Abstract
Purpose
Dimethyl fumarate (DMF) has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of relapsing-remitting multiple sclerosis (RRMS), a demyelinating autoimmune disease characterized by acute episodes of motor, sensory, and cognitive symptoms. Optic neuritis is an episodic sequela experienced by some patients with RRMS that typically presents as acute, monocular vision loss. Episodes of optic neuritis damage and kill retinal ganglion cells (RGCs), and can culminate in permanent vision loss. The purpose of these studies was to evaluate the capacity of DMF to mitigate optic neuritis. The work presented combines studies of a mouse model of MS and a retrospective chart analysis of files of patients with RRMS treated at the MS Center of Excellence within the Oklahoma Medical Research Foundation.
Methods
Experimental autoimmune encephalomyelitis (EAE) is a well-established mouse model that recapitulates cardinal features of somatic and visual MS pathologies. EAE was induced in female C57BL/6J mice by inoculation with myelin oligodendrocyte glycoprotein peptide (residues 35–55; MOG35–55). DMF or vehicle was administered twice a day by oral gavage. Visual acuity was measured longitudinally with optokinetic tracking. Post-mortem analyses included quantification of RGCs in retinal flatmounts and quantitative PCR (qPCR) of Nrf2 target genes and regulators of myelin. Retrospective chart analyses were performed using data obtained from deidentified files of patients with RRMS.
Results
In the EAE mouse studies, DMF decreased optic neuritis severity, preserved vision and RGCs, and concomitantly reduced motor deficits when administered by two different treatment regimens (prevention or interventional). DMF was more efficacious when administered as an interventional therapy, and the beneficial effects occurred independently of the induction of Nrf2 target genes. A complementary retrospective chart analysis demonstrated that DMF increased the time to a recurrence of optic neuritis, and protected against subsequent bouts of optic neuritis.
Conclusions
This work underscores the potential of DMF to mitigate the severity and recurrence of optic neuritis episodes in patients with RRMS.
Introduction
Dimethyl fumarate (DMF) is a small molecule approved by the U.S. Food and Drug Administration (FDA) to treat multiple sclerosis (MS), a neuroinflammatory demyelinating disease of the central nervous system (CNS). An estimated 85% of patients have relapsing-remitting MS (RRMS) [1], a classification characterized by acute episodes of symptoms with substantial recovery between flare-ups. The relapsing-remitting pattern is attributable to disrupted neuronal signal transduction caused by CNS inflammation and demyelination, followed by resolution and remyelination during remission. Most patients with RRMS accumulate significant axonal damage and neurodegeneration over their disease course, and a concomitant failure to recover between episodes. Incomplete remission between relapses constitutes a transition from RRMS to secondary progressive MS (SPMS).
RRMS treatment goals include preventing relapses and the resulting accumulation of neuronal damage to prolong remission, and thus, stave off progression to SPMS. DMF is an oral RRMS treatment that showed efficacy in decreasing relapse rate, as well as magnetic resonance imaging (MRI)-enhanced demyelinating lesions and disability progression in randomized, double-blind, placebo-controlled studies [2]. These studies included phase II and two phase III trials (namely, Determination of the Efficacy and Safety of Oral Fumarate in RRMS (DEFINE) and Comparator and an Oral Fumarate in RRMS (CONFIRM)) that cumulatively followed nearly 3,000 patients with RRMS. Notably, these trials did not take symptom or affected CNS compartment into account. Although the mechanisms of action for DMF are incompletely characterized, the compound has been shown to reduce CNS inflammation, and in some experimental paradigms, to activate the transcription factor, nuclear factor (erythroid-derived 2)-like 2 (Nrf2) [3]. Studies using the experimental autoimmune encephalomyelitis (EAE) mouse model of MS have shown Nrf2-independent efficacy of DMF (administered in a preventive regimen) in acute EAE [4], but a dependence on Nrf2 for mitigating motor deficits in chronic EAE when DMF was administered as an interventional therapy [5].
Greater than 50% of patients with MS experience optic neuritis (inflammation of the optic nerve) during disease progression, and it is the initial clinical event for about 20% of patients newly diagnosed with MS [6]. Optic neuritis onset can occur over the course of hours, and most commonly presents as painful vision loss in one eye that lasts several weeks. Episodes of optic neuritis cause retinal ganglion cells (RGCs), the axons of which comprise the optic nerve, to degenerate, resulting in decreased optic nerve conduction and incomplete visual recovery [7]. Notably, similar visual acuity deficits and RGC degeneration are observed in the murine EAE model (e.g., [8,9]). The current standard of care for optic neuritis is intravenous steroid administration, which can decrease the recovery time from a given flare-up, but has no effect on the subsequent relapse rate, final visual outcome, or RGC degeneration and survival [10].
The objective of the present study was to determine whether DMF confers protection against visual pathology and optic neuritis. We used the murine EAE model of MS to compare preventive and interventional administration regimens, and found that DMF was efficacious at mitigating the severity of optic neuritis in both regimens, but was more effective as an interventional treatment. A complementary retrospective chart analysis showed that patients with a previous history of optic neuritis episodes who were maintained on DMF were largely protected from recurrent episodes. Together, these findings underscore the potential of DMF to mitigate the severity and recurrence of optic neuritis in patients with RRMS.
Methods
Mice
All animal care and experimental procedures were performed in compliance with the Oklahoma Medical Research Foundation Institutional Animal Care and Use Committee–approved protocol, and complied with standards delineated by the Institute for Laboratory Animal Research. The studies herein adhered to the ARVO statement for the Use of Animals in Research. All studies used C57BL/6J female mice purchased from Jackson Laboratories (Bar Harbor, ME; stock # 000664). Mice were euthanized with CO2 asphyxiation followed by cardiac perfusion with PBS (10X; 1.37M NaCl, 27 mM KCl, 101 mM Na2HPO4, 18 mM KH2PO4, pH 7.4).
Induction of EAE
Fourteen-week-old mice were immunized subcutaneously with 150 μg of myelin oligodendrocyte glycoprotein peptide (residues 35–55; MOG35–55; Bio-Synthesis, TX) emulsified in complete Freund’s adjuvant (CFA; Chondrex, Redmond, WA) containing 2.5 mg/ml heat-killed Mycobacterium tuberculosis. Mice were injected intraperitoneally with 250 ng Bordetella pertussis toxin (Chondrex) the day of, and again, 2 days following MOG35–55 immunization. At 21–28 days post-immunization, mice were euthanized for histological analyses. The mice were euthanized by CO2 asphyxiation for 10 min from a compressed gas tank followed by decapitation or cardiac perfusion. Following perfusion, mice were immediately decapitated. EAE clinical manifestations of progressive ascending paralysis were assessed daily using a standard scoring system: 0 = no overt symptoms, 1= loss of tail tone, 2 = hind limb paresis, 3 = complete hind limb paralysis, 4 = hind limb paralysis and forelimb paresis, and 5 = moribund or dead. The mice were weighed daily to ensure weight loss did not exceed 25% of starting weight.
DMF administration by oral gavage
Dimethyl fumarate (DMF; Alfa Aesar, Tewksbury, MA) was freshly prepared in 0.08% methylcellulose (MC) before each dosing. Ten microliters per gram of mouse weight of DMF or MC (vehicle) was administered twice daily, at approximately 7 a.m. and 7 p.m., using plastic gavage needles (Instech Laboratories Inc., Plymouth Meeting, PA; Cat # FTP-20–38–250) to provide a total daily dose of 30 mg/kg.
Visual acuity assessment
Visual acuity threshold was measured daily by optokinetic tracking (OKT) response using Optometry software and apparatus (CerebralMechanics Inc., Medicine Hat, AB T1A 5K6, Canada), as previously described [8,9,11]. Briefly, the mice were surrounded by a virtual cylinder consisting of vertical lines rotating at varying frequencies, and tracking behavior was assessed. Visual acuity is represented as the highest spatial frequency at which the mice track the rotating cylinder. Because optokinetic tracking is a temporal-to-nasal reflex, counter-clockwise and clockwise rotations exclusively test the right and left eyes, respectively. Occasionally, EAE-induced motor deficits left mice without the balance and stability to adequately perform OKT testing. In these instances, a measurement was not recorded for the affected mouse on that day.
Retinal flatmount analysis
Eyes were enucleated and fixed in 10% neutral-buffered formalin (NBF) for up to 8 h. Retinas were isolated under a dissecting microscope (Olympus SZ-PT, Center Valley, PA), permeabilized in 1% Triton X-100/PBS for 30 min, and then incubated for 2 h in blocking solution (10% donkey serum + 3% bovine serum albumin [BSA] in PBS). Immunolabeling was done by overnight incubation with the RGC-specific antibody anti-Brn3a (goat; Santa Cruz Biotechnology, Santa Cruz, CA; sc31984; 1:500) in blocking solution at 4 °C. The following day, the retinas were washed in PBS, subjected to a series of relaxing cuts under a dissecting microscope, and incubated with Alexa546nm Fluor-conjugated donkey anti-goat immunoglobulin G (IgG; Molecular Probes, Eugene, OR; 1:1,000) and Hoechst 33342 (Molecular Probes; 2 µg/ml) in 3% BSA/PBS for 1 h. The retinas were washed in PBS before mounting with Prolong Gold mounting medium (Life Technologies, Grand Island, NY). Retinas were examined with a Nikon 80i microscope equipped with a 20X objective, and images were captured with a DXM1200C camera using NIS-Elements software (Nikon, Inc. Tokyo, Japan). Photomicrographs were captured from the four leaflets comprising the flatmount, with representative images captured from the peripheral, medial, and central retina within each quadrant, totaling 12 pictures per retina. Images were cropped to 325 μm2, and contrast-enhanced in Adobe Photoshop 8.0, using NIH ImageJ software. Brn3a-positive cells were counted manually.
Quantitative real-time PCR
After dissection, optic nerves and spinal cords were immersed in 250 µl of TRI Reagent® (Sigma-Aldrich, Co., St. Louis, MO) or 500 µl RNAlater® (Sigma-Aldrich), respectively, immediately frozen, and stored at −80 °C until processing. After thawing, the spinal cords were transferred into fresh 500 µl TRI Reagent. Total RNA was isolated with phenol-chloroform extraction, and further purified using Direct-zolTM RNA MicroPrep Plus (Zymo Research, Irvine, CA) or Direct-zolTM RNA MiniPrep Plus (Zymo Research) for optic nerve and spinal cord samples, respectively. RNA concentration was determined with a NanoDrop 2000 spectrophotometer (ThermoFisher Scientific, Waltham, MA). Approximately 200 ng of RNA from the optic nerve samples and 1 µg of RNA from the spinal cord samples were transcribed to first-stranded cDNA with qScriptTM cDNA SuperMix (Quanta Bio, Beverly, MA). Quantitative real-time PCR (qPCR) was performed using primers (see Table 1) and PowerUpTM SYBRTM Green Master Mix (ThermoFisher Scientific). mRNA levels were measured as the relative ratio to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. The specificity of the qPCR reactions was analyzed using melting curve analysis.
Table 1. Retrospective chart analysis.
| Gene symbol | Sequences (5′- 3′) |
|---|---|
| GAPDH |
F: GTGGAGTCTACTGGTGTCTTCA |
| R: TTGCTGACAATCTTGAGTGAGT | |
| HO-1 |
F: GTGATGGAGCGTCCACAGC |
| R: TGGTGGCCTCCTTCAAGG | |
| NQO1 | F: GGAAGCTGCAGACCTGGTGA |
| R: CCTTTCAGAATGGCTGGCA |
The retrospective chart analysis included in this manuscript was approved by the IRB committee at the Oklahoma Medical Research Foundation (Protocol 17–25 to Dr. Tania Reyna, MD). The study title is “Effects of Dimethyl Fumarate on Optic Neuritis and Gait Dysfunction in Patients with Multiple Sclerosis.” Chart analyses adhered to the tenets of the Declaration of Helsinki and the ARVO statement on human subjects.
Statistical analysis
Confidence intervals and p values for the statistical significance of each studied effect in the longitudinal binocular EAE mouse studies were determined by fitting the data to a linear mixed-effects model, using the lme function implemented in the nlme R package, as detailed in [8,12-14]. This function is an extended version of regular linear regression, but can accommodate complex data collection design features, such as longitudinal measurements, nested layers, and within-group correlation. Standard testing methods, such as the Student unpaired t test and the Mann–Whitney U test, were employed when no repeated measures or embedding was involved. The data from the retrospective chart analysis were subjected to a multivariate analysis with Cox regression to determine the impact of Tecfidera™ (Biogen, Cambridge, MA), along with the fixed covariates of sex and age at the time of diagnosis, on the time between recurrences of optic neuritis. The same data were subjected to Kaplan–Meier estimation and log-rank testing to determine differences in the time to a recurrence of optic neuritis before and after a positive diagnosis of MS and after initiation of Tecfidera™ treatment.
Results
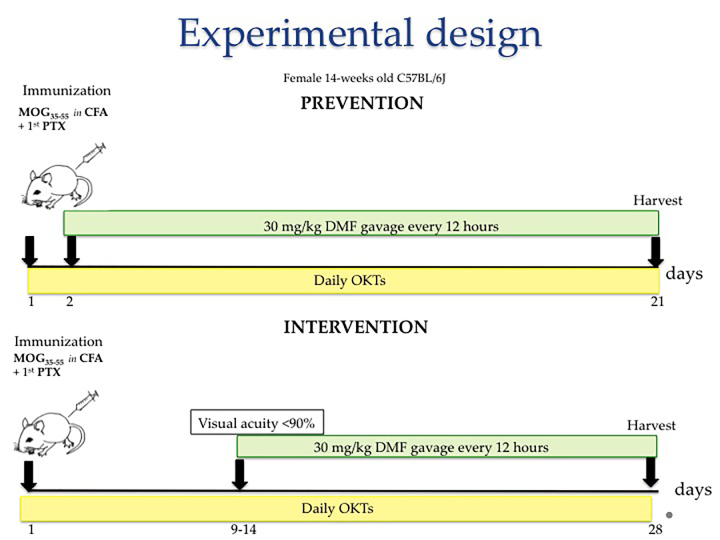
To investigate the capacity of DMF to mitigate optic neuritis, female mice were subjected to the acute EAE MOG35–55 disease model [4]. The focus on female mice was predicated on the finding that 77% of acute demyelinating optic neuritis episodes occur in women [15]. Fourteen-week-old, wild-type C57BL/6J female mice were subjected to EAE and either a prevention or intervention DMF treatment regimen (Figure 1). On day 1, the mice were immunized subcutaneously with 150 µg of myelin oligodendrocyte glycoprotein (MOG) peptide MOG35–55 emulsified in complete Freund’s adjuvant, and intraperitoneally with 250 ng Bordetella pertussis toxin. Two days later, the animals were again administered the toxin to further compromise the blood–brain barrier. Motor deficits were evaluated daily using an established scoring system from 0 to 5 described previously [8,9]. Visual acuity was measured daily by OKT response. For the prevention regimen, animals were administered DMF twice daily by oral gavage (at 7 a.m. and 7 p.m.) starting on day 2 (Figure 1, top). For the interventional regimen, DMF was administered twice daily starting the day after the first decrease in visual acuity exceeded 10% compared to the baseline (Figure 1, bottom). For both regimens, DMF treatment was continued until the experiments were terminated, typically 20–28 days post-immunization. Control mice in both regimens received 0.08% methylcellulose (vehicle) twice daily by oral gavage.
Figure 1.
Experimental design. Fourteen-week-old female C57BL/6J mice were subjected to an experimental autoimmune encephalomyelitis (EAE) protocol along with either a “Prevention” (top timeline) or “Intervention” (bottom timeline) treatment regimen of 30 mg/kg dimethyl fumarate (DMF) or methylcellulose (vehicle) administered twice a day by oral gavage. For the prevention regimen, administration of DMF was initiated the day after mice were immunized with myelin oligodendrocyte glycoprotein (residues 35–55; MOG35–55), whereas for the interventional regimen, treatment was initiated when motor deficits were observed, and visual acuity decreased by at least 10% from baseline. Once initiated, treatments were given until animals were euthanized. Optokinetic tracking (OKT) responses and motor scores were recorded daily.
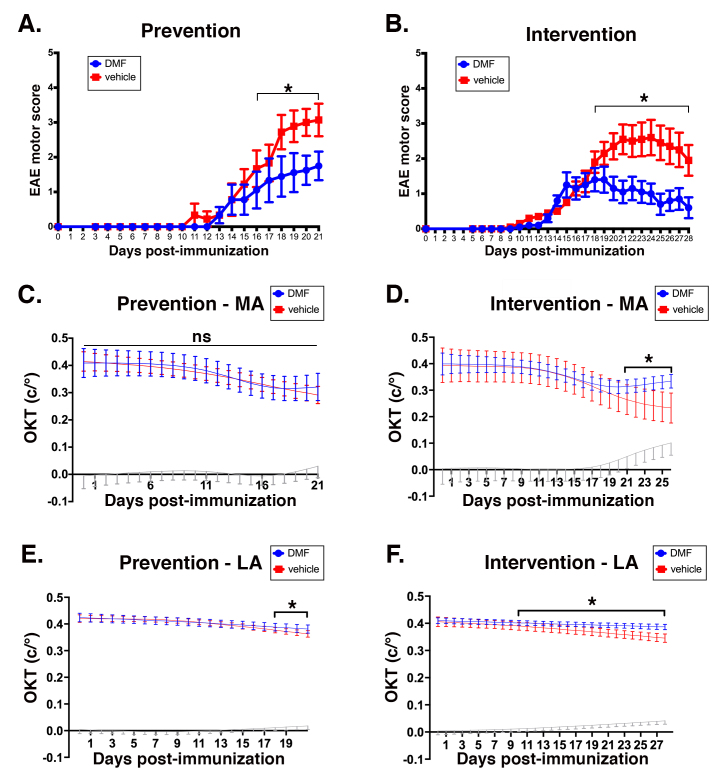
Motor deficits were initiated 10–14 days post-immunization, and were attenuated in severity by DMF under both regimens (Figure 2A,B). Optic neuritis and onset of visual impairment were concomitant with onset of motor deficits, as we reported previously [8,9]. Because optic neuritis is typically monocular, each eye was tracked separately by OKT, with the more affected eye designated “MA,” and the less affected eye designated “LA.” This monocular optic neuritis in EAE mirrors what is observed in patients with MS [15]. For the prevention protocol, although DMF mitigated the motor deficits, the compound provided minimal protection of vision, and surprisingly, only in the LA eye during the final few days of treatment (Figure 2C,E). In contrast, administration of DMF in the interventional protocol mitigated the motor deficits (Figure 2B), and preserved vision in both eyes (Figure 2D,F). As the graphs show mean OKT readings for all animals on a particular regimen as a function of days post-immunization, the decreases in visual acuity appear to be relatively small. However, the timing of the respective cycles of optic neuritis and recovery for individual animals varied, although all were immunized on the same day with the same antigenic mixture. A consequence of this asynchrony is that, on a given day, some mice were losing vision as others were recovering it [8,9].
Figure 2.
DMF treatment mitigates EAE-induced motor and visual deficits. A, B:Comparison of motor scores between dimethyl fumarate (DMF; blue) and methylcellulose (vehicle; red) in a prevention regimen (A) versus an interventional regimen (B). Motor scores were recorded daily, and are graphed as mean scores versus days post-immunization with the myelin oligodendrocyte glycoprotein (residues 35–55; MOG35–55) peptide. C, D:Mean optokinetic tracking (OKT) visual acuity recordings in the more affected (MA) eyes of mice on the DMF prevention protocol (C) or the interventional protocol (D) as a function of days post-immunization. Blue traces represent mean readings for DMF-treated mice, and red traces represent mean readings for vehicle-treated animals. E, F: Same as C and D, but for the less affected (LA) eyes. For C–F, the gray trace on each graph depicts the difference between DMF and vehicle, such that the difference is statistically significant when the lower value of the confidence bar is above the zero value on the axis of abscissa. OKT recordings were measured as cycles per degree (c/°). For all graphs, “ns” indicates not statistically significant, and an asterisk and bracket indicate a statistically significant (p<0.05) difference between DMF and vehicle for each time point under the bracket, as calculated with the linear mixed effects model. Error bars represent the standard error of the mean (SEM). n=9 mice for each group in the prevention regimen, and n=8 mice for each group in the interventional regimen.
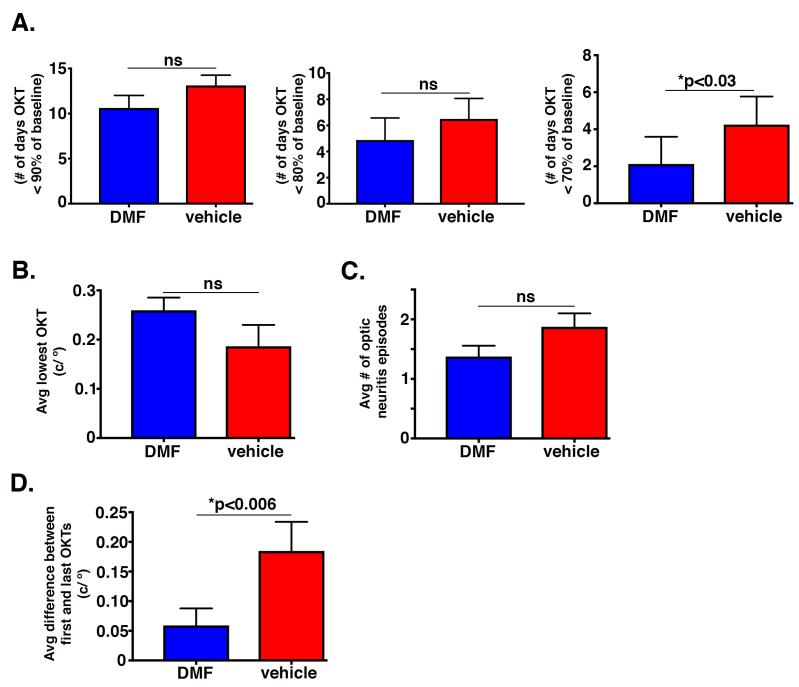
In addition to comparing mean OKTs, we compared interventional and preventive regimens for the [1] average number of days that mice registered an OKT deficit of 10%, 20%, or 30% below their individual respective baselines (Figure 3A and Appendix 1; left, center, and right graphs, respectively) [2], average lowest OKT recorded (Figure 3B and Appendix 1) [3], average number of episodes of optic neuritis (Figure 3C and Appendix 1), and [4] average difference between the first and last OKT readings (Figure 3D and Appendix 1). For both treatment regimens, each comparison trended with the beneficial effects of DMF, but due to the animal-to-animal variability inherent to the EAE model (e.g., [16]), only two of the comparisons reached statistical significance: (a) the number of days that DMF prevented the OKT readings from dropping 30% or greater below the baseline compared to vehicle (Figure 3A and Appendix 1) and (b) the average difference between the first and last OKT readings (Figure 3D and Appendix 1). Because the difference between the first and last OKT readings represents the capacity to preserve visual acuity over the course of treatment, we regard this difference as the most clinically relevant metric indicative of the efficacy of DMF to mitigate the severity of optic neuritis.
Figure 3.
Interventional DMF treatment preserves visual acuity. A: Graph of the average number of days that mice on the interventional regimen registered an optokinetic tracking (OKT) reading deficit of 10% (left graph), 20% (center graph), or 30% (right graph) below their respective individual baseline OKT readings as a function of dimethyl fumarate (DMF; blue bar) or vehicle (red bar). B: Graph of the average lowest OKT readings for mice on the interventional treatment with DMF or vehicle. C: Graph of the average number of optic neuritis episodes for mice on the interventional regimen with DMF or vehicle. D: Graph of the average difference between the baseline and final OKT readings for mice on the interventional regimen. Statistics for all graphs determined with the Mann–Whitney U test, an asterisk denotes statistical significance, “ns” denotes not significant, and error bars represent standard error of the mean (SEM); n=8 mice for each treatment group.
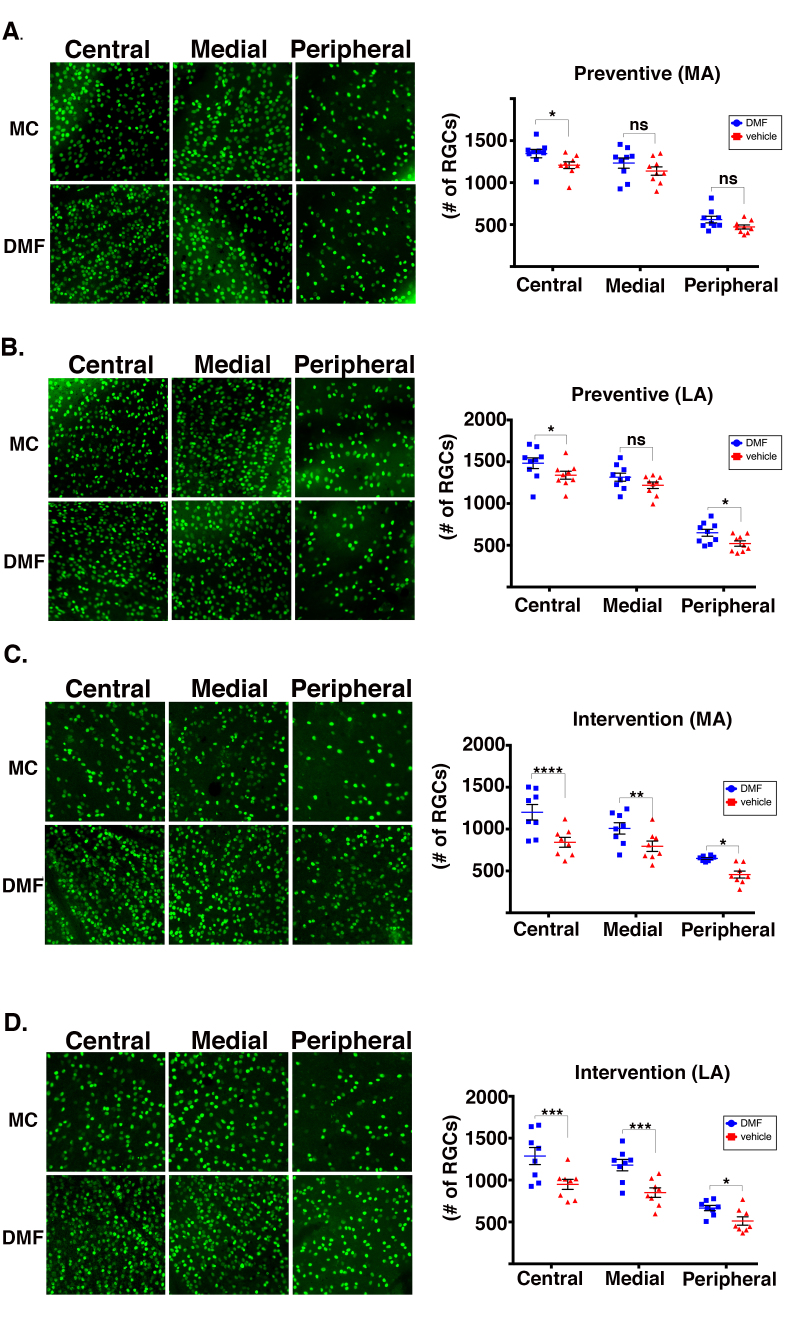
We next analyzed the protective effects of DMF on retinal ganglion cell (RGC) survival. RGC axons comprise the retinal nerve fiber layer and optic nerve. Repeated bouts of optic nerve inflammation cause RGC loss, and can culminate in irreversible visual deficits in humans [17-19] and mice [8,9]. The mice were euthanized at the termination of the treatment regimens, the eyes were enucleated, and retinal flatmounts were prepared and incubated with an anti-Brn3A antibody to immunolabel RGCs, as we reported previously [8]. For each retina, RGCs were counted from 12 representative fields encompassing the central, medial, and peripheral regions (four fields/region). Anatomically, the density of RGCs is central > medial > peripheral [9]. For the prevention regimen, DMF preserved RGCs in the central retina of the MA and LA eyes, and in the peripheral retina of the LA eyes (Figure 4A,B, respectively). DMF in the interventional regimen protected against RGC loss in all three regions of the MA and LA eyes (Figure 4C,D, respectively). Notably, the extent of RGC preservation in the central retina in both eyes was greater in the interventional regimen compared to the prevention regimen (Figure 4C,D graphs versus Figure 4A,B graphs), consistent with the relatively better preservation of visual acuity in the interventional scheme (Figure 2).
Figure 4.
DMF treatment preserves RGCs. Representative photomicrographs and accompanying graphs showing average total regional retinal ganglion cells (RGCs) in mice treated with either dimethyl fumarate (DMF; blue squares) or vehicle (red triangles). Data from the preventive regimen for the more affected eye (MA; A) and the less affected eye (LA; B). Data for the interventional regimen, the MA eye (C) and the LA eye (D). All photomicrographs were captured with a 20X inverted objective. Error bars represent the standard error of the mean (SEM). All statistical analysis performed with the linear mixed-effects model. **** p<0.0001; *** p<0.001; ** p<0.01; * p<0.05; ns p>0.05 by differential effect. n=9 mice per group for the preventive group and n=8 mice per group for the interventional group.
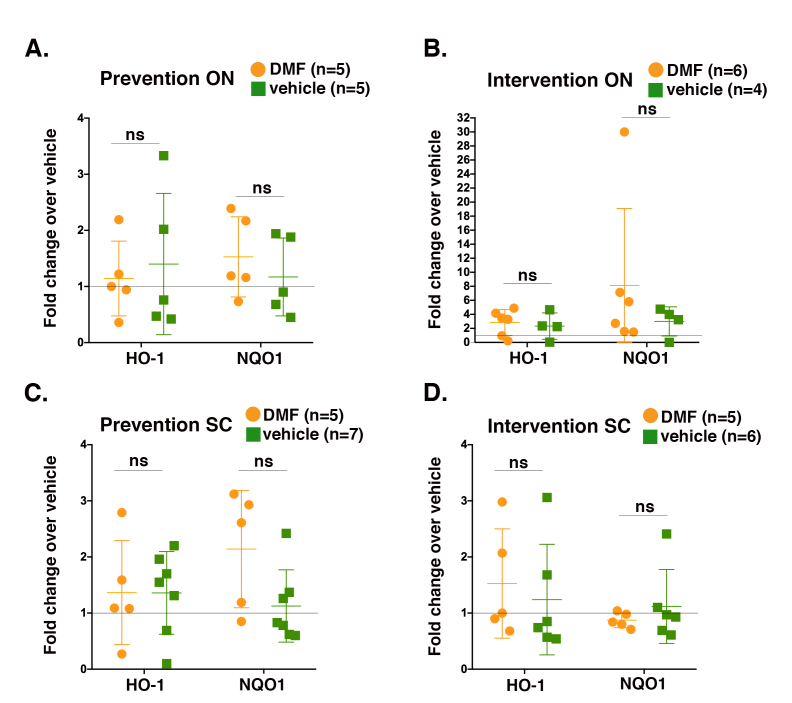
The efficacy of DMF at mitigating EAE-induced motor deficits has been attributed in part to the activation of Nrf2 [5]. Linker et al. reported that Nrf2 was required for the DMF-mediated reduction of motor deficits in a chronic EAE MOG35–55 disease model in which DMF was administered as an interventional therapy. However, the transcription factor was found to be dispensable for DMF efficacy against motor deficits in an acute version of the same mouse model in which the compound was given as a preventive regimen [4]. As neither study analyzed vision or optic nerves, we isolated RNA from the optic nerves of the MA eyes, as well as from the spinal cords of the same animals, and performed qPCR analysis to determine whether DMF induced the expression of prototypical Nrf2 target genes, namely, heme-oxygenase 1 (HO-1) and NAD(P)H:quinone oxidoreductase (NQO1). We did not detect statistically significant increases in the mRNA expression of either message in response to DMF in the optic nerves (Figure 5A,B) or spinal cords (Figure 5C,D) with either treatment regimen. These data are consistent with DMF conferring protection in acute EAE independently of Nrf2 [4].
Figure 5.
DMF does not induce the Nrf2 target genes HO-1 and NQO1 in the optic nerves or spinal cords of mice experiencing acute EAE. Quantitative real-time PCR (qPCR) data measuring the mRNA expression of the Nrf2 target genes HO-1 and NQO1 from either the optic nerves of the more affected (MA) eyes (A and B) or the spinal cords (C and D) of mice subjected to the indicated treatment regimens. Data graphed as the fold change in the expression of the dimethyl fumarate (DMF)-derived samples compared to that of the vehicle-derived samples. Each qPCR reaction was performed in triplicate (three technical replicates) on the indicated number of mice. Error bars represent ± standard deviation (SD). Data were analyzed with the Student t test, with p<0.05 indicating statistical significance; no values reached statistical significance. ON = optic nerve; SC = spinal cord.
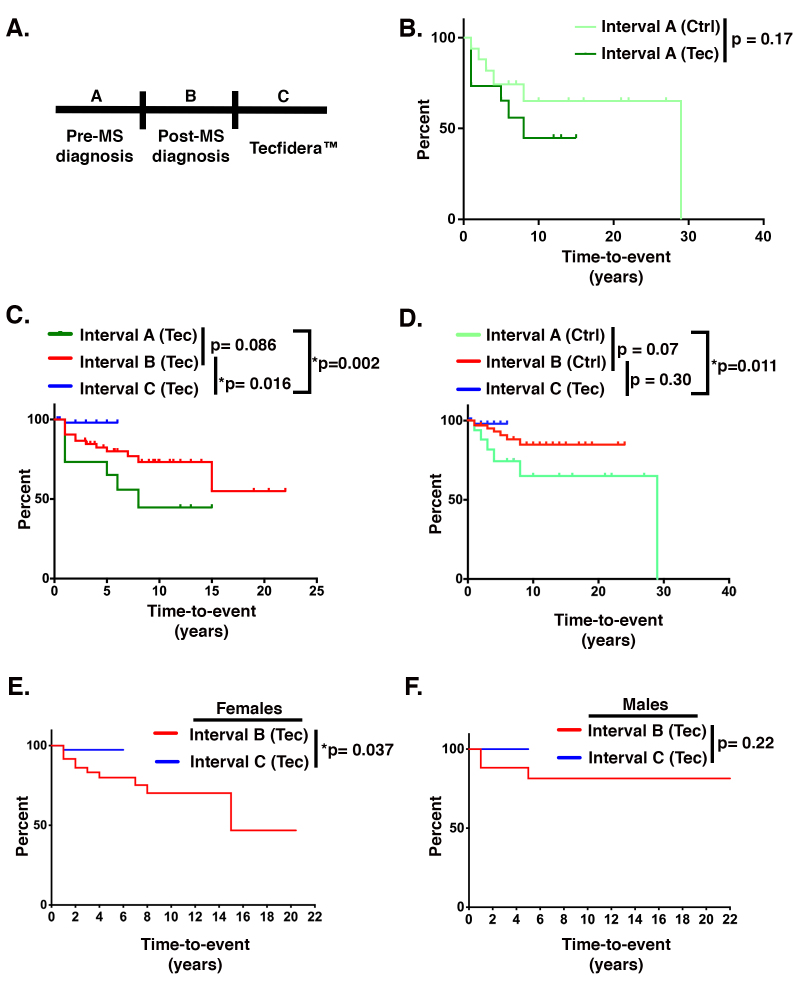
To investigate whether these results from the murine EAE model relate to the clinical efficacy of DMF, we performed a retrospective chart analysis of patients treated at the Oklahoma Medical Research Foundation Multiple Sclerosis Center of Excellence Clinic in Oklahoma City, Oklahoma, since 2013. Note, we refer to DMF by its trade name, Tecfidera™, for this retrospective chart study as all patients were administered the compound manufactured by Biogen. Table 2 details the exclusion criteria, and Table 3 lists the treatment modalities for all patients analyzed. All patients received an RRMS diagnosis based on the 2010 Revised McDonald Criteria for MS. In accordance with standard of care for treating MS, patients maintained on Tecfidera™ (240 mg twice daily) were not concomitantly taking other disease-modifying therapies. Using the Cox proportional hazards regression, we analyzed time-to-event dependence, with the event defined as an episode of optic neuritis. Sex and age at the time of diagnosis were included as fixed covariates, and durations between the last disease occurrence and last patient visits were included in the model as censored observations. Neither sex nor age showed statistically significant effects (p values of 0.58 and 0.1, respectively), and notably, did not diminish the impact significance of Tecfidera™ (p value=0.009). Further, time-to-event data were plotted as Kaplan–Meier curves. We compared the time-to-event distribution for this patient cohort during three intervals: (a) before a diagnosis of MS (Interval A), (b) following a diagnosis of MS and the beginning of any treatment (Interval B), and (c) following the initiation of Tecfidera™ (Interval C; Figure 6A). Likewise, we performed a similar time-to-event analysis on a cohort of DMF-naïve patients using only Intervals A and B. All patients in both cohorts experienced at least one episode of optic neuritis (Appendix 2). The Tecfidera™ treatment cohort consisted of 39 women and 12 men, and the control cohort had 40 women and 13 men. The average age of patients at the initiation of the administration of Tecfidera™ was 44±10 years for women and 46±11 years for men. The average age of the control patients at the time of the MS diagnosis and treatment initiation was 30±12 years for women and 38±12 years for men.
Table 2. Patient criteria for retrospective chart analysis.
| Description | # of patients |
|---|---|
| Patients taking Tecfidera™ |
369 |
| Patients that discontinued Tecfidera™ |
-207 |
| Optic neuritis-naïve patients |
-109 |
| Patients with unknown dates for |
-2 |
| optic neuritis episodes | |
| Patients taking Tecfidera™ analyzed in this study |
51 |
| (39 females, 12 males) | |
Exclusion criteria and patient numbers used for retrospective chart analysis. An initial analysis revealed that the OMRF Multiple Sclerosis Center of Excellence had treated 369 patients with Tecfidera™ since 2013. The exclusion criteria are accompanied by a minus sign to indicate the number of patients excluded. The final analysis included 51 Tecfidera™-treated patients (39 females and 12 males).
Table 3. Treatment modalities for each patient included in the retrospective chart analysis.
| Tecfidera™ (n=51) |
Control (n=53) |
|||
|---|---|---|---|---|
| Females (n=39) | Males (n=12) | Females (n=40) | Males (n=13) | |
| Average # of drugs before Tecfidera™ |
1.92 ± 1.46 |
2.08 ± 1.78 |
n/a^ |
n/a |
| Average # of drugs |
n/a |
n/a |
1.78 ± 0.89 |
1.69 ± 0.75 |
| Number of patients taking each of the following drugs | ||||
| None or IVS* |
3 |
1 |
1 |
0 |
| Interferon β-1a |
24 |
7 |
12 |
5 |
| Interferon β-1b |
8 |
3 |
4 |
1 |
| Glatiramer acetate |
24 |
7 |
37 |
13 |
| Fingolimod |
6 |
2 |
6 |
1 |
| Teriflunomide |
0 |
0 |
5 |
0 |
| Mitoxantron |
1 |
1 |
0 |
0 |
| Natalizumab |
4 |
1 |
4 |
1 |
| Ocrelizumab |
0 |
0 |
1 |
1 |
| Alemtuzumab |
0 |
0 |
0 |
0 |
| Glatiramer acetate + IVS* |
4 |
0 |
0 |
0 |
| Cladribine |
1 |
0 |
0 |
0 |
| Interferon β-1b + IVS* |
1 |
1 |
0 |
0 |
| CCT + Interferon β-1b + IVS* |
1 |
1 |
0 |
0 |
| Interferon β-1a + IVS* |
0 |
1 |
0 |
0 |
| Intravenous Immunoglobulin |
0 |
1 |
0 |
0 |
| Laquinimab | 0 | 0 | 1 | 0 |
* Intravenous steroids, Interferon β-1α: Avonex, Plegridy, Rebif, ^ not applicable
Figure 6.
Tecfidera™ maintenance therapy mitigates optic neuritis in patients with RRMS. A: Schematic of time-to-event intervals analyzed in the retrospective chart study. Interval A corresponds to the time before a diagnosis of multiple sclerosis (MS), Interval B corresponds to time following a diagnosis of MS and the beginning of any treatment, and Interval C corresponds to the time during which patients were maintained on Tecfidera™. All subsequent graphs in this figure (B–F) are cumulative time-to-event curves derived with the Kaplan–Meier nonparametric estimation. Percent on the y-axis indicates the estimated probability of a new episode of optic neuritis not to occur before a given time represented in years on the x-axis. As the overall percentages decrease, the likelihood of shorter times to a recurrence of optic neuritis increases. Consequently, the curves closer to the horizontal axis correspond to patients being sicker. The differences between the Kaplan–Meier curves were assessed with a nonparametric log-rank test. p values less than or equal to 0.05 are considered statistically significant and are marked with asterisks. B: Time-to-event curve comparing Interval A between the control patients (Ctrl) and the patients maintained on Tecfidera™ (Tec). C:Comparison within the Tecfidera™-treated cohort of the time to a recurrence of optic neuritis before an MS diagnosis (Interval A, dark green trace), after MS diagnosis (Interval B, red trace), and during maintenance on Tecfidera™ (Interval C, blue trace). P values are shown for comparisons of Interval A versus B, Interval B versus C, and Interval A versus C. D: Comparison within the control cohort of the time to a recurrence of optic neuritis before (Interval A, lime green trace) and after (Interval B, red trace) a definitive diagnosis of MS and the initiation of treatment. This graph also compares the time to a recurrence of optic neuritis for the Tecfidera™-treated cohort during maintenance therapy on Tecfidera™ (Interval C; blue trace) to each interval of the control cohort (Interval A; lime green trace and Interval B; red trace). E, F: Analysis of Interval B versus C for the Tecfidera™ patient cohort divided into women (E) and men (F). For all graphs, asterisks denote statistical significance for p0.05.
The control and Tecfidera™ groups were not statistically significantly different in time-to-event before a diagnosis of MS (i.e., comparing Interval A between the two cohorts; Figure 6B). When we compared the time to recurrence within each cohort before and after a diagnosis of MS and the initiation of treatment (i.e., Interval A versus Interval B within each cohort), the Tecfidera™-treated patients (Figure 6C) and the Tecfidera™-naïve patients (Figure 6D) had increased times to recurrence that strongly trended toward statistical significance, but did not reach a p value 0.05, likely due to the relatively small number of patients in the study. Most importantly, a significantly increased time to a recurrence of optic neuritis was observed when patients were switched from other therapies to Tecfidera™ (i.e., Interval B versus Interval C within the Tecfidera™-treated cohort; Figure 6C). Likewise, Tecfidera™ conferred an even greater increased time to a recurrence when compared to the interval before which patients received a positive MS diagnosis (i.e., Interval A versus Interval C; Figure 6C). Strikingly, only two out of 51 patients (one woman and one man) experienced a recurrence of optic neuritis while taking Tecfidera™ (Appendix 2). When the women and men in the Tecfidera™-treated cohort were evaluated separately for time to recurrence of optic neuritis before and after the administration of Tecfidera™ (i.e., Interval B versus Interval C), only the women reached statistical significance (Figure 6E,F, respectively). The lack of statistical significance for the men is likely due to the sample size (approximately fourfold fewer men than women in the study), as only one out of the 12 men had a single recurrence of optic neuritis while taking Tecfidera™ (Appendix 2). Last, a comparison of the time to a recurrence between the treatment of the control cohort for MS and the treatment of the Tecfidera™ cohort with Tecfidera™ did not reach statistical significance (Figure 6D; Control cohort Interval B versus Tecfidera™ cohort Interval C). A summation of these findings is provided in Table 4.
Table 4. Summary of statistical analyses from retrospective chart study.
| Control cohort intervals | Tecfidera™ cohort intervals | p value | Figure |
|---|---|---|---|
| A |
A |
0.17 |
6B |
| - |
A v. B |
0.086 |
6C |
| - |
B v. C |
0.016* |
6C |
| - |
A v. C |
0.002* |
6C |
| A v. B |
- |
0.07 |
6D |
| B |
C |
0.3 |
6D |
| A |
C |
0.011* |
6D |
| - |
B v. C (females) |
0.037* |
6E |
| - | B v. C (males) | 0.22 | 6F |
* indicates statistically significant (p ≤ 0.05)
Discussion
Tecfidera™ is an orally administered fumarate ester approved by the FDA in 2013 for treating patients with RRMS. The phase III clinical trials, DEFINE and CONFIRM, demonstrated that Tecfidera™ decreased the annualized relapse rates for patients with RRMS, the disability progression, and the formation of active lesions detected with brain MRI [2]. Tecfidera™ was tested as a potential therapeutic for MS based on the success of the compound in treating psoriasis, a skin disorder with immunological overlap to MS [20], along with a well-established safety profile in humans [21].
Retrospective analyses of data from the DEFINE and CONFIRM studies promoted the utility of Tecfidera™ as a first-line treatment option for patients with RRMS [22-26]. However, to date, whether Tecfidera™ impacts the recurrence or severity of optic neuritis has not been reported, as this sequela was not monitored in the DEFINE or CONFIRM trials. Likewise, the capacity of DMF to mitigate optic neuritis episodes or severity in the commonly used EAE MOG35–55 mouse model of MS had not been investigated. To bridge these gaps, we performed a set of studies using the acute EAE MOG35–55 mouse model coupled to a retrospective chart analysis comparing the recurrence of optic neuritis in patients maintained on Tecfidera™ versus patients who never received Tecfidera™.
The present mouse studies revealed that the twice daily administration of DMF, beginning the day after the initial episodes of motor deficits and visual loss and continued until the mice were euthanized, reduced subsequent motor deficits (Figure 2B), and preserved vision and RGCs in both eyes (Figure 2D,F and Figure 4C,D, respectively). In contrast, DMF administered as a prevention regimen (i.e., beginning the day after MOG35–55 immunization) was relatively less effective (Figure 2), but modestly preserved vision (Appendix 1), and protected RGCs in the central retina (Figure 4A,B). As these studies exclusively used the EAE MOG35–55 immunization model, it remains an open question as to whether the efficacy of DMF for optic neuritis extends to disease induced by other antigens. Continuing efforts to refine the molecular signatures of autoimmune disorders that share symptoms (e.g., MS, neuromyelitis optica [NMO], and acute disseminated encephalomyelitis) [27] hold the promise of enabling treatment modalities to be tested in sub-sets of patients stratified by autoantigen.
DMF reduced motor deficits to a greater extent than visual deficits in the acute EAE model. A recent analysis of adaptive and innate immune modulation showed that DMF efficacy derives, at least in part, from inducing anti-inflammatory M2 (type 2) monocytes and reducing B-cell major histocompatibility complex (MHC) class II expression [4]. It is possible that these modulatory functions and cell types are manifested in the brain and spinal cord, but are present to a lesser degree in the optic nerve. An additional explanation could relate to the failure of DMF to increase CD4+CD25+Foxp3+ T regulatory cells (Tregs; 4), a subset of cells critical for immune suppression to self [28]. Modalities that expand these Tregs attenuate EAE-evoked optic neuritis [29,30], implying that expansion of CD4+CD25+Foxp3+ Tregs may be necessary to ameliorate optic neuritis.
Oxidative stress and the antioxidant transcription factor Nrf2 have been strongly implicated in many neurodegenerative diseases, including MS [31,32], but there is still much to be learned and clarified. The dependence of DMF efficacy on Nrf2 activation has been demonstrated in the chronic EAE MOG35–55 model [5], but subsequent work in the acute version of this model showed comparable disease reduction by DMF in wild-type and Nrf2-knockout mice [4]. We previously showed that the absence of Nrf2 exacerbates the disease course and severity (motor and visual deficits) in the acute EAE MOG35–55 model [8], whereas efforts to increase Nrf2 levels via Adeno-associated virus (AAV)-mediated transgenesis conferred only partial EAE symptom reduction of optic neuritis. Specifically, RGC survival was increased, but visual deficits, demyelination, and inflammation were not mitigated [33]. A similar finding was reported for Nrf2 overexpression in the murine optic nerve crush model [34]. These discrepancies may, in part, be attributable to the modest transduction efficiency of RGCs [33], as well as the differential expression of Nrf2 in MS lesions (e.g., [35]). Interestingly, we did not detect DMF-induced increases of mRNA for the Nrf2 target genes, HO-1 and NQO1, with quantitative real-time PCR on RNA isolated from optic nerves or spinal cords (Figure 5). We also did not detect the induction in optic nerves of CNPase, PAD2, or ermin, markers of myelin status [36–38] (Appendix 3). A direction moving forward will be to determine whether sustained activation of Nrf2 in specific cell types (e.g., RGCs, oligodendrocytes, astrocytes, and neurons) confers preservation of vision, and promotes remyelination of the optic nerve. This knowledge could potentially lead to the incorporation of dietary Nrf2 activators, such as sulforaphane (found in cruciferous vegetables) into treatment regimens as an adjuvant to DMF or alternatively, as a replacement therapy for patients who cannot tolerate DMF due to adverse effects.
This retrospective study of Tecfidera™ efficacy for preventing or delaying recurrences of optic neuritis revealed new findings. First, Tecfidera™ eliminated recurrences of optic neuritis in all but two of 51 patients maintained on the drug (Figure 6C and Appendix 2). When women and men were analyzed separately, the efficacy of Tecfidera™ to delay the recurrence of optic neuritis remained statistically significant for women, but not for men (Figure 6E,F, respectively). As only one man in this cohort had a recurrence of optic neuritis while on Tecfidera™ (Appendix 2), the simplest explanation is that the lack of statistical significance derives from only having 12 men in the cohort. Second, robust statistical significance was reached within the Tecfidera™-treated cohort when the time to a recurrence during maintenance treatment with Tecfidera™ (i.e., Interval C) was compared to either pre-MS diagnosis (Interval A) or post-MS diagnosis (Interval B; Figure 6C). In contrast, the time to a recurrence of optic neuritis between Intervals A and B in this cohort trended toward, but did not reach, statistical significance. Together, these findings demonstrate that the patients in this cohort responded better to Tecfidera™ than to the initial therapy(ies) they received following a diagnosis of MS. Third, a comparison of the time to recurrence of optic neuritis between Interval C from the Tecfidera™-treated cohort to Interval B of the Tecfidera™-naïve cohort was not statistically significantly different (Figure 6D). This result contrasts with the comparison of Interval B to Interval C within the Tecfidera™-treated cohort (Figure 6C), and implies that although Tecfidera™ may not confer additional therapeutic advantages (with respect to optic neuritis) to all patients with RRMS, the compound does confer therapeutic benefit to patients who are refractory to or less responsive to other treatments.
We believe these findings warrant an expansion of such analyses to include larger patient populations, and that the continued use of Tecfidera™ will facilitate conducting such studies retrospectively. Additionally, we emphasize that a definitive MS diagnosis be secured before Tecfidera™ is administered, based on case reports that patients with NMO spectrum disorder (also known as Devic’s syndrome) can experience exacerbated symptoms if administered Tecfidera™ [39-41]. Although diagnostic tools and criteria for distinguishing NMO from MS are established (reviewed in [42]), differential diagnosis can still be challenging.
Acknowledgments
This work was supported by National Institutes of Health Grant R21EY0266684 (to SMP), grant HR16–068 from The Oklahoma Center for the Advancement of Science and Technology (to SMP), and a grant from the Presbyterian Health Foundation (to SMP). We are grateful to members of the Plafker laboratory for helpful discussions. Conflict-of-interest disclosure: Dr. Tania Reyna is on speaker’s bureau for Biogen, which manufacturers Tecfidera™. She is also on speaker’s bureau for Genentech, Genzyme, and Novartis.
Appendix 1. The preventive regimen with DMF modestly preserves visual acuity.
(A) Graph of the average number of days that mice on the preventive regimen registered an OKT reading deficit of 10% (left graph), 20% (center graph), or 30% (right graph) below their respective baseline OKT readings as a function of treatment with DMF (blue bar) or vehicle (red bar). (B) Graph of the average lowest OKT readings for mice on the preventive protocol with DMF or vehicle. (C) Graph of the average number of optic neuritis episodes for mice on preventive treatment with DMF or vehicle. (D) Graph of the average difference between baseline and final OKT readings for mice on preventive treatment with DMF or vehicle. Statistics for all graphs determined by Mann–Whitney U test, ‘ns’ denotes not significant, and asterisk denotes statistically significant (p<0.05). Error bars represent SEM; n=8 mice for each treatment group. To access the data, click or select the words “Appendix 1.”
Appendix 2. Optic neuritis history for patients in retrospective chart study. (A, B)
Control cohort of Tecfidera™-naïve patients with optic neuritis episodes for females (A) and males (B). Each hashmark on the y-axis corresponds to an individual patient with the aligned red squares representing the individual optic neuritis episodes for that patient. Years are depicted on the x-axis. The vertical line in each graph represents the initial MS diagnosis and initiation of treatment (Dx and Tx, respectively). Data points to the left of the vertical line indicate optic neuritis episodes before treatment for MS and those to the right of the vertical line represent episodes following diagnosis and treatment initiation. (C,D) Graphs of optic neuritis episodes for Tecfidera™-treated patients using initial diagnosis (Dx) and treatment (Tx) as a reference point (vertical dotted line) as described for (A,B). Females are depicted in (C) and males in (D). Each hashmark on the y-axis corresponds to a single patient with the horizontally-aligned green dots representing individual optic neuritis episodes for that patient. Years are depicted on the x-axis. (E,F) Replotted data from (C,D) for Tecfidera™-treated female (E) and male (F) patients using the initiation of Tecfidera™ treatment as a reference point (vertical dotted line). Data points to the left of the vertical line represent optic neuritis episodes before Tecfidera™ treatment and those to the right represent episodes during maintenance on Tecfidera™. To access the data, click or select the words “Appendix 2.”
Appendix 3. DMF does not induce myelin regulatory factors on the optic nerves of EAE mice.
Quantitative PCR data measuring the mRNA expression of myelin regulatory factors from the optic nerves (ON) of the MA eyes of mice subjected to the prevention regimen (A) or the interventional regimen (B). Data graphed as fold change in expression of DMF-derived samples over vehicle-derived samples. Each qPCR reaction was done in triplicate (3 technical replicates) on the indicated number of mice. Error bars represent ± SD. Data were analyzed by Mann–Whitney U Test with p<0.05 indicative of statistical significance; no values reached significance (ns). To access the data, click or select the words “Appendix 3.”
References
- 1.Goldenberg MM. Multiple sclerosis review. P&T. 2012;37:175–84. [PMC free article] [PubMed] [Google Scholar]
- 2.Bomprezzi R. Dimethyl fumarate in the treatment of relapsing-remitting multiple sclerosis: an overview. Ther Adv Neurol Disorder. 2015;8:20–30. doi: 10.1177/1756285614564152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox RJ, Kita M, Cohan SL, Henson LJ, Zambrano J, Scannevin RH, O’Gorman J, Novas M, Dawson KT, Phillips JT. BG-12 (dimethyl fumarate): a review of mechanism of action, efficacy, and safety. Curr Med Res Opin. 2014;30:251–62. doi: 10.1185/03007995.2013.849236. [DOI] [PubMed] [Google Scholar]
- 4.Schulze-Topphoff U, Varrin-Doyer M, Pekarek K, Spencer CM, Shetty A, Sagan SA, Cree BA, Sobel RA, Wipke BT, Steinman L, Scannevin RH, Zamvil SS. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc Natl Acad Sci USA. 2016;113:4777–82. doi: 10.1073/pnas.1603907113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, Ellrichmann G, Bruck W, Dawson K, Goelz S, Wiese S, Scannevin RH, Lukashev M, Gold R. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134:678–92. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- 6.Pinholt M, Frederiksen JL, Andersen PS, Christiansen M. Apo E in multiple sclerosis and optic neuritis: the apo E-epsilon4 allele is associated with progression of multiple sclerosis. Mult Scler. 2005;11:511–5. doi: 10.1191/1352458505ms1207oa. [DOI] [PubMed] [Google Scholar]
- 7.Halliday AM, McDonald WI, Mushin J. Visual evoked response in diagnosis of multiple sclerosis. BMJ. 1973;4:661–4. doi: 10.1136/bmj.4.5893.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larabee CM, Desai S, Agasing A, Georgescu C, Wren JD, Axtell RC, Plafker SM. Loss of Nrf2 exacerbates the visual deficits and optic neuritis elicited by experimental autoimmune encephalomyelitis. Mol Vis. 2016;22:1503–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Larabee CM, Hu Y, Desai S, Georgescu C, Wren JD, Axtell RC, Plafker SM. Myelin-specific Th17 cells induce severe relapsing optic neuritis with irreversible loss of retinal ganglion cells in C57BL/6 mice. Mol Vis. 2016;22:332–41. [PMC free article] [PubMed] [Google Scholar]
- 10.Beck RW, Gal RL. Treatment of acute optic neuritis: a summary of findings from the optic neuritis treatment trial. Arch Ophthalmol. 2008;126:994–5. doi: 10.1001/archopht.126.7.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–6. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 12.Axtell RC, de Jong BA, Boniface K, van der Voort LF, Bhat R, De Sarno P, Naves R, Han M, Zhong F, Castellanos JG, Mair R, Christakos A, Kolkowitz I, Katz L, Killestein J, Polman CH, de Waal Malefyt R, Steinman L, Raman C. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–12. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 14.Lindstrom ML, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46:673–87. [PubMed] [Google Scholar]
- 15.The clinical profile of optic neuritis. Experience of the Optic Neuritis Treatment Trial. Optic Neuritis Study Group. Arch Ophthalmol. 1991;109:1673–8. doi: 10.1001/archopht.1991.01080120057025. [DOI] [PubMed] [Google Scholar]
- 16.Bittner S, Afzali AM, Wiendl H, Meuth SG. Myelin oligodendrocyte glycoprotein (MOG35–55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J Vis Exp. 2014;(86):51275. doi: 10.3791/51275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, Freedman MS, Zackon DH, Kardon RH. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–9. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- 18.Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC, Calabresi PA, Maguire MG, Cutter GR, Balcer LJ. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–32. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Trip SA, Schlottmann PG, Jones SJ, Altmann DR, Garway-Heath DF, Thompson AJ, Plant GT, Miller DH. Retinal nerve fiber layer axonal loss and visual dysfunction in optic neuritis. Ann Neurol. 2005;58:383–91. doi: 10.1002/ana.20575. [DOI] [PubMed] [Google Scholar]
- 20.Egeberg A, Mallbris L, Gislason GH, Skov L, Hansen PR. Risk of Multiple Sclerosis in Patients with Psoriasis: A Danish Nationwide Cohort Study. J Invest Dermatol. 2016;136:93–8. doi: 10.1038/JID.2015.350. [DOI] [PubMed] [Google Scholar]
- 21.Hoefnagel JJ, Thio HB, Willemze R, Bouwes Bavinck JN. Long-term safety aspects of systemic therapy with fumaric acid esters in severe psoriasis. Br J Dermatol. 2003;149:363–9. doi: 10.1046/j.1365-2133.2003.05433.x. [DOI] [PubMed] [Google Scholar]
- 22.Arnold DL, Gold R, Kappos L, Bar-Or A, Giovannoni G, Selmaj K, Yang M, Zhang R, Stephan M, Sheikh SI, Dawson KT. Magnetization transfer ratio in the delayed-release dimethyl fumarate DEFINE study. J Neurol. 2014;261:2429–37. doi: 10.1007/s00415-014-7504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnold DL, Gold R, Kappos L, Bar-Or A, Giovannoni G, Selmaj K, Yang M, Zhang R, Stephan M, Sheikh SI, Dawson KT. Effects of delayed-release dimethyl fumarate on MRI measures in the Phase 3 DEFINE study. J Neurol. 2014;261:1794–802. doi: 10.1007/s00415-014-7412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Havrdova E, Hutchinson M, Kurukulasuriya NC, Raghupathi K, Sweetser MT, Dawson KT, Gold R. Oral BG-12 (dimethyl fumarate) for relapsing-remitting multiple sclerosis: a review of DEFINE and CONFIRM. Evaluation of: Gold R, Kappos L, Arnold D, Bar-Or A, Giovannoni G, Selmaj K, Tornatore C, Sweetser MT, Yang M, Sheikh SI, Dawson KT. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098–107; and Fox RJ, Miller DH, Phillips JT, Hutchinson M, Havrdova E, Kita M, Yang M, Raghupathi K, Novas M, Sweetser MT, Viglietta V, Dawson KT. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087–97. Expert Opin Pharmacother. 2013;14:2145–56. doi: 10.1517/14656566.2013.826190. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson M, Fox RJ, Miller DH, Phillips JT, Kita M, Havrdova E, O’Gorman J, Zhang R, Novas M, Viglietta V, Dawson KT. Clinical efficacy of BG-12 (dimethyl fumarate) in patients with relapsing-remitting multiple sclerosis: subgroup analyses of the CONFIRM study. J Neurol. 2013;260:2286–96. doi: 10.1007/s00415-013-6968-1. [DOI] [PubMed] [Google Scholar]
- 26.Kawalec P, Mikrut A, Wisniewska N, Pilc A. The effectiveness of dimethyl fumarate monotherapy in the treatment of relapsing-remitting multiple sclerosis: a systematic review and meta-analysis. Curr Neuropharmacol. 2014;12:256–68. doi: 10.2174/1570159X12666140115214801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salama S, Khan M, Pardo S, Izbudak I, Levy M. MOG antibody-associated encephalomyelitis/encephalitis. Mult Scler. 2019;•••:1352458519837705. doi: 10.1177/1352458519837705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan U, Ghazanfar H. T Lymphocytes and Autoimmunity. Int Rev Cell Mol Biol. 2018;341:125–68. doi: 10.1016/bs.ircmb.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda R, Kezuka T, Nishiyama C, Usui Y, Matsunaga Y, Okunuki Y, Yamakawa N, Ogawa H, Okumura K, Goto H. Suppression of murine experimental autoimmune optic neuritis by mature dendritic cells transfected with calcitonin gene-related Peptide gene. Invest Ophthalmol Vis Sci. 2012;53:5475–85. doi: 10.1167/iovs.12-9935. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz K, de Bruin N, Bishay P, Mannich J, Haussler A, Altmann C, Ferreiros N, Lotsch J, Ultsch A, Parnham MJ, Geisslinger G, Tegeder I. R-flurbiprofen attenuates experimental autoimmune encephalomyelitis in mice. EMBO Mol Med. 2014;6:1398–422. doi: 10.15252/emmm.201404168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McBean GJ, Lopez MG, Wallner FK. Redox-based therapeutics in neurodegenerative disease. Br J Pharmacol. 2017;174:1750–70. doi: 10.1111/bph.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohl K, Tenbrock K, Kipp M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp Neurol. 2016;277:58–67. doi: 10.1016/j.expneurol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDougald DS, Dine KE, Zezulin AU, Bennett J, Shindler KS. SIRT1 and NRF2 Gene Transfer Mediate Distinct Neuroprotective Effects Upon Retinal Ganglion Cell Survival and Function in Experimental Optic Neuritis. Invest Ophthalmol Vis Sci. 2018;59:1212–20. doi: 10.1167/iovs.17-22972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiong W, MacColl Garfinkel AE, Li Y, Benowitz LI, Cepko CL. NRF2 promotes neuronal survival in neurodegeneration and acute nerve damage. J Clin Invest. 2015;125:1433–45. doi: 10.1172/JCI79735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Licht-Mayer S, Wimmer I, Traffehn S, Metz I, Bruck W, Bauer J, Bradl M, Lassmann H. Cell type-specific Nrf2 expression in multiple sclerosis lesions. Acta Neuropathol. 2015;130:263–77. doi: 10.1007/s00401-015-1452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brockschnieder D, Sabanay H, Riethmacher D, Peles E. Ermin, a myelinating oligodendrocyte-specific protein that regulates cell morphology. J Neurosci. 2006;26:757–62. doi: 10.1523/JNEUROSCI.4317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabrese R, Zampieri M, Mechelli R, Annibali V, Guastafierro T, Ciccarone F, Coarelli G, Umeton R, Salvetti M, Caiafa P. Methylation-dependent PAD2 upregulation in multiple sclerosis peripheral blood. Mult Scler. 2012;18:299–304. doi: 10.1177/1352458511421055. [DOI] [PubMed] [Google Scholar]
- 38.Duncan GJ, Plemel JR, Assinck P, Manesh SB, Muir FGW, Hirata R, Berson M, Liu J, Wegner M, Emery B, Moore GRW, Tetzlaff W. Myelin regulatory factor drives remyelination in multiple sclerosis. Acta Neuropathol. 2017;134:403–22. doi: 10.1007/s00401-017-1741-7. [DOI] [PubMed] [Google Scholar]
- 39.Kira JI. Unexpected exacerbations following initiation of disease-modifying drugs in neuromyelitis optica spectrum disorder: Which factor is responsible, anti-aquaporin 4 antibodies, B cells, Th1 cells, Th2 cells, Th17 cells, or others? Mult Scler. 2017;23:1300–2. doi: 10.1177/1352458517703803. [DOI] [PubMed] [Google Scholar]
- 40.Popiel M, Psujek M, Bartosik-Psujek H. Severe disease exacerbation in a patient with neuromyelitis optica spectrum disorder during treatment with dimethyl fumarate. Mult Scler Relat Disord. 2018;26:204–6. doi: 10.1016/j.msard.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Yamout BI, Beaini S, Zeineddine MM, Akkawi N. Catastrophic relapses following initiation of dimethyl fumarate in two patients with neuromyelitis optica spectrum disorder. Mult Scler. 2017;23:1297–300. doi: 10.1177/1352458517694086. [DOI] [PubMed] [Google Scholar]
- 42.Mathey G, Michaud M, Pittion-Vouyovitch S, Debouverie M. Classification and diagnostic criteria for demyelinating diseases of the central nervous system: Where do we stand today? Rev Neurol (Paris) 2018;174:378–90. doi: 10.1016/j.neurol.2018.01.368. [DOI] [PubMed] [Google Scholar]