Abstract
The immune complement system protects against pathogens; however, excess activation results in disease like hemolytic uremic syndrome, a clinical imitator of preeclampsia. Vascular endothelial factor (VEGF) protects against aberrant complement activation and is inhibited by soluble fms-like tyrosine kinase-1 (sFLT1) in other organs. We hypothesize that sFLT1 promotes complement-mediated placental damage through VEGF inhibition in preeclampsia.
Objective:
Quantify placental complement activity and sFLT1 expression in preeclampsia, and the subgroup of preeclampsia with hemolysis elevated liver enzymes low platelets (HELLP) syndrome.
Methods:
Placental complement activation marker C4d, membrane attack complex (MAC), and sFLT1 expression was quantified using immunofluores cence microscopy.
Results:
Placentas from 18 controls, 25 preeclampsia, including 6 cases of HELLP syndrome were identified. Placental C4d expression was greater in PE (median 6.4 [IQR: 5.1, 8.3]) compared to controls (4.4 [3.6, 5.5]; p = 0.003). MAC expression was also increased in preeclampsia compared to controls (6.5 [5.8, 8.7]; 5.4 [2.9, 5.9], p = 0.001). Placental sFLT1 expression was also higher in preeclampsia (p <0.0001). C4d and MAC were strongly correlated with sFLT1 levels in the placenta (R = 0.72; p < 0.0001 and R = 0.59; p = 0.01, respectively). Complement and sFLT1 expression was elevated in HELLP compared to preeclampsia without laboratory abnormalities, but this difference did not reach statistical significance.
Conclusion:
Increased placental complement activation and damage was seen in preeclampsia and correlates with sFLT1 expression. Our findings support the importance of the complement pathway in preeclampsia.
Keywords: Preeclampsia, complement, sFLT1, HELLP
Introduction
Preeclampsia (PE) is a common pregnancy complication affecting 4–10% of all pregnancies with a heterogeneous clinical presentation. Some women develop early-onset PE while others will not develop preeclampsia until term or even postpartum (1). If PE is left untreated (by early identification and delivery), it can result in damage to multiple organs as in hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome. The pathophysiology of PE is multifactorial with both immunologic and vascular pathologic origins. Women with autoimmune disease and excessive complement activation, such as systemic lupus erythematosus (SLE) have a higher likelihood of developing preeclampsia, supporting an immunologic contribution to PE, whereby impaired maternal immune tolerance leads to inflammation and placental rejection (2).
The complement cascade system was evolved to protect against pathogens through activation of the host immune system and direct damage of pathogen cells through production of the terminal membrane attack complex (MAC) (3,4). The MAC complex assembles within the cell membranes of targets resulting in cell death due to perforation of cell membranes. The classical complement pathway is mediated by the formation of antigen-antibody immune complexes which initiates a cascade of complement component degradation. In that pathway C4 is degraded into C4b which combines with C2a to create the C3 convertase, a critical component that promotes further activation to the terminal MAC complex in both the classical pathway, alternate pathway, and lectin pathway of complement activation. C4 is also degraded into C4d, which is covalently cross-linked to cells where this proximal complement activation occurs – making it a useful marker for local sites of complement initiation through various initiating pathways (5). Excess complement activation leads to human diseases like hemolytic uremic syndrome – a clinical imitator of PE and HELLP. Systemic complement activation has been identified in numerous states of pregnancy pathologies including spontaneous preterm birth, fetal growth restriction, antiphospholipid antibody syndrome, and systemically in PE (6-10). Systemic complement elevation has been previously demonstrated in PE utilizing plasma C3a levels (9). Other groups have identified local complement activation within the placenta using C4d staining in patients with systemic lupus erythematosus and antiphospholipid antibody syndrome, however, this study did not specifically look for complement staining as it is associated with PE (11). Using the same cohort of women with autoimmune disease combined with a group of women without autoimmune disease, critical genetic mutations in complement inhibitory factors were identified in association with preeclampsia, further supporting the importance of complement activity in the pathogenesis of preeclampsia (12).
The vascular pathogenesis of PE stems from impaired spiral artery remodeling causing prolonged hypoxia within the placenta, inducing HIF1α transcription factor and leading to increased anti-angiogenic factor expression (1). Soluble fms-like tyrosine kinase-1 (sFLT1) elevation correlates strongly with PE and HELLP syndrome. However, the pathogenic mechanism leading to multisystem tissue damage in PE with severe features is unclear (1). sFLT1 is a circulating anti-angiogenic protein that acts by blocking the receptor-binding domains of vascular endothelial factor (VEGF) and placental growth factor, leading to endothelial dysfunction (13). In the kidney and retina, VEGF signaling in endothelial cells upregulates complement inhibitory factor H (CFH) and protects against damage from spontaneous complement activation (14). We hypothesize that similar mechanisms for protection exist to protect the fetal-derived placenta from destruction by the maternal complement activation and that in patients with PE, the VEGF-inhibitory properties of increased sFLT1 are associated with increased complement activation at the maternal-fetal interface. In this study, we evaluated for placental complement activation using C4d and MAC in pregnancies complicated by PE and HELLP syndrom and MAC correlated those with sFLT1 expression.
Methods
Ethics statement
The study was approved by the Beth Israel Deaconess Medical Center (BIDMC) institutional review board (Protocol#2008P-000061), and subjects gave informed consent.
Human subjects and sample collection
In this case-control study, we identified placentas from a placenta biorepository collected from women delivering at BIDMC in Boston, MA from January 2008 to December 2015. Demographic and obstetric data were collected and reviewed in order to identify participants meeting clinical criteria for our three study groups. Patients with PE were defined by elevated blood pressure ≥140/90 and proteinuria ≥300mg on a 24-hour urine according to guidelines in the ACOG 2013 Hypertension Task Force (15). Participants in the group with PE were included if they had severe features or had no severe features. The PE group was further subdivided into PE without laboratory abnormalities and women with laboratory abnormalities consistent with HELLP syndrome (defined as PE with both doubling of AST and ALT IU/L above the upper bounds of normal in our lab (40 IU/L) and platelet count less than 100k/μL). Control pregnancies without PE were also identified from this cohort.
Placental samples were collected from healthy and patients with PE as described previously (16,17). Briefly, several villous biopsies (2 cm3) were excised from the maternal surface midway between the chorionic and basal plates, within 30 minutes of delivery, and the decidua layer was carefully removed. The villous tissue collected was cut into 0.5 cm3 and rinsed twice in 50 ml of ice-cold phosphate buffer saline for two minutes.
Immunofluorescence staining
Fresh placental tissues were frozen in isopentane cooled in liquid nitrogen. For immunofluorescence (IF) staining, the tissues were cryosectioned (4 μm-thick) and stained for sFLT1, C4d and MAC. Expression of sFLT1 protein in cryosections of placenta was evaluated using an anti-human FLT1 antibody that recognizes the N-terminal region of FLT1/sFLT1 (catalog no. AF321; 1:600 dilution, R & D Systems, Minneapolis, MN), an anti-human C4d monoclonal antibody Clone # 10–11 (catalog no. 2222–8004; 1:500 dilution; Bio-Rad, Hercules, CA) and anti-human MAC monoclonal mouse antibody clone# Ae11; (catalog number. M077701-5; 1:50 dilution Dako, Carpentaria, CA). Secondary antibodies were used for sFLT1 – VectaFluor R.T.U. DyLight® 594 Anti-Goat IgG staining kit (catalog no. DI-3794) and for MAC and C4d VectaFluor R.T.U. DyLight® 488 Anti-Mouse IgG catalog no. DI-2798; Vector Laboratories, Burlingame, CA).
Morphometric quantification by fluorescence microscopy
Measurements were generated from IF microscopy images with an original magnification of 40X. Representative digital images of placental villi were acquired; 3 random images for each of the placentas were included. For assessment of the three antibodies, IF morphometric activity measurements were performed using NIH Image J software. JPEG images were used to quantitate the fluorescence histochemical product and the mean fluorescence intensity (MFI) of the reaction products were calculated. The mean intensity was divided by the tissue area to calculate positivity per area.
Statistical analysis
Data are presented as mean (standard deviation) and median with interquartile range (IQR) for non-parametric data. Categorical data were compared using Chi-square or Fisher’s exact test as appropriate, and Wilcoxon-rank sum test was used to compare medians. Median MFI for each group was compared using a Spearman correlation coefficient. All tests were two-sided and p-values <0.05 denoted statistical significance. Data were analyzed and graphically displayed using Prism (version 8, GraphPad Software).
Results
Eighteen healthy control placentas and 25 placentas from participants with PE meeting the clinical criteria defined above were identified from the placenta repository. Seventy-two percent (n = 18) of the PE group had PE with severe features (primarily severe blood pressure elevation) as outlined by the ACOG 2013 Task Force guidelines (15) (data not shown). Of the group with PE, 6 participants (24%) had laboratory abnormalities consistent with HELLP syndrome, while the remaining 19 had PE without laboratory abnormalities. Baseline characteristics of the participants are represented in Table 1.
Table 1.
Baseline characteristics of study participants.
| Control | All preeclampsia | HELLP syndrome | |
|---|---|---|---|
| n | 18 | 25 | 6 |
| Maternal age (year) | 33 ± 5 | 31 ± 6 | 35 ± 4 |
| Race | |||
| White | 72 (13) | 76 (19) | 83 (5) |
| Black | 22 (4) | 16 (4) | 17 (1) |
| Other/Mixed | 6 (1) | 8 (2) | 0 |
| Ethnicity | |||
| Non-Hispanic | 67 (12) | 80 (20) | 83 (5) |
| Hispanic | 17 (3) | 4 (1) | 17 (1) |
| Unknown | 17 (3) | 16 (4) | 0 |
| Gestational age (weeks) | 36 ± 3 | 34 ± 4 | 32 ± 6 |
| Multifetal gestation | 6 (1) | 8 (2) | 0 |
| Vaginal delivery | 39 (7) | 20 (5) | 17 (1) |
| SGA infant | 27 (5) | 24 (6) | 33 (2) |
| Pre-pregnancy BMI kg/m^2 | 24 ± 5 | 28 ± 8 | 31 ± 10 |
| Nulliparous | 56 (10) | 68 (17) | 83 (5) |
| Medical condition | |||
| Chronic HTN | 22 (4) | 44 (11) | 33 (2) |
| Pre-gestational DM | 0 | 8 (2) | 0 |
| Renal disease | 0 | 8 (2) | 17 (1) |
Values expressed as mean ± standard deviation or%(n). BMI = body mass index; HELLP = Hemolysis, elevated liver enzymes, low platelet syndrome; HTN = hypertension; DM = diabetes mellitus; SGA = small for gestational age
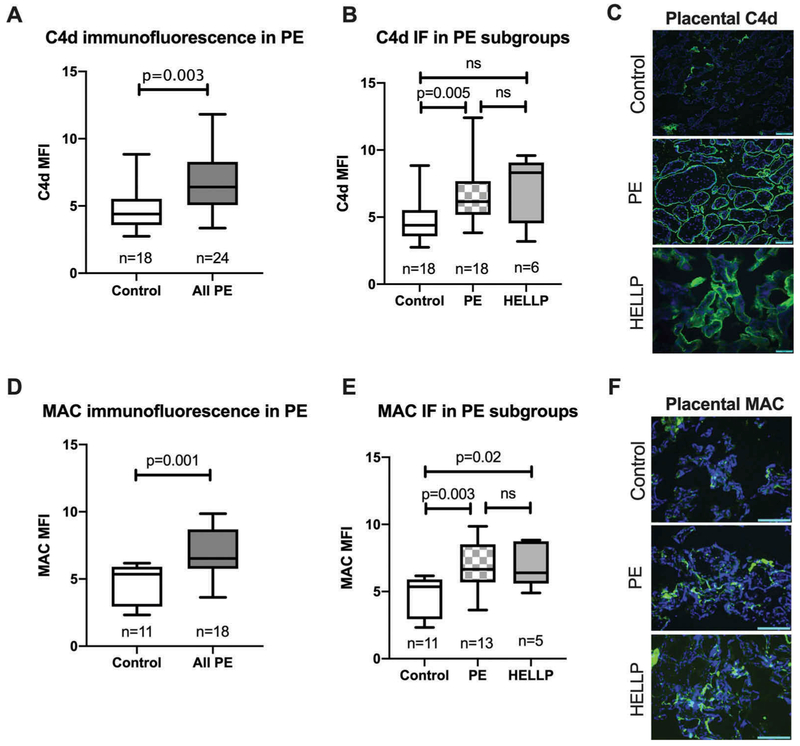
C4d complement staining and quantification by IF microscopy was first performed in the group of healthy control placentas compared to all patients with PE (representative images, Figure 1C). There was a significant difference in the median C4d MFI of the PE placentas (6.4; IQR of [5.1, 8.3]), compared to control placentas (4.4; IQR of [3.6, 5.5]), with a p-value of 0.003 (Figure 1A). Because we saw such a significant increase in complement activity in PE, we were curious to know whether the subgroup of PE with HELLP syndrome had a more profound complement activity by IF compared to PE without laboratory abnormalities. HELLP placentas tended to have a higher median C4d MFI (8.3, IQR [4.5, 9.1]) than PE (6.1, IQR [5.2, 7.7]). However, this did not reach statistical significance (Figure 1B).
Figure 1.
Placental complement activity by immunofluorescence studies in healthy pregnancies, preeclampsia, and HELLP syndrome. Comparison of placental complement C4d and membrane attack complex (MAC) mean fluorescence intensity (MFI) by immunofluorescence (IF) staining in control and all PE (including both PE without laboratory abnormalities and HELLP syndrome) displayed as box plots with median with 1st and 3rd interquartile range; whiskers at the 5th and 95th percentile (A, D). C4d and MAC MFI in control and subtypes of PE; PE without laboratory abnormalities and HELLP syndrome (B, E). Representative C4d (green) with DAPI nuclear staining (blue) in the placentas of control, PE without laboratory abnormalities, and HELLP syndrome imaged at 40X magnification (C, F). Scale bars in the figures represent 100 micrometers.
Because C4d is an early degradation product in the complement cascade, we wanted to know whether terminal MAC complexes could also be identified within the placental tissue as potential evidence for direct placental damage. Similar to C4d activity, MAC IF staining within the placentas was also significantly elevated in PE compared to healthy controls (Figure 1D, F). When comparing the subgroup of HELLP syndrome placentas, there was a significant increase in MAC complex activity compared to controls. However, no significant difference was found when comparing to PE without laboratory abnormalities (Figure 1E).
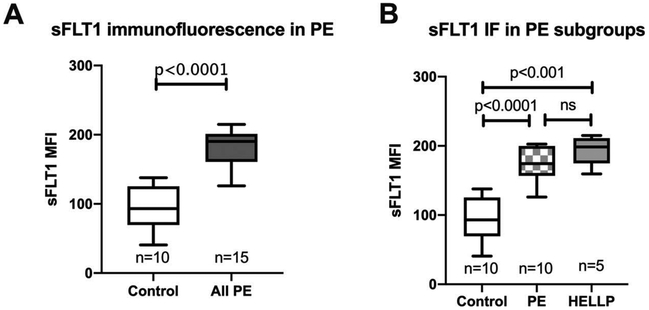
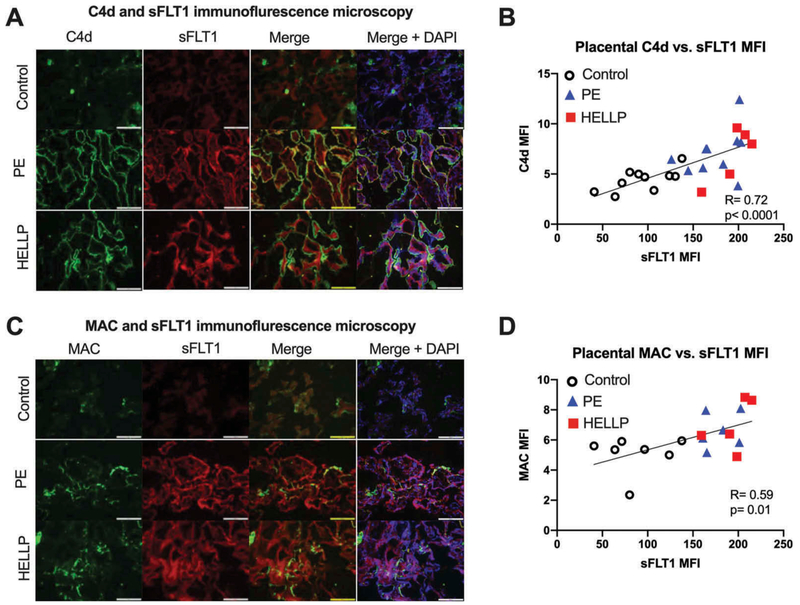
We have previously demonstrated increases in placental sFLT1 in preeclampsia and HELLP syndrome (13,18,19). We wanted to evaluate whether placental sFLT1 expression correlated with complement activation levels in the placentas of PE and healthy pregnancies. sFLT1 IF staining in the group of PE placentas was significantly elevated compared to healthy controls (Figure 2A). Similar to the pattern for C4d and MAC staining, there was significantly increased sFLT1 levels detected in HELLP syndrome compared to both healthy pregnancy but not when compared to the subgroup of PE without laboratory abnormalities (Figure 2B). The association between placental C4d and sFLTl in our entire cohort of placentas was highly correlated with an R = 0.72 (Figure 3B) with HELLP syndrome (red squares) tending to have both a higher sFLT1 and C4d expression than PE without laboratory abnormalities (blue triangles). Placental MAC and sFLT1 levels were also strongly associated with a correlation factor of R = 0.59 (Figure 3D). Also, placental sFLT1 signaling was also spatially correlated in close proximity to syncytiotrophoblast membrane C4d and MAC signal on IF (Figure 3A, C).
Figure 2.
Placental sFLT1 activity by immunofluorescence staining. Comparison of placental sFLT1 mean fluorescence intensity (MFI) by immunofluorescence (IF) staining in control and all PE (including both PE without laboratory abnormalities and HELLP syndrome) displayed as box plots with median with 1st and 3rd interquartile range; whiskers at the 5th and 95th percentile (A). sFLT1 MFI in control and subtypes of PE; PE without laboratory abnormalities and HELLP syndrome (B).
Figure 3.
Immunofluorescence of sFLT1 co-localization and correlation with complement markers in the placenta. Immunofluorescence microscopy for complement C4d (green; first column) in panel A and MAC (green; first column) in panel C, along with sFLT1 (red; second column), the merged image with areas of co-localization (yellow; third column), and merged with nuclear DAPI staining (blue; fourth column) for visualization of trophoblastic architecture. Images obtained at 40X magnification. Scatterplot of placental C4d MFI and sFLT1 MFI with controls (open circles), PE without laboratory abnormalities (blue triangles), and HELLP syndrome (red squares) all data on the same plot with the line of best fit for correlation displayed (B). Similar scatterplot of placental MAC MFI and sFLT1 MFI with the line of best fit for correlation (D)m. Scale bars represent 100 micrometers.
Discussion
Maintenance of the uteroplacental interface is crucial for placental and pregnancy health. The finding of enhanced complement activity in pregnancies affected by PE in our study is consistent with prior studies examining systemic peripheral blood complement activation. We demonstrate complement activity at the site of the maternal-fetal interface, within the placenta, is strongly associated with PE and HELLP syndrome. PE was associated with elevated complement degradation products as well as terminal MAC complex activity within the placenta tissue, supporting the role of complement in direct placental damage. Based on prior studies in kidney and retina, increases in sFLT1 may result in complement damage in the placenta of women with preeclampsia through VEGF inhibition (14). Future studies should be directed at the characterization of VEGF signaling and CFH in association with complement activation and placental ischemia.
For immunofluorescence detection of sFLT1 activity in the placenta, we utilized an antibody that recognizes both membrane bound soluble FLT1 splice variants in the placenta, thus we are unable to determine the differential contribution of soluble versus membrane-bound FLT1 protein in our study. We anticipate that the signal in our cohort is predominantly from soluble FLT1 based on prior studies which evaluated the relative transcripts of FLT1 splice variants by tissue type and demonstrated that sFLTl comprised >90% of the placental FLT1 transcripts (20,21). Follow-up studies using in situ hybridization are needed to confirm whether the soluble form of the FLT1 gene product is responsible for the signal seen in the placenta.
We demonstrated a strong correlation between placental complement and placental sFLT1 activity, however, as we are sampling at a single time point, we are unable to determine whether sFLT1 aberrations precede complement activation or rather, complement activation precedes sFLT1 activation. Prior studies in humans and animal models suggest that sFLT1 enables complement activation (8), but increased complement activation may also be a trigger for sFLT1 production (22). Placenta complement activation in the placenta preceded sFLT1 production in decidua monocytes in a mouse model of immunologic pregnancy rejection (22). Excessive complement activity in human syncytiotrophoblasts cultured in vitro resulted in increased sFLT1 transcripts and protein (23). In addition, increased peripheral blood complement activity detected early in pregnancy (12–14 weeks gestation) is associated with subsequent adverse pregnancy outcomes in patients with systemic lupus erythematosus and antiphospholipid antibody syndrome (2). These studies in combination with our data suggest that early complement activation in the placenta may provoke local sFLT1 production and promote adverse pregnancy outcomes such as preeclampsia.
HELLP syndrome was not clearly defined by an increase in complement activity over PE without laboratory abnormalities. However, we are limited by low sample size for the HELLP syndrome group. Further collection and analysis of placental complement activity in HELLP syndrome is ongoing as this may be a fundamental biological mechanism differentiating the pathophysiology of PE from HELLP syndrome. Eculizumab, a monoclonal antibody against complement, has recently been FDA-approved for use in paroxysmal nocturnal hemoglobinuria, has been suggested as a therapy for early-onset PE with laboratory abnormalities with limited data to guide patient selection (24). We plan future studies selecting principal subtypes of PE (e.g. PE characterized by fetal growth restriction or primarily renal injury) in order to evaluate which subtypes are most associated with excessive complement activation, and potentially amenable to complementblocking therapy to prolong pregnancy and improve maternal and neonatal outcomes.
Acknowledgments
We thank Ms. Lena Ellezian for cryosectioning the placental tissue samples.
Funding
This study was supported by funds from the BIDMC OB/GYN foundation. A. Y. C. was supported by the Reproductive Scientist Development Program (K12HD000849) by the Eunice Kennedy Shriver National Institute of Child Health & Human Development and Burroughs Wellcome Fund, as part of the Reproductive Scientist Development Program.
Footnotes
Disclosure statement
S. A. K. is co-listed as a coinventor on patents related to preeclampsia biomarkers and therapies that are held at Beth Israel Deaconess Medical Center. S.A.K. has a financial interest in Aggamin, LLC and also reports serving as a consultant to Thermo Fisher Scientific and Roche. S.A.K. has received research funding from Siemens. Z. Z. reports financial interests in Radikal Therapeutics. The remaining authors do not have any conflicts to disclose.
References
- [1].Rana S, Lemoine E, Granger J, et al. Preeclampsia. Circ Res. 2019;124(7):1094–1112. [DOI] [PubMed] [Google Scholar]
- [2].Kim MY, Guerra MM, Kaplowitz E, et al. Complement activation predicts adverse pregnancy outcome in patients with systemic lupus erythematosus and/or antiphospholipid antibodies. Ann Rheum Dis. 2018;77 (4):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344(14):1058–1066. [DOI] [PubMed] [Google Scholar]
- [4].Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344(15):1140–1144. [DOI] [PubMed] [Google Scholar]
- [5].Cohen D, Colvin RB, Daha MR, et al. Pros and cons for C4d as a biomarker. Kidney Int. 2012;81(7):628–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Girardi G, Bulla R, Salmon JE, et al. The complement system in the pathophysiology of pregnancy. Mol Immunol. 2006; 43(1–2): 68–77. [DOI] [PubMed] [Google Scholar]
- [7].Lee J, Romero R, Xu Y, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011. February 04;6(2):e16806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Penning M, Chua JS, van Kooten C, et al. Classical complement pathway activation in the kidneys of women with preeclampsia. Hypertension. 2015;66 (1):117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].He Y, Xu B, Song D, et al. Expression of the complement system’s activation factors in plasma of patients with early/late-onset severe pre-eclampsia. Am J Reprod Immunol. 2016. September;76(3):205–211. [DOI] [PubMed] [Google Scholar]
- [10].Song D, Yu XJ, Wang FM, et al. Overactivation of complement alternative pathway in postpartum atypical hemolytic uremic syndrome patients with renal involvement. Am J Reprod Immunol. 2015;74(4):345–356. [DOI] [PubMed] [Google Scholar]
- [11].Salmon JE, Heuser C, Triebwasser M, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011;8(3):e1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shamonki JM, Salmon JE, Hyjek E, et al. Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. Am J Obstet Gynecol. 2007. February;196(2):167e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. [DOI] [PubMed] [Google Scholar]
- [14].Keir LS, Firth R, Aponik L, et al. VEGF regulates local inhibitory complement proteins in the eye and kidney. J Clin Invest. 2017;127(1):199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].American College of, Obstetricians, Gynecologists and Task Force on Hypertension in, Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
- [16].Rajakumar A, Cerdeira AS, Rana S, et al. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension. 2012;59(2):256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang A, Zsengeller ZK, Hecht JL, et al. Excess placental secreted frizzled-related protein 1 in maternal smokers impairs fetal growth. J Clin Invest. 2015;125 (11):4021–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111 (5):649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–649. [DOI] [PubMed] [Google Scholar]
- [20].Jebbink J, Keijser R, Veenboer G, et al. Expression of Placental FLT1 Transcript Variants Relates to. Hypertension. 2011;70–76. [DOI] [PubMed] [Google Scholar]
- [21].Ashar-Patel A, Kaymaz Y, Rajukumar A, et al. FLT1 and transcriptome-wide polyadenylation site (PAS) analysis in preeclampsia. Sci Rep. 2017;7(1):12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Girardi G, Yarlin D, Thurman JM, et al. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203(9):2165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Banadakoppa M, Balakrishanan M, Yallampalli C. Upregulation and release of soluble fms-like tyrosine kinase receptor 1 mediated by complement activation in human syncytiotrophoblast cells. Am J Reprod Immunol. 2018;80(5):e13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta. 2013;34(2):201–203. [DOI] [PubMed] [Google Scholar]