Summary:
Biopharmaceuticals have become increasingly attractive therapeutic agents and are often PEGylated to enhance their pharmacokinetics and reduce their immunogenicity. However, recent human clinical trials have demonstrated that administration of PEGylated compounds can evoke anti-PEG antibodies. Considering the ubiquity of PEG in commercial products and the presence of pre-existing anti-PEG antibodies in patients in large clinical trials evaluating a PEG-modified aptamer, we investigated how anti-PEG antibodies effect the therapeutic activities of PEGylated RNA aptamers. We demonstrate that anti-PEG antibodies can directly bind to and inhibit anticoagulant aptamer function in vitro and in vivo. Moreover, in parallel studies we detected the presence of anti-PEG antibodies in nonhuman primates after a single administration of a PEGylated aptamer. Our results suggest that anti-PEG antibodies can limit the activity of PEGylated drugs and potentially compromise the activity of otherwise effective therapeutic agents.
Keywords: Aptamer, PEG (polyethylene glycol), PEGylation, hypersensitivity, anti-PEG antibodies, REGULATE-PCI, RB006, ELISA (enzyme-linked immunosorbent assay), aPTT (activated partial thromboplastin time), rhesus monkeys
Graphical Abstract
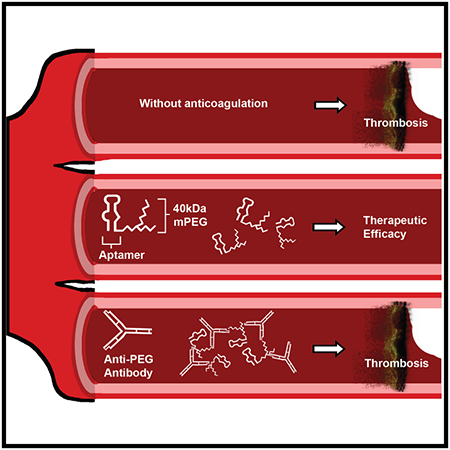
eTOC
The most common approach for pharmacokinetic enhancement of biologically inspired therapies is PEGylation; however, possible limitations of this formulation strategy have arisen. Here, we describe how anti-PEG antibodies can inhibit therapeutically efficacy of a PEGylated RNA aptamer. These findings further highlight emerging issues between the immune system and PEGylated therapeutics.
Introduction
Macromolecular drugs can offer substantial advantages over small molecular therapies due to their pronounced specificity and prolonged potency. Though these novel agents are promising; however, their clinical utility is often limited by their short circulating half-life. Of the nearly 900 bioactive compounds in the drug development pipeline (Mitragotri et al., 2014) many require conjugation to carrier molecules to overcome this shortcoming. One of the most common formulation strategies employed to date, utilizes polytheylene glycol (PEG) in a process known as PEGylation (Harris and Chess, 2003). Since its discovery in 1972, PEGylation is a common and successful approach for pharmacokinetic (PK) enhancement. Sixteen PEGylated therapies have been approved by the Food and Drug Administration (FDA), with 75 active trials with PEG-containing interventions underway (McSweeney et al., 2018; Swierczewska et al., 2015; Veronese and Pasut, 2005). These drugs have been in clinical use for nearly three decades starting with the first PEGylated protein therapy, Adagen®, which is used to treat severe combined immunodeficiency syndrome (Hershfield et al., 1987). PEG immunogenicity was first reported in 1983 by Richter and Ackerblom where the administration of a PEGylated protein produced anti-PEG antibodies in rabbits (Richter and Akerblom, 1983). However, we are just now beginning to understand the impact PEG may have on the immune system and more specifically how anti-PEG antibodies may impact the efficacy of PEGylated therapies.
While proteins are the most common agents modified with PEG, PEGylation has also been used for oligonucleotide therapies. A PEGylated aptamer, Macugen, has received FDA approval (Querques et al., 2009), and PEGylation was used to functionalize pegnivacogen (or RB006), a novel fast acting and rapidly reversible anticoagulant RNA aptamer targeting coagulation Factor IXa (FIXa) (Dyke et al., 2006) originally generated in our laboratory (Rusconi et al., 2002). After promising results in early clinical trials (Aberle et al., 2010; Chan et al., 2008; Dyke et al., 2006; Povsic et al., 2011), pegnivacogen was evaluated in large Phase 2b RADAR and Phase 3 REGULATE - PCI clinical trials (Cohen et al., 2010; Lincoff et al., 2016; Povsic et al., 2013). Unexpectedly, both of these large clinical studies had to be prematurely terminated due to serious adverse events (SAEs) that occurred in 0.6% of patients within minutes of the first exposure to pegnivacogen. Blinded analysis of pre-treatment samples from approximately 350 RADAR patients identified high levels of pre-existing IgG anti-PEG antibodies in those who experienced severe allergic reactions (Ganson et al., 2016). These findings were subsequently confirmed in the larger Phase 3 REGULATE trial (Povsic et al., 2016). In the present study, we employed our anticoagulant aptamer platform to better characterize the interactions between anti-PEG antibodies and PEGylated aptamers. Our findings shed additional light on the potential downside of PEG formulations and inform the future development of both oligonucleotide and protein-based therapeutics.
PEGs are hydrophilic polymers of differing sizes consisting of (-CH2-CH2-O-) in repeating units that are common in medical and commercial products (Wenande et al., 2015; Yamasuji et al., 2013). PEGylated compounds exhibit reduced renal clearance, which is predicated on inhibiting the adsorption of proteins (Zhang et al., 2016). PEG reduces protein adsorption by: 1) forming a hydration layer between water molecules which allow for stable hydrogen bonding, 2) maintaining flexibility in aqueous solutions, and 3) increasing the hydrodynamic size of conjugated compounds. Therefore, therapies that contain PEG have an increased circulation time and enhanced pharmacodynamic properties. These beneficial mechanisms underscore the reason for the pervasiveness of PEG in medicaments and everyday products.
PEGs have been generally considered as biologically inert and have been previously described as “non-fouling”; however, severe hypersensitivity reactions are being reported more frequently along with their continual use (Yamasuji et al., 2013). Early studies with PEGylated bovine serum albumin (BSA) in rabbits indicated that there were no signs of anti-PEG antibodies or hypersensitivities associated with the administration of PEGylated proteins (Abuchowski et al., 1977a; Abuchowski et al., 1977b). Since then, several reports have demonstrated that anti-PEG antibodies could be generated with repeated administration (Ishida and Kiwada, 2013; Koide et al., 2010), although the clinical relevance of these findings were unclear at the time. Currently, a growing body of work suggests that potential hazards behind PEGylated therapies exist for agents in the drug development pipeline as well as currently approved compounds. These emerging concerns are due, in part, to the unfortunate complications that arose in the Phase 3 clinical trial, REGULATE - PCI evaluating a PEGylated RNA aptamer against FIXa. These results exemplify a scenario where patient safety and drug efficacy can be deleteriously impacted by existing anti-PEG antibodies. Such concerns are not exclusive to aptamers because they can theoretically occur with any PEGylated compound.
Aptamers are single stranded nucleic acids that bind with high affinity to defined molecular targets ranging from cells to proteins akin to naturally occurring RNA-protein interactions (Nimjee et al., 2017). The concept of aptamers emerged in the 1980’s from the study of HIV where virally encoded RNAs were discovered to bind viral and cellular proteins with profound affinity and specificity (Cullen and Greene, 1989; Marciniak et al., 1990b). Further analysis revealed that these RNAs evolved to modulate cellular networks in order to ensure efficient virion production and dissemination (Cullen et al., 1989; Marciniak et al., 1990a; Marciniak et al., 1990b). This observation was the impetus to begin testing if RNA ligands could inhibit viral replication and be applicable to translational research (Sullenger et al., 1990). In 1990, SELEX (systematic evolution of ligands by exponential enrichment) was described by Ellington and Szostak and Tuerk and Gold, a combinatorial chemistry and molecular biology technique to generate short nucleic acids that bind to molecular targets (Ellington and Szostak, 1990; Tuerk and Gold, 1990). In addition to having high specificity, aptamers are easily reversed by complimentary oligonucleotides harnessing Watson-Crick base pairing, which allows for rapid control of aptamer activity in vitro and in vivo (Rusconi et al., 2004; Rusconi et al., 2002). Through SELEX, our laboratory has generated aptamers and reversal agents to target proteins in the coagulation cascade, a critical pathway that must be controlled during most surgical procedures (Cohen et al., 2010; Sullenger and Nair, 2016).
One such RNA aptamer, RB006 conjugated to a 40 kDa methoxy PEG (mPEG) targets coagulation FIX/IXa and exhibits potent anticoagulant activity by binding to an exosite on FIXa with ~3 nM affinity (Dyke et al., 2006). This binding inhibits the cleavage of FX to FXa which is a critical event involved in clot formation (Sullenger et al., 2012). RB006 is part of the REG1 system, a novel combination of the rapid onset anticoagulant RB006 and reversal antidote RB007 that was used in patients undergoing percutaneous coronary intervention (PCI) to tightly control blood coagulation (Povsic et al., 2016; Povsic et al., 2013). Phase 1 and 2a clinical trials demonstrated encouraging results. However, in a Phase 3 study, following a single intravenous bolus infusion of pegnivacogen (1 mg/kg), 0.6% of the patients (10 out of 1,605) experienced a SAE, resulting in early termination of the study. Subsequent investigation correlated the severity of the allergic reactions to the presence of pre-existing anti-PEG antibodies in these patients predominantly of the IgG subclass (Ganson et al., 2016; Povsic, 2016). These observations demonstrate how PEG, a molecule that was previously thought to be biologically inert, was likely responsible for the failure of a late-stage clinical trial apparently due to high levels of pre-existing anti-PEG antibodies. The REGULATE-PCI trial joins a growing number of studies that highlight how anti-PEG antibodies can impact patient safety and as demonstrated here, therapeutic efficacy (Fix et al., 2018; Judge et al., 2006).
In 2010, a PEGylated recombinant mammalian uricase (Pegloticase, Krystexxa®) was approved by the FDA for the treatment of refractory gout (Sundy et al., 2011). In Phase 1 studies it was discovered that injections of pegloticase, administered by both subcutaneous and intravenous routes, induced anti-PEG antibodies in about 40% of patients (Ganson et al., 2006; Sundy et al., 2007). In these and later Phase 2 and 3 clinical trials, the induction of anti-PEG antibodies was associated with rapid clearance of pegloticase from plasma, loss of efficacy, and with an increased frequency of infusion reactions (Hershfield et al., 2014; Lipsky et al., 2014; Sundy et al., 2011; Sundy et al., 2008). In one of these trials, pre-existing anti-PEG antibodies were detected in pre-treatment samples from half of the patients in whom treatment with pegloticase induced higher levels of anti-PEG antibodies (Hershfield et al., 2014).
In contrast to this study, the REGULATE-PCI trial illuminated a different issue where pre-existing anti-PEG antibodies were the apparent cause of anaphylactoid responses. Unlike other anti-drug antibodies, IgMs and IgGs to PEG can be found in patients who have seemingly never been exposed to a particular drug that has been PEGylated. This observation is particularly relevant in today’s world where upwards of 70% of the general public have anti-PEG antibodies compared to 0.2% two decades ago (Richter and Akerblom, 1984; Yang et al., 2016). The advancement of diagnostic techniques to some degree may explain this increase in percentage, but nevertheless, given the pervasiveness of PEG in everyday life and the cautionary tale of the REGULATE-PCI trial, a more detailed investigation of the impact of pre-existing anti-PEG antibodies is warranted. In this study, we utilized well-established models of blood coagulation and the PEGylated aptamer RB006 that was evaluated in the REGULATE-PCI Phase 3 clinical trial, to explore the effects of anti-PEG antibodies on a PEGylated-drug’s activity in vitro and efficacy in vivo. These data serve to inform the development of the many PEGylated drugs currently in clinical trials (Jevsevar et al., 2010; Kolate et al., 2014), the use of clinically available PEGylated drugs, and provide important insights into the complex interplay between the immune system and PEGylated compounds for future therapeutic development.
Results
Anti-PEG antibodies in patient samples and mice recognize PEGylated aptamers
We have previously shown that human anti-PEG antibodies induced by pegloticase, as well as pre-existing anti-PEG antibodies associated with first exposure reactions to pegnivacogen, recognize the PEGylated aptamer RB006 (Figure 1a) and several PEGylated therapeutic proteins. However, these antibodies do not recognize the unPEGylated version of the same aptamer, named RB005, or the cognate unPEGylated proteins (Ganson et al., 2016). In order to begin testing if anti-PEG antibodies can directly interfere with a PEGylated aptamer’s function, we once again evaluated patient samples from the Krystexxa clinical trial, which contained anti-PEG antibodies. These samples were compared to pooled normal human plasma as a negative control. Similar to the competition ELISA in the aforementioned study, we observed that anti-PEG IgGs from patients are capable of binding to PEGylated compounds, which corroborates our previous findings. These antibodies recognize two therapeutic PEGylated aptamers, RB006 and Macugen as well as two PEGylated proteins ADAgen (PEGylated adenosine deaminase) and PEGylated uricase. However, they did not recognize the control unPEGylated aptamer RB005, which is identical to RB006 in sequence and functionality but lacks the PEG moiety, nor the unPEGylated protein adenosine deaminase (ADA). By contrast, no signal above background was observed for PEGylated drugs when testing samples from patients that lacked anti-PEG antibodies (Figure 1b). See Star Methods for materials, methods, and experimental design for all results herein.
Figure 1-. PEGylated aptamers are recognized by anti-PEG IgGs in patient plasma.
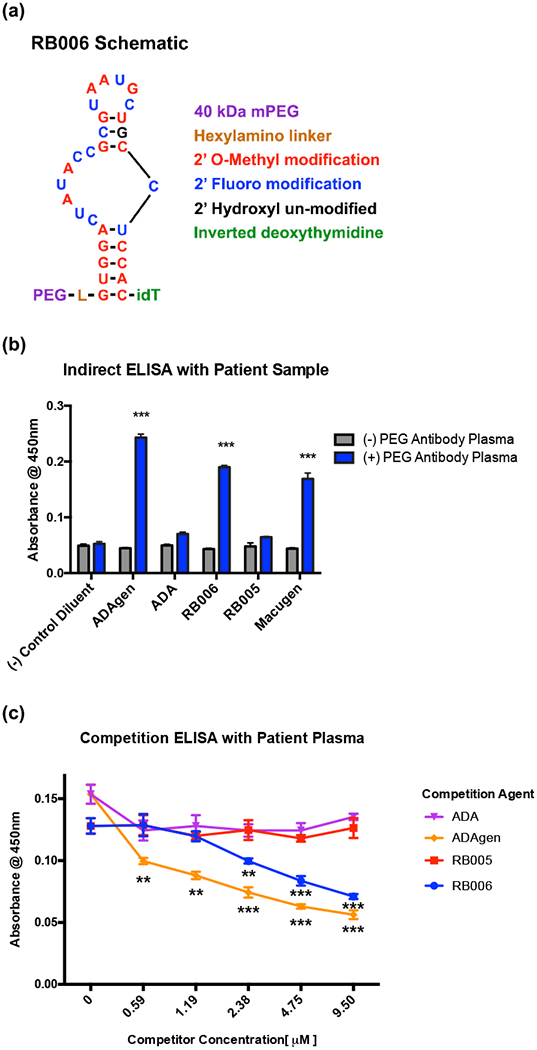
(a) Schematic representation of the PEGylated RNA aptamer RB006.
(b) Indirect ELISA demonstrates that plasma from anti-PEG IgG positive patients (blue) exclusively detects PEGylated compounds including the PEGylated RNA aptamers RB006 and Macugen and the PEGylated proteins ADAgen (PEGylated adenosine deaminase). However, patient plasma containing anti-PEG antibodies did not detect the non-PEGylated controls, including a non-PEGylated version of ADAgen, termed ADA, and a non-PEGylated version of RB006, termed RB005. Additionally, patient plasma that is negative for anti-PEG IgGs (grey) did not detect any of the PEGylated compounds. Data represent the mean ± SD of technical triplicates. *** denotes p-value < 0.001, using a t-test against (−) PEG plasma.
(c) Competition ELISA demonstrates that patient-derived anti-PEG antibodies bind with more affinity to PEGylated verses unPEGylated compounds, as shown by a decrease in absorbance with increasing concentrations of either the PEGylated aptamer RB006 (blue) or the PEGylated protein ADAgen (orange). No change was seen with the unPEGylated controls RB005 (red) and ADA (purple). Data represents the mean ± SD of technical triplicates. ** denotes p-value<0.01, *** denotes p-value < 0.001, using t-test verses unPEGylated compounds.
Competition ELISAs were used to verify these findings and assess apparent affinity of patient anti-PEG IgGs to the aptamer RB006. As shown in Figure 1c, only the PEGylated aptamer RB006 and PEGylated protein ADAgen can compete with patient antibody binding to RB006 and not the unPEGylated aptamer or unPEGylated protein. These results indicate that antibodies in the patient samples bind specifically to the PEG portion of the compounds and not to the nucleic acid or protein portions, which once again corroborates our previous findings (Ganson et al., 2016).
Since patient antibody samples are limited and cannot be easily evaluated in murine models of disease, we also evaluated murine anti-PEG antibodies for their binding to RB006 using the same indirect ELISA approach. As shown in Figure 2a, a murine anti-PEG antibody also binds RB006. Similar to the competition ELISA using patient samples, the murine antibody binds selectively to the PEGylated aptamer (Figure 2b). These data demonstrate that anti-PEG antibodies exclusively bind PEGylated compounds including aptamer RB006 but not the unPEGylated version RB005.
Figure 2-. PEGylated aptamers are recognized by monoclonal anti-PEG IgGs.
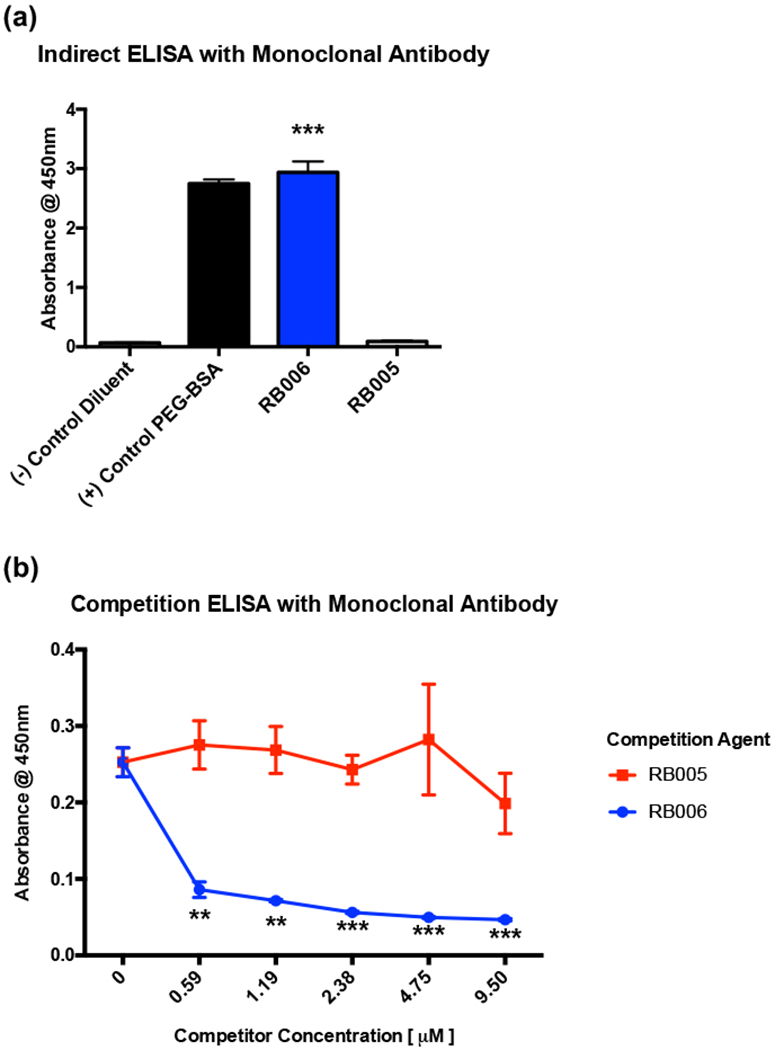
(a) A commercially available mouse monoclonal anti-PEG antibody specific for PEG recognizes PEGylated aptamer RB006 (blue) and control PEGylated-BSA (black) but does not recognize the unPEGylated aptamer RB005 (grey) through indirect ELISA. Data represent the mean ± SD of technical triplicates. ** denotes p-value <0.01, *** denotes p-value < 0.001, using a t-test against unPEGylated RNA aptamer RB005.
(b) Competition ELISA demonstrates that monoclonal anti-PEG IgGs bind to PEGylated aptamer RB006 (blue) with more affinity than to unPEGylated control aptamer RB005 (red). Data represent the mean ± SD of technical triplicates. ** denotes p-value<0.01, *** denotes p-value < 0.001, using a t-test against unPEGylated RNA Aptamer RB005.
The presence of anti-PEG antibody reduces aptamer binding to FIXa
After we confirmed the molecular recognition of PEGylated aptamers by anti-PEG antibodies, we hypothesized that the binding of an IgG (~150 kDa) to RB006 (~50 kDa) might hinder the ability of the aptamer to bind its target protein Factor IXa (FIXa). To better understand the binding characteristics of the aptamer - anti-PEG antibody complex to FIXa, we used surface plasmon resonance (SPR). We immobilized FIXa to a CM5 sensor chip and tested the binding of RB006 to the coagulation factor in the presence of varying concentrations of anti-PEG IgG. In Figure 3, the SPR competition studies revealed that the binding of RB006 to FIXa is inhibited when increasing concentrations of anti-PEG antibodies are added, as indicated by a reduction in Response Units (RU). When RB006 alone was injected over the FIXa coated surface we observed a robust increase in RU, in contrast to all samples that contain anti-PEG antibodies where only a limited increase in RUs is observed. Additionally, we detected lower binding as the molar ratio of anti-PEG IgG increased indicating that this effect is concentration dependent. Direct interaction between the aptamer and antibody is suggested by the similarities between the raw SPR sensorgram curves in each sample. Additionally, max binding was quantified as RUmax (inset). These data indicate that an anti-PEG IgG can inhibit RB006 binding to FIXa.
Figure 3-. Aptamer target binding is inhibited in the presence of anti-PEG antibody.
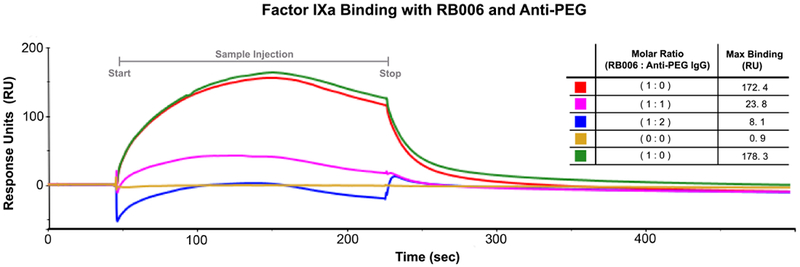
Surface plasmon resonance (SPR) was used to test the binding of RB006 to its target protein FIXa both alone and in the presence of anti-PEG IgG. Factor IXa was covalently attached to the chip surface and binding was measured in Response Units (RU) after injection of either RB006 alone (red and green, replicates) or RB006 in the presence of increasing molar ratios of monoclonal anti-PEG antibody (pink and blue respectively). Data represent a single experiment, though the effect was reproducible in two other replicate experiments. (Inset) A decrease in max binding (RU) was observed when aptamer and antibody were premixed and injected over the surface of the chip. The order of listed samples reflects the order of sample injections. The chip surface was uncompromised throughout the duration of the experiment as a similar Rmax was seen with the aptamer only samples injected at the beginning of the assay (red) and at the end of the assay (green).
Aptamer function is inhibited in the presence of anti-PEG antibodies in vitro
To date, no studies have been performed to determine if anti-PEG antibodies impact aptamer activity. In order to determine if the antibody-mediated inhibition of FIXa binding results in loss of anticoagulant activity, we performed an in vitro clinical coagulation assay, the activated partial thromboplastin time (aPTT), which is routinely employed to monitor FIXa activity. A dose titration curve was generated to quantify RB006-mediated clot-time extension (Figure 4a). Normal human plasma clots at approximately 30 seconds and RB006 dose-dependently increases aPTT with a clotting time of 125 seconds at high concentrations. As shown in Figure 4b, a significant decrease in RB006-mediated clotting time extension was observed when anti-PEG antibodies were titrated into the aPTT reaction. This inhibitory effect appears to be more pronounced as the molar ratio of anti-PEG antibodies increases while no changes in clotting time were observed with the antibody alone. In order to definitively test that the PEG portion of RB006 is responsible for aPTT inhibition, we utilized RB005 as a control. We observed no significant changes in clotting time extension when the anti-PEG antibody is included in the reaction with RB005, indicating that the inhibition of anticoagulant aptamer function only occurs when PEG is appended to the anti-FIXa aptamer (Figure 4c).
Figure 4-. Aptamer function is inhibited in the presence of anti-PEG antibodies in vitro.
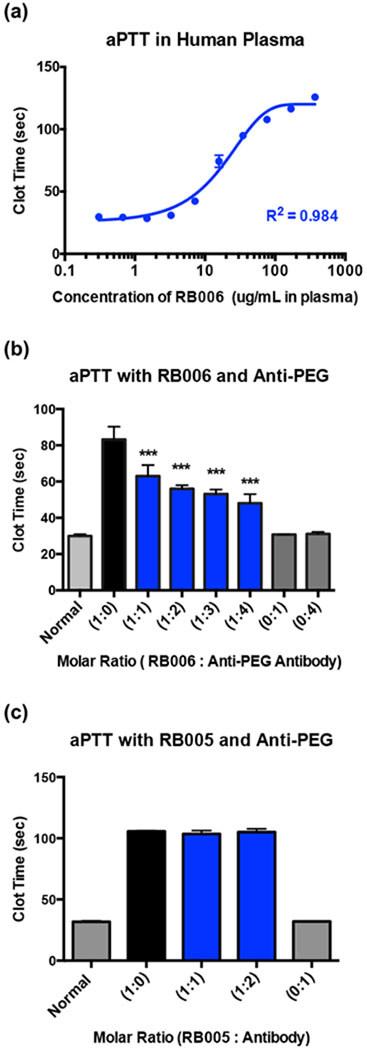
(a) Activated partial thromboplastin time (aPTT) measurement of the anticoagulant activity of RNA aptamer RB006 (blue) in pooled normal human plasma demonstrates aptamer mediated clotting time extension occurs in a dose dependent manner. A max clotting time of ~125 seconds is observed, which is significantly above the normal clotting time of approximately 30 seconds. Data represent the mean ± SD of technical replicates (N=4 wells).
(b) aPTT measurements of human plasma clotting time in the presence of RB006 with or without anti-PEG IgG in the reaction. Decreases in aptamer mediated clot-time extension with increasing concentrations of monoclonal anti-PEG IgG (blue) provide evidence for antibody mediated inhibition of RB006 function. Data represent the mean ± SD of technical replicates (N=6 wells) pooled from triplicate experiments. *** denotes p-value < 0.001, using t-test against aptamer only (1:0) (black).
(c) aPTT measurements of human plasma clotting time in the presence of the unPEGylated control aptamer RB005 with or without anti-PEG IgG demonstrates no change in clotting time, suggesting that anti-PEG IgG only inhibits the function of PEGylated aptamer. Data represent the mean ± SD of technical duplicates.
Antithrombotic and anticoagulant aptamer function is inhibited in the presence of anti-PEG antibodies in vivo
Although the aPTT is a standard clinical coagulation assay routinely used to measure clotting of plasma in vitro, it does not necessarily predict the effects of the anti-PEG antibody on the PEGylated aptamer in vivo. Therefore, we next evaluated whether the presence of the anti-PEG antibody impacts aptamer-mediated inhibition of clot formation in a well-established murine model of anticoagulation and thrombosis, the ferric chloride-induced damage model of the common carotid artery (Figure 5a). As shown in Figure 5b, administration of the RB006 aptamer at concentrations greater than or equal to 0.5 mg/kg were able to maintain the patency of the damaged vessels and limit occlusive thrombi. By contrast, mice that received no aptamer exhibited thrombosis and full occlusion of the common carotid artery in ~3 minutes. These data indicate that RB006 is capable of inhibiting FIXa-mediated clot formation in this carotid artery damage model.
Figure 5-. Anticoagulant aptamer activity is inhibited in the presence of anti-PEG antibodies in vivo.
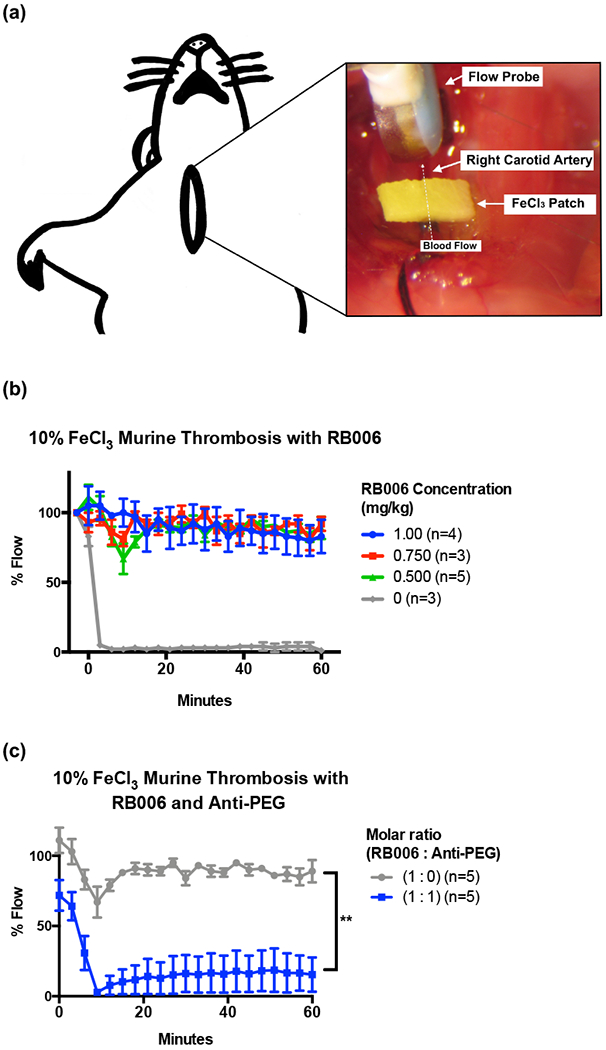
(a) Schematic represents the murine model of thrombosis used to analyze the antithrombotic / anticoagulant properties of aptamers and anti-PEG antibodies in real time. A ferric chloride (FeCl3) patch is used to induce endothelial damage to the carotid artery, and blood flow through the artery is measured via a distal flow probe. Percent blood flow at various time points post-intravenous (IV) administration of the aptamer was normalized to the blood flow prior to placement of the FeCl3 patch, with time zero indicating the time of patch removal.
(b) Increasing concentrations of PEGylated RB006 (green, red, blue) demonstrate the antithrombotic activity compared to buffer control (grey) in the murine model of thrombosis. Data represent the mean ± SEM of technical replicates as described in the figure legend.
(c) Addition of anti-PEG IgG at a 1:1 molar ratio to the PEGylated RB006 (blue) diminishes vessel patency in the murine thrombosis model as shown by a decrease in % Flow compared to aptamer alone (grey). For both treatment groups, RB006 was used at a concentration of 0.5 mg/kg. Data represent the mean ± SEM of technical replicates as described. **indicates p-value <0.01, using 2-way ANOVA vs. aptamer alone (1:0).
To test the potential impact of circulating anti-PEG antibodies on the aptamer’s anti-thrombotic activity in vivo, we utilized the same murine thrombosis model. Prior to intravenous administration of the aptamer, we infused the murine anti-PEG antibody, which was allowed to circulate for 15 minutes. A 1:1 molar ratio of aptamer to murine monoclonal anti-PEG antibody was chosen using the efficacious antithrombotic dose of RB006 (0.5 mg/kg). As shown in Figure 5c, we observed that the presence of circulating anti-PEG antibodies in mice dramatically limits the antithrombotic activity of the FIXa aptamer RB006. The significant difference in blood flow through the common carotid artery following trauma indicates that the presence of anti-PEG antibodies in these mice significantly limits aptamer-mediated inhibition of its target coagulation factor. For comparative purposes, we estimated that the amount of anti-PEG IgG that was administered in these experiments accounts for ~4% of all IgG in the mouse. These data corroborate our in vitro aPTT results.
After a single injection of PEGylated aptamer anti-PEG antibodies can be detected in rhesus monkeys
Up to this point we explored the impact of anti-PEG antibodies on a PEGylated drug’s activity. In order to expand on the concepts underlying PEG immunogenicity we chose to explore the prevalence of anti-PEG antibodies in humans. Unfortunately, additional patient samples from the REGULATE-PCI clinical trials were not available; therefore, we sought alternative avenues to address if the prevalence of anti-PEG in other primate species mimic findings observed in humans and if the administration of a PEGylated aptamer could generate anti-PEG antibodies. We explored these questions using the rhesus monkey model system. An ELISA was used to determine if a group of 73 healthy wild-type rhesus monkeys (35 males and 38 females) had detectable levels of anti-PEG antibodies prior to aptamer administration. These animals were naïve to RB006, although it could not be definitively confirmed that every subject had never received any drug treatments that may have included a PEG component due to the pervasiveness of PEG in common commercial products. However, we found only one of 73 with detectable levels of anti-PEG IgGs in plasma (1.3%) (Figure S1).
To further explore PEG antigenicity and immunogenicity in the nonhuman primate model, 18 young rhesus monkeys selected from above that did not contain detectable anti-PEG IgGs were administered RB006 either subcutaneously (N=9) or intravenously (N=9) as a component of a larger PK study. Anti-PEG IgG levels were measured before and after exposure to the PEGylated aptamer. The cohort of 18 rhesus monkeys were selected from the initial screen of 73 animals. As noted above, given the ubiquity of PEG in a myriad of consumables it cannot definitively be ruled out that pre-exposure to PEG had not occurred in the rhesus monkey cohort that was initially screened (N=73). The 18 animals were split into two groups with one group (N=9) administered an intravenous injection of RB006 (low, middle, or high dose; 0.5, 1.0, or 1.5 mg/kg, N=3 per dose) and the other group (N=9) a subcutaneous injection (low, middle, or high dose) (Table 1). After a single administration of RB006 we were able to detect the presence of anti-PEG IgG antibodies in 4 of 18 rhesus monkeys (22%) (Figure 6). Animals were considered positive based on two criteria: 1) if statistical significance was observed between the pre- and post-absorbance values, and 2) if the post-absorbance was greater than two standard deviations from the mean of all pre-absorbance values. One animal (#29), demonstrated substantially elevated levels of anti-PEG antibodies. Little to no correlation between the route of administration, time of sample collection, or dose was observed when compared to levels of anti-PEG IgG (Figure S2). It was observed that over 20% of naïve animals generate anti-PEG IgGs following a single administration of the aptamer by either the intravenous or subcutaneous route. These data further support that rhesus monkeys provide an important primate model system in which to test PEG immunogenicity as it pertains to aptamer drug design and PEGylated drug development.
Table 1-.
RB006 rhesus monkey dosing scheme and sample collection
| Animal Number | RB006 Administration Route - Dose (mg/kg) | Sample Collection Hours post injection (days) |
|---|---|---|
| 25 | IV - low dose (0.5) | 192 (8) |
| 27 | IV - low dose (0.5) | 192 (8) |
| 29 | IV - low dose (0.5) | 192 (8) |
| 30 | IV - mid dose (1.0) | 96 (4) |
| 55 | IV - mid dose (1.0) | 96 (4) |
| 38 | IV - mid dose (1.0) | 96 (4) |
| 36 | IV - high dose (1.5) | 216 (9) |
| 42 | IV - high dose (1.5) | 216 (9) |
| 43 | IV - high dose (1.5) | 216 (9) |
| 51 | SQ - low dose (0.5) | 216 (9) |
| 70 | SQ - low dose (0.5) | 216 (9) |
| 47 | SQ - low dose (0.5) | 216 (9) |
| 49 | SQ - mid dose (1.0) | 96 (4) |
| 61 | SQ - mid dose (1.0) | 96 (4) |
| 62 | SQ - mid dose (1.0) | 96 (4) |
| 66 | SQ - high dose (1.5) | 96 (4) |
| 68 | SQ - high dose (1.5) | 96 (4) |
| 69 | SQ - high dose (1.5) | 96 (4) |
Figure 6-. Anti-PEG antibodies can be detected in rhesus plasma after a single administration of RB006.
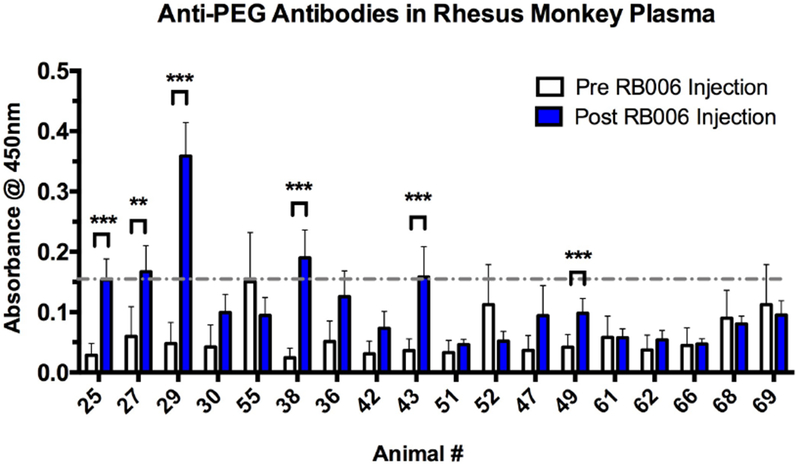
Indirect ELISA was used to measure the level of anti-PEG IgG in plasma before and after RB006 administration, with PEG-BSA coated 96-well plates followed by the addition of plasma for recognition and IgG rhesus monkey specific antibody for detection. Six out of 18 healthy rhesus monkeys tested had significant increases in anti-PEG IgG levels between the pre-aptamer injection samples (white) and post-aptamer injection samples (blue), with four of the animals reaching anti-PEG IgG levels greater than 2 SD from the mean of all pre-injection absorbance values (grey dashed line). The route of administration and dosing of the aptamer as well as the time of sample collection are described in Table 1. The animals selected and their numbers are from the original numerical designation in Figure S1. Data represent the mean ± SEM of technical triplicates. ** indicates p-value <0.01, ***indicates p-value <0.001, using a t-test.
Discussion
The assumption that PEG is non-immunogenic has recently been challenged in human studies which clearly demonstrate that PEGylated drugs can elicit antibodies to PEG. Moreover, in the RADAR and REGULATE-PCI trials, it was found that high levels of pre-existing anti-PEG antibodies could cause serious adverse events (SAEs) primarily related to a PEGylated aptamer. In the study described herein, we identified another potential issue with the use and development of PEGylated drugs in that the existence of anti-PEG antibodies can directly inhibit a PEGylated aptamer’s therapeutic effect. Taken together with previous reports that suggest antibody-mediated accelerated drug clearance can also diminish PEGylated drug potency, these results also suggest that anti-PEG antibodies can significantly and directly limit drug activity. The occurrence of anti-PEG antibodies in the general public is just now being appreciated and thoroughly investigated. Considering that at least 16 FDA approved PEGylated drugs are on the market and numerous unregulated products contain PEG, additional studies examining the effects of anti-PEG antibodies on drug efficacy and safety are required for the public well-being. Herein, we show that the presence of anti-PEG antibodies is capable of inhibiting the anticoagulant activity of a PEGylated aptamer in vitro as well as in vivo. These findings shed additional light on the potential limitations of conjugation and formulation strategies that employ PEG at least for inhibitors such as aptamers that act by blocking assembly of macromolecular complexes. Although macromolecular drugs are an attractive area of translational research the need for better pre-screening for PEG antibodies or a better understanding of PEG in relation to the immune system is required to ensure that novel PEGylated therapies can maximally benefit the health of the public.
The RADAR and REGULATE-PCI pegnivacogen trials were halted because of SAEs apparently caused by pre-existing anti-PEG antibodies. The severity of the allergic reactions seen in 0.6% of patients in each of these trials who received the aptamer therapy appear to be explained by the 40 kDa mPEG that was conjugated to the aptamer. The aptamer was delivered as a bolus intravenously, with a high probability of rapidly coming into contact with circulating anti-PEG antibodies as IgGs are the second most common protein found in blood (Gonzalez-Quintela et al., 2008). Most of the patients that experienced a SAE had the highest levels of anti-PEG antibodies. In addition to anti-PEG IgGs, anti-PEG IgMs are now also being explored as they have been implicated with enhanced clearance of other PEGylated therapies (Fix et al., 2018; Ganson et al., 2006; Hsieh et al., 2018) and can contribute to deleterious immunological effects. Based on the results from the murine thrombosis study we can extrapolate numerical inferences about anti-PEG IgG levels and PEGylated drug inhibition in vivo. The amount of anti-PEG IgG that was administered during the in vivo studies accounts for approximately 4% of total mouse IgG. Therefore, using the therapeutic dose of RB006 / pegnicavogen from the REGULATE-PCI trial and the average concentration of IgG in adults (Gonzalez-Quintela et al., 2008), we estimate that a low percentage of total IgGs (0.38% - 0.88%) would have to be targeted against PEG to thwart the anticoagulant properties of the aptamer in patients. Unfortunately, the levels of pre-existing anti-PEG IgGs from the patients in the RADAR and REGULATE-PCI trial are unavailable, therefore no direct quantitation can be made as these patient samples no longer exist.
To assess whether aptamer function was retained in the presence of anti-PEG antibodies, we first evaluated antibodies from patient samples that were enrolled in the Krystexxa trial and a murine anti-PEG antibody to determine if they can recognize the PEGylated aptamer RB006. We observed that anti-PEG antibodies can inhibit aptamer binding to its target protein and that this inhibition can result in diminished anticoagulant activity in clinical coagulation assays performed in vitro. For such studies, the relative affinities of the antibody and aptamer are worth noting. The addition of a 40 kDa mPEG to this aptamer results in a modest reduction in affinity to FIXa with the parent aptamer sequence 9.3t having a Kd = 0.58 ± 0.1 nM and the PEGylated version with a Kd = 2.83 (± 0.4 nM) (Rusconi et al., 2004; Rusconi et al., 2002). The anti-PEG IgG that was used in the inhibition experiments has an affinity to the PEG chain of 2.9 nM as per the manufacturer’s documentation. Since the affinity of the aptamer to the IgG and FIXa are very similar it would seem reasonable that the antibody could effectively compete with FIXa for binding to the aptamer. Moreover the bivalent antibody may bind with increased avidity to the PEGylated aptamer as multivalent binding is possible with antibody binding to polyvalent antigens such as large, branched PEGs. This possibility is supported by SPR binding data that illustrates the extent of reduced aptamer binding to its target FIXa by anti-PEG antibodies. The SPR data considered in combination with the affinities suggests a steric hinderance mechanism of antibody-based inhibition of RB006 binding to FIXa. This observation, at first glance, was surprising as the aptamer does tolerate the addition of a 40 kDa branched PEG; therefore, why would the binding of a ~150 kDa protein impede activity? As mentioned above the addition of the PEG to the aptamer does reduce affinity of the aptamer to FIXa by approximately 5-fold. Moreover, as the PEG contains two 20 kDa branches, it is entirely possible that two or more anti-PEG IgGs can simultaneously bind RB006 which would further sterically impede aptamer binding to its target. Finally, IgGs have been shown to bind two different antigens and link them forming a chain which seems particularly plausible given the bifunctional nature of both the IgG and the branched PEG in this instance (Yamaguchi and Harada, 2002). Thus, supramolecular IgG-antigen chains may also be forming, which would be expected to further limit the aptamers ability to bind FIXa. Finally, although not directly addressed herein, we would anticipate that anti-PEG IgMs would be even more effective at sterically interfering with PEGylated drug activity.
Somewhat surprisingly, we detected the presence of anti-PEG IgG in rhesus monkeys following only a single subcutaneous or intravenous injection of PEGylated aptamer RB006. Therefore, although aptamers are attractive novel therapeutics with numerous exciting emerging applications including as anticoagulants (Drolet et al., 2016; Gunaratne et al., 2018; Hirota et al., 2016; Nimjee et al., 2009; Nimjee et al., 2017; Powell Gray et al., 2018; Ramos et al., 2015; Sullenger and Nair, 2016; Woodruff et al., 2013; Zhou et al., 2018), our results suggest careful consideration is needed for bio-conjugation in the future to avoid the pitfalls that were found during pegnivacogen / RB006 clinical development. These findings are not limited to aptamers as they extend to any macromolecular therapies that contain PEG. However, the observation that anti-PEG IgGs can impede PEGylated drug activity may be a particular concern for therapeutic agents such as anticoagulant aptamers that induce their effects by binding large solvent exposed surfaces on their target molecules and thereby block macromolecular interactions (Gelinas et al., 2016; Gunaratne et al., 2018; Long et al., 2008). By contrast PEGylated protein enzymes, such as Adagen or pegloticase that function by binding small molecule substrates in active sites, may be less susceptible to IgG-based steric inhibition. Regardless, the possibility of such anti-PEG Ig-based steric inhibition should be explored for each PEGylated therapeutic agent given the prevalence of anti-PEG IgGs in the population.
PEG hypersensitivity as it pertains to drug development is just now being appreciated. Our work complements these studies and indicates that in addition to safety issues associated with anti-PEG antibodies, pre-existing anti-PEG antibodies may also reduce PEGylated drug efficacy. This outcome can lead to erroneous conclusions in clinical trials; a drug may be deemed ineffective because its efficacy is limited in the growing subset of patients that harbor antibodies against PEG even though it may be particularly efficacious in those that do not. Although these studies present general questions related to public health, they also point to the need for improved precision medicine-based approaches to exclude patients from studies and from taking PEGylated drugs if they harbor anti-PEG antibodies.
The beneficial attributes of PEG are responsible for its pervasiveness in everyday life with countless medical and commercial products with PEG in their ingredients. Contrary to its “non-fouling” nature, increasing evidence indicates that PEG can be harmful and moreover as this study illustrates, can also reduce drug efficacy making clinical development even more challenging. Despite the advances in biopharmaceutical therapies, a disconnect remains between translating drugs from the laboratory bench to the patient bedside. Our limited understanding of the complex immunological processes that are involved in medicament administration can inhibit the generation of improved therapeutics. Therefore, further investigation into PEG antibody generation and actions are required for the advancement and approval of many bona fide effective therapies.
Significance
PEGylation of biologically inspired therapies has become the gold standard of formulation due to increased solubility, stability, and ease of conjugation. Although generally considered to be “non-fouling” repeat administration of PEGylated compounds has been shown to generate anti-PEG antibodies. The presence of anti-PEG antibodies is not only pharmacologically detrimental but also can pose a potentially serious safety concern, especially with an estimated 70% of the general population with circulating anti-PEG antibodies compared to 0.2% two decades ago. The early termination of the PEGylated aptamer RB006 Phase 3 trial exemplifies the possible safety concerns that can be associated with the administration of PEGylated therapies. However, little is known about how these anti-PEG antibodies affect PEGylated aptamer function since PEG immunogenicity is just now being investigated. Given the pervasiveness of PEG and the recent reports of PEG-hypersensitivities, a critical understanding of the extent of antibody-mediated drug neutralization is warranted. Furthermore, if the trend in the number of people with anti-PEG antibodies continues to increase a critical question about PEG and its use as a stabilizing agent may be brought into question. Here, we address some of these concerns and begin to ask how these antibodies may affect PEGylated RNA aptamer therapeutic activity. The findings herein will inform future therapeutic development and encourage precision medicine-based approaches for humans with anti-PEG antibodies.
Star Methods:
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for reagents may be directed to, and will be fulfilled by the corresponding author Bruce A. Sullenger (bruce.sullenger@duke.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAIL
Mice
Experiments were approved by the Duke Institutional Animal Care and Use Committee and performed in accordance with NIH guidelines. Wild-type female C57BL/6J mice ages 7 – 13 weeks were used for in vivo thrombosis studies, mice were anesthetized and temperature was measured for the duration of the study. All mice were housed in accordance to standard operating protocols.
Rhesus monkeys (Macaca mulatta)
All animal procedures conformed to the requirements of the Animal Welfare Act and protocols were approved prior to implementation by the Institutional Animal Care and Use Committee at the University of California, Davis. Activities related to animal care (diet, housing) were performed per California National Primate Research Center standard operating procedures. Healthy, wild-type young rhesus monkeys (35 males and 38 females) housed according to standardized protocols (see below) were included in the study. 18 healthy young rhesus monkeys selected from the 73 above (10 females and 8 males) were used in the second rhesus monkey study and were housed according to standardizes and approved protocols.
METHOD DETAILS
Synthesis of RB006
RB006, a 31 nucleotide 2’ modified RNA aptamer conjugated to 40 kDa methoxy polyethylene glycol (Figure 1a), was manufactured by Samchully Pharm Co., LTD. (Seoul, Korea). All experiments, excluding aPTTs, with aptamer were performed with re-folded RB006 by heating the sample to 65°C for 5 minutes to unfold and 5 minutes at room temperature (RT) for re-folding.
Indirect enzyme linked immunosorbent assay (ELISA)
To test if antibodies in human plasma recognize PEGylated compounds PEGylated aptamers RB006 and Macugen, PEGylated proteins ADAgen (PEGylated adenosine deaminase), and unPEGylated aptamer RB005, protein ADA as controls (at 950 nM) were adsorbed to a 96-well plate in PBS overnight at 4°C. Plates were washed with 250 μL/well B-PBS buffer (1% BSA, PBS), and all other incubations were performed with 100 μL/well and all dilutions represent (v:v). Blocking was accomplished by adding B-PBS for 1 hour at RT. Next, patient plasma containing anti-PEG IgG was diluted (1:400) in B-PBS and incubated for 2 hours at room temperature (RT), with patient plasma negative for anti-PEG IgG as a control. Human anti-PEG IgGs were detected using chicken polyclonal antibody to human IgG conjugated to horseradish peroxidase (HRP) (Ca# 6864 Abcam, Cambridge, MA) diluted 1:5,000 incubated for one hour at RT. As a substrate, tetramethylbenzidine (TMB) (Ca# 7004 Cell Signaling, Danvers, MA) was added to each well and the reaction was stopped with STOP (Ca# 7002 Cell Signaling, Danvers, MA) after the plate was incubated for 15 min at RT on an orbital shaker at 100 rpm. Plate absorbance was read at A450 and all values were references to plate blank (buffer only), unless stated otherwise.
The presence of rhesus monkey anti-PEG IgGs was determined through ELISA. Mono mPEG (20 kDa) BSA coated plates (Ca # PBSA-01, Life Diagnostics, West Chester, PA) were washed with 250 μL/well B-PBS buffer (1% BSA, PBS), and all other incubations were performed with 100 μL/well and all dilutions represent (v:v). Blocking was accomplished by adding B-PBS for 1 hour at RT. Rhesus monkey plasma was diluted 1:10 in B-PBS and incubated for 2 hours at RT, following 4 plate washes with B-PBS. Rhesus anti-PEG IgGs were detected using rabbit anti-monkey IgG-HRP (Ca# 2054, Sigma, St. Louis, MO) diluted 1:20,000 in B-PBS. As a substrate, TMB was added to each well and the reaction was stopped with STOP (Ca# 7002 Cell Signaling, Danvers, MA) after the plate was incubated for 15 minutes at RT on an orbital shaker at 100 rpm. Plate absorbance was read at A450 and all values were referenced to plate blank (buffer only), unless stated otherwise.
Competition enzyme linked immunosorbent assay (ELISA)
Apparent anti-PEG IgG affinity to PEGylated aptamer was determined via competition ELISA beginning with 96-well plates coated with PEGylated uricase (identical concentration to indirect ELISA) in PBS overnight at 4°C. In separate tubes 100 μL anti-PEG containing patient plasma diluted (1:200) in B-PBS was combined with 100 μL of increasing concentrations of competitor ADAgen or RB006, and ADA or RB005 as controls which were diluted 2-fold with a starting concentration of 9.5 μM in B-PBS and incubated overnight at 4°C. Next, wells were washed and 50 μL of plasma with competitor was added to wells and incubated 1 hour at RT. Wells were washed and human anti-PEG IgGs were detected using chicken polyclonal antibody to human IgG conjugated to horseradish peroxidase (HRP) (Ca# 6864 Abcam, Cambridge, MA) diluted 1:5,000 incubated for one hour at RT, followed by the addition of TMB and STOP solution as described in the ELISA procedure.
Surface Plasmon Resonance (SPR)
All SPR studies were performed in collaboration with the Duke Human Vaccine Institute Biomolecular Interaction Analysis Facility with consultation from Dr. Brian Watts, using a Biacore T200 instrument, Biacore T200 Control Software, and T200 Evaluation Software version 2.0 (GE Healthcare, Stockholm, Sweden). The response signal (RU) of the BIAcore instrument is proportional to changes in the refractive index at the surface and is proportional to the mass of the substance bound to the chip.
For immobilization, a carboxymethylated dextran CM5 sensor chip (Ca# BR100012, GE Healthcare, Stockholm, Sweden) was primed with running buffer HBS-EP+buffer, which contained 0.01 M Hepes (pH 7.4), 0.15 M NaCl, 0.0034 M EDTA (Ethylenediaminetetraacetic acid) and 0.05% (v/v %), P20 (or Tween 20). The chip was subsequently activated with N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) at 5 μL/min for 7 minutes and recombinant human FIXa (Ca# HCIXA-0050, Haematologic Technologies, Essex Junction, VT) at 60 μg/mL in 10 mM sodium acetate (NaOAc) buffer pH 4.5 was injected over flow cell 2 at 1-minute increments until approximately 5,000 RU were achieved. After sufficient coupling the surface was inactivated with 1 M ethanolamine hydrochloride-NaOH (pH 8.5) for 12 minutes.
Binding experiments were performed at 37°C with a flow rate of 30 μL/min and all samples were diluted in binding buffer (20 mM HEPES pH 7.4, 150 mM NaCl, 2 mM CaCl2, 0.01% BSA), which also served as the running buffer. Each sample was kept at 37°C in the sample compartment. The resultant binding curves are reference subtracted where the flow cell 2 sensorgram values were subtracted from the sensogram values from control flow cell 1 resulting in the reported raw sensorgram. Each sample was injected at the same rate and with equivalent contact time. The order of samples on the inset in Figure 3 represents the order of sample injection. Samples containing anti-PEG antibody and RB006 were pre-mixed before injection. Interestingly, we observe a modest reduction in binding following the maximum binding during the association phase, prior to the dissociation phase. While this observation does not detract from the overall interpretation that the anti-PEG antibody inhibits aptamer binding to factor IXa, it does suggest that adding additional aptamer to formed aptamer-factor IXa complexes may modestly impact the ability of the aptamer to binds its target protein. As aptamers naturally contain self-complementary sequences due to their base paired regions, it is possible that they can serve as weak reversal agents for the aptamer at least at the high concentrations utilized in such SPR studies.
In vitro clotting assays
Activated partial thromboplastin time (aPTT) assays were performed using a STart 4 coagulometer (Diagnostica Stago Inc., Asnières sur Seine Cedex, France.) as described previously in (Soule et al., 2016). Pooled normal human plasma (50 μL) (George King Biomedical, Overland Parks, KS) was incubated with aptamer re-suspended in phosphate buffered saline (PBS) alone (5 μL) or aptamer pre-mixed with anti-PEG antibody IgG (5 μL) (Ca# ID9-6 Life Diagnostics, West Chester, PA) followed by TriniCLOT aPTT S (50 μL) (Trinity Biotech, Bray, Co Wicklow, Ireland) was added and incubated for 5 minutes at 37°C. Clotting was initiated by the addition of 0.02M CaCl2 (50 μL) and time to clot formation was determined. Each sample was tested in duplicate and each experiment was repeated in triplicate.
In vivo murine model of thrombosis
The carotid FeCl3 injury model was performed as previously described (Westrick et al., 2007). Briefly, C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) were intubated and the left jugular vein was cannulated. The right common carotid artery was isolated and a Transonic Nanoprobe (Transonic Systems Incorporated, Ithaca, NY) was placed around the vessel to measure blood flow for 5 minutes to ensure a stable baseline. Samples were administered intravenously either: 1) RB006 and circulated for 5 minutes in an aptamer dose titration study, or 2) anti-PEG antibody circulated for 15 minutes followed by RB006 and circulated for 5 minutes in the inhibition study. Carotid artery thrombosis was induced by placing a 10% ferric chloride (FeCl3) soaked 1×2 mm Whatman paper on the carotid artery for 3 minutes. Blood flow was measured for 60 minutes, and all animals were euthanized at the end of the study.
Rhesus monkey study
Blood samples were collected from a peripheral vessel in sedated animals (either ketamine hydrochloride at 5-30 mg/kg or telazol 5-8 mg/kg intramuscular), and RB006 administered (intravenous or subcutaneous injection; ~ 1 mL) under aseptic conditions. Samples were collected from a peripheral vessel as noted in Table 1, and plasma was obtained, frozen in aliquots, then used for indirect anti-PEG ELISA to test for the presence of anti-PEG IgG as previously described. Each sample was tested in triplicate and each experiment was performed in triplicate.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical parameters including n, the definition of center, dispersion and precision measures (mean ± SD) and statistical significance are reported in the Figures and Figure Legends. Data is considered to be statistically significant when p < 0.05 by Student’s t test. In figures, asterisks denote statistical significance as calculated by Student’s t-test or 2-way ANOVA (*, p < 0.05; **, p < 0.01; ***, p < 0.001). Statistical analysis was performed in GraphPad PRISM 6.
DATA AND SOFTWARE AVAILABILITY
Supplementary Material
Figure S1 - Related to Figure 6 - Preliminary screening of rhesus plasma for anti-PEG IgG.
(a) Preliminary screening was performed on plasma samples from 73 young healthy rhesus monkeys (~2-3 years of age, ~3-5 kg; 35 males and 38 females) for the presence of anti-PEG antibodies using indirect ELISA. Pre-coated PEG-BSA 96-well plates were incubated with diluted plasma followed by rhesus monkey IgG specific detection antibody and absorbance was measured. Data represent the mean ± SEM of N=4.
(b) Re-screening for validation of rhesus monkey plasma that was positive from pre-screening in (a) using the identical indirect ELISA procedure was performed. Rhesus #34 (blue) was significantly higher in absorbance suggesting the presence of anti-PEG antibody. Preliminary screening was performed in different plates and on a different day, therefore re-screening any potential positive animals in one plate was accomplished for thorough analysis. Data represent the mean ± SEM of technical triplicates. ** denotes p-value <0.01 using t-test vs. control.
Figure S2 - Related to Figure 6 - Comparing post-aptamer rhesus plasma anti-PEG IgG levels for possible correlates
(a) Post-aptamer administration rhesus monkey plasma IgG absorbances (blue) were determined by ELISA as described in Supplemental Figure 1 compared by dose and route of delivery. While there appeared to be a higher average anti-PEG IgG level in the intravenous-administered group the variation was not statistically significant. There was no apparent trend when comparing aptamer dose. Each bar represents mean of technical replicates, N=9 wells from three rhesus monkeys combined. Error bars represent standard deviation (SD) of the mean.
(b) Post-aptamer administered rhesus plasma IgG absorbances (blue) were plotted against time of sample collection. Using a two-parameter analysis no statistically relevant correlation was observed suggesting that the time of collection had no influence on IgG levels. Each dot represents the mean of technical triplicates of one rhesus monkey.
KEY RESOURCE TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Chicken polyclonal anti-human IgG-HRP | Abcam | Ca# 6864 |
| Rabbit anti-monkey IgG-HRP | Sigma-Aldrich | Ca# 2054 |
| Mouse anti-PEG | Life Diagnostics | Ca# ID9-6 |
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Rhesus monkey plasma | Tarantal, UC Davis | N/A |
| Human plasma samples | Duke University; Department of Medicine | N/A |
| Pooled normal human plasma | George King Biomedical | Ca# 0010-1 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Human Factor IXa | Haematologic Technologies | Ca# HCIXA-0050 |
| Adenosine deaminase bovine (ADA) | Sigma-Aldrich | A5168; CAS: 9026-93-1 |
| ADAGEN (pegademase bovine) | Lediant Biosciences | http://www.adagen.com/pdf/AdagenPI.pdf |
| Macugen (pegaptanib) | Bausch and Lomb | http://www.bausch.com/Portals/69/-/m/BL/United%20States/USFiles/Package%20Inserts/Pharma/macugen-package-insert.pdf |
| Critical Commercial Assays | ||
| TriniCLOT aPTT S | Tcoag Ireland Limited | Ca# T1201 |
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Mouse: C57/BL/6J | Jackson Laboratories | JAX: 000664 |
| Oligonucleotides | ||
| PEGylated RNA aptamer RB006 sequence: 40 kDa mPEG-GUGGAcuAuAccGcGuAAuGcU(G)CcuCCACidT: (uppercase-2’ F, lowercase-2’O Methyl, ()-unmodified) | Dyke et al., 2006 | N/A |
| UnPEGylated RNA aptamer RB005 sequence: GUGGAcuAuAccGcGuAAuGcU(G)CcuCCAC-idT: (uppercase-2’ F, lowercase-2’O Methyl, ()-unmodified) | Dyke et al., 2006 | N/A |
| Recombinant DNA | ||
| Software and Algorithms | ||
| Biacore T200 control software | GE Healthcare - Life Sciences | Ca# 29148695 |
| T200 evaluation software | GE Healthcare - Life Sciences | Ca# BR-1005-97 |
| LabChart v8.1.10 Mac | ADInstruments | https://www.adinstruments.com/support/downloads/mac/labchart |
| Other | ||
| PEG-BSA coated plates | Life Diagnostics | Ca# PBS-01 |
| Transonic flow probe | Transonic | Ca# MA0.5PSB |
| Biacore T200 instrument | GE Healthcare - Life Sciences | Ca# 28975001 |
| CM5 sensor chip | GE Healthcare - Life Sciences | Ca# BR-100012 |
Highlights:
Anti-PEG antibodies bind to PEGylated RNA aptamer RB006
Anti-PEG antibodies inhibit anticoagulant aptamer activity in vitro and in vivo
After one administration of RB006 anti-PEG antibodies were found in rhesus monkeys
Acknowledgements:
The authors thank Dr. Brian Watts from the Duke Human Vaccine Institute’s Biomolecular Interaction Analysis Core Facility for his assistance with and consultation of SPR experimental analysis and for use of the Biacore T200 platform.
Funding sources: U54-HL112307 and R01-HL065222 (BAS), P01-HL112761 (BAS/AFT), NHLBI Center for Gene Transfer for Heart, Lung, and Blood Disease R24-HL085794 (AFT), and the California National Primate Research Center base operating grant P51-OD011107 (AFT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
The authors have declared no conflict of interest exists. However, Duke University has submitted patent applications on the anticoagulant aptamers.
References:
- Aberle JH, Aberle SW, Redlberger-Fritz M, Sandhofer MJ, and Popow-Kraupp T (2010). Human metapneumovirus subgroup changes and seasonality during epidemics. Pediatr Infect Dis J 29, 1016–1018. [DOI] [PubMed] [Google Scholar]
- Abuchowski A, McCoy JR, Palczuk NC, van Es T, and Davis FF (1977a). Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem 252, 3582–3586. [PubMed] [Google Scholar]
- Abuchowski A, van Es T, Palczuk NC, and Davis FF (1977b). Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem 252, 3578–3581. [PubMed] [Google Scholar]
- Chan MY, Cohen MG, Dyke CK, Myles SK, Aberle LG, Lin M., Walder J, Steinhubl SR, Gilchrist IC, Kleiman NS, et al. (2008). Phase 1b randomized study of antidote-controlled modulation of factor IXa activity in patients with stable coronary artery disease. Circulation 117, 2865–2874. [DOI] [PubMed] [Google Scholar]
- Cohen MG, Purdy DA, Rossi JS, Grinfeld LR, Myles SK, Aberle LH, Greenbaum AB, Fry E, Chan MY, Tonkens RM, et al. (2010). First clinical application of an actively reversible direct factor IXa inhibitor as an anticoagulation strategy in patients undergoing percutaneous coronary intervention. Circulation 122, 614–622. [DOI] [PubMed] [Google Scholar]
- Cullen BR, and Greene WC (1989). Regulatory pathways governing HIV-1 replication. Cell 58, 423–426. [DOI] [PubMed] [Google Scholar]
- Cullen KW, Bleach NR, and Green DM (1989). Juvenile basal cell carcinoma. Br J Clin Pract 43, 419–420. [PubMed] [Google Scholar]
- Drolet DW, Green LS, Gold L, and Janjic N (2016). Fit for the Eye: Aptamers in Ocular Disorders. Nucleic Acid Ther 26, 127–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke CK, Steinhubl SR, Kleiman NS, Cannon RO, Aberle LG, Lin M, Myles SK, Melloni C, Harrington RA, Alexander JH, et al. (2006). First-in-human experience of an antidote-controlled anticoagulant using RNA aptamer technology: a phase 1a pharmacodynamic evaluation of a drug-antidote pair for the controlled regulation of factor IXa activity. Circulation 114, 2490–2497. [DOI] [PubMed] [Google Scholar]
- Ellington AD, and Szostak JW (1990). In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822. [DOI] [PubMed] [Google Scholar]
- Fix SM, Nyankima AG, McSweeney MD, Tsuruta JK, Lai SK, and Dayton PA (2018). Accelerated Clearance of Ultrasound Contrast Agents Containing Polyethylene Glycol is Associated with the Generation of Anti-Polyethylene Glycol Antibodies. Ultrasound Med Biol 44, 1266–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganson NJ, Kelly SJ, Scarlett E, Sundy JS, and Hershfield MS (2006). Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res Ther 8, R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganson NJ, Povsic TJ, Sullenger BA, Alexander JH, Zelenkofske SL, Sailstad JM, Rusconi CP, and Hershfield MS (2016). Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J Allergy Clin Immunol 137, 1610–1613 e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas AD, Davies DR, and Janjic N (2016). Embracing proteins: structural themes in aptamer-protein complexes. Curr Opin Struct Biol 36, 122–132. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, Fernandez-Merino C, and Vidal C (2008). Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 151, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratne R, Kumar S, Frederiksen JW, Stayrook S, Lohrmann JL, Perry K, Bompiani KM, Chabata CV, Thalji NK, Ho MD, et al. (2018). Combination of aptamer and drug for reversible anticoagulation in cardiopulmonary bypass. Nat Biotechnol 36, 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, and Chess RB (2003). Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2, 214–221. [DOI] [PubMed] [Google Scholar]
- Hershfield MS, Buckley RH, Greenberg ML, Melton AL, Schiff R, Hatem C, Kurtzberg J, Markert ML, Kobayashi RH, Kobayashi AL, et al. (1987). Treatment of adenosine deaminase deficiency with polyethylene glycol-modified adenosine deaminase. N Engl J Med 316, 589–596. [DOI] [PubMed] [Google Scholar]
- Hershfield MS, Ganson NJ, Kelly SJ, Scarlett EL, Jaggers DA, and Sundy JS (2014). Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther 16, R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota M, Murakami I, Ishikawa Y, Suzuki T, Sumida S, Ibaragi S, Kasai H, Horai N, Drolet DW, Gupta S, et al. (2016). Chemically Modified Interleukin-6 Aptamer Inhibits Development of Collagen-Induced Arthritis in Cynomolgus Monkeys. Nucleic Acid Ther 26, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh YC, Wang HE, Lin WW, Roffler SR, Cheng TC, Su YC, Li JJ, Chen CC, Huang CH, Chen BM, et al. (2018). Pre-existing anti-polyethylene glycol antibody reduces the therapeutic efficacy and pharmacokinetics of PEGylated liposomes. Theranostics 8, 3164–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T, and Kiwada H (2013). Anti-polyethyleneglycol antibody response to PEGylated substances. Biol Pharm Bull 36, 889–891. [DOI] [PubMed] [Google Scholar]
- Jevsevar S, Kunstelj M, and Porekar VG (2010). PEGylation of therapeutic proteins. Biotechnol J 5, 113–128. [DOI] [PubMed] [Google Scholar]
- Judge A, McClintock K, Phelps JR, and Maclachlan I (2006). Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther 13, 328–337. [DOI] [PubMed] [Google Scholar]
- Koide H, Asai T, Hatanaka K, Akai S, Ishii T, Kenjo E, Ishida T, Kiwada H, Tsukada H, and Oku N (2010). T cell-independent B cell response is responsible for ABC phenomenon induced by repeated injection of PEGylated liposomes. Int J Pharm 392, 218–223. [DOI] [PubMed] [Google Scholar]
- Kolate A, Baradia D, Patil S, Vhora I, Kore G, and Misra A (2014). PEG - a versatile conjugating ligand for drugs and drug delivery systems. J Control Release 192, 67–81. [DOI] [PubMed] [Google Scholar]
- Lincoff AM, Mehran R, Povsic TJ, Zelenkofske SL, Huang Z, Armstrong PW, Steg PG, Bode C, Cohen MG, Buller C, et al. (2016). Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE-PCI): a randomised clinical trial. Lancet 387, 349–356. [DOI] [PubMed] [Google Scholar]
- Lipsky PE, Calabrese LH, Kavanaugh A, Sundy JS, Wright D, Wolfson M, and Becker MA (2014). Pegloticase immunogenicity: the relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res Ther 16, R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SB, Long MB, White RR, and Sullenger BA (2008). Crystal structure of an RNA aptamer bound to thrombin. RNA 14, 2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak RA, Calnan BJ, Frankel AD, and Sharp PA (1990a). HIV-1 Tat protein trans-activates transcription in vitro. Cell 63, 791–802. [DOI] [PubMed] [Google Scholar]
- Marciniak RA, Garcia-Blanco MA, and Sharp PA (1990b). Identification and characterization of a HeLa nuclear protein that specifically binds to the trans-activation-response (TAR) element of human immunodeficiency virus. Proc Natl Acad Sci U S A 87, 3624–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney MD, Wessler T, Price LSL, Ciociola EC, Herity LB, Piscitelli JA, Zamboni WC, Forest MG, Cao Y, and Lai SK (2018). A minimal physiologically based pharmacokinetic model that predicts anti-PEG IgG-mediated clearance of PEGylated drugs in human and mouse. J Control Release 284, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitragotri S, Burke PA, and Langer R (2014). Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov 13, 655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimjee SM, Oney S, Volovyk Z, Bompiani KM, Long SB, Hoffman M, and Sullenger BA (2009). Synergistic effect of aptamers that inhibit exosites 1 and 2 on thrombin. RNA 15, 2105–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimjee SM, White RR, Becker RC, and Sullenger BA (2017). Aptamers as Therapeutics. Annu Rev Pharmacol Toxicol 57, 61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povsic TJ (2016). Current State of Stem Cell Therapy for Ischemic Heart Disease. Curr Cardiol Rep 18, 17. [DOI] [PubMed] [Google Scholar]
- Povsic TJ, Lawrence MG, Lincoff AM, Mehran R, Rusconi CP, Zelenkofske SL, Huang Z, Sailstad J, Armstrong PW, Steg PG, et al. (2016). Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J Allergy Clin Immunol 138, 1712–1715. [DOI] [PubMed] [Google Scholar]
- Povsic TJ, Vavalle JP, Aberle LH, Kasprzak JD, Cohen MG, Mehran R, Bode C, Buller CE, Montalescot G, Cornel JH, et al. (2013). A Phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the REG1 system in patients with acute coronary syndromes: results of the RADAR trial. Eur Heart J 34, 2481–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povsic TJ, Wargin WA, Alexander JH, Krasnow J, Krolick M, Cohen MG, Mehran R, Buller CE, Bode C, Zelenkofske SL, et al. (2011). Pegnivacogin results in near complete FIX inhibition in acute coronary syndrome patients: RADAR pharmacokinetic and pharmacodynamic substudy. Eur Heart J 32, 2412–2419. [DOI] [PubMed] [Google Scholar]
- Powell Gray B, Kelly L, Ahrens DP, Barry AP, Kratschmer C, Levy M, and Sullenger BA (2018). Tunable cytotoxic aptamer-drug conjugates for the treatment of prostate cancer. Proc Natl Acad Sci U S A 115, 4761–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querques G, Bux AV, Martinelli D, Iaculli C, and Noci ND (2009). Intravitreal pegaptanib sodium (Macugen) for diabetic macular oedema. Acta Ophthalmol 87, 623–630. [DOI] [PubMed] [Google Scholar]
- Ramos AM, Gonzalez-Guerrero C, Sanz A, Sanchez-Nino MD, Rodriguez-Osorio L, Martin-Cleary C, Fernandez-Fernandez B, Ruiz-Ortega M, and Ortiz A (2015). Designing drugs that combat kidney damage. Expert Opin Drug Discov 10, 541–556. [DOI] [PubMed] [Google Scholar]
- Richter AW, and Akerblom E (1983). Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int Arch Allergy Appl Immunol 70, 124–131. [DOI] [PubMed] [Google Scholar]
- Richter AW, and Akerblom E (1984). Polyethylene glycol reactive antibodies in man: titer distribution in allergic patients treated with monomethoxy polyethylene glycol modified allergens or placebo, and in healthy blood donors. Int Arch Allergy Appl Immunol 74, 36–39. [DOI] [PubMed] [Google Scholar]
- Rusconi CP, Roberts JD, Pitoc GA, Nimjee SM, White RR, Quick G Jr., Scardino E, Fay WP, and Sullenger BA (2004). Antidote-mediated control of an anticoagulant aptamer in vivo. Nat Biotechnol 22, 1423–1428. [DOI] [PubMed] [Google Scholar]
- Rusconi CP, Scardino E, Layzer J, Pitoc GA, Ortel TL, Monroe D, and Sullenger BA (2002). RNA aptamers as reversible antagonists of coagulation factor IXa. Nature 419, 90–94. [DOI] [PubMed] [Google Scholar]
- Soule EE, Bompiani KM, Woodruff RS, and Sullenger BA (2016). Targeting Two Coagulation Cascade Proteases with a Bivalent Aptamer Yields a Potent and Antidote-Controllable Anticoagulant. Nucleic Acid Ther 26, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullenger B, Woodruff R, and Monroe DM (2012). Potent anticoagulant aptamer directed against factor IXa blocks macromolecular substrate interaction. J Biol Chem 287, 12779–12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullenger BA, Gallardo HF, Ungers GE, and Gilboa E (1990). Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell 63, 601–608. [DOI] [PubMed] [Google Scholar]
- Sullenger BA, and Nair S (2016). From the RNA world to the clinic. Science 352, 1417–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundy JS, Baraf HS, Yood RA, Edwards NL, Gutierrez-Urena SR, Treadwell EL, Vazquez-Mellado J, White WB, Lipsky PE, Horowitz Z, et al. (2011). Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA 306, 711–720. [DOI] [PubMed] [Google Scholar]
- Sundy JS, Becker MA, Baraf HS, Barkhuizen A, Moreland LW, Huang W, Waltrip RW, 2nd, Maroli AN, Horowitz Z, and Pegloticase Phase 2 Study, I. (2008). Reduction of plasma urate levels following treatment with multiple doses of pegloticase (polyethylene glycol-conjugated uricase) in patients with treatment-failure gout: results of a phase II randomized study. Arthritis Rheum 58, 2882–2891. [DOI] [PubMed] [Google Scholar]
- Sundy JS, Ganson NJ, Kelly SJ, Scarlett EL, Rehrig CD, Huang W, and Hershfield MS (2007). Pharmacokinetics and pharmacodynamics of intravenous PEGylated recombinant mammalian urate oxidase in patients with refractory gout. Arthritis Rheum 56, 1021–1028. [DOI] [PubMed] [Google Scholar]
- Swierczewska M, Lee KC, and Lee S (2015). What is the future of PEGylated therapies? Expert Opin Emerg Drugs 20, 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk C, and Gold L (1990). Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510. [DOI] [PubMed] [Google Scholar]
- Veronese FM, and Pasut G (2005). PEGylation, successful approach to drug delivery. Drug Discov Today 10, 1451–1458. [DOI] [PubMed] [Google Scholar]
- Wenande E, Kroigaard M, Mosbech H, and Garvey LH (2015). Polyethylene glycols (PEG) and related structures: overlooked allergens in the perioperative setting. A A Case Rep 4, 61–64. [DOI] [PubMed] [Google Scholar]
- Westrick RJ, Winn ME, and Eitzman DT (2007). Murine models of vascular thrombosis (Eitzman series). Arterioscler Thromb Vasc Biol 27, 2079–2093. [DOI] [PubMed] [Google Scholar]
- Woodruff RS, Xu Y, Layzer J, Wu W, Ogletree ML, and Sullenger BA (2013). Inhibiting the intrinsic pathway of coagulation with a factor XII-targeting RNA aptamer. J Thromb Haemost 11, 1364–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, and Harada A (2002). Supramolecular formation of antibodies with viologen dimers: utilization for amplification of methyl viologen detection signals in surface plasmon resonance sensor. Biomacromolecules 3, 1163–1169. [DOI] [PubMed] [Google Scholar]
- Yamasuji Y, Higashi Y, Sakanoue M, Katsue H, Kawai K, Arai N, and Kanekura T (2013). A case of anaphylaxis caused by polyethylene glycol analogues. Contact Dermatitis 69, 183–185. [DOI] [PubMed] [Google Scholar]
- Yang Q, Jacobs TM, McCallen JD, Moore DT, Huckaby JT, Edelstein JN, and Lai SK (2016). Analysis of Pre-existing IgG and IgM Antibodies against Polyethylene Glycol (PEG) in the General Population. Anal Chem 88, 11804–11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Sun F, Liu S, and Jiang S (2016). Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J Control Release 244, 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Lazar D, Li H, Xia X, Satheesan S, Charlins P, O’Mealy D, Akkina R, Saayman S, Weinberg MS, et al. (2018). Receptor-targeted aptamer-siRNA conjugate-directed transcriptional regulation of HIV-1. Theranostics 8, 1575–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 - Related to Figure 6 - Preliminary screening of rhesus plasma for anti-PEG IgG.
(a) Preliminary screening was performed on plasma samples from 73 young healthy rhesus monkeys (~2-3 years of age, ~3-5 kg; 35 males and 38 females) for the presence of anti-PEG antibodies using indirect ELISA. Pre-coated PEG-BSA 96-well plates were incubated with diluted plasma followed by rhesus monkey IgG specific detection antibody and absorbance was measured. Data represent the mean ± SEM of N=4.
(b) Re-screening for validation of rhesus monkey plasma that was positive from pre-screening in (a) using the identical indirect ELISA procedure was performed. Rhesus #34 (blue) was significantly higher in absorbance suggesting the presence of anti-PEG antibody. Preliminary screening was performed in different plates and on a different day, therefore re-screening any potential positive animals in one plate was accomplished for thorough analysis. Data represent the mean ± SEM of technical triplicates. ** denotes p-value <0.01 using t-test vs. control.
Figure S2 - Related to Figure 6 - Comparing post-aptamer rhesus plasma anti-PEG IgG levels for possible correlates
(a) Post-aptamer administration rhesus monkey plasma IgG absorbances (blue) were determined by ELISA as described in Supplemental Figure 1 compared by dose and route of delivery. While there appeared to be a higher average anti-PEG IgG level in the intravenous-administered group the variation was not statistically significant. There was no apparent trend when comparing aptamer dose. Each bar represents mean of technical replicates, N=9 wells from three rhesus monkeys combined. Error bars represent standard deviation (SD) of the mean.
(b) Post-aptamer administered rhesus plasma IgG absorbances (blue) were plotted against time of sample collection. Using a two-parameter analysis no statistically relevant correlation was observed suggesting that the time of collection had no influence on IgG levels. Each dot represents the mean of technical triplicates of one rhesus monkey.


