Abstract
Total joint arthroplasty (TJA) of the hip or knee (THA, TKA) has become an increasingly common procedure. While TJA is a successful treatment for individuals experiencing degenerative joint diseases, it is well known that one of the most common perioperative complications of TJA is deep venous thrombosis (DVT). To profile tissue factor (TF), microparticle-tissue factor (MP-TF), thrombin-activatable fibrinolysis inhibitor (TAFI), and fibrinogen levels in patients undergoing TJA to determine potential preexisting Hemostatic dysregulation. De-identified blood samples were obtained from patients undergoing TJA 1 day pre- and 1 day postprocedure. Plasma samples were analyzed using enzyme-linked immunosorbent assay kits for fibrinogen, TAFI, TF, and MP-TF; fibrinogen levels were also assessed using a clot-based activity assay. In comparison with healthy controls, there were significant increases of fibrinogen and MP-TF levels, while there were significant decreases in TF and TAFI levels in the preoperative and postoperative patients. Comparing the pre versus postoperative patients, no significant differences were found; interestingly, however, surgical intervention exacerbated the changes found in the preoperative samples compared to the controls. The results of this study confirm that patients undergoing TJA have preexisting alterations in the fibrinolytic system. Surgical intervention tended to exacerbate these changes. The alterations observed in this study may provide insight as to why TJA is associated with higher rates of DVT and thromboembolism.
Keywords: arthroplasty, inflammation, coagulation, fibrinolysis
Introduction
Degenerative joint disease (DJD) is one of the most common medical ailments worldwide. The prevalence of DJD, particularly of the knee, has been estimated to be between 19.2% and 27.8% in individuals ≥45 years and increases to 37% in individuals >60 years.1 Once standard medical therapy (nonsteroidal anti-inflammatory drug use, intra-articular corticosteroid injection) is no longer effective in the symptomatic management of DJD, surgical intervention— total joint arthroplasty (TJA)— is commonly performed. With an aging population, DJD is becoming increasingly prevalent, thus leading to more TJA procedures performed. It is predicted that by 2030, there will be over 4 million TJA procedures performed.2 While there is a high success rate of TJA in the symptomatic treatment of DJD, this surgical intervention does not come without risk. It has been observed that orthopedic surgeries, in particular TJA, are associated with higher rates of postoperative venous throm-boembolism (VTE) such as deep venous thrombosis (DVT) and pulmonary embolism as well as bleeding events.3,4 It is well known that orthopedic surgeries have higher rates of VTE than procedures performed for other surgical procedures such as in obstetrics and gynecology, general surgery, and cardiothoracic procedures. It is not well understood as to why these postoperative coagulopathies occur at a higher incidence in TJA. Some have hypothesized that these patients have intrinsic, preexisting alterations in their coagulation profiles. Several fibrinolytic biomarkers have previously been identified as being altered in patients undergoing TJA.5
The current postoperative standard of care differs between institutions and may include ASA administration only or incorporate the use of various anticoagulant therapies such as tranexamic acid (TXA) or heparins. However, it is the accepted standard of practice that postoperative prophylactic anticoagulant therapy be continued for at least 10 days following surgery.4,6–8 The aim of this study was to profile several components of hemostasis that may also influence the inflammatory process of DJD. This study examined tissue factor (TF), microparticle-tissue factor (MP-TF), fibrinogen, and thrombin-activatable fibrinolysis inhibitor (TAFI).
Tissue factor initiates the extrinsic coagulation cascade leading to the activation of the common coagulation pathway and conversion of fibrinogen to fibrin. Upregulation of TF synthesis has been observed to occur in response to inflammation, thus potentially creating a hypercoagulable state.9 Both TF and fibrin have been identified on synovial tissue; fibrin deposition at the synovium has been demonstrated to potentiate local inflammation.10
Microparticles are vesicular structures derived from platelets, monocytes, endothelial cells, smooth muscle cells, and tumor cells. Only a small fraction of microparticles in the blood express TF.11 The MP-TF, acting just as TF, can initiate blood coagulation.12
Fibrinogen is one of the major components of the coagulation cascade and is also observed to be an acute phase reactant. Fibrinogen is acted upon by thrombin via proteolytic cleavage to yield insoluble fibrin, which is the ultimate product of the coagulation cascade. Activated fibrin binds to platelets, forming a platelet–fibrin meshwork clot.13,14
While thrombin is mainly known to activate fibrinogen to fibrin, it also concomitantly activates TAFI. The main function of TAFI is to stabilize the fibrin–platelet clot by inhibiting clot lysis.15 Thrombin-activatable fibrinolysis inhibitor circulates in plasma as a plasminogen-bound zymogen that is activated by thrombin. Once activated, TAFI inhibits the activation of fibrinolytic activity by the removal of fibrin C-terminal residues via carboxypeptidase activity.16,17 Not only does TAFI inhibit fibrinolysis, but it may also reduce the inflammation by inactivating kinins and anaphylatoxins,18 a mechanism that may relate to the pathophysiology of DJD. This study is based on previous evidence of hemostatic dysregulation in TJA patients and it is the primary hypothesis of this study that the biomarkers investigated here will show altered levels in these TJA patients when compared to healthy controls. Furthermore, this study represents the first assessment of these biomarkers in TJA patients and therefore it represents an exploratory investigation and does not include a prospective statistical power— and sample size assessment.
Patients and Methods
This study was designed to compare the circulating levels of various plasma biomarkers of hemostatic dysregulation in DJD patients in comparison to normal healthy controls. Deidentified blood samples were obtained from 45 patients undergoing TKA (n = 27) or THA (n = 18) day 1 preoperatively and day 1 postoperatively. The patient ages ranged from 48 to 85 years, with a mean age of 64.9 (10.5) years. Patient blood samples were collected into tubes containing 3.2% sodium citrate, and plasma was then isolated. Patient and control plasma samples were stored at −80°C. In order to reduce repeated freeze–thaw cycles, patient plasma samples were aliquoted into separate tubes. Citrated plasma samples from healthy individuals (n = 8, ages 18–35, nonsmokers) were obtained from George King Biomedical, Inc (Overland Park, Kansas) to serve as controls. Plasma samples were analyzed via ELISA kits for TF, MP-TF, fibrinogen, and TAFI levels (Hyphen Biomed, Neuville-sur-Oise, France). Fibrinogen clot–based activity was analyzed on the ACL300plus (Dade Innovin reagents, Erlangen, Germany). Based on the available information, the chart review indicated that these patients were asked not to take any anticoagulant or antiplatelet medications for 1 week prior to surgery. This study was approved by the institutional review board of Loyola University Chicago Health Sciences Division. Patients undergoing TJA revision were excluded from this study.
Statistical Analysis
Data were analyzed using the Microsoft Excel and in Graphpad Prism Software version 6. The results were expressed as mean (standard deviation). Comparisons between groups were evaluated using the nonparametric Mann-Whitney U test used for nonnormally distributed quantitative data. Correlation analysis was carried out using the nonparametric Spearman test. P values less than .05 were considered statistically significant.
Results
Measured values for the control, preoperative, and postoperative samples for fibrinogen activity and antigen, TF, MP-TF, and TAFI as well as the percentage changes for each biomarker are presented in Table 1. Figures 1–4 show the box plots for each of the investigated thrombotic and fibrinolytic biomarkers, respectively.
Table 1.
Control, Preoperative, and Postoperative Values and Percentage Change for the Measured Thrombotic Biomarkers, Tissue Factor (TF), Microparticles-Tissue Factor (MP-TF), Fibrinogen Activity and Antigen, and Thrombin-Activatable Fibrinolysis Inhibitor (TAFI).
| Control (C) | Preoperative | Postoperative | Preoperative Versus Postoperative | |
|---|---|---|---|---|
| TF (pg/mL) | 1096.0 (184.9) | 29.47 (38.6) | 22.4 (26.82) | −19.07 (38.14) |
| MP-TF (pg/mL) | 0.32 (0.20) | 0.48 (0.22) | 0.64 (0.57) | 47.79 (146.4) |
| Fibrinogen antigen (mg/dl) | 367.7 (52.18) | 424.0 (174.3) | 401.9 (187.6) | −4.41 (30.42) |
| Fibrinogen activity (mg/dl) | 262.6 (27.37) | 345.7 (98.25) | 391.2 (147.7) | 21.87 (59.31) |
| TAFI (%) | 172.0 (22.59) | 135.7 (28.85) | 137.5 (28.44) | 3.11 (18.66) |
Figure 1.
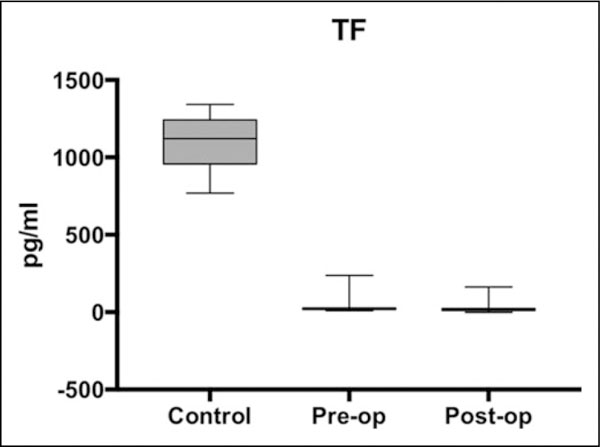
Box plot showing median levels of tissue factor (TF) measured in control, preoperative, and postoperative groups. Boxes show interquartile ranges and I bars demonstrate highest and lowest values.
Figure 4.

Box plot showing median levels of fibrinogen antigen (gray) and activity (white) measured in control, preoperative, and post-operative groups. Boxes show interquartile ranges and I bars demonstrate highest and lowest values.
There were significant differences in TF and MP-TF levels between the control samples and both the preoperative and postoperative samples; however, the changes were opposite of one another. TF levels were lower in both the preoperative (P < .0001) and the postoperative (P < .0001) samples compared to controls. Tissue factor levels were the same in pre-operative and postoperative samples (P = .1208). The levels of MP-TF exhibited the opposite change. Compared to the controls, MP-TF levels were increased in the preoperative (P =.0400) and in the postoperative (P = .0160) patients. While levels in the postoperative samples were marginally elevated compared to the preoperative samples, the difference was not statistically significant (P = .3822).
There were significant increases in both the fibrinogen antigen (control vs pre, P = .0016; control vs post, P = .0253) and fibrinogen activity (control vs pre, P = .0054; control vs post, P = .0001) in patients compared to controls. However, there was not a significant difference between the preoperative and postoperative samples (antigen, P = .5948; activity, P = .2957).
TAFI levels were significantly lower in both the preoperative (P = .0015) and postoperative (P = .0037) samples compared to the controls; no difference in mean TAFI levels between the preoperative and postoperative samples (P = .7415) was observed.
The impact of clinical parameters, such as type of surgery, diabetes, drug use, gender, and so on, on the levels of thrombotic and fibrinolytic biomarkers was assessed. The only significant correlation observed was between body mass index (BMI) and preoperative levels of fibrinogen activity (P = .0237, Spearman r = .3446) shown in Figure 5.
Figure 5.

Scatter plot demonstrating the positive correlation as measured by the Spearman correlation coefficient (r = .346, P = .0237).
The results of this study coincide with the results of previous studies performed in this laboratory— the previous studies demonstrated that TJA patients have preexisting alterations in the fibrinolytic cascade, which may preclude them to VTE or bleeding events. Those results are consistent with the findings of this study.5,19 Of the 45 TJA patients who participated in this study, 23 were anticoagulated with TXA perioperatively; there was no difference in the measured values for the studied biomarkers.
Discussion
Previous studies examining the aspects of coagulation and fibrinolysis in patients undergoing TJA have found that there is a tendency for these patients to show alterations of the coagulation and fibrinolytic biomarkers.19,20 One such study reported that patients undergoing TJA had a preexisting, profi-brinolytic profile as demonstrated by alterations in D-dimer and antiplasmin.19 The results of that study are consistent with the findings in this study. Although there is an increased risk of thrombus formation as demonstrated by the elevated fibrinogen and MP-TF, the stability of the clots may be diminished, due to decreased TAFI and TF levels. The results from this study as well as previous studies demonstrate that patients undergoing TJA may inherently have an elevated risk of perioperative thrombus formation in addition to a bleeding diathesis due to diminished clot stability.
This study examined both the antigenic and activity levels of fibrinogen. While there were increases of both antigen and activity levels in the patients undergoing TJA, there was not a difference in the activity: antigen ratio in the controls (1.38 [0.19]), compared to preoperative (1.27 [17.52], P = .65) and postoperative (1.24 [0.42], P = .21), nor when comparing preoperative versus postoperative levels (P = .22). This means that there is not a qualitative change in the fibrinogen synthesized in the patients undergoing TJA.
Interestingly, while TF was greatly decreased in the patients undergoing TJA, MP-TF demonstrated a mild, yet significant, increase. It was hypothesized that TF levels should be elevated in these patients because it is associated with coagulation, angiogenesis, and inflammation.5 This observation may be explained by the fact that the deterioration seen in osteoarthritis is so progressive that, rather than the larger TF molecule being exposed in blood, the MP-TF is elevated due to micro-insults to the synovium that accrue over time. An alternate hypothesis could be that TF is consumed during thrombogenesis, therefore providing falsely decreased measured values. Additionally, the observation that TF levels were lower in patients undergoing TJA than in the controls may be explained by increases in tissue factor pathway inhibitor (TFPI) brought about by upregulation of the inflammatory process.5 While Basavaraj et al showed that TF is regulated by TFPI on monocytes, it is possible that a similar process may also occur on endothelial cells or synovium in DJD as well as during other inflammatory states. It is also possible that TFPI, while holding a regulatory role in the TF coagulation pathway, may not interact with TF when it is embedded in the membrane of microparticles. Since there are several factors that contribute to the balance between TF and MP-TF levels, it is difficult to project the differential role of these biomarkers in the overall pathogenesis of DJD. Generated TF has a relatively short half-life and readily complexes with factor VII and other cell surfaces, therefore the observed lower level of measurable TF reflects its consumption in the mediation of thrombogenesis or hemostatic process.
While the main function of TAFI is to inhibit fibrinolysis via thrombus stabilization, it has also been implicated in reducing inflammation.18 In this study, it was shown that patients under-going TJA possess decreased levels of TAFI, which predisposes these patients to increased fibrinolysis and inflammation. Since there is decreased ability to stabilize the thrombi, this alteration may provide insight as to why there are higher rates of thromboembolism and perioperative blood loss.
The biomarkers of interest in this study were compared not only against each other but also with the clinical information of these patients. No correlations were identified with this information except for the positive correlation between fibrinogen activity and BMI. This finding is consistent with previous work which demonstrated that body fat was directly correlated with fibrinogen levels.21 Furthermore, it would make sense that these patients, many of whom have elevated BMI (33.8 [8.2]), would have an elevated fibrinogen level.
While the patients undergoing TJA are treated with multiple medications, in this study, the anticoagulant and antiplatelet drugs were stopped 1 week prior to the surgery. The possibility of the use of other pharmacologic agents, such as antihypertensives, diabetic medications, and antibiotics, along with other agents that are widely used cannot be ruled out. It is unlikely that such nonanticoagulant drugs taken by the patients who participated in this study would have any impact on the biomarker levels studied.
Conclusion
DJD is a disorder affecting millions of individuals worldwide. Medical treatment remains the first line management; however, many patients ultimately resort to surgical intervention in the form of TJA. Although highly successful in permitting patients to regain function coupled with reduction or elimination of pain, there is an inherent risk of perioperative coagulopathy. While orthopedic surgeries, TJA in particular, are associated with DVT and thromboembolism, it may be that there is a continuous thrombosis–fibrinolysis cycle that causes thrombus formation and adhesion followed by embolization. This study’s relevance lies in the potential to identify preexisting alterations in the hemostatic profiles of patients undergoing either TKA or THA and work toward normalizing coagulopathies in at-risk patients to reduce their risk of perioperative complications.
Limitations
One of the major limitations of this study was the lack of age-matched controls for comparison. While these control samples ranged from 18 to 35 years and our patient population had a mean age of 64.9 years, this should not discount the findings that patients undergoing TJA have preexisting dysregulation of the coagulation and fibrinolytic systems. While previous studies found that TF, fibrinogen, and TAFI have been positively correlated with age, this should not minimize the findings observed in this study either22–24; particularly since these findings were observed in females opposed to both males and females in general. Future studies involving age-matched controls may build on the findings presented here. Additionally, it may be of interest as to the information regarding postoperative DVT or VTE development. Unfortunately, this information was unavailable to be examined during this study.
Figure 2.

Box plot showing median levels of microparticles-tissue factor (MP-TF) measured in control, preoperative, and postoperative groups. Boxes show interquartile ranges and I bars demonstrate highest and lowest values.
Figure 3.
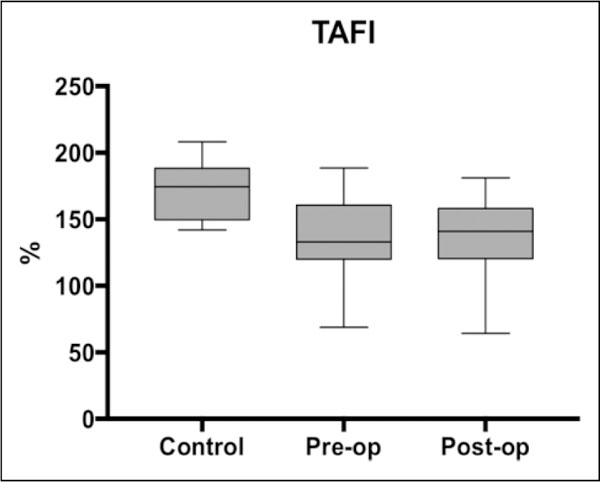
Box plot showing median levels of thrombin-activatable fibrinolysis inhibitor (TAFI) measured in control, preoperative, and postoperative groups. Boxes show interquartile ranges and 1 bars demonstrate highest and lowest values.
Acknowledgments
The authors gratefully acknowledge the technical support from the staff of the hemostasis and thrombosis laboratories for facilitating the logistics of the blood collection, storage, and the analysis of the samples included in this study. This research was a part of Students Training in Approaches to Research (STAR) program for medical students. We are thankful to Dr Gail Hecht and Dean Brubaker for their support and encouragement during this study. We are also thankful to Dr Wojick for providing support and resources to conduct this study.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4): 780–785. [DOI] [PubMed] [Google Scholar]
- 3.Fujita Y, Nakatsuka H, Namba Y, et al. The incidence of pulmonary embolism and deep vein thrombosis and their predictive risk factors after lower extremity arthroplasty: a retrospective analysis based on diagnosis using multidetector CT. J Anesth. 2015;29(2): 235–241. [DOI] [PubMed] [Google Scholar]
- 4.Sun Q, Yu X, Nie X, Gong J, Cai M. The efficacy comparison of tranexamic acid for reducing blood loss in total knee arthroplasty at different dosage time. J Arthroplasty. 2017;32(1): 33–36. [DOI] [PubMed] [Google Scholar]
- 5.Guler N, Burleson A, Syed D, et al. Fibrinolytic dysregulation in total joint arthroplasty patients: potential clinical implications. Clin Appl Thromb Hemost. 2016;22(4):372–376. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765–2775. [DOI] [PubMed] [Google Scholar]
- 7.Nurmohamed MT, Rosendaal FR, Buller HR, et al. Low-molecular-weight heparin versus standard heparin in general and orthopaedic surgery: a meta-analysis. Lancet. 1992;340(8812):152–156. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DR, Dunbar MJ, Bohm ER, et al. Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med. 2013;158(11):800–806. [DOI] [PubMed] [Google Scholar]
- 9.Camerer E, Kolstø AB, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation. Thromb Res. 1996;81(1): 1–41. [DOI] [PubMed] [Google Scholar]
- 10.Busso N, Morard C, Salvi R, Péclat V, So A. Role of the tissue factor pathway in synovial inflammation. Arthritis Rheum. 2003; 48(3):651–659. [DOI] [PubMed] [Google Scholar]
- 11.Zwicker JI, Trenor CC, Furie BC, Furie B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler Thromb Vasc Biol. 2011;31(4):728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel O, Toti F, Hugel B, et al. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26(12):2594–2604. [DOI] [PubMed] [Google Scholar]
- 13.Lowe GD, Rumley A, Mackie IJ. Plasma fibrinogen. Ann Clin Biochem. 2004;41(pt 6):430–440. [DOI] [PubMed] [Google Scholar]
- 14.Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. 2005;70: 247–299. [DOI] [PubMed] [Google Scholar]
- 15.Bouma BN, Mosnier LO. Thrombin activatable fibrinolysis inhibitor (TAFI)—how does thrombin regulate fibrinolysis? Ann Med. 2006;38(6):378–388. [DOI] [PubMed] [Google Scholar]
- 16.Boffa MB, Reid TS, Joo E, Nesheim ME, Koschinsky ML. Characterization of the gene encoding human TAFI (thrombin-activable fibrinolysis inhibitor; plasma procarboxypeptidase B). Biochemistry. 1999;38(20):6547–6558. [DOI] [PubMed] [Google Scholar]
- 17.Zhao L, Morser J, Bajzar L, Nesheim M, Nagashima M. Identification and characterization of two thrombin-activatable fibrinolysis inhibitor isoforms. Thromb Haemost. 1998;80(6): 949–955. [PubMed] [Google Scholar]
- 18.Craven S, Dewar L, Yang X, Ginsberg J, Ofosu F. Altered regulation of in-vivo coagulation in orthopedic patients prior to knee or hip replacement surgery. Blood Coagul Fibrinolysis. 2007; 18(3):219–225. [DOI] [PubMed] [Google Scholar]
- 19.Myles T, Nishimura T, Yun TH, et al. Thrombin activatable fibrinolysis inhibitor, a potential regulator of vascular inflammation. J Biol Chem. 2003;278(51):51059–51067. [DOI] [PubMed] [Google Scholar]
- 20.Basavaraj MG, Gruber FX, Sovershaev M, et al. The role of TFPI in regulation of TF-induced thrombogenicity on the surface of human monocytes. Thromb Res. 2010;126(5):418–425. [DOI] [PubMed] [Google Scholar]
- 21.Bo M, Raspo S, Morra F, Cassader M, Isaia G, Poli L. Body fat is the main predictor of fibrinogen levels in healthy non-obese men. Metabolism. 2004;53(8):984–988. [DOI] [PubMed] [Google Scholar]
- 22.Ariens RA, Coppola R, Potenza I, Mannucci PM. The increase with age of the components of the tissue factor coagulation pathway is gender-dependent. Blood Coagul Fibrinolysis. 1995; 6(5):433–437. [DOI] [PubMed] [Google Scholar]
- 23.Fu A, Nair KS. Age effect on fibrinogen and albumin synthesis in humans. Am J Physiol. 1998;275(6 pt 1):E1023-E1030. [DOI] [PubMed] [Google Scholar]
- 24.Juhan-Vague I, Renucci JF, Grimaux M, et al. Thrombin-activatable fibrinolysis inhibitor antigen levels and cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2000;20(9): 2156–2161. [DOI] [PubMed] [Google Scholar]


