Abstract
Background
Resistance among bacterial infections is increasingly well-documented in high-income countries; however, relatively little is known about bacterial antimicrobial resistance in low-income countries, where the burden of infections is high.
Methods
We prospectively screened all adult inpatients at a referral hospital in Rwanda for suspected infection for seven months. Blood, urine, wound and sputum samples were cultured and tested for antibiotic susceptibility. We examined factors associated with resistance and compared hospital outcomes for participants with and without resistant isolates.
Results
We screened 19,178 patient-days, and enrolled 647 unique participants with suspected infection. We obtained 942 culture specimens, of which 357 were culture-positive specimens. Of these positive specimens, 155 (43.4%) were wound, 83 (23.2%) urine, 64 (17.9%) blood, and 55 (15.4%) sputum. Gram-negative bacteria comprised 323 (88.7%) of all isolates. Of 241 Gram-negative isolates tested for ceftriaxone, 183 (75.9%) were resistant. Of 92 Gram-negative isolates tested for the extended spectrum beta-lactamase (ESBL) positive phenotype, 66 (71.7%) were ESBL positive phenotype. Transfer from another facility, recent surgery or antibiotic exposure, and hospital-acquired infection were each associated with resistance. Mortality was 19.6% for all enrolled participants.
Conclusions
This is the first published prospective hospital-wide antibiogram of multiple specimen types from East Africa with ESBL testing. Our study suggests that low-resource settings with limited and inconsistent access to the full range of antibiotic classes may bear the highest burden of resistant infections. Hospital-acquired infections and recent antibiotic exposure are associated with a high proportion of resistant infections. Efforts to slow the development of resistance and supply effective antibiotics are urgently needed.
Introduction
When accepting the Nobel Prize for the discovery of penicillin in 1945, Alexander Fleming cautioned that bacteria could become resistant to antibiotics that had revolutionized care for infected patients.[1] The World Health Organization (WHO) now credibly warns that we are headed toward a “post-antibiotic era” in which infections can no longer be treated with antibiotics.[2] Gram-negative bacterial infections resistant to broad-spectrum third-generation cephalosporins, carbapenems and now colistin have been identified.[3]
The world is divided into low, lower-middle, upper-middle and high income countries by the World Bank, based on gross national income per capita.[4] While it is clear that antimicrobial resistance (AMR) is a threat, most data on bacterial AMR are from high-income countries.[5] The resistance data that do arise from low-income countries (LICs) have been focused largely on tuberculosis, malaria, and human immunodeficiency virus (HIV).[6] In a recent international study examining bacterial infections among critically ill patients in 75 countries, only 1.1% of positive culture data originated from the African continent.[7] A review of the pediatric AMR literature from sub-Saharan Africa since 2005 identified only 18 articles, with evidence ranging from very-low to moderate quality.[8]
The scarce data that are available from LICs suggest that AMR is common.[9–12] A study of women with postpartum fever in Uganda found that among 25 blood and urine cultures with Gram-negative isolates, 80% were multi-drug resistant including cefepime-resistant.[13] In Rwanda, a retrospective study of cultures from patients in the internal medicine wards of a referral hospital revealed that almost a third of Escherichia coli isolates were resistant to one or more third-generation cephalosporins, and 82% of Staphylococcus aureus isolates were methicillin-resistant (MRSA).[14] A recent review of surgical-site infections in Rwanda found resistance to ceftriaxone in 53.3% of isolates and resistance to gentamicin in 92.6%.[15]
To determine the proportion of resistant isolates among patients with suspected infection in a LIC, we performed a prospective hospital-wide study of all adult patients with suspected infection in a referral hospital in Rwanda. We examined factors associated with third-generation cephalosporin resistance, and compared hospital length of stay and mortality outcomes for patients with and without resistant isolates.
Methods
Study oversight
The ethics committee of the University of Rwanda, College of Medicine and Health Sciences in Kigali, Rwanda, approved the study, as did the Committee on Clinical Investigations at Beth Israel Deaconess Medical Center (BIDMC) in Boston, Massachusetts. We asked for waiver of consent because collecting cultures in the setting of suspected infection is standard of care for the hospital (though it is often not achievable in clinical practice due to financial constraints.) The BIDMC IRB requested that we obtain verbal consent. Therefore, as approved by both ethics committees, patients with suspected infection were enrolled after verbal consent was obtained following a standard script in the patient’s primary language. Consent or refusal was documented on the patient screening enrollment log. Culture and sensitivity data from the study were made available to clinicians.
Study design and setting
We designed this prospective observational study to characterize antimicrobial resistance among all adult inpatients in a referral hospital in Rwanda. The University Teaching Hospital of Kigali (KUTH) is a government-funded academic tertiary referral hospital in Kigali, Rwanda. It is one of three public referral hospitals in a country of approximately twelve million people. It has 560 total beds of which seven beds are intensive care unit (ICU) beds, and approximately 12,000 admissions each year. Rwanda has achieved almost universal heath insurance coverage;[16] most patients pay 10% of hospital charges, including laboratory studies, when in this public hospital. The hospital’s microbiology laboratory is managed by a senior technician and university microbiologist, who oversee eight technicians. It has refrigerators for plate storage and a generator for emergency power supply. The hospital is currently undergoing its first accreditation process with The Council for Health Service Accreditation of Southern Africa (COHSASA); in the initial assessment by COHSASA, the laboratory received a passing score.
Study population and data collection
From January 25 through August 14, 2017, all adult patients (15 years or older, the criteria used to define adult ward admission at KUTH) were screened daily for inclusion criteria: temperature ≤35.0o C or ≥38.0o C and suspected infection, or undergoing surgery for an infectious process, regardless of temperature. Exclusion criteria included age<15, patient refusal to participate, and suspected viral or fungal source of infection, based on clinician judgment. Since many patients are unable to pay for laboratory studies, sample collection and testing costs were paid by the study. This allowed inclusion of data from all patients who met criteria for the study and consented, regardless of ability to pay.
Depending on the clinically suspected source of infection, blood (aerobic and anaerobic), urine, sputum, tracheal aspirate, wound, and/or surgical specimens were collected using standardized kits by the patient’s nurse, who sent specimens to the microbiology laboratory for processing. Urine samples were clean-catch or clean-catheterization samples collected after perineal cleansing. All surgical and open wound specimens were collected after sterile prep or saline cleaning. Tracheal aspirates were collected from intubated patients’ ventilation circuits. Blood, urine, and surgical sample collection was performed in an aseptic, sterile fashion; wound, sputum, and tracheal aspirate samples were non-sterile. Nurses were trained in standard sample collection, and re-trained throughout the study. A nurse educator provided additional training to locations in the hospital that were found to have higher than expected prevalence of contamination after an audit at the end of the pilot phase. We used standardized collection kits that included sterile alcohol wipes and single use gloves for collection.
Demographic and clinical data, including age, gender and co-morbidities, were collected from each participant’s chart and de-identified to protect privacy. Enrolled participants who developed a new fever or hypothermia and signs of infection after having been afebrile for >48 hours, and those who were taken back to the operating room for suspicion of a new infection, were re-enrolled with a new study identifier, with a crosswalk to link the two identifiers. Participants were followed through hospital discharge to determine length of stay and in-hospital mortality.
During the initial phase of the study, we recognized that a significant number of positive cultures in the laboratory had been drawn from patients who were not enrolled in our study because they had not been febrile, hypothermic, or undergone an infection source-control operation. We filed an amendment with both IRBs in March 2017. After approval, we added patients to the study who had positive bacterial cultures but had not been febrile on the ward and had not had an operation. We also began using the Center for Disease Control’s (CDC’s) two-step process for extended spectrum beta-lactamase (ESBL) confirmation[17] and added meropenem testing to the protocol to identify carbapenem resistance. All other protocols remained the same.
Active enrollment ended August 14, 2017 after the team reached our target of 300 positive cultures, and the remaining participants were followed until October 2017, when the last participant was discharged. Study data were entered into REDCap (Research Electronic Data Capture), a secure, web-based application, which was hosted at BIDMC.
Laboratory methods
Blood samples were collected and incubated in BD BACTEC (Becton-Dickinson, Franklin Lakes, USA) bottles. Urine, wound and sputum cultures were collected in sterile containers; cutaneous wounds and purulent surgical sites were incubated in Amies vials. Blood cultures were directly incubated at 37°C and processed with the BACTEC system to identify bacteria. Samples positive for bacterial growth were sub-cultured on appropriate media guided by Gram stain results: Gram-positive cocci were plated on mannitol salt agar (MSA) and blood agar, and Gram-negative bacilli were plated on MacConkey agar and Xylose Lysine Deoxycholate agar (XLD) media. Additional identification of Gram-positive cocci species was done using catalase and coagulase tests. Gram-negative bacilli were identified by colony morphology. In addition, biochemical tests were performed, including triple sugar iron (TSI), motility indole urea (MIU), and citrate tests to identify and differentiate Enterobacteriaceae species.
Urine samples, after wet mount examination, were cultured on blood agar, cysteine lactose electrolyte-deficient (CLED), and MacConkey agar. The number of colonies were counted after 18–24 hours of incubation at 37°C, with maximum incubation 48 hours. Urinary specimens with > 105 colony forming unit per milliliter (CFU/mL) urine were considered positive.[13, 18] For operative specimens, wound swabs and sputum specimens, the Gram stain morphology of principal pathogens dictated the selection of appropriate medium for culture, which was then incubated at 37°C for 24 hours. Similarly, identification of bacterial species was performed using a combination of colony morphology, growth characteristics, and biochemical tests.
Antibiotic susceptibility testing was performed by the Kirby Bauer disk diffusion method (S1 Table), per established laboratory protocol. Interpretation of the diameter of inhibition was done according to 2012 Clinical and Laboratory Standards Institute (CLSI) guidelines.[19]
For the second phase of the study, all laboratory technicians were trained in the CDC ESBL two-step screening and confirmation process[17] (S1 Table) We tested Proteus spp., Klebsiella spp. and E. coli isolates for ESBL production. Of note, we were unable to test all of these isolates for ESBL production due to supply chain limitations.
Definitions
Participants were considered to have hospital-acquired infections if their fever or suspected infection occurred >48 hours after presentation to the hospital.[20] Comorbidity was defined as known diabetes, hypertension, tuberculosis, HIV, cancer, and/or severe malnutrition at the time of presentation.
A culture set refers to a set of cultures drawn at one time point, from all suspected sites of infection. A participant could have more than one set of cultures if they had a new fever and suspected infection, with an afebrile period of at least 48 hours between culture sets. Each culture set could include one or more specimens (wound/surgical specimen, urine, blood, and/or sputum/tracheal aspirate), and each specimen could grow one or more isolates.
The laboratory considered a blood culture specimen contaminated if only one of two samples was positive for coagulase- negative Staphylococcus (CnS). A urine culture was considered contaminated if more than three microbes were identified. Tracheal aspirates and wound swabs were considered contaminants if they produced CnS or Gram-positive rods.
We defined resistant isolates as Gram-negative bacteria that were resistant to at least one third or fourth generation cephalosporin (ceftriaxone, cefotaxime, ceftazidime, and/or cefepime), or met criteria for ESBL positive phenotype using the CDC definition.[17] We defined an isolate as MRSA if it was S. aureus resistant to cefoxitin, consistent with CDC guidelines, which were in turn based upon CLSI international standards.[19, 21]
Statistical analysis
Data are presented as median (interquartile range) for continuous variables, mean (standard deviation) for discrete variables, and frequency (proportion) for categorical variables. Normality was assessed with the Shapiro-Wilk test. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC) with two-sided p-values < 0.05 considered statistically significant.
If a participant had multiple positive cultures in one culture set, only cultures with unique isolates were considered in the analysis. For example, if a participant had a blood culture positive for E. coli and a urine culture positive for E. coli and Klebsiella spp, the urine E. coli antibiogram was not included to prevent double-counting of isolates.
Because participants could have more than one culture set taken if they had new suspected infection after 48 hours of being afebrile, generalized estimating equations with robust variance estimators were used to assess for differences between culture type, accounting for within-subject correlation. In addition, a secondary analysis was performed with unique participants, using only the first set of cultures drawn for each participant (S2 Table).
We examined clinical and demographic characteristics of participants stratified by culture positivity and resistance: culture positive with a resistant isolate, culture positive but no resistant isolate, and culture negative.
Based on an historic hospital-wide mortality rate of approximately 10% and ICU mortality rate of 50%, we estimated a mortality rate of 30% among patients with antimicrobial resistant infections and 10% among patients with infections without resistance. Based on our clinical experience, we also estimated that two thirds of positive cultures would demonstrate resistance. Using these estimates, we found that we would need to collect 147 positive samples for the study to be adequately powered (power = 80%; alpha = 0.05) to detect a mortality difference between patients with resistant and sensitive isolates. To account for the possibility that mortality among patients with sensitive infections was higher than the hospital average (as high as 15%), we planned to collect a minimum of 279 positive cultures, with a target end point of 300 positive cultures.
Results
Between January 25 and August 14, 2017, we screened 19,178 patient-days for suspected infection. We enrolled 647 unique study participants who had suspected infection and were cultured; only one patient who met study criteria declined enrollment.
From 647 unique participants, 762 sets of cultures were taken, totaling 942 specimens. Two of 942 specimens had missing results. Of 940 specimens with results, 357 (38.0%) were positive for bacterial growth, 489 (52.0%) were negative, and 94 (10.0%) were contaminated (Table 1). The 357 positive culture specimens came from 338 unique participants, with 364 unique isolates. Of 357 culture-positive specimens, 155 (43.4%) were wound specimens, 83 (23.2%) urine, 64 (17.9%) blood, and 55 (15.4%) sputum/tracheal aspirates.
Table 1. Culture positivity by specimen type.
| Positive (%) n = 357* |
Negative (%) n = 489 |
Contaminated (%) n = 94 |
Total (%) n = 940** |
|
|---|---|---|---|---|
| Specimen Type | ||||
| Wound / surgical specimen | 155 (43.4) | 78 (16.0) | 9 (9.6) | 242 (25.8) |
| Urine | 83 (23.2) | 107 (21.9) | 0 (0.0) | 190 (20.2) |
| Blood | 64 (17.9) | 297 (60.7) | 83 (88.3) | 444 (47.2) |
| Sputum / Tracheal Aspirate | 55 (15.4) | 7 (1.4) | 2 (2.1) | 64 (6.8) |
Blood, urine, and surgical sample collection was performed in an aseptic, sterile fashion; wound, sputum, and tracheal aspirate samples were non-sterile.
* 357 specimens were positive, and some had more than one type of colony. The 357 cultures represent 364 unique organisms and were obtained from 338 unique patients.
** A total of 942 cultures were obtained, however two had missing results for culture positivity and are not presented here (n = 940).
Gram-negative bacteria made up 323 (88.7%) of the 364 cultured isolates, and Gram-positive isolates were 41 (11.3%). E. coli and Klebsiella species comprised nearly two-thirds of all cultured isolates (Table 2). Gram-negative isolates were tested for cephalosporin susceptibility, and 183 of the 241 Gram-negative isolates tested for ceftriaxone resistance (75.9%) were ceftriaxone-resistant; 184 of 252 isolates tested (73.0%) were ceftazidime-resistant (Table 3). Of 92 E. coli, Klebsiella spp., and Proteus spp. formally tested for ESBL production, 66 (71.7%) met criteria for ESBL positive phenotype. Of the 250 Gram-negative isolates tested for ciprofloxacin susceptibility, 165 (66.0%) were resistant. Of the 296 Gram-negative isolates tested for resistance to carbapenems, 12 (4.0%) were resistant. Of 22 S. aureus isolates tested for cefoxitin susceptibility, seven (31.8%) met criteria for MRSA (Table 4).
Table 2. Distribution of organisms by culture type.
| Total n = 364 |
Blood n = 66 |
Urine n = 74 |
Tracheal Aspirate / Sputum n = 62 |
Wound / surgical specimen n = 162 |
|
|---|---|---|---|---|---|
| Gram Negative Organisms | |||||
| Escherichia coli | 139 (38.2) | 18 (27.3) | 52 (70.3) | 10 (16.1) | 59 (36.4) |
| Klebsiella spp. | 91 (25.0) | 15 (22.7) | 15 (20.3) | 19 (30.6) | 42 (25.9) |
| Proteus spp. | 26 (7.1) | 1 (1.5) | 3 (4.1) | 5 (8.1) | 17 (10.5) |
| Acinetobacter spp. | 50 (13.7) | 11 (16.7) | 1 (1.4) | 21 (33.9) | 17 (10.5) |
| Pseudomonas spp. | 15 (4.1) | 0 (0.0) | 2 (2.7) | 7 (11.3) | 6 (3.7) |
| Salmonella spp. | 2 (0.5) | 2 (3.0) | 0 (0.0) | 0 (0) | 0 (0.0) |
| Gram Positive Organisms* | |||||
| Staphylococcus aureus | 34 (9.3) | 15 (22.7) | 1 (1.4) | 0 (0.0) | 18 (11.1) |
| Coagulase-negative Staphylococcus spp. | 2 (0.5) | 2 (3.0) | 0 (0) | 0 (0.0) | 0 (0) |
| Streptococcus spp. | 5 (1.4) | 2 (3.0) | 0 (0) | 0 (0.0) | 3 (1.9) |
Blood, urine, and surgical sample collection was performed in an aseptic, sterile fashion; wound, sputum, and tracheal aspirate samples were non-sterile.
*Testing was performed in order to identify Enterococcus spp; however, no positive cultures were observed.
Table 3. Proportion of gram-negative organisms resistant to tested antimicrobial agents.
| Antimicrobial Agent | Total (%) n = 323* |
E. coli n = 139 |
Klebsiella spp. n = 91 |
Proteus spp. n = 26 |
Acinetobacter spp. n = 50 |
Pseudomonas spp. n = 15 |
Salmonella spp. n = 2 |
|---|---|---|---|---|---|---|---|
| Amikacin, n = 234 | 17 (7.3) | 9 (9.7) | 2 (2.7) | 2 (9.1) | 1 (3.0) | 3 (25.0) | 0 (0.0) |
| Cefepime, n = 183 | 98 (53.5) | 41 (51.2) | 32 (64.0) | 7 (36.8) | 13 (56.5) | 4 (40.0) | 1 (100.0) |
| Ceftazidime, n = 252 | 184 (73.0) | 72 (66.7) | 57 (82.6) | 15 (68.2) | 33 (86.8) | 7 (46.7) | 0 (0.0) |
| Ceftriaxone, n = 241 | 183 (75.9) | 75 (67.0) | 67 (85.9) | 14 (63.6) | 24 (92.3) | 1 (100.0) | 2 (100.0) |
| Cefotaxime, n = 227 | 166 (73.1) | 81 (66.4) | 65 (82.3) | 16 (72.7) | 2 (100.0) | 0 (0.0) | 2 (100.0) |
| Cefuroxime, n = 126 | 99 (78.6) | 50 (68.5) | 33 (89.2) | 13 (100.0) | 2 (100.0) | 0 (0.0) | 1 (100.0) |
| Ciprofloxacin, n = 250 | 165 (66.0) | 73 (63.5) | 52 (69.3) | 16 (69.6) | 17 (80.9) | 5 (35.7) | 2 (100.0) |
| Trimethoprim/sulfamethoxazole (Cotrimoxazole), n = 212 | 191 (90.1) | 92 (93.9) | 58 (90.6) | 17 (85.0) | 22 (78.6) | 0 (0.0) | 2 (100.0) |
| Gentamicin, n = 274 | 191 (69.7) | 71 (59.7) | 66 (82.5) | 18 (81.8) | 29 (78.4) | 6 (40.0) | 1 (100.0) |
| Carbapenem,**n = 296 | 12 (4.0) | 0 (0) | 4 (4.6) | 0 (0.0) | 3 (7.3) | 5 (35.7) | 0 (0.0) |
| Piperacillin, n = 49 | 37 (75.5) | 12 (75.0) | 6 (75.0) | 3 (50.0) | 12 (92.3) | 3 (60.0) | 1 (100.0) |
| Piperacillin / Tazobactam, n = 168 | 49 (29.2) | 14 (19.7) | 20 (40.8) | 3 (15.8) | 9 (47.4) | 3 (30.0) | 0 (0.0) |
| ESBL positive phenotype,***n = 92 | 66 (71.7) | 37 (68.5) | 24 (82.8) | 5 (55.6) |
* The total number of unique Gram-negative organisms cultured was 323. Each organism was tested for resistance to some but not all antibiotics. See Supplemental S1 Table for laboratory protocols. The total n tested for each antibiotic is listed with each antibiotic name. The n tested by bacteria type is available upon request.
** This includes either Imipenem or Meropenem resistance.
*** E. coli (N = 54), Klebsiella (n = 29), and Proteus (n = 9) species were tested for ESBL using CDC guidelines when testing supplies were available; see methods for detailed protocol.
Table 4. Proportion of gram-positive organisms resistant to tested antimicrobial agents*.
| Antimicrobial Agent | Total n = 41** |
S. aureus n = 34 |
Streptococcus spp n = 5 |
Coag negative staph spp n = 2 |
|---|---|---|---|---|
| Ampicillin, n = 22 | 15 (68.2) | 13 (68.4) | 2 (66.7) | 0 (0.0) |
| Cefoxitin,***n = 22 | 7 (31.8) | 7 (31.8) | 0 (0.0) | 0 (0.0) |
| Cephalothin, n = 6 | 1 (16.7) | 1 (16.7) | 0 (0.0) | 0 (0.0) |
| Ciprofloxacin, n = 33 | 13 (39.4) | 10 (34.5) | 2 (66.7) | 1 (100.0) |
| Clindamycin, n = 32 | 8 (25.0) | 6 (23.1) | 2 (40.0) | 0 (0.0) |
| Erythromycin, n = 36 | 14 (38.9) | 11 (36.7) | 3 (60.0) | 0 (0.0) |
| Carbapenem,****n = 24 | 3 (12.5) | 3 (13.6) | 0 (0.0) | 0 (0.0) |
| Linezolid, n = 15 | 2 (13.3) | 1 (8.3) | 1 (33.3) | 0 (0.0) |
| Penicillin, n = 30 | 27 (90.0) | 24 (92.3) | 3 (75.0) | 0 (0.0) |
| Tetracycline, n = 36 | 18 (50.0) | 14 (45.2) | 4 (100.0) | 0 (0.0) |
| Vancomycin, n = 37 | 5 (13.5) | 3 (9.4) | 2 (50.0) | 0 (0.0) |
* Testing was performed in order to identify enterococcus spp; however, no positive cultures were observed.
** The total number of unique Gram-positive organisms cultured was 41. Each organism was tested for resistance to some but not all antibiotics. See Supplemental S1 Table for laboratory protocols. The total n tested for each antibiotic is listed with each antibiotic name. The n tested by bacteria type is available upon request.
*** Cefoxitin resistance is an indicator of MRSA.
**** This includes either Imipenem or Meropenem resistance.
The median age of the 647 unique participants with suspected infection was 35 years (IQR 27, 51) (S2 Table). Of these, 22.1% had a known co-morbidity, including 10.5% with known HIV infection. In addition, 47.6% had undergone an operation within the 30 days prior to enrollment, and 65.2% had been given antibiotics within the 30 days prior to enrollment. Data on the type and duration of antibiotics prior to enrollment were not collected. More than half (57.8%) had hospital-acquired infections.
Age and gender were not associated with culture positivity or isolate resistance. Resistant isolates were significantly more likely to be present in participants transferred from another hospital (p = 0.01), those who had surgery within the prior 30 days (p<0.0001) and those who had been given antibiotics in the prior 30 days (p<0.0001). Isolates from hospital-acquired infections were also more likely to be antibiotic-resistant (p<0.0001) (Table 5).
Table 5. Patient characteristics stratified by culture positivity and resistance.
| All culture sets* n = 760 |
At least one positive culture with a resistant organism** n = 232 |
At least one positive culture but no resistant organisms** n = 106 |
All Cultures Negative n = 424 |
P-Value | |
|---|---|---|---|---|---|
| Male gender, n (%) | 423 (55.5) | 129 (55.6) | 50 (47.2) | 244 (57.5) | 0.11 |
| Age***, median (IQR) | 35 (27, 52) | 35 (27, 52) | 38 (30, 57) | 35 (27, 51) | 0.38 |
| Patient lives outside Kigali***, n (%)2 | 437 (57.6) | 155 (66.8) | 50 (47.2) | 232 (55.1) | 0.02 |
| Patient transferred from outside facility, n (%) | 495 (65.0) | 168 (72.4) | 59 (55.7) | 268 (63.2) | 0.01 |
| Outside facility length of stay (days), median (IQR) | 2 (1, 5) | 2 (1, 5) | 1 (1, 4) | 2 (1, 5) | 0.60 |
| Surgery within 30 days, n (%) | 364 (47.8) | 156 (67.2) | 49 (46.2) | 159 (37.5) | <0.0001 |
| Antibiotic use in prior 30 days, n (%) | 517 (67.8) | 191 (82.3) | 58 (54.7) | 268 (63.2) | <0.0001 |
| the -associated infection****, n (%) | 481 (63.1) | 188 (81.0) | 54 (50.9) | 239 (56.4) | <0.0001 |
| Comorbidity***** | 171 (22.4) | 45 (19.4) | 34 (32.1) | 92 (21.7) | 0.94 |
| HIV | 73 (9.6) | 13 (5.6) | 9 (8.5) | 51 (12.0) | 0.04 |
| Temperature ≤ 35.0o C or ≥ 38.0o C | 582 (76.4) | 149 (64.2) | 62 (58.5) | 371 (87.5) | <0.0001 |
| Heart rate > 100, n (%) | 428 (56.2) | 124 (53.4) | 55 (51.9) | 249 (58.7) | 0.23 |
| Systolic blood pressure < 90 mmHg, n (%) | 42 (5.5) | 6 (2.6) | 6 (5.7) | 30 (7.1) | 0.02 |
| On oxygen or oxygen saturation < 90%, n (%) | 261 (34.2) | 79 (34.0) | 34 (32.1) | 148 (34.9) | 0.97 |
| Intubated, n (%) | 128 (16.8) | 55 (23.7) | 15 (14.1) | 58 (13.7) | 0.07 |
* This includes culture sets taken at different times from the same patient. A total of 762 culture sets were taken from 647 unique patients. Two culture sets were missing data, for a total of 760 culture sets to analyze. The within-subjects correlation was accounted for in the reported p-value by using generalized estimating equations with robust variance.
** Resistance is defined as any of the following: resistance to a third- or fourth-generation cephalosporin (ceftriaxone, cefotaxime, ceftazidime and/or cefepime), and/or confirmed ESBL-producer.
*** Missing data for age for seven culture sets. N = 755 for age. Missing data for three culture sets for whether a patient lives outside Kigali. N = 759 for this variable.
**** Defined as in the study hospital (KUTH) > 48 hours when culture set taken for suspected infection.
***** Includes patients who had any of the following documented co-morbidities: diabetes, hypertension, tuberculosis, cancer, and/or severe malnutrition.
Among non-hospital-acquired infections, isolates from participants on the obstetrics ward made up a significantly higher proportion of resistant isolates than other wards (31.8%; p = 0.03) (S3 Table).
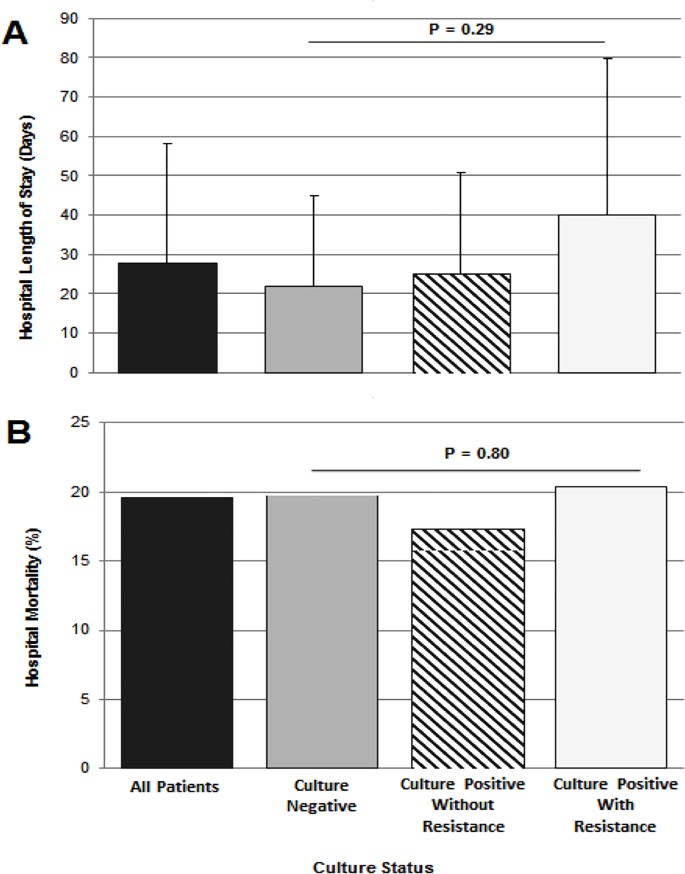
The mean length of stay for participants with at least one resistant isolate was 40.0 days (SD 39.8) whereas it was 25.5 days (SD 25.3) for participants with positive cultures but no resistant isolates and 21.9 days (SD 23.0) for those with negative cultures (p = 0.29, Fig 1). Hospital mortality for all participants was 19.6% and did not differ significantly by culture positivity or resistance status (p = 0.80).
Fig 1. Hospital outcomes by culture status.
Part (A) indicates hospital length of stay in days, and part (B) indicates hospital mortality rate, each stratified by culture type. Resistance is defined as any of the following: resistance to a third or fourth generation cephalosporin (ceftriaxone, cefotaxime, ceftazidime and/or cefepime), and/or confirmed ESBL positive phenotype.
Discussion
We prospectively performed bacterial cultures and antibiotic susceptibility testing on samples from all adult patients with suspected infection in a referral hospital in a LIC over a seven-month period. We identified an alarming rate of antimicrobial resistance in a population with limited and variable access to the full range of antibiotic classes. We found that nearly 90% of infections were due to Gram-negative isolates and that approximately 75% of these were resistant to third- or fourth-generation cephalosporins. Of isolates specifically tested for ESBL positive phenotype, 72% met criteria. Antimicrobial resistance was more common in patients who were transferred from another facility, had undergone recent surgery, had taken antibiotics recently, and those with hospital-acquired infections. In-hospital mortality among all participants with suspected infection was high, near 20%, but antimicrobial resistance was not associated with higher mortality.
Among patients with suspected infection, a higher proportion of bacterial isolates were Gram-negative than Gram-positive bacteria. One possible reason for this is the high proportion of positive cultures that came from wounds, which were most often operative samples for intra-abdominal infections. In an international study of predominantly high- and middle-income countries that found a more even distribution of Gram-positive and Gram-negative infections, 63% of isolates were from a respiratory source; our study had only 17% of specimens originating from sputum/tracheal aspirates.[7] In addition, variability by geographical location has been noted before, with Latin America demonstrating a significantly higher prevalence of Gram-negative bacterial infections than North America.[22, 23]
Our most striking finding was an extremely high proportion of third- and fourth-generation cephalosporin resistance. This finding is consistent with prior studies that suggest more resistance in poorer areas. An international study of 10,069 ICU patients in 84 almost entirely high- and middle-income countries found only 3% of Gram-negative infections to have an ESBL positive phenotype, though it is not clear what proportion of isolates were tested for ESBL positivity. [24] Another study examined over 5,000 isolates from 72 hospitals across the United States, and found ESBL resistance in 16% of Klebsiella pneumoniae isolates.[25] The same laboratory surveillance group[23] looked at over 5,000 Gram-negative isolates from 10 hospitals in four Latin American countries and found much higher rates of ESBL positive phenotypes: 60% of Klebsiella spp. isolates in Argentina, 50% in Brazil, 59% in Chile, and 33% in Mexico.[22] A recent study at another Rwandan referral hospital looked at intestinal carriage of ESBL positive Enterobacteriaceae and found a 50% carriage rate among patients on admission that rose to 65% on discharge, with 37% carriage among caregivers on admission, rising to 47% at time of discharge.[26]
A few studies in Africa suggest lower rates of antimicrobial resistance, but these studies’ methodologies were unlikely to capture the current rate of resistance. In a global point prevalence survey of antibiotic use, only 5% of patients in Africa who were receiving antibiotics and had a positive culture were receiving antibiotics for ESBL positive bacteria, though this result may have been driven by carbapenem scarcity rather than ESBL prevalence.[27] A retrospective review of wound cultures from 2004–2016 from six African countries found only 24% of Enterobacteriaceae isolates resistant to ceftriaxone.[12] However, the results were presented in aggregate for all twelve years, and it is likely that resistance has changed significantly over time. A study of blood samples drawn between 1998 and 2016 in a hospital in Malawi demonstrated increasing resistance over time, with the proportion of ESBL positive phenotype specimens reaching levels similar to what we found by 2016.[28]
Possible mechanisms for the high proportion of resistance seen in our study include those that have been hypothesized for all resource-constrained settings: widespread access to antibiotics within the community, use of antibiotics in agriculture, minimal regulation of the content of antibiotics, fewer resources for infection control measures within hospitals, and a lack of diagnostic modalities that enable de-escalation of antibiotics.[29] Some have also proposed a possible relationship between high humidity and high temperatures with certain Gram-negative bacterial infections that are prone to develop resistance.[22]
The impact of high rates of resistant isolates in a LIC setting is potentially devastating. Though ceftriaxone is one of the most commonly used antibiotics for serious infections in many settings, including Rwanda, we found 76% of Gram-negative isolates were ceftriaxone-resistant. We also found 72% of tested Gram-negative isolates to be ESBL positive phenotype. Currently, the only recommended treatments for ESBL positive infections are antibiotics from the carbapenem class (and in limited situations, cefepime).[30–33] Meropenem is the only carbapenem listed on the WHO essential medicine lists, and it is only on the complementary, not the core list.[34] In Rwanda, carbapenems are prescribed restrictively and are too expensive for most patients to afford. Although our study suggests a carbapenem would be appropriate empiric therapy among hospitalized patients in whom a Gram-negative infection is suspected, it is very rarely available even as targeted therapy. In our study, infection with a resistant isolate did not correlate with an increase in mortality. One possible explanation for this is that almost half of our positive culture specimens were wound cultures, for which source control may have reduced the need for antimicrobial therapy. It may also be that clinicians were able to use culture data to tailor antibiotic therapy to resistance patterns during the study. It is also possible that bacterial isolates acquiring resistance mutations did so at a cost to their virulence, resulting in eventual clearance of the infection by the participants’ own immune systems.[35]
Our study has several strengths. The laboratory is led by an experienced microbiologist and technician, and we were able to maintain a cold chain supply with stable electricity including a backup generator. This study is one of the first in East Africa that was able to perform confirmatory ESBL testing in specimens from multiple infection sites. In addition, we were able to analyze factors associated with resistance, compare risks and outcomes between hospital-acquired and non-hospital-acquired infections, and evaluate trends within different wards. All of these enable a more targeted approach to future interventions that might reduce infections with resistant isolates.
Our study also has several limitations. First, we did not collect data on duration and type of antibiotic use prior to enrollment. We also did not collect data on livestock exposure prior to admission, or chronic prophylactic antibiotic use (such as trimethoprim/sulfamethoxazole for participants living with HIV). This limited our ability to make inferences about some factors that may be contributing to resistance. Second, while we developed standard protocols for antibiotic testing, availability of antibiotic discs was limited early in the study. Thus, each isolate was not always tested for all recommended antibiotics per CLSI guidelines.[19] Unusual patterns of vancomycin-resistance were detected among isolated Streptococci samples; due to limited positivity, we are unable to definitively characterize this trend. Finally, our study was limited to one referral hospital in one LIC. It is not clear how generalizable the results are to other hospitals in other LICs; however, other studies suggest that our results may be applicable to many sub-Saharan African hospitals.[9–11, 13, 14, 26, 28] While our definitions of resistance to cephalosporins and MRSA do not include all isolates resistant to any antibiotic, they capture an important and clinically relevant subset of resistant isolates.
Conclusion
Our study of adult inpatients with suspected infection in a Rwandan referral hospital found an alarming proportion of cephalosporin resistance among Gram-negative bacterial isolates. This suggests that, just as with multi-drug resistance in tuberculosis, low resource settings with the least access to alternative antibiotics may bear the highest burden of resistant infections.[36]
Our results imply an urgent need for pre-hospital and in-hospital interventions to slow the spread of antimicrobial resistance.
Supporting information
(XLSX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank Valerie Banner-Goodspeed for her help with study design and Susan Weir for her assistance in designing laboratory protocols. We are grateful to the staff of the University Teaching Hospital of Kigali’s microbiology lab, who adapted protocols and increased productivity to perform the hospital’s first antibiogram. We are also thankful to the hospital’s physician and nursing staff for assisting with study protocol implementation and to Jean-Paul Mvukiyehe for research assistance. We are grateful to Niaz Banaei and Eric Rosenberg for their generous donations of supplies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work received internal funding from the Department of Surgery, University of Minnesota (https://www.surgery.umn.edu/) to JR. This work also received internal department funding from the Beth Israel Deaconess Anesthesiology Foundation (https://www.bidmc.org/centers-and-departments/anesthesia-critical-care-and-pain-medicine) to TS/EDR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rosenblatt-Farrell N. The landscape of antibiotic resistance. Environ Health Perspect. 2009;117(6):A244–50. 10.1289/ehp.117-a244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Global action plan on antimicrobial resistance. Geneva: WHO, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Tian GB, Zhang R, Shen Y, Tyrrell JM, Huang X, et al. Prevalence, risk factors, outcomes, and molecular epidemiology of mcr-1-positive Enterobacteriaceae in patients and healthy adults from China: an epidemiological and clinical study. Lancet Infect Dis. 2017;17(4):390–9. 10.1016/S1473-3099(16)30527-8 . [DOI] [PubMed] [Google Scholar]
- 4.The World Bank. World Bank Country and Lending Groups 2019 [cited 2019 June 4]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 5.World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance. Geneva: 2014. Available at url: http://www.who.int/drugresistance/documents/surveillancereport/en/.
- 6.Dheda K, Gumbo T, Maartens G, Dooley KE, McNerney R, Murray M, et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017. 10.1016/S2213-2600(17)30079-6 . [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–9. 10.1001/jama.2009.1754 . [DOI] [PubMed] [Google Scholar]
- 8.Williams PCM, Isaacs D, Berkley JA. Antimicrobial resistance among children in sub-Saharan Africa. Lancet Infect Dis. 2018;18(2):e33–e44. Epub 2017/10/17. 10.1016/S1473-3099(17)30467-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernabe KJ, Langendorf C, Ford N, Ronat JB, Murphy RA. Antimicrobial resistance in West Africa: a systematic review and meta-analysis. Int J Antimicrob Agents. 2017;50(5):629–39. Epub 2017/07/15. 10.1016/j.ijantimicag.2017.07.002 . [DOI] [PubMed] [Google Scholar]
- 10.Tadesse BT, Ashley EA, Ongarello S, Havumaki J, Wijegoonewardena M, Gonzalez IJ, et al. Antimicrobial resistance in Africa: a systematic review. BMC Infect Dis. 2017;17(1):616 10.1186/s12879-017-2713-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ampaire L, Muhindo A, Orikiriza P, Mwanga-Amumpaire J, Bebell L, Boum Y. A review of antimicrobial resistance in East Africa. Afr J Lab Med. 2016;5(1):432 Epub 2017/09/08. 10.4102/ajlm.v5i1.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai PS, Bebell LM, Meney C, Valeri L, White MC. Epidemiology of antibiotic-resistant wound infections from six countries in Africa. BMJ Glob Health. 2017;2(Suppl 4):e000475 Epub 2018/03/29. 10.1136/bmjgh-2017-000475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bebell LM, Ngonzi J, Bazira J, Fajardo Y, Boatin AA, Siedner MJ, et al. Antimicrobial-resistant infections among postpartum women at a Ugandan referral hospital. PLoS One. 2017;12(4):e0175456 Epub 2017/04/14. 10.1371/journal.pone.0175456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ntirenganya C, Manzi O, Muvunyi CM, Ogbuagu O. High prevalence of antimicrobial resistance among common bacterial isolates in a tertiary healthcare facility in Rwanda. Am J Trop Med Hyg. 2015;92(4):865–70. 10.4269/ajtmh.14-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukagendaneza MJ, Munyaneza E, Muhawenayo E, Nyirasebura D, Abahuje E, Nyirigira J, et al. Incidence, root causes, and outcomes of surgical site infections in a tertiary care hospital in Rwanda: a prospective observational cohort study. Patient Saf Surg. 2019;13:10 Epub 2019/03/02. 10.1186/s13037-019-0190-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chemouni B. The political path to universal health coverage: Power, ideas andcommunity-based health insurance in Rwanda. World Development 2018; 106: 87–98. [Google Scholar]
- 17.(CDC) CfDCaP. Healthcare-associated infections: Laboratory detection of Extended-Spectrum Beta-Lactamases (ESBLs). Accessed on 1 January 2017 at url: https://www.cdc.gov/hai/settings/lab/lab_esbl.html.
- 18.Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2004;38(8):1150–8. Epub 2004/04/20. 10.1086/383029 . [DOI] [PubMed] [Google Scholar]
- 19.Clinical & Laboratory Standards Institute (CLSI). M100: Performance Standards for Antimicrobial Suceptibility Testing, 27th Edition Wayne: 2017. [Google Scholar]
- 20.World Health Organization. Prevention of hospital-acquired infections: a practical guide; 2002. https://www.who.int/csr/resources/publications/drugresist/en/whocdscsreph200212.pdf?ua=1. Accessed June 4, 2019. [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC). Laboratory Testing for MRSA 2017 [updated October 12; cited 2018 May 18]. Available from: https://www.cdc.gov/mrsa/lab/index.html.
- 22.Gales AC, Castanheira M, Jones RN, Sader HS. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010). Diagn Microbiol Infect Dis. 2012;73(4):354–60. Epub 2012/06/05. 10.1016/j.diagmicrobio.2012.04.007 . [DOI] [PubMed] [Google Scholar]
- 23.JMI Laboratories. SENTRY Antimicrobial Surveillance Program 2017 [cited 2018 January 1]. Available from: https://www.jmilabs.com/sentry-surveillance-program/.
- 24.Vincent JL, Marshall JC, Namendys-Silva SA, Francois B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–6. 10.1016/S2213-2600(14)70061-X . [DOI] [PubMed] [Google Scholar]
- 25.Castanheira M, Farrell SE, Krause KM, Jones RN, Sader HS. Contemporary diversity of beta-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent beta-lactamase groups. Antimicrob Agents Chemother. 2014;58(2):833–8. Epub 2013/11/20. 10.1128/AAC.01896-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurz MS, Bayingana C, Ndoli JM, Sendegeya A, Durst A, Pfuller R, et al. Intense pre-admission carriage and further acquisition of ESBL-producing Enterobacteriaceae among patients and their caregivers in a tertiary hospital in Rwanda. Trop Med Int Health. 2017;22(2):210–20. 10.1111/tmi.12824 . [DOI] [PubMed] [Google Scholar]
- 27.Versporten A, Zarb P, Caniaux I, Gros MF, Drapier N, Miller M, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6):e619–e29. Epub 2018/04/24. 10.1016/S2214-109X(18)30186-4 . [DOI] [PubMed] [Google Scholar]
- 28.Musicha P, Cornick JE, Bar-Zeev N, French N, Masesa C, Denis B, et al. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998–2016): a surveillance study. Lancet Infect Dis. 2017;17(10):1042–52. Epub 2017/08/19. 10.1016/S1473-3099(17)30394-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bebell LM, Muiru AN. Antibiotic use and emerging resistance: how can resource-limited countries turn the tide? Global heart. 2014;9(3):347–58. Epub 2015/02/11. 10.1016/j.gheart.2014.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paterson DL, Ko WC, Von Gottberg A, Casellas JM, Mulazimoglu L, Klugman KP, et al. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol. 2001;39(6):2206–12. Epub 2001/05/29. 10.1128/JCM.39.6.2206-2212.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, et al. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum beta-lactamase bacteremia. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;60(9):1319–25. Epub 2015/01/15. 10.1093/cid/civ003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimhony O, Chmelnitsky I, Bardenstein R, Goland S, Hammer Muntz O, Navon Venezia S, et al. Endocarditis caused by extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae: emergence of resistance to ciprofloxacin and piperacillin-tazobactam during treatment despite initial susceptibility. Antimicrob Agents Chemother. 2006;50(9):3179–82. Epub 2006/08/31. 10.1128/AAC.00218-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker KJ, Lee YR, Klar AR. Clinical Outcomes of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae Infections with Susceptibilities among Levofloxacin, Cefepime, and Carbapenems. Can J Infect Dis Med Microbiol. 2018;2018:3747521 10.1155/2018/3747521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. WHO Model List of Essential Medicines: 20th List (March 2017) [cited 2018 January 1]. Available from: http://www.who.int/medicines/publications/essentialmedicines/20th_EML2017.pdf?ua=1.
- 35.Da Silva GJ, Mendonca N. Association between antimicrobial resistance and virulence in Escherichia coli. Virulence. 2012;3(1):18–28. 10.4161/viru.3.1.18382 . [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization (WHO) Global Tuberculosis Report 2017. Available from: http://apps.who.int/iris/bitstream/10665/259366/1/9789241565516-eng.pdf?ua=1.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.