Abstract
A targeted delivery of defined antigens in vivo allows for the probing of relevant functions of the immune system. Recombinant chimeric antibodies, produced by genetically modifying original monoclonal antibodies specific for molecules expressed on dendritic cells and other immune cells, have paved the way for the development of such strategies and have become reliable tools for achieving a specific immunomodulation. These antibodies have proven important in both basic research and clinical applications, extending data obtained in disease models of autoimmunity and cancer. Here we will describe the advances gained from the experimental and therapeutic strategies based on the targeting of the specific antigens by recombinant chimeric antibodies to the multilectin receptor DEC205 and other cell surface molecules.
Keywords: Recombinant Chimeric Antibody, Immunotherapy, Immunomodulation, Autoimmunity, Cancer, DEC205
Defined Delivery of Peptide Antigens to DCs
Conventional dendritic cells (cDCs or DCs) play integral roles in both the innate and adaptive immune responses due to their unique capacities to uptake, process, and present multiple foreign and self-antigens to T cells. Therefore, harnessing these functions of DCs by applying the methods of defined antigen delivery proves important in both basic scientific research and therapy for patients. The concept of delivering defined peptide antigens to DCs in order to promote desired cognate T cell responses was first based on early observations showing DCs’ efficient uptake and processing of the immunoglobulin specifically binding to these cells [1]. However, to obtain specific T cell responses, the cognate T cell epitopes were subsequently introduced to original monoclonal antibodies specific for molecules expressed on the surfaces of DCs and their subsets. This can be achieved by chemically-coupling such antigens to antibody molecules, as well as by genetically fusing the specific antigens within recombinant antibodies. Other options include formation of single-chain fragment variable region (scFv) molecules [2–5]. Additionally, in a chimeric antibody design, the species-specific constant regions are used to minimize off-target effects. Therefore, among multiple options, recombinant chimeric antibodies may be more favorable in certain contexts, as they can be readily modified based on the desired solubility and specific molecular interaction capacities for in vivo administration in the organism of choice. Additionally, production allows for known numbers of antigenic peptides to be included in the reagent construct as well as for the minimization of contamination by endotoxin, rendering these reagents ideal for basic science and clinical applications [2, 6].
The evolutionary conservation of CD11c+ DCs, plasmacytoid DCs (pDCs), and their development-governing transcription factors provides scientific justification for murine studies including those employing antigen targeting by recombinant chimeric antibodies [2, 7–9]. Briefly, the CD11c+ DCs are subdivided into the DC1 and DC2 developmental lineages governed by the transcription factors IRF8/Batf3 and IRF4/Notch2, respectively. Murine DC1s are characterized by their expression of XCR1, and some also express CD8a, DEC205, BTLA, Langerin, Treml4, and Clec9a. In contrast, murine DC2s are generally characterized by their expression of CD172a (SIRPa) and additional expression of DCIR2 and CD11b, while pDCs are characterized by expression of DC-SIGN, Siglec-H, B220, and Ly6c [2, 8–11]. Whereas DC2s can preferentially promote T helper 2 (Th2), T helper 17 (Th17), and follicular helper T (Tfh) cell differentiation, DC1s have crucial roles in the cross-priming of CD8+ T cells and the priming of CD4+ T helper 1 (Th1) cells, as well as in the induction of CD4+CD25+Foxp3+ peripheral regulatory T (pTreg) cells [2, 10, 12, 13]. Importantly, human DC1s, defined as CD141+ (BDCA-3+) XCR1+ BTLA+, and human DC2s, defined as CD1c+ CD172a+ CD11b+, as well as BDCA-2+ neuropiiin+ pDCs, share many developmental, phenotypical, and functional similarities with their murine counterparts [7, 14–19].
DEC205 and the First Recombinant Chimeric Antibodies
The first recombinant chimeric antibodies that were initially designed to faithfully deliver defined peptide antigens to DCs targeted the endocytic receptor DEC205 (CD205, LY75) [20, 21]. DEC205 is expressed at high levels on murine DC1s [22]. Antigens targeted to DEC205 can be presented both in the context of MHCI and MHCII leading to very efficient cross priming of CD8+ T cells and also activation of CD4+ T cells. Although an activation of CD4+ T cells by DC1s appears as less efficient than that mediated by DC2s, DC1s nevertheless have central roles in the promotion and maintenance of both immunogenic and tolerogenic CD4+ T cell responses [2, 8–10, 23, 24].
The basic design of the anti-DEC205 chimeric antibody is comprised of the variable (V) regions specific for DEC205 that were cloned from the original hybridomas producing the rat anti-mouse antibody (NLDC-145). These V regions were genetically combined with modified murine lgG1 constant regions containing additional mutations to ensure minimal non-specific interactions with host cells in vivo [20]. Importantly, the C-termini of the constant regions of such DEC205-specific chimeric antibodies may be genetically fused with a defined antigen of choice. The entire recombinant chimeric antibody molecule is produced in a eukaryotic in vitro expression system [20], The first antigens that were included in this chimeric antibody construct were peptides: hen egg lysozyme (HEL46–61) (anti-DEC205-HEL) and myelin oligodendrocyte glycoprotein (MOG35–55) (anti-DEC205-MOG) [20, 21]. The administration of these novel chimeric antibodies demonstrated that defined antigen may be presented by DCs to cognate CD4+ T cells in vivo and also revealed that, under “steady state” (non-inflammatory) conditions, presentation by DCs of such targeted antigens induced T cell tolerance in the periphery [20]. Particularly, experiments using anti-DEC205-MOG demonstrated the potential therapeutic benefits, as its administration blocked autoimmune responses and disease symptoms of experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis (MS) [21]. Furthermore, by using MHCI-restricted and cross-presented antigens, subsequent experiments extended the initially established tolerogenic functions of the DEC205+ DCs to the induction of tolerance among CD8+ T cells [25], The effectiveness of targeting antigens to DEC205 to promote antigen-specific immune tolerance and to ameliorate disease severity was subsequently extended to other models of autoimmune disease, including diabetes, inflammatory bowel disease (IBD), and arthritis, as well as to a model of graft-versus-host disease [Figure 1] [26–31].
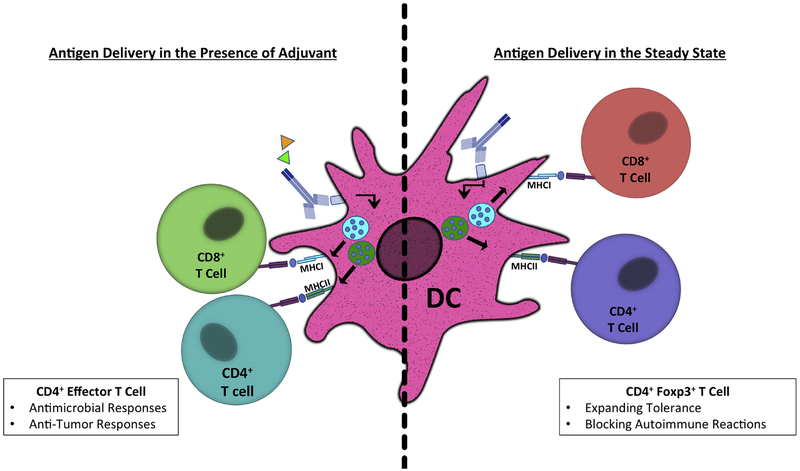
Figure 1: Administration of antigen-targeting antibodies to DCs directs antigen-specific T cell responses.
The delivery of antigens (Ag) to DCs or their subsets results in the processing and presentation of antigenic peptides on MHCII to CD4+ T cells as well as in the crosspresentation of such peptides on MHCI to CD8+ T cells [2, 8–10, 23, 24, 54], In the absence of pro-immunogenic stimuli (“steady state”), DCs induce a deletion and other forms of T cell tolerance including a conversion of CD4+ T cells to Ag-specific peripheral regulatory T (pTreg) cells [20, 21], In the presence of pro-immunogenic stimuli such as the adjuvants Poly(l:C), CpG, or anti-CD40 (represented as triangles), DCs can induce Ag-specific effector and cytotoxic T cells [32–41].
Further, the targeting of antigens to DEC205 has also been successfully applied as a promising vaccine approach in both infectious and tumor models. For example, intranasal administration of the adjuvant polyinosinic:polycytidylic acid (Poly(l:C)) together with recombinant anti-DEC205 antibodies targeting the Yersinia pestis virulence protein LcrV to DEC205-expressing cells induced IgG and IgA antibodies as well as IFNy-secreting CD4+ T cells in the lung [32, 33]. Moreover, in the presence of anti-CD40 adjuvant, administration of recombinant anti-DEC205-HIVgag-p24 led to cross presentation and also Th1 responses [34]. The delivery of antigens to DEC205 has also been applied to tumor models, particularly melanoma and breast cancers. Additional studies employing recombinant chimeric antibodies, chemically-coupled conjugates, and scFv molecules have demonstrated that the delivery of tumor-associated antigens (such as tyrosinase-related protein 2) or other antigens known to be involved in tumorigenesis (such as survivin) to DEC205-expressing cells may lead to decreased tumor burden when such antigens are co-administered with adjuvants such as CpG, Poly(l:C), or anti-CD40. Tumor shrinkage is generally associated with enhanced immune responses, including antigen-specific CD4+ and CD8+ T cell responses and increases in pro-inflammatory cytokine production [35–41]. The capacity of anti-DEC205-mediated antigen targeting to increase T cell responses to infectious agents and tumors renders these antibodies central to the design of new vaccine-based immunotherapies [Figure 1].
Notably, especially under some pro-inflammatory conditions, expression of murine DEC205 is not limited to DCs. Upon administration of specific adjuvants such as alum in vivo or stimulation with IL-4, anti-CD40, or lipopolysaccharide (LPS) in vitro, germinal center B cells upregulate expression of DEC205. This upregulation of DEC205 by germinal center B cells may render antigen delivery to DEC205-expressing cells broader in scope and could serve as a vehicle for enhancing additional humoral responses [24, 42–46].
Human DC1s also highly express DEC205, although an expression of human DEC205 is not limited to DC1s [47, 48]. Such broader expression of DEC205 on human cells may additionally account for the promising effects of treatments with anti-human(h)DEC205 chimeric antibodies. The in vivo administration of anti-hDEC205 antibodies fused to Epstein-Barr virus or human immunodeficiency (HIV) antigens together with the adjuvant Poly(l:C) elicits antiviral T cell responses [46, 49–51]. Moreover, cancer patients who were vaccinated with anti-hDEC205-NY-ESO-1 tumor antigen in conjunction with Toil-like receptor (TLR) agonists experienced increases in cellular and humoral immunity against the tumor, and some patients even experienced tumor regression [52, 53]. Given the successes of anti-hDEC205-Ag chimeric antibody administration, it is likely that additional pathogen-and tumor-derived antigens will be included in future vaccine strategies based on targeting through DEC205.
Additional Cell Surface Molecules Used For Antigen Targeting
The methodological and immune-modulating successes of targeting antigen via DEC205 prompted the introduction of antibodies targeting other molecules. These designs, which are produced via similar genetic techniques, employ corresponding V regions specific for other cell surface molecules present on DCs and pDCs [2, 23, 34, 54–59]. The CD11c integrin, which is expressed by murine DCs, has been frequently targeted with antigens by various strategies, demonstrating the capacity of anti-CD11c targeting to induce cellular and humoral responses in vivo [60–62]. More recently, the recombinant chimeric anti-CD11c-MOG antibody was produced based on the design containing murine lgG1 constant regions, as in the case of anti-DEC205 [54]. Importantly, as such anti-CD11c delivers antigens to all DCs irrespectively of subsets, the in vivo administration of this chimeric antibody in genetically modified mice that lack specific subsets of DCs can further advance the understanding of the functions of individual DC1 and DC2 subsets. For example, in the steady state, DC1s robustly induce pTreg cells, and such pTreg cells are crucial for ameliorating autoimmune responses such as those in EAE [63–65]. This induction of pTreg cells depends on functions of the BTLA molecule that is specifically expressed on some DC1s but not on DC2s [2, 12, 54]. In contrast, when antigens are delivered via anti-CD11c, only inefficient pTreg cell differentiation ensues because the majority of such antigen is presented by BTLAneg DCs. However, in mice with a DC-specific deletion in IRF4, the DC1:DC2 ratio is increased, and pTreg cell differentiation is restored [2, 54]. Overall, the combination of genetic models with a targeted antigen delivery by recombinant chimeric antibodies can help clarify the specific functions of DC subsets including those applicable to human immunology [2, 7, 12, 14, 15, 54, 66, 67].
Many other antigen-targeting antibodies have been produced. Anti-Langerin-Ag [34, 55, 56], anti-Treml4-Ag [56, 57], and anti-Clec9a-Ag [68] have been produced and employed as alternatives to anti-DEC205-Ag to deliver antigen to DC1s, while anti-DCIR2-Ag delivers antigen to DC2s [23] and anti-Siglec-H-Ag [58] and anti-BST2-Ag [59] deliver antigen to pDCs. Though the cell-specific targeting of these antibodies may differ, their ability to enhance cognate T cell responses has been demonstrated in a wide variety of studies, some of which are discussed in greater detail below.
Langerin (CD207) is a transmembrane protein that functions as an endocytic receptor by binding various sugars and certain pathogens such as HIV and Candida albicans [69–75]. It is expressed at relatively low levels on the cell surfaces of Langerhans cells and some CD8a+ DEC205+ DC1s of the spleen and skin draining lymph nodes in mice and at similarly-low levels on human lymphoid and tissue-resident DCs [67, 69–74, 76–80]. Anti-Langerin-Ag antibodies target antigen to Langerin+ DCs of the spleen and peripheral lymph nodes in vivo, resulting in long-lasting antigen presentation on MHCI and MHCII molecules to CD8+ and CD4+ T cells, respectively. The targeting of MOG35–55 peptide by anti-Langerin-MOG to skin Langerin+ migratory DCs also lessened EAE symptom severity in a manner similar to that observed following administration of anti-DEC205-MOG [2, 55, 56].
A more recently discovered cell surface receptor, Trem-like 4 (Treml4), is a member of the “triggering receptor expressed on myeloid cells” family that binds apoptotic or necrotic cells. Though the expression of Treml4 in human DCs remains unclear, the expression of Treml4 in mice primarily occurs on CD8a+ DCs and macrophages of the spleen [57, 81]. The targeting of diverse antigens to Treml4+ DC1s by anti-Treml4 antibodies has been shown to elicit both CD4+ and CD8+ T cell responses; however, the effects of antigen delivery on disease severity in various models including tumor transplantation and EAE remain to be fully elucidated [56, 57].
C-type lectin domain family 9A, also known as DC NK lectin group receptor-1 (Clec9a, DNGR-1), is an endocytic C-type lectin receptor that binds necrotic cells and presents the processed antigens on MHCI and MHCII. It is primarily expressed by murine CD8a+ DCs and pDCs and by human BDCA3+ DCs, with additional low-level expression on human monocytes and B cells [82–89]. Under steady state conditions, antigen delivered to DCs via anti-Clec9a chemically-conjugated antibodies and presented on MHCII prompted the differentiation of Foxp3+ T cells [68], Moreover, as antigen targeting to Poly(l:C)-matured DCs using anti-Clec9a-Ag antibodies results in Th1 CD4+ and CD8+ T cell priming further comparable to that observed following administration of anti-DEC205-Ag or anti-Langerin-Ag, the therapeutic capacities of these reagents may overlap under some immunological conditions, potentially providing additional options for immunotherapy [2, 34, 56, 57].
Currently, fewer options exist for targeting antigen to DCs of the DC2 lineage. The prime example of such an antibody is anti-DCIR2-Ag, which targets the DC inhibitory receptor 2 lectin. DCIR2 (also known as Clec4a4) is expressed by some murine CD8neg DC2s localized in the splenic marginal zone and red pulp, while Clec4a (DCIR), the sole human DCIR family member, is broadly expressed on myeloid DCs, pDCs, and other professional antigen presenting cells (APCs) [23, 90–95]. As demonstrated, altering the extracellular immune conditions during antigen delivery to DCIR2 or Clec4a may mount pro-immunogenic or tolerogenic CD4+ T cell responses, leading to desirable outcomes such as prolonged survival in tumor models [23, 35, 94, 96].
Murine pDCs may also be specifically targeted with recombinant chimeric antibodies against molecules including sialic acid binding lg-like lectin H (Siglec-H) and bone marrow stromal cell antigen 2 (BST2), which correlate with human pDC-expressed molecules, to elicit pro-immunogenic or tolerogenic T cell responses [11, 58, 59, 97–101], Notably, under steady state conditions, targeting antigen to pDCs blocks autoimmune reactions, whereas such targeting may lead to antiviral and anti-tumor T cell responses in the presence of adjuvant [58, 59].
The targeted delivery of antigens through surface molecules such as DEC205 did not result in altered immune phenotypes in vivo, illustrating the absence of specific intrinsic signaling leading to changes in the DC’s inherent functions initiated by a ligation of anti-DEC205-Ag [2, 20, 21]. In contrast, alterations in external immune conditions, such as those brought about by the addition of adjuvants, affected the balance of pro-immunogenic responses against and tolerogenic responses toward the delivered antigen [2, 20, 21], However, some other surface receptors used for antigen targeting, including DCIR2 and DCIR (Clec4a2), may exhibit intrinsic immunoregulatory signaling properties, when crosslinked by antibodies, such as the inhibition of IFNa production despite the presence of adjuvants [95, 102–104], Additionally, the engagement of the “lectin-like” scavenger receptor DC-asialoglycoprotein receptor (DC-ASGPR), whose expression is limited to human and non-human primate cells, can lead to increased production of IL-10 [105, 106], Moreover, a deficiency of Treml4, another receptor used for antigen targeting, has been associated with a decrease in pro-inflammatory cytokine production and autoimmunity-inducing antibodies, further indicating the potential plasticity in the regulatory or pro-immunogenic responses of certain cell surface molecules. It has not been established, though, whether such effects are directly correlated with antibody-mediated antigen delivery [57, 107], In contrast to the proposed inherent immunomodulatory properties of certain DC cell surface molecules, targeting to the Clec9a receptor has been shown to elicit potent humoral responses [82, 108–110], Similarly, the original anti-CD11c hamster or rat IgG and Fab fragments introduced to mice could boost the humoral response to the antigen [62], However, targeting of antigen to mouse CD11c by a recombinant chimeric antibody containing murine constant regions is not immunogenic [54].
As stated previously, recombinant chimeric antibodies may generally be a superior choice for antigen targeting purposes because they are modified in a manner that reduces off-target immune effects. The use of recombinant chimeric antibodies containing mutated, species-matched constant regions minimizes the possibility of non-specific cross-linking in vivo. Further, studies combining murine genetic models lacking specific subsets of DCs and the in vivo targeting of antigens to multiple cell surface molecules can provide additional confirmation that the observed results represent the intrinsic functions of DCs unaffected by the signaling elicited by ligation of the surface molecule used for targeting.
Conclusions
In conclusion, targeting antigens, particularly to dendritic cells and by using recombinant chimeric antibodies, has become an important tool in both basic science and clinical applications. Though the expression of specific cell surface molecules may differ between murine and human DCs, the conservation of DCs and their subsets provides scientific justification for further studies in animal models of disease as well as for additional expansion of such applications in the immunotherapeutic contexts.
Highlights:
Recombinant chimeric antibodies faithfully deliver antigens in vivo.
Targeted antigen delivery achieves specific immunomodulation.
DEC205 remains an important target for therapeutic antigen delivery.
Acknowledgments
This work was supported in part by grants from the National Multiple Sclerosis Society (RG5019A) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01AI113903) (both to D.H.).
This publication is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Finkelman FD, et al. , Dendritic cells can present antigen in vivo in a tolerogenic or immunogenic fashion. J Immunol, 1996157(4): p. 1406–14. [PubMed] [Google Scholar]
- 2.Iberg CA, Jones A, and Hawiger D, Dendritic Cells As Inducers of Peripheral Tolerance. Trends Immunol, 2017. 38(11): p. 793–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ring S, et al. , Targeting of autoantigens to DEC205(+) dendritic cells in vivo suppresses experimental allergic encephalomyelitis in mice. J Immunol, 2013191(6): p. 2938–47. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad ZA, et al. , scFv antibody: principles and clinical application. Clin Dev Immunol, 2012. 2012: p. 980250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahnke K, et al. , Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood, 2003101(12): p. 4862–9. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann CH, et al. , Direct Delivery of Antigens to Dendritic Cells via Antibodies Specific for Endocytic Receptors as a Promising Strategy for Future Therapies.Vaccines (Basel), 2016. 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collin M and Bigley V, Human dendritic cell subsets: an update. Immunology, 2018. 154(1): p. 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilliams M, et al. , Unsupervised High-Dimensional Analysis Aligns Dendritic Cells across Tissues and Species. Immunity, 201645(3): p. 669–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merad M, et al. , The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol, 2013. 31: p. 563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durai V and Murphy KM, Functions of Murine Dendritic Cells. Immunity, 2016. 45(4): p. 719–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, et al. , Characterization ofSiglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood, 2006107(9): p. 3600–8. [DOI] [PubMed] [Google Scholar]
- 12.Bourque J and Hawiger D, Immunomodulatory Bonds of the Partnership between Dendritic Cells and TCells. 2018. 38(5): p. 379–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnaswamy JK, et al. , Determination ofT Follicular Helper Cell Fate by Dendritic Cells. Frontiers in Immunology, 2018. 9(2169). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzionek A, et al. , BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol, 2000165(11): p. 6037–46. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald KP, et al. , Characterization of human blood dendritic cell subsets. Blood, 2002100(13): p. 4512–20. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler-Heitbrock L, et al. , Nomenclature of monocytes and dendritic cells in blood. Blood, 2010116(16): p. e74–80. [DOI] [PubMed] [Google Scholar]
- 17.Ju X, et al. , CD300a/c regulate type I interferon and TNF-alpha secretion by human plasmacytoid dendritic cells stimulated with TLR7 and TLR9 ligands. Blood, 2008. 112(4): p. 1184–94. [DOI] [PubMed] [Google Scholar]
- 18.Heidkamp GF, et al. , Human lymphoid organ dendritic cell identity is predominantly dictated by ontogeny, not tissue microenvironment. Sei Immunol, 20161(6). [DOI] [PubMed] [Google Scholar]
- 19.Murphy TL, et al. , Transcriptional Control of Dendritic Cell Development. Annu Rev Immunol, 2016. 34: p. 93–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawiger D, et al. , Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo.J Exp Med, 2001194(6): p. 769–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawiger D, et al. , Immunological unresponsiveness characterized by increased expression ofCD5 on peripheral T cells induced by dendritic cells in vivo. Immunity, 2004. 20(6): p. 695–705. [DOI] [PubMed] [Google Scholar]
- 22.Jiang W, et al. , The receptor DEC-205 expressed by dendritic cells and thymic epithelial cells is involved in antigen processing. Nature, 1995. 375(6527): p. 151–5. [DOI] [PubMed] [Google Scholar]
- 23.Dudziak D, et al. , Differential antigen processing by dendritic cell subsets in vivo. Science, 2007. 315(5808): p. 107–11. [DOI] [PubMed] [Google Scholar]
- 24.Steinman RM, Hawiger D, and Nussenzweig MC, Tolerogenic dendritic cells. Annu Rev Immunol, 2003. 21: p. 685–711. [DOI] [PubMed] [Google Scholar]
- 25.Bonifaz L, et al. , Efficient Targeting of Protein Antigen to the Dendritic Cell Receptor DEC-205 in the Steady State Leads to Antigen Presentation on Major Histocompatibility Complex Class I Products and Peripheral CD8(+] T Cell Tolerance. J Exp Med, 2002196(12): p. 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukherjee G, et al. , DEC-205-mediated antigen targeting to steady-state dendritic cells induces deletion of diabetogenic CD8(+) T cells independently ofPD-1 and PD-L1. Int Immunol, 2013. 25(11): p. 651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petzold C, et al. , Dendritic cell-targeted pancreatic beta-cell antigen leads to conversion of self-reactive CD4(+) T cells into regulatory T cells and promotes immunotolerance in NOD mice. Rev Diabet Stud, 2010. 7(1): p. 47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wadwa M, et al. , Targeting Antigens to Dec-205 on Dendritic Cells Induces Immune Protection in Experimental Colitis in Mice. Eur J Microbiol Immunol (Bp), 2016. 6(1):p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiering R, et al. , DEC205+ Dendritic Cell-Targeted Tolerogenic Vaccination Promotes Immune Tolerance in Experimental Autoimmune Arthritis. J Immunol, 2015. 194(10): p. 4804–13. [DOI] [PubMed] [Google Scholar]
- 30.Ettinger M, et al. , Targeting of the hNC16A collagen domain to dendritic cells induces tolerance to human typeXVII collagen. Exp Dermatol, 2012. 21(5): p. 395–8. [DOI] [PubMed] [Google Scholar]
- 31.Bruder D, et al. , On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes, 2005. 54(12): p. 3395–401. [DOI] [PubMed] [Google Scholar]
- 32.Do Y, et al. , Induction of pulmonary mucosal immune responses with a protein vaccine targeted to the DEC-205/CD205 receptor. Vaccine, 2012. 30(45): p. 6359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Do Y, et al. , Targeting ofLcrV virulence protein from Yersinia pestis to dendritic cells protects mice against pneumonic plague. Eur J Immunol, 2010. 40(10): p. 2791–6. [DOI] [PubMed] [Google Scholar]
- 34.Idoyaga J, et al. , Comparable T helper 1 (Thl) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A. Proc Natl Acad Sei USA, 2011108(6): p. 2384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neubert K, et al. , Antigen delivery to CDllc+CD8-dendritic cells induces protective immune responses against experimental melanoma in mice in vivo. J Immunol, 2014. 192(12): p. 5830–8. [DOI] [PubMed] [Google Scholar]
- 36.Mahnke K, et al. , Targeting of antigens to activated dendritic cells in vivo cures metastatic melanoma in mice. Cancer Res, 2005. 65(15): p. 7007–12. [DOI] [PubMed] [Google Scholar]
- 37.Johnson TS, et al. , Inhibition of melanoma growth by targeting of antigen to dendritic cells via an anti-DEC-205 single-chain fragment variable molecule. Clin Cancer Res, 200814(24): p. 8169–77. [DOI] [PubMed] [Google Scholar]
- 38.Charalambous A, et al. , Dendritic cell targeting ofsurvivin protein in a xenogeneic form elicits strong CD4+ T cell immunity to mouse survivin.J Immunol, 2006177(12): p. 8410–21. [DOI] [PubMed] [Google Scholar]
- 39.Wang B, et al. , The human cancer antigen mesothelin is more efficiently presented to the mouse immune system when targeted to the DEC-205/CD205 receptor on dendritic cells. Ann N Y Acad Sei, 20091174: p. 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang B, et al. , Targeting of the non-mutated tumor antigen HER2/neu to mature dendritic cells induces an integrated immune response that protects against breast cancer in mice. Breast Cancer Res, 201214(2): p. R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonifaz LC, et al. , In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves Tcell vaccination. J Exp Med, 2004199(6): p. 815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Victora GD and Nussenzweig MC, Germinal centers. Annu Rev Immunol, 2012. 30: p. 429–57. [DOI] [PubMed] [Google Scholar]
- 43.Victora GD, et al. , Germinal center dynamics revealed by multiphoton microscopy with a photoactivatablefluorescent reporter. Cell, 2010143(4): p. 592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasqual G, Angelini A, and Victora GD, Triggering positive selection of germinal center B cells by antigen targeting to DEC-205. Methods Mol Biol, 20151291: p. 125–34. [DOI] [PubMed] [Google Scholar]
- 45.Kamphorst AO, et al. , Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol, 2010185(6): p. 3426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheong C, et al. , Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood, 2010116(19): p. 3828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macri C, et al. , Targeting dendritic cells: a promising strategy to improve vaccine effectiveness. Clin Transi Immunology, 2016. 5(3): p. e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohn L, et al. , Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation. J Exp Med, 2013. 210(5): p. 1049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gurer C, et al. , Targeting the nuclear antigen 1 of Epstein-Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood, 2008. 112(4): p. 1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trumpfheller C, et al. , Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity.J Intern Med, 2012. 271(2): p. 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park CG, Vaccine strategies utilizing C-type lectin receptors on dendritic cells in vivo. Clin Exp Vaccine Res, 2014. 3(2): p. 149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sehgal K, Dhodapkar KM, and Dhodapkar MV, Targeting human dendritic cells in situ to improve vaccines. Immunol Lett, 2014162(1 Pt A): p. 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhodapkar MV, et al. , Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transi Med, 2014. 6(232): p. 232ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones A, et al. , Immunomodulatory Functions ofBTLA and HVEM Govern Induction of Extrathymic Regulatory T Cells and Tolerance by Dendritic Cells. Immunity, 2016. 45(5): p. 1066–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Idoyaga J, et al. , Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHCI and II products in vivo. J Immunol, 2008. 180(6): p. 3647–50. [DOI] [PubMed] [Google Scholar]
- 56.Idoyaga J, et al. , Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest, 2013123(2): p. 844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hemmi H, et al. , Treml4, an Ig superfamily member, mediates presentation of several antigens to T cells in vivo, including protective immunity to HER2 protein. J Immunol, 2012188(3): p. 1147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loschko J, et al. , Antigen targeting to plasmacytoid dendritic cells via Siglec-H inhibits Th cell-dependent autoimmunity. J Immunol, 2011187(12): p. 6346–56. [DOI] [PubMed] [Google Scholar]
- 59.Loschko J, et al. , Antigen delivery to plasmacytoid dendritic cells via BST2 induces protective Tcell-mediated immunity. J Immunol, 2011186(12): p. 6718–25. [DOI] [PubMed] [Google Scholar]
- 60.Castro FV, et al. , CDllc provides an effective immunotargetfor the generation of both CD4 and CD8 T cell responses. Eur J Immunol, 2008. 38(8): p. 2263–73. [DOI] [PubMed] [Google Scholar]
- 61.Chappell CP, et al. , Controlling immune responses by targeting antigens to dendritic cell subsets and B cells. Int Immunol, 2014. 26(1): p. 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White AL, et al. , Ligation of CDllc during vaccination promotes germinal centre induction and robust humoral responses without adjuvant. Immunology, 2010. 131(1): p. 141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kretschmer K, et al. , Inducing and expanding regulatory T cell populations by foreign antigen. Nature Immunology, 2005. 6(12): p. 1219–27. [DOI] [PubMed] [Google Scholar]
- 64.Jones A, et al. , Peripherally Induced Tolerance Depends on Peripheral Regulatory T Cells That Require Hopx To Inhibit Intrinsic IL-2 Expression. J Immunol, 2015195(4): p. 1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones A and Hawiger D, Peripherally Induced Regulatory T Cells: Recruited Protectors of the Central Nervous System against Autoimmune Neuroinflammation. Front Immunol, 2017. 8: p. 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dzionek A, et al. , BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med, 2001194(12): p. 1823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haniffa M, et al. , Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity, 2012. 37(1): p. 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joffre OP, et al. , Efficient and versatile manipulation of the peripheral CD4+ T-cell compartment by antigen targeting to DNGR-1/CLEC9A. Eur J Immunol, 2010. 40(5): p. 1255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Witte L, et al. , Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med, 200713(3): p. 367–71. [DOI] [PubMed] [Google Scholar]
- 70.Galustian C, et al. , High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int Immunol, 200416(6): p. 853–66. [DOI] [PubMed] [Google Scholar]
- 71.Stambach NS and Taylor ME, Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cells. Glycobiology, 200313(5): p. 401–10. [DOI] [PubMed] [Google Scholar]
- 72.Takahara K, et al. , Identification and expression of mouse Langerin (CD207’) in dendritic cells. Int Immunol, 200214(5): p. 433–44. [DOI] [PubMed] [Google Scholar]
- 73.Takahara K, et al. , Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int Immunol, 200416(6): p. 819–29. [DOI] [PubMed] [Google Scholar]
- 74.Valladeau J, et al. , Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity, 2000. 12(1): p. 71–81. [DOI] [PubMed] [Google Scholar]
- 75.De Jesus M, et al. , Sampling of Candida albicans and Candida tropicalis by Langerin-positive dendritic cells in mouse Peyer’spatches. Immunol Lett, 2015168(1): p. 64–72. [DOI] [PubMed] [Google Scholar]
- 76.Kissenpfennig A, et al. , Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity, 2005. 22(5): p. 643–54. [DOI] [PubMed] [Google Scholar]
- 77.Cheong C, et al. , Production of monoclonal antibodies that recognize the extracellular domain of mouse langerin/CD207.J Immunol Methods, 2007. 324(1–2): p. 48–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Douillard P, et al. , Mouse lymphoid tissue contains distinct subsets of langerin/CD2 07 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol, 2005125(5): p. 983–94. [DOI] [PubMed] [Google Scholar]
- 79.Flacher V, et al. , Expression oflangerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology, 2008123(3): p. 339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bigley V, et al. , Langerin-expressing dendritic cells in human tissues are related to CDlc+ dendritic cells and distinct from Langerhans cells and CD141high XCR1 + dendritic cells. J Leukoc Biol, 2015. 97(4): p. 627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hemmi H, et al. , A new triggering receptor expressed on myeloid cells (Trem) family member, Trem-like 4, binds to dead cells and is a DNAX activation protein 12-linked marker for subsets of mouse macrophages and dendritic cells. J Immunol, 2009. 182(3): p. 1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caminschi I, et al. , The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood, 2008112(8): p. 3264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huysamen C, et al. , CLEC9A is a novel activation C-type lectin-like receptor expressed on BDCA3+ dendritic cells and a subset of monocytes. J Biol Chem, 2008. 283(24): p. 16693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sancho D, et al. , Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest, 2008118(6): p. 2098–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sancho D, et al. , Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature, 2009. 458(7240): p. 899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tacken PJ, et al. , Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol, 2007. 7(10): p. 790–802. [DOI] [PubMed] [Google Scholar]
- 87.Jongbloed SL, et al. , Human CD141+ (BDCA-3J+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med, 2010. 207(6): p. 1247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poulin LF, et al. , Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med, 2010. 207(6): p. 1261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schreibelt G, et al. , The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood, 2012. 119(10): p. 2284–92. [DOI] [PubMed] [Google Scholar]
- 90.Vremec D, et al. , The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med, 1992176(1): p. 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Witmer MD and Steinman RM, The anatomy of peripheral lymphoid organs with emphasis on accessory cells: light-microscopic immunocytochemical studies of mouse spleen, lymph node, and Peyer’spatch. Am J Anat, 1984170(3): p. 465–81. [DOI] [PubMed] [Google Scholar]
- 92.Klechevsky E and Banchereau J, Human dendritic cells subsets as targets and vectors for therapy. Ann N Y Acad Sei, 20131284: p. 24–30. [DOI] [PubMed] [Google Scholar]
- 93.Nagae M, et al. , Crystal structure of human dendritic cell inhibitory receptor C-type lectin domain reveals the binding mode with N-glycan. FEBS Lett, 2016. 590(10): p. 1552. [DOI] [PubMed] [Google Scholar]
- 94.Meyer-Wentrup F, et al. , DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production.J Leukoc Biol, 2009. 85(3): p. 518–25. [DOI] [PubMed] [Google Scholar]
- 95.Uto T, et al. , Clec4A4 is a regulatory receptor for dendritic cells that impairs inflammation and T-cell immunity. Nat Commun, 2016. 7: p. 11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Price JD, et al. , DCIR2+ cDC2 DCs and Zbtb32 Restore CD4+ T-Cell Tolerance and Inhibit Diabetes. Diabetes, 2015. 64(10): p. 3521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Asselin-Paturel C, et al. , Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J Immunol, 2003. 171(12): p. 6466–77. [DOI] [PubMed] [Google Scholar]
- 98.Blasius A, et al. , A cell-surface molecule selectively expressed on murine natural interferon-producing cells that blocks secretion ofinterferon-alpha. Blood, 2004. 103(11): p. 4201–6. [DOI] [PubMed] [Google Scholar]
- 99.Cao W, et al. , Regulation ofTLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med, 2009. 206(7): p. 1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Collison J, Autoimmunity: Siglec-H protects mice from lupus-like disease. Nat Rev Rheumatol, 201612(9): p. 498. [DOI] [PubMed] [Google Scholar]
- 101.Sapoznikov A, et al. , Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J Exp Med, 2007. 204(8): p. 1923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujikado N, et al. , Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med, 200814(2): p. 176–80. [DOI] [PubMed] [Google Scholar]
- 103.Kanazawa N, et al. , DCIR acts as an inhibitory receptor depending on its immunoreceptor tyrosine-based inhibitory motif. J Invest Dermatol, 2002118(2): p. 261–6. [DOI] [PubMed] [Google Scholar]
- 104.Meyer-Wentrup F, et al. , Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood, 2008. 111(8): p. 4245–53. [DOI] [PubMed] [Google Scholar]
- 105.Li D, et al. , Targeting self-and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ Tcells. J Exp Med, 2012. 209(1): p. 109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valladeau J, et al. , Immature human dendritic cells express asialoglycoprotein receptor isoforms for efficient receptor-mediated endocytosis. J Immunol, 2001. 167(10): p. 5767–74. [DOI] [PubMed] [Google Scholar]
- 107.Ramirez-Ortiz ZG, et al. , The receptor TREML4 amplifies TLR7-mediated signaling during antiviral responses and autoimmunity. Nat Immunol, 201516(5): p. 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kato Y, et al. , Targeting Antigen to Clec9A Primes Follicular Th Cell Memory Responses Capable of Robust Recall.J Immunol, 2015195(3): p. 1006–14. [DOI] [PubMed] [Google Scholar]
- 109.Li J, et al. , Antibodies targeting Clec9A promote strong humoral immunity without adjuvant in mice and non-human primates. Eur J Immunol, 201545(3): p. 854–64. [DOI] [PubMed] [Google Scholar]
- 110.Lahoud MH, et al. , Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype.J Immunol, 2011187(2): p. 842–50. [DOI] [PubMed] [Google Scholar]