Abstract
Background & Aims:
Little is known about mechanisms of perineural invasion (PNI) by pancreatic ductal adenocarcinomas (PDAs) or other tumors. Annexin A2 (ANXA2) regulates secretion of SEMA3D, an axon guidance molecule, which binds and activates the receptor PLXND1 to promote PDA invasion and metastasis. We investigated whether axon guidance molecules promote PNI and metastasis by PDA cells in mice.
Methods:
We performed studies in a dorsal root ganglion (DRG) invasion system, wild-type C57BL/6 mice (controls), mice with peripheral sensory neuron-specific disruption of PlxnD1 (PLAC mice), LSL-KRASG12D/+;LSL-TP53R172H/+;PDX-1-CRE+/+ (KPC) mice, and KPC mice crossed with ANXA2-knockout mice (KPCA mice). PDA cells were isolated from KPC mice and DRG cells were isolated from control mice. Levels of SEMA3D or ANXA2 were knocked down in PDA cells with small hairpin and interfering RNAs and cells were analyzed by immunoblots in migration assays, with DRGs and with or without antibodies against PLXND1. PDA cells were injected into the pancreas of control and PLAC mice, growth of tumors was assessed, and tumor samples were analyzed by histology. DRG cells were incubated with SEMA3D and analyzed by live imaging. We measured levels of SEMA3D and PLXND1 in PDA specimens from patients with PNI and calculated distances between tumor cells and nerves.
Results:
DRG cells increase the migration of PDC cells in invasion assays; knockdown of SEMA3D in PDA cells or antibody blockade of PLXND1 on DRG cells reduced this invasive activity. In mice, orthotopic tumors grown from PDA cells with knockdown of SEMA3D, and in PLAC mice, orthotopic tumors grown from PDA cells, had reduced innervation and formed fewer metastases than orthotopic tumors grown from PDA cells in control mice. Increased levels of SEMA3D and PLXND1 in human PDA specimens associated with PNI.
Conclusions:
DRG cells increase the migratory and invasive activities of pancreatic cancer cells, via secretion of SEMA3D by pancreatic cells and activation of PLXND1 on DRGs. Knockdown of SEMA3D and loss of neural PLXND1 reduces innervation of orthotopic PDAs and metastasis in mice. Increased levels of SEMA3D and PLXND1 in human PDA specimens associated with PNI. Strategies to disrupt the axon guidance pathway mediated by SEMA3D and PLXND1 might be developed to slow progression of PDA.
Keywords: neuron-derived factors, motility, pancreas, tumor microenvironment
“Lay Summary”
This study links the axon guidance pathway to pancreatic cancer invasion toward nerves and also describes a role of axon guidance molecules in pancreatic cancer metastasis.
Introduction:
Pancreatic ductal adenocarcinoma (PDA) is a devastating malignant disease in which the 5-year stage combined survival rate is lower than any other cancer type in the United States at 9% and it is the fourth leading cause of cancer death1. A major attribute to the poor prognosis is the lack of effective treatments in preventing and controlling metastasis. Many patients present with metastatic disease upon diagnosis; and, nearly all patients with localized disease recur and die from uncontrolled metastatic disease, even if their local disease had been controlled by surgical resection and radiation therapy2. Accumulating evidence suggests the PDA tumor microenvironment (TME) consisting of stroma, fibroblasts, immune cells, endothelial cells, nerves, and the extracellular matrix attributes to tumor growth and metastasis 3–7. However, among all the TME components, the tumor-neural paracrine signaling interaction is the least understood.
Perineural invasion (PNI), the neoplastic invasion of tumor cells into or surrounding the nerves, is a common histological feature among cancer types, but has the highest incidence in PDA at 80-100% and is an indicator of aggressive tumor behavior and poor prognosis8–13. PNI can occur in the absence of lymphatic or vascular invasion and is thought to represent the initial steps of metastasis14,15. Patients with PNI have been found to have an overall survival of over two years shorter than patients without PNI16. Moreover, neuroplastic changes such as increased neural hypertrophy and sprouting occur early in PDA development and, along with PNI, these factors have been implicated to provide a route for cancer dissemination3,8,17.
Whole exome sequencing of PDA samples demonstrated the axon guidance gene family was the most frequently altered gene family, including mutations and copy number changes18. Several neuron-derived factors have been identified to regulate PNI such as neural growth factor (NGF), glial cell-derived neurotrophic factor (GDNF), Artemin and SLIT signaling11,14,19–22; however, the role of semaphorin-plexin signaling, molecules involved in axon guidance, has not been determined in PNI. Multiple semaphorins and plexins, such as Semaphorin 3D (SEMA3D), SEMA3E, SEMA3A, Plexin A1, and Plexin D1 (PLXND1), have been found overexpressed in tumorigenesis and aid disease progression and metastasis23–25. We recently reported SEMA3D expression is upregulated during PDA development and is an essential secreted protein involved in PDA metastasis. SEMA3D secretion was found to be regulated by Annexin A2 (ANXA2), a phospholipid binding protein involved in exocytosis26. Secreted SEMA3D acts in an autocrine manner to increase PDA invasion and metastasis through binding to its co-receptors, PLXND1 and Neuropilin-1 (NRP1)25. Moreover, in human PDA SEMA3D expression is associated with poor survival and metastasis25. Since SEMA3D and PLXND1 are axon guidance molecules involved in neuroplasticity during development, we hypothesized paracrine SEMA3D and PLXND1 signaling could contribute to the increased PNI and neuroplasticity observed in PDA leading to increased invasive tumor capabilities and increased metastatic disease. Further elucidation of tumor-nerve signaling may provide new therapeutic targets that preclude PNI and aid survival.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal Experiments
All animal experiments were performed in line with the Animal Care and Use Committee of Johns Hopkins University and maintained according to the American Association of Laboratory Animal Care guidelines. The KPC mouse is a genetically engineered mouse model of PDA, previously established though a pancreatic specific knock-in of conditional alleles of the KrasG12D and TP53R172H mutations on a mixed background of 129/SvJae/C57Bl/627. Once these mice are crossed with PDX-1-CRE+/+ mice, they develop PanIN lesions that progress stepwise to full PDA development comparable to human disease with the LSL-KRASG12D/+;LSL-TP53R172H/+;PDX-1-CRE+/+ genotype. ANXA2 homozygous knockout mice (ANXA2−/−) with C57Bl/6 background were previously crossed with KPC mice to generate LSL-KRASG12D/+;LSL-TP53R172H/+;PDX-1-CRE+/+;ANXA2−/− (KPCA)25. Conditional PLXND1 mice with loxP sites flanking exon one of the PLXND1 gene were purchased from The Jackson Laboratory (stock number: 018319). Conditional PLXND1 mice were crossed with ADV-CRE (EMMA mouse repository) mice to create PLXND1 ADV-CRE+/− mice (PLAC).
The mouse pancreatic orthotopic injection model was previously described28. In summary, mice ages 8-12 weeks were anesthetized, the abdomen was opened and KPC tumor cells (1×105) suspended in 20uL PBS were injected into the pancreas.
Cell Culture
The development and culture method of primary murine KPC cell lines was previously described25. COS7 cells were cultured in DMEM with 10% FBS at 37°C in 5% CO2.
Primary DRG cells were collected from postnatal day 1-7 mice. Mice were euthanized and the vertebral column was removed and transferred to Leibovitz’s L15 medium (Gibco) with the dorsal side facing up under a microscope (Nikon SMZ Stereo Zoom) and DRGs were individually extracted. After collection, DRGs were resuspended in 5mg/mL collagenase and 2mg/mL dispase for 40 minutes shaking at 37°C. Cells were washed and cultured in DMEM with 10% FBS at 37°C in 5% CO2.
Human PDA specimens:
All human PDA specimens were obtained by patients who underwent pancreaticoduodenectomy between 1998-2004 at Johns Hopkins Hospital and received only adjuvant chemotherapy in accordance with Johns Hopkins Medial Institution Institutional Review Board approved protocol as previously described29. Patients who were primarily followed at JHMI and whose archived paraffin-embedded tissue blocks were in suitable condition were included. PNI was scored directly by a blinded pathologist using Hematoxylin & Eosin (H&E) stained slides. Tumor presence in the perineurium/endoneurium region or a minimum of 120 degree encasement of the nerves by tumor cells was considered to be PNI.
Western Blot Analysis:
Western Blot analysis with rabbit anti-ANXA2 (Santa Cruz Biotechnology), rabbit anti-SEMA3D (Abcam), mouse β-actin (Santa Cruz Biotechnology), rabbit anti-PLXND1 (Novus) and rabbit anti-GAPDH (Cell Signaling) was described previously25.
Immunohistochemistry:
Immunohistochemistry(IHC) on mouse tissue was performed manually with anti-rabbit IgG ImmPRESS Excel Staining Kit (Vector) per manufacture instruction. Detail method is provided in supplement. IHC on human tissue for SEMA3D and PLXND1 was performed on an automatic stainer (Leica Microsystems) as previously described25,26.
Multiplex and duplex IHC was performed using the sequential staining and striping method on mouse and human tissue as previously described30 and detailed method is provided in supplement.
Plasmid transfection, RNA interference and shRNA knockdown of SEMA3D in KPC cells:
Plasmid transfections and RNA interference, lentiviral expressing mouse SEMA3D shRNA (GeneCopeia) KPC cells, and recombinant AP-SEMA3D, AP-SEMA3E and AP-control was produced as described previously25.
Neurite Outgrowth Analysis:
DRG cells were isolated from WT C57Bl/6 mice 1-7 days after birth. Following culture, DRG cells were plated at 3,000 cells/well in 96-well plate and treated with KPC conditioned medium, AP-Ctrl, AP-S3D, anti-PLXND1 antibody, and/or IgG isotype antibody. Phase contrast images were taken every 3-4 hours for 5 days by IncuCyte ® Live imaging. After imaging, the IncuCyte ® NeuroTrack Software Module (Cat. No. 9600-0010) was utilized to determine neurite length and neurite branch points per cell body cluster area. Analysis was defined as cell-body cluster segmentation adjustment=0.3, Hole fill (μm2)=0.00, Minimum cell width (μm:10.00). cell-body cluster filters: Area (μm2)=min-500 max-4000. Neurite Parameters: filtering=better, neurite sensitivity=0.25, and neurite width=1 μm.
Halo Image Analysis:
Stained slides were scanned using the Hamamatsu (Nano Zoomer) at 20-40x magnification and distance analysis was performed using HALO 2.0 software as described in detail in the supplement methods.
Quantification and Statistical Analysis:
Statistical analysis was performed using GraphPad Prismx7.0c (GraphPad Software). Data was presented as means±SEM. A P value of < 0.05 was considered statistically significant.
Results:
Reduction of SEMA3D decreases tumor cell invasion in vitro and nerve density in murine PDA tumor model.
Accumulating evidence on the biological function of semaphorins and plexins in tumorigenesis 23,24,31, as well as, our previous report on SEMA3D in PDA25, provided clues to direct our attention to a potential role in PNI of PDA. We investigated if tumoral SEMA3D could regulate invasion towards nerves in a paracrine manner. To model PNI in vitro, we used a modified Boyden chamber invasion assay, in which Dorsal Root Ganglion (DRG) cells obtained from postnatal wild-type C57BL/6 (WT) mice were used as a source of nerves, and as a chemoattractant to tumor cells32. Murine PDA cells were previously derived from a KPC (LSL-KRASG12D/+;LSL-TP53R172H/+;PDX-1-CRE+/+) mouse that develops PDA spontaneously25,27. Using this assay, we demonstrated KPC tumor cells have an inherent migratory ability which is significantly increased upon co-culture with DRG cells (Supplementary Fig. S1A). SEMA3D (shSEMA3D) or scramble control (shCTRL) was knocked down in KPC tumor cells using a lentiviral system and confirmed by western blot (Supplementary Fig. S1B). ShSEMA3D KPC cells had decreased invasive potential compared shCTRL cells. In addition, the invasion of KPC cells towards DRG is SEMA3D dependent, as cells with SEMA3D knock down lose their invasive potential enhanced by DRGs. The invasion of shSEMA3D cells tends to be lower in the absence of DRG, suggesting secreted factors from DRGs can partially rescue the invasion of shSEMA3D cells (Fig. 1A). These results demonstrate tumoral SEMA3D acts in a paracrine fashion important for invasion towards nerves in vitro.
Figure 1: Reduction of SEMA3D decreases tumor cell invasion in vitro and nerve density in murine PDA tumor model.
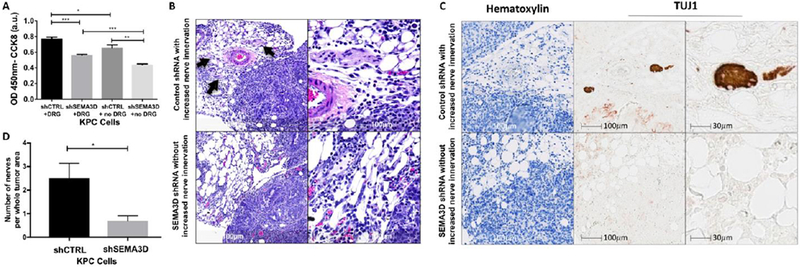
(A) Invasion potential of shSEMA3D or shCTRL KPC cells was measured in the presence and absence of DRG. Tumor cells were plated on the top chamber while DRG were plated on the bottom chamber and invasion was measured 24 hours later by CCK8 at 450nm. Data are means ± SEM from 4 technical replicates and representative of at least 3 experiments. (B) Mice with orthotopic tumors from shSEMA3D or shCTRL PDA cells were utilized. H&E images of the pancreas tumor area on day 10 post-injection. Arrows indicate nerve innervation into the tumor area. Images representative of 10 mice in each group. (C) Duplex immunohistochemical staining of tumor tissue was examined using hematoxylin and nerve specific marker, TUJ1 to analyze nerve density. (D) The number of nerves per whole tumor area from orthotopic tumors from shSEMA3D or shCTRL PDA cells was analyzed by H&E. Data are means ± SEM from 10 mice in each group *p<0.05, **p<.01, ***p<0.001 (unpaired student t-test).
To further establish the paracrine role of tumoral SEMA3D on neural invasion in PDA, we utilized a murine orthotopic injection model, previously described28 to examine changes in neural innervation in vivo. KPC shSEMA3D or shCTRL tumor cells were orthotopically injected into the pancreas of WT mice. After 10 days of growth, the time found optimal to view nerves within the tumor environment before the expansive tumor growth crushed the nerves, tumors were collected. Nerve innervation was analyzed by H&E, and previously described neuron-specific class III β-tubulin TUJ1 immunohistochemistry33 (Fig. 1B and 1C). Mice who received shSEMA3D cells had significantly less nerve innervation present in the tumor area compared to control (Fig. 1D). These results suggest decreased tumoral SEMA3D is associated with less nerve innervation in the TME.
Blockade of neural PLXND1 reduces tumor cell invasion towards nerve.
Since our data demonstrates nerve presence increases the invasion capability of PDA tumor cells in vitro, we next examined if axon guidance molecules expressed by the nerves are important for paracrine mediated tumor invasion. PLXND1 has been identified as a co-receptor for SEMA3D signaling25,34,35 and is expressed during development and in adult murine peripheral nervous tissue36,37. Therefore, we hypothesized the receptor PLXND1 expressed by the nerves is important for paracrine mediated tumor cell invasion. We confirmed PLXND1 protein expression on postnatal murine DRG through immunohistochemical staining (Supplementary Fig. S2). Next, KPC cell invasion was measured after co-culture with DRG cells treated with PLXND1 neutralizing antibodies to block the putative SEMA3D receptor or isotype control antibodies. Blockade of PLXND1 resulted in diminished invasion of KPC tumor cells towards DRG cells compared to control treatment (Fig. 2A). KPC tumor cells still maintained their baseline motility, implicating enhanced invasion of tumor cells towards DRGs is PLXND1 dependent.
Figure 2: Blockade of neural PLXND1 reduces tumor cell invasion towards nerve.
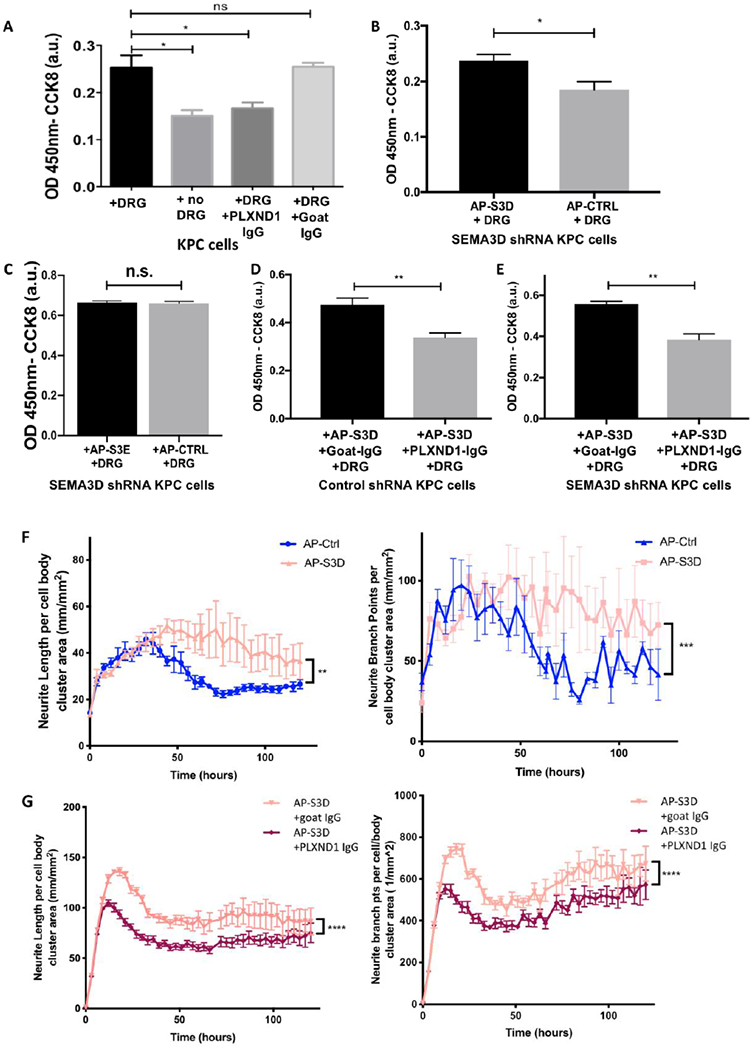
(A) Invasion assay using KPC tumor cells were plated in the top chamber and untreated, IgG isotype control or PLXND1 neutralizing antibody treated DRGs were plated in the bottom chamber, invasion was quantified 24 hours later by CCK8. (B) Recombinant AP-Ctrl or AP-S3D was added to shSEMA3D KPC cells and invasion was measured after 24 hours. (C) Invasion capability of shSEMA3D KPC cells was measured after 24 hour treatment with AP-Ctrl or AP-SEMA3E (AP-S3E). (D) KPC shCTRL or (E) shSEMA3D were plated on the top chamber with AP-S3D and DRG was plated on the bottom chamber with either control or PLXND1 neutralizing antibody, invasion was measured 24 hours later (unpaired, student t-test). (F) DRG cells were treated with AP-Ctrl or AP-S3D and (G) AP-S3D and control antibody (goat-IgG) or PLXND1 antibody (PLXND1-IgG). DRG cells were phase contrast imaged every 3-4 hours and images were used for analysis of neurite length or neurite branch point per cell body cluster area over time (Kruskal-Wallis Test). Data are means ± SEM from 4 technical replicates and representative of at least 2 experiments. ns− not significant, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
Next, we tested if exogenous recombinant SEMA3D protein could rescue the decreased invasion capabilities in shSEMA3D KPC cells and neuronal PLXND1 blockade. Exogenous addition of alkaline phosphatase tagged-SEMA3D recombinant protein(AP-S3D), prepared as previously described38, to shSEMA3D KPC tumor cells rescued the enhanced invasive potential compared to AP-Ctrl treatment (Fig. 2B), confirming the role of SEMA3D in increased invasion. Furthermore, we examined if SEMA3E, previously been found to also bind PLXND1, could compensate for loss of SEMA3D in invasion 25,38. Alkaline phosphatase tagged-SEMA3E recombinant protein (AP-S3E), and AP-Ctrl was added to shSEMA3D cells in the presence of DRG cells. However, SEMA3E treatment did not significantly increase KPC cell invasion in shSEMA3D KPC cells (Figure 2C). These results suggest that SEMA3E cannot compensate for the loss of SEMA3D to increase invasion capabilities of KPC cells. Moreover, when shCTRL or shSEMA3D KPC cells were co-cultured with DRG cells treated with PLXND1 neutralizing antibody, exogenous addition of AP-S3D did not restore tumor cell invasion (Fig. 2D and 2E). These results indicate blockade of neuronal PLXND1 reduces tumoral invasion in vitro even in the presence of exogenous SEMA3D.
SEMA3D increases neurite outgrowth on DRG cells.
Previous studies have shown PDA tumor conditioned media is capable of increasing neurite outgrowth of DRG cells, implicating secreted factors are partially responsible for increased neural hypertrophy and sprouting detected in the PDA TME39. We also found KPC conditioned medium significantly increases DRG cell neurite outgrowth; which, is diminished upon addition of PLXND1 neutralizing antibodies (Supplemental Fig 3A and B). To test the role of SEMA3D in increased neural remodeling, recombinant AP-S3D and AP-Ctrl was added to DRG cells and live imaged every 3-4 hours. AP-S3D significantly increased DRG neurite length and neurite branch points per cell body cluster areas which was significantly decreased upon the addition of PLXND1 neutralizing antibodies (Fig. 2F and 2G). This data suggests, SEMA3D signaling through PLXND1 impacts neuronal outgrowth implicating reciprocal signaling between neural and PDA cells.
Presence of perineural invasion and increased nerve innervation in murine PDA is ANXA2 dependent.
We previously found ANXA2 to regulate the tumoral secretion of SEMA3D; therefore, next we investigated if loss of ANXA2 expression in PDA tumors, and consequently low amounts of secreted SEMA3D25, impacted the incidence of PNI. To evaluate role of ANXA2 in the mechanism of PNI in PDA development we utilized two mouse models, LSL-KRASG12D/+;LSL-TP53R172H/+;PDX-1-CRE+/+ (KPC) mice, which are genetically engineered to develop spontaneous PDA tumors, and KPCA mice, obtained by crossing KPC and ANXA2 knockout mice. PDA development in KPC and KPCA mice recapitulates human disease progression starting with low-grade pancreatic intraepithelial neoplasms stepwise to invasive PDA; however, the KPCA mice lack metastatic potential in their primary tumors25. H&E and TUJ1 stained primary tumor slides of similar size from both KPC and KPCA mice were scored for PNI (Fig 3A and 3B). We found 31% of the KPC mice (5/16) had PNI present, whereas PNI was not found in the KPCA mice (0/26) (Fig. 3C), suggesting the presence of PNI is ANXA2 dependent. Next, we investigated the loss of ANXA2 on intratumoral nerve density. Nerve density in H&E stained tissue from KPC and KPCA mice were analyzed. Overall nerve density was generally low in the KPC model, potentially explained by the aggressive tumor cell expansion and consequent nerve crushing during tumor growth. Nevertheless, tumors from KPC mice had significantly higher nerve density compared to KPCA mice (Fig. 3D) implicating a role for ANXA2 in regulating innervation of PDA tumors.
Figure 3: Presence of perineural invasion and increased nerve innervation in murine PDA is ANXA2 dependent.
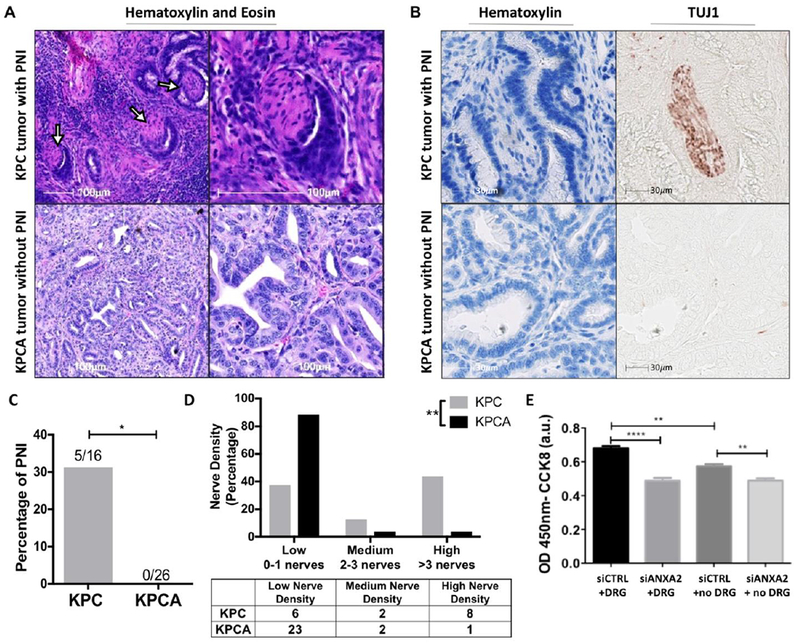
(A) H&E staining of PDA tissue from KPC and KPCA mice indicating PNI (arrows) in KPC mouse. Images representative of 16 KPC mice and 26 KPCA mice. (B) Duplex immunohistochemical staining of KPC and KPCA mouse PDA tissue was performed with hematoxylin and TUJ1. Representative images are shown. (C) Percentage of perineural invasion found in KPC versus KPCA mice, Fisher’s Exact Test. (D) Nerves were counted in the pancreas tumor area of KPC and KPCA mice on H&E slide. The samples were defined as having low (0-1), medium (2-3), or high (>3) nerve density. Differences in KPC and KPCA nerve density is shown by overall percentage, Fisher’s exact test. (E) Cell invasion was quantified in KPC cells with siRNA targeting ANXA2 (siANXA2) or scramble control (siCTRL) in the presence and absence of DRGs. Data are means ± SEM from 4 technical replicates and representative of at least 3 experiments, unpaired student’s T-test. * p<0.05, **p<0.01, ****p<0.0001
Next, we used the DRG invasion assay in vitro model to confirm the role of ANXA2 in tumor cell invasion towards nerves. Small interfering RNA targeting ANXA2 (siANXA2) or scrambled RNA (siCTRL) was used to knock down ANXA2 expression in KPC cells (Supplementary Fig. S1C). Knockdown of ANXA2 significantly reduced tumor cell invasion compared to control cells in the absence of DRG, confirming its importance in the invasion process (Fig. 3E). Moreover, in the presence of DRG, ANXA2 knockdown decreased tumor cell invasion toward nerve, whereas control transfected tumor cells showed significant DRG dependent increased invasion (Fig. 3E). These results support a role for tumoral ANXA2 in the paracrine signaling mechanism of tumor cells migrating towards nerves.
Reduction of neuronal PLXND1 expression decreases intratumoral nerve density and metastasis in murine model of PDA.
To further establish the role of neuronal PLXND1 in the paracrine tumor cell invasion signaling we created a mouse model to study the nerve innervation and tumor cell metastasis in vivo. Conditional PLXND1, PlxnD1f/f (PL) mice, with loxP sites flanking exon one of the PLXND1 gene, were crossed with Advillin-Cre (Adv-Cre+/−) to create PlxnD1f/f ADV-CRE+/− (PLAC) mice. Advillin is expressed in almost all peripheral sensory neurons of the DRG and trigeminal ganglia40; therefore, in PLAC mice PLXND1 expression is knocked out in peripheral sensory neurons to study paracrine neuron-tumor signaling. PLXND1 knock out was confirmed by immunohistochemical staining in DRG and pancreas tissue (Supplementary Fig. S4). The orthotopic PDA tumor cell injection model was used study the role of neuronal PLXND1 on nerve density. Upon histological examination of nerves present per whole tumor area, we found PLAC mice had a significantly lower intratumoral nerve density in comparison to their littermate controls (Fig. 4A), while no innervation differences were observed in the pancreas of mice without pancreas tumors (data not shown). This result suggests neural PLXND1 is involved in regulating intratumoral nerve density.
Figure 4: Reduction of neuronal PLXND1 expression decreases intratumoral nerve density and metastasis in murine model of PDA.
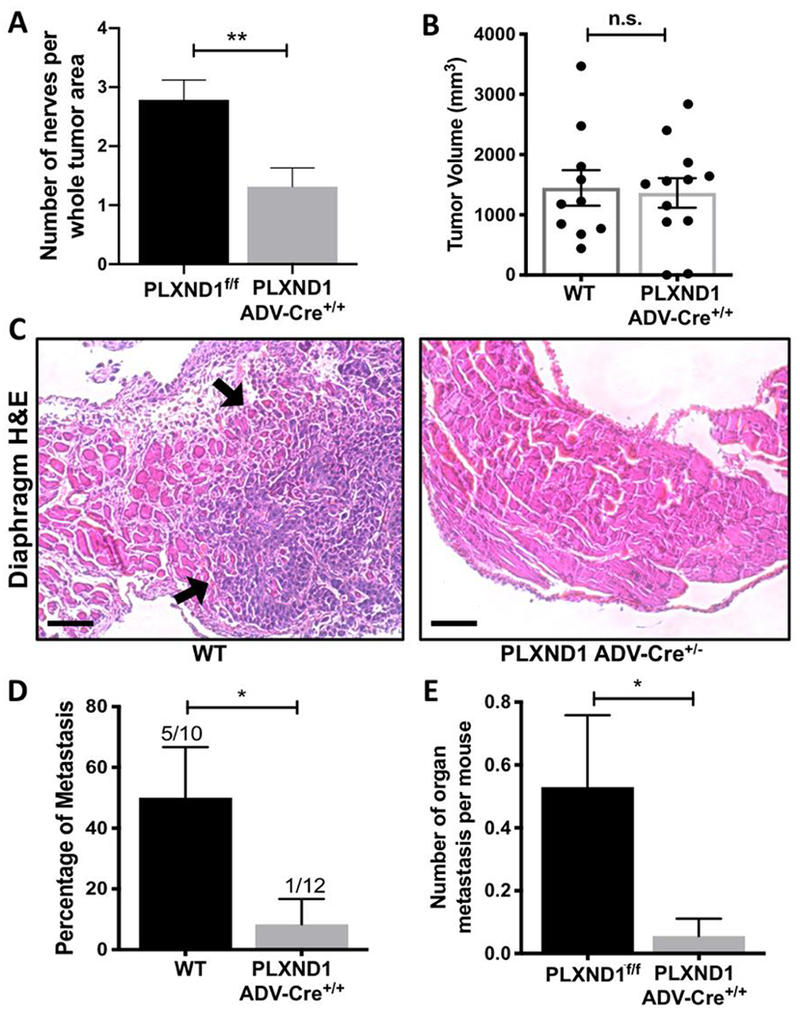
(A) PLXND1f/f mice and PLXND1 Adv-Cre+/− mice underwent orthotopic injection of KPC cells into the pancreas. Nerves per whole tumor area were quantified. Data are means ± SEM from n=14 PLXNDf/f and n=19 PLXND1 Adv-Cre+/− mice in each group, unpaired, two-tailed student t-test. (B) Orthotopic tumors grown from PDA tumor cells were collected and measured in WT and PLXND1 Adv-Cre+/− mice 28 days later. Data are means ± SEM from at least 10 mice, unpaired, two-tailed student t-test. (C) Representative H&E staining of the diaphragm from WT and PLXND1 Adv-Cre+/− mice indicating metastasis in the WT tissue (metastasis indicated with arrows). Scale bars=50μm. (D) Micrometastasis formation was assessed by H&E staining of the liver, gut, and diaphragm tissue collected 28 days after injection (Fisher’s Exact Test). (E) Number of metastatic organ sites/mouse in liver, gut and diaphragm was analyzed in organs collected upon autopsy. PLXND1f/f n=17, PLXND1 Adv-Cre+/− n=18 mice. Note: both WT and PL mice were used as control. n.s.− not significant, *p<0.05
PNI has recently been appreciated as an emerging route for tumor cell dissemination14. Studies indicate reciprocal tumor-nerve signaling may contribute to PNI41. We hypothesized neural PLXND1 involved in paracrine tumoral SEMA3D signaling may influence tumor cell metastasis. To test this hypothesis, orthotopic tumors grown from PDA cells from age and gender-matched WT and PLAC mice were evaluated for tumor size and metastasis. Tumor size measured at necropsy was not significantly different (Fig. 4B), in line with previous research that PLXND1 expression does not change tumor growth 25. In the control group of 10 mice, 5 mice were identified with liver, diaphragm or gut metastasis by histological examination; however, only 1 out of 12 PLAC mice were identified with metastasis (Fig. 4C and 4D). Furthermore, PDA tumors in littermate PL and PLAC were also examined. Twenty-nine percent of control (PL) mice developed liver, diaphragm or gut metastasis; whereas, only 6% of PLAC developed metastasis. In addition, the number of metastasis per organ was significantly higher in PL mice comparing to PLAC mice (Fig. 4E), suggesting neuronal PLXND1 is important for paracrine signaling to promote tumor metastasis. These results indicate decreased neuronal PLXND1 decreases murine PDA metastasis but does not alter primary tumor growth.
High SEMA3D and PLXND1 expression and shorter distance between SEMA3D/PLXND1-high tumor cells and nerves in human PDAs is associated with PNI.
We have previously shown PDA tumor cells increase expression of SEMA3D and PLXND1 over disease progression25; however, we sought to investigate if nerves innervating murine PDA tumors expressed these axon guidance proteins. Multiplex immunohistochemical staining was performed on KPC PDA tissue for hematoxylin, TUJ1, PLXND1 and SEMA3D on a single tissue slide. SEMA3D and PLXND1 expression co-localizes on tumor cells and peripheral nerves in the adult KPC tumor pancreas tissue (Fig. 5A and Supplemental Fig. S5). Next, we wanted to examine SEMA3D and PLXND1 expression and PNI in primary human PDA specimens. PNI was analyzed through multiplex staining of epithelial maker EpCam and neural marker TUJ1 (Figure 5B) in banked human paraffin-embedded resected PDA tissue. SEMA3D and PLXND1 were determined to have semi-quantitative high or low immunohistochemical staining (Fig. 5C and Supplementary Fig. S6). Tissue with histological confirmation of PNI was significantly associated with high SEMA3D staining compared to tissue without PNI (Fishers Exact Test p=0.002) (Fig. 5C, Supplementary Fig. S6, and Table 1). Furthermore, high staining of PLXND1 was significantly associated in tissue with PNI compared to without PNI (Fishers Exact Test p=0.002) (Fig. 5C and Table 1). Co-localization of SEMA3D and PLXND1 was also found in tumor cells and nerves in PNI positive samples (Fig. 5D). These results suggest SEMA3D and PLXND1 expression is increased in PNI and co-localizes in nerve and tumor cells.
Figure 5: High SEMA3D and PLXND1 expression and shorter distance between SEMA3D/PLXND1-high tumor cells and nerves in human PDAs is associated with PNI.
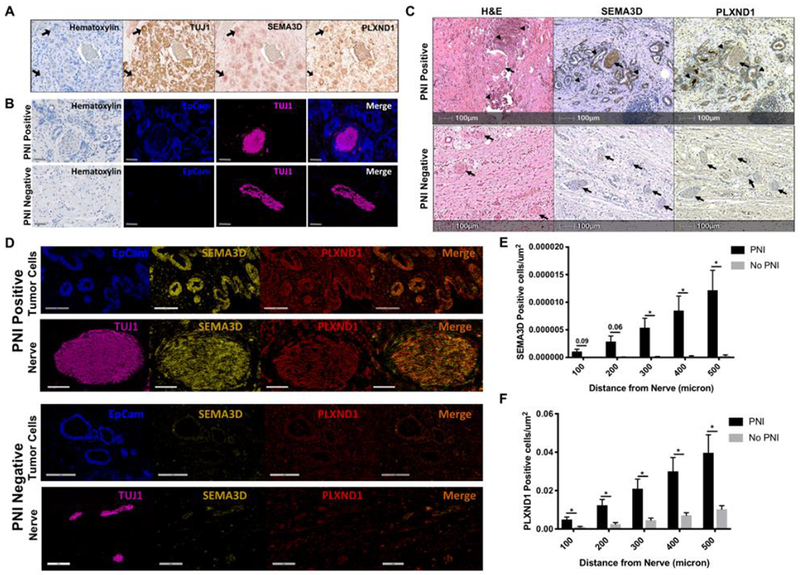
(A) Multiplex IHC was performed on a single slide of mouse PDA tissue for Hematoxylin, TUJ1, SEMA3D and PLXND1. An intratumoral nerve bundle is shown and arrows indicate single nerve cells stained with respective markers. (B) Multiplex IHC was used to identify PNI positive and negative human PDA tissue with epithelial marker, EpCam, staining tumor cells and nerve marker, TUJ1, staining neural cells. Representative images are shown, scale bar =100μm. (C) Banked human paraffin-embedded resected PDA tissue with or without PNI was stained for H&E, SEMA3D and PLXND1. Arrows indicate nerves and arrowheads indicate tumor cells. Images representative of n=16 with PNI and n=8 without PNI. (D) Co-localization of SEMA3D and PLXND1 on tumor cells and nerves in human PDA was analyzed using multiplex IHC. Respective images are from PNI positive and negative samples. Tumor cells and nerves are from different areas of the same PNI positive or negative slide. EpCam identifies tumor cells, TUJ1identifies nerves, and the merge image displays SEMA3D and PLXND1 co-localization. Scale bar =300pm for tumor cells and =200μm for nerve images. (E) Halo image analysis software was used to measure the amount of SEMA3D or (F) PLXND1 positively stained cells per μm2 within 100-500 microns of nerves in human PDA tissue with and without PNI, unpaired two-tailed student’s t-test. *p<0.05
Table 1:
Analysis of High SEMA3D and PLXND1 positive staining in human PDA tissue shows significant association with PNI positive samples compared to PNI negative samples. Fishers Exact Test p=0.002
| PNI Positive (n=16) | PNI Negative (n=8) | ||
|---|---|---|---|
| SEMA3D staining | High | 13 (81%) | 0 (0%) |
| Low | 3 (19%) | 8 (100%) | |
| PLXND1 staining | High | 15 (94%) | 1 (13%) |
| Low | 1 (6%) | 7 (87%) | |
Moreover, we used Halo image analysis software to quantitatively measure the distance of positively stained SEMA3D or PLXND1 cells to nerves in human PDA tissue with and without PNI (Supplementary Fig. S7). The number of SEMA3D positively stained tumor cells per μm2 was significantly higher in tissue with PNI when the tumor cells were within 300-500 microns of the nerves compared to tissue without PNI. A strong trend towards increased SEMA3D positive tumor cells within 0-200 microns of nerves in tissue with PNI was also observed (Fig. 5E). In addition, the number of PLXND1 positively stained cells per μm2 was significantly higher in tissue with PNI when tumor cells were within 0 to 500 microns of the nerves compared to tissue without PNI (Fig. 5F). Increase in SEMA3D or PLXND1 positive cells was not due to an increase in overall tumor cellularity as there is no significant difference in cell density between PNI positive and negative samples (Supplementary Fig. S7). These results suggest that shorter distance between tumor cells, which express high-level SEMA3D/PLXND1, and nerves in PDAs is associated with PNI.
Decrease in both tumoral SEMA3D and neural PLXND1 DRG shows further reduction in tumor invasion and nerve density.
To better elucidate the mechanism of paracrine SEMA3D/PLXND1 signaling, we analyzed tumor cell invasion with tumoral SEMA3D knockdown and DRG cells treated with a PLXND1 neutralizing antibody. We found when both tumoral SEMA3D is decreased and DRG cells are treated with PLXND1 neutralizing antibody, there is a significant further reduction in the KPC tumor cell invasion capability compared to shSEMA3D or PLXND1 antibody treatment alone (Fig. 6A). In addition, the orthotopic PDA tumor cell injection model was used to study neuronal PLXND1 and tumoral SEMA3D in PDA invasion in vivo in littermate PL and PLAC mice. Ten days after orthotopic cell injection, tumors were collected. Upon histological examination of nerve abundance in the whole tumor area, we found PL mice receiving shSEMA3D tumors had a similar nerve density compared to PLAC mice with shCTRL tumors and, shSEMA3D knockdown tumors in PLAC mice exhibited a further reduction in intratumoral nerve innervation (Fig. 6B). Possible explanation for the combined further reduction in tumor cell invasion and intratumoral nerves could result from incomplete antibody blockade of DRG cells or incomplete PLXND1 sensory nerve knock out (Supplementary Fig. S4C); however, we do not rule out the possibility of involvement of other secreted semaphorins in this pathway (Fig. 6C). Further investigation is necessary to determine if reduction of both tumoral SEMA3D and sensory neural PLXND1 can further inhibit tumor cell metastasis. Taken together, these results suggest that both neural PLXND1 and tumoral SEMA3D are involved in regulating increased tumor cell invasion and intratumoral nerve density.
Figure 6: Decrease in both tumoral SEMA3D and neural PLXND1 shows further reduction in tumor invasion and nerve density.
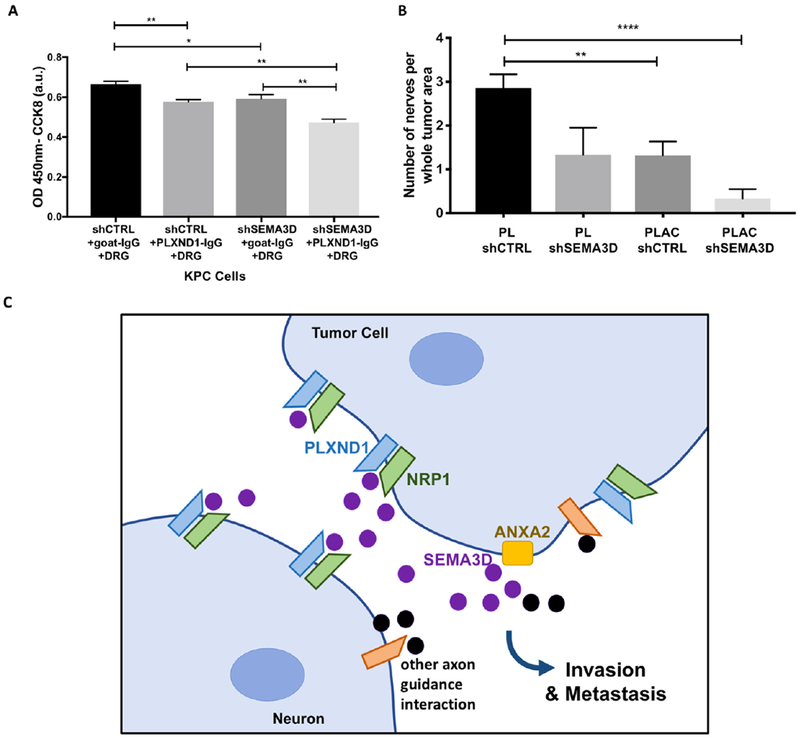
(A) ShSEMA3D or shCTRL KPC cells were plated on the top chamber and DRG cells were plated on the bottom with control goat antibody or neutralizing PLXND1 antibody, invasion was measured after 24 hours. Data are means ± SEM from 4 technical replicates, unpaired, two-tailed students t-test. (B) Orthotopic tumors were grown from shSEMA3D or shCTRL PDA cells in PLXND1 f/f and PLXND1 Adv-Cre+/− mice. The number of nerves per whole tumor area from mice pancreas H&E slides were quantified. Note PL and PLAC shCTRL data from Figure 4A is combined for comparison. Data are means±SEM from at least 6 mice in each group, unpaired, two-tailed student t-test. *p<0.05, **p<0.01, ***p<0.0001 (C) Model presenting the proposed paracrine signaling mechanism. ANXA2 (yellow block) controls the extracellular release of SEMA3D (purple circle) allowing interaction with its receptor PLXND1 (blue) on neurons aiding in increased tumor cell invasion and metastatic capabilities. The black circles and orange receptors indicate other axon guidance molecules potentially contributing to this signaling pathway.
Discussion:
Perineural invasion is a well-established pathological feature of PDA associated with negative prognostic outcomes after surgical resection10–12,42; however, the mechanistic relationship between tumor cells and nerves leading to disease progression remains unclear. ANXA2, SEMA3D and PLXND1 have previously been linked to tumor growth and metastasis through an autocrine pathway; however, this study, for the first-time links ANXA2-SEMA3D-PLXND1 signaling to PNI in PDA through a paracrine pathway. Here, we have found PDA tumor cell invasion towards DRG cells is dependent on tumoral SEMA3D and ANXA2 expression. We believe functional ANXA2 allows for the exocytosis of SEMA3D, therefore regulating SEMA3D secretion25. As ANXA2 expression is increased during PDA development26, it will be interesting to explore how this process impacts PNI more in the future. Reduced expression of the SEMA3D co-receptor, PLXND1, on DRG cells led to reduced invasion of tumor cells toward nerves. Hyper-innervation is another well-established characteristic of PDA associated with PNI9,17; we found decreased tumoral SEMA3D decreased tumoral nerve innervation in our murine model of PDA. In addition, SEMA3D increased neurite outgrowth of DRG cells in a PLXND1-dependent manner. Moreover, transgenic mice which lack PLXND1 expression on sensory nerves presented a reduction in tumoral neural innervation, as well as, a significant decrease in PDA tumor metastasis compared to control mice. We found high tumoral expression of SEMA3D and PLXND1 and shorter distance between tumor cells, which express high-level SEMA3D/PLXND1, and nerves to be associated with PNI in human PDAs. These results provide further mechanistic understanding of paracrine interactions between tumor cells and nerves in PDA.
SEMA3E has been implicated in tumor cell migration24 and can bind to PLXND1 independently of NRP138, providing a possible paracrine interaction. Surprisingly, our study demonstrated SEMA3E did not increase shSEMA3D KPC cell invasion capability, suggesting SEMA3E is not involved in the paracrine function of regulating PNI. SEMA3A expression has also been negatively implicated in PDA23; however, is unlikely involved in this particular paracrine interaction because SEMA3A does not bind PLXND1. Nevertheless, we cannot exclude the possibility of other semaphorin involvement other than SEMA3E. Additionally, reduction of the autocrine SEMA3D pathway, previously established25, can potentially contribute to decreased invasion and intratumoral innervation. Consistent with this notion, Figure 1A demonstrates knockdown of SEMA3D from KPC cells lowered the migratory capability of KPC cells to a larger extent than just losing the capability of migrating toward DRG. Therefore, the autocrine effect of SEMA3D likely also plays a role in PNI. A future study on how SEMA3D coordinates the tumor intrinsic migratory capability with its capability of migrating toward the nerve in vivo is warranted.
Human PDA immunohistochemistry demonstrated the distance of SEMA3D-positive cells were found in close proximity to nerves, supporting our in vitro data that tumor cells migrate toward nerves; however, the impact of tumor cell invasion in other co-culturing models with tumor and nerve cells remains to be investigated. In addition, to define tumor cellularity differences between samples, we were limited to use weak SEMA3D or PLXND1 staining to identify tumor cell populations per area of tissue. In the future studies, it will be interesting to co-stain tumor cells and axon guidance markers to further investigate the spatial role of SEMA3D and PLXND1 expression in relation to PNI. Intratumoral nerve density was measured in orthotopic tumors from PDA cells as a surrogate for PNI, as the rapid tumor cell growth often crushed nerves in the later stages of this disease model. However, the murine KPC model is capable of developing PNI during disease progression43; therefore, we utilized KPCA tumors to confirm the role of ANXA2 in vivo. The rate of PNI is much lower in KPC mice compared to humans, which is an anticipated characteristic of the KPC model. KPC mice primarily die from primary tumor growth, not metastasis; therefore, KPC tumors may not have as high metastatic potential as human PDAs. Moreover, because SEMA3D knock out mice cannot survive past birth, AnxA2 knock out mice were utilized.
SEMA3D is dually expressed by the tumor cells and by the intrapancreatic nerves (Fig. 5C), implicating two possible mechanisms of PNI: the tumor cells travel towards the nerves or the nerves sprout towards the tumor. While both options may be happening synergistically, our DRG in vitro data supports the hypothesis that tumor cells expressing SEMA3D can travel towards PLXND1 expressing neurons. Similarly, without DRG in the invasion chamber, SEMA3D positive tumor cells still exhibit increased motility, possibly due to the autocrine effect of SEMA3D25. Furthermore, if this perineural invasion activity is a route for metastasis or just a surrogate marker for increased metastasis needs to be further explored. The celiac plexus is adjacent to the pancreas and is often invaded by PDA tumors before surgical resection. However, after primary and marginal surgical resection, there is no specific recurrence pattern along the nerve, suggesting the classical route of systemic hematogenous spread of cancer cells44. While tumor cell invasion into the nerves might provide a route for metastasis, based on recurrence pattern data, it seems nerves are providing a reservoir for tumor cells after resection, allowing tumor cells to thrive and then become a source of hematogenous dissemination and peritoneal metastasis.
This is the first time a PNI mechanism is associated with the semaphorin and plexin gene family that is genetically altered in PDA. In future studies, it will be important to link the role of axon guidance molecules in PNI to specific genetic alterations in human PDAs. It will be interesting to explore how the ANXA2-SEMA3D-PLXND1 signaling pathway fits in with other signaling molecules currently identified to be involved in PNI in PDA such as NGF, GDNF, Artemin and SLIT12/ROBO19–22. Furthermore, the exact signaling mechanism of SEMA3D inducing increased tumor cell invasion remains to be explored.
Supplementary Material
“What You Need to Know”.
Background and Context:
Why pancreatic cancer is associated with high incidence of perineural invasion and the underlying mechanism involved is not fully understood.
New Findings:
The Sema3D-PlexinD1 axon guidance pathway mediates paracrine signaling between tumor cells and nerves to enhance innervation and perineural invasion in pancreatic cancer.
Limitation:
This study has not examined the relationship between the role of axon guidance molecules in perineural invasion with specific pancreatic cancer genetic alterations.
Impact:
Increased elucidation of tumor-nerve signaling will provide new therapeutic targets that preclude perineural invasion and enhance patient survival.
Grant support:
This study was supported by the NIH RO1 CA169702 grant, NIH grant R01 CA197296; Sidney Kimmel Comprehensive Cancer Center Grant P30 CA006973, the Viragh Foundation, the Skip Viragh Pancreatic Cancer Center at Johns Hopkins and the Japan Society for the Promotion of Science Overseas Research Fellowship.
We want to thank Dr. Alex Kolodkin for his expectional guidance in our SEMA3D and PLXD1 signaling models. We also want to thank Dr. Peter Calabresi and Matthew Smith for lending their Neurotrack Analysis software and guidance during our neurite outgrowth studies.
Disclosures: LZ has grant support from Bristol-Meyer Squibb, Merck, iTeos, Amgen, Gradalis, Halozyme, and NovaRock. LZ is a paid consultant at Biosynergies, Merck, NovaRock, AstroZeneca, Oncorus, Alphamab, Fosun Biopharma, and Sound Biologics. LZ and EMJ receive royalty for licensing GVAX to Aduro Biotech. NJ, AAR, SM, ET, RS, DD, JRE, RAA, and KF have no relevant conflict of interest to report.
Abbreviations:
- PNI
(perineural invasion)
- PDA
(pancreatic ductal adenocarcinoma)
- ANXA2
(annexin a2)
- SEMA3D
(semaphorin 3D)
- PLXND1
(plexin D1)
- NRP1
(neuropilin-1)
- NGF
(neural growth factor)
- GDNF
(glial cell-derived neurotrophic factor)
- DRG
(dorsal root ganglion)
- WT
(wild type)
- H&E
(hematoxylin and eosin)
- SEMA3A
(semaphorin 3A)
- SEMA3E
(semaphorin 3E)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics , 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Wolfgang CL, Herman JJM, Laheru DA, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63(5):318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jobling P, Pundavela J, Oliveira SMR, Roselli S, Walker MM, Hondermarck H. Nerve-cancer cell cross-talk: A novel promoter of tumor progression. Cancer Res. 2015;75(9):1777–1781. doi: 10.1158/0008-5472.CAN-14-3180. [DOI] [PubMed] [Google Scholar]
- 4.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2013;18(16):4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi K, Ehata S, Koinuma D, et al. Pancreatic tumor microenvironment confers highly malignant properties on pancreatic cancer cells. Oncogene. 2018;37(21):2757–2772. doi: 10.1038/s41388-018-0144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin HJ, Lin J. Seed-in-soil: Pancreatic cancer influenced by tumor microenvironment. Cancers (Basel). 2017;9(7):1–13. doi: 10.3390/cancers9070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rucki AA, Zheng L. Pancreatic cancer stroma: Understanding biology leads to new therapeutic strategies. World J Gastroenterol. 2014;20(9):2237–2246. doi: 10.3748/wjg.v20.i9.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amit M, Na’Ara S, Gil Z. Mechanisms of cancer dissemination along nerves. Nat Rev Cancer. 2016;16(6):399–408. doi: 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- 9.Stopczynski RE, Normolle DP, Hartman DJ, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74(6):1718–1727. doi: 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systemic review of the literature. J Pancreas. 2008;9(2):99–132. [PubMed] [Google Scholar]
- 11.Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11(10):695–707. doi: 10.1038/nrc3131. [DOI] [PubMed] [Google Scholar]
- 12.Shimada K, Nara S, Esaki M, Sakamoto Y, Kosuge T, Hiraoka N. Intrapancreatic nerve invasion as a predictor for recurrence after pancreaticoduodenectomy in patients with invasive ductal carcinoma of the pancreas. Pancreas. 2011;40(3):464–468. doi: 10.1097/MPA.0b013e31820b5d37. [DOI] [PubMed] [Google Scholar]
- 13.Demir IE, Ceyhan GO, Liebl F, D’Haese JG, Maak M, Friess H. Neural invasion in pancreatic cancer: The past, present and future. Cancers (Basel). 2010;2(3):1513–1527. doi: 10.3390/cancers2031513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liebig C, Ayala G, Wilks JA, Berger DH, Albo D. Perineural invasion in cancer: A review of the literature. Cancer. 2009;115(15):3379–3391. doi: 10.1002/cncr.24396. [DOI] [PubMed] [Google Scholar]
- 15.Chang A, Kim-Fuchs C, Le C, Hollande F, Sloan E. Neural regulation of pancreatic cancer: A novel target for intervention. Cancers (Basel). 2015;7(3):1292–1312. doi: 10.3390/cancers7030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee D, Katz MH, Rashid A, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36(3):409–417. doi: 10.1097/PAS.0b013e31824104c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demir IE, Friess H, Ceyhan GO. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2015;12(11):649–659. doi: 10.1038/nrgastro.2015.166. [DOI] [PubMed] [Google Scholar]
- 18.Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491(7424):399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gohring A, Detjen KM, Hilfenhaus G, et al. Axon guidance factor SLIT2 inhibits neural invasion and metastasis in pancreatic cancer. Cancer Res. 2014;74(5):1529–1540. doi: 10.1158/0008-5472.CAN-13-1012. [DOI] [PubMed] [Google Scholar]
- 20.Ketterer K, Rao S, Friess H, Weiss J, Büchler MW, Korc M. Reverse transcription-PCR analysis of laser-captured cells points to potential paracrine and autocrine actions of neurotrophins in pancreatic cancer. Clin Cancer Res. 2003;9(14):5127–5136. doi: 10.1158/1078-0432.ccr-03-0820. [DOI] [PubMed] [Google Scholar]
- 21.Gil Z, Cavel O, Kelly K, et al. Paracrine regulation of pancreatic cancer cell invasion by peripheral-nerves. J Natl Cancer Inst. 2010;102(2):107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceyhan GO, Giese NA, Erkan M, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244(2):274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller MW, Giese NA, Swiercz JM, et al. Association of axon guidance factor Semaphorin 3A with poor outcome in pancreatic cancer. Int J Cancer. 2007;121(11):2421–2433. doi: 10.1002/ijc.22949. [DOI] [PubMed] [Google Scholar]
- 24.Yong L-K, Lai S, Liang Z, et al. Overexpression of Semaphorin-3E enhances pancreatic cancer cell growth and associates with poor patient survival. Oncotarget. 2016;7(52):87431–87448. doi: 10.18632/oncotarget.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley K, Rucki AA, Xiao Q, et al. Semaphorin 3D autocrine signaling mediates the metastatic role of annexin A2 in pancreatic cancer. Sci Signal. 2015;8(388):ra77. doi: 10.1126/scisignal.aaa5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng L, Foley K, Huang L, et al. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One. 2011;6(4):e19390. doi: 10.1371/journal.pone.0019390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Wanglong Q, Gloria S. Development of orthotopic pancreatic tumor mouse models. Methods Mol Biol. 2013;980(14):215–223. doi: 10.1007/978-1-62703-287-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bever KM, Sugar EA, Bigelow E, et al. The prognostic value of stroma in pancreatic cancer in patients receiving adjuvant therapy. Hpb. 2015;17(4):292–298. doi: 10.1111/hpb.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsujikawa T, Kumar S, Borkar RN, et al. Quantitative multiplex immunohistochemistry reveals myeloidinflamed tumor-immune complexity associated with poor prognosis. Cell Rep. 2017;19(1):203–217. doi: 10.1016/j.celrep.2017.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neufeld G, Mumblat Y, Smolkin T, et al. The role of the semaphorins in cancer. Cell Adhes Migr. 2016;10(6):652–674. doi: 10.1080/19336918.2016.1197478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binmadi NO, Yang YH, Zhou H, et al. Plexin-B1 and semaphorin 4D cooperate to promote perineural invasion in a RhoA/ROK-dependent manner. Am J Pathol. 2012;180(3):1232–1242. doi: 10.1016/j.ajpath.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Schmitd LB, Beesley LJ, Russo N, et al. Redefining perineural invasion : Integration of biology with clinical. Neoplasia. 2018;20(7):657–667. doi: 10.1016/j.neo.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamm MJ, Kirchmaier BC, Herzog W. Sema3d controls collective endothelial cell migration by distinct mechanisms via nrp1 and plxnD1. J Cell Biol. 2016;215(3):415–430. doi: 10.1083/jcb.201603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worzfeld T, Offermanns S. Semaphorins and plexins as therapeutic targets. Nat Rev Drug Discov. 2014;13(8):603–621. doi: 10.1038/nrd4337. [DOI] [PubMed] [Google Scholar]
- 36.Gay CM, Zygmunt T, Torres-Vázquez J. Diverse functions for the semaphorin receptor PlexinD1 in development and disease. Dev Biol. 2011;349(1):1–19. doi: 10.1016/j.ydbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeDoux MS, Xu L, Xiao J, Ferrell B, Menkes DL, Homayouni R. Murine central and peripheral nervous system transcriptomes: Comparative gene expression. Brain Res. 2006;1107(1):24–41. doi: 10.1016/j.brainres.2006.05.101. [DOI] [PubMed] [Google Scholar]
- 38.Gu C, Yoshida Y, Livet J, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science (80-). 2005;307(5707):265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 39.Demir IE, Ceyhan GO, Rauch U, et al. The microenvironment in chronic pancreatitis and pancreatic cancer induces neuronal plasticity. Neurogastroenterol Motil. 2010;22(480):e113. doi: 10.1111/j.1365-2982.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- 40.Zurborg S, Piszczek A, Martínez C, et al. Generation and characterization of an Advillin-Cre driver mouse line. Mol Pain. 2011;7(1):66. doi: 10.1186/1744-8069-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li S, Sun Y, Gao D. Role of the nervous system in cancer metastasis. Oncol Lett. 2013;5(4):1101–1111. doi: 10.3892/ol.2013.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B, Lu KY. Neural invasion in pancreatic carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1(3):469–476. [PubMed] [Google Scholar]
- 43.Bakst RL, Wong RJ. Mechanisms of Perineural Invasion. J Neurol Surgery, Part B Skull Base. 2016;77(2):96–106. doi: 10.1055/s-0036-1571835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hishinuma S, Ogata Y, Tomikawa M, Ozawa I. Stomach-preserving distal pancreatectomy with combined resection of the celiac artery: Radical procedure for locally advanced cancer of the pancreatic body. J Gastrointest Surg. 2007;11(6):743–749. doi: 10.1007/s11605-007-0143-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


