Abstract
Background & Aims:
Although pancreatic cystic lesions (PCLs) are frequently and incidentally detected, it is a challenge to determine their risk of malignancy. In immunohistochemical and ELISA analyses of tissue and cyst fluid from pancreatic intraductal papillary mucinous neoplasms (IPMNs), the monoclonal antibody Das-1 identifies those at risk for malignancy with high levels of specificity and sensitivity. We aimed to validate the ability of Das-1 to identify high-risk PCLs, in comparison to clinical guidelines and clinical features, using samples from a multicenter cohort.
Methods:
We obtained cyst fluid samples of 169 PCLs (90 IPMNs, 43 mucinous cystic neoplasms, and 36 non-mucinous cysts) from patients undergoing surgery at 4 tertiary referral centers (January 2010 through June 2017). Histology findings from surgical samples, analyzed independently and centrally re-reviewed in a blinded manner, were used as the reference standard. High-risk PCLs were those with invasive carcinomas, high-grade dysplasia, or intestinal-type IPMNs with intermediate-grade dysplasia. An ELISA with Das-1 was performed in parallel using banked cyst fluid samples. We evaluated the biomarker’s performance, generated area under the curve (AUC) values, and conducted multivariate logistic regression using clinical and pathology features.
Results:
The ELISA for Das-1 identified high-risk PCLs with 88% sensitivity and 99% specificity, and 95% accuracy, at a cut-off optical density value of 0.104. In 10-fold cross validation analysis with 100 replications, Das-1 identified high-risk PCLs with 88% sensitivity and 98% specificity. The Sendai, Fukuoka, and American Gastroenterological Association guideline criteria identified high-risk PCLs with 46%, 51.5%, and 73.7% accuracy (P for comparison to Das-1 ELISA<.001). When we controlled for Das-1 in multivariate regression, main pancreatic duct dilation >5 mm (odds ratio [OR], 14.98; 95% CI, 2.63–108; P<.0012), main pancreatic duct dilation ≥1cm (OR, 47.9; 95% CI, 6.39–490; P<.0001), and jaundice (OR, 6.16; 95% CI, 1.08–36.7; P=.0397) were significantly associated with high-risk PCLs.
Conclusions:
We validated the ability of an ELISA with the monoclonal antibody Das-1 to detect PCLs at risk for malignancy with high levels of sensitivity and specificity. This biomarker might be used in conjunction with clinical guidelines to identify patients at risk for cancer.
Keywords: mAb, MCN, Tumor, diagnostic
Graphical Abstract
Lay Summary
Pancreatic cystic lesions are commonly detected but it is not clear which are at risk for malignancy. We validated the ability of an assay that uses the monoclonal antibody Das-1 to detect high-risk cystic lesions with high levels of sensitivity and selectivity.
INTRODUCTION
Pancreatic cystic lesions (PCL) have been increasingly recognized to have malignant potential and are readily detectable on cross sectional imaging.1–3 The overall prevalence of pancreatic cysts is estimated to be 2.6–9.3% of asymptomatic patients undergoing abdominal CT4 and MRI5 scans, with their resections accounting for up to 20% of pancreatic resections in referral centers.6 However, while a small proportion has malignant potential, the vast majority of these lesions are either benign or indolent.7,8 Several clinical guidelines have been adopted to assist clinicians in determining when a lesion should be surgically resected. 7,9–11 However, validation studies have demonstrated that these guidelines either have inadequate sensitivity (7.3%−35.2%)9,10 or inadequate specificity (23%–30%).11 Given the prevalence of asymptomatic cysts and possible morbidity associated with surgical interventions, there is an unmet need for molecular tools to risk-stratify lesions.
PCL can be broadly divided into non-mucinous and mucinous lesions. Non-mucinous PCL include pseudocysts (PC) and serous cystadenoma (SCA) that have no malignant potential, and cystic neuroendocrine tumors and solid pseudopapillary neoplasm (SPN), both of which have low-grade malignant potential. Mucinous PCL consist of intraductal papillary mucinous neoplasms (IPMN) and mucinous cystic neoplasms (MCN) that are of varying malignant potential.12 IPMN are divided into three broad anatomic subtypes (main duct, branch duct, mixed) based on their involvement of the pancreatic duct and four epithelial subtypes (gastric [IPMN-G], intestinal [IPMN-I], pancreatobiliary and oncocytic) with varying degrees of dysplasia (low-[LGD], intermediate-[IGD], and high-grade [HGD]).1–3,13 While main-duct and mixed-type IPMNs have a 48% and 42% likelihood of harboring invasive carcinoma, in branch duct lesions it is only 11%.1,2 IPMN-G comprise the majority of branch-duct IPMN, and rarely exhibit HGD. Conversely, IPMN-I make up the majority of the main-duct IPMN and frequently exhibit HGD/invasive carcinoma.13 Pancreatobiliary and oncocytic subtypes are rare, high-grade lesions, and typically present with large cystic tumors involving the main duct. The majority of the latter two subtypes contain invasive or minimally invasive components, respectively.2
We have previously developed a novel murine monoclonal antibody, mAb Das-1, which reacts specifically with normal non-goblet and goblet colonic epithelium but not with normal small intestinal enterocytes.14–17 While absent in normal esophageal, gastric, and pancreatic epithelium, we have demonstrated that it is present in precancerous and cancerous conditions of these same tissues.14,15,18–20 In a preliminary single center study, we reported on the specific immunoreactivity of mAb Das-1 against resected tissue and cyst fluid from patients with high-risk IPMN and associated invasive carcinomas.15 Indeed, in a small cohort of patients with resected PCL, evaluation of mAb Das-1 in perioperatively aspirated cyst fluid samples by ELISA assay demonstrated a sensitivity of 89% and specificity of 100% in detecting high-risk lesions.15
Here, we explore the ability of mAb Das-1 to segregate PCLs with high-risk for malignant behavior in a large, multicenter, cohort of cyst fluid samples aspirated at the time of surgical resection. With blinded, centrally verified pathological review of all cases we ensured a comparison to a uniform gold standard. Finally, we evaluated the performance of mAb Das-1 against current available clinical guidelines and their constituent components.
MATERIALS AND METHODS
Study design and subjects
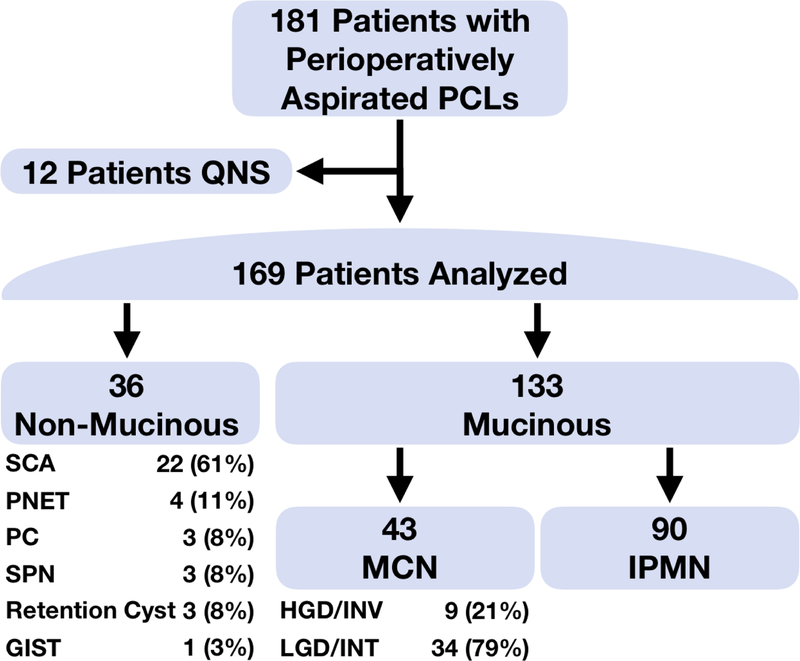
The institutional review boards of all centers approved this study and it is reported in accordance with STARD and REMARK guidelines (Supplementary Statistical Checklists). Patients underwent surgical resection for PCL with peri-operative cyst fluid collection between January 2010 and June 2017 at four, tertiary referral centers (MGH [n=94], JHU [n=46], MSKCC [n=37], WU [=4]) with multidisciplinary PCL programs. The decision to resect a pancreatic cyst is multifactorial, and includes not only an assessment of the risk of the presence of high-grade dysplasia or invasive cancer within a cyst, but also the presence of other features, including symptoms secondary to the cyst, the patient’s age and/or comorbidities. All pancreatic cyst fluid was aspirated, aliquoted, and flash frozen (−80°C) at the time of surgical resection and stored in the respective institutions’ biobanks. Retrospective inclusion of patients into the current study was on the basis of the availability of frozen, banked cyst fluid. Of the 181 patients with available cyst fluid for analysis, 12 patients did not have a sufficient quantity to analyze (Figure 1). Each institution provided clinical data on review of the enrolled patients’ records.
Figure 1. Flow diagram of patients evaluated.
PCL – Pancreatic Cystic Lesion, QNS – Quantity Not Sufficient, SCA – Serous Cyst Adenoma, PNET – Pancreatic Neuroendocrine Tumor, PC – Pseudocyst, SPN – Solid Pseudopapillary Neoplasm, GIST – Gastrointestinal Stromal Tumor, MCN – Mucinous Cystic Neoplasm, HGD/INV – High Grade Dysplasia/Invasive Carcinoma, LGD/INT – Low Grade- /Intermediate Grade- Dysplasia, IPMN – Intraductal Papillary Mucinous Neoplasm
Pathologic evaluation of tissue specimens
De-identified, coded slides of all available patients were reviewed by one of the authors specialized in the field (MM-K). Analysis was performed blinded to the original pathologic diagnosis and immunoreactivity to mAb Das-1. Of those, all cases of IPMN were histopathologically classified by main/branch duct involvement and by dysplastic grade. We used a three-tiered grading system (LGD, IGD and HGD) for the purpose of this study, and the LGD and IGD correspond to LGD in the recently recommended two-tiered grading system.21 Epithelial subtypes were determined on the basis of their epithelial morphology on routine H&E staining and, when available, immunoreactivity against mucin glycoproteins and/or CDX2 according to previously established criteria.22,23 IPMN lesions were classified on a per-patient basis, based on the most predominant epithelial subtype, and the highest-grade lesion demonstrated. All cases of MCN were also classified by dysplastic grade.
For the purposes of this study, high-risk lesions (i.e. those warranting definitive surgical management) were those pathologically verified to have invasive carcinoma in association with a PCL, HGD either arising in an MCN or IPMN, or IPMN-I with IGD. IPMN-I with IGD were included in this high risk category as we2 and others23 have found these cases frequently harbor multiple, small foci of HGD and present in patients with (recurrent) pancreatitis24 both warranting surgical management. Low-risk lesions were defined as all other PCL, including non-mucinous PCL, and IPMN-G and MCN with LGD or IGD. A separate analysis, defining high-risk lesions as “advanced neoplasia,” meaning those lesions with HGD or invasive carcinoma (not including IPMN-I with IGD), was also performed in parallel.
Analyses of cyst fluid aspirates for mAb Das-1
De-identified, frozen samples were processed blinded to their pathological diagnosis. Fluid was assayed for total protein concentration and all samples were normalized to equal protein amount. Sandwich ELISA was performed with mAb Das-1 IgM and mAb Das-1 IgG isotypes as previously described.15 All experiments were conducted at least in duplicate and normalized with respect to reactivity of the positive control.
Statistical Analysis
Based on our preliminary evaluation of PCL cyst fluid,15,25 we expected that both the sensitivity and specificity of the assay would be approximately 92%. We performed sample size calculations demonstrating that for a sample of n=50 patients with high-risk lesions and n=50 with low-risk lesions, the 95% confidence interval for an observed sensitivity or specificity of 92% would be (81%, 98%). Our cohort includes 101 low-risk and 68 high-risk patients.
Optical Density (OD) values are displayed with standard deviations and compared across patient groups through the Mann-Whitney test. The performance of the continuous mAb-Das-1 OD values in predicting high-risk PCL was described through receiver operating curves (ROC) and the area under the receiver operating curves (AUC). The optimal cut-point for predicting high-risk PCL with mAb-Das-1 was determined from the ROC utilizing Youden’s statistic. The performance of the dichotomized mAb-Das-1 and other clinical criteria for high-risk PCL (Sendai guidelines, Fukuoka guidelines, and AGA guidelines) is described through sensitivity, specificity, and accuracy (the percent correctly identified by the screen, or the sum of the true positives and true negatives). Exact 95% confidence intervals are given, and performance of these guidelines and mAb Das-1 are compared through exact paired-sample McNemar’s tests for proportions. All the tests were two-sided and the significance level was set at 0.05. The analyses were performed with STATA 14 (STATACorp College Station, TX, USA) and SAS 9.2
Examination of clinical risk factors controlling for mAb Das-1
We used logistic regression to examine associations between clinical risk factors and high-risk PCL, first examining unadjusted associations (odds ratios and 95% confidence intervals) and then examining associations between clinical risk factors and high-risk PCL after controlling for the dichotomized mAb Das-1. Clinical factors examined were cyst size, MPD dilation (>5mm, ≥1cm), enhancing mural nodule, solid component, multifocality, any symptoms, weight loss, abdominal pain, jaundice, pancreatitis, as well as a previously validated composite clinical marker (jaundice or MPD dilation or cyst size ≥4cm).26,27 Because of the high sensitivity and specificity of mAb Das-1, which lead to a small number of false positives (n=1) and negatives (n=8), we used exact logistic regression to estimate the adjusted associations.
K-fold Validation
We used repeated k fold cross-validation to estimate the sensitivity, specificity, and accuracy of the mAb Das-1 screen when applied to an independent sample of subjects. First, we randomly divided the sample into 10 equal sized subsamples. Then, for each subsample, we 1) took the subsample as a holdout validation data set, 2) took the remaining subsamples as a training data set, 3) determined the Das-1 cut-off in the training data set, using Youden’s index, 4) using the cut-off from the training data set, determined the performance of the cut-off in the held out validation data set. Performance results in the 10 validation data sets are then pooled to calculate sensitivity, specificity, accuracy, and their 95% confidence intervals. Since these performance estimates depend on the original random sample division, we repeated this process 100 times and present average sensitivity, specificity, and accuracy across the 100 replications.
RESULTS
Study Cohort
Demographic and clinical information on the examined study cohort are displayed in Table 1. Of the 181 patients with PCL in the study, 169 patients had sufficient cyst fluid for analysis. Of these PCL, 36 were non-mucinous and 133 were mucinous (43 MCN and 90 IPMN) (Figure 1). As expected, patients with MCN tended to be younger and have a female predominance.
Table 1 –
Patient and Cyst Characteristics
| All Samples | IPMN | MCN | Non-Mucinous Cystic Lesions | |
|---|---|---|---|---|
| n=169 | n=90 | n=43 | n=36 | |
| Female Sex, n (%) | 112 (66%) | 43 (48%) | 41 (95%) | 28 (78%) |
| Age at Surgery, mean (SD) | 58.9 (15.2) | 66.6 (12.1) | 48.4 (14.2) | 52.3 (12.5) |
| Symptoms, n (%) | 76 (45%) | 44 (49%) | 22 (51%) | 10 (28%) |
| Weight Loss | 9 (5%) | 7 (8%) | 1 (2%) | 1 (3%) |
| Abdominal Pain | 50 (30%) | 24 (27%) | 18 (42%) | 8 (22%) |
| Pancreatitis | 33 (20%) | 21 (23%) | 7 (16%) | 5 (14%) |
| Jaundice | 7 (4%) | 7 (8%) | 0 (0%) | 0 (0%) |
| Cyst Size cm mean (SD) | 5.04 (3.59) | 4.07 (3.23) | 6.30 (4.32) | 5.88 (2.72) |
| Mural Nodule n (%) | 26 (15%) | 18 (20%) | 6 (14%) | 2 (6%) |
Preoperative cyst fluid CEA was available in 49 patients. Utilizing the previously established threshold of 192ng/mL to discriminate a potential mucinous PCL,28 there was a 50% sensitivity (0.329–0.671) and 92.3% specificity (0.640–0.998) for CEA accurately identifying a mucinous lesion (Supplementary Table 1). Cyst fluid cytology (n=57) was not readily available in a large enough subgroup of the patients included in this cohort to provide a meaningful evaluation of its performance in parallel.
Among the 90 patients with IPMN examined, 44 (49%) involved the main duct and 46 (51%) were exclusively branch duct lesions, with all epithelial subtypes represented (Supplementary Table 2). Preoperative assessment of main duct involvement was observed in 42 (46%) on cross sectional imaging. Of the IPMN lesions, 19 harbored LGD, 21 IGD, 32 HGD, and 18 showed an invasive component. Only 9 of the MCN harbored HGD or invasive features.
Cyst Fluid Protein Analysis
The median cyst fluid protein concentration was 4.1µg/µl (IQR 1.61 – 9.56µg/µl) and reflected the pathology of the resected specimens with low-grade IPMN-G/SCA and high-grade IPMN-I/Colloid carcinoma at the extremes. Considering the lower end of the IQR, the vast majority of samples could be processed in duplicate (requiring 200µg protein) with less than 150µl of cyst fluid.
mAb Das-1 identifies high risk PCL
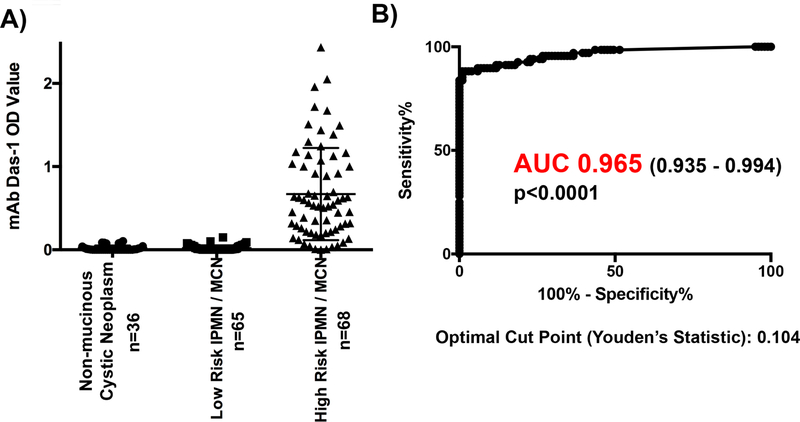
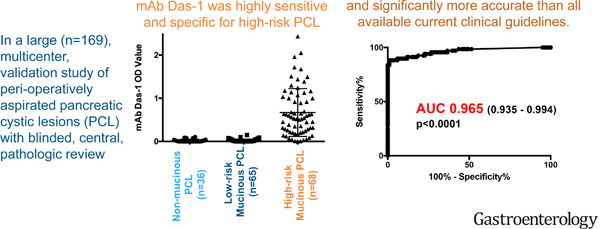
Cyst fluid from non-mucinous PCL (n=36) demonstrated very little reactivity with mAb Das-1 by sandwich ELISA assay (OD 0.019±0.032). Similarly, low-risk IPMN and MCN (n=65) had minimal reactivity (OD 0.019±0.034). Conversely, high-risk IPMN and MCN lesions (n=68) expressed a significantly higher amount of reactivity (OD 0.670±0.555) when compared with low-risk IPMN and MCN (p<0.0001) and non-mucinous PCL (p<0.0001) (Figure 2A). Plotting the overall sensitivity and specificity of mAb Das-1 for high-risk PCL as a continuous variable, AUC was 0.965 (0.935–0.994) (Figure 2B).
Figure 2. mAb Das-1 is highly sensitive and specific for high risk pancreatic cystic lesions.
(A) Cyst fluid immunoreactivity against mAb Das-1 by ELISA. Optical density values as determined by ELISA in high-risk intraductal papillary mucinous neoplasm (IPMN) (invasive IPMN, high grade dysplasia (HGD) of any epithelial subtype, and intermediate dysplasia of intestinal subtype) and mucinous cystic neoplasm (MCN) with HGD (n=68), low-risk IPMN and MCN (n=65), non-mucinous cystic neoplasm (serous cyst adenoma, pancreatic neuroendocrine tumors, pseudocysts, solid pseudopapillary neoplasm, retention cysts, gastrointestinal stromal tumor) (n=36). Bars indicate the mean and SD in each column. Reactivity of mAb Das-1 from fluid from high-risk IPMN and MCN was significantly higher than that from low-risk IPMN/MCN (p<0.0001) and non-mucinous cystic lesions (p<0.0001). (B) Receiver Operating Curve Analysis of mAb Das-1 for the identification of high-risk pancreatic cystic lesions. The area under the curve (AUC) was 0.965 which was highly significant (p<0.0001). Utilizing Youden’s statistic, an optimal binary cut point of 0.104 was selected and the sensitivity and specificity of Das-1 for segregating high-risk PCL from low-risk PCL was 88.2% and 99% respectively.
While low-risk IPMN (n=31) had an OD 0.025±0.042, non-invasive high-risk IPMN (n=41) had an OD 0.610±0.493 (p<0.0001), and IPMN with an invasive component (n=18) had an OD 0.680±0.555 (p<0.0001), demonstrating a progressive increase in reactivity to mAb Das-1. mAb Das-1 had a strong ability to segregate IPMN-G with LGD/IGD (n=31, OD 0.025±0.042), which represent the majority of indolent branch duct IPMN, from IPMN-G with HGD or invasive tubular carcinoma arising from IPMN-G (n=14, OD 0.636±0.486) (p<0.0001) or IPMN of any type with HGD or invasive carcinoma arising therefrom (n=50, OD 0.658±0.473) (p<0.0001). The reactivity of the biomarker with the various histologic subtypes and dysplastic grades of IPMN are displayed in Supplementary Table 3. Among MCN, lesions with LGD/IGD (n=34) were non-reactive (OD 0.014±0.023) in comparison to MCN with HGD (n=9, OD 0.924±0.825) (p<0.0001).
Evaluation of a cut-off for identification of high risk PCL by mAb Das-1
We have previously reported proposed preliminary OD cut offs in our initial descriptions of an ELISA for mAb Das-1 in IPMN and in a subsequent pilot abstract.15,25 Our initial report of a cut-off15 was based on the mean of only 9 samples of low-risk gastric-type IPMN and twice their standard deviation. Subsequently, improving our assay and utilizing a small, preliminary, previously reported cohort, we had initially estimated an optimal cut-off OD for high-risk lesions to be 0.120, based on maximization of sensitivity and specificity (data presented at DDW 2014).25 With this cut-off we reported a sensitivity of 84% and specificity of 100% for identifying high risk IPMN. However, this cohort did not include any non-mucinous cystic lesions, MCN, or other PCL and represented only a single center experience. Regardless, utilizing this cut-off, and the unique cases in the current study cohort (n=126) as a validation set, this previously suggested cut off (0.120) had a sensitivity and specificity of detecting high-risk lesions very similar to the prior report: 83.0% (0.679–0.928) and 100% (0.958–1.00) respectively.
Given our current much larger dataset (n=169), that encompass all PCL subtypes, a statistically valid, optimal cut-off was calculated by Youden’s index of ≥0.104. Utilizing this cut-off, the sensitivity and specificity for segregating high-risk PCL from low-risk PCL was 88.2% (0.781–0.948) and 99.0% (0.946–1.00), respectively. In our cohort of 169 patients there was only one case with an OD value between the 0.120 and 0.104 cut-offs – a patient with a high-risk MCN that had an OD value of 0.119. The small differences in sensitivity and specificity between the cut offs is due to this single case.
Utilizing a cut-off of 0.104, it should be noted, that there is only one patient who had a positive ELISA for mAb Das-1 (OD 0.149) with a low risk lesion. This patient had a 3cm PCL with a mural nodule with a gastric type IPMN with intermediate grade dysplasia on final surgical pathology. Similarly, there were 8 high-risk cases, non-reactive to mAb Das-1. Of these lesions, 2 had malignant cytology identified on pre-operative EUS and 5 presented with an MPD dilated >1cm or a focal mass on imaging.
When defining high-risk lesions strictly as those with HGD/Invasive component, mAb Das-1 continued to have strong diagnostic performance with a sensitivity and specificity of 88.3% (77.4–95.2) and 92.7% (0.860–0.968), respectively (Supplementary Table 4).
K fold validation of sensitivity and specificity
To estimate the performance of mAb Das-1 on an independent sample, we performed 10-fold cross validation with 100 replications. Cross-validated sensitivity and specificity were 88.0% (77.9%−94.5%) and 97.6% (92.5%−99.4%), respectively.
mAb Das-1 is superior to current clinical guidelines for identifying high-risk PCLs
We next compared the performance of mAb Das-1 to the available clinical guidelines7,29,30 (Table 2). The Sendai guidelines had an overall sensitivity, specificity and accuracy of 94.1% (85.6–98.4), 13.9% (7.79–22.2), and 46.2% (38.5–54.0), respectively for identifying high-risk lesions. In comparison, the Fukuoka Guidelines were significantly more accurate (p<0.012) with a sensitivity, specificity and accuracy of 97.1% (89.8–99.6), 20.8% (13.4–30.0), and 51.5% (43.7–59.2), respectively. The revised International Association of the Pancreas consensus guidelines from 2017 have few changes for the indications for surgery from the Fukuoka Guidelines, and thus the performance of these updated guidelines were identical to the Fukuoka guidelines.31 The AGA guidelines were significantly more accurate than the Sendai guidelines (p<0.001) as well as the Fukuoka guidelines (p<0.001), with a sensitivity, specificity and accuracy of 50.0% (37.6–62.4), 89.1% (81.4–94.4), and 73.7% (66.0–79.9), respectively. A validated composite clinical risk indicator27 was also significantly more accurate than the Sendai guidelines (p<0.003) (Supplementary Table 5). However, with a sensitivity, specificity, and accuracy of 88.2%, 99.0%, and 94.7% respectively, the performance of mAb Das-1 was significantly (p<0.001) more accurate than that of the Sendai, Fukuoka, or AGA guidelines, or the composite risk indicator (Table 2). The same was true when defining high-risk lesions strictly as those with a HGD/Invasive component (Supplementary Table 4).
Table 2 -.
Performance of Clinical Guidelines and mAb Das-1 in Segregating High Risk PCL
| Mucinous PCL (n=133) | All PCL (n=169) | |||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | Accuracy | Sensitivity | Specificity | Accuracy | |
| mAb Das-1 | 88.2% (78.1–94.8) Ref |
98.5% (91.7–100) Ref |
93.2% (87.5–96.9) Ref |
88.2% (78.1 – 94.8) Ref |
99% (94.6 – 100) Ref |
94.7% (90.1–97.5) Ref |
| Sendai Guidelines | 94.1% (85.6–98.4) p=0.3877 |
15.4% (7.63–26.5) p<0.0001 |
55.6% (46.8–64.3) p<0.001 |
94.1% (85.6 – 98.4) p=0.3877 |
13.9% (7.79–22.2) p<0.0001 |
46.2% (38.5–54.0) p<0.001 |
| Fukuoka Guidelines | 97.1% (89.8–99.6) p=0.1094 |
23.1% (13.5–35.2) p<0.0001 |
60.9% (52.1–69.2) p<0.001 |
97.1% (89.8–99.6) p=0.1094 |
20.8% (13.4–30.0) p<0.0001 |
51.5% (43.7–59.2) p<0.001 |
| AGA Guidelines | 50.0% (37.6–62.4) p<0.0001 |
93.8% (85–98.3) p=0.3750 |
71.4% (62.9–78.2) p<0.001 |
50.0% (37.6–62.4) p<0.0001 |
89.1% (81.4–94.4) p=0.0063 |
73.7% (66.0–79.9) p<0.001 |
Note: p-values reported in comparison to various guideline performance as compared to mAb Das-1. p-values calculated using exact paired-sample McNemar’s test of proportions
Evaluation of clinical risk factors associated with high risk PCL in association with mAb Das-1
In performing a univariate analysis to identify high-risk PCL in our cohort utilizing all of the component clinical indicators in the current guidelines, significant predictors included: mAb Das-1 (OR750 [91.5–6145.1]; p<0.0001), MPD dilation >5mm (OR13.0 [5.77–29.28]; p<0.0001) and ≥1cm (OR15.6 [4.45–54.9]; p<0.0001), solid component (OR4.07 [1.77–9.34]; p=0.0009), any symptoms (OR2.01 [1.08–3.76]; p=0.0280), jaundice (OR2.41 [1.11–5.23]; p=0.0260), and the composite risk indicator (OR3.81 [1.63–8.86]; p=0.0013) (Table 3).
Table 3 –
Univariate and Multivariate Analysis of m Ab Das-1 and Clinical Indicators for Predicting High Risk PCL
| Univariate Analysis | Multivariate Analysis Controlling for Das-1 | |||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | OR (95% CI) | p Value | aOR (95% CI) | p Value | |
| mAb Das-1 | 88.2% (78.1–94.8) |
99.0% (94.6–100) |
750 (91.5–6145.1) |
p<0.0001 | --- | --- |
| Cyst Size ≥3cm | 65.7% (53.1–76.8) |
28.7% (20.1–38.6) |
0.738 (0.382–1.43) |
p=0.3665 | 0.597 (0.116–3.33) |
p=0.7001 |
| Cyst Size ≥4cm | 47.1% (34.8–59.6) |
47.5% (37.5–57.7) |
0.805 (0.435–1.49) |
p=0.4900 | 1.76 (0.358–11.4) |
p=0.6624 |
| MPD >5mm | 52.9% (40.4–65.2) |
90.1% (82.5–95.1) |
13.0 (5.77 – 29.28) |
p<0.0001 | 14.98 (2.63–108) |
p=0.0012 |
| MPD ≥1cm | 32.4% (21.5–44.8) |
97% (91.6–99.4) |
15.6 (4.45–54.9) |
p<0.0001 | 47.9 (6.39–490) |
p<0.0001 |
| Mural Nodule | 20.6% (11.7–32.1) |
88.0% (80.0–93.6) |
1.92 (0.829 – 4.46) |
p=0.1280 | 1.28 (0.084–9.76) |
p=1.0000 |
| Solid Component | 30.9% (20.2–43.3) |
90.0% (82.4–95.1) |
4.07 (1.77–9.34) |
p=0.0009 | 3.24 (0.384–20.3) |
p=0.3258 |
| Multifocal | 28.8% (17.8–42.1) |
87.1% (79–93) |
2.26 (1.01–5.02) |
p=0.0463 | 2.41 (0.290–14.6) |
p=0.5022 |
| Symptoms | 55.9% (43.3–67.9) |
61.2% (50.8–70.9) |
2.01 (1.08 – 3.76) |
p=0.0280 | 5.25 (0.946–53.9) |
p=0.0604 |
| Weight Loss | 5.97% (1.65–14.6) |
95.0% (88.8–98.4) |
1.20 (0.310–4.64) |
p=0.7916 | 6.16 (0.51–49.3) |
p=0.1628 |
| Abdominal Pain | 32.8% (21.8–45.4) |
72.3% (62.5–80.7) |
1.25 (0.639–2.44) |
p=0.5182 | 1.06 (0.154–5.62) |
p=1.0000 |
| Jaundice | 27.9% (17.7–40.1) |
85.9% (77.4–92.0) |
2.41 (1.11–5.23) |
p=0.0260 | 6.16 (1.08–36.7) |
p=0.0397 |
| Pancreatitis | 10.3% (4.24–20.1%) |
100.0% (96.3–100.0) |
- - | p=0.9752 | -- | -- |
| Composite Risk (jaundice or MPD dilation or cyst size ≥4cm) | 88.2% (78.1–94.8) |
34.7% (25.5–44.8) |
3.81 (1.63–8.86) |
p=0.0019 | -- | -- |
We then examined the associations between clinical risk factors and high-risk PCL after controlling for mAb Das-1 reactivity. Using exact logistic regression, with only two independent variables (the clinical variable of interest and mAb Das-1), MPD dilation >5mm (OR14.98 [2.63–108]; p<0.0012) and ≥1cm (OR47.9 [6.39–490]; p<0.0001) and jaundice (OR6.16 [1.08–36.7]; p=0.0397) were still significantly associated with high risk even after controlling for mAb Das-1 reactivity. Given the highly sensitive and specific nature of mAb Das-1 in being able to detect high risk lesions with only 9 misclassifications in our cohort of 169 patients, a statistically valid multivariate model or the creation of a risk prediction model was not feasible.
DISCUSSION
PCL are very frequently, incidentally identified in patients,4,5 without clinical guidelines or biomarkers that can reliably identify lesions that necessitate definitive management. While data from long-term cohorts of PCL have identified a small, but ongoing risk to the development of carcinoma,32,33 this must be balanced against the increasing data demonstrating low yield, and high potential morbidity of surgical intervention in elderly patients with non-worrisome PCL.8,34 In our present study, we demonstrate that mAb Das-1 reliably identifies high-risk PCL lesions in a large, pathologically verified, multicenter cohort of patients. With a sensitivity of 88.2%, specificity of 99%, and overall accuracy of 94.7%, the biomarker was significantly more accurate than currently available guidelines (p<0.001).
There are several limitations of our study. Our study cohort is retrospectively collected, prospectively banked, surgical specimens, from large, tertiary care centers, which introduces surgical selection, referral, and treatment access biases. While several validation and exploration studies in PCL biomarkers have reported larger non-pathologically verified cohorts, few have demonstrated a similarly sized and powered surgical resection cohort as the one utilized herein from four, high-volume, geographically diverse centers.35 Without a prospective cohort of patients followed, it is impossible to assess the malignant potential of a PCL within a patient’s lifetime, however evaluation against a gold standard of blinded, pathology review is ultimately superior to surrogate end points and assumptions of indolence based on clinical/radiographic follow-up. As such, this study does not address the optimal approach of integrating mAb Das-1 into PCL surveillance programs. We are currently studying the utilization of this marker on a prospective basis to validate its use in this fashion. With multiple promising biomarkers becoming available to risk stratify PCL, further studies are currently ongoing, to prospectively validate these various markers against one another, in the same cohorts.
While the specific identity of the antigen that mAb Das-1 is reactive to currently remains under investigation, limited by its large molecular weight (>200kDa) and heavy glycosylation, previous examination of the biomarker in fetal tissues has demonstrated expression of the Das-1 antigen in organs arising from the primitive gut (oropharynx, lung, esophagus, stomach, biliary tree, pancreas, liver, intestine).36 Several investigators have demonstrated that while Das-1 is expressed in the fetal esophagus, stomach, small bowel, and pancreas, it is lost in the respective adult organs, and reappears in precancerous and cancerous conditions like Barrett’s esophagus/esophageal adenocarcinoma,14,37 incomplete-type gastric intestinal metaplasia/gastric adenocarcinoma,18,38 small intestinal adenomas/adenocarcinoma,19 and PanIN/pancreatic adenocarcinoma.20 This pattern of expression, loss, and reemergence appears to suggest its role as an oncofetal marker. Previous in vitro experiments have demonstrated that the Das-1 antigen is externalized and released from cells into the culture medium,39 which may explain the intense staining of extracellular mucin we have observed in colloid carcinomas previously15 and abundant presence in cyst fluid here. It is also promising that even in acellular cyst fluid aspirates, mAb Das-1 is still readily assayable. Ultimately, the presence of Das-1 in fetal pancreatic tissue, loss in normal pancreas and pancreatitis,15 and re-emergence in dysplastic PCL suggests that identification of this antigen may both help advance our knowledge of pathophysiological mechanisms of malignant transformation and improve our diagnostic capacity for these lesions.
IPMN progress from LGD and IGD to HGD (carcinoma in situ) and invasive carcinoma. We have previously demonstrated in a cohort of 94 patients with resected IPMN that by immunohistochemistry, mAb Das-1 expression was preferentially expressed in higher-grade lesions.15 We confirmed in our present study our initial observation that mAb Das-1 is minimally reactive to IPMN-G with LGD/IGD (3% [1/31]), that most frequently represent indolent, branch duct lesions. In addition, among the 43 MCN in the current cohort, mAb Das-1 had a 100% sensitivity and specificity for identifying MCN harboring HGD. While guidelines typically recommend the resection of all MCN lesions, the ability to reliably assess the development of dysplasia in MCN prospectively may improve the timing of surgical intervention and avoid morbid interventions in those with significant comorbidities. Of the 8 high-risk lesions that were non-reactive with mAb Das-1, there was no clear bias to histologic subtype, and of these cases, 2 had pre-operative EUS FNA with positive cytology and 5 of the lesions had a MPD >1cm or a focal mass on imaging. Thus, in practice, 7/8 of these would have likely been referred to resection on clinical grounds alone given the very high specificity of positive cytology and severe MPD dilation for high-risk lesions (cytology >90%,40 MPD≥1cm – 97%).
Classically, CEA has been utilized to discriminate mucinous from non-mucinous PCL, traditionally with a cut off of 192ng/mL28 though there has been considerable controversy regarding an optimal cut-off value with only moderate reported accuracy.41,42 In our available cohort, CEA was only 50% sensitive for identifying mucinous lesions. Other techniques including cyst fluid glucose43 and combinations of clinical indicators27 have been utilized to distinguish mucinous from non-mucinous lesions. While specific genetic alterations identified with next generation sequencing (NGS) can subtype PCL with moderate accuracy,26,44,45 mAb Das-1 is completely non-reactive with the entire gamut of non-mucinous PCL by ELISA (Figure 2).
Given the high prevalence of PCL in clinical practice, clinical guidelines have been adopted7,29,30 to aid clinicians in attempting to risk stratify patients in the absence of adequate biomarkers. Validation cohorts have proven these to be either inadequate in their sensitivity or specificity, especially among branch duct lesions.9–11 While these guidelines are not meant to be applied retroactively to surgical series given the inherent biases associated with this, ideally a clinical risk model would have reasonable accuracy even in these cohorts. In our cohort of 169 resected PCL, as expected, while the Sendai guideline had a high sensitivity (94.1%), it was non-specific (13.9%) for high-risk lesions, which was improved upon in the Fukuoka guidelines (specificity 20.8%) without sacrificing sensitivity. Given their similarity, the performance of the 2012 Fukuoka guidelines and the 2017 IAP updated guidelines in identifying high-risk lesions was identical. The AGA guidelines (AUC 0.696) were considerably more specific (89.1%), but at the cost of reduced sensitivity (50.0%). Overall, the AGA & Fukukoa guidelines were significantly more accurate than the Sendai guidelines and the AGA guidelines were more accurate than the Fukuoka guidelines (Table 2). In comparison, mAb Das-1 reactivity was significantly more accurate than any of the available guidelines (p<0.001). Utilizing univariate regression modeling, several of the same clinical factors that are constituent elements of the current clinical guidelines remained significant predictors of risk (MPD dilation, MPD involvement, solid component, symptoms, jaundice). While several of these traditional “high-risk” clinical features like dilated MPD >1cm, mural nodule, solid component, pancreatitis, jaundice, or weight loss were highly specific (97.0%, 88.0%, 90.0%, 100.0%, 85.9%, 95.0%, respectively) they were highly insensitive (32.4%, 20.6%, 30.9%, 10.3%, 27.9%, 5.97%). Therefore, their presence clinically should not be discounted, but they are insufficient to identify all high-risk lesions. Multivariate regression modeling controlling for mAb Das-1 and these clinical indicators demonstrated the presence of MPD dilation or jaundice to still be significantly associated with high-risk PCL, independent of mAb Das-1 reactivity. Interestingly, in examining patients without any high-risk features (no dilated pancreatic duct >1cm, weight loss, jaundice, pancreatitis, mural nodule, or solid component (n=84)), the sensitivity, specificity, and AUC of mAb Das-1 is 94.7% (74.0–99.9), 100.0% (94.5–100.0), and 0.97 (0.92–1.00). Therefore, among these lesions without obvious clinical “high-risk” features, mAb Das-1 may be of particular benefit.
There have been several studies attempting to identify new biomarkers for high-risk PCLs, including microRNAs, NGS, and telomerase activity.45–48 Singhi et al reported a panel of NGS targets with specific thresholds of mean allele frequency (MAF) that demonstrate high accuracy for advanced neoplasia.44 Similarly, targeted mass spectrometry analysis of cyst fluid identified mucin-5AC and mucin-2 has been reported as accurate in identifying advanced neoplasia.49 While these are promising initial studies, their results need to be confirmed by multi-center validation cohorts. In this study, we conducted multi-center validation on mAb Das-1 as a biomarker for high-risk PCL. As mAb Das-1 is non-reactive to normal gastric mucosa and duodenal mucosa,16 it is not susceptible to contamination that can effect cytology interpretation or even MAF in NGS. Also, in comparison to the technical expertise required for NGS or targeted mass spectrometry, ELISA is simple, highly reproducible, and inexpensive. Indeed, the non-commercialized cost of performing the assay for an entire plate (40 samples) is $50–100. In addition, we found that the vast majority (81%) could be analyzed with as little as 125µl of cyst fluid, and 94% could be completed with 500µl. As the ELISA assay is further refined, likely smaller volumes of fluid will be required.
In conclusion, mAb Das-1 is a sensitive and highly specific biomarker for the detection of high-risk and malignant PCL. The inclusion of the Das-1 marker into the analysis of cyst fluid may aid in the preoperative diagnosis and risk stratification of patients with PCL.
Supplementary Material
What You Need to Know.
BACKGROUND AND CONTEXT:
Pancreatic cystic lesions (PCLs) are frequently found but there are no biomarkers to identify those at high risk for malignancy. We aimed to validate the ability of the monoclonal antibody Das-1 to identify high-risk lesions, in comparison to criteria from clinical guidelines and clinical features, using cyst fluid from 169 PCLs.
NEW FINDINGS:
In an analysis of cyst fluid, the monoclonal antibody Das-1 identified high-risk PCLs with 88% sensitivity and 99% specificity; this was a higher level of accuracy than criteria from clinical guidelines.
LIMITATIONS:
This was a retrospective study of surgical samples. Prospective studies are needed.
IMPACT:
An ELISA with Das-1 might be used to analyze fluid from pancreatic cysts and determine their risk for malignancy.
Acknowledgements
We would like to thank Dr. Timothy Heeren, Professor of Biostatistics at the Boston University School of Public Health for his invaluable assistance in the statistical analysis and preparation of this manuscript.
Grant Support:
This work was supported by the American Society for Gastrointestinal Endoscopy (KKD), National Pancreas Foundation (KMD), National Institutes of Health NIH/NIDDK T32DK007130–41 and DDRCC Pilot & Feasibility Grant as part of P30 DK052574 (JWB), NIH/NCI P50CA196510–01A1 Washington University SPORE in Pancreatic Cancer, and NIH/NIDDK P30 DK052574 Digestive Diseases Research Core Centers. Development of mAb Das-1 was supported in part by research grants NIDDK, R01 DK47673 and R01 DK63618 to KMD.
Abbreviations:
- PCL
Pancreatic Cystic Lesions
- IPMN
Intraductal Papillary Mucinous Neoplasm
- MCN
Mucinous Cystic Neoplasm
- SCA
Serous Cyst Adenoma
- SPN
Solid Pseudopapillary Neoplasm
- NET
Neuroendocrine Tumor
- OD
Optical Density
- mAb
Monoclonal Antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentations: Data from this study was presented in part at the American Gastroenterological Association, Digestive Disease Week 2017 on 5/8/2017 in Chicago, IL.
Writing Assistance: None.
Conflict of Interest Statement: A patent for the use of mAb Das-1 in the detection of cancerous lesions of pancreas has been granted (KKD, MMK, KMD). This patent has not been licensed and these authors hold no commercial interests at this time. No other authors have any conflicts of interest to disclose.
Writing Assistance: None.
Author names in bold designate shared co-first authorship
REFERENCES
- 1.Crippa S, del Castillo CF, Salvia R, et al. Mucin-Producing Neoplasms of the Pancreas: An Analysis of Distinguishing Clinical and Epidemiologic Characteristics. YJCGH 2010;8:213–219.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mino-Kenudson M, Fernandez-del Castillo C, Baba Y, et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut 2011;60:1712–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castillo CF, Adsay NV. Intraductal Papillary Mucinous Neoplasms of the Pancreas. Gastroenterology 2010;139:708–713.e2. [DOI] [PubMed] [Google Scholar]
- 4.Laffan TA, Horton KM, Klein AP, et al. Prevalence of Unsuspected Pancreatic Cysts on MDCT. American Journal of Roentgenology 2008;191:802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Oliveira PB, Puchnick A, Szejnfeld J, et al. Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS ONE 2015;10:e0121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrell JJ, Brugge WR. Intraductal papillary mucinous tumor of the pancreas. Gastrointest. Endosc 2002;55:701–714. [DOI] [PubMed] [Google Scholar]
- 7.Vege SS, Ziring B, Jain R, et al. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Asymptomatic Neoplastic Pancreatic Cysts. Gastroenterology 2015;148:819–822. [DOI] [PubMed] [Google Scholar]
- 8.Crippa S, Bassi C, Salvia R, et al. Low progression of intraductal papillary mucinous neoplasms with worrisome features and high-risk stigmata undergoing non-operative management: a mid-term follow-up analysis. Gut 2017;66:495–506. [DOI] [PubMed] [Google Scholar]
- 9.Xu M-M, Yin S, Siddiqui AA, et al. Comparison of the diagnostic accuracy of three current guidelines for the evaluation of asymptomatic pancreatic cystic neoplasms. Medicine 2017;96:e7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma GK, Goldberg DS, Thiruvengadam N, et al. Comparing American Gastroenterological Association Pancreatic Cyst Management Guidelines with Fukuoka Consensus Guidelines as Predictors of Advanced Neoplasia in Patients with Suspected Pancreatic Cystic Neoplasms. Journal of the American College of Surgeons 2016;223:729–737.e1. [DOI] [PubMed] [Google Scholar]
- 11.Tang R, Weinberg B, Dawson D, et al. Evaluation of the Guidelines for Management of Pancreatic Branch-Duct Intraductal Papillary Mucinous Neoplasm. Clinical Gastroenterology and Hepatology 2008;6:815–819. [DOI] [PubMed] [Google Scholar]
- 12.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N. Engl. J. Med 2004;351:1218–1226. [DOI] [PubMed] [Google Scholar]
- 13.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am. J. Surg. Pathol 2001;25:26–42. [DOI] [PubMed] [Google Scholar]
- 14.Das KM, Prasad I, Garla S, et al. Detection of a shared colon epithelial epitope on Barrett epithelium by a novel monoclonal antibody. Ann. Intern. Med 1994;120:753–756. [DOI] [PubMed] [Google Scholar]
- 15.Das KK, Xiao H, Geng X, et al. mAb Das-1 is specific for high-risk and malignant intraductal papillary mucinous neoplasm (IPMN). Gut 2014;63:1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das KM, Sakamaki S, Vecchi M, et al. The production and characterization of monoclonal antibodies to a human colonic antigen associated with ulcerative colitis: cellular localization of the antigen by using the monoclonal antibody. J. Immunol 1987;139:77–84. [PubMed] [Google Scholar]
- 17.Halstensen TS, Das KM, Brandtzaeg P. Epithelial deposits of immunoglobulin G1 and activated complement colocalise with the M(r) 40 kD putative autoantigen in ulcerative colitis. Gut 1993;34:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirza ZK, Das KK, Slate J, et al. Gastric intestinal metaplasia as detected by a monoclonal antibody is highly associated with gastric adenocarcinoma. Gut 2003;52:807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onuma EK, Amenta PS, Jukkola AF, et al. A phenotypic change of small intestinal epithelium to colonocytes in small intestinal adenomas and adenocarcinomas. Am. J. Gastroenterol 2001;96:2480–2485. [DOI] [PubMed] [Google Scholar]
- 20.Das KK, Das KM, Mino Kenudson M. MAb DAS-1, a Monoclonal Antibody Reactive Against a Colonic Phenotype, Identifies Pancreatic Adenocarcinoma and High-Grade Pancreatic Intraepithelial Neoplasm (PanIN) With High Specificity. Gastroenterology 2011;140:S–341. [Google Scholar]
- 21.Basturk O, Hong S-M, Wood LD, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas In: Vol 39 2015:1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furukawa T, Klöppel G, Volkan Adsay N, et al. C lassification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch 2005;447:794–799. [DOI] [PubMed] [Google Scholar]
- 23.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an” intestinal” pathway of carcinogenesis in the pancreas. Am. J. Surg. Pathol 2004;28:839. [DOI] [PubMed] [Google Scholar]
- 24.Morales-Oyarvide V, Mino Kenudson M, Ferrone CR, et al. Acute pancreatitis in intraductal papillary mucinous neoplasms: A common predictor of malignant intestinal subtype. Surgery 2015;158:1219–1225. [DOI] [PubMed] [Google Scholar]
- 25.Das KK, Marchegiani G, Geng X, et al. Comparison of the International Consensus Guidelines for Management of Intraductal Papillary Mucinous Neoplasm (IPMN) With Analysis of Pancreatic Cyst Fluid Aspirates for mAb-Das-1 Reactivity in Identifying High-Risk and Malignant IPMN. Gastroenterology 2014;146:S-25-25. [Google Scholar]
- 26.Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015;149:1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masica DL, Dal Molin M, Wolfgang CL, et al. A novel approach for selecting combination clinical markers of pathology applied to a large retrospective cohort of surgically resected pancreatic cysts. J Am Med Inform Assoc 2016;24:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004;126:1330–1336. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka M, Chari S, Adsay V, et al. International Consensus Guidelines for Management of Intraductal Papillary Mucinous Neoplasms and Mucinous Cystic Neoplasms of the Pancreas. Pancreatology 2006;6:17–32. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka M, Fernández Del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas In: Vol 12 2012:183–197. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka M, Castillo CF-D, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017:1–16. [DOI] [PubMed] [Google Scholar]
- 32.Tanno S, Nakano Y, Sugiyama Y, et al. Incidence of synchronous and metachronous pancreatic carcinoma in 168 patients with branch duct intraductal papillary mucinous neoplasm. Pancreatology 2010;10:173–178. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence SA, Attiyeh MA, Seier K, et al. Should Patients With Cystic Lesions of the Pancreas Undergo Long-term Radiographic Surveillance?: Results of 3024 Patients Evaluated at a Single Institution. Annals of Surgery 2017;266:536–544. [DOI] [PubMed] [Google Scholar]
- 34.Kwok K, Chang J, Duan L, et al. Competing Risks for Mortality in Patients With Asymptomatic Pancreatic Cystic Neoplasms: Implications for Clinical Management. Am. J. Gastroenterol 2017;112:1330–1336. [DOI] [PubMed] [Google Scholar]
- 35.Efishat Al MA, Attiyeh MF, Eaton AA, et al. Multi-Institutional Validation Study of Pancreatic Cyst Fluid Protein Analysis for Prediction of High-Risk Intraductal Papillary Mucinous Neoplasms of the Pancreas. Annals of Surgery 2017:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badve S, Lôgdberg L, Sokhi R, et al. An antigen reacting with das-1 monoclonal antibody is ontogenically regulated in diverse organs including liver and indicates sharing of developmental mechanisms among cell lineages. Pathobiology 2000;68:76–86. [DOI] [PubMed] [Google Scholar]
- 37.Piazuelo MB, Haque S, Delgado A, et al. Phenotypic differences between esophageal and gastric intestinal metaplasia. Mod Pathol 2003;17:62–74. [DOI] [PubMed] [Google Scholar]
- 38.Watari J, Das KK, Amenta PS, et al. Effect of eradication of Helicobacter pylori on the histology and cellular phenotype of gastric intestinal metaplasia. Clin. Gastroenterol. Hepatol 2008;6:409–417. [DOI] [PubMed] [Google Scholar]
- 39.Kesari KV, Yoshizaki N, Geng X, et al. Externalization of tropomyosin isoform 5 in colon epithelial cells. Clin Exp Immunol 1999;118:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Genevay M, Mino Kenudson M, Yaeger K, et al. Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Annals of Surgery 2011;254:977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frossard J Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am. J. Gastroenterol 2003;98:1516–1524. [DOI] [PubMed] [Google Scholar]
- 42.Jin DX, Small AJ, of CVJJ, et al. A lower cyst fluid CEA cut-off increases diagnostic accuracy in identifying mucinous pancreatic cystic lesions. JOP 2015;16:271–277. [Google Scholar]
- 43.Zikos T, Pham K, Bowen R, et al. Cyst Fluid Glucose is Rapidly Feasible and Accurate in Diagnosing Mucinous Pancreatic Cysts. Am. J. Gastroenterol 2015;110:909–914. [DOI] [PubMed] [Google Scholar]
- 44.Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2017:gutjnl-2016-313586-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones M, Zheng Z, Wang J, et al. Impact of next-generation sequencing on the clinical diagnosis of pancreatic cysts. Gastrointest. Endosc 2016;83:140–148. [DOI] [PubMed] [Google Scholar]
- 46.Farrell JJ, Toste P, Wu N, et al. Endoscopically Acquired Pancreatic Cyst Fluid MicroRNA 21 and 221 Are Associated With Invasive Cancer. Am. J. Gastroenterol 2013;108:1352. [DOI] [PubMed] [Google Scholar]
- 47.Kanda M, Knight S, Topazian M, et al. Mutant GNAS detected in duodenal collections of secretin-stimulated pancreatic juice indicates the presence or emergence of pancreatic cysts. Gut 2013;62:1024–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hata T, Molin MD, Suenaga M, et al. Cyst Fluid Telomerase Activity Predicts the Histologic Grade of Cystic Neoplasms of the Pancreas. Clin. Cancer Res 2016;22:5141–5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jabbar KS, Arike L, Verbeke CS, et al. Highly Accurate Identification of Cystic Precursor Lesions of Pancreatic Cancer Through Targeted Mass Spectrometry: A Phase IIc Diagnostic Study. Journal of Clinical Oncology 2017:JCO2017737288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.