SUMMARY
Despite the relevance of Argonaute proteins in RNA silencing, little is known about the structural steps of small-RNA loading to form RNA-induced silencing complexes (RISCs). We report the 1.9 Å crystal structure of human Argonaute4 with guide RNA. Comparison with the previously determined apo structure of Neurospora crassa QDE2 revealed that the PIWl domain has two subdomains. Binding of guide RNA fastens the subdomains, thereby rearranging the active-site residues and increasing the affinity for TNRC6 proteins. We also identified two water pockets beneath the nucleic acid-binding channel that appeared to stabilize the mature RISC. Indeed, mutating the water-pocket residues of Argonaute2 and Argonaute4 compromised RISC assembly. Simulations predict that internal water molecules are exchangeable with the bulk solvent, but always occupy specific positions at the domain interfaces. These results suggest that after guide RNA-driven conformational changes, water-mediated hydrogen-bonding networks tie together the converged domains to complete the functional RISC structure.
Keywords: Argonaute, RNAi, TNRC6, protein folding, water molecules
In Brief
Park et al. present the crystal structure of human Argonaute4 bound to guide RNA, revealing water molecules that form two clusters and that tie together its converged domains to stabilize the bilobal RISC scaffold. This water reservoir formation essential for duplex loading is common among Argonaute proteins but not PIWIs.
Graphical Abstract

INTRODUCTION
Humans have four Argonaute paralogs (AG01-AG04). They load microRNAs (miRNAs) to form RNA-induced silencing complexes (RISCs) that target complementary mRNAs for gene silencing (Bartel, 2018; Kobayashi and Tomari, 2016). RNA sequencing analyses revealed a large overlap of small RNAs between different AGOs, with a limited population of small RNAs unique to each AGO (Azuma-Mukai et al., 2008; Beitzinger et al., 2007; Dueck et al., 2012; Hafner et al., 2010), suggesting the redundancy and specificity of their target mRNAs. To date, characteristic features of each AGO have been reported. For example, AGOI interacts with RNA polymerase II bound to the active promoters (Huang et al., 2013) and controls alternative splicing through transcriptional enhancers (Allo et al., 2014). Only AG02 cleaves precursor miR-451 and generates the mature mi RNA important for erythropoiesis (Cheloufi et al., 2010). AG03 shares the same catalytic tetrad with AG02, but activation for RNA cleavage requires specific guides (Park et al., 2017b). AG04 regulates entry into meiosis and influences silencing of sex chromosomes in male mouse germlines (Modzelewski et al., 2012), and is exploited for the replication of hepatitis delta virus (Haussecker et al., 2008). To elucidate the molecular bases for the common and unique roles of each AGO, a comprehensive structural comparison is indispensable. The previously determined RISC structures of AGOI, AG02, and AG03 showed their unique local structures (Elkayam et al., 2012; Faehnle et al., 2013; Nakanishi et al., 2013; Park et al., 2017b; Schirle and MacRae, 2012). In contrast, no structural information is currently available for AG04, although a model structure was proposed (Hauptmann et al., 2014).
Since the discovery of RNA interference (Fire et al., 1998), tremendous advancements have identified the mi RNA biogenesis pathways, along with its key players. The genes of miRNAs are transcribed and processed by a complex of RNase III enzyme Drosha and DGCR8 in the nucleus (Lee et al., 2003) and by Dicer in the cytoplasm (Bernstein et al., 2001; Zhang et al., 2002). Lastly, miRNA duplexes are loaded into AGOs (Gregory et al., 2005; MacRae et al., 2008). This loading step requires chaperone machinery, showing drastic conformational changes of AGOs coupled with ATP hydrolysis (Iwasaki et al., 2010; Iwasaki et al., 2015a). In contrast, the subsequent passenger-strand ejection is thermodynamically favored and managed by AGO alone (Kawamata et al., 2009). Thus, the loading step is known to involve drastic AGO conformational changes, but details of AGO’s structural transitions during RISC assembly are still poorly understood.
Previous structural studies have dissected the bilobal RISC scaffold (Nakanishi, 2016). The C-terminal lobe consists of MID and PIWI domains while the N-terminal lobe is composed of N, L1, PAZ, and L2 domains (conventionally L1 and L2 have been named “linkers” but we label them as “domains” here) (Figure 1A). The N-terminal lobe is essential for the catalytic C-terminal lobe to modulate the slicing activity when there is a mismatch between the guide and target strands (Dayeh et al., 2018). There are two regions within the PIWI domain, labeled cluster 1 (CL1) and cluster 2 (CL2), which are known to impact the catalytic activity of AGOs (Hauptmann et al., 2013). Both lobes, which form globular structures with mainly α-helices and β-strands, are connected by a hinge portion where loops and domain linkers shape the intervening nucleic acid-binding channel. Although one of these loops can be phosphorylated at Ser798 (Rudel et al., 2011), this residue is not accessible in the bilobal RISC structure (Nakanishi, 2016), suggesting that the loop must be solvent accessible before AGO incorporates a guide RNA.
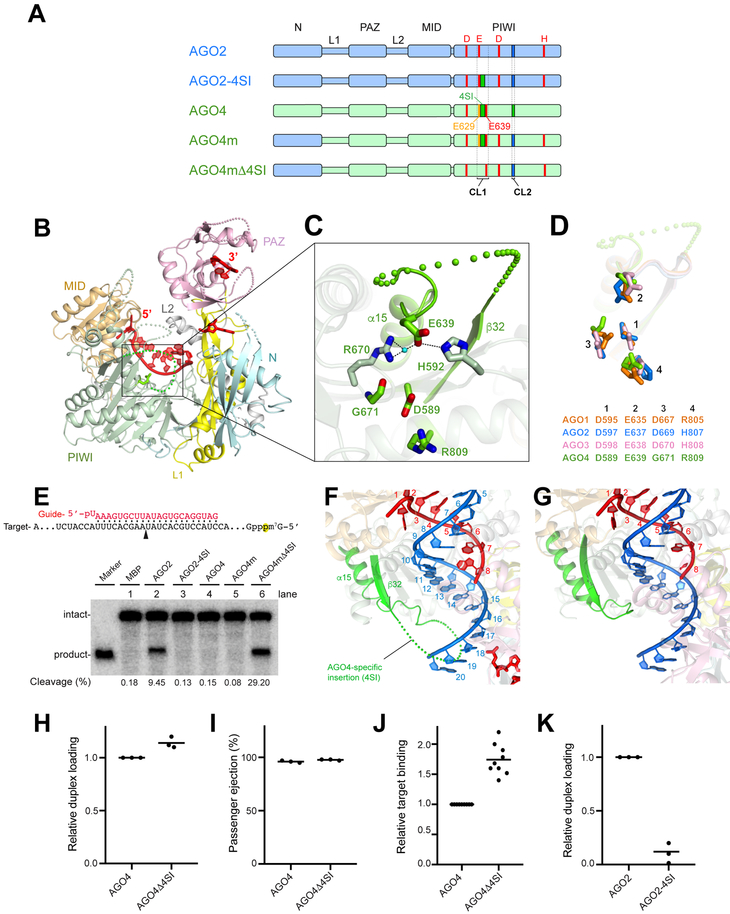
Figure 1. Structure of human AG04-RISC.
(A) Domain architectures of AG02 (light blue), AG04 (light green) and their chimeras. The catalytic residues are shown in red. 4SI within CL1 is colored green. AG02- and AG04-specific CL2s are highlighted in blue and green, respectively. The previously proposed glutamate finger of AG04, E629, is colored yellow.
(B) Overall structure of guide-bound AG04 with the N (cyan), L1 (yellow), PAZ (pink), L2 (gray), MID (wheat), and PIWI (green) domains. The bound guide RNA is colored red.
(C) A blow-up of the pseudo-catalytic tetrad of AG04.
(D) Catalytic and pseudo-catalytic tetrads of four human AGOs.
(E) Target cleavage assay with AG02, AG04 and their chimeras are shown in (A). See also Figure S1C.
(F-G) A docked target or passenger strand (blue) on the current AG04-RISC (F) and the previously determined AG02-RISC (PDB ID: 40LA) (G). See also Figure S1D.
(H-l) Duplex loading (H) and passenger ejection (I) of AG04 and AG04Δ4SI. See also Figure S1E.
(J) Target binding to AG04 and AG04Δ4SI. See also Figure S1F.
(K) Duplex loading of AG02 and AG02–4SI. See also Figure S1G.
Black bars on panels H to K represent averages with independent replicates shown as dots.
ApohumanAG02is known to be proteolyzed into several fragments but resistant after loading a guide RNA (Elkayam et al., 2012). A recent single-molecule Forster resonance energy transfer (FRET) study revealed that apo fly Ago2 takes many different conformations, but after loading guide RNA, the structure is restricted to a unique conformation (Tsuboyama et al., 2018). These results suggest that a bound guide RNA stitches all of the AGO domains together, likely stabilizing the bilobal structure. Meanwhile, recent studies revealed that extensive pairing between guide RNA and non-canonical target RNAs strips the guide from AGO (De et al., 2013; Park et al., 2017a). Thus, RISCs work as stable effector complexes and retain control of guide RNA release. The molecular mechanism behind RISC disassembly remains elusive.
Here, we report the 1.9 Å crystal structure of human AG04 in complex with guide RNA. The high-resolution structure enabled us to locate pockets with many water molecules trapped in the protein interior. These water pockets are also found in high-resolution structures of other AGOs. Our combined approach of molecular dynamics (MD) simulation and functional analyses suggests that internal water molecules in these pockets confer structural integrity to all AGOs, and presumably facilitate reversible conformational changes, which are essential for RISC function.
RESULTS
Structure of AG04 reveals its true glutamate finger
Among the four human AGO paralogues, AG02 and AG03 are the only catalytically active enzymes (Liu et al., 2004; Meister et al., 2004; Park et al., 2017b). Both RISC structures share the same conformation in which their second catalytic residue, known as a “glutamate finger,” is arranged to complete the catalytic DEDH tetrad (Elkayam et al., 2012; Park et al., 2017b; Schirle and MacRae, 2012). Despite its slicing deficiency, the RISC structure of AGOI shows the same conformation with its pseudo-catalytic DEDR tetrad (Faehnle et al., 2013; Nakanishi et al., 2013). Due to a lack of structural information, it remains unclear whether the same is true for AG04, another slicing-deficient paralog. A model structure of AG04, based on the crystal structure of AG02, suggested that E629 of AG04 served as the glutamate finger (Hauptmann et al., 2014). Indeed, AG04 was successfully converted into a catalytic enzyme (AG04mΔ4SI’) when part of CL1 within the PIWI domain, S631-V640, was deleted while the N domain, the third and fourth catalytic residues, and CL2 were swapped with their counterparts in AG02 (Figure S1A bottom left). Accordingly, E629 and the adjacent S631-V640 region were proposed to be the glutamate finger and the AG04-specific insertion (4ST’), respectively (Hauptmann et al., 2014).
In this context, we determined the 1.9 Å crystal structure of human AG04 in complex with guide RNA that was co-purified from insect cells (Figures 1B, S1B, and Table 1). This RISC structure shows that AG04 forms a pseudo-catalytic DEGR tetrad with D589, E639, G671, and R809 (Figure 1C). Structural alignment revealed that E639 occupied the position of the corresponding glutamate finger in other human AGOs (Figure 1D) (Elkayam et al., 2012; Faehnle et al., 2013; Nakanishi et al., 2013; Park et al., 2017b; Schirle and MacRae, 2012). This observation was not consistent with the model structure, in which E629 was assigned as the glutamate finger (Hauptmann et al., 2014). To investigate the role of E639, we converted AG04 into an active slicer. First, we swapped the N domain, the third and fourth catalytic residues, and the CL2 of AG04 with the counterparts of AG02 as previously reported (Figures 1A and S1A bottom right) (Hauptmann et al., 2014). The resultant chimera (FLAG-AG04m) purified from HEK293T cells did not show slicing activity (Figure 1E lane 5). However, further removal of E629-Q638 (AG04-specific insertion, 4SI) conferred significant slicing activity (AG04mΔ4SI) (Figures 1A and 1E lane 6). Thus, our structural and functional studies demonstrate that E639 serves as the glutamate finger of AG04, while the region E629-Q638 is the actual 4SI.
Table 1.
Data collection and refinement 660 statistics
| AGO4-RISC (PDB ID: 600N) |
|
|---|---|
| Data collection | |
| Space group | C2 |
| Cell dimensions | |
| a, b, c (Å) | 213.98, 68.01, 83.27 |
| α, β, γ (°) | 90.0, 102.7, 90.0 |
| Resolution (Å) | 72.26–1.90 |
| Rpim | 0.047 (0.394) |
| I / σI | 10.3 (2.1) |
| Completeness (%) | 99.1 (98.4) |
| Redundancy | 4.1 (4.2) |
| Refinement | |
| Resolution (Å) | 49.08–1.90 |
| No. reflections | 91128 |
| Rwork / Rfree | 0.171/ 0.202 |
| No. atoms | |
| Protein | 6314 |
| RNA | 243 |
| Water | 464 |
| B-factors | |
| Protein | 47.05 |
| RNA | 69.30 |
| Water | 44.23 |
| R.M.S. deviations | |
| Bond lengths (Å) | 0.008 |
| Bond angles (°) | 1.117 |
| Ramachandran plot | |
| Favored (%) | 96.88 |
| Allowed (%) | 2.73 |
| Disallowed (%) | 0.39 |
The AG04-specific insertion may modulate target RNA recognition
The current structure showed clear, continuous electron density maps of the bound guide RNA at nucleotide positions 1–8 (g1–g8) and the 3’ end 1–2 nt, whereas the middle portion was disordered except for a single nucleotide (Figure S1B). This observation indicates that AG04 recognizes guide RNA similarly to other human AGOs (Elkayam et al., 2012; Faehnle et al., 2013; Nakanishi et al., 2013; Park et al., 2017b; Schirle and MacRae, 2012), except that a 19 residue loop between p32 and a15, including 4SI, protruded towards the nucleic acid-binding channel (Figures 1B–C). When an ideal A-form RNA, mimicking a guide-passenger or guide-target duplex, was manually docked on the g2–g7 of AG04-bound guide RNA, 4SI seemed to interfere with the interaction between the two strands (Figure 1F). In contrast, the counterpart in AG02 has a kink-turn (~9 residues) that is too short to contact the docked strand (Figure 1G). These observations prompted us to hypothesize that 4SI affects either RISC assembly, guide-dependent target recognition, or both.
To test this idea, we removed 4SI from AG04 (AG04Δ4SI) and used this mutant in a RISC maturation assay (see STAR Methods) (Iwasaki and Tomari, 2018). AG04Δ4SI showed comparable abilities of duplex loading and passenger ejection (Figure 1H–I). On the other hand, our filter binding assay indicated that AG04Δ4SI bound to target RNA more efficiently than the wild type (WT; Figure 1J). Therefore, the 4SI within AG04 is not necessary for RISC assembly, but it modulates the guide-dependent target recognition. Reciprocally, when 4SI was implanted at the corresponding position of FLAG-AG02 (AG02–4SI) (Figure 1 A), the mutant completely lost slicing activity (Figure 1E lane 3). To examine whether the cleavage deficiency was due to the impact of 4SI on duplex loading or passenger ejection, we performed the RISC maturation assay. AG02–4SI showed very low duplex loading (Figure 1K), indicating that the implant of 4SI alone is devastating to RISC maturation of AG02. This could explain why the mutant showed no RNA cleavage (Figure 1E lane 3). Altogether, these results suggest that 4SI modulates target RNA recognition by AG04.
Previous structural studies revealed that AGOI and AG03 possess unique local structures, CL2 (also known as cS7) and AG03-specific insertion (3SI), respectively (Faehnle et al., 2013; Nakanishi et al., 2013; Park et al., 2017b). Like 4SI, CL2 and 3SI protrude toward the nucleic acid-binding channel, which affects the interaction between the middle portions of a guide RNA and its target RNAs. The differences in those AGO-specific motifs suggest that the four human AGOs recognize the middle part of a guide RNA in different manners. Therefore, all four paralogs loaded with the same guide RNA may have different specificities and affinities for target RNAs, which could explain the previously reported specialized roles of each AGO (Haussecker et al., 2008; Hu et al., 2012; Modzelewski et al., 2012; Winter and Diederichs, 2013). Intriguingly, the N domain that does not contain any of the catalytic residues needed to be swapped to generate a catalytic AG04mΔ4SI (Figure 1E). Since the N domain and the abovementioned AGO-specific motifs on the C-terminal lobe sandwich the middle part of the guide, their specific combinations may be necessary to confer catalytic activity.
Requirements of TNRC6 proteins for binding to AGOs
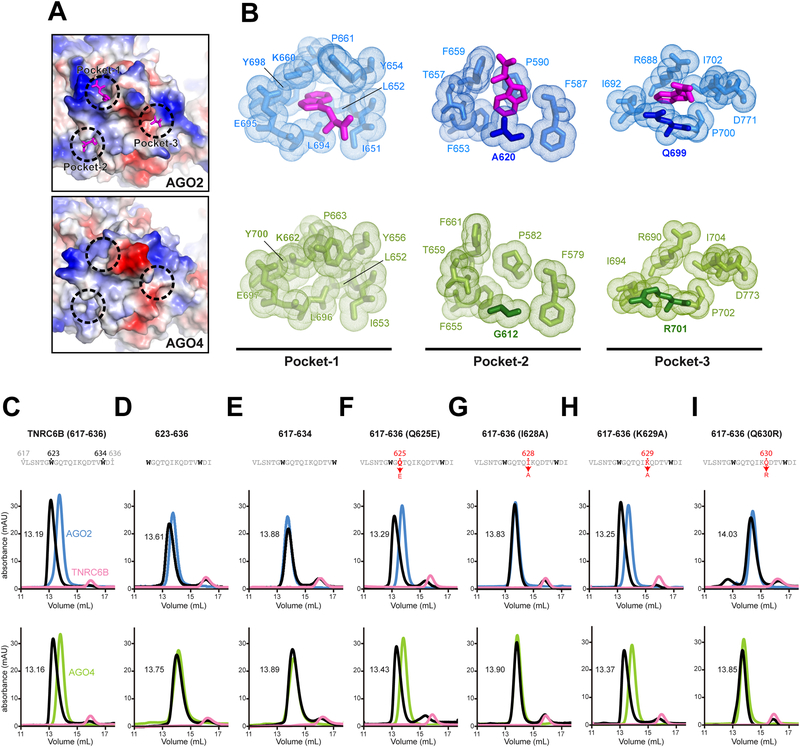
Human AGOs bind to trinucleotide repeat containing 6 proteins, TNRC6A, −6B and −6C, to form miRNA-mediated RISCs that degrade target mRNAs by recruiting a CCR4-NOT complex (Baillat and Shiekhattar, 2009; Huntzinger and Izaurralde, 2011; Jakymiw et al., 2005; Jonas and Izaurralde, 2015; Liu et al., 2005a; Liu et al., 2005b). TNRC6 proteins interact with AGOs using their N-terminal region, which includes more than 30 tryptophan residues. The regions called motif I, motif II, and AGO-hook are especially known as strong AGO-binding sites (Hauptmann et al., 2015; Lazzaretti et al., 2009; Pfaff et al., 2013). A recent structural study identified three tryptophan-binding pockets on the surface of the AG02 PIWI domain (Figure 2A) (Sheu-Gruttadauria and MacRae, 2018). Pockets 1 and 2 are used to interact with TNRC6B motif II, while Pockets 1 and 3 are required for binding to TNRC6B motif I (Figure S2A) (Sheu-Gruttadauria and MacRae, 2018). Another structural study showed that, through its Pockets 1 and 2, AGOI binds to the AGO hook of TNRC6A (Elkayam et al., 2017). Our current structure showed that AG04 forms the corresponding pockets composed of the same set of residues, except for G612 in Pocket-2 and R701 in Pocket-3, which are substituted for alanine and glutamine, respectively, in the other paralogs (Figures 2B and S3).
Figure 2. Requirements of TNRC6 for AGO binding.
(A) Electrostatic surfaces of three Trp-binding pockets on the PIWI domain of AG02 (top, PDB ID: 6CBD) and AG04 (bottom).
(B) Residues forming the Trp-binding pockets of AG02 in complex with tryptophans (pink) (top) and apo-AG04 (bottom).
(C-l) Gel filtration analyses of AG02 (blue), AG04 (green), TNRC6B fragment (pink), and a mixture of the TNRC6B fragment and either AG02 or AG04 (black).
To test if AG04 binds to specific regions within TNRC6 proteins like AG02, we made two TNRC6B fragments, 599–683 and 869–916, including the motifs I and II, respectively (Figure S2A) (Pfaff et al., 2013 Sheu-Gruttadauria and MacRae, 2018). We also designed three other fragments, 507–598, 684–770, and 771–860, none of which includes the known strong AGO-binding sites. Due to low solubility, these fragments were expressed as SUMO-tag fused proteins and purified from E. coli cells. After preincubation with either of the TNRC6B fragments, AG02 or AG04 was analyzed for binding by size-exclusion chromatography. Both AGOs interacted only with the two motif-containing TNRC6B fragments, 599–683 and 869–916 (Figure S2B). These results indicated that, despite the differences in Pockets-2 and −3, AG04 distinguishes motifs I and II from other tryptophan repeat regions.
Previous studies reported that AGOI and AG02 recognize TNRC6 proteins by binding to two tryptophan residues within AGO-binding sites (Elkayam et al., 2017; Pfaff et al., 2013). The two tryptophans need to be separated by 10 amino acids or longer so that they occupy their binding pockets at the same time (Pfaff et al., 2013). However, in the N-terminal domain of TNRC6 proteins, many segments meet the requirements for a strong AGO-binding site. Nevertheless, our current study, as well as others, revealed that human AGOs recognize only specific tryptophan repeat motifs (Figure S2B) (Elkayam et al., 2017; Pfaff et al., 2013; Sheu-Gruttadauria and MacRae, 2018). These results indicate a possibility that the motif I, motif II, and AGO hook must possess as-yet-unidentified determinants that make them different from other tryptophan repeat motifs. To validate this idea, we tested the influence of residues in the vicinity of two tryptophans, W623 and W634, on the AGO binding of the TNRC6B V617-I636 fragment, including its well-studied motif (Hauptmann et al., 2015; Pfaff et al., 2013). The V617-I636 fragment bound to AG04 as well as AG02 did (Figure 2C). Deletion of either region outside the two tryptophans, V617-G622 or D635-I636, dramatically affected the binding to AG02 or AG04 (Figures 2D–E). These results demonstrate the significance of the flanking regions outside a two tryptophan-including segment for binding to AGO. We also made single mutations to residues located between the two tryptophans. While both Q625E and K629A mutants bound to AG02 and AG04 comparably to the WT, the I628A mutant interacted with neither (Figures 2F–H). Given that the corresponding residue in TNRC6 protein homologs and paralogs (i.e., GW182 proteins) is isoleucine or valine, a β-branched, non-polar residue at position 628 seems to be essential for the motif I to interact with AGOs. When a strictly conserved Q630 was replaced with arginine, the mutant completely lost the ability to bind to AG02 or AG04 (Figure 21). These results indicate that the flanking regions outside of and the specific residues between two tryptophan residues are also determinants of AGO-binding sites.
Two PIWI subdomains fasten together upon RNA binding
A previous study determined the RNA-free structure of the C-terminal lobe of the Neurospora crassa Argonaute homolog, QDE2 (NcQDE2 C-lobe), which is the only structure reflecting the apo-form of a eukaryotic Argonaute protein thus far (Boland et al., 2011). To investigate any structural differences, we superposed the RISC structure of AG04 and the apo structure of NcQDE2 C-lobe on their RNase H motifs within the PIWI domain (hereafter referred to as PIWI-catalytic subdomain) (Figure 3A). Notably, the rest of their PIWI domains encompassing α17 to the C-terminus (hereafter referred to as PIWI-helical subdomain) are arranged differently, suggesting that the interaction between two PIWI subdomains differs in the absence of a guide strand. On the other hand, the MID domain and the PIWI-helical subdomain in the AG04-RISC and apo-NcQDE2 interact with each other quite similarly, involving similar conserved residues as well as their corresponding water molecules (Figure S4) (Boland et al., 2011). In apo-NcQDE2, a sulfate ion occupying the interlace between the MID domain and PIWI-helical subdomain seems to mimic a 5’ monophosphate of guide RNA, reinforcing their interaction (this will be further addressed later).
Figure 3. Rearrangement of PIWI subdomains during RISC assembly.
(A) Different arrangements of PIWI-helical subdomain and MID domain. (Top) The crystal structures of the guide-bound AG04 and the apo-form NcQDE2 (PDB ID: 2YHA) are superimposed on their PIWI-catalytic subdomains (gray). The pseudo-catalytic residue of AG04, R809 (green), and the catalytic residue of NcQDE2, D890 (pink), are depicted as a stick model on α18 (see also Figure 1D). The transition from the apo-NcQDE2 to that of the guide-bound AG04 is shown by red arrows. For clarity, loops L31 and L32 and the N-PAZ lobe of AG04 are not shown. (Bottom) Schematic of the MID and PIWI domains.
(B) Interactions between the two PIWI subdomains of NcQDE2 (top), AG04 (middle), and AG02 (bottom). Residues involved in the hydrophobic interaction are shown as stick models with their van der Waals radii (dots). Colors as in (A).
(C) Seed region of guide RNA tying together two PIWI subdomains of AG04. For clarity, the N-PAZ lobe is not shown. The PIWI-catalytic subdomain is shown as a surface model. The MID domain and the PIWI-helical subdomain are depicted as ribbon models. The guide RNA is colored in red. Colors as in (A).
(D) Target cleavage assay of AG02 and mutants. The amount of AGOs used for target cleavage assays (right) was adjusted based on the western blot with anti-FLAG antibody (left).
(E) Duplex loading of AG02 and mutants. See also Figure S4E.
(F) Co-immunoprecipitation of TNRC6A with AG02 and mutants. The amount of AGOs used to detect co-immunopurified TNRC6A with anti-TNRC6A antibody (right) was adjusted based on the western blot with anti-FLAG antibody (left).
Experiments in D and F were performed at least three times. A representative gel is shown.
Our structural inspection of the guide-bound AG04 and apo-NcQDE2 C-lobe revealed a difference in the interface between their two PIWI subdomains. In the apo-NcQDE2 C-lobe, the helical subdomain is tilted away from the β-sheet of the PIWI catalytic subdomain, and therefore there is a gap between the subdomains (Figure 3B top). In contrast, the counterparts of the guide-bound AG04 mesh in a shape-complementary manner (Figure 3B middle). Note that the residues involved in the van der Waals interactions are conserved throughout eukaryotic AGOs (Figure S3). The same interactions are seen in the RISC structure of AG02 (Figure 3B bottom) (Schirle and MacRae, 2012) as well as AGOI and AG03 (Elkayam et al., 2012; Nakanishi et al., 2013; Park et al., 2017b). This structural difference between the presence and absence of guide RNA suggests that the bound guide RNA pushes the PIWI-helical subdomain towards the PIWI-catalytic subdomain during RISC assembly (Figure 3C). The current RISC structure shows that the seed region of guide RNA is mainly recognized by loop L4 of the PIWI-helical subdomain and loops L31 and L32 of the PIWI-catalytic subdomain, suggesting that the bound guide RNA ties the two PIWI subdomains together to form the composite PIWI domain.
Next, we tested the influence of this rearrangement of the two PIWI subdomains on the RISC assembly by inhibiting their shape-complementary interaction. For this purpose, we exploited the slicer enzyme activity of AG02, whose RISC assembly is prerequisite for target cleavage. First, we expressed FLAG-AG02 (Y529E), a well-known mutant deficient in duplex loading (Iwasaki et al., 2015a; Phan et al., 2018; Rudel et al., 2011), in HEK293T cells and tested it for target cleavage. Like the catalytic mutant AG02 (E637A), the Y529E mutant failed to cleave target RNA (Figure 3D), demonstrating the utility of this assay to evaluate the impact of a mutation on the RISC assembly. Then, G595 and V615 of AG02 were mutated to isoleucine and alanine, respectively, to disrupt the shape-complementary interaction between the two PIWI subdomains. As a result, the double mutant completely lost its slicing activity (Figure 3D). To examine the impact of the mutations on duplex loading and passenger ejection, we performed the RISC maturation assay. AG 02 (E637A) loaded the siRNA duplex and ejected the passenger strand as efficiently as did the WT (Figure 3E). In contrast, AG02 (G595/V615A) failed to load even a miR-20a duplex, like AG02 (Y529E) (Figure 3E). This result suggests that the interaction between two PIWI subdomains is indispensable for loading of the small RNA duplex. These results support a molecular mechanism in which the bound guide RNA fastens the hydrophobic interface of the two PIWI subdomains.
It is well known that the glutamate finger is poised over the other three catalytic residues upon binding of guide RNA to complete the catalytic tetrad (Nakanishi et al., 2012). Catalytically active AG02 and AG03, as well as naturally slicer-deficient AGOI and AG04, have their fourth catalytic (or pseudo-catalytic) residue on α18, which is part of the PIWI-helical subdomain (Figure 3A) (Faehnle et al., 2013; Hauptmann et al., 2014; Liu et al., 2004; Meister et al., 2004; Nakanishi et al., 2013; Park et al., 2017b). Therefore, the conformational changes during RISC assembly rearrange two out of the four catalytic (or pseudo-catalytic) residues.
Although loading of small RNAs increases the affinity of AGOs for TNRC6 proteins (Boland et al., 2011; Elkayam et al., 2017), little is known about the molecular mechanisms behind this. To investigate how formation of the composite PIWI domain affects binding to TNRC6 proteins, we expressed either of FLAG-AG02, the E637A single mutant, or the G595I/V615A double mutant in HEK293T cells. Then, we tested if they are co-immunopurified with TNRC6A using an anti-FLAG antibody. The catalytic mutant, E637A, bound to TNRC6A comparably, whereas the double mutant drastically reduced its binding affinity (Figure 3F). This result suggests that the interaction between the two PIWI subdomains is prerequisite for the binding of TNRC6A.
Water reservoirs inside AGO proteins
Our 1.9 Å resolution crystal structure of AG04 allowed us to identify two clusters of 13 and 4 water molecules, named LAKE1 and LAKE2 (Loop-Associated Key Estuary), respectively, located beneath the nucleic acid-binding channel (Figure 4A and Video 1). The water molecules that we located inside AG04 are different than those previously found at the catalytic site (Schirle et al., 2014) and the target nucleotide-1 pocket (Schirle et al., 2015) (Figure S5A). At the LAKEs, all residues whose side chain participates in the hydrogen-bonding network with these water molecules are conserved throughout the four human AGOs, except that AG02 has N623 instead of Ser (S615 in AG04) (Figure S3). This finding suggests that the other human AGOs also accommodate water molecules in the same manner. Indeed, we observed water molecules at the corresponding positions in other available AGO-RISC structures, albeit the visibility of these water molecules varied depending on the resolution (Figure 4B) (Elkayam et al., 2012; Faehnle et al., 2013; Nakanishi et al., 2013; Schirle and MacRae, 2012; Schirle et al., 2015; Schirle et al., 2014). The internal water clusters are seen also in the AG02-RISC complex structure with a target RNA (PDB ID: 4Z4D) (Schirle et al., 2014), suggesting that LAKEs exist even after a guide-target duplex is propagated along the nucleic acid-binding channel.
Figure 4. Two water clusters inside AG04-RISC.
(A) Cross sections of AG04-RISC. Water molecules (cyan) are shown as spheres. The guide RNA (red) is displayed as a stick model.
(B) Conservation of internal water molecules among human AGOs. Water molecules equivalent to W9 and W12 in AG04 have slightly different positions, W9’ and W12’, in AG02, due to N623.
(C) Water molecules in LAKE1 (right) and LAKE2 (left). The simulated annealing Fo-Fc map (blue mesh) is contoured at 3.0 σ around the internal water molecules. See also Figures S5C–D.
The B-factors of water molecules in LAKEs (23 – 42 Å2) are lower than the averaged B-factors of AG04 protein atoms (Table 1), a hallmark of buried water molecules (Koellner et al., 2000). We investigated the crystallographic internal water molecules using ProACT2, a program that classifies water molecules based on their solvent accessibility (Williams et al., 1994). All water molecules in LAKE1 and two in LAKE2 were categorized as buried water molecules that are completely sequestered from solvent (Figure S5B). Generally speaking, for soluble globular proteins, the hydrophobic effect is a major driving force of protein folding and excludes water molecules from the protein interior. Although sometimes a few water molecules are incorporated inside a folded structure to neutralize charged areas, they are isolated from each other (Carugo, 2016). In contrast, water molecules in the LAKEs form clusters. Therefore, it is unlikely that the observed 13 and 4 water molecules are trapped inside during the primary protein folding of AG04.
LAKEs are not pre-organized in apo AGO
LAKE1 is located at the interfaces of three domains (L2, MID, and PIWI) where α14, α17, and four loops come together (Figure 4C right). Two of the four loops are the domain linkers between L2 and MID domains (linker LM) and between MID and PIWI domains (linker MP). A previous study reported that apo-AG02 was cleaved within these two domain linkers by thermolysin, but became resistant after loading guide RNA (Elkayam et al., 2012). The third loop is loop L4, and is flexible in the absence of guide RNA, as seen in the apo structure of NcQDE2 (Figure S4B) (Boland et al., 2011). Loop L4 uses one side to enclose 13 water molecules and the other side to interact with the guide nucleotide positions 2–5 (g2–g5) (Figure 4C right). The fourth loop connecting β31 and β32 includes conserved serine (S) and aspartate (D) residues (hereafter, this loop is referred to as loop SD) (Figure 4C right).
LAKE2 is formed by the helix-loop-helix motif (α7 and α8) of the L2 domain and loops L31 and L32 of the PIWI-catalytic subdomain (Figure 4C left). Both loops are disordered in the absence of guide RNA (Boland et al., 2011), but fold into this unique shape once a guide RNA binds. Loops L31 and L32 recognize the phosphate backbone of g6–g8. Furthermore, loop L32 interacts with loop L4 and linker LM, directly or indirectly, via non-LAKE water molecules (Figure 4C left). Thus, loops L31, L32, and L4 pave the composite nucleic acid-binding channel, suggesting that the LAKE formation is part of the RISC assembly process.
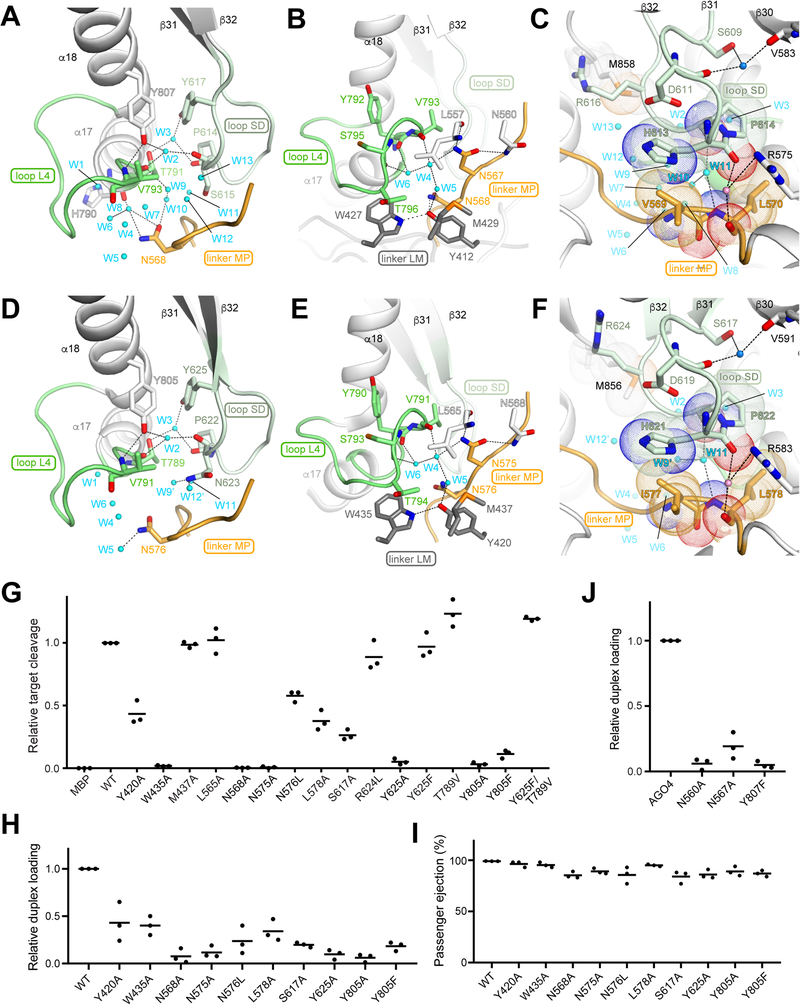
Domain linkers and loops build LAKE1
As we have shown earlier (Figure 3B middle), Y617 on loop SD and Y807 on a18 participate in van der Waals interactions between the two PIWI subdomains. These tyrosines also hydrogen bond with loop L4, directly or indirectly, via water molecules in LAKE1 (Figure 5A). As a result, the two tyrosine residues anchor loop SD and α18 to loop L4. To examine the influence of the tyrosine-centered interactions on RISC assembly, we replaced either of the corresponding tyrosines of AG02 with alanine or phenylalanine, and the mutants were tested for target cleavage. The Y625A mutation (Y617 of AG04) almost completely lost slicer activity whereas the Y625F mutant retained comparable activity (Figure 5G), indicating that the aromatic ring of Y625, not the hydroxyl group, is essential for RISC assembly. Supporting this, the hydroxyl group of Y625 interacts with T789 (T791 in AG04) via a water molecule, W3 (Figures 5A and 5D), but the T789V mutation did not affect slicer activity (Figure 5G). Even the double mutant of Y625F/T789V showed comparable activity to the WT (Figure 5G). In contrast, Y805A and Y805F (Y807 of AG04) drastically decreased the slicer activity (Figure 5G), indicating that both the hydroxyl group and aromatic ring of Y805 are critical for RISC assembly.
Figure 5. Water-mediated hydrogen-bonding network of LAKE1.
(A-F) Different views of water-mediated loop-loop interactions in LAKE1 involving the conserved residues of AG04 (A-C) and AG02 (D-F). Colors as in Figure 4C. Water molecules (cyan) and hydrogen bonds are depicted as spheres and dotted lines, respectively.
(G) Target cleavage assays with AG02 and mutants. See also Figures S6A–C.
(H-l) Duplex loading (H) and passenger ejection (I) of AG02 mutants that drastically reduced their target cleavage in (G). See also Figure S6F.
(J) Duplex loading of AG04 mutants. See also Figure S6G.
Black bars on panels G to J represent averages with independent replicates shown as dots.
In AG04-RISC, W427 on linker LM is stacked with T796 on loop L4 while Y412 on linker LM hydrogen-bonds with N568 on linker MP (Figure 5B). These interactions anchor both loop L4 and linker MP to linker LM. When the corresponding tryptophan (W435) of AG02 (Figure 5E) was mutated to alanine, the mutation drastically decreased slicer activity (Figure 5G). On the other hand, alanine mutations of Y420 (Y412 of AG04) and N576 (N568 of AG04) reduced slicer activity to about half of the WT (Figure 5G). These results suggest the significance of these residues for RISC assembly.
The current AG04 structure has shown that N567 on linker MP hydrogen-bonds with the side chain of N560 and the carbonyl oxygen of L557, both of which are located on a14 of the MID domain (Figures 5B and S6C). The same interactions are seen in AG02-RISC (PDB ID: 40LA) (Figure 5E), but not in the previously reported crystal structure of an isolated AG02 MID domain that has no interaction with the flanking linkers LM and MP (Figures S6D–E) (Frank et al., 2010). This difference suggests that linkers LM and MP are free to move until they form LAKE1 with loop L4 upon binding of guide RNA. To investigate the influence of the asparagine-centered interactions on RISC assembly, single-point mutants of N568 and N575 of AG02 (N560 and N567 of AG04, respectively) were tested for target cleavage. Both alanine mutations diminished slicer activity (Figure 5G).
Our structural observation indicates that the L2 and MID domains and two PIWI subdomains converge to form LAKE1 through the interactions of the two loops (L4 and SD), two linkers (LM and MP) and two helices (α14 and α18). Since LAKE1 is located far from the catalytic site, it seemed unlikely that the replacement of even a single residue forming LAKE1 directly affected the target cleavage. Rather, it would be plausible that the single mutations had a negative impact on duplex loading or passenger ejection, thereby lowering the target cleavage activity. To validate this idea, all AG02 mutants that poorly cleaved target RNAs in Figure 5G were tested for RISC assembly. The results showed that most of these mutants failed to load duplexes (Figure 5H). However, once loaded with a duplex, those mutants efficiently ejected the passenger strand to became the mature RISC (Figure 5I), suggesting that LAKE1 needs to be established prior to passenger ejection. Lastly, to see whether LAKE1 formation is also essential for AG04 to load duplexes, we made three single mutations N560A, N567A, and Y807F on AG04, which correspond to N568A, N575A, and Y805F on AG02, respectively, and tested them for RISC maturation. These AG04 mutants failed to load duplexes (Figure 5J). Altogether, these results demonstrate the significance of LAKE formation for the duplex-loading step.
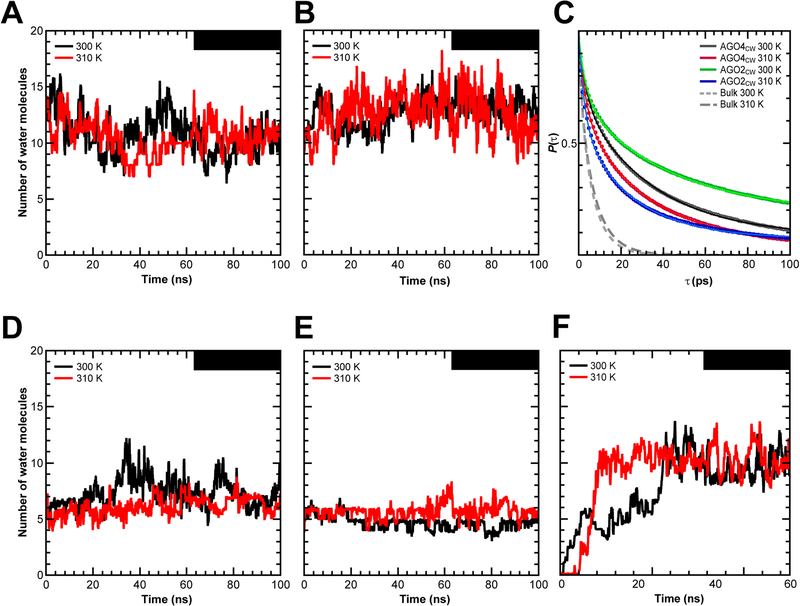
Confined LAKE water molecules are exchangeable
To investigate the dynamics of the LAKE water molecules, we performed all-atom MD simulations of AG04 and AG02 keeping all crystallographic water molecules in their initial positions. The simulations performed at 300 K and 310 K revealed that the occupancy of LAKE1 fluctuated, yet no dehydration was observed in the simulated time-scale (~100 nanoseconds) (Figures 6A–B). This result indicates that water molecules are confined inside the cavity, but are continuously exchanged with the bulk solvent. The exchange rate can be quantified by estimating the survival probability P(τ), i.e., the probability of a water molecule to remain inside the cavity after a certain amount of time. As shown in Figure 6C, the decay in P(τ) for water molecules in LAKE1 is slower than the bulk, and determination of a decay rate required a biexponential fit of the P(τ) curve with a fast and a slow decay constant (Table S1). This further suggests that water is highly confined inside LAKE1, and that water molecules in different regions of LAKE1 may experience different degrees of confinement given by geometry and/or favorable interactions. Interestingly, despite AG04 showing a greater number of water molecules in the crystallographic structure (13 versus 8~10 from AG02), this trend is reversed during the simulations (average occupancy of AG04 10 ± 2 versus AG02 13 ± 1; Table S1).
Figure 6. Dynamical behavior of simulated water molecules in LAKE1 and LAKE2.
(A-B, and D-E) Water occupancy vs. time in LAKE1 of AG04 (A) and AG02 (B) and in LAKE 2 of AG04 (D) and AG02 (E) simulated with crystallographic water molecules in their initial positions (AG04CW, AG02CW) at 300 K (black) and 310 K (red).
(C) Survival probability P(x) for water molecules inside LAKE1 of AG04CW and AG02CW simulated at 300 K and 310 K. Solid and dotted lines correspond to biexponential fits to the AG04 and AG02 data and to exponential fits to the bulk data, respectively (Table S1).
(F) Water occupancy vs. time in LAKE1 of AG04 simulated without crystallographic water molecules at the start of the simulation (AG04NCW) at 300 K (black) and 310 K (red).
Lines in panels A, B, and D-F correspond to 100 ps moving window averages.
Structures of both AG02 and AG04 have 4 crystallographic water molecules inside LAKE2 (Figure 4C left) and an average of ~6 water molecules during the simulations (Figure 6D–E). The analysis of LAKE2 dynamics for AG04 showed a marked increase in occupancy of the cavity at tsim ~30 ns. Visual inspection of the trajectory of AG04 at 300 K revealed that the interaction between loops L31 and L32 was transiently disrupted, thereby allowing more water molecules to enter LAKE2. Subsequently, the conformation was partially reverted while the water occupancy remained high until tsim ~60 ns.
These simulation predictions suggest that internal water molecules in LAKEs are confined, but move and are exchangeable with solvent.
LAKE water molecules maintain the composite RISC
Most of the conserved residues on the domain linkers and loops around LAKE1 directly interact with the internal water molecules (Figure S6C). This observation prompted us to raise the hypothesis that water molecules in LAKEs offer an adhesive property to maintain the domain-domain interactions within the mature RISC. Supporting this, single mutations of the corresponding conserved residues of AG02 severely affected the RISC assembly and thus slicing activity (Figures 5G–I). Meanwhile, using their nonpolar side chains, M429 and L557 form part of the interior wall of LAKE1 (Figure 5B), but mutants of their corresponding residues in AG02, M437A and L565A, retained a comparable slicing activity to WT (Figures 5E and 5G). These results indicate that internal water molecules are recognized by hydrogen bonds, consistent with our MD simulations in which water molecules transfer from one place to another within LAKE1, but always occupy specific positions, rather than randomly moving around (Video 2). To validate our hypothesis that RISCs proactively incorporate solvent water molecules into the cavity to stabilize the composite structure, we started MD simulations in an initial state where the crystallographic water molecules were removed from AG04 LAKE1. Immediately after simulations start, solvent water molecules filled the cavity (Figure 6F), which is strong evidence that the uptake of water molecules into AG04 is energetically preferable. These results suggest that the water-mediated hydrogen-bonding network stabilizes the composite RISC.
MD trajectories also showed two gates through which water molecules enter and exit the AG04 LAKE1 (Figures 5C and S6I). The first of these (Gate1), is composed of M610 and R616 on loop SD and H856 and M858 on the C-terminal loop. Our MD analysis indicated that R616 located at the edge of Gate1 seemed to control the rate of entry and exit of water molecules. To investigate the significance of this arginine residue, we mutated the corresponding R624 of AG02 to leucine (Figure 5F). The mutant retained a comparable activity (Figure 5G), suggesting that the flow rate of water molecules does not affect the function of RISC. The second gate (Gate2) is formed by V569 and L570 on linker MP and H613 and P614 on loop SD (Figures 5C and S6I). When L578 of AG02, corresponding to L570 of AG04, was mutated to alanine, the slicer activity was reduced to less than half of that of the WT (Figure 5G). Residue S609 forms tripartite hydrogen bonds, which uniquely kink loop SD, and thus shapes the two gates (Figure 5C). An AG02 alanine mutant of the corresponding residue, S617, largely decreased the slicer activity (Figures 5F–G). In our RISC maturation assay, both L578A and S617A mutations drastically affected duplex loading of AG02 (Figure 5H). These results suggest that a fully functional RISC needs to shape these two gates properly.
DISCUSSION
In this study, our combined approach revealed that the seed region of guide RNA ties together the two PIWI subdomains, forming the composite PIWI domain and two LAKEs. These data provide the following insights.
Update of RISC loading mechanism
A previously reported crystal structure of Thermus thermophilus AGO (PBD ID: 3D LB) (Wang et al., 2008) captured a state where the 10-nt guide strand was recognized at its 3’ end by the PAZ domain, but its length was too short to reach the 5’ monophosphate-binding site on the MID domain. As a result, the PIWI-helical subdomain was completely disordered, while the apo MID domain directly interacted with the PIWI-catalytic subdomain (Video 3). This conformation was not well understood, but now it can be explained by considering the existence of two PIWI subdomains that are free to move independently unless they are tied by the seed region of the guide strand. In support of this, the crystal structure of apo-NcQDE2 C-lobe showed a loose interaction between the two PIWI subdomains due to the absence of a guide RNA (Boland et al., 2011). Nevertheless, the PIWI-helical subdomain tightly interacts with the MID domain, as do the counterparts of AG04-RISC (Video 3). Given that, in the NcQDE2 C-lobe, a sulfate ion is recognized at the 5’ monophosphate-binding site between the MID domain and PIWI-helical subdomain, binding of the 5’ monophosphate of the guide strand may reinforce their interaction.
Based on our data and previous studies, we propose here a model of RISC loading (Video 4). Loops L4 and SD and linkers LM and MP would be solvent-exposed and flexible prior to the binding of guide RNA, which allows the MID domain and two PIWI subdomains to move independently. Then, the MID domain and PIWI-helical subdomain capture a 5’ monophosphate of the duplex and flip out the base of g1. The resultant ternary complex would last longer if the first nucleotide base is adenine or uracil, due to the high affinity of the MID domain for those bases (Frank et al., 2010). Subsequently, loop L4 and a18 of the PIWI-helical subdomain recognize the phosphate backbone of g2–g5 and load the duplex to the rest of AGO, such that loops L31 and L32 recognize the g6–g8. As a result, the two PIWI subdomains are tied together by the seed region (g2–g8). Before binding to a duplex, the MID domain and the PIWI subdomains solvent-expose their hydrophobic interfaces, providing good clients for the heat-shock proteins (HSPs) that are required for sufficient RISC loading (Iki et al., 2010; Iwasaki et al., 2010; Iwasaki et al., 2015a). Since binding of a duplex occludes the hydrophobic areas, HSPs would then be released and no longer involved in the subsequent passenger ejection step, which could explain a previous report that HSPs are essential only for the duplex-loading step (Iwasaki et al., 2010).
PIWI proteins have no LAKEs
Like AGOs, PIWI proteins (PIWIs), together with PIWI-interacting RNAs (piRNAs), form piRNA-induced silencing complexes (piRISCs) (Czech and Hannon, 2016; Ghildiyal and Zamore, 2009; Iwasaki et al., 2015b). To see whether piRISCs also possess internal water molecules, we investigated the previously determined crystal structure of piRNA-bound silkworm PIWI protein, Siwi (Matsumoto et al., 2016). Siwi replaces the residues corresponding to N568, T791, and V793 of AG04 with tryptophan (W655), leucine (L856), and phenylalanine (F858), respectively (Figures 7A–B). These bulky, nonpolar residues occupy the space where the internal water molecules are found in AG04. As a result, Siwi-piRISC has only two internal water molecules corresponding to W1 and W3 of AG04 LAKE1 (Figure 7B). The two buried water molecules are isolated from each other, like those trapped during the primary protein folding. In addition, Siwi substitutes non-polar residues for the polar ones that recognize the internal water molecules in AG04 LAKE2 (Figures 7C–D). Accordingly, Siwi-piRISC forms a tightly-packed hydrophobic core without the aid of internal water molecules. Notably, the above-mentioned hydrophobic residues are conserved throughout PIWIs (Figure S7), suggesting that other PIWIs also assemble into pi RISC without LAKEs. This discrepancy between AGOs and PIWIs may reflect the differences in their RISC assemblies. The nucleic acid-binding channel of eukaryotic AGOs is not wide enough to load a duplex, suggesting that drastic conformational changes are essential for duplex loading. In contrast, PIWIs incorporate single-stranded precursor piRNAs. Therefore, apo PIWIs may pre-organize the composite nucleic acid-binding channel and thereby may not require a large conformational change for piRISC assembly.
Figure 7. AGOs, but not PIWIs, possess LAKEs.
(A-B) Interactions between loop L4 and linker MP in AG04 (A) and Siwi (B) (PDB ID: 5GUH). The van der Waals surfaces are shown as dots.
(C-D) Interactions of loops L31 and L32 in AG04 (C) and Siwi (D). The AGO-specific insert (yellow) makes space for four water molecules (LAKE2).
(E) Model of the water-mediated RISC assembly and disassembly (see the main text for details). The MD simulation predicts that mature RISC exchanges water molecules in LAKEs with the solvent (blue arrows). The water-mediated RISC disassembly is hypothetical based on our and previous studies.
The possession of LAKEs may also reflect the difference in the turnover mechanisms between AGOs and PIWIs. It is well known that AGOs are expressed ubiquitously in different cells (Farazi et al., 2008; Meister et al., 2004; Sasaki et al., 2003) and that the half life of RISCs is in the order of days to weeks (De et al., 2013). Therefore, AGOs have lots of opportunities to exchange guide RNAs. Supporting this idea, the MacRae group reported that highly complementary target RNAs promote the release of guide RNAs from human AG02 (De et al., 2013). A subsequent detailed study by the Shin group revealed that loaded guide RNAs can be released by hybridization with non-canonical target RNAs that are fully complementary to the guide except for its 5’ nucleotides (Park et al., 2017a). These studies demonstrate that AGOs can unload guide RNAs. Although the molecular mechanism remains elusive, it can be imagined that topological stress is accumulated on the seed region (g2–g8) as the guide-noncanonical target duplex is propagated toward the 5’ end of the guide strand. In this study, we have revealed that internal water molecules connect the L2 and MID domains along with the PIWI subdomains through their water-mediated hydrogen bonds. In addition, our MD simulations predict that internal water molecules move, indicating that their hydrogen bonds are transiently broken without disrupting the conformation of the RISC. While direct experimental validation of these predictions is yet to come, our functional analyses are consistent with all simulation results. Therefore, we hypothesize that the transient rupture of water-mediated loop-loop interactions would enable the RISCs to collapse when the topological stress reaches a threshold (Figure 7E). In contrast, PIWIs stabilize their piRISC conformation by forming a hydrophobic core without LAKEs (Matsumoto et al., 2016). Such tight hydrophobic interactions would make piRISC difficult to disassemble. Given that PIWI proteins are predominantly expressed in germ cells only during a short term (Farazi et al., 2008), they do not seem to have enough time to exchange piRNAs, if any, and their exchange efficiency may be much lower than that of AGOs. These properties could explain why pi RISCs do not incorporate internal water molecules.
Internal water molecules have been identified in high-resolution structures (Meyer, 1992; Park and Saven, 2005). Functional studies and MD simulations have revealed that trapped water molecules contribute to the structural stability and affinity for ligands (Ball, 2017; Levy and Onuchic, 2006; Park and Saven, 2005). A recent structural study of GPCR reported that the internal water molecules work as a “lubricating oil” for the movement of a transmembrane helix (Yuan et al., 2014). In the current study, we discovered the significance of the internal water molecules for RISC assembly and discussed their possible involvement in RISC disassembly. Our simulation predictions and functional assays suggest another property of internal water molecules, one which facilitates a drastic, yet reversible, conformational change of globular proteins comprising multi domains.
STAR METHOD
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact Kotaro Nakanishi (nakanishi.9@osu.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% (v/v) Fet al Bovine Serum (FBS, Sigma-Aldrich) and 1% (v/v) Penicillin-Streptomycin (Gibco) at 37°C with 5% CO2. Plasmids were transfected by TranslT-X2 reagent (Mirus). For the AG04 overexpression, Sf9 and Trichoplusia ni (Tni) cells were used for Bac-to-Bac Baculovirus Expression System. Sf9 and Tni cells were grown in Grace’s Insect Medium (Gibco) and ESF 921 (Expression Systems). Refer the KEY Resources Table for detail information of cell strains and medium.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-FLAG | Sigma-Aldrich | Cat # F1804 |
| Anti-TNRC6A | Bethyl Laboratories Inc. | Cat # A302–329A |
| Mouse monoclonal anti-α tubulin | Cell Signaling Technology | Cat # 3873T |
| Goat anti-mouse | LI-COR | Cat # 925–33210 |
| Goat anti-rabbit | LI-COR | Cat # 925–33211 |
| Anti-FLAG M2 beads | Sigma-Aldrich | Cat # A2220 |
| Bacterial and Virus Strains | ||
| DH5α competent cells | Invitrogen | Cat # 18258012 |
| One Shot MACH I™ TI | Invitrogen | Cat # 1493679 |
| BL21(DE3) Rosetta2 | Novagen | Cat # 71397–3 |
| DH10bac Mar Efficiency | Invitrogen | Cat # 1492020 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Prime STAR Max premix(2x) | Takara | Cat # R045 |
| Rapid DNA ligation kit | Roche | Cat # 11635379001 |
| Gibson assembly master mix | NEB | Cat # E2611S |
| Takara Z-taq™ | Takara | Cat # R006A |
| CellFectin® II reagent | Invitrogen | Cat # 10362–100 |
| TransIt-X2®system | Mirus | Cat # MIR 6006 |
| DpnI | NEB | Cat # R01768 |
| EcoRI | NEB | Cat # R0101T |
| EcoRV | NEB | Cat # R0195T |
| NotI | NEB | Cat # 0189S |
| SigmaFAST™ Protease inhibitor cocktail tablets, EDTA-free | Sigma-Aldrich | Cat # S8830–20TAB |
| T4PNK | Thermo Scientific | Cat # EK0031 |
| 4–12% gradient Bis-Tris gels | Life Technologies | Cat # NW04125BOX |
| γ−32P-ATP | Parkin-Elmer | Cat # BLU002A250UC |
| α−32P-GTP | Parkin-Elmer | Cat # BLU006H250UC |
| Phosphatase, alkaline (AP) | Roche | Cat # 11097975001 |
| Thermo Scientific RiboLock RNase Inhibitor | Thermo Scientific | Cat # E00381 |
| 3X FLAG peptides | Sigma-Aldrich | Cat # F4799 |
| Grace’s Insect Medium (1x) | Gibco, Life Technologies | Cat # 11605–094 |
| Penicillin Streptomycin | Gibco, Life Technologies | Cat # 15070–063 |
| DMEM (Dulbecco’s Modified Eagle Medium) | Gibco, Life Technologies | Cat # 11965–092 |
| FBS (Fetal Bovine Serum, Qualified) | Gibco, Life Technologies | Cat # 10437–028 |
| Opti-MEM® I | Gibco, Life Technologies | Cat # 31985–070 |
| 0.25% Trypsin-EDTA (1x) | Gibco, Life Technologies | Cat # 25200–056 |
| ESF 921 | Expression Systems | Cat # 96-001-01 |
| Deposited Data | ||
| Human AGO4 Structural data | This paper | PDB: 6OON |
| Human AGO1 (4KXT) | Nakanishi et al., 2013 | PDB: 4KXT |
| Human AGO1 (4KRE) | Faehnle et al., 2013 | PDB: 4KRE |
| Human AGO1 (4KRF) | Faehnle et al., 2013 | PDB: 4KRF |
| Human AGO2 (4F3T) | Elkayam et al., 2012 | PDB: 4F3T |
| Human AGO2 (4OLA) | Schirle et al., 2012 | PDB: 4OLA |
| Human AGO2 (4OLB) | Schirle et al., 2012 | PDB: 4OLB |
| Human AGO2 (4Z4D) | Schirle et al., 2015 | PDB: 4Z4D |
| Human AGO2 (6CBD) | Sheu-Gruttadauria et al., 2018 | PDB: 6CBD |
| AGO2 MID domain (3LUC) | Frank et al., 2010 | PDB: 3LUC |
| NcQDE2 (2YHA) | Boland et al., 2011 | PDB: 2YHA |
| Silkworm PIWI-clade Argonaute Siwi (5GUH) | Matsumoto et al., 2016 | PDB: 5GUH |
| Experimental Models: Cell Lines | ||
| Sf9 | Expression System | 94–001F |
| Tni | Expression System | 94–002F |
| HEK293T | ATCC | CRL-1573 |
| Oligonucleotides | ||
| AGO4WT-FW-GCGCGCCCATGGAGGCGCTGGGACC | This paper | N/A |
| AGO4WT-RV-GCGCGCGTCGACTCAGGCAAA ATAC | This paper | N/A |
| AGO2Y529E-FW- CGGCAAGACGCCCGTGGAAGCCGAG | This paper | N/A |
| AGO2Y529E-RV- ACGCGCTTGACCTCGGCTTCCACGGGC | This paper | N/A |
| AGO2E637A-FW- GCAGCAGCACCGGCAGGCGATCATACAAG |
This paper | N/A |
| AGO2E637A-RV -GGCGGCCAGGTCTTGTATGATCGCCTGCCGG | This paper | N/A |
| miR-20a-5p UAAAGUGCUUAUAGUGCAGGUAG | Dharmacon | N/A |
| miR-20a-3p ACCUGCACUAUAAGCACUUUAAG | Dharmacon | N/A |
| 60-nt miR-20a target RNA GGGAGAAACAAAAAUACCUACCUGCACUAUAAGCACUUUACCAUCUCAAACUUACUCAGA | Park et al., 2017b | N/A |
| 60-nt miR-20a mismatch target RNA GGGAGAAACAAAAAUACCUACCUGCACUAAUAGCACUUUACCAUCUCAAACUUACUCAGA | Dayeh et al., 2018 | N/A |
| Recombinant DNA | ||
| pFB-HTB | Invitrogen | Cat # 10584–027 |
| pCAGEN | Addgene | Cat # 11160 |
| SUMO fused pRSFDuet™−1 | Dayeh et al., 2018 | N/A |
| Software and Algorithms | ||
| iMOSFLM | https://www.mrc-lmb.cam.ac.uk/harry/imosflm/ | N/A |
| PHASER | McCoy et al., 2007 | N/A |
| PHENIX | www.phenix-online.org/ | N/A |
| PyMol | https://pymol.org/ | N/A |
| Coot | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ | N/A |
| Chimera | https://www.cgl.ucsf.edu/chimera/ | N/A |
| imageQuant | https://www.gelifesciences.com/en/us/shop/protein…/imagequant-tl-8-1-p-00110 | N/A |
| Image studio | https://www.licor.com/bio/image-studio/ | N/A |
| Prism | https://www.graphpad.com/scientific-software/prism/ | N/A |
| imageJ | https://imagej.nih.gov | N/A |
METHOD DETAILS
Cloning, expression, and purification of AGO proteins and TNRC6B fragments
Recombinant AG02 and AG04 were purified from insect cells as previously reported (Park et al., 2017b). TNRC6B fragments were cloned into a SUMO-fused pRSFDuet™−1 vector (Novagen) and plasmids were transformed into BL21(DE3) E. coli cells. SUMO-fused TNRC6B fragments were purified by two steps. After the homogenized cells were centrifuged for 1 hour, the supernatant was loaded onto 5 mL HisTrap HP column (GE Healthcare) equilibrated with Buffer A (10 mM phosphate buffer pH 7.3, 1 M NaCl, 40 mM imidazole, 10 mM β-mercaptoethanol, 5% glycerol). The column was washed with Buffer A, followed by elution of the fragments over a linear gradient to 50% of Buffer B (10 mM phosphate buffer pH 7.3, 1 M NaCl, 1.5 M imidazole, 10 mM β-mercaptoethanol, 5% glycerol). The eluted samples were dialyzed against Buffer C (200 mM NaCl, 20 mM Tris-HCI pH 7.5, 10 mM β-mercaptoethanol) and then concentrated by ultrafiltration. The concentrated proteins were loaded onto HiLoad 16/600 Superdex 75 column (GE Healthcare) equilibrated with Buffer D (20 mM Tris-HCI pH 7.5, 200 mM NaCl, 2 mM Dithiothreitol (DTT)). After concentration by ultrafiltration, the purified SUMO-fused TNRC6B fragments were flash-frozen in liquid nitrogen and stored at −80°C. For in vitro cleavage assays, a gene of FLAG-tagged AG02 was cloned into a pCAGEN vector (Addgene). Mutants were subsequently generated from this construct by site-directed mutagenesis with primers that were designed to anneal to the mutation site. The PCR products were digested by Dpn1 (NEB) and then transformed into DH5α E. coli cells (Invitrogen™). The mutations were verified by sequencing. In our western blot analyses, all chimeras and mutants of AGO showed comparable expression levels in HEK293T cells without degradation, indicating that they were properly folded.
Crystallization, structure determination and refinement
The purified AG04 in Buffer E (10 mM Tris-HCI pH 8.0, 200 mM NaCl, 5 mM DTT) was crystallized at 20°C using the sitting-drop vapor diffusion method in 450 mM lithium chloride, 100 mM MES buffer pH 6.0, 16% PEG6,000, 15 mM trimethylamine. Crystals were soaked in cryoprotectant buffer (450 mM lithium chloride, 100 mM MES buffer pH 6.0, 16% PEG6,000, 15 mM trimethylamine, 20% (v/v) glycerol) and cryo-cooled in liquid nitrogen. Data were collected at the N E-CAT beamlines (Advanced Photon Source, Chicago) and processed with iMOSFLM (Battye et al., 2011). Molecular replacement was performed with PHASER (McCoy et al., 2007) using the crystal structure of AG02 (PDB ID: 40LA) as the search model. The structural model was refined using PHENIX (Adams et al., 2010). All figures of structures and electrostatic surfaces were generated using PyMol (http://www.pymol.org/) and Chimera (https://www.cgl.ucsf.edu/chimera/).
Cell culture, transfection, cell lysis and western blot
HEK293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% (v/v) Fet al Bovine Serum (FBS, Sigma-Aldrich) and 1% (v/v) Penicillin-Streptomycin (Gibco) under standard condition (37°C, 5% CO2). 10 μg of pCAGEN plasmid encoding the FLAG-tagged AGO protein was transfected into one 10 cm plate using the TranslT-X2 reagent (Mirus). Cells were harvested and weighed 48 hours after transfection. Cell pellets were resuspended in five-fold excess volume (v/w) of Lysis Buffer (30 mM HEPES-KOH pH 7.4, 100 mM KOAc, 2 mM Mg(OAc)2, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, EDTA-free protease inhibitor cocktail), and lysed by ultrasonication. Cell lysate (7 μl) was used for western blot analysis. 5 μl of SDS loading buffer (125 mM Tris-HCl pH 6.8, 4% sodium dodecyl sulfate (SDS), 20% Glycerol, 0.04% Bromophenol blue, and 100 mM β-mercaptoethanol) was mixed with the same volume of the cell lysates and incubated at 90°C for 1 min. The sample was separated on 4–12% gradient Bis-Tris gels (Life Technologies) and transferred to Amersham Hybond ECL nitrocellulose membranes (GE Healthcare). Anti-FLAG antibody (F1804, Sigma-Aldrich, 1:1000 dilution), anti-TNRC6A antibody (A302–329A, BETHLY, 1:1000 dilution), anti-mouse antibody (IRDye 800CW Goat anti-Mouse IgG, LI-COR, 1:16 000 dilution), and anti-rabbit antibody (IRDye 800CW Goat anti-Rabbit IgG, LI-COR, 1:16 000 dilution) were used as the primary and the secondary antibodies. The amount of FLAG-AGO proteins in the cell lysate was normalized by western blot result, AGO was quantified by using a standard curve generated with known amounts of recombinant FLAG-AG03.
In vitro cleavage assay
An siRNA-like duplex of miR-20a and a 60-nt 5’ cap-labeled target RNA were prepared as previously reported (Park et al., 2017b). Based on the western blot data, 50 pmol of AGO mutants were incubated with 0.5 pmol of an siRNA duplex of miR-20a at 37°C for 1 hour for the RISC assembly, followed by immunoprecipitation (IP) with 20 μl of anti-FLAG M2 beads (Sigma-Aldrich) for 2 hours at room temperature. The beads were washed eight times with IP-Wash Buffer (30 mM HEPES-KOH pH 7.4, 300 mM NaCl, 100 mM KOAc, 2 mM Mg(OAc)2, 1% Triton X-100) and two times with 1x Cleavage Buffer (25 mM HEPES-KOH pH 7.4, 50 mM KOAc, 5 mM Mg(OAc)2, 5 mM DTT). The cleavage reaction was initiated by adding 0.5 pmol of 5’ cap-labeled target RNAs in 1x cleavage buffer and 5 U/μL RiboLock RNase Inhibitor (ThermoFisher) at 37°C for 1 hour. The reaction was stopped by add ing 20 μl of quenching dye (7 M urea, 1 mM EDTA, 0.05% (w/v) xylene cyanol, 0.05% (w/v) bromophenol blue, 10% (v/v) phenol). Cleavage products were resolved on a 7 M urea denaturing 16% (w/v) polyacrylamide gel. The gels were dried on filter paper and analyzed by phosphorimaging.
RISC maturation assay
pCAGEN vector encoding either of FLAG-AGO mutants were transfected into HEK293T cells as previously reported (Park et al., 2017b). After 48 hours, 50 mg of the harvested cells were resuspended in 300 μL of Lysis Buffer (1% NP-40, 150 mM NaCl, 25 mM Tris-HCI pH 7.5, 2 mM EDTA, 0.5 mM DTT, 0.1% SDS, and 0.1% sodium deoxycholate). The cells were lysed with ultrasonication and centrifuged at 15,000 × g for 20 min at 4°C. 5 μL of the lysate was resolved on an SDS-PAGE, and the FLAG-AGO mutant was detected by western blot with anti FLAG antibody (Sigma-Aldrich). The amount of the FLAG-AGO mutant was quantified using a standard curve of recombinant FLAG-AG03. The lysate was adjusted so as to contain 50 pmol of FLAG-AGO mutant and then incubated with 5 pmol of siRNA-like duplex of miR-20a whose guide strand was radiolabeled at its 5’-end for 1 hour at 37°C. Then, FLAG-AGO mutant was immunoprecipitated with 60 μl of anti-FLAG M2 beads (Sigma-Aldrich) for 2 hours at room temperature. The beads were washed 8 times with Wash Buffer (300 mM NaCl, 50 mM Tris-HCI pH 7.5, 5 mM MgCl2, and 0.05% NP-40) and 2 times with Cleavage Buffer (25 mM HEPES-KOH pH 7.5, 50 mM KCI, 5 mM MgCI2, 5 mM DTT, and 2 mM EDTA). AGO-bound RNAs were extracted from the beads using 200 μl of phenol-chloroform and then precipitated with ethanol. After centrifugation, the RNA pellets were dissolved with 10 μl of 1x Annealing Buffer (30 mM HEPES-KOH pH 7.5, 100 mM KOAc, 1 mM EDTA) and native loading dye (15% w/v of Ficoll, 2 mM MgCl2, 0.5 TBE). The samples were resolved on a 10% native gel to separate the singlestranded guide RNA from the duplex. The intensity of pre-RISC (i.e., a complex of AGO with a duplex) and of mature RISC (i.e., a complex of AGO with a guide) are lpre-RISC and lRISC, respectively. The relative duplex loading and passenger ejection were calculated with these equations;
Filter binding assay
FLAG-AGO mutant (50 pmol) was incubated with 5 pmol of siRNA-like duplex of miR-20a for 1 hour at 37°C and immunoprecipitated with anti-FLAG M2 beads for 2 hours at room temperature. After the beads were thoroughly washed with Wash Buffer as described in the RISC maturation assay, FLAG-AGO mutant was eluted with 250 μg/ml of FLAG peptide (Sigma-Aldrich) overnight at 4°C. The eluted AGO mutant was incubated with a 60-nt 5’ cap-labeled mismatch target (GGGAGAAACAAAAAUACCUACCUGCACUAAUAGCACUUUACCAUCUCAAACUUACUCAG A) for 1 hour at 37°C. After the reactions were put on Hybond ECL nitrocellulose membranes (GE Healthcare) and Zeta-Probe Blotting Membranes (BIO-RAD), the membranes were washed with 1x Binding Buffer (25 mM HEPES-KOH pH 7.5, 10 mM MgCI2 3 mM DTT, and 125 mM NaCl2) and analyzed by phosphorimager.
Binding assay with TNRC6 fragments
The binding assays were performed with a Superdex 200 increase 10/300 GL column (GE Healthcare) equilibrated with Buffer F (150 mM NaCl, 20 mM Tris-HCI pH 7.5, 2 mM DTT). The column was injected with 2 nmol of AGO protein, of a SUMO-fused TNRC6 fragment, or their mixture incubated on ice for 30 min.
Simulation systems preparation
The crystallographic structures of human AG04 (PDB ID: 600N) and AG02 (PDB ID: 40LA) were used to build the systems for simulations (Table S2). Missing loops were not added and their ends were treated as non-neutral N and C termini. Hydrogen atoms were automatically added to protein structures and crystallographic water molecules. Residues aspartate, glutamate, lysine, and arginine were assumed to be charged. Histidine residues were assumed neutral, and their protonation state was chosen to favor the formation of evident hydrogen bonds. Additional water molecules and randomly placed ions were used to solvate the systems with 150 mM NaCl. Two systems were built for AG04: i) AG04CW protein + RNA + crystallographic water molecules, and ii) AG04NCW protein + RNA and no crystallographic waters inside of the LAKE1 cavity. For AG02 only one system was built: AG02CW protein + RNA + crystallographic waters. All systems were built using the psfgen, solvate and ionize plugins of VMD (Humphrey et al., 1996).
MD simulations
All MD simulations were performed using NAMD 2.11 (Phillips et al., 2005), the CHARMM36 force field for proteins and nucleic acids with the CMAP correction and the TIP3P model for water (Huang and MacKerell, 2013). A cutoff of 12 Å (with a switching function starting at 10 Å) was used for van der Waals interactions along with periodic boundary conditions. The Particle Mesh Ewald method was used to compute long-range electrostatic forces without cutoff and with a grid point density of >1 Å−3. A uniform 2 fs integration time step was used together with SHAKE. Langevin dynamics was utilized to enforce constant temperature (T = 300 or T= 310 K) with a damping coefficient of 0.1 ps−1 unless otherwise stated. Constant pressure simulations (A/p7”) at 1 atm were conducted using the hybrid Nosé-Hoover Langevin piston method with a 200 fs decay period and a 50 fs damping time constant. Initially the AG04NCW, AG04CW and AG02CW systems were subjected to 5000 steps of minimization followed by 200 ps of dynamics with the backbone of the protein and the C2’ atoms of the RNA restrained and by 1 ns of free dynamics in the NpT ensemble (T = 300 K, p = 1 atm, Langevin damping coefficient γ = 1 ps−1).
Definition of LAKE1 and LAKE2 for estimation of water dynamics parameters from the MD trajectories
Carefully tailored selections were defined for AG04 and AG02 in order to precisely monitor the water molecules that reside inside LAKE1 and LAKE2 during the MD simulations and to avoid the inclusion of water molecules that are outside of the cavities. Water molecules were considered to be inside the cavity if their oxygen atom was located within 4 Å of a selection of atoms. For LAKE1 of AG04 this selection includes the side-chain atoms of residues 567, 568 and 858, the side-chain OH group of residue 617 or the backbone oxygen atoms of residues 565, 614, 615 and 795. Similarly for LAKE1 of AG02 the selection includes the side-chain atoms of residue 576 and 623, the side-chain OH group of residues 625 and 805 or the backbone oxygen of residues 573, 622, 787, 789, 790, 791, 793, and 795. LAKE2 of AG04 includes the side-chain atoms of residues 719, 725, 732, 761, and 764, the Cβ atom of residue 362 or the backbone atoms of residue 716. For AG02, LAKE2 includes side-chain atoms of residue 730, the Cβ atom of residue 372 or the backbone atoms of residues 714, 715, 717 and 728.
Computation of survival probability of water molecules in MD simulations
Local translational and rotational mobility of water molecules can be related to the amount of time that a molecule is likely to remain in a given region (Liu, 2004). By measuring which fraction of molecules were in a given region at time f and remain there at a later time t+τ, the survival probability P(τ) can be computed. The decay rate of P(τ) depends on the size of the region and the mobility of molecules within it and is calculated as follows:
where N(t, τ) is the number of particles that remain in the selection at time t + τ, N(t) is the number of particles at time t, and Tn is the number of time steps contributing to P(τ). The survival probability was calculated from our trajectories using the water dynamics module of the MD-analysis library (Araya-Secchi et al., 2014; Gowers et al., 2016; Michaud-Agrawal et al., 2011). Each P(τ) was estimated for all the available time windows (τ = 1 to 100 ps) from four 20 ns segments of the 100 ns simulations excluding the first 20 ns. The data presented in Figure 3C corresponds to P(τ) averaged for each time window.
QUANTIFICATION AND STATISTICAL ANALYSIS
All the assay data were quantified using ImageJ (https://imaaei.nih.aov/ii/) and ImageQuant (GE Healthcare life Sciences). The dot plots in Figures 1, 3, and 5 were generated by Prism software (GraphPad) and black bars on the graph show averages with independent replicates shown as dots. The detail calculations were in the method section.
DATA AND SOFTWARE AVAILABILITY
Coordinates and structure factors for human Argonaute 4 in complex with guide RNA have been deposited in the Protein Data Bank under accession code 600N.
Supplementary Material
Video 1. Water molecules trapped in LAKE1, Related to Figure 4.
Video 2. Water molecules are exchangeable with solvent, Related to Figure 5. Water molecules occupying LAKE1 (yellow spheres) are exchanged with solvent water molecules (red spheres).
Video 3. Differences of the domain-domain interactions, Related to Figure 3.
HIGHLIGHTS.
Crystal structure of AG04-RISC completes the structural catalogs of four human AGOs
Guide-RNA binding ties together two PIWI subdomains and allows the RISC to bind TNRC6
Confining water molecules inside AGO is essential to form functional RISC
Interior water clusters named LAKEs are conserved in AGOs but not in PIWIs
ACKNOWLEDGMENTS
We are grateful to K. Okamura (NAIST) for useful advice and insights. This work was supported by Pelotonia Fellowships (to M.S.P., R.A-S., and D.M.D.), The Ohio State University startup funds (to M.S. and K.N.), PRESTO (JPMJPR13L7 to K.N.) and NIH (R01GM124320 to K.N. and R01DC015271 to M.S.). The research was supported by the Office of the Director, NIH (S100D023582). This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the NIH (P41 GM103403). The Eiger 16M detector on 24-ID-E beamline is funded by a NIH-ORIP HEI grant (S100D021527). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02–06CH11357. All MD simulations were carried out at the Ohio Supercomputer Center using the Owens cluster (grant PAS1037 to M.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alio M, Agirre E, Bessonov S, Bertucci P, Gomez Acuna L, Buggiano V, Bellora N, Singh B, Petrillo E, Blaustein M, et al. (2014)]. Argonaute-1 binds transcriptional enhancers and controls constitutive and alternative splicing in human cells. Proc Natl Acad Sci U S A 111, 15622–15629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya-Secchi R, Perez-Acle T, Kang SG, Huynh T, Bernardin A, Escalona Y, Garate JA, Martinez AD, Garcia IE, Saez JC, et al. (2014). Characterization of a novel water pocket inside the human Cx26 hemichannel structure. Biophys J 107, 599–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma-Mukai A, Oguri H, Mituyama T, Qian ZR, Asai K, Siomi H, and Siomi MC (2008). Characterization of endogenous human Argonautes and their miRNA partners in RNA silencing. Proc Natl Acad Sci U S A 105, 7964–7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillat D, and Shiekhattar R (2009). Functional dissection of the human TNRC6 (GW182-related) family of proteins. Mol Cell Biol 29, 4144–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P (2017). Water is an active matrix of life for cell and molecular biology. Proc Natl Acad Sci U S A 114,13327–13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2018). Metazoan MicroRNAs. Cell 173, 20–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battye TG, Kontogiannis L, Johnson O, Powell HR, and Leslie AG (2011). iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr D Biol Crystallogr 67, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitzinger M, Peters L, Zhu JY, Kremmer E, and Meister G (2007). Identification of human microRNA targets from isolated argonaute protein complexes. RNA Biol 4, 76–84. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, and Hannon GJ (2001). Role fora bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366. [DOI] [PubMed] [Google Scholar]
- Boland A, Huntzinger E, Schmidt S, Izaurralde E, and Weichenrieder O(2011). Crystal structure of the MID-PI WI lobe of a eukaryotic Argonaute protein. Proc Natl Acad Sci U S A 108,10466–10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carugo O (2016). Statistical survey of the buried waters in the Protein Data Bank. Amino Acids 48,193–202. [DOI] [PubMed] [Google Scholar]
- Cheloufi S, Dos Santos CO, Chong MM, and Hannon GJ (2010). A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465, 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech B, and Hannon GJ (2016). One Loop to Rule Them All: The Ping-Pong Cycle and piRNA-Guided Silencing. Trends Biochem Sci 41, 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayeh DM, Kruithoff BC, and Nakanishi K (2018). Structural and functional analyses reveal the contributions of the C- and N-lobes of Argonaute protein to selectivity of RNA target cleavage. J Biol Chem 293, 6308–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De N, Young L, Lau PW, Meisner NC, Morrissey DV, and MacRae IJ (2013). Highly complementary target RNAs promote release of guide RNAs from human Argonaute2. Mol Cell 50, 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueck A, Ziegler C, Eichner A, Berezikov E, and Meister G (2012). microRNAs associated with the different human Argonaute proteins. Nucleic Acids Res 40, 9850–9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkayam E, Faehnle CR, Morales M, Sun J, Li H, and Joshua-Tor L (2017). Multivalent Recruitment of Human Argonaute by GW182. Mol Cell 67, 646–658 e643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkayam E, Kuhn CD, Tocilj A, Haase AD, Greene EM, Hannon GJ, and foshua-Tor L (2012). The structure of human argonaute-2 in complex with miR-20a. Cell 150,100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faehnle CR, Elkayam E, Haase AD, Hannon GJ, and Joshua-Tor L (2013). The making of a slicer: activation of human Argonaute-1. Cell Rep 3,1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazi TA, Juranek SA, and Tuschl T (2008). The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development 135,1201–1214. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, and Mello CC (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811. [DOI] [PubMed] [Google Scholar]
- Frank F, Sonenberg N, and Nagar B (2010). Structural basis for 5’-nucleotide base-specific recognition of guide RNA by human AG02. Nature 465, 818–822. [DOI] [PubMed] [Google Scholar]
- Ghildiyal M, and Zamore PD (2009). Small silencing RNAs: an expanding universe. Nat Rev Genet 10, 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowers RJ, Linke M, Barnoud J, Reddy TJE, Melo MN, Seyler SL, Domanski, Dotson DL, Buchoux S, Kenney IM, et al. (2016). MDAnalysis: A Python Package for the Rapid Analysis of Molecular Dynamics Simulations In Benthall S and Rostrup S, editors, Proceedings of the 15th Python in Science Conference, 98–105. [Google Scholar]
- Gregory RI, Chendrimada TP, Cooch N, and Shiekhattar R (2005). Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 123, 631–640. [DOI] [PubMed] [Google Scholar]
- Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M Jr., Jungkamp AC, Munschauer M, et al. (2010). Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141, 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann J, Dueck A, Harlander S, Pfaff J, Merkl R, and Meister G (2013). Turning catalytically inactive human Argonaute proteins into active slicer enzymes. Nat Struct Mol Biol 20, 814–817. [DOI] [PubMed] [Google Scholar]
- Hauptmann J, Kater L, Loffler P, Merkl R, and Meister G (2014). Generation of catalytic human Ago4 identifies structural elements important for RNA cleavage. RNA 20,1532–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann J, Schraivogel D, Bruckmann A, Manickavel S, Jakob L, Eichner N, Pfaff, Urban M, Sprunck S, Hafner M, et al. (2015). Biochemical isolation of Argonaute protein complexes by Ago-APP. Proc Natl Acad Sci U S A 112,11841–11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussecker D, Cao D, Huang Y, Parameswaran P, Fire AZ, and Kay MA (2008). Capped small RNAs and MOVIO in human hepatitis delta virus replication. Nat Struct Mol Biol 15, 714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Tanasa B, Trabucchi M, Li W, Zhang J, Ohgi KA, Rose DW, Glass CK, and Rosenfeld MG (2012). DICER- and AG03-dependent generation of retinoic acid-induced DR2 Alu RNAs regulates human stem cell proliferation. Nat Struct Mol Biol 19,1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, and MacKerell AD (2013). CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J Comput Chem 34, 2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang V, Zheng J, Qi Z, Wang J, Place RF, Yu J, Li H, and Li LC (2013). Ago 1 Interacts with RNA polymerase II and binds to the promoters of actively transcribed genes in human cancer cells. PLoS Genet 9, e1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, and Schulten K (1996). VMD: visual molecular dynamics. J Mol Graph 14, 33–38, 27–38. [DOI] [PubMed] [Google Scholar]
- Huntzinger E, and Izaurralde E (2011). Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 12, 99–110. [DOI] [PubMed] [Google Scholar]
- Iki T, Yoshikawa M, Nishikiori M, Jaudal MC, Matsumoto-Yokoyama E, Mitsuhara I, Meshi T, and Ishikawa M (2010). In vitro assembly of plant RNA-induced silencing complexes facilitated by molecular chaperone HSP90. Mol Cell 39,282–291. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kobayashi M, Yoda M, Sakaguchi Y, Katsuma S, Suzuki T, and Tomari Y (2010). Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol Cell 39, 292–299. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Sasaki HM, Sakaguchi Y, Suzuki T, Tadakuma H, and Tomari Y (2015a). Defining fundamental steps in the assembly of the Drosophila RNAi enzyme complex. Nature 521, 533–536. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, and Tomari Y (2018). Reconstitution of RNA Interference Machinery. Methods Mol Biol 1680,131–143. [DOI] [PubMed] [Google Scholar]
- Iwasaki YW, Siomi MC, and Siomi H (2015b). PIWI-Interacting RNA: Its Biogenesis and Functions. Annu Rev Biochem 84, 405–433. [DOI] [PubMed] [Google Scholar]
- Jakymiw A, Lian S, Eystathioy T, Li S, Satoh M, Hamel JC, Fritzler MJ, and Chan EK (2005). Disruption of GW bodies impairs mammalian RNA interference. Nat Cell Biol 7, 1267–1274. [DOI] [PubMed] [Google Scholar]
- Jonas S, and Izaurralde E (2015). Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16, 421–433. [DOI] [PubMed] [Google Scholar]
- Kawamata T, Seitz H, and Tomari Y (2009). Structural determinants of miRNAs for RISC loading and slicer-independent unwinding. Nat Struct Mol Biol 16, 953–960. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, and Tomari Y (2016). RISC assembly: Coordination between small RNAs and Argonaute proteins. Biochim Biophys Acta 1859, 71–81. [DOI] [PubMed] [Google Scholar]
- Koellner G, Kryger G, Millard CB, Silman I, Sussman JL, and Steiner T (2000). Active-site gorge and buried water molecules in crystal structures of acetylcholinesterase from Torpedo californica. J Mol Biol 296, 713–735. [DOI] [PubMed] [Google Scholar]
- Lazzaretti D, Tournier I, and Izaurralde E (2009). The C-terminal domains of human TNRC6A, TNRC6B, and TNRC6C silence bound transcripts independently of Argonaute proteins. RNA 15,1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, et al. (2003). The nuclear RNase III Drosha initiates micro RNA processing. Nature 425, 415–419. [DOI] [PubMed] [Google Scholar]
- Levy Y, and Onuchic JN (2006). Water mediation in protein folding and molecular recognition. Annu Rev Biophys Biomol Struct 35, 389–415. [DOI] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, and Hannon GJ (2004). Argonaute2 is the catalytic engine of mammalian RNAi. Science 305,1437–1441. [DOI] [PubMed] [Google Scholar]
- Liu J, Rivas FV, Wohlschlegel J, Yates JR, Parker R, and Hannon GJ (2005a). A role for the P-body component GW182 in microRNA function. Nat Cell Biol 7,1261–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Valencia-Sanchez MA, Hannon GJ, and Parker R (2005b). MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol 7, 719–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.a.H., Edward and Berne BJ (2004). On the Calculation of Diffusion Coefficients in Confined Fluids and Interfaces with an Application to the Liquid-Vapor Interface of Water. J Phys Chem B 108, 6595–6602. [Google Scholar]
- MacRae IJ, Ma E, Zhou M, Robinson CV, and Doudna JA (2008). In vitro reconstitution of the human RISC-loading complex. Proc Natl Acad Sei U S A 105, 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto N, Nishimasu H, Sakakibara K, Nishida KM, Hirano T, Ishitani R, Siomi H, Siomi MC, and Nureki O (2016). Crystal Structure of Silkworm PIWI-Clade Argonaute Siwi Bound to piRNA. Cell 167, 484–497.e489. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, and Read RJ (2007). Phaser crystallographic software. J Appl Crystallogr 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Landthal er M, Patkaniowska A, Dorsett Y, Teng G, and Tuschl T (2004). Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15, 185–197. [DOI] [PubMed] [Google Scholar]
- Meyer E (1992). Internal water molecules and H-bonding in biological macromolecules: a review of structural features with functional implications. Protein Sei 1,1543–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud-Agrawal N, Denning EJ, Woolf TB, and Beckstein O (2011). MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J Comput Chem 32, 2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modzelewski AJ, Holmes RJ, Hilz S, Grimson A, and Cohen PE (2012). AG04 regulates entry into meiosis and influences silencing of sex chromosomes in the male mouse germline. Dev Cell 23, 251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K (2016). Anatomy of RISC: how do small RNAs and chaperones activate Argonaute proteins? Wiley Interdiscip Rev RNA 7, 637–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Ascano M, Gogakos T, Ishibe-Murakami S, Serganov AA, Briskin D, Morozov P, Tuschl T, and Patel DJ (2013). Eukaryote-specific insertion elements control human ARGONAUTE slicer activity. Cell Rep 3,1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Weinberg DE, Bartel DP, and Patel DJ (2012). Structure of yeast Argonaute with guide RNA. Nature 486, 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Shin SY, and Shin C (2017a). Non-canonical targets destabilize microRNAs in human Argonautes. Nucleic Acids Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MS, Phan HD, Busch F, Hinckley SH, Brackbill JA, Wysocki VH, and Nakanishi K (2017b). Human Argonaute3 has slicer activity. Nucleic Acids Res 45,11867–11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, and Saven JG (2005). Statistical and molecular dynamics studies of buried waters in globular proteins. Proteins 60, 450–463. [DOI] [PubMed] [Google Scholar]
- Pfaff J, Hennig J, Herzog F, Aebersold R, Sattler M, Niessing D, and Meister G (2013). Structural features of Argonaute-GW182 protein interactions. Proc Natl Acad Sei U S A 110, E3770–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan HD, Li J, Poi M, and Nakanishi K (2018). Quantification of miRNAs Co-Immunoprecipitated with Argonaute Proteins Using SYBR Green-Based qRT-PCR. Methods Mol Biol 1680, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, and Schulten K (2005). Scalable molecular dynamics with NAMD. J Comput Chem 26,1781–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel S, Wang Y, Lenobel R, Korner R, Hsiao HH, Urlaub H, Patel D, and Meister G (2011). Phosphorylation of human Argonaute proteins affects small RNA binding. Nucleic Acids Res 59,2330–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Shiohama A, Minoshima S, and Shimizu N (2003). Identification of eight members of the Argonaute family in the human genome. Genomics 82, 323–330. [DOI] [PubMed] [Google Scholar]
- Schirle NT, and MacRae IJ (2012). The crystal structure of human Argonaute2. Science 336,1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirle NT, Sheu-Gruttadauria J, Chandradoss SD, Joo C, and MacRae IJ (2015). Water-mediated recognition of t1-adenosine anchors Argonaute2 to microRNA targets. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirle NT, Sheu-Gruttadauria J, and MacRae IJ (2014). Structural basis for microRNA targeting. Science 346, 608–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu-Gruttadauria J, and MacRae IJ (2018). Phase Transitions in the Assembly and Function of Human miRISC. Cell 173, 946–957.e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboyama K, Tadakuma H, and Tomari Y (2018). Conformational Activation of Argonaute by Distinct yet Coordinated Actions of the Hsp70 and Hsp90 Chaperone Systems. Mol Cell 70, 722–729.e724. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sheng G, Juranek S, Tuschl T, and Patel DJ (2008). Structure of the guide-strand-containing argonaute silencing complex. Nature 456, 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Goodfellow JM, and Thornton JM (1994). Buried waters and internal cavities in monomeric proteins. Protein Sci 3,1224–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J, and Diederichs S (2013). Argonaute-3 activates the let-7a passenger strand microRNA. RNA Biol 10,1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Filipek S, Palczewski K, and Vogel H (2014). Activation of G-protein-coupled receptors correlates with the formation of a continuous internal water pathway. Nat Commun 5, 4733. [DOI] [PubMed] [Google Scholar]
- Zhang H, Kolb FA, Brondani V, Billy E, and Filipowicz W (2002). Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J 21, 5875–5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Water molecules trapped in LAKE1, Related to Figure 4.
Video 2. Water molecules are exchangeable with solvent, Related to Figure 5. Water molecules occupying LAKE1 (yellow spheres) are exchanged with solvent water molecules (red spheres).
Video 3. Differences of the domain-domain interactions, Related to Figure 3.
Data Availability Statement
Coordinates and structure factors for human Argonaute 4 in complex with guide RNA have been deposited in the Protein Data Bank under accession code 600N.