Abstract
Introduction
Atopic eczema affects 20% of UK children, and environmental factors are important in its aetiology. Several observational studies suggest an increased risk of atopic eczema in children living in hard water areas. The Softened Water for Eczema Prevention pilot trial tests the feasibility of installing domestic ion-exchange water softeners around the time of birth to reduce the risk of atopic eczema in children with a family history of atopy. A further aim is to explore the pathophysiological mechanisms for this in an embedded mechanistic study.
Methods and analysis
Multicentre parallel group assessor-blinded randomised controlled pilot trial. Participants are newborn babies (n=80) living in a hard water (>250 mg/L calcium carbonate) area at risk of developing atopic eczema because of a family history of atopy. Participants will be randomised prior to birth in a 1:1 ratio. The intervention group will have an ion-exchange water softener installed prior to birth. The control group will receive their usual domestic hard water supply. Follow-up will be until 6 months of age. Data will be collected at birth (baseline), 1, 3 and 6 months of age. The main outcome is the proportion of eligible families screened who are willing and able to be randomised. Several secondary feasibility and clinical endpoints will also be evaluated, alongside mechanistic outcomes. Data will be analysed on an intention-to-treat basis. There will be no hypothesis testing for the clinical outcomes. Study acceptability will be evaluated through semistructured interviews.
Ethics and dissemination
This study has been reviewed and given a favourable opinion by the North West–Liverpool East Research Ethics Committee (Ref: 17/NW/0661). The results of the study will be reported at international conferences and in peer-reviewed scientific journals. We will send participating families a summary of the pilot trial results.
Trial registration number
Keywords: atopic eczema, water hardness, pilot trial, dermatology
Strengths and limitations of this study.
This pilot study comprehensively tests the design of a randomised controlled trial for the primary prevention of atopic eczema using a mixture of quantitative and qualitative methods.
In addition, various mechanistic measurements will further our understanding of the mechanisms by which water softeners might prevent atopic eczema.
This study is being performed in two centres to gain a wider understanding of potential barriers and facilitators to recruitment and retention in a multicentre definitive trial.
The study is outcome assessor blinded as participant blinding is not possible to achieve.
Follow-up is only for 6 months and so may not provide full information on postrandomisation losses to follow-up that could occur in a future definitive trial with a longer duration of follow-up.
Introduction
Background & Rationale
Atopic eczema (synonym atopic dermatitis, hereinafter referred to as ‘eczema’) is a common inflammatory skin condition affecting around 20% of UK children.1 It is associated with significant morbidity and affects health-related quality of life. The cause of eczema is not fully understood. It is likely to be multifactorial, and several genetic and environmental factors have been identified.2 No primary prevention strategy has been established.3 However, several approaches have been proposed such as probiotics during pregnancy, dietary supplementation, house dust mite avoidance, intensive emollient use and domestic water softening.4
Relationship between hard water and atopic eczema
Hard water is the result of dissolved minerals, mainly calcium carbonate and magnesium carbonate, from the percolation of water through rock in the environment. England, especially in the south, has very hard (>250 mg/L calcium carbonate) domestic water. A cross-sectional study conducted in the 1990s found that primary school-age children living in hard water areas had an increased risk of eczema compared with children living in softer water areas around Nottingham, UK.5 Two further cross-sectional studies among schoolchildren conducted in Japan and Spain confirmed this association.6 7 Subsequently, a cross-sectional analysis from a cohort study among over 1300 infants in England and Wales has also confirmed this relationship in early life, even after adjusting for likely confounders.8 The same study suggested a possible interaction with loss-of-function mutations in the skin barrier gene, filaggrin (FLG). Most recently, a large study from a Danish birth cohort found a 5% increase in prevalence of eczema within the first 18 months of life for each 5 unit increase in domestic water hardness (equivalent to 89.2 mg/L calcium carbonate)9 over a range of 6.60–35.90 German degrees of hardness (118–641 mg/L calcium carbonate).10
Potential mechanisms for hard water causing atopic eczema
Several potential mechanisms have been proposed for the way in which hard water may lead to eczema development: increased deposition of detergents such as sodium lauryl sulfate (SLS) on the skin, altered calcium signalling in the epidermis and a rise in skin surface pH, resulting in increased protease activity, could all have a detrimental effect on skin barrier function.8 Experimental work has demonstrated an increased deposition of SLS in skin washed with hard water versus softened water.11 In animal studies using a hairless mouse model, low extracellular concentrations of calcium ions in the upper epidermis led to exocytosis of lamellar bodies, required for skin barrier repair, independent of skin barrier disruption.12
Eczema is associated with a preponderance of Staphylococcus aureus and a reduction in microbial diversity.13 Recent work has identified a synergistic relationship between the human cathelicidin-related antimicrobial peptide LL-37 and antimicrobial peptides produced by coagulase negative staphylococcal species that selectively kill S. aureus.14 Human LL-37 activity against some bacterial species is decreased by the presence of calcium, but not magnesium, ions.15
Trials of water softeners in atopic eczema
The multicentre Softened Water Eczema Trial (SWET), completed in 2011, examined the role of water softeners in treating children with established, moderate to severe eczema and found no overall benefit in terms of eczema severity reduction.16 However, the factors that drive the development of eczema may not be the same as those that determine disease exacerbations and severity and the negative result in the SWET does therefore not exclude a role for water softeners in prevention trials. Early life is likely to be an important time in the development of eczema, particularly as most eczema develops before 2 years of age. Early interactions between genes and the environment may be crucial in instigating the cycle of inflammation and skin barrier dysfunction seen in eczema. Indeed, skin barrier dysfunction at just 1 week of age, as measured by transepidermal water loss (TEWL), is a predictor of subsequent eczema risk.17 A small pilot randomised controlled double-blind cross-over trial of 12 patients aged 3–6 years with mild to moderate eczema compared ultrapure soft water with tap water. After 6 weeks, no statistically significant differences in Eczema Area and Severity Index (EASI) or TEWL were observed between the groups, although there was a statistically significant improvement in pruritus as measured by visual analogue score (−2.10, 95% CI −4.14 to 0.063).18 To date, there are no published studies examining the role of water softeners in the prevention of eczema.19
Rationale
The overall rationale is that by installing a domestic water softener around the time of birth, the infant will be exposed to softened water rather than hard water for bathing and that this will be less irritating to the skin than hard water and so associated with a lower risk of eczema development.
This pilot trial builds on the experience gained from the National Institute for Health Research (NIHR)-funded SWET16 and a trial of emollients in early life (Barrier Enhancement for Eczema Prevention, BEEP),20 both of which were coordinated by KST and JRC, our collaborators on this trial, who are based at the Centre of Evidence Based Dermatology in Nottingham. This is a pilot trial, as defined by the UK NIHR in that it is a ‘version of the main study that is run in miniature to test whether the components of the main study can all work together.’21 This pilot trial will therefore not definitively answer the question of whether installation of a domestic water softener will prevent eczema. The rationale for performing this pilot study is that a definitive trial on eczema prevention using domestic water softening devices would require a much larger number of participants and so prior to embarking on a larger multicentre trial it will be important to determine whether the planned trial recruitment and assessment procedures are possible and workable, or whether they require adapting or changing.22
Trial objectives
The aim of this pilot trial is to determine the feasibility of undertaking a large-scale definitive trial to determine whether installation of domestic ion-exchange water softeners around the time of birth reduces the risk of high-risk children developing eczema. A further aim is to explore the pathophysiological mechanisms for this in an embedded mechanistic study.
Methods and analysis
Trial design
This is a multicentre parallel group assessor-blinded randomised controlled pilot trial of an ion-exchange water softener for the prevention of eczema in neonates at high risk of developing eczema, with an embedded mechanistic study. Eighty newborn infants will be enrolled into the trial for a period of 6 months. The end of study is defined as the final assessment visit of the last participant to enter the trial. Recruitment commenced in February 2018 and is due to end in autumn 2019.
Pregnant women will be randomised prior to delivery to one of two groups in a 1:1 ratio:
Control group: usual domestic hard water supply.
Intervention group: softened domestic water through installation of an ion-exchange water softener.
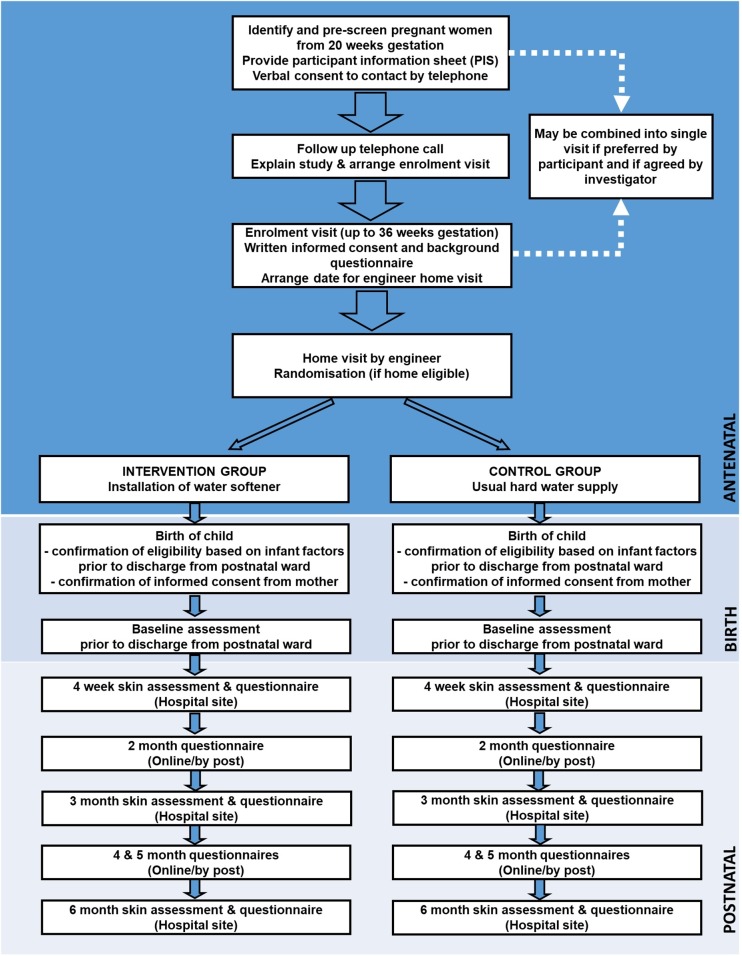
The study design is summarised as a flow chart in figure 1.
Figure 1.
Study flow chart.
Patient involvement
Patients were involved in the design of this study. Members of the Patient Panel at the Centre of Evidence-Based Dermatology reviewed the participant material and provided feedback on the online questionnaires. During the trial, a parent of a child with eczema will join the independent trial steering committee. Once the trial has been published, participants will be sent details of the results in a study newsletter suitable for a non-specialist audience.
Trial population
The study will recruit pregnant women living in hard water areas who will be identified from antenatal services at local National Health Service Trust sites. Women will be approached at the 20-week anomaly scan and asked if they would be interested in participating. We will also publicise the study through posters and by making clinical midwifery teams aware of the study. Full inclusion and exclusion criteria are given in table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | |
| Antenatal maternal | |
| 1 | History of doctor-diagnosed atopy (atopic eczema, asthma or hay fever) in the woman, her partner or other child the couple have parented. |
| 2 | Woman aged 18 years or older. |
| 3 | Woman able to understand English. |
| 4 | Lives in a hard water area (>250 mg/L calcium carbonate). |
| 5 | Lives in a property suitable to have a water softener fitted. |
| 6 | If in a rented property—agrees to seek consent of landlord for fitting of water softener device. |
| 7 | Agrees to have water softener±additional tap for drinking water fitted at home. |
| 8 | Agrees to researchers accessing pregnancy and pregnancy outcome data for the mother and child. |
| 9 | Able and willing to give informed consent. |
| Postnatal maternal | |
| 10 | Maternal consent for her neonate to participate in the study. |
| Exclusion criteria | |
| 11 | Preterm birth (defined as birth prior to 37 weeks’ gestation). |
| 12 | Significant inflammatory skin disease at birth not including seborrheic dermatitis (‘cradle cap’). |
| 13 | Sibling (including twin) previously randomised to this trial. If multiple birth, the first child will be randomised into the trial. |
| 14 | Any immunodeficiency disorder or severe genetic skin disorder. |
| 15 | Child has any other serious health issue which, at parent or investigator discretion, would make it difficult for the family to take part in the trial. |
| 16 | Planned stays away from home for a continuous period of more than 2 weeks or a total of 1 month out of the 6-month follow-up period. |
| 17 | Water softening or filtration device already installed. |
| 18 | Concurrent enrolment in any other skin-related intervention study. |
| 19 | Other medical condition that in the opinion of the chief investigator could interfere with the conduct of the trial. |
Intervention
A domestic ion-exchange water softener will be installed in the homes of participants randomised to the intervention group after enrolment and before the child’s birth. Ion-exchange water softeners exchange calcium and magnesium, among other divalent cations, for monovalent sodium cations, typically reducing downstream water hardness to close to zero. The sodium ions come from common salt which needs to be topped up every 3–4 weeks. The water softeners to be used in this trial will be supplied and funded by Harvey Water Softeners, Woking, UK. Standard procedure will be to soften all water in the home except the drinking water tap, as the water softening process exchanges calcium with sodium ions and will therefore increase the water sodium concentration, making the water unsuitable for drinking purposes. Unsoftened mains drinking water will be delivered through the existing kitchen tap wherever possible, or otherwise through an extra (faucet-style) tap installed at the side of the kitchen sink. At the end of the study, all participants will be given the option to purchase the water softener from Harvey Water Softeners at a reduced price of £399.00 inclusive of value-added tax, installation and warranty; this is approximately a quarter of the full retail price of £1678.80. Alternatively, it will be removed.
Potential risks
This is a low-risk trial as the intervention involves a commercially available ion-exchange water softener that has no known clinical side effects. There is a potential, but low risk, of damage to participants’ properties during installation of the water softening unit. However, these will be installed by a qualified water engineer according to the Code of Practice produced by British Water and in accordance with the recommendations of the Water Regulations Advisory Service.
Concomitant medications and skincare
No restrictions will be placed on the use of concomitant treatment a child might receive. All concomitant medications will be recorded at baseline and updated at follow-up visits. Given the lack of a consistent approach to neonatal skincare, and the possible interaction between some wash products and hard water, we will not provide specific skincare advice to participants. However, data on such use will be sought from parents via monthly online questionnaires.
Coenrolment guidelines
To avoid potentially confounding issues, neonates should not be recruited into other prevention of eczema or allergy intervention trials.
Participant compliance
Compliance with treatment does not represent a large problem for this trial as long as the participants are not absent from home for long periods of time and remember to replenish the salt. Reminders about salt replenishment will be sent. To check that the units are working correctly, participants will be asked to send weekly water samples to Harvey Water Softeners using prepaid envelopes. Any samples with a reading of >20 mg/L calcium carbonate will be referred back to the engineer for investigation. If participants move home during the trial, attempts will be made to reinstall the device in the new property. It is anticipated that loss to follow-up will be <15%.
Primary outcome
Proportion of eligible families screened who are willing and able to be randomised. This is key to the determination of the likely success of a future, large-scale definitive randomised controlled trial (RCT).
Secondary feasibility outcomes
The secondary objectives are designed to further facilitate the design of a larger, definitive multicentre RCT. Namely, to determine the proportion of:
Pregnant women approached who agree to be screened.
Families eligible on screening that cannot have a water softener installed (eg, due to landlord or local authority refusal, technical (plumbing) reasons).
Families randomised that withdraw due to infant ineligibility.
Families in intervention arm who found the intervention acceptable.
Participants in control arm who become exposed to softened water (eg, by moving to a new home in a soft water area, or moving to a home with an active water softener installed, before the end of follow-up).
Participants that have the water softening unit removed or disabled prior to end of follow-up.
Participants with visible eczema status (yes/no) recorded at each time point: baseline, 4 weeks, 3 and 6 months.
Water samples with hardness >20 mg/L calcium carbonate in the intervention arm.
Participants who withdraw from the trial prior to end of follow-up.
Median number of nights spent away from the participant’s main home during follow-up.
Clinical outcome assessments that have remained blinded at 4 weeks, 3 and 6 months.
Secondary clinical outcomes
Proportion with patient-reported, doctor-diagnosed atopic eczema by 6 months of age.
Proportion with visible eczema according to the UK diagnostic criteria-based photographic protocol.23
Severity of eczema (if present) using EASI.
Patient-reported eczema symptoms (Patient-Oriented Eczema Measure) score.
Time to onset of patient-reported doctor-diagnosed eczema.
Additional mechanistic outcomes
The following will be assessed as change from baseline until follow-up at 6 months in the intervention compared with the control group:
TEWL.
Cutaneous cytokine profiles (eg, interleukin-1 (IL-1) levels).
Natural moisturising factor levels.
Shannon Diversity Index and other skin and upper respiratory microbiota parameters.
Median domestic water hardness level (calcium carbonate concentration).
Skin surface hydration.
Adverse events
This trial involves use of a commonly available domestic water softening unit with provision for separate mains drinking water during the time when the water softening unit is installed. Accordingly, we do not anticipate any adverse events or adverse reactions related to the trial intervention. A contact number for the service department at Harvey Water Softeners will be provided and an engineer will be sent to resolve the issue, if needed. Details of technical issues reported to the service department will be reported to the study principal investigators. Events of particular relevance such as plumbing difficulties, floods or difficulties with the units will be logged and reported to the Research Ethics Committee and relevant Research and Development departments annually.
Sample size
This is a pilot study and therefore not powered to establish the efficacy of the intervention. A total of 80 families (40 per group) is judged to provide a sufficiently precise (within 10 percentage points for a 95% CI) estimate of the proportion of families who are willing to be randomised and who will go on to complete the trial.
Findings from the Enquiring About Tolerance (EAT) study8 and the BEEP feasibility study24 allowed us to make a conservative estimate that approximately 70% of families screened will have a history of atopy that predisposes to a high risk of eczema in their offspring. Of these, 40%–60% would be expected to be willing and able to participate. Home factors also need to be considered: in SWET, 27% of eligible families could not participate because their home was not suitable for installation.16
Informed consent
Written informed antenatal consent will be obtained at the enrolment visit from the mother. The consent form will be signed and dated before entering the trial and participants are reminded that they may withdraw from the trial at any time. Mothers will be reconsented postnatally to ensure they agree to their child taking part in the study.
Randomisation and blinding
Participants will be randomised antenatally at the time of the engineer home visit to receive either a water softener or their usual water supply, once:
Antenatal eligibility criteria have been fulfilled.
Fully informed written consent has been obtained.
The engineer is satisfied that the softener can be installed.
Participants will be randomised in a 1:1 ratio to one of the two treatment arms based on a computer-generated code. The randomisation result will be relayed to the installation engineer by telephone as either an ‘INSTALL’ or ‘DO NOT INSTALL’ instruction. The randomisation service will be provided by researchers at King’s College London who are not involved in the study, via telephone.
Experience from the SWET has shown that the effects of a functional water softener are too noticeable to allow participants to be blinded.16 Skin examinations and measurements will be performed by research team members who will be blinded to treatment allocation. Participants will be encouraged not to disclose allocation. Results will be analysed based on treatment code, using a statistical analysis plan (SAP) finalised prior to database lock and any data analysis. Only after the analyses are completed will the actual treatment arms corresponding to treatment codes be revealed. Study team members in direct contact with study participants will be trained in the study protocol and importance of demonstrating equipoise.
Visit schedule and study procedures
The schedule for assessments during the study is shown in table 2.
Table 2.
Schedule of study assessments and procedures
| Screening* | Enrolment* | Home screen and installation | Birth | Baseline (±1 week) | 4 weeks (±1 week) | 3 months (±2 weeks) | 6 months (±2 weeks) | |
| Confirm eligibility | X | X | X | |||||
| Verbal consent to collect contact details and access antenatal records | X | |||||||
| Written informed consent | X | X | ||||||
| Demographic data | X | X | ||||||
| Engineer home assessment | X | |||||||
| Install water softener | X | |||||||
| Randomisation | X | |||||||
| Visible eczema status | X | X | X | |||||
| Blinded eczema severity assessment (EASI) | X | X | X | |||||
| DNA collection from buccal swab | X | |||||||
| Antenatal factors questionnaire | X | |||||||
| Acceptability and feedback questionnaire | X | |||||||
| Invite to participate in semistructured interview about study | X | |||||||
| Collection of skin and nasal microbiome swabs | X | X | X | X | ||||
| TEWL measurement‡ | X | X | X | X | ||||
| Cutaneous tape stripping‡ | X | X | X | X | ||||
| Skin pH measurement‡ | X | X | X | X | ||||
| ATR-FTIR measurement‡ | X | X | X | X | ||||
| Skin surface hydration‡ | X | X | X | X | ||||
| Monthly infant skin and health† questionnaire, including Patient-Oriented Eczema Measure (POEM) score | From 4 weeks to 6 months of age | |||||||
| Weekly water samples (in intervention arm) | From installation to 6 months of age | |||||||
*Screening and enrolment may occur at the same visit if participant prefers and if investigator agrees.
†Any other concomitant illnesses that develop during the study including episodes of respiratory, gastrointestinal and other acute illnesses.
‡At Guy’s and St Thomas’ Hospital site only.
ATR-FTIR, attenuated total reflectance Fourier transform infrared; EASI, Eczema Area and Severity Index; POEM, Patient-Oriented Eczema Measure; TEWL, transepidermal water loss.
Enrolment visit (up until 36 weeks’ gestation)
Confirm eligibility (see table 1).
Answer questions about study.
Written consent is taken.
Antenatal history (including antibiotic exposure, probiotic and omega 3 use), family medical history and environment questionnaire.
Arrange water softener installation.
The enrolment visit may occur at the same time as the screening visit, if preferred by the participant and deemed appropriate by the investigator.
Water softener engineer home visit (up until 40 weeks’ gestation)
Assessment by the engineer to check the home’s suitability for water softener installation.
If home eligible, the engineer will telephone a given number at King’s College London to determine the randomisation group.
Install water softener and provide water sample materials if randomised to the intervention arm.
Baseline visit (on postnatal ward or within 1 week of birth)
Confirm infant eligibility criteria.
Postnatal written consent.
Skin examination.
Infant skincare questions.
Record concomitant infant medications including systemic antibiotic use.
Systemic antibiotic use in mother during pregnancy, including prophylactic antibiotic use during delivery.
Infant comorbidities.
Topical medication use in infant.
Delivery questions.
Pregnancy outcomes and birth details.
-
Additional mechanistic assessments (procedures marked ‡ will be performed only at the Guy’s and St Thomas’ Hospital site):
Tape stripping (forearm).
TEWL measurement (forearm)‡.
Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy measurement (forearm)‡.
Skin pH measurement (forearm)‡.
Skin surface hydration (forearm) ‡.
Microbiome swabs from skin (antecubital fossa and cheek) and nares.
Four-week visit (±1 week)
As per baseline, plus optional buccal swab for DNA for FLG mutation analyses.
Three-month visit (±2 weeks)
As per baseline, plus optional buccal swab for DNA for FLG mutation analyses (if unable to collect at 4-week visit).
Six-month visit (±2 weeks)
As per baseline, plus:
Acceptability questionnaire.
Optional buccal swab for DNA for FLG mutation analyses (if unable to collect at 3-month visit).
Arrange date for removal of water softener within 1 month if not planning to purchase (intervention group) or installation of water softener for 6 months period, if desired, in control arm.
Invite to participate in semistructured qualitative interview about study (via telephone or in person) and seek verbal consent for this.
Monthly email messages (intervention group)
Remind participants to complete questionnaires.
Remind participants’ parents to refill the unit with salt.
Invite to contact Harvey Water Softeners for any issues with water softener.
Monthly email messages (control group)
Remind participants to complete questionnaires.
Monthly electronic questionnaires
A secure web-based questionnaire link using the Snap Surveys platform will be emailed to participants’ parents monthly to determine whether the child has received a diagnosis of eczema from a healthcare professional and to check current skincare and hygiene, and confirm residence/time away from main residence and infant general health. A paper copy of the questionnaire can be posted to participants if they prefer or if they do not have internet access.
At the end of the study, that is, 6-month visit, a date will be arranged for removal of the water softener within 1 month if the participating family does not want to purchase the device (intervention group). Control arm participants can purchase the same water softener at the end of the study. Participants will also be invited to participate in a semistructured qualitative interview about the study.
Embedded mechanistic substudy
The embedded mechanistic substudy will help elucidate the mechanisms by which softened water might affect skin barrier function and therefore eczema risk. It will also provide more general insights into the pathogenesis of eczema in young infants with resulting opportunities for hypothesis generation.
ATR-FTIR spectroscopy measurements will be performed using the Agilent 4300 ATR-FTIR device (Agilent Technologies, USA) to measure the deposition of detergents, such as SLS, on the skin surface.25
TEWL provides a standardised measure of skin barrier dysfunction26 and there is evidence to suggest that elevated TEWL in neonates is predictive of subsequent eczema.17 27 We will use the AquaFlux AF200 condensing chamber probe (Biox Systems, London, UK) to measure TEWL. Epidermal stratum corneum hydration will be measured using a CM 825 Corneometer (Courage and Khazaka, Cologne, Germany).
Skin surface pH has an important role in the regulation of epidermal and microbial cellular process and will be measured using a PH905 Skin-Surface-pH probe fitted with a Mettler and Toledo flat surface electrode (Courage and Khazaka).28
Skin protein samples will be collected from the skin by applying and removing eight consecutive D-Squame cutaneous stripping discs (CuDerm, Dallas, USA). Samples will be collected to perform the protein and cytokine analyses, such as IL-1ɑ levels.29
The FLG gene encodes profilaggrin protein and its subsequent breakdown products are key to normal barrier function of the skin. Loss-of-function sequence variants in FLG are associated with an increased risk of eczema (Palmer, 2006). EAT study suggests that there might be an interaction between FLG and water hardness.8 We will examine the relationship between FLG mutation status and risk of eczema in a subgroup analysis and explore the relationship between FLG mutation status and skin microbiome diversity, TEWL and cutaneous cytokine expression. DNA will be extracted by standard techniques and FLG whole gene sequencing will be used for genotyping FLG loss-of-function sequence variants and the presence of intragenic copy number variations (Wong et al JACI, 2017). Buccal swab for DNA will be collected once, during the 4-week visit if consent is given. If unable to collect at the 4-week visit, collection will be attempted at 3-month visit or 6-month visit.
We will sample the antecubital fossa and the cheek using Isohelix bacterial DNA swabs (Cell Projects, Harrietsham, UK). A further swab will be taken from each nostril using an Ultra Minitip Flocked Swab (Copan Flock Technologies, Brescia, Italy). A control sample is taken for each participant. We will use Illumina MiSeq V1–V3 Amplicon sequencing of the 16S ribosomal subunit to characterise both skin and nasal bacteria communities with regard to species diversity. We will examine within-participant changes in microbial diversity over time and explore the relationship between water hardness, microbial diversity and FLG gene mutational profile as well as taking into account environmental factors such as mode of delivery and antibiotic use in the mother and infant.
Statistical analysis
The feasibility parameters of this pilot trial will not be formally statistically tested. The focus will therefore be on descriptive statistics and CIs. A separate and full SAP for the feasibly trial and analysis of mechanistic data will be developed prior to unblinding and any analysis of the data.
Randomisation will be in a 1:1 ratio using randomly permuted blocks. Participants’ data will be analysed on an intention-to-treat basis. There will be no hypothesis testing for the clinical outcomes except in relation to the embedded mechanistic study. Results will be presented as estimates with 95% CIs, where appropriate.
Supplementary Material
Acknowledgments
JLP is a National Institute for Health Research (NIHR) senior investigator. We thank the members of the Centre of Evidence-Based Dermatology Patient Panel for their contributions to the development of the study.
Footnotes
Contributors: CF is the chief investigator with overall responsibility for the SOFTER pilot trial. He conceived the idea for the study, contributed to the writing of the trial protocol and reviewed this manuscript. ZKJL is the senior coinvestigator responsible for designing and running the SOFTER pilot trial. He led on the writing of the trial protocol and drafted the manuscript. JLP is the senior statistical advisor and contributed to the writing of the trial protocol and reviewed the manuscript. NG coordinates the study, reviewed the trial protocol and drafted the manuscript. DG, AB, JRC, KST, TF, SK, HHK, JAS, JEAC, SD and MJC were involved in finalising the study protocol and reviewing of the manuscript.
Funding: Funding to conduct the trial is provided by the NIHR through a Career Development Fellowship (CF, chief investigator (CDF-2014-07-037)). The SOFTER trial is also supported by the NIHR Clinical Research Network, the NIHR Biomedical Research Centre at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London as well as the Harvey Water Softeners, Woking, UK, who have agreed to cover the following costs: supply and installation of the water softening units, salt supplies and testing of the water samples.
Disclaimer: The views expressed are those of the authors and not necessarily those of the UK National Health Service, the UK Department of Health and Social Care or the National Institute of Health Research.
Competing interests: CF has received investigator-led research funding from Sanofi. His department has received clinical trial funding from Sanofi and AbbVie to test novel therapeutics in patients with paediatric atopic eczema. MJC is an investigator and consultant for Regeneron, Sanofi Genzyme, Pfizer, Leo, Galapagos, Novartis, Boots, L’Oreal, Dermavant, Menlo, Reckitt Benckiser, Oxagen, Johnson & Johnson, Hyphens, Astellas, Amlar, AbbVie, Galderma and Procter & Gamble. Harvey Water Softeners (HWS) contributed to the design and operational running of the study (supply and installation of water softeners, testing of water samples). Final decisions around design and conduct were made independently by investigators. HWS will not be involved in the analysis or interpretation of the results.
Patient consent for publication: Not required.
Ethics approval: North West–Liverpool East Research Ethics Committee (Ref: 17/NW/0661)
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International study of asthma and allergies in childhood. J Allergy Clin Immunol 1999;103:125–38. 10.1016/S0091-6749(99)70536-1 [DOI] [PubMed] [Google Scholar]
- 2. Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014;69:3–16. 10.1111/all.12270 [DOI] [PubMed] [Google Scholar]
- 3. Weidinger S, Novak N, dermatitis A. Atopic dermatitis. Lancet 2016;387:1109–22. 10.1016/S0140-6736(15)00149-X [DOI] [PubMed] [Google Scholar]
- 4. Flohr C, Mann J. New approaches to the prevention of childhood atopic dermatitis. Allergy 2014;69:56–61. 10.1111/all.12343 [DOI] [PubMed] [Google Scholar]
- 5. McNally NJ, Williams HC, Phillips DR, et al. Atopic eczema and domestic water hardness. Lancet 1998;352:527–31. 10.1016/S0140-6736(98)01402-0 [DOI] [PubMed] [Google Scholar]
- 6. Arnedo-Pena A, Bellido-Blasco J, Puig-Barbera J, et al. [Domestic water hardness and prevalence of atopic eczema in Castellon (Spain) school children]. Salud Publica Mex 2007;49:295–301. [DOI] [PubMed] [Google Scholar]
- 7. Miyake Y, Yokoyama T, Yura A, et al. Ecological association of water hardness with prevalence of childhood atopic dermatitis in a Japanese urban area. Environ Res 2004;94:33–7. 10.1016/S0013-9351(03)00068-9 [DOI] [PubMed] [Google Scholar]
- 8. Perkin MR, Craven J, Logan K, et al. Association between domestic water hardness, chlorine, and atopic dermatitis risk in early life: A population-based cross-sectional study. J Allergy Clin Immunol 2016;138:509–16. 10.1016/j.jaci.2016.03.031 [DOI] [PubMed] [Google Scholar]
- 9. dGH: Wikipedia , 2018. Available: https://en.wikipedia.org/wiki/DGH
- 10. Engebretsen KA, Bager P, Wohlfahrt J, et al. Prevalence of atopic dermatitis in infants by domestic water hardness and season of birth: cohort study. J Allergy Clin Immunol 2017;139:1568–74. 10.1016/j.jaci.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 11. Danby SG, Brown K, Wigley AM, et al. The effect of water hardness on surfactant deposition after washing and subsequent skin irritation in atopic dermatitis patients and healthy control subjects. J Invest Dermatol 2018;138:68-77 10.1016/j.jid.2017.08.037 [DOI] [PubMed] [Google Scholar]
- 12. Menon GK, Price LF, Bommannan B, et al. Selective obliteration of the epidermal calcium gradient leads to enhanced lamellar body secretion. J Invest Dermatol 1994;102:789–95. 10.1111/1523-1747.ep12377921 [DOI] [PubMed] [Google Scholar]
- 13. Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 2012;22:850–9. 10.1101/gr.131029.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nakatsuji T, Chen TH, Narala S, et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 2017;9:eaah4680 10.1126/scitranslmed.aah4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turner J, Cho Y, Dinh NN, et al. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother 1998;42:2206–14. 10.1128/AAC.42.9.2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas KS, Koller K, Dean T, et al. A multicentre randomised controlled trial and economic evaluation of ion-exchange water softeners for the treatment of eczema in children: the softened water eczema trial (SWET). Health Technol Assess 2011;15:1–156. 10.3310/hta15080 [DOI] [PubMed] [Google Scholar]
- 17. Kelleher M, Dunn-Galvin A, Hourihane Jonathan O'B, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol 2015;135:930–5. 10.1016/j.jaci.2014.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Togawa Y, Kambe N, Shimojo N, et al. Ultra-pure soft water improves skin barrier function in children with atopic dermatitis: a randomized, double-blind, placebo-controlled, crossover pilot study. J Dermatol Sci 2014;76:269–71. 10.1016/j.jdermsci.2014.10.009 [DOI] [PubMed] [Google Scholar]
- 19. Jabbar-Lopez Z, Phongphit V, Ung CY, et al. The role of domestic water hardness in the development of skin barrier dysfunction and atopic eczema: a systematic review of the literature. Br J Dermatol 2017;177:159–59. [Google Scholar]
- 20. Chalmers JR, Thomas KS, Montgomery A, et al. A protocol for a randomized controlled trial to determine whether application of emollient from birth can prevent eczema in high-risk children (BEEP trial). Br J Dermatol 2014;170:E32. [Google Scholar]
- 21. NIHR Feasibility and pilot studies, 2017. Available: http://www.nets.nihr.ac.uk/glossary/pilot-studies [Accessed 21 Feb 2017].
- 22. Eldridge SM, Lancaster GA, Campbell MJ, et al. Defining feasibility and pilot studies in preparation for randomised controlled trials: development of a conceptual framework. PLoS One 2016;11:e0150205 10.1371/journal.pone.0150205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiland SK, Björkstén B, Brunekreef B, et al. Phase II of the International study of asthma and allergies in childhood (Isaac II): rationale and methods. Eur Respir J 2004;24:406–12. 10.1183/09031936.04.00090303 [DOI] [PubMed] [Google Scholar]
- 24. Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014;134:818–23. 10.1016/j.jaci.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saad P, Flach CR, Walters RM, et al. Infrared spectroscopic studies of sodium dodecyl sulphate permeation and interaction with stratum corneum lipids in skin. Int J Cosmet Sci 2012;34:36–43. 10.1111/j.1468-2494.2011.00678.x [DOI] [PubMed] [Google Scholar]
- 26. Imhof RE, De Jesus MEP, Xiao P, et al. Closed-chamber transepidermal water loss measurement: microclimate, calibration and performance. Int J Cosmet Sci 2009;31:97–118. 10.1111/j.1468-2494.2008.00476.x [DOI] [PubMed] [Google Scholar]
- 27. Horimukai K, Morita K, Narita M, et al. Transepidermal water loss measurement during infancy can predict the subsequent development of atopic dermatitis regardless of filaggrin mutations. Allergol Int 2016;65:103–8. 10.1016/j.alit.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 28. Danby SG, Cork MJ. pH in atopic dermatitis. Curr Probl Dermatol 2018;54:95–107. 10.1159/000489523 [DOI] [PubMed] [Google Scholar]
- 29. Dapic I, Jakasa I, Yau NLH, et al. Evaluation of an HPLC method for the determination of natural Moisturizing factors in the human stratum corneum. Anal Lett 2013;46:2133–44. 10.1080/00032719.2013.789881 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.