Abstract
Introduction
Chronic pancreatitis (CP) is thought to present the end stage of a continuous disease process evolving from acute pancreatitis (AP), over recurrent AP, to early and end-stage CP. Due to the irreversible nature of CP, early detection and prevention is key. Prospective assessment based on advanced imaging modalities as well as biochemical markers of inflammation, fibrosis and oxidative stress may provide a better understanding of the underlying pathological processes and help identify novel biomarkers of disease with the ultimate goal of early diagnosis, intervention and prevention of disease progression. This paper describes the protocol of a prospective multicentre cohort study investigating the fibroinflammatory process involved in progression from acute to CP using state-of-the-art diagnostic imaging modalities and circulating biomarkers of inflammation, fibrosis and oxidative stress.
Methods and analysis
Adult control subjects and patients at different stages of CP according to the M-ANNHEIM system will be recruited from outpatient clinics at the participating sites and form three cohorts: controls (n=40), suspected CP (n=60) and definitive CP (n=60). Included patients will be followed prospectively for 15 years with advanced MRI and contrast-enhanced endoscopic ultrasound with elastography, assessment of endocrine and exocrine pancreatic function, biochemical and nutritional assessment, and evaluation of pain processing using quantitative sensory testing. Blood samples for a biobank will be obtained. The purpose of the biobank is to allow analyses of potential circulating biomarkers of disease progression, including markers of inflammation, fibrosis and oxidative stress.
Ethics and dissemination
Permissions from the Regional Science Ethics committee and the Regional Data Protection Agency have been obtained. We will submit the results of the study for publication in peer-reviewed journals regardless of whether the results are positive, negative or inconclusive.
Keywords: chronic pancreatitis, inflammation, fibrosis, oxidative stress, pain processing
Strengths and limitations of this study.
The prospective and repetitive assessment of imaging, functional and biochemical parameters in the present study may provide clinical useful biomarkers for the identification of patients at increased risk of developing chronic pancreatitis.
The relatively homogeneous population, both genetically, socially and economically provide us with a unique and unbiased framework for studying the natural course of pancreatitis.
A limitation of the study is that ‘state-of-the art’ imaging, functional, endoscopic and biochemical assessment parameters may change during the study period, thus making comparisons over time difficult and potentially based on outdated technology.
Introduction
Chronic pancreatitis (CP) is a progressive fibroinflammatory disease associated with persistent pathological response to parenchymal injury or stress.1 Over time, the fibroinflammatory process can lead to irreversible fibrosis with morphological changes and loss of pancreatic function. Due to the irreversible nature of end-stage pancreatitis, early detection and prevention is key.
The global pooled incidence of CP is 10 per 100 000 general population per year2 and the prevalence rates are reported to be between 40 and 50 per 100 000 persons.3 However, cumulating evidence suggests that the incidence of CP is rising4 5 and a Danish nationwide population-based cohort study showed a fivefold increased mortality rate with a life expectancy that was approximately 8 years less among patients with CP compared with population controls.6 Taken together, this illustrates that the great impact CP has on health, economic and social aspects.
Alcohol and smoking are the major aetiological risk factors of CP,7 and there seems to be an additive effect of alcohol and smoking. Other causes include hypercalcaemia, hyperlipidaemia, obstruction of the biliary or pancreatic duct, autoimmune conditions and hereditary conditions. About 20% of cases are idiopathic, but this number is likely to decrease as the understanding of the pathophysiology underlying CP evolves.
Currently, the diagnosis of CP is based on a combination of symptoms, biochemical tests and imaging parameters. Abdominal pain is the cardinal symptom of CP and present in the majority of patients during their disease course, but the intensity and temporal pattern varies substantially. At later disease stages, patients may develop diabetes, steatorrhoea and weight loss, but the disease course is unpredictable in the majority of patients, thus making symptom-based assessment unreliable. The biochemical tests used for assessment of CP include glycated haemoglobin (HbA1c) (endocrine pancreatic function) and faecal elastase (FE), breath test or direct pancreatic function tests (exocrine pancreatic function tests). Diagnostic imaging is used for characterisation of typical morphological changes including parenchymal lobulation, calcifications, parenchymal atrophy, pseudocysts and pancreatic duct abnormalities. Various imaging modalities are used for assessment including CT, MRI and endoscopic ultrasound (EUS).8
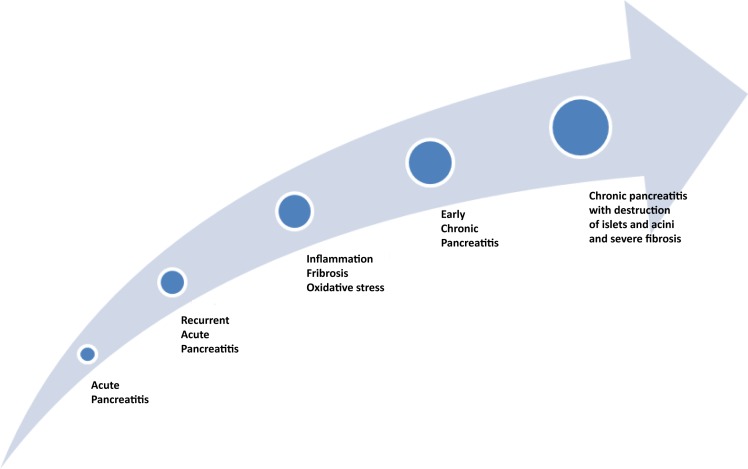
Some patients are diagnosed with CP without preceding attacks of acute pancreatitis (AP). These patients typically present with symptoms of end-stage disease including steatorrhoea, diabetes and chronic abdominal pain. However, a large proportion of patients diagnosed with CP have a history of AP or recurrent AP (RAP) (figure 1). In keeping with this, a meta-analysis found that 10%of patients with a first episode of AP and 36% of patients with RAP develop CP, with a higher risk of disease progression among smokers, alcoholics and men.2 3 9 These findings attest to the understanding of CP as a continuous disease process evolving from AP, over RAP, to early and end-stage CP as also outlined in a recent international consensus draft on a mechanistic definition of CP.1 Importantly, this understanding provides a theoretical framework for studying the fibroinflammatory processes involved in the development of CP by prospectively assessing patients admitted with AP and RAP during their course of disease towards early and end-stage CP. Hence, prospective assessment based on state-of-the-art imaging modalities as well as biochemical markers of inflammation, fibrosis and oxidative stress may provide a better understanding of the underlying pathological processes and help identifying novel biomarkers of disease with the ultimate goal of early diagnosis, intervention and prevention of progression. Along these lines, a holistic framework for prevention of pancreatitis has recently been introduced and outlined preventive measures at both primary, secondary and tertiary levels based on current knowledge on the natural course of disease progression.3
Figure 1.
A large proportion of patients develop CP after an episode of acute pancreatitis. The development of CP is associated with continued or recurrent inflammation and progressive development of fibrosis. CP, chronic pancreatitis.
Current standards and challenges in assessment of pancreatitis
Imaging
Various imaging techniques can be used to characterise pancreatic morphology. CT is in most cases used for the initial workup and is particularly useful for detection of advanced disease characteristics of CP including parenchymal and intraductal calcifications, gross duct pathology and parenchymal atrophy.10 Furthermore, CT is key in ruling out relevant differential diagnosis including pancreatic cancer. Despite CT being the imaging modality of choice for initial investigation, it cannot exclude a diagnosis of CP, nor can it be used to exclusively diagnose ‘early CP’.
MRI-based techniques, including MR cholangiopancreatography, are more accurate for detection of pancreatic duct abnormalities (dilatations and strictures) and subtle changes of the pancreatic parenchyma and side branches, which can be attributed to early signs of CP.11 The MR-based techniques can be further refined by assessment of pancreatic gland volume and administration of secretin whereby a combined quantitative morphological and functional assessment of the pancreatic gland can be obtained. This includes characterisation of subtle ductal abnormalities and pancreatic secretion. In addition, diffusion weighted imaging can provide a proxy of fibrotic changes and emerging methods like the DIXON technique can be used to assess fat signal fraction for evaluation of fatty infiltration of the pancreatic parenchyma.
The gold standard for establishing a diagnosis of early CP imaging is currently EUS. Diagnostic criteria related to the parenchyma and the pancreatic duct have been established, and in 2009, a weighted scoring system was proposed by a panel of experts, the Rosemont score.12 Emerging endoscopic methods with assessment of pancreatic blood flow and tissue stiffness by elastography may further improve the diagnostic utility of EUS.13
Taken together, a multimodal approach combining advanced MRI and EUS techniques will likely provide novel and complementary information on pancreatic morphology and function that may reflect unique aspects of the pathology underlying progressive pancreatitis disorders (AP → RAP → CP) that have not previously been identified using conventional imaging modalities.
Circulating biomarkers of inflammation, fibrosis and oxidative stress
Most of our knowledge about physiological and pathophysiological mechanisms in healthy and diseased pancreas comes from experimental studies. Due to the anatomy, complexity and risk associated with direct sampling from pancreatic tissue, most of our information on human pancreas arises from circulating marker of pancreatic physiology or injury.
Inflammation
The inflammatory profile characterising early stages of pancreatitis may reflect the trajectory of disease and subsequent development of fibrosis. Hence, patients with CP have increased serum levels of interleukin (IL)−6, IL-4 and IL-18,14–16 which are implicated in the wound healing response and the progression of fibrotic disease. Also, tumour necrosis factor-α may be implicated in disease progression.17
Fibrosis
Patients with CP have elevated plasma levels of fibrogenesis markers such as transforming growth factor beta-1 (TGF-β1), soluble fractalkine and monocyte chemoattractant protein-1 (MCP-1).18–20 Decreasing MCP-1 and TGF-β1 levels with anti-inflammatory drugs was shown to reduce the severity of CP in a caerulein-induced CP mouse model.21 Matrix metalloproteinases (MMPs) are a group of enzymes involved in the degradation of collagen, proteoglycans, elastin and fibronectin, all of which play an important role in the remodelling of the extracellular matrix. The role of MMPs in the development of progressive fibrosis and cirrhosis is well established22 and emerging evidence suggests that MMPs may have a similar role in CP.23 Elevated serum MMP-9 levels have been found in patients with both AP and CP, and active form of MMP-9 could be involved in the development of diabetes mellitus associated with CP.24
Oxidative stress is a result of imbalance between reactive oxygen species and their neutralising mechanisms, which may result in cell or tissue damage. Increased oxidative stress with the resulting formation of free radicals has been implicated in the pathophysiology of CP25 and treatment with antioxidants has been shown to reduce disease and pain severity in randomised controlled trials,26 27 although the effects may be related to the underlying aetiology.28 29 Recent studies have shown that oxidative stress may also be implicated in RAP.30 Free radicals do not only exert local effect in pancreas but are also released in systemic circulation and can thus be quantified.
Pain processing and quantitative sensory testing
The processing of pain in central pain pathways undergoes changes in a large proportion of patients with painful CP. These include sensitisation of central pain pathways, impaired capacity of pain modulation as well as structural and functional changes in the brain.31 Although the presence of these changes is well documented in numerous cross-sectional studies, the temporal aspects of changes in pain processing during disease progression have not previously been investigated in the context of pancreatitis. Quantitative sensory testing (QST) can be used to map the pain system; the technique is based on the rationale that different neural pathways and networks can be explored using standardised stimulation with simultaneous recording of the evoked pain response by psychophysical and/or objective methods.32 Due to spinal convergence between visceral afferents from the pancreas and somatic afferents from upper abdominal skin dermatomes, somatic QST can be used to assess if the pain system is sensitised by nociceptive input from the pancreas.33 Together with specific test paradigms (temporal summation and assessment of pain modulation), QST can be used to characterise the state of the pain system and to document if patients have abnormal central pain processing.
Nutrition
The risk factors associated with underweight and malnutrition in patients with CP are complex and likely multifactorial with the most frequently reported being pancreatic exocrine insufficiency. However, many patients lose weight early in their disease course and before evolution of pancreatic exocrine insufficiency. For example, postprandial pain, which is seen in many patients, may also limit food intake and lead to underweight and malnutrition. There is a paucity of data on this important topic, and continuous characterisation of nutritional status in patients going through phases of AP, to RAP to CP may provide us with essential knowledge on metabolic processes related to inflammation and fibrosis. A combined use of bioelectrical impedance, hand grip strength (HGS), timed up-and-go test (TUG), assessment of endocrine (HbA1c) and exocrine insufficiency (FE), and biochemical tests enables qualitative and quantitative assessment of nutritional status.
Hypotheses
Current knowledge on the progression of CP is mostly based on cross-sectional studies including patients with different stages of RAP and CP. By prospectively following well-characterised cohorts of patients with pancreatitis at different stages (AP → RAP → CP) with serial imaging (advanced MRI and EUS), profiling of fibroinflammatory pathways using circulating biomarkers and assessment of nutritional status and pain processing using QST, this study will help elucidate the underlying pathophysiology of pancreatitis.
The hypotheses of this study are:
Prospective assessment of patients progressing from AP to CP, using advanced imaging techniques and circulating biomarkers of inflammation, fibrosis and oxidative stress, can provide new mechanistic insight to the underlying fibroinflammatory process. This knowledge may be used to design the future clinical studies focusing on prevention of inflammation and fibrosis in the pancreas and other organ systems.
Changes in pain processing over time are poorly understood in patients progressing from AP to CP. This information may be used to identify patients at risk for developing a chronic pain syndrome with irreversible neuroplastic changes in the central nervous system.
Changes in nutritional state over time are poorly understood in patients progressing from AP to CP. This information may be used to identify patients at risk for developing malnutrition.
Aims
Primary aim
To prospectively investigate the fibroinflammatory process involved in progression from acute to CP using state-of-the-art diagnostic imaging modalities (contrast-enhanced EUS, EUS-guided elastography and advanced MRI) and circulating biomarkers of inflammation, fibrosis and oxidative stress.
Secondary aim
To prospectively characterise pain processing and nutritional status in patients progressing from acute to CP.
Methods
Study design
Prospective cohort study including adult patients with RAP, probable or definitive CP recruited from outpatient clinics at the three participating sites (Hvidovre, Bispebjerg and Aalborg University Hospitals). The study is non-interventional; all participants will be treated and monitored according to the current best clinical evidence-based practice. The patient inclusion began in February 2019. We anticipate to complete the inclusion of all cohorts during a 2-year period. We will follow the patient cohorts for 15 years, and the anticipated completion date is 1 February 2035.
Inclusion criteria
Age 18–75 years
Cohort 1 (definitive CP): Patients with definitive CP (n=60) of any aetiology except gallstone-induced CP. The M-ANNHEIM diagnostic criteria will be used for the definition of definitive CP. Hence, patients will be included in this cohort if they fulfil one or more of the following criteria: pancreatic calcifications, moderate or marked pancreatic duct changes (Cambridge III or IV) or typical histology of CP.34
Cohort 2 (suspected CP): Patients with RAP except gallstone-induced RAP and patients with probable CP according to the M-ANNHEIM criteria (n=60). RAP is defined as two or more cases of AP as diagnosed by the revised Atlanta Criteria.35 Probable CP is defined as patients with a typical history of CP (persistent abdominal pain and/or a history of a single AP episode) in combination with any of the following: mild pancreatic duct changes (Cambridge I or II), recurrent or persistent pseudocysts, abnormal exocrine pancreatic function test or post pancreatitis diabetes mellitus (elevated glycated haemoglobin >3 months after diagnosis of pancreatitis).3 34
Cohort 3 (control subjects): Subjects without any previous history of pancreatic or gastrointestinal diseases will be included in the control cohort (n=40).
Exclusion criteria
Patients with the following conditions will be excluded from participation in the study:
Pregnant or lactating patients.
Patients in whom MRI is contraindicated (metallic implants, pacemaker, implantable cardioverter defibrillator and claustrophobia).
Patients with chronic liver disease, chronic renal failure, malignancy, chronic inflammatory bowel syndrome, chronic obstructive lung disease and pulmonary fibrosis. If the patient develops any of the listed diseases during the study period, they will continue their participation in the study.
Patients treated with anti-inflammatory drugs of any kind at the time of inclusion: local or systemic corticosteroids, non-steroidal anti-inflammatory drugs (NSAID’s), salazopyrin or other. However, once included, they will continue their participation even though they receive treatment with anti-inflammatory drugs.
Sample size, time schedule and follow-up
The present study is observational. No previous long-term prospective studies have evaluated the fibroinflammatory process associated with the development of CP and we were, therefore, unable to undertake a valid sample size calculation. Consequently, the sample size was set to 60 participants in the patient cohorts, assuming that 36% of participants in cohort 2 will progress to definitive CP.3 36 37 Similar sample size estimates apply for a recently published study protocol from the Consortium for the Study of Chronic Pancreatitis, Diabetes and Pancreatic Cancer (CPDPC) that is also based on MRI assessment parameters.38 We expected that we will be able to reach our sample size during an inclusion period of 2 years, based on experience from previous cohort studies from our departments. We anticipate that all three centres will include approximately equal numbers of participants.
Included patients will be followed up prospectively for 15 years. An interim analysis, assessing both clinical, functional, imaging and biochemical parameters will be performed after 4 years.39 Patient cohorts will follow the same time schedule and plan for follow-up as outlined in table 1.
Table 1.
Schedule of events
| On inclusion | Yearly | Every second year | ||
| Informed consent and assessment of eligibility criteria | X | |||
| Review of medical history including medication | X | X | ||
| Physical examination | Anthropometrics | X | X | |
| Vital signs | X | X | ||
| Clinical laboratory and biobank | X | X | ||
| F-elastase-1 and HbA1c | X | X | ||
| Diagnostic imaging |
Advanced MRI | X | X | |
| Contrast-enhanced EUS and elastography | X | X | ||
| Pain processing |
Quantitative sensory testing | X | X | |
| Questionnaires | X | X | ||
| Body composition: Bioelectrical impedance | X | X | ||
| Muscle strength and function: Hand grip strength and timed up-and-go test | X | X | ||
EUS, endoscopic ultrasound; HbA1c, glycated haemoglobin.
Included patients will be evaluated during a quiescent phase of disease; consequently, an acute attack of pancreatitis or exacerbation in CP requiring hospital admission will lead to a quarantine period of 4 weeks before evaluation by the protocol can be performed. Resolution of pancreatitis will be documented by assessment of plasma amylase and C reactive protein (CRP).
On inclusion, review of medical history including medication, physical examination will be performed. The following patient characteristics will be recorded: age at inclusion; gender; date the patient was diagnosed with AP/RAP/CP; aetiology of pancreatitis; previous and current patterns of tobacco and alcohol consumption; presence of diabetes mellitus and other comorbidities; medications. Advanced MRI and contrast-enhanced EUS with elastography will be used to characterise pancreatic morphology40 41 (table 2). Endocrine and exocrine pancreatic function will be characterised by HbA1c and FE and routine biochemical tests including white cell count, haemoglobin, platelets, sodium, potassium, urea, creatinine, CRP, albumin, alanine aminotransferase (ALT), amylase, alkaline phosphatase, bilirubin, INR, calcium, phosphate, magnesium, cobalamin, vitamin D, PTH will be obtained. In addition to routine biochemical tests, blood samples (see later) will be deposited in a biobank for the future evaluation of biomarkers of inflammation, fibrosis and oxidative stress (table 3). Nutritional assessment and evaluation of pain processing by QST will be performed, and the measurement variables reported in table 4 will be recorded.
Table 2.
Imaging assessment parameters
| Method | Modality | Measurement variables |
| MRI | T1, T2, MRCP | Cambridge classification with modification for MRCP40
Main pancreatic duct diameter Pancreatic dimensions and volume |
| DWI | Apparent diffusion coefficient | |
| DIXON | Fat signal faction | |
| EUS | EUS B-mode | Rosemont score12 |
| Contrast enhanced | 90 s film recorded from stomach of pancreatic body will be recorded for later analysis | |
| Elastography | Histogram analysis and measurement of strain ratio41 |
DWI, diffusion weighted imaging; EUS, endoscopic ultrasound; MRCP, MR cholangiopancreatography.
Table 3.
Circulating biomarker assessment parameters
| Measurement variable | |
| Inflammation | Interelukin(IL)−4, IL-6, IL-8, IL-10, IL-12, IL-18, TNF-alpha |
| Fibrosis | Transforming growth factor beta-1, soluble fractalkine, monocyte chemoattractant protein 1, matrix metalloproteinases |
| Oxidative stress | Glutathione peroxidase, vitamin C, ferric reducing ability of plasma, malondialdehyde, 4-hydroxynonenal, superoxide dismutase, nitric oxide |
TNF, tumour necrosis factor.
Table 4.
Nutritional and QST assessment parameters
| Method | Measurement variable | |
| Nutritional assessment | Muscle strength: Han- held dynamometer | Muscle strength (kg) |
| Muscle function: Timed up-and-go test | Seconds | |
| Body composition: Bioelectrical impedance |
Various bioelectrical impedance parameters | |
| Assessment of pain processing | Pressure algometry | Pressure pain and tolerance thresholds (kPa) |
| Temporal summation | Absolute and relative change in pain scores (NRS) to single and repetitive pinprick stimulations | |
| Conditioned pain modulation | Absolute and relative change in pressure pain tolerance thresholds (kPa) before and after cold pressor test |
NRS, Numerical Rating Scale; QST, quantitative sensory testing.
On subsequent yearly visits review of medical history including medication, patterns of smoking and alcohol consumption during the past year and physical examination will be performed. Endocrine and exocrine pancreatic function will be reassessed together with the routine biochemical tests outlined above. Blood samples will be deposited in a biobank for future evaluation of circulating biomarkers and nutritional assessment and evaluation of pain processing by QST will be performed as described for the inclusion visit. In addition to the yearly assessments, patients will undergo advanced MRI and contrast-enhanced EUS as described at the inclusion visit every second year.
During the study period, patients will be counselled and treated according to best clinical practice, including advice on alcohol and smoking cessation.
Biobank
Ninety millilitres of blood (processed as plasma, serum, buffy coat and cells) will be deposited in a biobank on the day of inclusion, and subsequently on a yearly basis for 15 years. The purpose of the biobank is to allow analyses of potential biomarkers for disease progression. The analyses will be conducted before 1 January 2040 (the study termination date). Any remaining blood will be destroyed. The deposited blood material will also enable us to perform genetic studies if deemed relevant. The genetic analyses will only be performed following approval from the Regional Committee on Health Research Ethic.
Patient and public involvement
Patients and public were not involved in the design and recruitment of the present study. As the study is designed as a part of a clinical setup and monitoring, the results of the study will be continuously discussed with the participating patients.
Study procedures
MRI
MRI is performed in collaboration with the radiological departments of the participating institutions. A common MRI protocol is used at all participating sites; the protocol has been described in detail previously.8 42
Endoscopic ultrasound
EUS is performed under conscious sedation with fasting (min 6 hours) patient lying on the left side, and under continuous monitoring of pulse, blood pressure and oxygenation. EUS B-mode is applied to calculate Rosemont score (performed before further examination). Ten seconds sequences from the body and head of the pancreas are recorded. From stomach, elastography is applied on pancreatic body: 10 s loop will be recorded with three images and a histogram. Contrast-enhanced EUS is performed by a bolus administration of 2.4 mL SonoVue. A film sequence of 90 s is recorded.
Circulating biomarkers
Venous blood samples are drawn from an antecubital vein. Following this procedure, the blood is processed as plasma, serum, buffy coat and cells, and stored at −80°C until subsequent analysis (biobank).
Quantitative sensory testing
Pressure algometry
The pressure pain detection threshold (pPDT) and pressure pain tolerance threshold (pPTT) are determined on six different sites, corresponding to the somatic dermatomes above the clavicle (C5), middle of the anterior axillary fold (T4), dorsum (T10(D)), spina illiaca anterior superior (L1), rectus femoris (L4) and the abdominal pancreatic area (T10(P)). Pressure algometry is performed using an electronic pressure algometer (Somedic AB, Stockholm, Sweden), with a surface area of 1 cm2. Pressure is increased at a rate of 30 kPa/sec until pPDT and pPTT is reached, and subjects are instructed to press a button at this point, which stops the stimulation, and the corresponding pressure (kPa) is recorded.
Conditioned pain modulation
To induce conditioned pain modulation (CPM), a cold pressor test is applied as the conditioning stimulus and pressure stimulations are used as test stimuli. Pressure stimulation is applied at the on the non-dominant rectus femoris until subjects reach the PTT using the equipment and procedure described above. The cold pressor test is performed by asking the subject to immerse their dominant hand in cooled water (2°C) for 2 min, or less if the evoked pain is considered to be intolerable. The CPM effect is assessed as the absolute and relative change in PTT before and after the cold pressor test.
Temporal summation
In this test, the perceived intensity of a single pinprick stimulus of 256 mN, is tested over the epigastric area (T10) and the dominant forearm and compared with that of a series of 10 repetitive pinprick stimuli of the same physical intensity (1/s applied within an area of 1 cm2). The subject is asked to give a pain rating representing the single stimulus, and the estimated mean over the whole series of 10 stimuli using a ‘0–10’ Numerical Rating Scale (NRS). The temporal summation ratio is calculated as the absolute and relative change in pain scores to single and repetitive pinprick stimulations. Pin pricks are applied using a modified von Frey hairs (Optihair2-Set, Marstock Nervtest, Germany).
Nutritional assessment
Body composition
Bioelectrical impedance analysis enables a fast and accurate measurement of body compartments. For the purpose of this study, bioelectrical impedance will be assessed by the seca medical Body Composition Analyzer 514/515 (Seca, Hamburg) or BioScan 920-II (Maltron, Essex, UK). The analyser consists of a platform with an integrated scale, a handrail system and a display and operation unit. The device uses four pairs of electrodes that are positioned at each hand and foot, with one electrode in each pair through which the electrical current enters the limb and the other electrode detects the voltage drop. Analysis time is 20–30 s.
Hand grip strength
Muscle strength is determined by HGS measured to the nearest kilogram using a hydraulic hand dynamometer (NC70142, North Coast Medical, Arcata, California, USA or Jamar Smart Hand Dynamometer, Patterson medical, Warrenville, Illinois, USA). The dynamometer is held in the second handle position and the patient is instructed to sit on a chair with the shoulder neutrally rotated, holding the elbow bend 90° and the wrist in neutral position. HGS is measured three times for each hand; assessments are separated by intervals of approximately 10 s. The highest value for each hand is recorded and the mean value is calculated.
Timed up-and-go test
Muscle function is characterised by the TUG. This test is performed and reported (seconds) as the time it takes a patient to get up from sitting position on a chair, walk 3 m, turn around, walk back to the chair and sit down.
Questionnaires
Quality of life
The European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORCT QLQ-C30) is used to document life quality, physical function and a number of other health-related parameters.43 The questionnaire has been validated specifically for assessment of patients with CP and is composed of single-item measures and multi-item scales with scores ranging from 0 to 100 after linear transformation of the raw score.44 A high score for a functional scale represents a high level of functioning, as does a high score for the global health status, while a high score for the symptom items represents a high level of symptomatology.
Pain symptoms
The brief pain inventory short form is used to document the patients pain symptoms and its interference with daily activities (interference score).45
Ethics and dissemination
This study will be conducted according to the Danish national legislation on health. The project will lead to better understanding of fibrogenic process that leads to CP and better understanding of changes in pain perception and nutritional aspects during the course of the disease. The combination of diagnostic imaging and biochemical parameters will enable improved identification of those patients who are at particular risk of developing early CP. The improvement in identification is vital, as there are emerging inhibitors of fibrosis, which potentially could impair the ongoing fibrogenesis of pancreas. Hence, the project will lead to new strategies for patient management. Socioeconomically, the results of this study may lead to reduced costs for hospitalisations and accidents related to CP. The techniques developed in the project period may also be used to understand and evaluate the fibrogenic processes in other organs, primarily the liver.
The investigations and examinations that are planned in present protocol are all a part of existing routine workup. The major change associated with the participation in the study is that the examinations are scheduled at regular time intervals. Blood for the purpose of biobank will be drawn simultaneously with the routine blood tests. FE, bioelectrical impedance, hand grip test, TUG test, QST and MRI are not associated with any risks. The EUS can be associated with discomfort and in very rare cases with serious complications such as perforation of oesophagus, stomach or duodenum.
The study may contain several combinations of data that may be written as papers in peer-reviewed international journals. We will publish the study results regardless of whether the results are positive, negative or inconclusive.
Discussion
Recently the US-based consortium for the Study of CPDPC has published their study protocol for the Prospective Evaluation of Chronic Pancreatitis for Epidemiologic and Translational Studies (PROCEED).39 Although the PROCEED study is mainly based on CT-based imaging parameters, as opposed to the MRI-based approach in our study, the design of the PROCEED study is in many ways comparable to ours. Hence, both studies focus on prospective assessment of disease progression parameters in well-defined cohorts of patients with CP at different disease stages using cross-sectional imaging and circulating biomarkers. The patient cohorts have been characterised using comparatively similar criteria, which will allow comparison across studies.
A unique characteristic of our study, compared with the PROCEED study, is the annual assessment of pain processing and modulation based on a QST protocol specifically designed for pancreatic pain. In addition, detailed nutritional assessment, using bioelectrical impedance and muscle function tests, distinct our protocol from that of the PROCEED study.39 Finally, our study population comprises of a very homogeneous population, both genetically, socially and economically, which provides us with an opportunity to study the natural course of CP with reduced bias from diverse patient characteristics.
Supplementary Material
Footnotes
Contributors: All authors made substantial contributions to the design of the study. SN, LG and SSO contributed to drafting the protocol and revising it critically for important intellectual content. ABJ, JBF and FKJ contributed with important aspects on diagnostic imaging. DK, PNS and EFH contributed with important aspects on endoscopic techniques. MW, AB, CN, MBH, LNJ and AMD contributed critical revisions to the draft for important intellectual content. All authors reviewed and approved the final version submitted for publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study is approved by the Danish Data Protection Agency (VD-2018–298; I-suite no.: 6542) and the Regional Committee on Health Research Ethics (Journal-no.: H-18017705). The study will be conducted according to the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Whitcomb DC, Frulloni L, Garg P, et al. Chronic pancreatitis: an international draft consensus proposal for a new mechanistic definition. Pancreatology 2016;16:218–24. 10.1016/j.pan.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xiao AY, Tan MLY, Wu LM, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016;1:45–55. 10.1016/S2468-1253(16)30004-8 [DOI] [PubMed] [Google Scholar]
- 3. Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol 2019;16:175–84. 10.1038/s41575-018-0087-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jupp J, Fine D, Johnson CD. The epidemiology and socioeconomic impact of chronic pancreatitis. Best Pract Res Clin Gastroenterol 2010;24:219–31. 10.1016/j.bpg.2010.03.005 [DOI] [PubMed] [Google Scholar]
- 5. Nøjgaard C, Bendtsen F, Becker U, et al. Danish patients with chronic pancreatitis have a four-fold higher mortality rate than the Danish population. Clin Gastroenterol Hepatol 2010;8:384–90. 10.1016/j.cgh.2009.12.016 [DOI] [PubMed] [Google Scholar]
- 6. Bang UC, Benfield T, Hyldstrup L, et al. Mortality, cancer, and comorbidities associated with chronic pancreatitis: a Danish nationwide matched-cohort study. Gastroenterology 2014;146:989–94. 10.1053/j.gastro.2013.12.033 [DOI] [PubMed] [Google Scholar]
- 7. Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology 2001;120:682–707. 10.1053/gast.2001.22586 [DOI] [PubMed] [Google Scholar]
- 8. Madzak A, Olesen SS, Wathle GK, et al. Secretin-Stimulated magnetic resonance imaging assessment of the benign pancreatic disorders: systematic review and proposal for a standardized protocol. Pancreas 2016;45:1092–103. 10.1097/MPA.0000000000000606 [DOI] [PubMed] [Google Scholar]
- 9. Ahmed Ali U, Issa Y, Hagenaars JC, et al. Risk of recurrent pancreatitis and progression to chronic pancreatitis after a first episode of acute pancreatitis. Clin Gastroenterol Hepatol 2016;14:738–46. 10.1016/j.cgh.2015.12.040 [DOI] [PubMed] [Google Scholar]
- 10. Löhr JM, Dominguez-Munoz E, Rosendahl J, et al. United European gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J 2017;5:153–99. 10.1177/2050640616684695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson PJA, Sheridan MB. Abdominal radiology Review article Pancreatitis : computed tomography and magnetic resonance imaging. Eur Radiol 2000;408:401–8. [DOI] [PubMed] [Google Scholar]
- 12. Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc 2009;69:1251–61. 10.1016/j.gie.2008.07.043 [DOI] [PubMed] [Google Scholar]
- 13. Kawada N, Tanaka S. Elastography for the pancreas: current status and future perspective. World J Gastroenterol 2016;22:3712–24. 10.3748/wjg.v22.i14.3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pedersen N, Larsen S, Seidelin JB, et al. Alcohol modulates circulating levels of interleukin-6 and monocyte chemoattractant protein-1 in chronic pancreatitis. Scand J Gastroenterol 2004;39:277–82. 10.1080/00365520310008296 [DOI] [PubMed] [Google Scholar]
- 15. Sandström A, Andersson R, Segersvärd R, et al. Serum proteome profiling of pancreatitis using recombinant antibody microarrays reveals disease-associated biomarker signatures. Proteomics Clin Appl 2012;6:486–96. 10.1002/prca.201200051 [DOI] [PubMed] [Google Scholar]
- 16. Schneider A, Haas S-L, Hildenbrand R, et al. Enhanced expression of interleukin-18 in serum and pancreas of patients with chronic pancreatitis. World J Gastroenterol 2006;12:6507–14. 10.3748/wjg.v12.i40.6507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sri Manjari K, Jyothy A, Shravan Kumar P, et al. A single-nucleotide polymorphism in tumor necrosis factor-α (−308 G/A) as a biomarker in chronic pancreatitis. Gene 2014;539:186–9. 10.1016/j.gene.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 18. Kamath MG, Pai CG, Kamath A, et al. Monocyte chemoattractant protein-1, transforming growth factor-beta1, nerve growth factor, resistin and hyaluronic acid as serum markers: comparison between recurrent acute and chronic pancreatitis. Hepatobiliary Pancreat Dis Int 2016;15:209–15. 10.1016/S1499-3872(15)60029-7 [DOI] [PubMed] [Google Scholar]
- 19. Ceyhan GO, Deucker S, Demir IE, et al. Neural fractalkine expression is closely linked to pain and pancreatic neuritis in human chronic pancreatitis. Lab Invest 2009;89:347–61. 10.1038/labinvest.2008.170 [DOI] [PubMed] [Google Scholar]
- 20. Ito T. Can measurement of chemokines become useful biological and functional markers of early-stage chronic pancreatitis? J Gastroenterol 2007;42(Suppl 17):72–7. 10.1007/s00535-006-1929-4 [DOI] [PubMed] [Google Scholar]
- 21. Bai H, Chen X, Zhang L, et al. The effect of sulindac, a non-steroidal anti-inflammatory drug, attenuates inflammation and fibrosis in a mouse model of chronic pancreatitis. BMC Gastroenterol 2012;12:115 10.1186/1471-230X-12-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roeb E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol 2018;68-69 10.1016/j.matbio.2017.12.012 [DOI] [PubMed] [Google Scholar]
- 23. Kurzepa J, Mądro A, Czechowska G, et al. Role of MMP-2 and MMP-9 and their natural inhibitors in liver fibrosis, chronic pancreatitis and non-specific inflammatory bowel diseases. Hepatobiliary Pancreat Dis Int 2014;13:570–9. 10.1016/S1499-3872(14)60261-7 [DOI] [PubMed] [Google Scholar]
- 24. Descamps FJ, Van den Steen PE, Martens E, et al. Gelatinase B is diabetogenic in acute and chronic pancreatitis by cleaving insulin. FASEB J 2003;17:887–9. 10.1096/fj.02-0725fje [DOI] [PubMed] [Google Scholar]
- 25. Tandon RK, Garg PK. Oxidative stress in chronic pancreatitis: pathophysiological relevance and management. Antioxid Redox Signal 2011;15:2757–66. 10.1089/ars.2011.4115 [DOI] [PubMed] [Google Scholar]
- 26. Uden S, Bilton D, Nathan L, et al. Antioxidant therapy for recurrent pancreatitis: placebo-controlled trial. Aliment Pharmacol Ther 1990;4:357–71. 10.1111/j.1365-2036.1990.tb00482.x [DOI] [PubMed] [Google Scholar]
- 27. Bhardwaj P, Garg PK, Maulik SK, et al. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology 2009;136:149–59. 10.1053/j.gastro.2008.09.028 [DOI] [PubMed] [Google Scholar]
- 28. Forsmark CE, Liddle RA. The challenging task of treating painful chronic pancreatitis. Gastroenterology 2012;143:533–5. 10.1053/j.gastro.2012.07.029 [DOI] [PubMed] [Google Scholar]
- 29. Siriwardena AK, Mason JM, Sheen AJ, et al. Antioxidant therapy does not reduce pain in patients with chronic pancreatitis: the ANTICIPATE study. Gastroenterology 2012;143:655–63. 10.1053/j.gastro.2012.05.046 [DOI] [PubMed] [Google Scholar]
- 30. Bopanna S, Nayak B, Prakash S, et al. Increased oxidative stress and deficient antioxidant levels may be involved in the pathogenesis of idiopathic recurrent acute pancreatitis. Pancreatology 2017;17:529–33. 10.1016/j.pan.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 31. Poulsen JL, Olesen SS, Malver LP, et al. Pain and chronic pancreatitis: a complex interplay of multiple mechanisms. World J Gastroenterol 2013;19:7282–91. 10.3748/wjg.v19.i42.7282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bouwense SAW, de Vries M, Schreuder LTW, et al. Systematic mechanism-orientated approach to chronic pancreatitis pain. World J Gastroenterol 2015;21:47–59. 10.3748/wjg.v21.i1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Olesen SS, Graversen C, Bouwense SAW, et al. Quantitative sensory testing predicts pregabalin efficacy in painful chronic pancreatitis. PLoS One 2013;8:e57963 10.1371/journal.pone.0057963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schneider A, Löhr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol 2007;42:101–19. 10.1007/s00535-006-1945-4 [DOI] [PubMed] [Google Scholar]
- 35. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 2013;62:102–11. 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 36. Kalaria R, Abraham P, Desai DC, et al. Rate of recurrence in Indian patients presenting with acute pancreatitis and identification of chronicity on follow up: possible risk factors for progression. Indian J Gastroenterol 2018;37:92–7. 10.1007/s12664-018-0818-0 [DOI] [PubMed] [Google Scholar]
- 37. Sankaran SJ, Xiao AY, Wu LM, et al. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology 2015;149:1490–500. 10.1053/j.gastro.2015.07.066 [DOI] [PubMed] [Google Scholar]
- 38. Tirkes T, Shah ZK, Takahashi N, et al. Consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. reporting standards for chronic pancreatitis by using CT, MRI, and MR cholangiopancreatography. Radiology 2019;290:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yadav D, Park WG, Fogel EL, et al. Consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer (CPDPC). prospective evaluation of chronic pancreatitis for epidemiologic and translational studies: rationale and study design for PROCEED from the Consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 2018;47:1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schreyer AG, Jung M, Riemann JF, et al. S3 guideline for chronic pancreatitis - Diagnosis, classification and therapy for the radiologist. RoFo Fortschritte Auf Dem Gebiet Der Rontgenstrahlen Und Der Bildgeb Verfahren 2004;186:1002–8. [DOI] [PubMed] [Google Scholar]
- 41. Kuwahara T, Hirooka Y, Kawashima H, et al. Quantitative diagnosis of chronic pancreatitis using EUS elastography. J Gastroenterol 2017;52:868–74. 10.1007/s00535-016-1296-8 [DOI] [PubMed] [Google Scholar]
- 42. Madzak A, Olesen SS, Lykke Poulsen J, et al. MRI assessed pancreatic morphology and exocrine function are associated with disease burden in chronic pancreatitis. Eur J Gastroenterol Hepatol 2017;29:1269–75. 10.1097/MEG.0000000000000955 [DOI] [PubMed] [Google Scholar]
- 43. Aaronson NK, Ahmedzai S, Bergman B, et al. The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 44. Fitzsimmons D, Kahl S, Butturini G, et al. Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol 2005;100:918–26. 10.1111/j.1572-0241.2005.40859.x [DOI] [PubMed] [Google Scholar]
- 45. Mendoza T, Mayne T, Rublee D, et al. Reliability and validity of a modified brief pain inventory short form in patients with osteoarthritis. Eur J Pain 2006;10:353–61. 10.1016/j.ejpain.2005.06.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.