Abstract
Sexual selection promotes the evolution of conspicuous animal ornaments. To evolve as signals, these traits must reliably express the “quality” of the bearer, an indicator of individual fitness. Direct estimates of individual fitness may include the contribution of longevity and fecundity. However, evidence of a correlation between the level of signal expression and these two fitness components are scarce, at least among vertebrates. Relative fitness is difficult to assess in the wild as age at death and extra-pair paternity rates are often unknown. Here, in captive male red-legged partridges, we show that carotenoid-based ornament expression, i.e., redness of the bill and eye rings, at the beginning of reproductive life predicts both longevity (1–7 years) and lifetime breeding output (offspring number and hatching success). The recently proposed link between the individual capacity to produce red (keto) carotenoid pigments and the efficiency of cell respiration could, ultimately, explain the correlation with lifespan and, indirectly, fecundity. Nonetheless, in males of avian species, carotenoid-based coloration in bare parts is also partially controlled by testosterone. We also manipulated androgen levels throughout life by treating males with testosterone or antiandrogen compounds. Treatments caused correlations between signal levels and both fitness components to disappear, thus making the signals unreliable. This suggests that the evolution of carotenoid-based sexual signals requires a tightly-controlled steroid metabolism.
Introduction
The ultimate and proximate mechanisms involved in the evolution of animal signals have been the subject of intense debate for decades (see e.g. recently [1–3]). Theoretical evolutionary models predict that signals must be reliable to evolve (e.g. [4, 5]). These reliable signals evolve when the signal production or maintenance costs are disproportionally high for low-quality individuals [6–8], or alternatively, when a direct causal link between signaling level and individual quality exists, making signals uncheatable [4, 9]. In both cases, those individuals producing larger or more intense signals should be higher-quality individuals, and be able to survive better and/or reproduce at a higher rate. In other words, higher signal expression should be linked to superior fitness, and not only as a result of direct benefits (fitness returns) of signaling (i.e. leading to a circular reasoning; [10]), but also to intrinsic genetic quality [4, 5, 11, 12]. The ornaments would thus became “honest” signals of high fitness [11–13].
Recently, the oxidative stress concept has gained empirical support, and is considered a ubiquitous selective pressure able to shape individual phenotypes from conception to death [14, 15]. Thus, it has been hypothesized that oxidative stress could be an important cost of reproduction influencing fecundity vs. survival trade-off [16–19]. That cost could not only be the consequence of increasing cell metabolism during reproduction (but see [19]) but also due to resource investments in sexual signals (e.g. [20]) that would then favor mating and reproductive success [15]. Among potential signals involved in the oxidative cost of reproduction, colored ornaments generated by carotenoids (many yellow-to-red traits) are probably the best known in vertebrates (e.g. [20–22]).
Carotenoid pigments are usually considered as antioxidants that can also be bleached by free radicals [22–24] (but see [25–27]). Accordingly, the production of carotenoid-based signals would risk the individual’s antioxidant status when no other compensatory mechanisms are available [22]. This would make carotenoid-based ornaments reliable signals of individual quality [22, 28]. Nevertheless, the positive links between the level of signal expression and longevity or fecundity has not definitively been established, but mostly inferred from other indicators of health status (reviewed in [24, 29, 30]) or, in the case of longevity, deduced from overwinter survival rates in avian species [31–36]. Although survival estimates in the wild are valuable as obtained under natural conditions, they are also subject to imperfect detection [37].
In captive conditions, zebra finches (Taeinopygia guttata) have been studied to understand the relationship between bill redness (a carotenoid-based signal) and longevity [38, 39]. One study reported no correlation among males, but a negative link in females [38], while another showed a positive association in both sexes [39]. Unfortunately, more than 40% of birds remained alive at the end of that study, and thus, an important part of individual variability (the oldest birds) was excluded (see [24] for additional analyses and data). As far as we know, the only longitudinal study that examined the association between carotenoid-based coloration and total lifetime longevity was performed by Pike et al. [40] in captive male sticklebacks (Gasterosteus aculeatus). In a one-year-long study, males with the reddest ventral skin at early life lived longer than paler ones in a population study of 34 individuals.
To our knowledge, the association between carotenoid-based signaling and lifetime reproductive success remains to be tested directly. In males, the association can be expected if we consider that a positive correlation between carotenoid-based color intensity and sperm quality has been detected [41, 42]. We must note that carotenoids are also present in semen [43], apparently protecting spermatozoids and germline DNA from oxidation [44].
In this framework, we consider the role of sex steroids. In males, testosterone is able to control/intensify carotenoid-based signal expression by increasing circulating carotenoid levels [45–48]. Sustaining high testosterone levels to favor carotenoid-based signaling theoretically implies costs derived from steroid pleiotropic effects such as injuries due to increased aggressiveness, immunosuppression or oxidative stress [49–51]. Alternatively, the level of expression of a sexual signal may predict the individual capacity to synthesize testosterone. McGlothlin et al. [52] found that male dark-eyed juncos (Junco hyemalis) with larger white plumage patches were more able to increase testosterone synthesis at a higher rate when triggered by gonadotropin injections. They argued that this capacity reinforced the link between signal size and fitness because testosterone improves reproductive success via increased mating effort, thus leading to a genetic correlation between signal size and testosterone levels (see also [53, 54]). However, testosterone levels may be subject to environmental influences that diminish this relationship. High population density and/or interaction rates among individuals can lead to sustained high testosterone levels that have been well demonstrated in mammals (see review in [55]) and birds [56–60]. Contrarily, breeding in dense populations can induce low testosterone levels in reptiles [55]. These studies illustrate the potential influence of environmental variability on testosterone levels, and theory suggests that environmental factors can alter the reliability of sexual signals, promoting or preventing their evolution (see [58, 61, 62]).
Here, we test whether male red carotenoid-based ornaments act as signals of fitness in the red-legged partridge, Alectoris rufa. The red-legged partridge is considered a monogamous species with long-term pair-bonds where intraspecific nest parasitism or polygamy can also occur [63, 64]. We previously found that wild birds mate assortatively according to trait redness [65], and captive females that mated with males whose carotenoid-based ornaments (red eye rings and bill) were artificially intensified laid more eggs [66]. These results suggest that these traits evolved as sexual signals involved in mate choice, and influenced post-mating resource allocation decisions in females. Here, we estimated the correlation between the expression level of these red traits at the first breeding age (one-year-old approx.) and total lifetime reproductive output over seven years. We must note that reproductive decisions at the first breeding opportunity may exert a crucial influence on life-history trajectories and fitness [67, 68]. Captive conditions allowed us to reliably establish both lifespan and paternity, as each male was individually housed with a female every reproductive season. Some of these males were treated throughout reproductive life with testosterone, vs those with anti-androgen compounds. The treatments allowed us to test the role of individual variability in testosterone levels in the link between signal level and lifetime fitness, mimicking the influence of environmental factors. We predicted that the intensity of the carotenoid-based coloration in controls should be positively correlated with longevity and fecundity, thus revealing individual quality [11–13]. We also predicted that lifetime testosterone action should disrupt that relationship. Based on previous evidence from this and other galliforms displaying carotenoid-based traits (e.g. [46, 58, 69, 70]), we predicted that if sustaining high testosterone levels implies higher costs for poor quality (paler) males [6, 7, 71], this correlation should increase in testosterone-treated males and, consequently, decrease in those receiving anti-androgens.
Materials and methods
Experimental protocol
This work was carried out at the Dehesa Galiana experimental facility (IREC, Ciudad Real, Spain) on captive-born, one-year old (born in spring 2005) red-legged partridges kindly provided by a large governmental breeding facility dedicated to restocking of wild populations (Chinchilla de Montearagón, Albacete, Spain; 38°55'13.6"N 1°42'40.3"W). The size of the population in this facility (850 breeding pairs and 10000 hatchlings produced per year) allowed us to choose males and females for this study, avoiding siblings in the sample. The same birds and dataset analyzed in Alonso-Alvarez et al. [72] were used here, but we assessed longevity and reproductive variables that were studied afterwards ending in 2013. Commercial pelleted food and water were provided ad libitum. Randomly formed pairs (N = 117) were kept in outdoor cages (1 × 0.5 × 0.4 m, length, width, height) under natural photoperiod and temperature conditions. Birds were acclimated from outdoor aviaries to cages for a minimum of 15 days before starting the experiment each year in April.
On April 10, 2006, a blood sample, body mass, and a color measurement (below) of each ornament, bill and eye ring, from each male partridge were taken. These were the initial values of this lifetime study. Blood samples were taken from the jugular vein within 2 min of the removal of a bird from its cage and stored at 4° C until centrifugation at 10,000 g for 10 min at 4°C to separate plasma from the cellular fraction. On April 20, 2006, males were subcutaneously implanted in the back 10 days after blood sampling with two silicone tubes (40 mm length, 1.47 mm i.d., 1.96 mm o.d.; Silastic tubing, Dow Corning, Midland, MI, USA). The date coincided with the time when the highest blood carotenoid concentrations were expected and when red carotenoid-based ornaments are displayed at the highest level and before egg laying began [73]. Males were randomly divided into four groups that received one of the four treatments. C-males (n = 30) received two empty implants. T-males (n = 29) received one of the implants packed with testosterone (Steraloids Inc.; Newport, RI, USA) plus the other empty one. F-treated males (n = 28) received an implant packed with flutamide (Sigma-Aldrich, St. Louis, MO, USA) and another empty one. Flutamide is a testosterone receptor blocker (e.g [74]). Finally, FA-males (n = 30) received an implant packed with flutamide plus an implant packed with 1,4,6-androstatriene-3,17-dione (ATD; Steraloids Inc.). ATD is a blocker of testosterone enzymatic transformation to estrogens (e.g [75]). The aim of the two anti-androgen treatments was to inhibit testosterone effects on target tissues. The FA implants would prevent not only direct effects on receptors but those due to estrogens (e.g [76]), which may increase as a result of testosterone-to-estradiol conversion when androgen receptors are neutralized (e.g [75, 77]). The tubes were closed at both ends with silicon glue (1 mm in each side; Nusil Technology, Carpinteria, CA, USA). The males were again sampled for blood and weighed 25 d and 70 d after the implant to control the effectiveness of hormonal treatments (15 May and 30 June, respectively; [72]).
The dimensions of the silicone tube were chosen from previous work on the same species that included a pilot study with same-sized implants (40 mm length) [45]. Blood hormone levels (see Hormonal phenotypes below) were within ranges described for non-manipulated red-legged partridges [78]. Testosterone and estradiol were measured by radioimmunoassay at the Centre d’Etudes Biologiques (Chizé, France; [79]).
The birds were released in two large adjacent outdoor aviaries (35 × 6 × 3 m; length, width, height; randomly distributed, with all groups represented) when the egg-laying period ended (10 July). Birds were again placed in the cages in March of the next year, sampled on 10 April and implanted on 20 April as in the preceding year. The same hormonal treatment and schedule was used for each bird during its entire life span. Implants were removed before being released to the aviaries. During the reproductive period, males were coupled with the same partner every year unless the female died or was injured and hence removed from the study. In these situations, the female was replaced by a yearling female without previous breeding experience obtained from the original population, i.e. Chinchilla de Montearagón (see above). The number of different females that mated with each male did not differ among treatments (Kruskal-Wallis test: χ2 = 3.13, df = 3, P = 0.372; range 1–6), or when that number was divided by the number of breeding events throughout the male’s life (χ2 = 0.57, df = 3, P = 0.904; see additional information in the supplementary material). The latter variable was also unrelated to lifetime hatching success of males (Spearman’s r = 0.079, P = 0.459).
Survivorship was checked twice weekly throughout the study. Egg laying was recorded, and eggs were moved from the cages to incubators daily (supplementary material). The number of chicks per cage was determined at the hatching date. “Lifetime hatching success” was the sum of all the hatchlings sired by a male divided by the sum of all the eggs produced by their mates. This variable was not calculated for males whose female(s) did not lay any egg throughout the experiment (supplementary material). We also determined “total chick survival” as the total number of chicks that reached 14d of life divided by the total number of hatchlings sired by the same male. Egg mass, hatchling mass and tarsus length, and chick body mass and tarsus length at 14d old were also measured.
Color assessment
All individuals were photographed following Alonso-Alvarez and Galván [80] to analyze their ornamentation (see also [81]). We took digital photographs of red masks of birds with a digital camera (Olympus E-510) under standard bird position and light conditions. For each photo, the same standard gray reference scale (Kodak Gray Scale, Kodak, New York) was placed next to the bird’s neck. The color intensity of the bill and eye-rings of red-legged partridges was measured in photographs using Adobe Photoshop 7.0. For each individual, we calculated the RGB (red, green, blue) components of the eye ring and bill separately. Mean RGB values obtained for each trait per duplicate were repeatable (r > 0.82, P < 0.001; see [82]) and therefore, average values were used (see also [83] for repeatabilities and correlations between this and spectrophotometry-based methods). Hue values were determined by applying the Foley & van Dam algorithm [84] on RGB data. The covariation between trait hue values and those obtained from the reference chip was never significant (all P > 0.10). High values of hue indicated paler traits. For this reason, we reversed these hue values (multiplying by -1) to obtain a more intuitive variable, i.e. “redness”. To obtain positive values the variable was rescaled by adding 25, the minimum negative value. Thus, high eye ring or bill redness values indicate redder traits. Despite their similar carotenoid composition [85], eye ring and beak redness were treated separately because they differ in their responsiveness to variations in physiological status (e.g [86]). The photographs from two FA birds were lost when storing the digital file, and the bill of two control and one F bird could not be measured due to damage on the integument surface and were excluded from the analyses.
Statistical analyses
Analyses were performed with IBM SPSS Statistics 24.0 (SPSS, Inc.). Cox proportional hazards models were applied to evaluate the influence of redness at the start of the reproductive life on longevity, and how hormone treatments may influence this potential association. Three birds (one C and two T birds) were excluded from the analyses as they escaped during the course of the experiment (also excluded in the dataset). In addition, another 14 individuals died due to accidents or their date of death could not be well established during the eight years of the study. The survivorship of these 14 birds until their last record was considered as censored data in log-rank tests and Cox models. The sample sizes of censored vs non-censored individuals were not biased among treatments (χ2 = 3.22, df = 3, P = 0.360). Linear and quadratic terms of the bill and eye ring redness relationship with survival were tested. The quadratic term was, however, always removed as it never reached the significance threshold (all P-values > 0.11).
The total number of eggs, lifetime hatching success and chick survivorship at 14 days of age showed zero-inflated distributions. Accordingly, Spearman’s correlation coefficients were used to analyze the relationship between redness and these reproductive variables. Moreover, we estimated the correlations between ornament redness and egg mass, hatchling and chick mass and tarsus length, all analyzed as mean values from lifetime data. Pearson’s correlation coefficients were used as these variables were normally distributed. The correlations between trait redness and reproductive parameters did not include censored data as we aimed to infer causal relationships between longevity and reproductive output. In any event, the same results were found when censored birds were included (S1 Table). In addition, correlations P-values were controlled for multiple testing by using the sequential Bonferroni procedure proposed by Benjamini and Hochberg [87], which considers the false discovery rate (FDR). In this case, the significance threshold moved from 0.05 to 0.009, thus only rejecting two tests from significance. We must, nonetheless, consider that we conventionally used two-tailed tests, whereas a priori predictions (see Introduction) would have allowed dividing the original P-values by two. This would only have removed from significance the correlation between eye-ring redness and number of eggs among F-males, thus not affecting the main conclusions of these results.
Ethics
This investigation was approved by the institutional animal welfare committee (University of Castilla-La Mancha’s Committee on Ethics and Animal Experimentation) in accordance with pertinent Spanish legislation under Protocol number: 1011.01. The implanting procedure was performed under veterinary supervision. We used a planned humane endpoint where any bird would be immediately euthanized by cervical dislocation when it rapidly lost more than 20% of body mass.
Results
Hormonal phenotypes
Testosterone-treated males (S1A Fig) showed significantly higher testosterone values compared to all the other groups 25 d after the implant (see [72] for full statistical results). However, values of C- and F-males did not differ significantly [80], which was expected if no negative feedback on testosterone synthesis is triggered by androgen receptor blockage (e.g. [88]). In contrast, FA-males showed significantly higher testosterone concentrations than F- and C-males (S1A Fig; both P-values < 0.001; also in [72]). Higher plasma testosterone values in FA-males have repeatedly been reported in avian studies where the two compounds have simultaneously been used [75, 89–91]. Testosterone is used as the substrate to estradiol synthesis, and it is possible that a temporary decrease in estradiol levels due to ATD blockage could have triggered the production of its precursor (i.e. testosterone). The involvement of estradiol levels in this feedback would explain why F-only-males did not show higher testosterone levels. An increase in testosterone synthesis in FA-males could also explain their higher estradiol circulating levels compared to controls (S1B Fig). Regardless, the effect on estradiol was short-lived, disappearing in the following sampling event (see legend in S1 Fig).
Redness and longevity
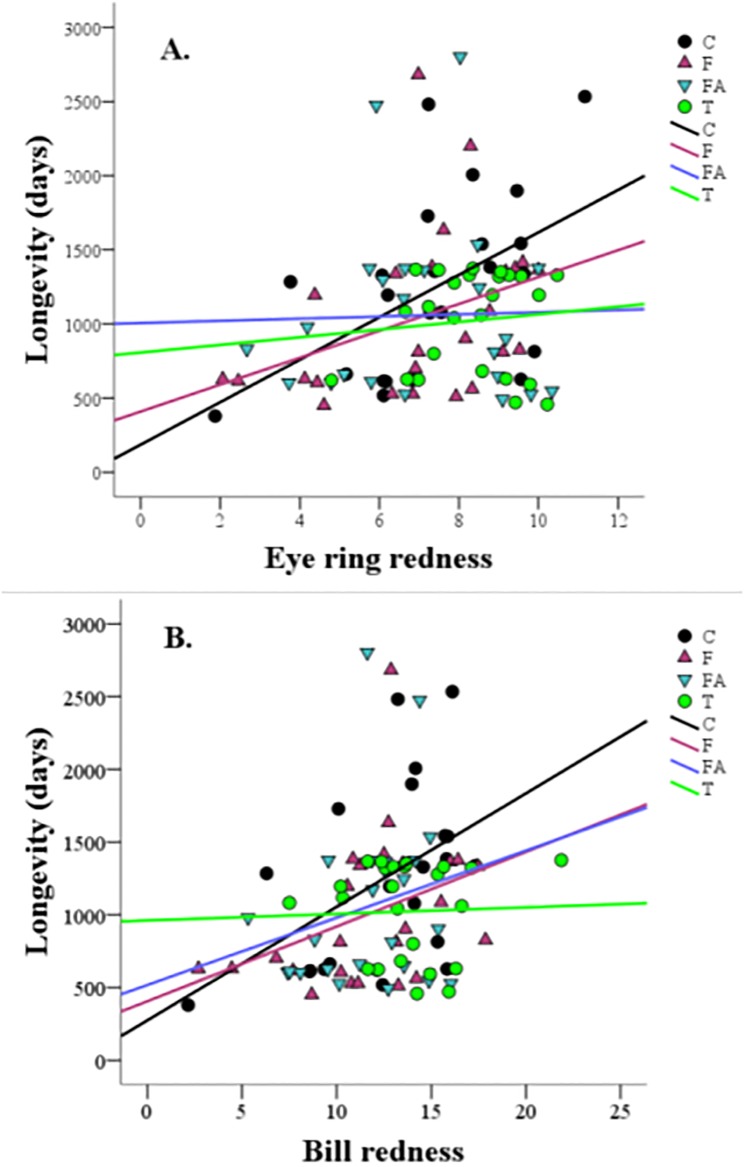
We detected a positive correlation between the intensity of the red-carotenoid based coloration in both head ornaments and lifespan (Table 1; omnibus tests: χ2 = 19.67, df = 7, P = 0.006 and χ2 = 18.77, df = 7, P = 0.009 for eye rings and bill models, respectively). Moreover, we found a significant interaction between hormonal treatments and redness in the eye ring (Table 1, P = 0.047). Pairwise comparisons among treatments revealed that individuals with redder eye rings and bills survived better than paler individuals among control males, and the slope of that relationship was significantly different from that of T-males, but from F- or FA-birds (Table 1).
Table 1. Cox proportional-hazards regression for survival.
| Eye ring redness | B | SE | Wald | df | P | Hazard ratio Exp(B) | 95% CI | |
| Lower | Upper | |||||||
| Treatment | 5.682 | 3 | 0.128 | |||||
| Eye ring redness | 0.317 | 0.122 | 6.701 | 1 | 0.010 | 0.729 | 0.573 | 0.926 |
| Eye ring redness*treatment | 7.961 | 3 | 0.047 | |||||
| Eye ring redness*treatment (F) | -0.061 | 0.156 | 0.154 | 1 | 0.695 | 1.063 | 0.783 | 1.444 |
| Eye ring redness*treatment (FA) | -0.129 | 0.167 | 0.594 | 1 | 0.441 | 1.138 | 0.819 | 1.580 |
| Eye ring redness*treatment (T) | -0.479 | 0.184 | 6.761 | 1 | 0.009 | 1.615 | 1.125 | 2.318 |
| Bill redness | B | SE | Wald | df | P | Hazard ratio Exp(B) | 95% CI | |
| Lower | Upper | |||||||
| Treatment | 2.985 | 3 | 0.394 | |||||
| Bill redness | 0.205 | 0.068 | 9.142 | 1 | 0.002 | 8.15 | 0.713 | 0.930 |
| Bill redness*treatment | 4.851 | 3 | 0.183 | |||||
| Bill redness*treatment (F) | -0.065 | 0.090 | 0.523 | 1 | 0.470 | 1.067 | 0.895 | 1.273 |
| Bill redness*treatment (FA) | -0.094 | 0.109 | 0.737 | 1 | 0.391 | 1.098 | 0.887 | 1.360 |
| Bill redness*treatment (T) | -0.197 | 0.092 | 4.585 | 1 | 0.032 | 1.218 | 1.017 | 1.459 |
*The relationship between trait redness and survival within flutamide (F), flutamide plus ATD (FA) or testosterone (T) groups using the same relationship in the control group as a reference.
P-values below 0.05 are shown in bold.
Table 1 shows the tests when using the controls as the reference group for pairwise comparisons. When the T-group is used instead (S2 Table), the relationship between eye ring redness and longevity also differs between T- and F-males (Wald test: 6.04, df: 1, P = 0.014), and showed a trend toward significance between the T- and FA-males (Wald test: 3.76, df: 1, P = 0.053). In the case of the bill, the slope of T-males did not significantly vary from that of F- or FA-treated males (both P > 0.13; also S2 Table). All of these differences lead to slopes that are ordered from steeper to flatter in the following way: C, F, FA and T (see Table 1). Finally, when Cox regressions were calculated separately for each treatment, the slope was significantly different from zero in C- and F-males, but not in FA- and T-treated males (Table 2), and the slopes were ordered in the same way.
Table 2. Cox proportional-hazards regression for survival and ornament (above eye ring, below bill) redness analyzed separately for each treatment.
Control (C), flutamide (F), flutamide plus ATD (FA) and testosterone (T).
| Eye ring redness | B | SE | Wald | df | P | Hazard ratio Exp(B) | 95% CI | |
| Lower | Upper | |||||||
| Control | 0.368 | 0.133 | 7.688 | 1 | 0.006 | 0.692 | -.368 | 0.133 |
| Flutamide (F) | 0.250 | 0.107 | 5.471 | 1 | 0.019 | 0.779 | -.250 | 0.107 |
| Flutamide + ATD (FA) | 0.121 | 0.114 | 1.115 | 1 | 0.291 | 0.886 | -.121 | 0.114 |
| Testosterone (T) | -0.194 | 0.140 | 1.915 | 1 | 0.166 | 1.214 | .194 | 0.140 |
| Bill redness | B | SE | Wald | df | P | Hazard ratio Exp(B) | 95% CI | |
| Lower | Upper | |||||||
| Control | 0.223 | 0.073 | 9.266 | 1 | 0.002 | 0.800 | 0.693 | 0.924 |
| Flutamide (F) | 0.140 | 0.067 | 4.390 | 1 | 0.036 | 0.869 | 0.762 | 0.991 |
| Flutamide + ATD (FA) | 0.086 | 0.083 | 1.062 | 1 | 0.303 | 0.918 | 0.780 | 1.080 |
| Testosterone (T) | 0.026 | 0.063 | 0.178 | 1 | 0.673 | 0.974 | 0.861 | 1.102 |
Control (C), flutamide (F), flutamide plus ATD (FA) and testosterone (T).
Additionally, Fig 1 shows raw data and ordinary least squares linear regression slopes for illustrative purposes. The non-parametric Cox regression statistics were used to avoid the lack of normality and take into account censored data. The figure shows similar trends described by Cox regression statistics. Finally, survival plots dividing the sample by redness tertiles are also reported in the supplementary material (S2 and S3 Figs).
Fig 1. Relationship between longevity and eye ring (A) or bill (B) redness in male partridges with different hormonal treatments.
Redness was measured before the first breeding opportunity. Slopes were obtained from ordinary least squares linear regressions. However, Cox regression slopes should be considered (see Results and Tables 1 and 2). Control (C), flutamide (F), flutamide plus ATD (FA) and testosterone (T) treatments. Censored data were excluded here.
Redness and lifetime reproductive success
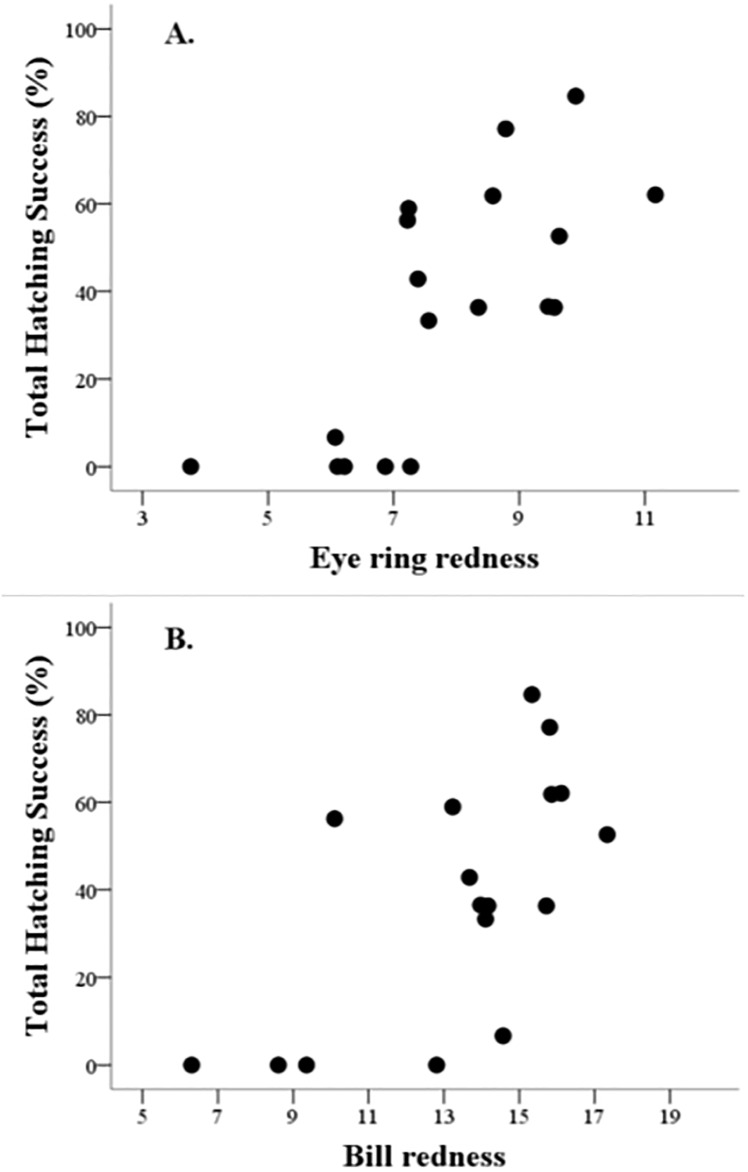
Control birds showed significant positive correlations between bill and eye ring redness and the total number of eggs, hatchlings and 14 d-old chicks sired during life (Table 3). Interestingly, other groups did not show any of these relationships (Table 3), except F-males where the eye ring redness was also positively related to the total number of eggs and hatchlings (though the later at P = 0.071; Table 3). Moreover, lifetime hatching success also positively correlated with ornament redness, but again only among control males (Table 3 and Fig 2A and 2B). Finally, no significant correlation (all P > 0.10) was detected when between eye ring or bill redness and the mean value of egg, hatchling and chick mass and tarsus length during the lifetime in any group.
Table 3. Correlation between ornament redness and reproductive output in male red-legged partridges.
| C-males | Number of eggs | Number of hatchlings | Number of 14d old chicks | Hatching success | Chick survivorship | |
|---|---|---|---|---|---|---|
| Eye ring redness | r | 0.538 | 0.638 | 0.555 | 0.717 | -0.278 |
| P | 0.008 | 0.001 | 0.006 | 0.001 | 0.357 | |
| n | 23 | 23 | 23 | 18 | 13 | |
| Bill redness | r | 0.510 | 0.629 | 0.577 | 0.654 | 0.044 |
| P | 0.015 | 0.002 | 0.005 | 0.004 | 0.886 | |
| n | 22 | 22 | 22 | 17 | 13 | |
| F-males | ||||||
| Eye ring redness | r | 0.417 | 0.360 | 0.249 | -0.353 | -0.363 |
| P | 0.034 | 0.071 | 0.221 | 0.117 | 0.116 | |
| n | 26 | 26 | 26 | 21 | 20 | |
| Bill redness | r | 0.216 | 0.311 | 0.258 | 0.073 | -0.174 |
| P | 0.301 | 0.131 | 0.213 | 0.760 | 0.477 | |
| n | 25 | 25 | 25 | 20 | 19 | |
| FA-males | ||||||
| Eye ring redness | r | 0.075 | -0.040 | -0.030 | -0.301 | 0.111 |
| P | 0.729 | 0.852 | 0.890 | 0.211 | 0.683 | |
| n | 24 | 24 | 24 | 19 | 16 | |
| Bill redness | r | 0.211 | 0.088 | 0.063 | -0.290 | -0.068 |
| P | 0.322 | 0.683 | 0.770 | 0.228 | 0.803 | |
| n | 24 | 24 | 24 | 19 | 16 | |
| T-males | ||||||
| Eye ring redness | r | 0.110 | -0.069 | -0.125 | -0.006 | -0.163 |
| P | 0.602 | 0.742 | 0.550 | 0.979 | 0.632 | |
| n | 25 | 25 | 25 | 20 | 11 | |
| Bill redness | r | 0.111 | -0.023 | -0.043 | -0.095 | 0.060 |
| P | 0.598 | 0.915 | 0.840 | 0.692 | 0.860 | |
| n | 25 | 25 | 25 | 20 | 11 |
Spearman’s correlation coefficients. P-values below 0.05 are shown in bold. P-values shown here are not controlled for multiple testing (see Statistical analyses).
Fig 2. Relationship between total hatching success and eye ring (A) or bill (B) redness among control males.
The total hatching success during lifetime was predicted by the redness of the eye ring (A) or bill (B) at the start of the first breeding season among control males.
Discussion
We show that carotenoid-based colored ornaments can act as fitness signals because their intensity is positively correlated to both lifespan and lifetime fecundity. To our knowledge, this is the first study in any vertebrate species reporting a significant link between the expression level of a carotenoid-based colored ornament and longevity and lifetime fecundity. Simons et al. [39] reported these correlations but during a shorter period of life (part of the sample was still alive at the end of the study), and reproductive success in their study period, i.e. not lifetime, was estimated assuming that extrapair paternity did not affect their results (also [38]). However, they recognized that zebra finch extrapair paternity may reach 29% in similar aviary conditions (citing [92, 93], also Ana Angela Romero-Haro & Carlos Alonso-Alvarez, unpublished data). Here, we avoided this problem as only one pair was housed per cage, and the eggs were immediately removed and artificially incubated. Moreover, we show that the testosterone function throughout life may reduce the link between signal level and fitness, providing further support for a role of sex steroids in the evolution of carotenoid-based signals [45].
The mechanistic link between signal expression and longevity may rely on specific pathways leading to the production of carotenoid-based ornaments. The red traits of partridges are the result of the metabolic transformation of dietary yellow hydroxycarotenoids, mostly zeaxanthin and lutein, to red ketocarotenoids (i.e. astaxanthin and papilioerythrinone, respectively; [94]). It has been hypothesized that this type of transformation depends on oxidase (ketolase) enzymes [95, 96]. Johnson and Hill [97] proposed that ketolases could be located in the inner mitochondrial membrane, sharing catalytic reactions with cell respiration. This should establish a causal link between the level of signal expression and cell metabolism. Thus, rather than signals being sustained by their production costs, the red carotenoid-based ornaments would become indices of quality [9, 26, 27, 98, 99]. The well-known link between cell respiration and aging (e.g [100–102]) could, at least partially, explain the correlation between carotenoid-based signaling and longevity. A connection between red carotenoid-based coloration and cell respiration has been recently supported by a study in which male zebra finches exposed to a mitochondria-specific (targeted) antioxidant (mitoQ) produced redder bills [103]. Interestingly, the same antioxidant is able to extend lifespan in Caenorhabditis elegans [104].
In regard to fecundity, the correlation between redness and reproductive variables can arise as a consequence of longevity, as redder birds live longer and thus have more breeding opportunities. Accordingly, if the association between redness and egg or offspring numbers is controlled for longevity (i.e. by non-parametric partial correlations; see S3 and S4 Tables), all the tests became non-significant (all P > 0.18). This suggests that red ornaments signal fitness by informing about the individual capacity to live longer, which in turn allows higher fecundity. Thus, although an effect of male redness on female resource allocation to egg numbers has been reported in this species [66], this should not be the explanation for the present results. Instead, intrinsic genetic quality associated with longevity and probably supported by the mechanisms of ketocarotenoid production could be involved [9, 97, 103].
Less clear, however, is the mechanism underlying the link between redness and hatching success. Hatching success would control for the individual variability in the number of eggs and hence, longevity. Moreover, partial correlations controlling for longevity did not affect the statistical significance of the correlation (control birds, both ornaments: P < 0.017; see S3 Table). Here, candidate mechanisms may involve (1) variability in sperm quality perhaps related to carotenoid levels in semen (see e.g. [42]) and/or (2) maternal effects allowing females to produce eggs with higher quality when mated with redder males (see [66]). Nonetheless, our data do not allow to address any of these mechanisms.
Interestingly, the relationship between signal expression and fitness components disappeared when testosterone function was altered throughout the lifetime. The results followed the predictions in the case of anti-androgen treatments (particularly FA-birds) as the slope of the relationship became flatter. Contrarily, exogenous testosterone did not reinforce the link, i.e. promoting a steeper slope (see [69]). Paler males did not survive less than redder males when exposed to high testosterone levels, which should be expected if the hormone imposes disproportionally higher costs to the former [7, 51].
The lack of a reinforcing positive effect of the testosterone treatment on the correlation between signal expression and fitness may, nonetheless, be due to the effect of the exogenous hormone on fitness parameters. Testosterone implants reduced lifespan in this sample of birds (Carlos Alonso-Alvarez et al., unpublished results), which is consistent with the existence of physiological costs linked to sustaining high testosterone levels, such as immunosuppression [51, 105] (but see [106]) or oxidative stress [50, 69] (but see also [107]). This is consistent with reduced survival in wild birds treated with testosterone implants [49, 108–110]. However, male partridges implanted with testosterone also showed lower hatching success (Alonso-Alvarez et al., unpublished results) probably due to feedback inhibiting endogenous testosterone synthesis. This feedback was known to induce testes involution in different avian species [111–113] (but see [114]). A shorter variability in both lifespan and hatching success among T-males may have prevented statistically detection of a correlation between signal level and fitness in these birds, which would preclude definitive conclusions from this group of birds.
In any event, whatever the proximate mechanisms involved, the results show that variation in testosterone levels or activity during a lifetime can interfere with the reliability of signals. This implies that testosterone function should be tightly regulated to allow the evolution of red ketocarotenoid based signals. This suggests that the expression of these traits and testosterone/androgen receptor levels should be genetically correlated (see [53]). The demonstration of testosterone-mediated interference with carotenoid-based fitness signaling, while recognizing that it was performed under experimental hormone conditions, supports the hypothesized interconnection between pigment and sexual steroid-based mechanisms in the expression of sexual signals (see [45, 47, 115]). We should also note that selective pressures under free-living conditions would strongly differ from those in captivity. Thus, attaining extra-pair offspring would alter the result of the cost/benefit equations.
To summarize, our study reports a positive correlation between the level of expression of carotenoid-based ornaments and both the whole lifespan and lifetime fecundity probably for the first time in any vertebrate species (but see [40], for a correlation with full longevity in fishes). The interfering role of testosterone in these associations highlights the complexity of carotenoid-based signaling mechanisms and the involvement of different physiological pathways to sustain signal reliability. Further work is needed to determine if the carotenoid-based signal expression or the expression of those genes involved in the mechanism of signal production (e.g [95, 96, 116]) are genetically correlated to the capacity to synthesize testosterone or regulate its function (e.g. via androgen receptor expression/mutations, etc.).
Supporting information
Testosterone levels significantly differed among groups (see also Alonso-Alvarez et al. 2009), whereas only the difference between FA-males and controls in estradiol levels reached significance (P = 0.025, other comparisons P > 0.19). Estradiol levels at the end of the breeding season (70 days after the implant date) did not differ among groups (all contrasts: P-values > 0.27).
(DOC)
When testing differences among tertiles within each treatment group, significant rank tests (P < 0.05) were found for controls and F-treated males, and P = 0.068 in the case of FA-males.
(DOC)
When testing differences among tertiles within each treatment group, significant rank tests (P < 0.05) were found for controls and F-treated males only.
(DOC)
Spearman’s correlation coefficients. P-values below 0.05 are shown in bold.
(DOC)
The relationship between trait redness and survival within control (C), flutamide (F) or flutamide plus ATD (FA) groups is compared to the same relationship when tested in the testosterone (T) group. P-values below 0.05 are shown in bold.
(DOC)
P-values below 0.05 are shown in bold. Degrees of freedom are reported.
(DOC)
P-values below 0.05 are shown in bold. Degrees of freedom are reported.
(DOC)
(DOC)
(DOC)
(DOCX)
Acknowledgments
We are grateful to Carlos Cano and Francisco Perez from Consejería de Medio Ambiente, Junta de Comunidades de Castilla-La Mancha (JCCM), Spain, for kindly supplying partridges for the study. Thanks to Emiliano Sobrino Fernando Dueñas and Luis Montó for bird maintenance, and Amalia Molinero, Ester Ferrero, Laura Ramírez and Clara Rico for technical support at the sampling work. At the CEBC, we thank C. Trouvé and C. Parenteau for their assistance with the hormone assays. We also acknowledge Sandra Díaz-Sánchez and Ursula Höfle for veterinary support, including parasitological and bacteriological analyses. Finally, we acknowledge Mirre Simons for suggestions on statistics and alternative figures and Sarah Young for the English review.
Data Availability
Data are available from the public digital repository Digital CSIC: http://digital.csic.es/handle/10261/187926.
Funding Statement
Financial support was obtained from the projects PII1I09-0271-5037, PII1C09-0128-4724, SBPLY/17/180501/000468 from the JCCM (co-financed with European Regional Development Fund –ERDF), CGL2009-10883-C02-02 from Ministerio de Ciencia e Innovación (MICIN, Spain) and CGL2015-69338-C2-2-P from Ministerio de Economía, Industria y Competitividad (MINECO). AC and LP-R were supported by a “Juan de la Cierva-formación” postdoctoral grant (MINECO, FJCI-2015-23536) and a SECTI postdoctoral contract from the University of Castilla-La Mancha (UCLM), respectively. AC is currently supported by a postdoctoral fellowship from Fundación Ramón Areces.
References
- 1.Laidre ME, Johnstone RA. Animal signals. Curr Biol. 2013;23: R829–R33. 10.1016/j.cub.2013.07.070 [DOI] [PubMed] [Google Scholar]
- 2.Irschick DJ, Briffa M, Podos J. Animal signaling and function: John Wiley & Sons, Inc; 2015. [Google Scholar]
- 3.Bradbury JW, Vehrencamp SL. Principles of animal communication: Sinauer Associates Inc.,U.S.; 2011. [Google Scholar]
- 4.Maynard Smith J, Harper D. Animal signals Oxford University Press O, U.K., editor. Oxford University Press, Oxford, U.K. 2003. i-ix, 1–166 p. [Google Scholar]
- 5.Searcy WA, Nowicki S. The evolution of animal communication: reliability and deception in signaling systems. Princeton University Press: Princeton, New Jersey; 2005. i-xii, 1–270 p. [Google Scholar]
- 6.Zahavi A. The cost of honesty (further remarks on the handicap principle). J Theor Biol. 1977;67: 603–5. 10.1016/0022-5193(77)90061-3 [DOI] [PubMed] [Google Scholar]
- 7.Grafen A. Biological signals as handicaps. J Theor Biol. 1990;144: 517–46. 10.1016/s0022-5193(05)80088-8 [DOI] [PubMed] [Google Scholar]
- 8.Davies NB, Krebs JR, West SA. An introduction to behavioural ecology, 4th Edition: Wiley-Blackwell; 2012. [Google Scholar]
- 9.Hill GE. Condition-dependent traits as signals of the functionality of vital cellular processes. Ecol Lett. 2011;14: 625–34. 10.1111/j.1461-0248.2011.01622.x [DOI] [PubMed] [Google Scholar]
- 10.Acosta MJs, Vazquez Fonseca L, Desbats MA, Cerqua C, Zordan R, Trevisson E, et al. Coenzyme Q biosynthesis in health and disease. Biochim Biophys Acta. 2016;1857: 1079–85. 10.1016/j.bbabio.2016.03.036 [DOI] [PubMed] [Google Scholar]
- 11.Andersson M. Sexual selection: Princeton University Press, Princeton, NJ; 1994. i-xx, 1–599 p. [Google Scholar]
- 12.Kokko H, Brooks R, Jennions Michael D, Morley J. The evolution of mate choice and mating biases. Proc R Soc Lond B. 2003;270: 653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid JM, Arcese P, Cassidy AL, Hiebert SM, Smith JN, Stoddard PK, et al. Fitness correlates of song repertoire size in free-living song sparrows (Melospiza melodia). Am Nat. 2005;165: 299–310. 10.1086/428299 [DOI] [PubMed] [Google Scholar]
- 14.Monaghan P, Metcalfe NB, Torres R. Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett. 2009;12: 75–92. 10.1111/j.1461-0248.2008.01258.x [DOI] [PubMed] [Google Scholar]
- 15.Metcalfe NB, Alonso-Alvarez C. Oxidative stress as a life-history constraint: the role of reactive oxygen species in shaping phenotypes from conception to death. Funct Ecol. 2010;24: 984–96. [Google Scholar]
- 16.Salmon AB, Marx DB, Harshman LG. A cost of reproduction in Drosophila melanogaster: stress susceptibility. Evolution. 2001;55: 1600–8. [DOI] [PubMed] [Google Scholar]
- 17.Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol Lett. 2004;7: 363–8. [Google Scholar]
- 18.Alonso-Alvarez C, Canelo T, Romero-Haro AÁ. The oxidative cost of reproduction: theoretical questions and alternative mechanisms. BioScience. 2017;67: 258–70. [Google Scholar]
- 19.Speakman JR, Garratt M. Oxidative stress as a cost of reproduction: beyond the simplistic trade-off model. Bioessays. 2014;36: 93–106. 10.1002/bies.201300108 [DOI] [PubMed] [Google Scholar]
- 20.von Schantz T, Bensch S, Grahn M, Hasselquist D, Wittzell H. Good genes, oxidative stress and condition-dependent sexual signals. Proc R Soc B 1999;266: 1–12. 10.1098/rspb.1999.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill GE. Cellular respiration: the nexus of stress, condition, and ornamentation. Integr Comp Biol. 2014;54: 645–57. 10.1093/icb/icu029 [DOI] [PubMed] [Google Scholar]
- 22.Hartley RC, Kennedy MW. Are carotenoids a red herring in sexual display? Trends Ecol Evol. 2004;19: 353–4. 10.1016/j.tree.2004.04.002 [DOI] [PubMed] [Google Scholar]
- 23.Britton G, Liaaen-Jensen S, Pfander H. Carotenoids Volume 5: Nutrition and Health 2009. XXXVI, 431 p. [Google Scholar]
- 24.Simons MJP, Cohen AA, Verhulst S. What does carotenoid-dependent coloration tell? plasma carotenoid level signals immunocompetence and oxidative stress state in birds-a meta-analysis. Plos One. 2012;7: e43088 10.1371/journal.pone.0043088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pérez-Rodríguez L. Carotenoids in evolutionary ecology: re-evaluating the antioxidant role. Bioessays. 2009;31: 1116–26. 10.1002/bies.200900070 [DOI] [PubMed] [Google Scholar]
- 26.Koch RE, Hill GE. Do carotenoid-based ornaments entail resource trade-offs? An evaluation of theory and data. Funct Ecol. 2018;32: 1908–20. [Google Scholar]
- 27.Koch RE, Staley M, Kavazis AN, Hasselquist D, Toomey MB, Hill GE. Testing the resource trade-off hypothesis for carotenoid-based signal honesty using genetic variants of the domestic canary. J Exp Biol 2019;222: jeb188102. [DOI] [PubMed] [Google Scholar]
- 28.Weaver RJ, Koch RE, Hill GE. What maintains signal honesty in animal colour displays used in mate choice? Proc R Soc B. 2017;372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blount JD, Metcalfe NB, Birkhead TR, Surai PF. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science. 2003;300: 125–7. 10.1126/science.1082142 [DOI] [PubMed] [Google Scholar]
- 30.McGraw KJ, Ardia DR. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am Nat. 2003;162: 704–12. 10.1086/378904 [DOI] [PubMed] [Google Scholar]
- 31.Hill GE. Plumage coloration is a sexually selected indicator of male quality. Nature. 1991;350: 337. [Google Scholar]
- 32.Nolan PM, Hill GE, Stoehr AM. Sex, size, and plumage redness predict house finch survival in an epidemic. Proc R Soc B. 1998;265: 961–5. [Google Scholar]
- 33.Hõrak P, Ots I, Vellau H, Spottiswoode C, Møller AP. Carotenoid-based plumage coloration reflects hemoparasite infection and local survival in breeding great tits. Oecologia. 2001;126: 166–73. 10.1007/s004420000513 [DOI] [PubMed] [Google Scholar]
- 34.Figuerola J, Carlos Senar J. Serins with intermediate brightness have a higher survival in the wild. Oikos. 2007;116: 636–41. [Google Scholar]
- 35.van Oort H, Dawson RD. Carotenoid ornamentation of adult male Common Redpolls predicts probability of dying in a salmonellosis outbreak. Funct Ecol. 2005;19: 822–7. [Google Scholar]
- 36.Crary AL, Rodewald PG. Plumage coloration and ornamentation as predictors of nest survival and number of young fledged in Yellow Warblers. J Field Ornithol. 2012;83: 130–40. [Google Scholar]
- 37.Kellner KF, Swihart RK. Accounting for imperfect detection in ecology: a quantitative review. Plos One. 2014;9: e111436 10.1371/journal.pone.0111436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Price DK, Burley NT. Constraints on the evolution of attractive traits—selection in male and female zebra finches. Am Nat. 1994;144: 908–34. [Google Scholar]
- 39.Simons MJP, Briga M, Koetsier E, Folkertsma R, Wubs MD, Dijkstra C, et al. Bill redness is positively associated with reproduction and survival in male and female zebra finches. Plos One. 2012;7: e40721 10.1371/journal.pone.0040721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pike TW, Blount JD, Bjerkeng B, Lindström J, Metcalfe NB. Carotenoids, oxidative stress and female mating preference for longer lived males. Proc R Soc B. 2007;274: 1591–6. 10.1098/rspb.2007.0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowe M, Tourville EA, McGraw KJ. Carotenoids in bird testes: links to body carotenoid supplies, plumage coloration, body mass and testes mass in house finches (Carpodacus mexicanus). Comp Biochem Physiol. 2012;163: 285–91. [DOI] [PubMed] [Google Scholar]
- 42.Tomášek O, Albrechtová J, Němcová M, Opatová P, Albrecht T. Trade-off between carotenoid-based sexual ornamentation and sperm resistance to oxidative challenge. Proc R Soc B 2017;284: 20162444 10.1098/rspb.2016.2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowe M, McGraw KJ. Carotenoids in the seminal fluid of wild birds: interspecific variation in fairy-wrens. Condor. 2008;110: 694–700. [Google Scholar]
- 44.Helfenstein F, Losdat S, Møller AP, Blount JD, Richner H. Sperm of colourful males are better protected against oxidative stress. Ecol Lett. 2010;13: 213–22. 10.1111/j.1461-0248.2009.01419.x [DOI] [PubMed] [Google Scholar]
- 45.Blas J, Pérez-Rodríguez L, Bortolotti GR, Viñuela J, Marchant TA. Testosterone increases bioavailability of carotenoids: Insights into the honesty of sexual signaling. P Natl Acad Sci. 2006;103: 18633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alonso-Alvarez C, Pérez-Rodriguez L, García JT, Viñuela J. Testosterone-mediated trade-offs in the old age: a new approach to the immunocompetence handicap and carotenoid-based sexual signalling. Proc R Soc B 2009;276: 2093–101. 10.1098/rspb.2008.1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGraw KJ, Correa SM, Adkins-Regan E. Testosterone upregulates lipoprotein status to control sexual attractiveness in a colorful songbird. Behav Ecol Sociobiol. 2006;60: 117–22. [Google Scholar]
- 48.Martínez-Padilla J, Pérez-Rodríguez L, Mougeot F, Ludwig SC, Redpath SM. Intra-sexual competition alters the relationship between testosterone and ornament expression in a wild territorial bird. Horm Behav. 2014;65: 435–44. 10.1016/j.yhbeh.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 49.Dufty AM. Testosterone and survival: a cost of aggressiveness? Horm Behav. 1989;23: 185–93. [DOI] [PubMed] [Google Scholar]
- 50.Alonso-Alvarez C, Bertrand S, Faivre B, Chastel O, Sorci G. Testosterone and oxidative stress: the oxidation handicap hypothesis. Proc R Soc B 2007;274: 819–25. 10.1098/rspb.2006.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am Nat. 1992;139: 603–22. [Google Scholar]
- 52.McGlothlin JW, Jawor JM, Greives TJ, Casto JM, Phillips JL, Ketterson ED. Hormones and honest signals: males with larger ornaments elevate testosterone more when challenged. J Evol Biol. 2008;21: 39–48. 10.1111/j.1420-9101.2007.01471.x [DOI] [PubMed] [Google Scholar]
- 53.McGlothlin JW, Ketterson ED. Hormone-mediated suites as adaptations and evolutionary constraints. Philos Trans R Soc Lond B Biol Sci 2008;363: 1611–20. 10.1098/rstb.2007.0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGlothlin JW, Parker PG, Nolan V Jr., Ketterson ED. Correlational selection leads to genetic integration of body size and an attractive plumage trait in dark-eyed juncos. Evolution. 2005;59: 658–71. [PubMed] [Google Scholar]
- 55.Hirschenhauser K, Oliveira RF. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim Behav. 2006;71: 265–77. [Google Scholar]
- 56.Wingfield JC, Hahn TP. Testosterone and territorial behaviour in sedentary and migratory sparrows. Anim Behav. 1994;47: 77–89. [Google Scholar]
- 57.Silverin B. Territorial behaviour and hormones of pied flycatchers in optimal and suboptimal habitats. Anim Behav. 1998;56: 811–8. 10.1006/anbe.1998.0823 [DOI] [PubMed] [Google Scholar]
- 58.Vergara P, Martinez-Padilla J, Mougeot F, Leckie F, Redpath SM. Environmental heterogeneity influences the reliability of secondary sexual traits as condition indicators. J Evol Biol. 2012;25: 20–8. 10.1111/j.1420-9101.2011.02399.x [DOI] [PubMed] [Google Scholar]
- 59.Beletsky LD, Orians GH, Wingfield JC. Year-to-year patterns of circulating levels of testosterone and corticosterone in relation to breeding density, experience, and reproductive success of the polygynous red-winged blackbird. Horm Behav. 1992;26: 420–32. [DOI] [PubMed] [Google Scholar]
- 60.Cantarero A, Laaksonen T, Järvistö PE, Gil D, López-Arrabé J, Redondo AJ, et al. Nest defense behaviour and testosterone levels in female Pied Flycatchers. Ethology. 2015;121: 946–57. [Google Scholar]
- 61.Greenfield MD, Rodriguez RL. Genotype–environment interaction and the reliability of mating signals. Anim Behav. 2004;68: 1461–8. [Google Scholar]
- 62.Ingleby FC, Hunt J, Hosken DJ. The role of genotype-by-environment interactions in sexual selection. J Evol Biol. 2010;23: 2031–45. 10.1111/j.1420-9101.2010.02080.x [DOI] [PubMed] [Google Scholar]
- 63.Cramp S, Simmons KEL. The Birds of the Western Palearctic, Vol. 2 Cramp S, Simmons KEL, editors. Oxford: Oxford University Press; 1980. [Google Scholar]
- 64.Casas F, Mougeot F, Viñuela J. Double-nesting behaviour and sexual differences in breeding success in wild Red-legged Partridges Alectoris rufa. Ibis. 2009;151: 743–51. [Google Scholar]
- 65.Casas F, Mougeot F, Pérez-Rodríguez L. Ornamentación sexual, estatus territorial y emparejamiento concordante en una población silvestre de perdiz roja (Alectoris rufa). XIII Congreso Nacional y X Iberoamericano de Etología; Ciudad Real, Spain2010.
- 66.Alonso-Alvarez C, Pérez-Rodriguez L, Ferrero ME, García de-Blas E, Casas F, Mougeot F. Adjustment of female reproductive investment according to male carotenoid-based ornamentation in a gallinaceous bird. Behav Ecol Sociobiol. 2012;66: 731–42. [Google Scholar]
- 67.Stearns SC. The evolution of life histories. Oxford University Press, Oxford: 1992. [Google Scholar]
- 68.Edward DA, Chapman T. Mechanisms underlying reproductive trade-offs: costs of reproduction in mechanisms of life history evolution. Flatt T, Heyland A, editors: Oxford University Press; 2011. 137–52 p. [Google Scholar]
- 69.Mougeot F, Martínez-Padilla J, Webster LMI, Blount JD, Pérez-Rodríguez L, Piertney SB. Honest sexual signalling mediated by parasite and testosterone effects on oxidative balance. Proc R Soc B 2009;276: 1093–100. 10.1098/rspb.2008.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Velando A, Noguera JC, da Silva A, Kim S-Y. Redox-regulation and life-history trade-offs: scavenging mitochondrial ROS improves growth in a wild bird. Sci Rep. 2019;9: 2203 10.1038/s41598-019-38535-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Getty T. Sexually selected signals are not similar to sports handicaps. Trends Ecol Evol. 2006;21: 83–8. 10.1016/j.tree.2005.10.016 [DOI] [PubMed] [Google Scholar]
- 72.Alonso-Alvarez C, Pérez-Rodríguez L, Mateo R, Chastel O, Viñuela J. The oxidation handicap hypothesis and the carotenoid allocation trade-off. J Evol Biol. 2008;21: 1789–97. 10.1111/j.1420-9101.2008.01591.x [DOI] [PubMed] [Google Scholar]
- 73.Pérez-Rodríguez L. Carotenoid-based ornamentation as a dynamic but consistent individual trait. Behav Ecol Sociobiol. 2008;62: 995–1005. [Google Scholar]
- 74.Schwabl H, Kriner E. Territorial aggression and song of male European robins (Erithacus rubecula) in autumn and spring: effects of antiandrogen treatment. Horm Behav. 1991;25: 180–94. [DOI] [PubMed] [Google Scholar]
- 75.Moore IT, Walker BG, Wingfield JC. The effects of combined aromatase inhibitor and anti-androgen on male territorial aggression in a tropical population of rufous-collared sparrows, Zonotrichia capensis. Gen Comp Endocrinol. 2004;135: 223–9. 10.1016/j.ygcen.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 76.Badeau M, Adlercreutz H, Kaihovaara P, Tikkanen MJ. Estrogen A-ring structure and antioxidative effect on lipoproteins. J Steroid Biochem Mol Biol. 2005;96: 271–8. 10.1016/j.jsbmb.2005.04.034 [DOI] [PubMed] [Google Scholar]
- 77.Mougeot F, Pérez-Rodríguez L, Martínez-Padilla J, Leckie F, Redpath SM. Parasites, testosterone and honest carotenoid-based signalling of health. Funct Ecol. 2007;21: 886–98. [Google Scholar]
- 78.Bottoni L, Massa R, Lea RW, Sharp PJ. Mate choice and reproductive success in the red-legged partridge (Alectoris rufa). Horm Behav. 1993;27: 308–17. 10.1006/hbeh.1993.1023 [DOI] [PubMed] [Google Scholar]
- 79.Chastel O, Lacroix A, Kersten M. Pre-breeding energy requirements: thyroid hormone, metabolism and the timing of reproduction in house sparrows Passer domesticus. J Avian Biol. 2003;34: 298–306. [Google Scholar]
- 80.Alonso-Alvarez C, Galván I. Free radical exposure creates paler carotenoid-based ornaments—a possible interaction in the expression of black and red traits. Plos One. 2011;6: e19403 10.1371/journal.pone.0019403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cantarero A, Carrasco Naranjo J, Casas F, Mougeot F, Viñuela J, Alonso-Alvarez C. The fractal dimension of a conspicuous ornament varies with mating status and shows assortative mating in wild red-legged partridges (Alectoris rufa). Sci Nat. 2018;105: 45. [DOI] [PubMed] [Google Scholar]
- 82.Lessells CM, Boag PT. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104: 116–21. [Google Scholar]
- 83.Alonso-Alvarez C, Galván I. Free radical exposure creates paler carotenoid-based ornaments: a possible interaction in the expression of black and red traits. Plos One. 2011;6: e19403 10.1371/journal.pone.0019403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foley JD, Van Dam A. Fundamentals of interactive computer graphics: Addison-Wesley, Reading, MA; 1982. [Google Scholar]
- 85.García-de Blas E, Mateo R, Viñuela J, Pérez-Rodríguez L, Alonso-Alvarez C. Free and esterified carotenoids in ornaments of an avian species: the relationship to color expression and sources of variability. Physiol Biochem Zool. 2013;86: 483–98. 10.1086/671812 [DOI] [PubMed] [Google Scholar]
- 86.Pérez-Rodriguez L, Viñuela J. Carotenoid-based bill and eye ring coloration as honest signals of condition: an experimental test in the red-legged partridge (Alectoris rufa). Naturwissenschaften. 2008;95: 821–30. 10.1007/s00114-008-0389-5 [DOI] [PubMed] [Google Scholar]
- 87.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57: 289–300. [Google Scholar]
- 88.Vleck CM, Dobrott SJ. Testosterone, antiandrogen, and alloparental behavior in bobwhite quail foster fathers. Horm Behav. 1993;27: 92–107. 10.1006/hbeh.1993.1007 [DOI] [PubMed] [Google Scholar]
- 89.Soma KK, Sullivan K, Wingfield J. Combined aromatase inhibitor and antiandrogen treatment decreases territorial aggression in a wild songbird during the nonbreeding season. Gen Comp Endocrinol. 1999;115: 442–53. 10.1006/gcen.1999.7334 [DOI] [PubMed] [Google Scholar]
- 90.Mougeot F, Piertney SB, Leckie F, Evans S, Moss R, Redpath SM, et al. Experimentally increased aggressiveness reduces population kin structure and subsequent recruitment in red grouse Lagopus lagopus scoticus. J Anim Ecol. 2005;74: 488–97. [Google Scholar]
- 91.van Duyse E, Pinxten R, Snoeijs T, Eens M. Simultaneous treatment with an aromatase inhibitor and an anti-androgen decreases the likelihood of dawn song in free-living male great tits, Parus major. Horm Behav. 2005;48: 243–51. 10.1016/j.yhbeh.2005.02.013 [DOI] [PubMed] [Google Scholar]
- 92.Burley NT, Lundy K, Parker PG. Sexual selection and extrapair fertilization in a socially monogamous passerine, the zebra finch (Taeniopygia guttata). Behav Ecol. 1996;7: 218–26. [Google Scholar]
- 93.Forstmeier W, Martin K, Bolund E, Schielzeth H, Kempenaers B. Female extrapair mating behavior can evolve via indirect selection on males. Proc Natl Acad Sci. 2011;108: 10608–13. 10.1073/pnas.1103195108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.García-de Blas E, Mateo R, Alonso-Alvarez C. Specific carotenoid pigments in the diet and a bit of oxidative stress in the recipe for producing red carotenoid-based signals. PeerJ. 2016;4: e2237 10.7717/peerj.2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lopes Ricardo J, Johnson James D, Toomey Matthew B, Ferreira Mafalda S, Araujo Pedro M, Melo-Ferreira J, et al. Genetic basis for red coloration in birds. Curr Biol. 2016;26: 1427–34. 10.1016/j.cub.2016.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mundy Nicholas I, Stapley J, Bennison C, Tucker R, Twyman H, Kim K-W, et al. Red carotenoid coloration in the zebra finch is controlled by a cytochrome P450 gene cluster. Curr Biol. 2016;26: 1435–40. 10.1016/j.cub.2016.04.047 [DOI] [PubMed] [Google Scholar]
- 97.Johnson JD, Hill GE. Is carotenoid ornamentation linked to the inner mitochondria membrane potential? A hypothesis for the maintenance of signal honesty. Biochimie. 2013;95: 436–44. 10.1016/j.biochi.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 98.Emlen DJ, Warren IA, Johns A, Dworkin I, Lavine LC. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science. 2012;337: 860–4. 10.1126/science.1224286 [DOI] [PubMed] [Google Scholar]
- 99.Biernaskie JM, Grafen A, Perry JC. The evolution of index signals to avoid the cost of dishonesty. Proc R Soc B. 2014;281: 20140876 10.1098/rspb.2014.0876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee S-J, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20: 2131–6. 10.1016/j.cub.2010.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144: 79–91. 10.1016/j.cell.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hartmann N, Reichwald K, Wittig I, Dröse S, Schmeisser S, Lück C, et al. Mitochondrial DNA copy number and function decrease with age in the short-lived fish Nothobranchius furzeri. Aging Cell. 2011;10: 824–31. 10.1111/j.1474-9726.2011.00723.x [DOI] [PubMed] [Google Scholar]
- 103.Cantarero A, Alonso-Alvarez C. Mitochondria-targeted molecules determine the redness of the zebra finch bill. Biol Lett. 2017;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, et al. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic Biol Med. 2014;71: 390–401. 10.1016/j.freeradbiomed.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 105.Mills SC, Grapputo A, Jokinen I, Koskela E, Mappes T, Oksanen TA, et al. Testosterone-mediated effects on fitness-related phenotypic traits and fitness. Am Nat. 2009;173: 475–87. 10.1086/597222 [DOI] [PubMed] [Google Scholar]
- 106.Roberts ML, Buchanan KL, Goldsmith AR, Evans MR. The role of testosterone in bib size determination in the male house sparrow Passer domesticus, is age dependent. J Avian Biol. 2012;43: 264–72. [Google Scholar]
- 107.Taff CC, Freeman-Gallant CR. An experimental test of the testosterone mediated oxidation handicap hypothesis in a wild bird. Horm Behav. 2014;66: 276–82. 10.1016/j.yhbeh.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 108.Nolan V, Ketterson ED, Ziegenfus C, Cullen DP, Chandler CR. Testosterone and avian life histories: effects of experimentally elevated testosterone on prebasic molt and survival in male dark-eyed juncos. Condor. 1992;94: 364–70. [Google Scholar]
- 109.Saino N, Møller AP, Bolzerna AM. Testosterone effects on the immune system and parasite infestations in the barn swallow (Hirundo rustica): an experimental test of the immunocompetence hypothesis. Behav Ecol. 1995;6: 397–404. [Google Scholar]
- 110.Reed WL, Clark ME, Parker PG, Raouf SA, Arguedas N, Monk DS, et al. Physiological effects on demography: a long-term experimental study of testosterone’s effects on fitness. Am Nat. 2006;167: 667–83. 10.1086/503054 [DOI] [PubMed] [Google Scholar]
- 111.Turek F, Desjardins C, Menaker M. Antigonadal and progonadal effects of testosterone in male House Sparrows. Gen Comp Endocrinol. 1976;28: 395–402. 10.1016/0016-6480(76)90147-7 [DOI] [PubMed] [Google Scholar]
- 112.Foerster K, Kempenaers B. Experimentally elevated plasma levels of testosterone do not increase male reproductive success in blue tits. Behav Ecol Sociobiol. 2004;56: 482–90. [Google Scholar]
- 113.Fusani L. Endocrinology in field studies: Problems and solutions for the experimental design. Gen Comp Endocrinol. 2008;157: 249–53. 10.1016/j.ygcen.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 114.Martínez-Padilla J, Pérez-Rodríguez L, Mougeot F, Ludwig SC, Redpath SM. Experimentally elevated levels of testosterone at independence reduce fitness in a territorial bird. Ecology. 2014;95: 1033–44. [DOI] [PubMed] [Google Scholar]
- 115.Peters A. Testosterone and carotenoids: an integrated view of trade-offs between immunity and sexual signalling. Bioessays. 2007;29: 427–30. 10.1002/bies.20563 [DOI] [PubMed] [Google Scholar]
- 116.Walsh N, Dale J, McGraw KJ, Pointer MA, Mundy NI. Candidate genes for carotenoid coloration in vertebrates and their expression profiles in the carotenoid-containing plumage and bill of a wild bird. Proc R Soc B 2012;279: 58–66. 10.1098/rspb.2011.0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Testosterone levels significantly differed among groups (see also Alonso-Alvarez et al. 2009), whereas only the difference between FA-males and controls in estradiol levels reached significance (P = 0.025, other comparisons P > 0.19). Estradiol levels at the end of the breeding season (70 days after the implant date) did not differ among groups (all contrasts: P-values > 0.27).
(DOC)
When testing differences among tertiles within each treatment group, significant rank tests (P < 0.05) were found for controls and F-treated males, and P = 0.068 in the case of FA-males.
(DOC)
When testing differences among tertiles within each treatment group, significant rank tests (P < 0.05) were found for controls and F-treated males only.
(DOC)
Spearman’s correlation coefficients. P-values below 0.05 are shown in bold.
(DOC)
The relationship between trait redness and survival within control (C), flutamide (F) or flutamide plus ATD (FA) groups is compared to the same relationship when tested in the testosterone (T) group. P-values below 0.05 are shown in bold.
(DOC)
P-values below 0.05 are shown in bold. Degrees of freedom are reported.
(DOC)
P-values below 0.05 are shown in bold. Degrees of freedom are reported.
(DOC)
(DOC)
(DOC)
(DOCX)
Data Availability Statement
Data are available from the public digital repository Digital CSIC: http://digital.csic.es/handle/10261/187926.