Abstract
Acute stress is generally thought to impair performance on tasks thought to rely on selective attention. This effect has been well established for moderate to severe stressors, but no study has examined how a mild stressor—the most common type of stressor—influences selective attention. In addition, no study to date has examined how stress influences the component processes involved in overall selective attention task performance, such as controlled attention, automatic attentional activation, decision-making, and motor abilities. To address these issues, we randomly assigned 107 participants to a mild acute stress or control condition. As expected, the mild acute stress condition showed a small but significant increase in cortisol relative to the control condition. Following the stressor, we assessed attention with two separate flanker tasks. One of these tasks was optimized to investigate component attentional processes using computational cognitive modeling, whereas the other task employed mouse-tracking to illustrate how response conflict unfolded over time. The results for both tasks showed that mild acute stress decreased response time (i.e., increased response speed) without influencing accuracy or interference control. Further, computational modeling and mouse-tracking analyses indicated that these effects were due to faster motor action execution time for chosen actions. Intriguingly, however, cortisol responses were unrelated to any of the observed effects of mild stress. These results have implications for theories of stress and cognition, and highlight the importance of considering motor processes in understanding the effects of stress on cognitive task performance.
Keywords: acute stress, attention, reaction time, response speed, stress severity, motor control
1. Introduction
The narrative is so common that it borders on public knowledge: Stress forcefully narrows your attention onto what is stressing you out at the expense of other things in your surroundings. That elegant narrative reflects the synthesis of numerous studies examining effects of stress on attention (Joëls et al., 2006; LeBlanc et al., 2015; Mather and Sutherland, 2011; Sänger et al., 2014; Shields et al., 2016; Wiemers et al., 2013). However, a limitation of this work is that it has been restricted to examining relatively severe acute stressors, whereas the stressors we experience most commonly and most frequently are relatively mild (Almeida et al., 2002). Importantly, not all stressors influence cognitive processes in the same ways (Hupbach and Fieman, 2012; Shields et al., 2017; Shields and Yonelinas, 2018), and there is some evidence that stress may influence some cognitive abilities in an in an inverted-U shape function, with mild stress at or near the peak of the inverted-U (Amsten, 2009). Therefore, it is unclear whether the stressors we are most likely to encounter impair or enhance selective attention. In the current study we addressed this issue by examining how a mild acute stressor influences performance on two implementations of a selective attention task.
Mild stress is typically defined in human acute stress literature as a stressor that produces only a small cortisol response (e.g., Buchanan and Tranel, 2008; Kunz-Ebrecht et al., 2003; van Stegeren et al., 2008), For clarity, we define a mild acute stressor as a stressor that, on average, produces an increase in cortisol relative to not having undergone that stressor (i.e., relative to a control condition) but an increase less than 1.5-fold. We adopt such a definition because laboratory inductions of moderate stress produce a two- to threefold increase in cortisol (e.g., Oei et al., 2006; PreuB and Wolf, 2009; Schoofs et al., 2008; Shields et al., 2017b). Although some studies have examined the effect of mild stress on some cognitive processes (e.g., Gagnon et al., 2018), the effects of mild stress on most cognitive processes are relatively unknown.
One straightforward route to attenuating the stress of more acute manipulations is to provide concurrent social support, which robustly diminishes biological responses to and self-reported distress following a stressor (Häusser et al., 2012; Hostinar, 2015). Notably, the stress-diminutive effects of social support are present even among strangers, as long as those strangers feel part of a group while undergoing a stressor (Häusser et al., 2012). Based on this literature, we sought to use one of the most commonly used stress manipulations—the cold pressor (Shields et al., 2017)—in concert with social support in the form of peers experiencing it together.
In a different vein, when considering the effects of stress on cognition, it is important to move beyond broad behavioral measures of performance to measuring the cognitive construct of interest. No performance task designed to assess cognitive function is process pure. That is, performance on cognitive tasks is the result of both neurocognitive processes relevant to the construct of interest as well as processes that are irrelevant to the construct of interest (Calanchini et al., 2018). For example, performance on the flanker task (i.e., a selective attention task wherein participants respond to a central stimulus and ignore flanking stimuli) is influenced by the extent to which goal-irrelevant stimuli capture attention, how well a person can suppress irrelevant information that has been attended to, and decisional and motor processes (Ulrich et al., 2015). Generally, broad behavioral outcomes such as reaction time cannot distinguish between simultaneous contributions of multiple neurocognitive processes, and therefore, these measures can sometimes only crudely approximate the cognitive construct of interest (Farrell and Lewandowsky, 2018). In this manuscript, we draw on two methods from cognitive science that facilitate more precise cognitive assessment: computational cognitive modeling and mouse-tracking.
Computational modeling and mouse-tracking both also offer another insight into the effects of stress that has been largely unstudied in stress and cognition work: the ability to quantify the effects of stress on decision and motor processes involved in cognitive task performance (Freeman et al., 2011; Ulrich et al., 2015). Although stress influences both decision and motor processes (Metz et al., 2005; Starcke and Brand, 2012), to date no work has considered the role of these processes in understanding the effects of stress on selective attention.
1.1. Current Research
The present study sought to address the issues outlined above by examining the effects of a mild stressor on selective attention. Participants were first randomly assigned to a mild stress induction or a control task. Saliva samples were taken pre- and post-manipulation to assess the success of our mild stress manipulation via cortisol assays. Selective attention was then assessed with two separate implementations of the flanker task. First, the classic flanker task, which enabled us to use computational cognitive modeling in order to determine which component processes were affected by the mild stressor. Second, a mouse-tracking flanker, which served both as a within-study replication of any effects observed in the classic flanker task and to provide continuous motor data on flanker task trials, allowed us to determine how effects of the mild stressor on selective attention develop over time. Because moderate-to-severe stress impairs selective attention, we expected the mild stressor to impair performance on the flanker, and to do so by impairing controlled attention rather than increasing automatic attentional activation, though we expected stress to alter motor and decisional processes also involved in task performance.
2. Method
2.1. Participants
107 (79 female) young adults (Mage=21.50, SDage=339, range: 18–46) attending UC Davis participated in this study. A sample size of 50 participants per group was chosen because it provided 80% power to detect a moderate effect (d=0.50) in an expected direction. Participants were randomly assigned to the mild acute stress induction (n=50; 37 female) or control condition (n=57; 42 female). Due to random chance (i.e., no significant difference between stress and control conditions,χ2(l)=0.46, p=499), fewer participants came to stress induction timeslots than control timeslots, and to ensure no differences in condition constituency by time of quarter, we continued running control participant timeslots until the stress induction condition reached 50 participants. Excluding the last seven control participants or randomly sampling seven control participants to exclude produced identical results to keeping the full sample, so we retained the full sample. The sample was fairly racially/ethnically diverse; 44.9% identified as Asian, 28.0% White, 22.4% Hispanic, 3.7% Black/African American, and 0.9% as Native Hawaiian/Pacific Islander.
2.2. Materials
2.2.1. Stress manipulation.
Participants in the stress condition completed a modified cold pressor test (Hines and Brown, 1932). An experimenter wearing casual clothing (i.e., not a laboratory coat) instructed participants to submerge their arm in ice water (M=2.37 °C) up to the wrist joint without making a fist for 3min. They were further reminded immediately before the stressor that they had the option to discontinue their study participation at any time—no participants opted to discontinue. Participants in each timeslot were seated next to each other during the stressor and could interact with each other during that time (Häusser et al., 2012). These modifications functioned to reduce the stressfulness of the cold pressor (see Häusser et al., 2012).
Participants in the control condition were instructed as a group by an experimenter wearing normal clothing that they were required to submerge their arm in lukewarm water (M=21.84°C) up to the wrist joint without making a fist for 3min. They were also reminded that they had the option to discontinue their study participation at any time. Participants in each timeslot in the control condition were also seated next to each other during the control task and could interact with each other during that time.
2.2.2. Cortisol.
Participants provided saliva samples via passive drool, which were immediately placed in a −20°C freezer until being assayed. Cortisol assays were conducted by G.S.S. and M.M.R. at the Behavioral Neuroendocrinology Lab at UC Davis using high-sensitivity ELISA kits from Salimetrics. Conditions were counterbalanced across plates; each plate contained an approximately equal number of timepoints from participants in the stress and control conditions. Intra-assay CV was 5.20% (range: 2.82–7.70%); inter-assay CV was 7.21%. All controls were in the expected ranges. As say sensitivity was <0.007μg/dL. Values are given in nmol/L.
2.2.3. Negative affect.
To verify the manipulation, current negative affect was assessed using the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). Participants were asked to report the extent to which they currently felt 10 negative and 10 positive emotions (20 items total). Responses to each item were provided on a 1 (Very slightly or not at all) to 5 (Extremely) scale, and responses to the 10 questions assessing negative affect were then averaged to create an overall index of negative affect, with higher scores indicating more negative affect. Internal consistency for the scale was good both pre- and post-manipulation, αs≥.85.
2.2.4. Classic flanker task.
Selective attention was assessed in part by completion of the Eriksen flanker task, coded by G.S.S. in PEBL (Mueller and Piper, 2014). The task began with a series of instruction screens informing participants to report the direction of the center arrow using the left or right arrow key on a keyboard. The instructions further stated that the center arrow would be flanked by two arrows on both sides pointing in the same direction (congruent trials), two arrows on both sides pointing in a different direction (incongruent trials), or nothing (neutral trials), and that any flanking arrows should be ignored. The instructions further stated to respond as quickly and as accurately as possible. After the instructions, 20 practice trials were presented and accuracy feedback was given for 400ms after responses. The test phase began after a brief reminder of instructions; no accuracy feedback was given during the test phase. The test phase consisted of 520 trials divided into four blocks (130 trials per block). This number of trials is sufficient for good to excellent parameter recovery for the Diffusion Model for Conflict Tasks (White et al., 2018). Each block contained 52 congruent trials, 52 incongruent trials, and 26 neutral trials, for a total of 208 congruent trials, 208 incongruent trials, and 104 neutral trials. The center arrow pointed left in half the trials of each type (i.e., congruent, incongruent, and neutral) and right in the other half of the trials of each type. After each block of trials, participants reached a screen that displayed their progress, were told to take as long of a break as they wanted, and to press “c” to continue when ready.
In each trial, a fixation cross was presented in the center of the screen for 500ms and was subsequently replaced by the target and flanking stimuli (if not a neutral trial) until a response was provided or 2000ms had elapsed. If the response to a trial took longer than 800ms, “try to respond faster” was displayed during the intertrial interval (ITI); if the response to a trial occurred within 800ms, no additional text was displayed during the ITI. The ITI lasted for 500ms.
2.2.5. MouseTracker flanker task.
Participants completed the second flanker task within MouseTracker, which is an extensively validated mouse-tracking program (Freeman and Ambady, 2010). To our knowledge, this is the first study to implement a mouse-tracker flanker task, but numerous mouse-tracking studies have implemented similar tasks (e.g., Hermens, 2018).
Participants received instructions asking them to “quickly and accurately” respond to the letter (either “A” or “B”) appearing in the center of a string of 5 letters. Letters were used rather than arrows to help distinguish this task from the traditional flanker task, helping to ensure that any converging results were not due to stimulus processing or familiarity. Following instructions, participants completed 8 practice trials and then a single test block of 48 trials for a total of 56 trials (though only the 48 test trials were analyzed). Half of the trials (i.e., 4 practice and 24 test trials) consisted of congruent stimulus arrays—where both flanker and target letters converged on the same response (e.g., “AAAAA”). The other half of trials (i.e., 4 practice and 24 test trials) consisted of incongruent stimulus arrays—where flanker and target letters were associated with different responses (e.g., “BBABB”). Letter stimuli were presented in 44px Times New Roman font.
To begin each trial, participants clicked a box at the bottom-center of the screen. The flanker stimulus array appeared immediately upon clicking start and was centered both horizontally and vertically. To indicate their response, participants had to click a box located in the upper-left or upper-right comer of the screen. For half of participants, response choice “A” was located in the upper left and “B” was located in the upper right of the screen, and this response mapping was counterbalanced between-participants.
Participants were required to respond within 1500ms of stimulus onset and were told to “Please respond faster!” if they exceeded this limit. Additionally, initiation of mouse movement was required within 500ms of stimulus onset with a warning to “Please start moving earlier” if participants exceeded this limit. Trials were terminated if either of these requirements was not met. Following errors, participants were shown a red “X” for 2000ms. Each trial began after a 500ms ITI.
2.3. Procedure
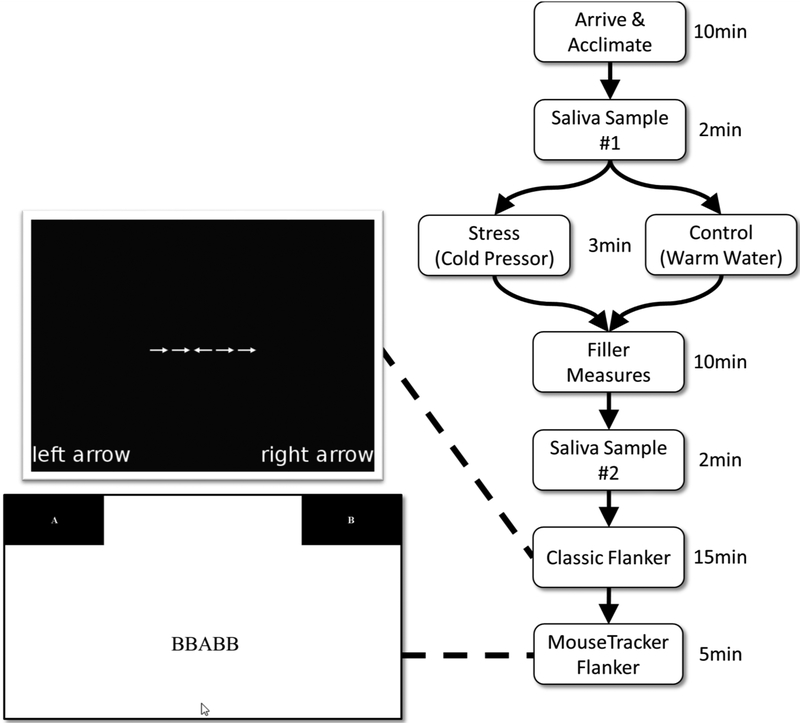
Figure 1 illustrates the study procedure. Participants came to the lab at either 10am or 11am in groups of two-to-four for a one-hour timeslot. Upon arrival, each participant was greeted by an experimenter who instructed the participant to rinse their mouth out with a provided glass of purified water. Once all participants arrived, they provided informed consent and subsequently completed filler questionnaires for 10min to allow acclimation to the lab. The experimenter then collected a saliva sample (baseline) from each participant. Participants then completed the pre-manipulation affect assessment. Once all participants had finished the pre-manipulation affect assessment, the experimenter introduced the stress or control task. After completing the stress or control task, participants immediately completed the post-manipulation affect assessment and then subsequently completed a novel time perception task as a filler task, which took approximately 10min. A time perception task was chosen to retain participants’ study engagement without making demands on executive control. After 10min post-manipulation-offset (13min post-manipulation-onset) had elapsed, the experimenter collected a second saliva sample (post-manipulation) from each participant. Participants then completed the classic flanker task (15min post-manipulation-onset). After completing the classic flanker task, participants completed the MouseTracker flanker task. Upon completion, participants were debriefed outside of the experiment room and dismissed.
Figure 1.
Graphical illustration of the study procedure. Post-manipulation and after saliva collection, participants completed two separate implementations of the Flanker task: the classic flanker, wherein only a single behavioral response was assessed for each trial, followed by the MouseTracker flanker, wherein mouse trajectories in responses were assessed.
2.4. Data Reduction and Analysis
2.4.1. Computational modeling.
Classic flanker task data were fit to the diffusion model for conflict tasks (DMC; Ulrich et al., 2015)—a task-general model designed to fit any response-competition task, such as the flanker. This model posits that response selection is driven by superimposed automatic (i.e., bottom-up) and controlled (i.e., top-down) attentional processes. The automatic attentional process is modeled as a scaled gamma function, which begins the trial at zero, reaches a maximum after a short delay, and approaches zero thereafter—fitting empirical literature showing that task-irrelevant information has its strongest effect early and less effect as a trial continues (Dyer, 1971; Ulrich et al., 2015). The peak amplitude (i.e., the size, A), shape (a), and scale (τ) of this gamma function are fit as model parameters. The controlled attentional process is modeled as a Wiener diffusion process with a constant drift rate (μc), indicating the strength of the controlled process (i.e., higher μc equal stronger controlled attention). Once evidence accumulation for a response reaches a certain threshold (i.e., the decision boundary, b), the selected response is encoded into a motor action and executed during some nondecision time (μR) that exhibits trial-to-trial variability (σR). The model further fits variability in the starting point of the decision process with a final parameter describing the shape of the beta distribution of response starting points (α) that is constrained by the decision boundary (b), reflecting the fact that on some trials participants are primed with a certain response (e.g., after three left-arrow trials in a row, participants are primed to respond with “left” on a fourth trial). The standard deviation of this starting point distribution (σX(o)) is calculable given α and b.
The model was fit to each participant’s data. Due to space limitations, the model fitting procedure is described within the Supplemental Material.
2.4.2. Analytic strategy.
Following Ulrich et al. (2015), trials from the classic flanker with response times shorter than 200ms or longer than 1,200ms were discarded (0.26% of all trials); although the model was only fit to these trimmed data, raw behavioral results were virtually identical when all trials were included. All analyses were conducted in R, version 3.4.3. The function for simulating DMC trials was coded in C++ and called using the Repp package, version 0.12.17. The subplex optimization algorithm was called from the subplex package, version 1.5–4. Because of the randomization of timeslots, rather than participants, to conditions, all models including between-subjects effects (i.e., analyses not solely restricted to examining flanker effects within subjects) were linear mixed models nesting participants within timeslots. Linear mixed models were fit using the ImerTest package, version 3.0–1, and model estimated marginal means and corresponding standard errors were derived using the lsmeans package, version 2.27–62. To facilitate future meta-analyses, effect sizes were calculated from raw values. Residualized changes in cortisol for use in correlational analyses—used because they are more reliable than simple difference scores (Cronbach and Furby, 1970)—were calculated by regressing log-transformed post-manipulation cortisol on log-transformed pre-manipulation cortisol. Using simple difference scores (i.e., Δ-cortisol) in analyses produced virtually identical results.
3. Results
3.1. Preliminary Analyses
3.1.1. Negative affect.
We first examined whether our mild stress manipulation was successful by examining changes in negative affect from pre- to post-manipulation. As hypothesized, the Time×Stress (i.e., experimental condition) interaction was significant, F(1,107.0)=23.40, P<.0001. Decomposing this interaction, we found that participants in the stress induction condition increased from baseline (M=1.37, SE=0.07) to post-manipulation (M=1.65, SE=0.07), t(107.0)=4.72, p<.0001, whereas participants in the control condition decreased in negative affect from baseline (M=1.49, SE=0.07) to post-manipulation (M=1.35, SE=0.07), t(107.0)=2.04, p=.044.
3.1.2. Cortisol.
We then examined the effect of our mild stress manipulation on cortisol. As hypothesized, the Time×Stress interaction was significant, F(1,99.8)=8.70, p=.004. Decomposing this interaction, we found that participants in the stress condition increased from baseline (M=7.76, SE=0.72) to post-manipulation (M=9.31, SE=0.12), t(99,7)=3.64, p<.001, whereas participants in the control condition did not change from baseline (M=8.29, SE=0.61) to post-manipulation (M=8.11, SE=0.67), t(99.8)=−0.44, p=.659. However, consistent with expectations of the mildness of the stressor, the stress condition did not show the standard twofold-or-greater increase in cortisol, but instead showed a small 1.20-fold increase in cortisol. In fact, the effect of our manipulation on cortisol was significantly smaller than the effect of stress on cortisol within other studies of stress and cognition: The effect size of our stress manipulation on cortisol (gppc=0.296) was outside the 95% confidence interval (gppc=0.777, CI95%[0.354, 1.201]) of the effect size of the cold pressor on cortisol at the same delay between stress and saliva collection in a recent meta-analysis (Shields et al., 2017).1 Thus, our mild stress manipulation was successful.
3.2. Primary Analyses2
3.2.1. Classic flanker task.
We analyzed the effects of stress on flanker performance in a mixed-model ANOV A, nesting trials within participants and participants within timeslots, and examined the fixed effects of Trial, Congruence, and Stress (i.e., experimental condition) on response times and errors. We observed classic flanker effects: Participants made, on average, 14.20 more errors on incongruent trials (M=19.49) than congruent trials (M=5.29), t(106)=6.45, p<.0001, dz=0.62, CI95% [9.83, 18.56], and they correctly responded, on average, 40.76ms slower on incongruent trials (M=447.16ms) than congruent trials (M=487.92ms), t(106)=26.08,P<.0001, dz=2.52, CI95% [37.66, 43.86].
3.2.1.1. Errors.
We found a significant Stress×Congruence effect on the likelihood of committing an error, χ2(l)=18.03, p<.0001. However, a closer examination of the data revealed that two control participants performed worse than chance on incongruent trials3 (see Supplementary Figure 1), each having committed over 130 errors to incongruent trials (out of a total of 260). Therefore, this Stress effect on the likelihood of committing an error was likely driven by including these two outliers. As such, we excluded these participants and reran the analyses. After excluding the outliers, the Stress×Congruence interaction was no longer significant, χ2 (1)=0.13, p=.714, and no main effect of or interaction with Stress was significant, ps>.159. Participants in the stress induction group committed the same number of errors as participants in the control condition on both incongruent trials (Mstress=16.27, Mcontrol=17.06), t(54.8)=−0.28, p=.783, d=−0.06, CI95% [−6.49, 4.92], and congruent trials (Mstress=5.22, Mcontrol=5.31), t(105.0)= −0.07, p=.944, d=−0.01, CI95% [−2.61, 2.43]. There were no interactions of Stress or Stress×Congruence with Trial, ps>.420, indicating that accuracy did not differentially change over the course of the task by condition. Thus, our stress manipulation did not influence error rates on the flanker task.
3.2.1.2. Response time.
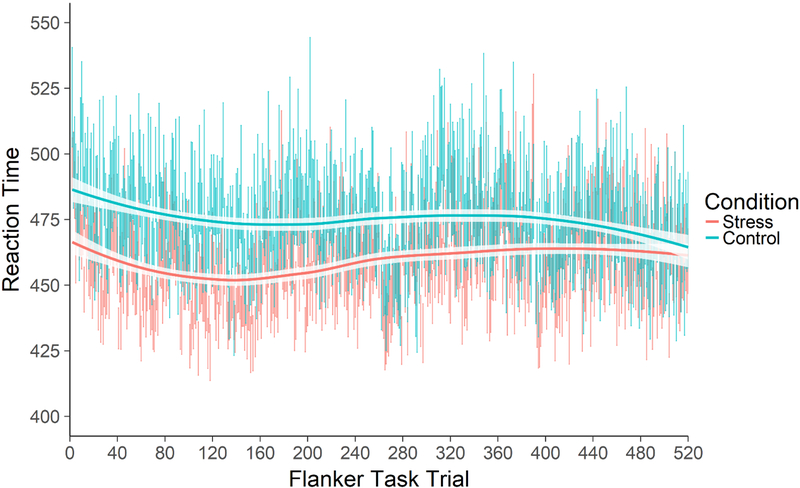
The nonsignificant effect of Stress on response time, F(1,45.0)=2.59, p=.114, was qualified by a significant Stress×Trial interaction, F(1,44263)=33.35,p<.0001 (Figure 2). The Stress×Congruence and Stress×Trial×Congruence interaction effects were not significant, ps>.439, indicating that flanker interference effects did not differ between stress and control groups. Decomposing the Stress×Trial interaction, participants in the stress condition (M=456.39, SE=6.82) responded approximately 22.65ms faster to trials at the beginning of the task (i.e., the model-estimated first test block trial) than did control participants (M=479.04, SE=6.52, t(46.4)=−2.40, p=.020, d=−0.50,95% CIdiff [−41.64, −3.67], but this difference was no longer detectable by the end of the task (Mstress=464.67, SEstress=6.82; Mcontrol=472.11, SEcontrol=6.52), t(46.4)=−0.79, p=434, d=−0.16, 95% CIdiff [−26.43, 11.55] (see Figure 2). Sensitivity analyses determined that participants in the stress condition responded significantly faster than participants in the control condition for approximately the first 129 trials (ps ≤ .0499), then did not differ in subsequent trials (ps≥.0500). Removing the participants mentioned above who committed an exorbitant number of errors to incongruent trials did not affect the results; the Stress×Trial interaction effect remained significant, F(1,43458)=22.14, p <.0001, and decomposing the interaction showed the same pattern of results. In sum, regardless of trial congruency, participants in the stress condition responded significantly faster than participants in the control condition during the first half of the task.
Figure 2.
Response time by trial by condition. Participants in the stress condition responded faster than participants in the control condition at the beginning of the task, but the groups converged to statistical equivalence by the end of the task. Bars for each trial represent mean ± SE for each condition on each trial. Lines are loess smooths fit to each condition’s trial data.
3.2.2. Computational modeling.
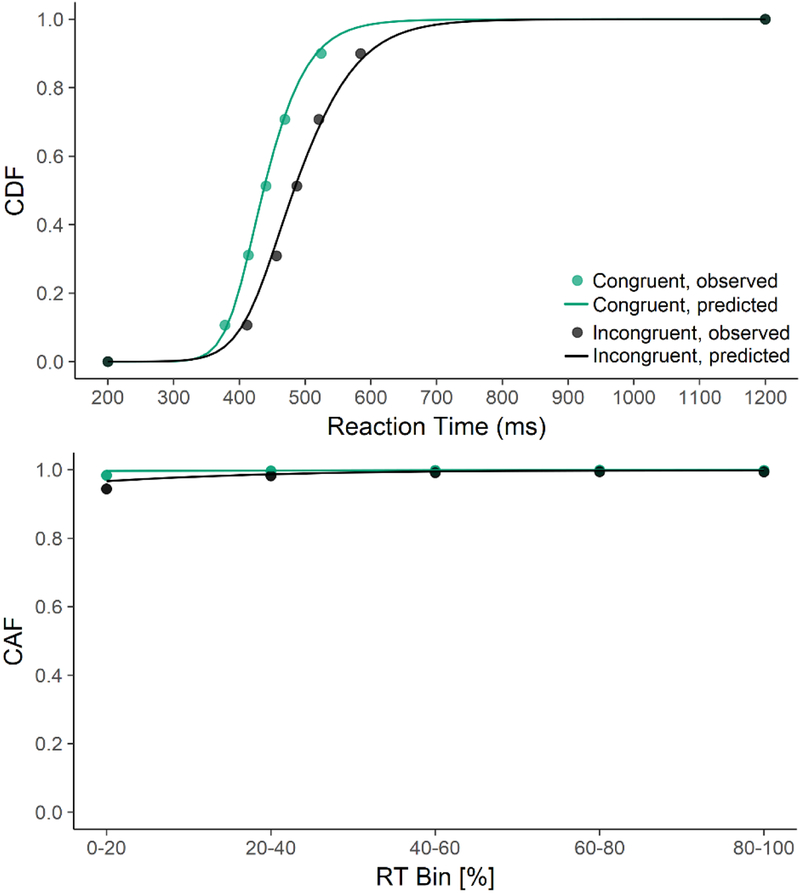
The diffusion model for conflict tasks (DMC) provided an excellent fit to the data (see Figure 3), with the average parameter values explaining over 99% of the variance in flanker response distributions (i.e., R2congruent=.999 and R2incongruent= .997) and over 90% of the variance in flanker error distributions (i.e., R2congruent=.900 and R2incongruent =.991) across participants’ average Vincentized distributions.
Figure 3.
Model fits for the average diffusion model for conflict (DMC) to the Vincent average cumulative distribution function (CDF) and conditional accuracy function (CAF). The DMC was an excellent fit to the data.
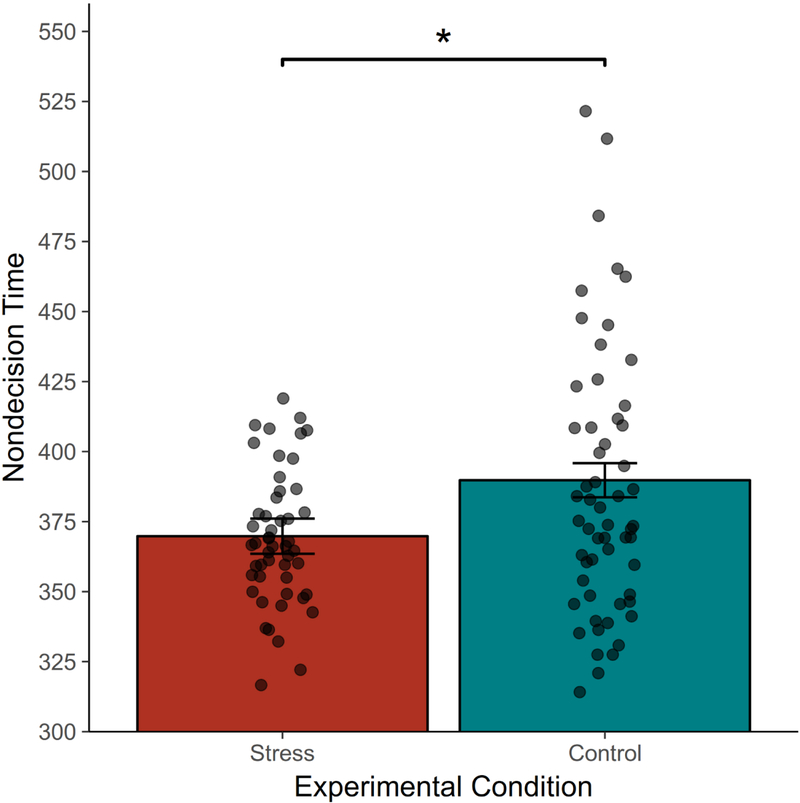
After fitting the DMC, we examined effects of the mild stress manipulation on DMC parameters. Critically, nondecision time (i.e., time taken to encode and execute a chosen motor action) was significantly faster in the stress group (M=369.80ms, SE=6.28) than in the control group (M=389.77ms, SE=6.10), t(37.8)=−2.28, p=.028, d=−0.46, 95% Cldiff [−32.02ms, −2.69ms] (Figure 4). No other model parameter differed between the stress and control groups, ps>. 136 (Table 1). Therefore, the mild stress manipulation enhanced motor action execution time without significantly influencing any other component process in the flanker task.
Figure 4.
Effects of the stress manipulation on nondecision time (i.e., selected motor action execution time) as fit by the DMC. Participants in the stress condition showed faster nondecision times, indicating a faster execution of chosen motor actions. No other model parameter differed between groups.*p < .05.
Table 1.
Parameter Means and Standard Errors for Each Condition
| Parameter | Stress Condition | Control Condition | Pdiff | ||
|---|---|---|---|---|---|
| Mean | (SE) | Mean | (SE) | ||
| A | 24.48 | (1.75) | 26.62 | (1.64) | .375 |
| a | 1.77 | (0.05) | 1.85 | (0.05) | .262 |
| τ | 215.74 | (12.60) | 208.09 | (11.80) | .659 |
| μc | 0.673 | (0.023) | 0.659 | (0.022) | .663 |
| b | 62.56 | (1.81) | 59.01 | (1.70) | .157 |
| μR | 369.80 | (6.28) | 389.77 | (6.10) | .028 |
| σR | 26.81 | (2.59) | 32.32 | (2.52) | .137 |
| α | 3.09 | (0.28) | 3.00 | (0.26) | .819 |
Note: A=size of automatic attention gamma function, a=shape of automatic attention gamma function, τ=scale of automatic attention gamma function, μc=drift rate of controlled attention (larger means better controlled attention), b=decision boundary (larger means a preference for accuracy in speed/accuracy tradeoff), μR=mean nondecision time, σR=standard deviation of nondecision time, α=shape parameter for beta distribution of variability in starting point of evidence accumulation (higher means less variability in starting point of evidence accumulation). More detail on each of these parameters is given in the Method section and Supplemental Material.
We present analyses examining the effects of stress on nondecision time during the first half and the second half of the task in Supplemental Material.
3.2.3. MouseTracker.
For analyses of participants’ mouse-tracking data, we analyzed trials with correct identifications and the response latency criteria for decisions and movement initiation were met (97.88% of trials). We observed classic flanker effects within this task: participants made, on average, 0.22 more errors on incongruent trials (M=0.29) than congruent trials (M=0.07), t(106)=2.97, p=.004, dz=0.29, CI95% [0.07, 0.37], and they responded, on average, 99.57ms slower on incongruent trials (M=944.69ms) than congruent trials (M=845.12ms), t(106)=21.37, p<.0001, dz =2.07, CI95% [90.33, 108.80]. We also observed novel flanker effects within the MouseTracker flanker implementation: Mouse-tracking measures indicative of response conflict showed flanker interference effects. In particular, participants’ area under the curve (AUC; i.e., the geometric area between an actual trajectory and the ideal trajectory) was significantly larger on incongruent trials (M= 1.71) than congruent trials (M=0.68), t(106)=20.74, p<000.1, dz =2.01, CI95% [0.93, 1.13]. Similarly, participants’ maximum deviation (i.e., the largest perpendicular deviation between an actual trajectory and the ideal trajectory to the correct response) was significantly larger on incongruent trials (M=0.80) than congruent trials (M=0.38), t( 106)=22.59,p<.0001, dz =2.18,CI95% [0.38, 0.46]. Additionally, although not a measure of spatial attraction to the opposite response, the time to maximum deviation was significantly greater in incongruent trials (M=475.25ms) than congruent trials (M=405.73ms), t(106)=23.05, p<.0001, dz =2.23, CI95% [62.63, 74.41]. Participants’ time to begin moving the mouse did not significantly differ between incongruent (M= 109.45ms) and congruent trials (M=110.03ms), t(106)= −0.28,p=.779, dz=−0.03, CI95% [−4.66, 3.51].
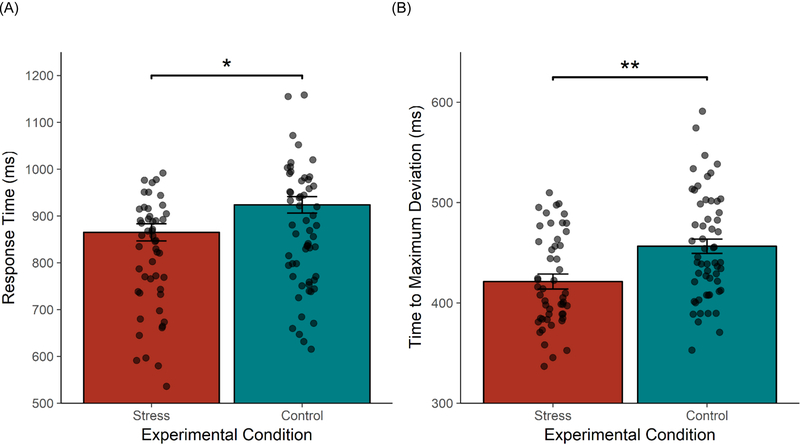
For our critical tests, we compared effects of stress in a mixed-model ANOV A, nesting congruence type within participants and participants within timeslots, and examined the fixed effects of Congruence and Stress on the above MouseTracker flanker dependent variables. We found significant effects of Stress on response time, F(1,44.8)=5.35,p=.025, and time to maximum deviation, F(l, 48.3)=11.40,P =.001, but not on error rates, AUC, maximum deviation, or time to begin mouse movement, ps>.245. All StressxCongruence interaction effects were nonsignificant, ps>.243, indicating that flanker interference effects did not differ between groups. Examining effects of Stress on response time, participants in the stress group responded faster than participants in the control group to both incongruent trials (Mstrtss=913.39ms, McontroL=975.38ms), t(47.8)=−2.40, p=.020, d=−0.48, CI95% [−113.91, −10.06], and congruent trials (Mstress=817.28ms, Mcontrol=872.78ms), t(47.8)= −2.15, p=.037, d=−0.44, CI95% [−107.43, −3.58] (Figure 5a). Similarly, examining effects of Stress on time to maximum deviation, participants in the stress group reached their maximum deviation points faster than control participants on both incongruent trials (Mstress=456.63ms, Mcontrol=490.83ms), t(56.3)= −3.17, p=.002, d=−0.63, CI95% [−55.81, −12.58], and congruent trials (Mstress=387.21,Mcontrol=423.09ms), t(56.3)= −3.32, p=.002, d=−0.67, CI95% [−57.49, −14.26] (Figure 5b). In sum, participants in the stress group moved the mouse in the same way as participants in the control group, but participants in the stress group moved it—and therefore responded—faster.
Figure 5.
Significant group differences from MouseTracker analyses. Although there were no differences in indices of response conflict (e.g., area under the curve) or in the time taken to begin moving the mouse, participants in the stress condition showed shorter response times to the entire trial and a faster time to maximum deviation than participants in the control condition. *p < .05, **p < .01.
3.2.4. Correlations with cortisol.
Changes in cortisol from pre- to post-manipulation were not correlated with any variable influenced by the mild stress manipulation. Changes in cortisol were unassociated with response times to congruent and incongruent trials in the classic flanker task, |r|s<.05, ps>.622, motor action execution time quantified by computational modeling, r=.01,p=.920, total response time on the MouseTracker flanker, r=−.14, p=. 144, and time to maximum deviation on the MouseTracker flanker, r=−.09, p=.384. Removing the two outlying participants on incongruent errors did not change any of these results, ps>.183. Examining these associations within the stress group alone did not change any of these results, ps >.114. Further exploratory analyses found no associations between cortisol changes and interference effects (in response times, errors, or novel MouseTracker flanker interference effects), ps>.274.
4. Discussion
Stress narrows your attention onto whatever is most stressful at the expense of attentional control—or so the synthesis of prior literature suggests (Shields et al., 2016). Our results show that this simple yet elegant account of stress effects on attention is perhaps too simple. In particular, we found that a mild stressor decreased response times in two implementations of the flanker task without influencing accuracy, response caution (i.e., speed-accuracy tradeoffs), or controlled or automatic attention. Moreover, as shown by computational cognitive modeling and analyses of mouse-tracking data, this improvement is specifically due to an enhancement of motor action execution time. These results therefore show that acute stress does not universally impair controlled attentional processes by forcibly narrowing attention onto stress-relevant information. Instead, mild acute stress—the stress that we experience most often (Almeida et al., 2002)—can decrease response times in selective attention tasks. Intriguingly, however, pre- to post-manipulation changes in cortisol were unassociated with any of these effects.
These results have numerous implications for both theories and assessments of stress effects on attention (and on executive functions). In particular, theories of stress effects on attention have focused primarily or exclusively on the cognitive effects of stress without acknowledging the role that motor response execution plays in tasks designed to assess attention (Easterbrook, 1959; LeBlanc et al., 2015; Mather and Sutherland, 2011; Shields et al., 2016, 2015). Our results demonstrate that stress has important effects on these overlooked motor processes. Without a process-level investigation using computational cognitive modeling or mouse-tracking—such that the processes underlying overt responding can be decomposed—it may have been tempting to speculate, based on raw behavioral data (i.e., error rates and mean latency), that stress-induced improvements in response time were due to improvements in sustained attention. For example, if selective attention was unaffected, better sustained attention would result in earlier stimulus detection without altering measures of response conflict (i.e., flanker effects) or errors. These results would have then been taken to show that mild stress can improve sustained attention, suggesting an inverted-U effect of stress on controlled attention. However, because we utilized more precise measures, we showed that this interpretation is unlikely. Namely, the lack of difference between the groups in variability in the starting point of evidence accumulation (quantified by computational modeling) and the lack of group difference in the initial time to move the mouse (quantified by MouseTracker) both suggest that our results were not driven by differences in sustained attention (Bonnelle et al., 2011; Shields, 2017; Stuss et al., 1989). These results therefore entail that theories of stress and cognition should consider motor processes involved in tasks designed to assess cognitive function and whether effects on those processes may explain any observed results. Additionally, these results suggest that outcomes that help disambiguate motor from cognitive processes—such as computational cognitive modeling, mouse-tracking, and eye-tracking (Farrell and Lewandowsky, 2018; Freeman et al., 2011; Freeman and Ambady, 2010; Glaholt and Reingold, 2011; Ramey et al., 2019)—are important for understanding stress effects on attention, and, more broadly, on cognition as a whole.
The observed result of an initial effect of stress decreasing response time on the classic flanker that converged to statistical equivalence by the task’s end coupled with an effect of stress decreasing response time on the shorter MouseTracker flanker task could support the idea that novelty is important for producing the effects of stress on cognitive tasks. Stress effects on cognition differ when tasks are well rehearsed (Arnsten, 2009). Because stress shifts actions from controlled to automatic (e.g., Schwabe et al., 2011), the mild stress manipulation may have caused a bypass of the normal gradual shift from monitoring the execution of chosen motor actions to an unmonitored, automatic execution of chosen motor actions. In short, our results suggest that novelty might influence the effects of stress on cognition.
Although these results diverge from prior analyses examining effects of moderate-to-severe stress on selective attention, which generally find that moderate-to-severe stress impairs selective attention (Sänger et al., 2014; Shields et al., 2016; Vinski and Watter, 2013), they are in line with findings that acute stress can improve control over motor actions (Schwabe et al., 2013; Shields et al., 2016). It is tempting to speculate that the effects of stress on motor actions require a lower stress threshold than effects of stress on cognition, given the obvious benefit of greater and faster motor control for fight-or-flight. However, we did not assess outcomes indicative of response inhibition, which is the component of control over motor actions prior research has found to be enhanced by stress (Dierolf et al., 2018; Schwabe et al., 2013; Shields et al., 2016). As such, it is unknown whether the enhancing effects of mild stress on motor action execution stem from the same mechanism as effects of stress on response inhibition.
Like with studies examining effects of moderate-to-severe stress on selective attention, these results differ from prior studies examining effects of moderate-to-severe stress on reaction time, which have generally found that moderate-to-severe stress slows reaction time on a variety of task types (Banks et al., 2014; Mahoney et al., 2007; McMorris et al., 2006; Sänger et al., 2014). However, because these studies did not utilize computational cognitive modeling or a similar method to decompose performance into underlying processes, it is difficult to know whether these impairing effects are due to impairing effects of stress on sustained attention or on motor action execution time per se. As such, it is possible that mild and moderate-to-severe stress differentially influence motor action execution time. Alternatively, it is possible that stressors of all severity improve motor action execution time, but this improvement to motor execution time is dwarfed by impairments in sustained attention (leading to slower stimulus detection times and therefore slower response times) caused by more severe stress. These possibilities suggest that parametrically manipulating stress severity is an important next step in understanding differential effects of stressors of differing severity on attention and motor performance.
Some study limitations should be noted. First, although our results show what the effects of a mild stressor on flanker performance are, we did not parametrically manipulate stress severity. These results are thus informative for understanding the effects of mild stress, but they cannot conclusively speak to how these effects differ from more severe stress. Second, due to timing constraints, we did not capture the cortisol peak post-stressor. Although our mild acute stress manipulation produced a cortisol response significantly smaller than the standard cold pressor task at the same delay between stressor onset and saliva sample collection, it is possible that cortisol may have been related to the effects of stress had we sampled it at a different time post-stressor. Third, the tasks used in this study were presented in a fixed order, which may have resulted in different effects of stress on one or both tasks than would have been observed if these tasks were switched, given the time-dependent nature of the stress response. Fourth, we did not explicitly instruct participants to develop a social identity as did Häusser et al. (2012), which may have contributed to individual differences in how the stressor was construed and may have resulted in some participants experiencing the stressor as more stressful than others. Fifth, we did not assess women’s menstrual cycles, nor did we exclude women taking hormonal contraceptives, which limits our ability to determine how either of these factors may alter the effects we observed; future research should therefore attempt to determine if phase of the menstrual cycle or contraceptive usage alters any of the effects we observed. Finally, the participants in this study were all undergraduates. Although we have no strong a priori reason to expect stress effects on motor and cognitive processes differ within different populations, these effects may differ in other populations (Henrich et al., 2010).
4.1. Conclusion
In conclusion, we found that mild acute stress improves response speed without affecting accuracy or interference control in two implementations of a selective attention task within a large sample of healthy young adults. At the process level, we found that this improvement in response speed was due to mild stress improving motor action execution. These same process-level analyses indicated that mild stress did not impact other component decision processes, such as automatic or controlled attention, response caution, or variability in motor or attentional processes. These findings come with numerous theoretical implications, including that future work examining effects of stress on cognitive processes should not ignore effects of stress on motor processes as is often done, nor should it ignore the severity of the stress manipulation used, again as is often the case.
Supplementary Material
Highlights.
Mild acute stress improved response speed in selective attention tasks
Mild acute stress did not influence accuracy or interference control in these tasks
Cortisol was unrelated to any of the behavioral effects of stress
Acknowledgments
This research was supported by a Provost Dissertation Year Fellowship to Grant S. Shields, NIH Grant R01 MH103322 to Brian C. Trainor, and NEIEY025999 to Andrew P. Yonelinas
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflict of interest in this work.
This confidence interval was not published in Shields et al. (2017), but is a secondary analysis estimating the effect of the cold pressor on cortisol at our 13min delay between stressor onset and saliva sample collection. Of note, one study in that meta-analysis used this exact delay, and the effect size in that study (gPPc=0.738) was nearly identical to the meta-analytic estimated effect at that delay (gPPc=0.777), indicating that the estimated meta-analytic effect is vexy accurate with this stressor and delay.
Controlling for sex did not influence any of these results.
Curiously, these participants were not outliers in congruent errors, nor did they only select one response option, fail to respond to multiple trials, or respond so fast as to indicate random button pressing. Similarly, these participants were not outliers in flanker interference effects on response time.
References
- Almeida DM, Wethington E, Kessler RC, 2002. The Daily Inventory of Stressful Events: An interview-based approach for measuring daily stressors. Assessment 9, 41–55. 10.1177/1073191102091006 [DOI] [PubMed] [Google Scholar]
- Amsten AFT, 2009. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci 10, 410–422. 10.1038/nm2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks JB, Tartar JL, Welhaf MS, 2014. Where’s the impairment: An examination of factors that impact sustained attention following a stressor. Cogn. Emot 28, 856–866. 10.1080/02699931.2013.857643 [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, Greenwood RJ, Sharp DJ, 2011. Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J. Neurosci 31, 13442–13451. 10.1523/JNEUROSCI.1163-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, 2008. Stress and emotional memory retrieval: Effects of sex and cortisol response. Neurobiol. Learn. Mem 89, 134–141. https://doi.Org/10.1016/j.nlm.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calanchini J, Rivers AM, Klauer KC, Sherman JW, 2018. Multinomial processing trees as theoretical bridges between cognitive and social psychology. Psychol. Learn. Motiv 69, 39–65. 10.1016/bs.plm.2018.09.002 [DOI] [Google Scholar]
- Cronbach LJ, Furby L, 1970. How we should measure “change”: Or should we? Psychol. Bull 74, 68–80. 10.1037/h0029382 [DOI] [Google Scholar]
- Dierolf AM, Schoofs D, Hessas E-M, Falkenstein M, Otto T, Paul M, Suchan B, Wolf OT, 2018. Good to be stressed? Improved response inhibition and error processing after acute stress in young and older men. Neuropsychologia. https://doi.Org/lO.lOl6/J.NEUROPSYCHOLOGIA.2Ol8.08.02O [DOI] [PubMed] [Google Scholar]
- Dulaney CL, Rogers WA, 1994. Mechanisms underlying reduction in Stroop interference with practice for young and old adults. J. Exp. Psychol. Learn. Mem. Cogn 20, 470–484. https://doi.Org/10.1037/0278-7393.20.2.470 [DOI] [PubMed] [Google Scholar]
- Dyer FN, 1971. The duration of word meaning responses: Stroop interference for different preexposures of the word. Psychon. Sci 25, 229–231. 10.3758/BF03329102 [DOI] [Google Scholar]
- Easterbrook JA, 1959. The effect of emotion on cue utilization and the organization of behavior. Psychol. Rev 66, 183–201. 10.1037/h0047707 [DOI] [PubMed] [Google Scholar]
- Farrell S, Lewandowsky S, 2018. Computational modeling of cognition and behavior. Cambridge University Press, New York, NY: 10.1017/9781316272503 [DOI] [Google Scholar]
- Freeman JB, Ambady N, 2010. MouseTracker: Software for studying real-time mental processing using a computer mouse-tracking method. Behav. Res. Methods 42, 226–241. https://doi.Org/10.3758/BRM.42.l.226 [DOI] [PubMed] [Google Scholar]
- Freeman JB, Dale R, Farmer TA, 2011. Hand in motion reveals mind in motion. Front. Psychol 2 10.3389/fpsyg.2011.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon SA, Waskom ML, Brown TI, Wagner AD, 2018. Stress Impairs Episodic Retrieval by Disrupting Hippocampal and Cortical Mechanisms of Remembering. Cereb. Cortex 10.1093/cercor/bhyl62 [DOI] [PubMed] [Google Scholar]
- Glaholt MG, Reingold EM, 2011. Eye movement monitoring as a process tracing methodology in decision making research. J. Neurosci. Psychol. Econ 4, 125–146. 10.1037/a0020692 [DOI] [Google Scholar]
- Hausser JA, Kattenstroth M, van Dick R, Mojzisch A, 2012. “We” are not stressed: Social identity in groups buffers neuroendocrine stress reactions. J. Exp. Soc. Psychol 48, 973–977. 10.1016/j.jesp.2012.02.020 [DOI] [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A, 2010. Most people are not WEIRD. Nature 466, 29 10.1017/S0140525X0999152X [DOI] [PubMed] [Google Scholar]
- Hermens F, 2018. When do arrows start to compete? A developmental mouse-tracking study. Acta Psychol. (Amst) 182, 177–188. https://doi.Org/10.1016/j.actpsy.2017.ll.015 [DOI] [PubMed] [Google Scholar]
- Hines EA, Brown GE, 1932. A standard stimulus for measuring vasomotor reactions: Its application in the study of hypertension. Proc. Staff Meet. Mayo Clin 7, 332. [Google Scholar]
- Hostinar CE, 2015. Recent developments in the study of social relationships, stress responses, and physical health. Curr. Opin. Psychol 5, 90–95. https://doi.Org/10.1016/j.copsyc.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Fieman R, 2012. Moderate stress enhances immediate and delayed retrieval of educationally relevant material in healthy young men. Behav. Neurosci 126, 819–825. 10.1037/a0030489 [DOI] [PubMed] [Google Scholar]
- Joels M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ, 2006. Learning under stress: How does it work? Trends Cogn. Sci 10, 152–158. https://doi.Org/10.1016/j.tics.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Mohamed-Ali V, Feldman PJ, Kirschbaum C, Steptoe A, 2003. Cortisol responses to mild psychological stress are inversely associated with proinflammatory cytokines. Brain. Behav. Immun 17, 373–383. 10.1016/S0889-1591(03)00029-1 [DOI] [PubMed] [Google Scholar]
- LeBlanc VR, McConnell MM, Monteiro SD, 2015. Predictable chaos: A review of the effects of emotions on attention, memory and decision making. Adv. Heal. Sci. Educ 20, 265–282. 10.1007/s10459-014-9516-6 [DOI] [PubMed] [Google Scholar]
- Mahoney CR, Castellani J, Kramer FM, Young A, Lieberman HR, 2007. Tyrosine supplementation mitigates working memory decrements during cold exposure. Physiol. Behav 92, 575–582. 10.1016/j.physbeh.2007.05.003 [DOI] [PubMed] [Google Scholar]
- Mather M, Sutherland MR, 2011. Arousal-biased competition in perception and memory. Perspect. Psychol. Sci 6, 114–133. 10.1177/1745691611400234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris T, Swain J, Smith M, Corbett J, Delves S, Sale C, Harris RC, Potter J, 2006. Heat stress, plasma concentrations of adrenaline, noradrenaline, 5-hydroxytryptamine and cortisol, mood state and cognitive performance. Int. J. Psychophysiol 61, 204–215. 10.1016/j.ijpsycho.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Metz GA, Jadavji NM, Smith LK, 2005. Modulation of motor function by stress: A novel concept of the effects of stress and corticosterone on behavior. Eur. J. Neurosci 22, 1190–1200. 10.1111/j.1460-9568.2005.04285.X [DOI] [PubMed] [Google Scholar]
- Mueller ST, Piper BJ, 2014. The Psychology Experiment Building Language (PEBL) and PEBL test battery. J. Neurosci. Methods 222, 250–259. 10.1016/jjneumeth.2013.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei NYL, Everaerd WTAM, Elzinga BM, van Well S, Bermond B, 2006. Psychosocial stress impairs working memory at high loads: An association with cortisol levels and memory retrieval. Stress 9, 133–141. 10.1080/10253890600965773 [DOI] [PubMed] [Google Scholar]
- PreuB D, Wolf OT, 2009. Post-learning psychosocial stress enhances consolidation of neutral stimuli. Neurobiol. Learn. Mem 92, 318–326. https://doi.Org/10.1016/j.nlm.2009.03.009 [DOI] [PubMed] [Google Scholar]
- Ramey MM, Yonelinas AP, Henderson JM, 2019. Conscious and unconscious memory differentially impact attention: Eye movements, visual search, and recognition processes. Cognition, 185, 71–82. 10.1016/j.cognition.2019.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sänger J, Bechtold L, Schoofs D, Blaszkewicz M, Wascher E, 2014. The influence of acute stress on attention mechanisms and its electrophysiological correlates. Front. Behav. Neurosci. Neurosci 8, 353 10.3389/fnbeh.2014.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs D, PreuB D, Wolf OT, 2008. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology 33, 643–653. 10.1016/j.psyneuen.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Schwabe L, Hoffken O, Tegenthoff M, Wolf OT, 2011. Preventing the stress-induced shift from goal-directed to habit action with a (3-adrenergic antagonist. J. Neurosci 31, 17317–17325. 10.1523/JNEUROSCI.3304-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L, Hoffken O, Tegenthoff M, Wolf OT, 2013. Stress-induced enhancement of response inhibition depends on mineralocorticoid receptor activation. Psychoneuroendocrinology 38, 2319–2326. 10.1016/j.psyneuen.2013.05.001 [DOI] [PubMed] [Google Scholar]
- Shields GS, 2017. Response: Commentary: The effects of acute stress on core executive functions: A meta-analysis and comparison with cortisol. Front. Psychol 8 10.3389/fpsyg.2017.02090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Bonner JC, Moons WG, 2015. Does cortisol influence core executive functions? A metaanalysis of acute cortisol administration effects on working memory, inhibition, and set-shifting. Psychoneuroendocrinology 58, 91–103. https://doi.Org/10.1016/j.psyneuen.2015.04.017 [DOI] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, McCullough AM., Yonelinas AP, 2017. The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychol. Bull 143, 636–675. 10.1037/bul0000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Sazma MA, Yonelinas AP, 2016. The effects of acute stress on core executive functions: A meta-analysis and comparison with effects of cortisol. Neurosci. Biobehav. Rev 68, 651–688. 10.1016/j.neubiorev.2016.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Yonelinas AP., 2018. Balancing precision with inclusivity in meta-analyses: A response to Roos and colleagues (2017). Neurosci. Biobehav. Rev 84, 193–197. https://doi.Org/10.1016/j.neubiorev.2017.ll.013 [DOI] [PubMed] [Google Scholar]
- Starcke K, Brand M, 2012. Decision making under stress: A selective review. Neurosci, Biobehav. Rev 36, 1228–1248. https://doi.Org/10.1016/j.neubiorev.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Hugenholtz H, Picton T, Pivik J, Richard MT, 1989. Reaction time after head injury: Fatigue, divided and focused attention, and consistency of performance. J. Neurol. Neurosurg. Psychiatry 52, 742–748. https://doi.Org/10.1136/jnnp.52.6.742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich R, Schroter H, Leuthold H, Bimgruber T, 2015. Automatic and controlled stimulus processing in conflict tasks: Superimposed diffusion processes and delta functions. Cogn. Psychol 78, 148–174. 10.1016/J.COGPSYCH.2015.02.005 [DOI] [PubMed] [Google Scholar]
- van Stegeren AH., Wolf OT, Kindt M, 2008. Salivary alpha amylase and cortisol responses to different stress tasks: Impact of sex. Int. J. Psychophysiol 69, 33–40. https://doi.Org/10.1016/j.ijpsycho.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Vinski MT, Watter S, 2013. Being a grump only makes things worse: A transactional account of acute stress on mind wandering. Front. Psychol 4, 730 10.3389/fpsyg.2013.00730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A, 1988. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol 54, 1063–1070. https://doi.Org/10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- White CN, Servant M, Logan GD, 2018. Testing the validity of conflict drift-dififusion models for use in estimating cognitive processes: A parameter-recovery study. Psychon. Bull. Rev 25, 286–301. 10.3758/sl3423-017-1271-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemers US, Sauvage MM, Schoofs D, Hamacher-Dang TC, Wolf OT, 2013. What we remember from a stressful episode. Psychoneuroendocrinology 38, 2268–2277. https://doi.Org/10.1016/j.psyneuen.2013.04.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.