few reports exist on the class II receptor family domains involved in receptor internalization (20, 21).
EXPERIMENTAL PROCEDURES
Materials
125I-PACAP-27 was purchased from PerkinElmer Life Sciences (50 mCi/mmol); [3H]adenine, and [3H]myo-3-inositol were from Amersham Pharmacia Biotech; and anti-hemagglutinin monoclonal antibody 12CA5 was from Roche Molecular Biochemicals. Dulbecco’s modified Eagle’s medium (DMEM), calf serum, penicillin G, and streptomycin sulfate were purchased from Life Technologies, Inc. Bovine serum albumin was obtained from ICN Biomedicals, Inc. (Aurora, OH), and forskolin was from Sigma.
cDNA Constructs for the hPAC1 with C-terminal Truncation
An XbaI site, Kozack sequence and hemagglutinin (HA) epitope sequence were used to prepare the WT and truncated mutants of hPAC1. Primers with the following sequence were were used to construct the 3’ ends of the Tr406, Tr416, Tr420, Tr424, and Tr433 mutants respectively: 5’-ACTGACTAGGAATTCTCAACCATTCAGAAAACAGTAGAGAAC-3’, 5’-ACTGACTAGGAATTCTCACCATTTTCGCTTGATCTCCGCTTGTACCAC-3’, 5’-ACTGACTAGGAATTCTCACCTCCAGCTTCGCCATTTTCGCTTGATCTCCGCTTG-3’, 5’-ACTGACTAGGAATTCTCAGTAACGGTTCACCTTCCAGCTTCGCCATTT-3’, and 5’-ACTGACTAGGAATTCTCAGTGTCGGTGCTTGAAGTCCAC-3’. The single and double amino acid mutations for HopTr420 (either Tr420A416 or Tr420A417 or Tr420A418 or Tr420A418A419) were performed by replacing the codon of original amino acid with AGC for mutation to Ala. Using a full-length hPAC1 cDNA (nucleotides 1–1591 for Null variant and nucleotide 1–1675 for Hop variant), the truncated mutants of hPAC1 cDNA were amplified by polymerase chain reaction (PCR). Briefly, the following PCR conditions were used: extension at 94 °C for 45 s, annealing at 60 °C for 25 s, and extension at 72 °C for 2 min (30 cycles). Following amplification, the DNA was digested with both XbaI and EcoRI and subcloned into the vector pCDL-SRa/Neo at an XbaI site and EcoRI site, as described previously (9).
Sequence Analysis
All PCR-generated mutants and chimeras were sequenced using the Applied Biosystem model 373A (PerkinElmer Life Sciences). DNA and amino acid sequence analysis was performed using the Geneworks® software package.
Establishment of Stably Transfected NIH/3T3 Cells Expressing HA- tagged WT and Truncated Mutants
Following linearization of the vector at the AatII restriction site, within the ampicillin resistance gene of the vector pCDL-SRa/Neo, 20 μg of recombinant plasmid were stably transfected into NIH/3T3 fibroblasts by using a BTX electroporator (model T820, San Diego, CA) at 475 V for 1 ms, 4 pulses on 2 × 107 cells, in a volume of 0.25 ml with 20 μg of the linearized recombinant vector containing the respective WT or mutant cDNA in the presence of 500 μg/ml salmon sperm DNA as a carrier. Clones of stably transfected cells were selected using Geneticin® (250 mg/ml; Life Technologies, Inc.). At least 10 stably transfected clones were assayed for 125I-PACAP-27 radioligand binding for each WT and transfected mutant. Cell clones with similar receptor densities were chosen for further study.
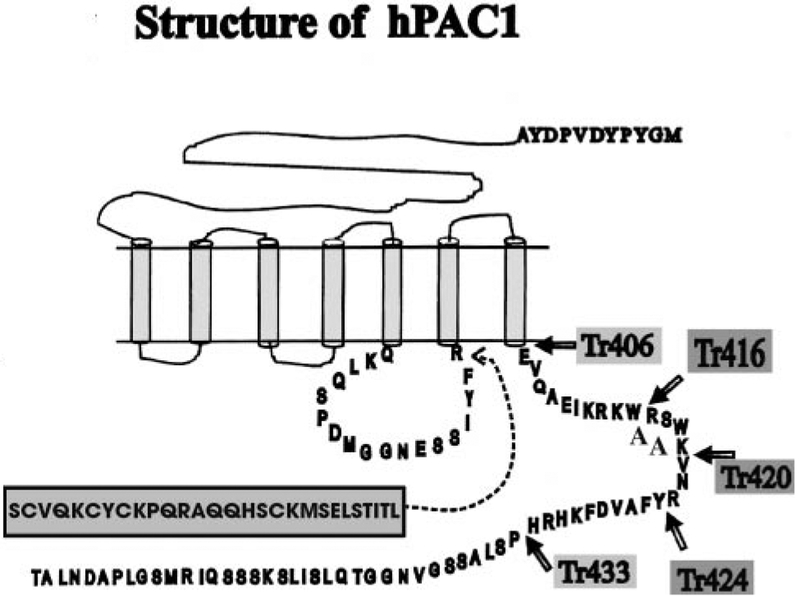
Structure of the PAC1 Truncation Mutants
A model depicting the wild type Null (NullWT) and Hop (HopWT) variants with an HA epitope tag is shown in Fig. 1. The HopWT contains additional 28 amino acids (including Ser365 as a putative protein kinase C phosphorylation site) in the 3rd intracellular loop compared with the Null splice variant, as described previously (8, 9). Five truncation mutants were generated: one lacked the entire 63 amino acids of the C terminus (Tr406), whereas the other mutants were reconstituted with additional 10, 14, 18, or 26 amino acids, added to the carboxyl tail, (Tr416, Tr420, Tr424, and Tr433, respectively) as shown by the arrows in Fig. 1. The putative protein kinase C phosphorylation site, Ser417, within the C terminus of the hPAC1 (Fig. 1) was deleted in the Tr416 and Tr406 receptor mutants, but it was present in the Tr420 mutant. Point mutations to the full-length receptor were performed as described above using as a template for the PCR reaction the full length of the HopWT receptor. The WT and truncated mutants of the PAC1 were constructed with an HA epitope (YPYDVPDYA) on the N terminus of the receptor to detect them, by using a monoclonal antibody directed against this epitope. The presence of an epitope tag on the N terminus was designed to confirm surface expression. The radioligand binding, cAMP, and inositol phosphate dose responses of the transfected cells expressing this construct were compared with untransfected cells and found to be identical (data not shown).
FIG. 1. Schematic representation structure of the Null hPAC1, Hop hPAC1, and the C-terminal deletion mutants, Tr406, Tr416, Tr420, Tr424, and Tr433.
Mutants Tr406 (−63 amino acids), Tr416 (−53 amino acids), Tr420 (−49 amino acids), Tr424 (−44 amino acids), and Tr433 (−37 amino acids) were constructed using PCR by truncation of the amino acids of the C-terminal tail, as indicated by the arrows. An HA epitope tag is present on the N terminus as indicated. An additional 28 amino acids contained in the third intracellular loop are the human Hip and Hop splice variant compared with the Null splice variant.
Polyclonal Antibody Synthesis
Peptides from the C terminus of the human and rat PACAP-1 receptors (EVQAEIKRKWRSWKVNRY) were synthesized by G. Poy (NIDDK, National Institutes of Health) and purified by the antibody and peptide CORE of the CURE:VA/UCLA Digestive Diseases Center. Conjugation of the peptide was performed with the keyhole limpet hemocyanin using bis-diazotized benzidine. Rabbits were immunized at 5–6-week intervals intradermally. Antibodies were screened using enzyme-linked immunosorbent assay and Western blot analysis to assess their specificity.
Immunofluorescent Detection of hPAC1 Expression on NIH/3T3 Cells
Cells were plated overnight at a low density on polylysine-coated coverslips. The following day, the cells were washed twice with phosphate-buffered saline (PBS) and incubated at a dilution of 1:1000 with polyclonal anti-PAC1 antibodies overnight at 4 °C. Then the cells were incubated with Alexa 488-conjugated anti-rabbit polyclonal antibodies (Molecular Probes) for 1 h at 4 °C. Results were visualized by fluorescent microscopy.
Preparation of Membrane Fractions
Cells were grown until ~80% confluence and harvested by washing with PBS containing 2.7 mM EDTA. Harvested cells were homogenized with homogenization buffer (100 mM NaCl, 2 mM MgCl2, 4 mM EGTA, 0.5 mM phenylmethylsulfonyl fluoride, 20 μg/ml leupeptin, and 10 μg/ml pepstatin A, 200 kIU/ml aprotinin, 8% sucrose, pH 7.4) using a Dounce homogenizer (Wheaton Inc., Millville, NJ) and centrifuged at 800 × g for 10 min. The pellet containing intact cells and nuclei was discarded, and the supernatant was centrifuged at 80,000 × g for 45 min in an ultracentrifuge (Beckman). The resultant membrane pellet was resuspended in the homogenization buffer and stored at −70 °C. The protein concentration was carried out by the method described by Lowry et al. (22).
Scatchard Analysis
125I-PACAP-27 binding experiments were performed by the previously described method (23). Briefly, 100 μg of membrane protein, isolated as above, were dissolved in 0.05% digitonin and mixed with 50 nM 125I-PACAP-27 either with or without the indicated concentrations of PACAP-27 at a final volume of 250 ml and incubated at 37 °C. After a 75-min incubation, the reaction was terminated by adding 800 ml of chilled phosphate-buffered saline containing 1% bovine serum albumin (1% BSA/PBS). Tracer binding was measured after filtration through a GF/C glass fiber filter (Whatman) soaked with 0.1% polyethyleneimine (Sigma) and washed three times with 3 ml of 1% BSA/PBS. Kd and Bmax were determined by Scatchard analysis by GraphPad Prism 2.01.
125I-PACAP-27 Radioligand Binding Inhibition
Stably transfected cells were plated overnight on 24-well plates in DMEM with 10% calf serum, at a density of ~50,000 cells/well. The following day, the cells were washed once in 1% BSA/PBS at 4 °C and incubated in DMEM containing 1% BSA for 60 min at 37 °C with 50 pM of 125I-PACAP-27 (2200 Ci/mmol) (PerkinElmer Life Sciences) either with or without the indicated concentrations of unlabeled peptides, PACAP-27 (Bachem, Torrance, CA). The density of cell surface receptors expressed for each stably transfected cell line was determined by measuring the amount of saturable binding (total binding in the presence of 125I-PACAP-27 alone minus nonspecific binding in the presence of 1 μM PACAP-27). Nonspecific binding was defined as total binding in the presence of 1 μM unlabeled PACAP. After termination of the binding reaction by washing the cells twice with cold 1% BSA/PBS, the cells were solubilized with 1 ml of 0.1 N NaOH, and radioactivity was detected by a g counter. For each mutant at least three experiments were performed in triplicate. Binding data were analyzed using the equation y 5 Bottom 1 [Top − Bottom]/(1 + 10 [x-log EC50]), where x represents the log concentration of PACAP-27 and y is the percentage of binding of control. Similar levels of 125I-PACAP-27 binding displacement by PACAP-27 were observed in five independent clones for each mutant receptor. Binding data were analyzed using the nonlinear least squares curve fitting program Prism 2.01 (GraphPad Software, Inc., San Diego). To normalize signal transduction data for receptor density, we estimated the receptor density by ligand binding using a concentration of free radioligand [L] > 3 × Kd, a concentration of the receptor [Rtotal] < 0.1Kd and the relationship [Rtotal] = [RL] [Kd + L ]/[L] (23).
Radioligand Stripping Studies
Transfected cells were prepared as described above and incubated with 50 pM 125I-PACAP at 37 °C for the indicated time intervals between 3 and 90 min. At the time intervals, cells were washed twice with 1% BSA/PBS and treated with 0.5 M KSCN for 8 min at room temperature. Radioactivity within the cells was measured after solubilization with 0.1 N NaOH. In all experiments, parallel incubations were performed in the presence of 1 μM unlabeled PACAP-27 to determine nonsaturable binding at each time point. Internalized receptor was defined as cell-associated radioactivity after stripping and expressed as a percentage of total binding. At the indicated time points, cells were washed with a 0.5 M KSCN solution. Internalized radioligand is defined as the amount of 125I-PACAP-27 that could not be stripped by KSCN washing and expressed as a percentage of the total bound 125I-PACAP-27 in control cells processed in parallel without KSCN exposure. At least three experiments were performed in triplicate.
Adenylyl Cyclase Assay
Stably transfected NIH/3T3 cells were plated on 24-well culture dishes overnight (~50,000 cells/well) with DMEM/10% calf serum in the presence of [3H]adenine (Amersham Pharmacia Biotech) at a concentration of 2 mCi/ml. The cells were washed with DMEM, incubated with or without the indicated concentrations of PACAP-27 in DMEM containing BSA (1 mg/ml) and 2.5 mM 3-isobutyl, 1–1-methyl-xanthine for 30 min, and then aspirated. 100 μl of 2% SDS, 1 mM cAMP solution was used to lyse the cells. cAMP was assayed by consecutive Dowex AG-50W-X4 resin (Bio-Rad) and aluminum oxide (Sigma) column chromatography as described by Salomon et al. (24). Elutes were collected in vials and counted using a Beckman Liquid Scintillation Counter (Beckman, Fullerton, CA).
Inositol Phosphate Assay
Transfected NIH/3T3 cells were plated on 24-well culture plates (~50,000 cells/well) with DMEM/10% calf serum in the presence of 100 μCi/ml myo-2-[3H]inositol (PerkinElmer Life Sciences) overnight. Following aspiration of the myo-2-[3H]-inositol, cells were incubated with PI buffer (20 mM HEPES, 2 mM CaCl2, 1.2 mM MgSO4, 10 mM LiCl, 11.1 mM, glucose, 0.5% BSA) in the presence of the indicated peptide concentrations. Total [3H]inositol phosphate was measured using strong anion exchange chromatography (Dowex AG 1-X8) according to a modification of the method of Berridge et al. (25).
RESULTS
Detection of hPAC1 Expression on NIH/3T3 Cells by Immu- nofluorescence
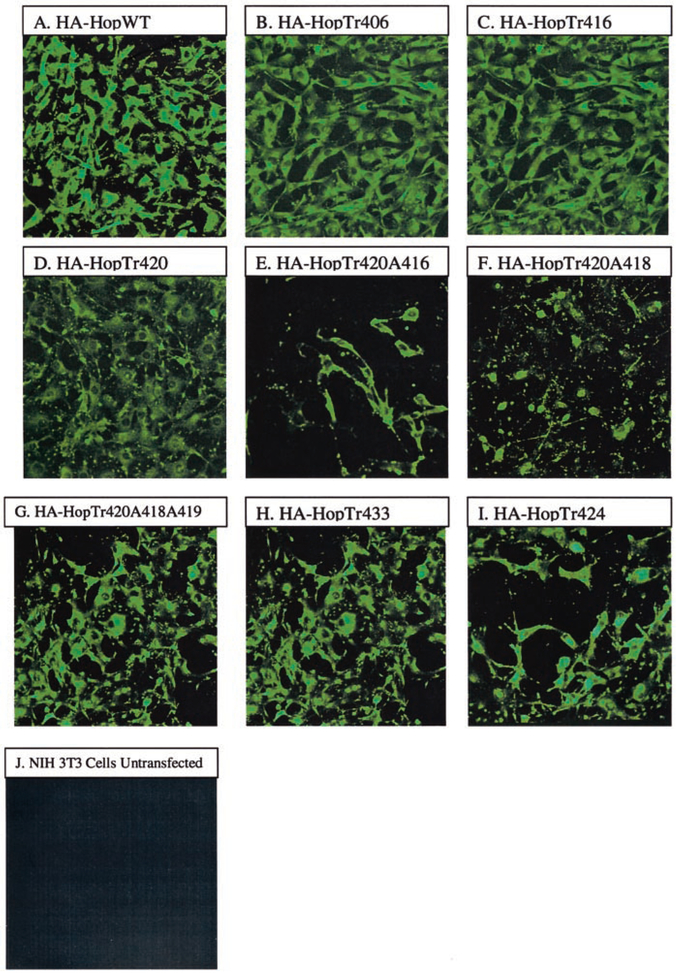
Immunofluorescence analysis by using a polyclonal anti-PAC1 antibody directed against the C terminus confirmed cell surface expression of wild type and mutant constructs for representative Hop splice variants as shown in Fig. 2. These results are consistent with the detection of rat and human WT PAC1s on transfected NIH/3T3 cells by confocal microscopy using a monoclonal anti-HA antibody directed against the HA epitope constructed on the N terminus of the receptor (results not shown). In addition, a fluorescent PACAP hormone (FluoPACAP) was also used to demonstrate cell surface expression using confocal microscopy for each of the constructs by confocal microscopy (data not shown). Similarly, FACS analysis using the polyclonal anti-PAC1 conjugated to Alexa488 showed that .90% of the stably transfected cells showed cell surface expression (data not shown).
FIG. 2. Immunofluorescence microscopy using the anti-PAC1 polyclonal-antibody expressing the indicated WT or truncated receptor on NIH/3T3 cells.
The anti-PAC1 antibody, which recognizes the C-terminal region of the receptor, was used as a primary antibody. The cells were then incubated with Alexa 488-conjugated anti-rabbit polyclonal antibodies, for 1 h at 4 °C. A, HA-HopWT; B, HA-HopTr406; C, HA-HopTr416; D, HA-HopTr420; E, HA-HopTr420A416; F, HA-HopTr420A418; G, HA-HopTr420A418A419; H, HA-HopTr433; I, HA-HopTr424; J, NIH/3T3 cells untransfected. Magnifica- tion is 400×.
Scatchard Analysis
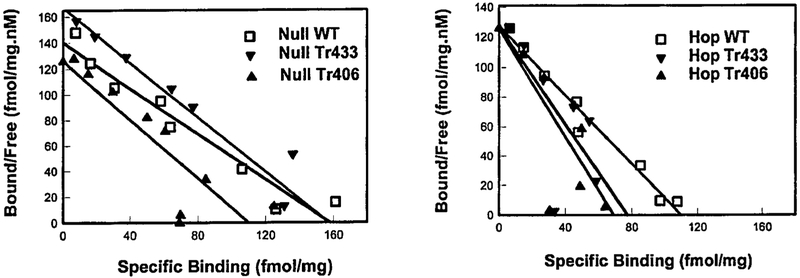
To investigate the ligand binding properties (Kd, Bmax) and expression levels of mutant receptors, we studied the 125I-PACAP-27 binding to membranes of NIH/3T3 cells expressing the WT or the truncated receptors (Fig. 3). The binding of 125I-PACAP-27 to cells expressing WT Null variant (right panel) and Hop variant (left panel) or truncated mutant receptors (Tr406 or Tr433) was performed at 37 °C. Linear regression reveals a Kd of 0.87, 0.91, and 1.12 nM and a Bmax of 129, 118, and 157 fmol/mg of protein for the NullWT, NullTr406, and NullTr433 (left panel), respectively and a Kd = 1.25, 0.55, and 0.63 nM and a Bmax ~130, 69, and 73 fmol/mg of protein for the HopWT, Tr406, and HopTr433 (Fig. 3, right panel), respectively. The Scatchard plot showed only small differences in Kd and Bmax between the truncated and wild type receptors (see Table II).
FIG. 3. Scatchard plot analysis of 125I-PACAP-27 binding on membranes of NIH/3T3 cells expressing either the WT or truncated mutant receptor.
Scatchard plot analysis using 50 pM of 125I-PACAP on membrane preparations was performed on the indicated WT or truncation mutants (Tr406 or Tr433). Each value represents the mean 6 S.E. of at least two experiments performed in triplicate. Specific binding on membranes for the Null variant (left panel) or Hop variant (right panel) and their mutants were fit to a one-site model.
TABLE II. Summary of the KD, Bmax, potencies and efficacies for both cAMP and inositol phosphate efficacy for the wild type and PAC1 mutant constructs.
The data presented are the means ± S.E. for at least three experiments performed in triplicate for each of the indicated constructs.
| PAC1 mutant construct | KD | Bmax | cAMP | Inositol phosphate | ||
|---|---|---|---|---|---|---|
| Efficacy | EC50 | Efficacy | EC50 | |||
| nM | pmol/mg | fold stimulation over basal | nM | fold stimulation over basal | nM | |
| WT Hop | 0.9 ± 0.05 | 109.9 ± 3.1 | 45.0 ± 1.41 | 15.5 ± 6.84 | 6.3 ± 0.32 | 28.7 ± 0.51 |
| Tr406 | 0.6 ± 0.52 | 73.8 ± 1.97 | 0 | 0 | 0 | 0 |
| Tr416 | 0.6 ± 0.32 | 69.3 ± 1.54 | 0 | 0 | 0 | 0 |
| Tr420 | 1.7 ± 0.24 | 241.9 ± 5.96 | 0 | 0 | 0 | 0 |
| Tr420A416 | 2.2 ± 0.91 | 242.8 ± 6.55 | 0 | 0 | 0 | 0 |
| Tr420A417 | 1.7 ± 0.16 | 316.9 ± 7.21 | 0 | 0 | 0 | 0 |
| Tr420A418 | 0.3 ± 0.05 | 79.8 ± 8.20 | 45.3 ± 2.67 | 7.1 ± 3.35 | 8.4 ± 0.38 | 30.0 ± 0.00 |
| Tr420A418A419 | 0.4 ± 0.39 | 101.4 ± 1.26 | 34.0 ± 0.94 | 15.5 ± 6.84 | 8.2 ± 0.70 | 53.3 ± 9.47 |
| Tr424 | 0.3 ± 0.47 | 58.1 ± 9.30 | 29.7 ± 2.14 | 0.40 ± 0.17 | 8.4 ± 0.57 | 23.3 ± 3.85 |
| Tr433 | 0.5 ± 0.29 | 129.8 ± 1.03 | 44.8 ± 2.10 | 1.1 ± 0.07 | 6.2 ± 0.54 | 90.0 ± 8.82 |
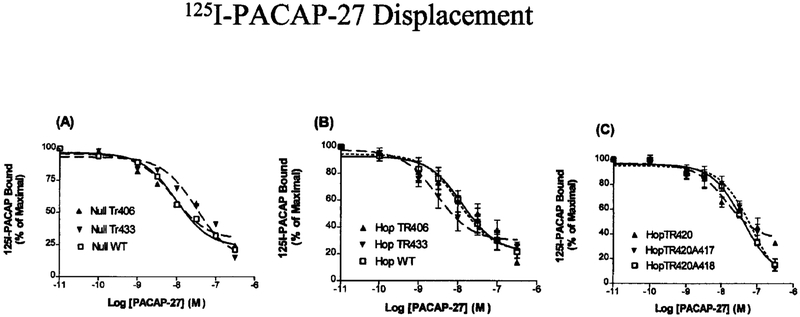
Radioligand Displacement of 125I-PACAP-27
Radioligand competitive binding was analyzed by specific binding of 125I-PACAP-27 at 37 °C for 1 h in the presence or absence of the indicated concentration of PACAP-27. Because previous studies using NIH/3T3 cells stably expressing hPAC1 have shown that PACAP-27 and PACAP-38 are equipotent, only PACAP-27 was used (9). A similar level of 125I-PACAP binding was observed for both the WT and truncated mutants. Half-maximal inhibition (IC50) of 125 I-PACAP-27 was shown on intact NIH/3T3 cells expressing NullWT, NullTr406, or NullTr433 (Fig. 4A) and HopWT, HopTr406, and Tr433 (Fig. 4B), whereas Hop Tr420, Hop Tr420A417, and Tr420A418 (Fig. 4C) were all nearly identical (IC50 = 3–13 nM). These data were fit to a one-site model for ligand binding and are in close agreement with the results obtained from the Scatchard analysis of membrane preparations; they suggest that truncation or substitution of amino acids within the C terminus does not alter the ligand binding to the receptor.
FIG. 4. Displacement of 125I-PACAP 27 on NIH/3T3 cells stably expressing Null or Hop WT receptor or the truncated mutant receptors Tr406, Tr420, Tr420A417, Tr420A418, and Tr433.
NIH/3T3 cells stably expressing the indicated hPAC1 NullWT, NullTr406 and NullTr433 (A); hPAC1 HopWT, HopTr406, and HopTr433 (B); and HopTr420, Tr420A417, and Tr420A418 (C) were incubated with 125I-PACAP (50 pM) either alone or with the indicated concentration of unlabeled PACAP-27. Each value represents the mean ± S.E. of at least three experiments performed in triplicate. Binding data were analyzed using the equation y = Bottom + [Top + Bottom]/(1 + 10 [x log EC50]), where x represents the log concentration of PACAP-27 and y is the percentage of binding of control. Similar levels of 125I-PACAP-27 binding displacement by PACAP-27 were observed for five independent clonal cell lines for each mutant receptor.
Radioligand Internalization
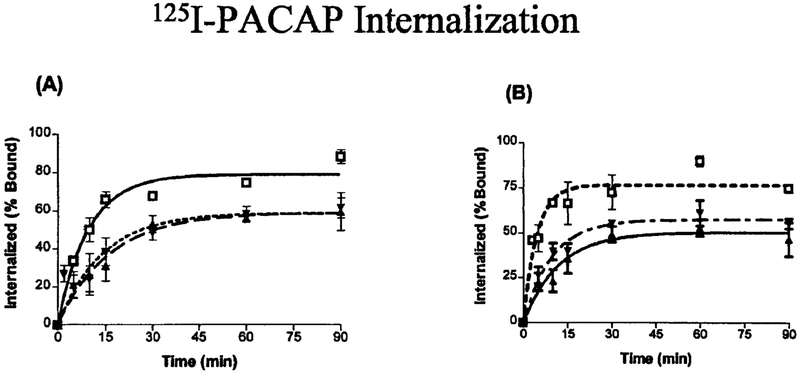
To investigate the role of the PAC1 C terminus in receptor internalization, we created truncated receptor mutants and performed 125I-PACAP-27 internalization studies. Shown in Fig. 5 are the results of 125I-PACAP-27 internalization in NIH/3T3 cells expressing NullWT, NullTr406, or NullTr433 (Fig. 5A) and the HopWT, HopTr406 or HopTr433 (Fig. 5B). Radioligand internalization was rapid in the NullWT and HopWT splice variants, where more than 50% of the radioligand was internalized in less than 5 min. As illustrated in Table I, the internalization process reached a plateau after 60 min, with a maximal radioligand internalization of 80 ± 2 and 90 ± 3% for NullWT and HopWT, respectively. The half-life (t1/2) was 4.4 min in the NullWT and 3.1 min in the HopWT (Table I). In contrast, 125I-PACAP in- ternalization was significantly decreased for both NullTr406 and NullTr433, which had nearly identical values. In all cases the internalization time course was longer (half-life about 8–9 min), and only 56.7 ± 4.2% and 58.4 ± 4.0% of cell-associated ligand was internalized following a 60-min incubation. Similarly, both the truncated mutants, HopTr406 and HopTr433, displayed a longer internalization time (half-life about 7–8 min), and only 60 ± 4%, 53 ± 4% of ligand was internalized for HopTr433 and HopTr406, respectively (Table I). It was noted that a small but significant difference in internalization rate and extent between the NullWT (90% at 60 min) and the HopWT (80% at 60 min). A shorter internalization time course was also observed among the truncated Hop mutants compared with truncated Null mutants.
FIG. 5. Internalization of 125I-PACAP-27 by NIH/3T3 cells stably expressing WT or truncated mutant receptor.
NIH/3T3 cells stably expressing the indicated hPAC1 NullWT, NullTr406, and NullTr433 (A) and hPAC1 HopWT, HopTr406, and HopTr433 (B) were incubated with 50 pM of 125I-PACAP-27. At the indicated time intervals, cells were subjected to a 0.5 M KSCN solution. Internalized radioligand is defined as 125I-PACAP-27 that could not be stripped by KSCN and is expressed as a percentage of the total saturable bound 125I-PACAP-27 in control cells, processed in parallel without KSCN exposure. Each value represents the means of at least three experiments performed in triplicate.
TABLE I. Internalization of radiolabeled PACAP for the wild type and truncated mutants.
The t1/2 (half-life) is the time to reach half-maximum internalization and is expressed in min. The percentage of internalization refers to the percentage of receptor at 60 min. Data are shown for both the null and Hop splice variants. The data presented are the means ± S.E. for at least three experiments performed in triplicate for each of the indicated constructs.
| Receptor | t1/2 of PACAP | Percentage of PACAP internalized at 60 min |
|---|---|---|
| min | % | |
| Null WT | 4.41 ± 0.02 | 90.1 ± 3.0 |
| Null Tr406 | 8.24 ± 0.02 | 56.7 ± 4.2a |
| Null Tr433 | 9.65 ± 0.02 | 58.4 ± 4.0a |
| Hop WT | 3.12 ± 0.03 | 80.2 ± 3.6 |
| Hop Tr406 | 8.23 ± 0.02 | 53.0 ± 4.0a |
| Hop Tr433 | 6.67 ± 0.01 | 61.3 ± 7.2a |
p < 0.001.
Adenylyl Cyclase Assay
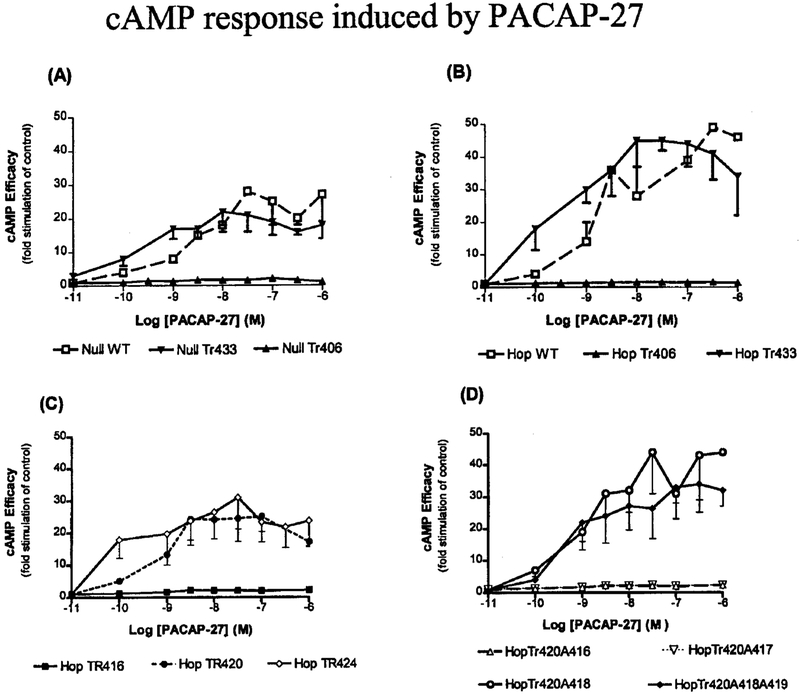
We next investigated the effects of the C terminus of the PAC1 on signal transduction. The AC stimulation for the WT receptors was 20–45-fold above base line (Fig. 6, A and B). Despite normal 125I-PACAP-27 binding for the WT receptor expressing cells, NIH/3T3 cells expressing the NullTr406 (Fig. 6A) and HopTr406 (Fig. 6B) showed no coupling to AC in response to PACAP-27. Longer incubation times with 1 μM PACAP-27 or PACAP-38 for up to 3 h did not result in a significant increase of intracellular cAMP levels for the NullTr406 or HopTr406 mutants (data not shown). For the HopTr406 and NullTr406 mutants, despite their inability to couple to cAMP following ligand exposure, application of forskolin (1 μM) for 1 h resulted in stimulation of cAMP. Reconstitution of 14, 18, or 26 amino acids, respectively, distal to the Tr406 mutation (HopTr420, HopTr424, and HopTr433) led to recovery of normal cAMP responses, as illustrated in Fig. 6 and Table II, whereas reconstitution of only 10 amino acids distal to the Tr406 (i.e. Tr416 mutant) did not recover the response. Mutation of Arg416 and Ser417 to Ala resulted in a loss of AC response compared with the HopWT or the HopTr420 truncation mutant (Fig. 6D). A double mutation of amino acid residues Trp418 and Lys419 to Ala did not affect the cAMP response in the HopTr420 truncation mutant (Fig. 6D).
FIG. 6. Efficacy of stimulation of intracellular cAMP by PACAP-27 in NIH/3T3 cells stably expressing either WT or truncated mutant receptors.
NIH/3T3 cells stably expressing the indicated hPAC1 NullWT, NullTr406, and NullTr433 (A); hPAC1 HopWT, HopTr406, and HopTr433 (B); HopTr416, HopTr420, and HopTr424 (C); and HopTr420A416, HopTr420A417, HopTr420A418, and HopTr420A418A419 (D) were incubated alone or in the presence of increasing concentrations of PACAP-27. Data are shown as fold stimulation of cAMP compared with basal and represent the means of at least three experiments performed in triplicate. To normalize signal transduction data for receptor density, the cells were plated at the same density (~50,000 cells/well) and assayed on the same day. Similar levels of adenylyl cyclase stimulation were observed for five independent clonal cell lines for each mutant receptor.
Inositol Phosphate Assay
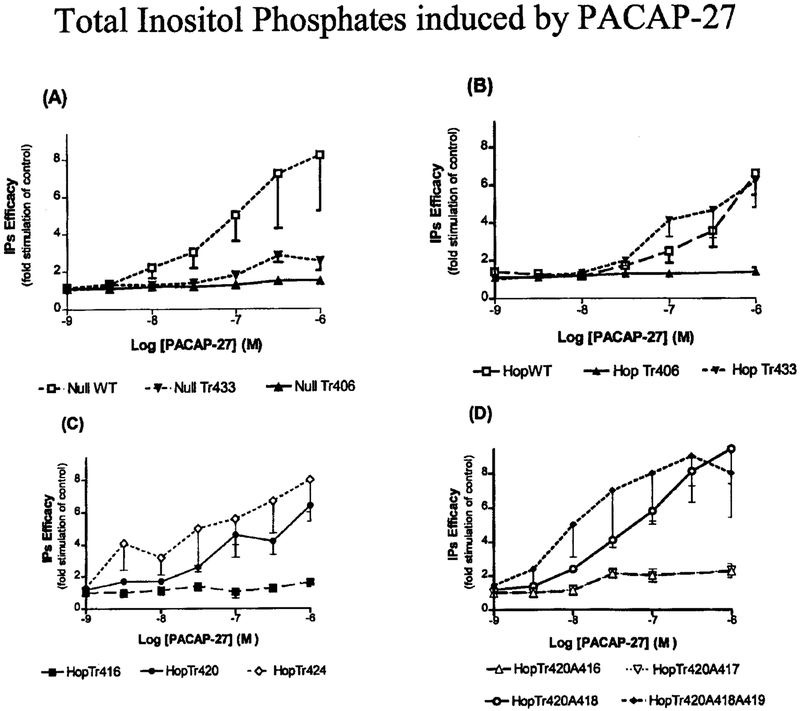
We investigated the effect of these receptor mutations on signal transduction pathway coupled to phospholipase C (Fig. 7). NullWT and HopWT showed a dose-dependent increase in inositol phosphate accumulation, with nearly identical efficacies (8- and 7-fold, respectively) and equivalent potencies (EC50 = 300 and 800 nM, respectively). Similar to the observed AC dose response experiments, the NullTr406 and HopTr406 mutants (Fig. 7, A and B) did not stimulate total inositol phosphates in response to up to 1 μM PACAP-27. Addition of 14, 18, and 26 amino acids, distal to the Tr406 mutation (HopTr420, HopTr424 and HopTr433), resulted in the reconstitution of the dose-dependent stimulation of inositol phosphates in the range of 2.6–10-fold over base-line values, respectively, in response to PACAP-27 (Table II). Mutation of Arg416 or Ser417 to Ala resulted in the loss of inositol phosphate accumulation in response to PACAP-27 when compared with wild type and HopTr420 expressing cells (Fig. 7D). Double mutation of Trp418 and Lys419 amino acid residues did not abolish the receptor response to PACAP-27 (Fig. 7D).
FIG. 7. Efficacy of PACAP-27 to increase intracellular inositol phosphates in NIH/3T3 cells stably expressing WT or truncated mutant of the receptor.
NIH/3T3 cells stably expressing the indicated hPAC1 NullWT, NullTr406, and NullTr433 (A); the hPAC1 HopWT, HopTr406, and HopTr420 (B); HopTr416 and HopTr420 (C); and HopTr420A416, HopTrA417, HopTr420A418, and HopTr420A418A419 (D) were incubated alone or in the presence of increasing concentrations of PACAP-27. Data are shown as fold stimulation of total inositol phosphates compared with basal or unstimulated inositol phosphate turnover and represent the means of at least three experiments performed in triplicate. Similar levels of inositol phosphate stimulation were observed for five independent clonal cell lines for each mutant receptor.
DISCUSSION
The third intracellular loop splice variants of PAC1 were chosen (Null and Hop) to investigate receptor/G protein coupling because they represented relative extremes in signal transduction coupling to PLC with the Hop variant a 7-fold greater efficacy for coupling to inositol phosphate turnover (9). Because little is known about the role of the PAC1 C terminus in receptor internalization and receptor/G protein interactions, the initial study was to determine whether these interactions would lead to differences in receptor internalization and to further define the structure-function correlates for PAC1 -induced G protein activation.
The potential influence of receptor/G protein coupling on receptor internalization was investigated by generating truncation mutants of the PAC1. The relationship between G protein coupling and receptor internalization has not been clarified, although studies of the β-adrenergic, muscarinic, and calcitonin receptors suggest a functional relationship between G protein coupling and receptor internalization (13, 26, 28). It has been shown, previously, that approximately 37% of the cholecystokinin receptor are internalized by a specific ligand (e.g. antagonist) into the intracellular compartment, a process that is independent from G protein coupling or receptor phosphorylation (29–31). As shown in Fig. 5, a significant decrease in the amount of receptor internalization occurs in both the PAC1 Tr406 and Tr433 mutants. In contrast to the Tr406 mutant that lost functional coupling to both AC and PLC, the Tr433 mutant revealed a normal efficiency in AC coupling and a minimal impairment in PLC coupling but had a significant decrease in internalization. This suggests that for the PAC1 there is no correlation between the activation of the signaling cascade and receptor internalization and are different from those reported previously for the calcitonin receptor where the two processes are coupled more tightly (21).
The Tr433 mutants exhibited normal PACAP-27 affinity and signal transduction responses but internalized only 58–61% of surface-bound receptors and ligands, compared with 80–90% internalization in the wild type (Table I). The decrease of 20–30% in internalization and the increase in t1/2 (half-life) of PAC1 support the concept that the distal region of the C terminus of PAC1 may contain a specific internalization motif important in ligand-activated internalization. We can speculate that the internalization rate was lower because nine Ser and two Thr residues contained in the C terminus of the PAC1 were deleted for the TR433 mutants resulting in the loss of potential phosphorylation sites. Alternatively, the presence of 28 amino acids in the third intracellular loop, containing a serine residue and potential phosphorylation site, may have also contributed to receptor internalization. However, because the different C terminus truncations only account for a 20–40% change in internalization, the study was extended to an investigation of the role of the C terminus in activating intracellular signaling. Accordingly, we set out to establish whether any amino acids or structure motifs contained within the C terminus of PAC1 plays a crucial role in the cAMP and inositol phosphate accumulation in response to PACAP stimulation.
It has been previously shown that the efficacy of ligand activation on signal transduction processes is, in part, dependent on the density of the receptors targeted on the cell surface (32). To ensure that the levels of receptor expressed on transfected NIH/3T3 cells were similar for both PAC1 WT and truncated mutants, immunofluorescence staining and radioligand binding studies were performed. Immunocytochemistry (Fig. 2) as well as confocal microscopy using a fluorescent PACAP ligand and FACS analysis indicate that all of the truncated mutants expressed receptors (data not shown). Further analysis by radioligand binding and Scatchard analysis indicates that similar amounts of receptor molecules are expressed on the membranes of both the WT and truncated mutant receptor transfected cells (Fig. 3 and Table II). To exclude the potential influence that different receptor densities may have on the efficacy of coupling to second messenger signaling pathways, we selected for binding and signaling studies transfected cell clones expressing similar receptor densities (approximately 60,000 receptors/cell).
Although all members of the type II GPCR family are coupled to AC activation, the structural sites for the interaction between receptors and G proteins has not been thoroughly investigated. One study indicated that the amino acids Arg233 and Gln451 in the second and seventh transmembrane regions of the PTH/PTH-related peptide receptor play an important role in cAMP and inositol phosphate signaling in response to PTH stimulation (33). However, the results of our current study support the evidence that the proximal region of the PAC1 receptor is critical for this interaction. As shown in Fig. 1, of the 63 amino acids that were deleted in the Tr406 mutants, only the proximal region exhibits conservation of amino acids compared with other members of the type II GPCR family. Because the Tr406 mutant does not exhibit coupling to either AC or PLC, compared with the Tr433 mutant as shown in Table II (5, 6), it appears likely that proximal region of the PAC1 C terminus contains domains critically involved in G protein activation.
The addition of 14 amino acids to the C terminus of the Tr406 mutant resulted in reconstitution of both AC and PLC signaling, clearly suggesting that G protein activation must require these amino acids. Substitution mutants were constructed using the full-length PAC1 as a backbone, which confirmed the results obtained using the shortened receptor (data not shown). Cell surface expression was confirmed using a polyclonal antibodies directed against the C terminus of the PAC1 as well as with a fluorescent PACAP hormone and confocal microscopy and by FACS analysis. These immunolocalizing studies confirm that the receptor constructs are expressed by the cells on the cell surface and further confirm the Scatchard analysis data. Taken together, these studies using site-directed mutagenesis analysis indicated that, of the mutants constructed only Arg416 and Ser417 are essential for signal transduction coupling (Table II). These results support the existence of specific Gs protein-coupling regions within the C terminus of PAC1, similar to observations that have been made for members of the Type I GPCR family. The carboxyl tail of the NPY-Y1 receptor (34) and prostaglandin E receptor (35) have both been shown to possess structural domains that are required for Gs coupling; however, for other members of the class I receptor family, other receptor domains have been shown to be important for activation, such as the first intracellular domain for the cholecystokinin receptor (36), the second and third intracellular domains for muscarinic m5 (3), β2-adrenergic receptors (26), and thyrotropin receptor (37).
To explore whether homology exists within the C terminus of receptors belonging to the class II GPCR family, the GenBank™ data base was analyzed, and amino acid alignments were made. An analysis of position alignment revealed that the amino acids Arg and Ser are conserved among members of the class II receptor family (Table III). In addition, we performed an alignment between the C terminus of the PAC1 and the carboxyl regions of the subunits 1 and 2 of GABAB receptor (Table III) and also showed conservation of the same amino acids. This region of the GABAB receptor was selected because it was previously shown to be an important site for functional heterodimer formation and because the deletion of this region (amino acids 785–797) failed to mediate the activation of outward potassium currents through a K+ channel (6).
TABLE III. Sequence alignment of residues of the proximal region within the C termini of the indicated receptors.
Identical amino acid motifs are shown in bold type. GBR1 and GBR2, GABAg receptor subunits: VPAC2R, human vasoactive intestinal polypeptide receptor type 2; Growth RF-R, hormone-releasing factor receptor; hPTH-R, human parathyroid hormone receptor.
| PAC1 | TM VII | …414KWRSWK419… |
| VPAC2 | TM VII | …509KWRSWK514… |
| Calcitonin-R | TM VII | …422SNRSAR427… |
| Growth RF-R | TM VII | …411PSRSLA416… |
| hPTHl-R | TM VII | …480SWSRWT485… |
| hPTH2-R | TM VII | …427MWSRWN432… |
| Calcitonin-Like-R | TM VII | …426ALRSAS431… |
| GBR1 | TM VII | …887SRL. .LEKEN814 |
| GBR2 | TM VII | …785SRLEGLQSEN794 |
The hypothesis that these Arg and Ser amino acids may be important for receptor-G protein interaction is supported by the observation that the deletion of the entire carboxyl tail of the porcine calcitonin receptor results in a loss of intracellular cAMP and Ca2+ signaling without altering the binding affinity (21). Truncation of amino acids distal to this putative motif has failed to result in receptor inactivation, as demonstrated by studies of the rat glucagon receptor (38), parathyroid hormone receptor (39), and the rat secretin receptor (20). Therefore, the conserved Arg and Ser residues, shown in Table III (bold type) appear to be important for PAC1 coupling to G proteins and may be similar to other members of the class II GPCR. This RS motif is reversed (SR) in many other GPCRs (Table III). The presence of a possible phosphorylation site at Ser417 of PAC1 may also be very important for G protein coupling by affecting phosphoserine-regulated assembly of cellular proteins (40).
Several different structural determinants within transmembrane or cytoplasmic domains have been shown to influence Gq/11 coupling. The increased efficacy of PLC coupling by the HopTr433 compared with the NullTr433 (Fig. 7, A and B) is consistent with the previous observation that the human HopWT is coupled with nearly a 7-fold greater efficacy to the stimulation of total inositol phosphates (5). The addition of 14 amino acids distal to the Tr406 truncation mutant of PAC1 reconstitutes PLC stimulation in response to PACAP similar to cAMP response (Fig. 7). Similar results have been observed for the calcitonin receptor, in which the truncation of the C terminus abolishes coupling to both PLC and AC (21). We can speculate that the Arg416-Ser417 identified in the PAC1 has a partial Gq protein-activation function because of its association with other critical receptor domains. This is supported by the recent discovery of the existence in the m5 muscarinic receptor that several cytoplasmic domains are involved as a “switch” to permit G protein activation (3). The involvement of other cytoplasmic domains in signal transduction coupling have been observed in other members of the class I receptor family such as the muscarinic m2 receptor, whereby the Tyr254 and Ala488, Ala489, Leu492, and Ser493 residues of the third intracellular loop have been shown to have a predominant role in the recognition and activation of G(i/o) (41). Other studies indicate that the second intracellular loop of m3 muscarinic receptor and PTH receptor may also be involved in Gq coupling (27, 42).
In conclusion, our results indicate that the proximal C terminus region of PAC1 is critical for both Gs and Gq activation and, to a lesser extent, for receptor internalization. The distal region of the C terminus of the hPAC1 is partially responsible for internalization. Because the complete deletion of the carboxyl-terminal does not abolish internalization, these results suggest that other domains of the hPAC1 are required for internalization. More importantly, in studying the role of the C terminus for receptor internalization, we identified that this region is critical for the signaling of the hPAC1 and identified Arg416 and Ser417 as potential sites of G protein coupling. Furthermore, Arg and Ser are also present in the C terminus of other members of the class II GPCR (Table III), and there exists some, albeit small, sequence homology with the GABAB receptor. Taken together, these results suggest that this motif may be involved in receptor-G protein interaction. The present study provides an initial basis from which future investigations can be conducted to determine the molecular and structural interactions underlying type II GPCR and G protein activation.
Acknowledgments
We thank James Stalter and Dr. Yan-Ping Wang for technical assistance. We also thank Drs. Mariel Birnbaumer, Patrizia Germano, John H. Walsh, and Stephen Wank for insightful comments and critical review of this manuscript.
This work was supported by National Institutes of Health Grant DK 52274–01, an American Gastroenterological Association Industry Research Scholar Award, and a Veterans Affairs Career Development Award. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Abbreviations:
- PAC1
pituitary adenylate cyclase-activating polypeptide type 1 receptor
- PACAP
pituitary adenylate cyclase-activating polypeptide
- AC
adenylyl cyclase
- PLC
phospholipase C
- GPCR
G protein-coupled receptor
- WT
wild type
- GABA
g-aminobutyric acid
- VIP
vasoactive intestinal peptide
- HA
hemagglutinin
- DMEM
Dulbecco’s modified Eagle’s medium
- PCR
polymerase chain reaction
- PBS
phosphate-buffered saline
- BSA
bovine serum albumin
- PTH
parathyroid hormone
REFERENCES
- 1.Cotecchia S, Exum S, Caron MG, and Lefkowitz RJ (1990) Proc. Natl. Acad. Sci. U. S. A 87, 2896–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Dowd BF, Hnatowich M, Regan JW, Leader WM, Caron MG, and Lefkowitz RJ (1988) J. Biol. Chem 263, 15985–15992 [PubMed] [Google Scholar]
- 3.Burnstein ES, Spalding TA, and Brann MR (1998) J. Biol. Chem 273, 24322–24327 [DOI] [PubMed] [Google Scholar]
- 4.Konig B, Arendt A, McDowell JH, Kahlert M, Hargrave PA, and Hofmann KP, (1989) Proc. Natl. Acad. Sci. U. S. A 86, 6878–6882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisegna JR, and Wank SA (1996) J. Biol. Chem 271, 17267–17274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, and Kornau HC (1998) Science 283, 74–77 [DOI] [PubMed] [Google Scholar]
- 7.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, and Coy DH (1989) Biochem. Cell Biol 164, 567–574 [DOI] [PubMed] [Google Scholar]
- 8.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, and Journot L (1993) Nature 365, 170–175 [DOI] [PubMed] [Google Scholar]
- 9.Pisegna JR, and Wank SA (1993) Proc. Natl. Acad. Sci. U. S. A 90, 6345–6349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harmar AJ, Arimura A, Gozes I, Journot L, laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, and Waschek JA (1998) Pharmacol. Rev 50, 265–270 [PMC free article] [PubMed] [Google Scholar]
- 11.Go WY, Holicky EL, Hadac EM, Rao RV, and Miller LJ (1998) Am. J. Physiol 275, G56–G62 [DOI] [PubMed] [Google Scholar]
- 12.Benya RV, Fathi Z, Battey JF, and Jensen RT (1993) J. Biol. Chem 268, 20285–20290 [PubMed] [Google Scholar]
- 13.Cheung AH, Sigal IS, Dixon RAF, and Strader CD (1989) Mol. Pharmacol 34, 132–138 [PubMed] [Google Scholar]
- 14.Fredericks ZL, Pitcher JA, and Lefkowitz RJ (1996) J. Biol. Chem 271, 13796–13803 [DOI] [PubMed] [Google Scholar]
- 15.Thomas WG, Thekkumkara TJ, Motel TJ, and Baker KM (1995) J. Biol. Chem 270, 207–213 [DOI] [PubMed] [Google Scholar]
- 16.Huang Z, Chen Y, and Nissenson RA (1995) J. Biol. Chem 270, 151–156 [DOI] [PubMed] [Google Scholar]
- 17.Prado GN, Suzuki H, Wilkinson N, Cousins B, and Navarro J (1996) J. Biol. Chem 271, 19186–19190 [DOI] [PubMed] [Google Scholar]
- 18.Pohl M, Silvente-Poirot S, Pisegna J, Tarasova NI, and Wank SA (1997) J. Biol. Chem 272, 18179–18184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pals-Rylaarsdam R, Xu Y, Witt-Enderby P, Benovic JL, and Hosey MM (1995) J. Biol. Chem 270, 29004–29011 [DOI] [PubMed] [Google Scholar]
- 20.Holtmann MH, Roettger BF, Pinon DI, and Miller LJ (1996) J. Biol. Chem 271, 23566–23571 [DOI] [PubMed] [Google Scholar]
- 21.Findlay DM, Houssami S, Lin HY, Myers DE, Brady CL Darcy PK, Ikeda K, Martin TJ, and Sexton PM (1994) Mol. Endocrinol 8, 1691–1700 [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Fatt AL, and Randall RJ, (1951) J. Biol. Chem 193, 265–275 [PubMed] [Google Scholar]
- 23.Varrault A, Journot L, Audigier Y, and Bockaert J (1992) Mol. Pharmacol 41, 999–1007 [PubMed] [Google Scholar]
- 24.Salomon Y, Londos C, and Rodbell M (1974) Anal. Biochem 58, 541–548 [DOI] [PubMed] [Google Scholar]
- 25.Berridge MJ, Dawson RM, Downes CP, Heslop JP, and Irvine RF (1983) Biochem. J 212, 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strader CD, Sigal IS, Blake AD, Cheung AH, Register RB, Rands E, Zemcik BA, Candelore MR, and Dixon RAF (1987) Cell 49, 855–863 [DOI] [PubMed] [Google Scholar]
- 27.Iida-Klein Akiko, Guo J, Takemura M, Drake MT, Potts JT, Abou-Samra A, Bringhurst FR, and Segre GV (1997) J. Biol. Chem 272, 6882–6889 [DOI] [PubMed] [Google Scholar]
- 28.Thompson AK, Mostafapour SP, Denlinger LC, Bleasdale JE, and Fisher SK (1991) J. Biol. Chem 266, 23856–23862 [PubMed] [Google Scholar]
- 29.Zanolari B, Raths S, Singer-Kruger B, and Riezman H (1992) Cell 71, 755–763 [DOI] [PubMed] [Google Scholar]
- 30.Hunyady L, Baukal AJ, Balla T, and Catt KJ (1994) J. Biol. Chem 269, 24798–24804 [PubMed] [Google Scholar]
- 31.Roettger BF, Ghanekar D, Rao R, Toledo C, Yingling J, Pinon D, and Miller LJ (1997) Mol. Pharmacol 51, 357–362 [PubMed] [Google Scholar]
- 32.Zhu X, Gilbert S, Birnbaumer M, and Birnbaumer L (1994) Mol. Pharmacol 46, 460–469 [PubMed] [Google Scholar]
- 33.Gardella TJ, Luck MD, Fan M-H, and Lee CW (1996) J. Biol. Chem 271, 12820–12825 [DOI] [PubMed] [Google Scholar]
- 34.Nakamura M, Sakanaka C, Aoki Y, Ogasawara H, Takashi T, Kodama H, Matsumoto T, Shimizu T, and Noma M (1995) J. Biol. Chem 270, 30102–30110 [DOI] [PubMed] [Google Scholar]
- 35.Namba T, Sugimoto Y, Negishi M, Irie A, Ushikubi F, Kakizuka A, Ito S, Ichikawa A, and Narumiya S (1993) Nature 365, 166–170 [DOI] [PubMed] [Google Scholar]
- 36.Wu V, Yang M, McRoberts JA, Ren J, Seensalu R, Dagarag M, Birnbaumer M, and Walsh J (1997) J. Biol. Chem 272, 9037–9042 [DOI] [PubMed] [Google Scholar]
- 37.Chazenbalk GD, Nagayama Y, Russo D, Wadsworth HL, and Rapoport B (1990) J. Biol. Chem 265, 20970–20975 [PubMed] [Google Scholar]
- 38.Unson CG, Cypess AM, Kim HN, Goldsmith PK, Carruthers CJL, Merrifield RB, and Sakmar TP (1995) J. Biol. Chem 270, 27720–27727 [DOI] [PubMed] [Google Scholar]
- 39.Huang Z, Chen Y, Pratt S, Chen T-H, Bambino T, Shoback DM, and Nissenson RA (1995) Mol. Pharmacol 9, 1240–1249 [DOI] [PubMed] [Google Scholar]
- 40.Lu P-J, Zhou XZ, Shen M, and Lu KP (1998).) Science 283, 1325–1328 [DOI] [PubMed] [Google Scholar]
- 41.Wess J (1997) FASEB J 11, 346–354 [PubMed] [Google Scholar]
- 42.Blin N, Yun J, and Wess J (1995) J. Biol. Chem 270, 17741–17748 [DOI] [PubMed] [Google Scholar]