Abstract
Purpose:
Necrotizing enterocolitis is associated with decreased intestinal perfusion and ischemia. Paneth cells, specialized epithelial cells, have been shown to regulate the intestinal vasculature and disruption of these cells has been associated with NEC. We hypothesized that Paneth cell disruption in immature mice intestine would decrease the perfusion of the intestinal microvasculature.
Methods:
Paneth cells were disrupted in P14–16 mice using chemical (dithizone) and transgenic (diphtheria toxin) methodology. Six hours after Paneth cell disruption, Dylight 488 was injected directly into the left ventricle and allowed to perfuse for five minutes prior to intestinal harvesting. Tissue samples were evaluated with confocal fluorescence microscopy to quantify intestinal perfusion and samples were quantified by real time RTPCR for gene expression.
Results:
Dithizone treatment significantly decreased intestinal perfusion compared to controls (p<0.01). However, diphtheria toxin treatment demonstrated no significant difference in perfusion (p>0.21). Intestines from all treatment groups had similar PECAM staining, but intestines treated with dithizone had significantly decreased nNOS and iNOS gene expression compared to controls (p<0.007).
Conclusions:
Paneth cell disruption significantly decreases the perfusion of the small intestinal microvasculature in a dithizone-specific manner. Dithizone has no effect on the amount of microvasculature, but does impact genes critical to nitric oxide signaling likely contributing to mesenteric vasoconstriction.
Keywords: Necrotizing enterocolitis, Animal model, Intestinal microvasculature, Nitric oxide
Introduction:
Necrotizing enterocolitis (NEC) remains one of the most common gastrointestinal emergencies acquired by premature infants. It is currently estimated that 30% of patients who develop NEC will go on to require surgical intervention and of those, 50% will die from the disease [1]. While the exact pathophysiology of NEC remains unclear, decreased blood flow in the microvasculature of the small intestine has been postulated to play a critical role in the pathogenesis, as >90% of intestinal tissue affected by NEC have histopathologic evidence of coagulation necrosis [1–4]. Animal models of experimental NEC have demonstrated significantly altered intestinal microvascular blood flow, decreased size of intestinal arterioles, and disrupted villous microvasculature compared to controls [2, 4–7]. In addition, studies that looked directly at the blood flow in the superior mesenteric artery (SMA) in infants found that infants who later developed NEC had an initial high-resistance pattern of blood flow in the SMA on the first day of life [8]. These studies suggest that intestinal microcirculatory dysfunction and altered blood flow may contribute to the subsequent development of NEC. However, the underlying mechanisms by which intestinal hypoperfusion occurs and induces intestinal damage and inflammation that lead to NEC remain unclear.
One possible cause of altered small intestinal blood flow is through Paneth cell disruption. Paneth cells are specialized epithelial cells that were first described in the late 1800’s as a granular cell type located within the small intestine at the base of the Crypts of Lieberkühn [9]. This location places Paneth cells just several cell diameters away from the microvasculature as it enters and exits small intestinal villi [10]. The dense granules in Paneth cells contain multiple antimicrobial peptides, angiogenins, cytokines, and vasoactive substances which are released into the crypt lumen constitutively, as well as at higher rates in response to exposure to bacterial antigens [11]. In doing so, Paneth cells are critical for many normal intestinal processes including regulation of the intestinal microbiome, prevention of bacterial translocation, maintenance of intestinal epithelial stem cells, and for regulating the intestinal microvasculature [12, 13]. Disruption of these important cells has significant adverse consequences such as decreased clearance of bacterial pathogens [14, 15], and development of inflammatory bowel disease [16–21]. Several studies have demonstrated a decrease in lysozyme-staining Paneth cells in the intestines affected by NEC compared to controls [20, 22–24]. However, mechanisms linking Paneth cell disruption or dysfunction to altered intestinal microvasculature and the development of NEC have not been previously studied. Taken together, given the close proximity of Paneth cells to the intestinal microvasculature, the vasoactive substances secreted by Paneth cells, and proposed involvement of Paneth cell dysfunction in NEC pathogenesis, we hypothesized that a Paneth cell disruption would significantly decrease the intestinal microvascular perfusion in immature mice.
Material and Methods:
1.1. Mice
All animal experiments were performed according to protocols approved by the Institutional Animal Care and Usage Committees at the University of Iowa (Iowa City, IA) and all mice were housed in standard conditions until experimentation. All experiments used P14–16 day old C57Bl/6J mice or mice on a C57Bl/6J background. PC-DTR mice were generated by inserting a HA-tagged human diphtheria toxin receptor into the cryptdin-2 promotor as previously described [25]. Td Tomato mice were purchased from Jackson Laboratories (stock 007576 mT/mG).
1.2. Paneth cell disruption
Paneth cells were disrupted as previously described [22, 25] via intraperitoneal injection with either 75mg/kg body weight dithizone (Sigma) dissolved in 25mM Lithium carbonate solution (C57Bl/6J mice) or 40ng/g body weight diphtheria toxin in phosphate buffered saline (PC-DTR mice). Sham animals were injected with an equivalent volume of buffer alone. All mice were monitored for 6 hours following injection.
1.3. Evaluation of intestinal microvasculature
Intestinal microvasculature perfusion was measured as previously described by multiple laboratories including Besner and Hackam [4, 26, 27]. Briefly, mice were anesthetized with a one-time intraperitoneal injection of ketamine (80mg/kg) and Xylazine (10mg/kg). Mice were then placed on a warming pad and a midline chest wall incision was made to visualize the left cardiac ventricle. Using a 30g needle, 50μL of 20mg/mL Dylight-488 (AbCam) was injected into the left ventricle over 10 seconds. The heart rate was monitored for 3–5 minutes following the injection to ensure adequate perfusion through the intestinal microvasculature. Mice were then euthanized and the terminal third of the ileum was identified and harvested. Harvested sections were evaluated by cross sectional and whole mount preparations [4]. For whole mount intestines, ileal segments were washed with PBS and opened longitudinally before fixation in a 4% paraformaldehyde solution shaken gently at 4°C overnight. Tissue samples were rinsed with cold PBS three times followed by incubation with 10% sucrose/PBS for 4 hours at 4°C and then incubated in 30% sucrose/PBS overnight at 4°C. Whole mount preparations were mounted on glass slides with the villous side up. Cross sectional tissue samples were fixed as above, frozen in OCT medium, and cut in 50μm thick sections for further evaluation.
1.4. Immunohistochemistry and microscopic quantification
Whole mount samples were washed with PBS and blocked with 5% normal goat serum. Anti-CD31 (BD Biosciences) was applied (at 1:50 dilution) and incubated at 4°C overnight in 1% NSG/TBST prior to secondary incubation with goat anti rat A-568 (BD Biosciences) (1:500 dilution). Whole mount and cross-sectional tissues were viewed using a Zeiss LSM 710 confocal microscope. Image J was used to quantify the Dylight 488 fluorescence per high powered field using a 10x objective to obtain a quantitative measurement of the circulating intestinal blood volume. To determine tissue quantity of eNOS, cross-sectional samples were washed with PBS and blocked with 5% normal goat serum. eNOS polyclonal antibody (Thermo Fischer Scientific) was applied (at 1:100 dilution) and incubated at 4°C overnight in 1% NSG/TBST prior to secondary incubation with anti rat IgG (Vector Laboratories).
1.5. Real Time RT-PCR
Ileal samples were homogenized using a TissueLyser LT (Qiagen) as previously described [22, 28, 29]. RNA was isolated using RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s directions. RNA concentration and quality were determined using a NanoDrop 1000 Spectrophotometer (Thermos Fisher Scientific). Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) was performed using Taqman Gene Expression Assays for nNOS(1), iNOS(2), and eNOS(3) (Life Technologies). qRT-PCR reactions were run in C1000 Thermal Cycler (Eppendorf) using the CFX96 Real-Time PCR Detection System (Bio-Rad). Fold change in gene expression was determined by normalizing gene expression to β-actin in each sample. The 2ΔΔ-CT method was used to compare gene expression levels between samples.
1.6. Statistical Analysis
All experiments were performed in at least triplicate and specific sample sizes are noted in the results section. Microvasculature blood volume in the whole mount and villi were compared using non-parametric Student T-test to determine statistical significance using Graph Pad Prism v7.03. Quantitative RT-PCR was analyzed as described above. In all experiments, differences were considered to be statistically significance if p<0.05.
Results
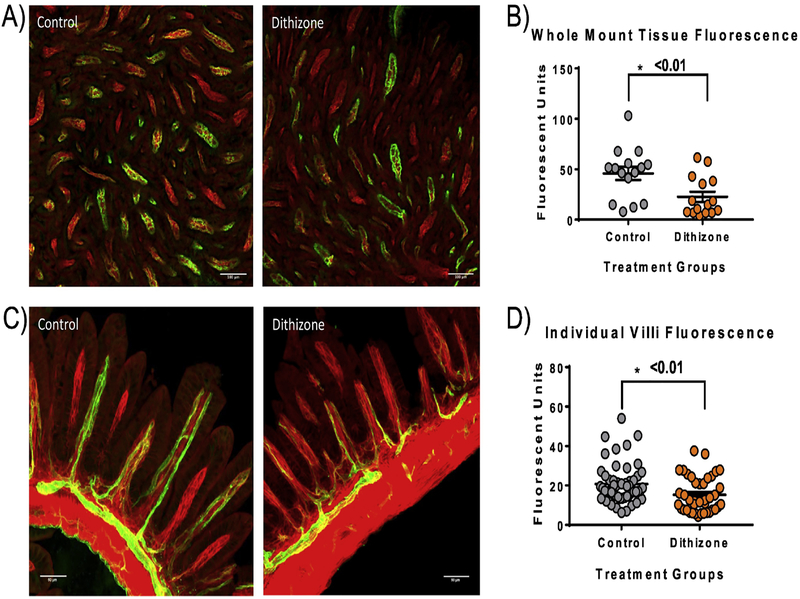
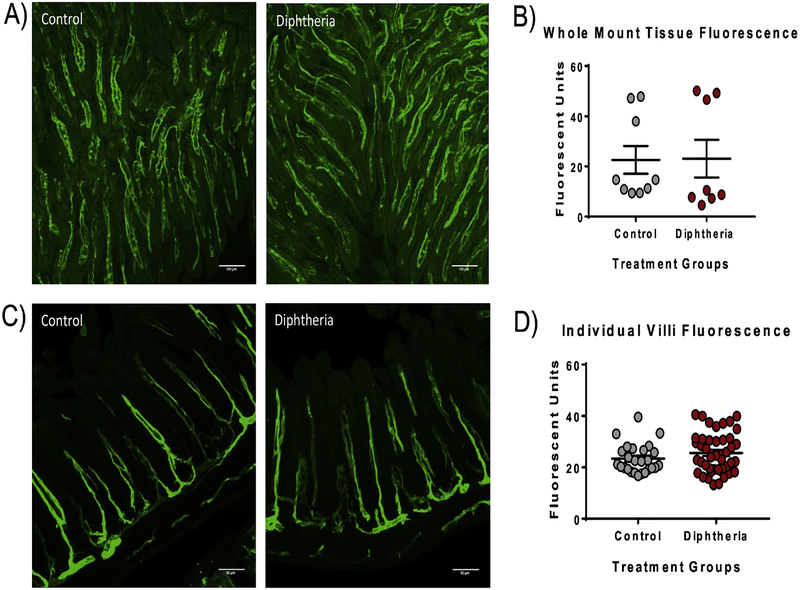
Dithizone-induced disruption of Paneth cells resulted in a significant decrease in intestinal microvascular perfusion as demonstrated by decreased Dylight 488 fluorescence of the small intestine compared to controls. This decrease was found in both the whole mount ileal tissue (dithizone 22.64 ± 5.03 average fluorescent units vs. control 45.89 ± 6.55 average fluorescent units, p<0.01) as well as in the individual villi when measured in cross section (dithizone 15.28 ± 1.43 average fluorescent units vs. control 20.77 ± 1.36 average fluorescent units, p<0.01) (Figure 1). To determine if the decreased perfusion following dithizone treatment was due to Paneth cell disruption, we used a second, complimentary method of Paneth cell disruption. Transgenic mice with a human diphtheria toxin receptor inserted in their Paneth cells (PC-DTR) [25] were given a single intraperitoneal injection with diphtheria toxin to induce selective necrosis of their Paneth cells. Interestingly, mice treated with diphtheria toxin had no significant difference in the Dylight 488 fluorescence in either whole mount or cross section compared to controls (diphtheria 23.09 ± 7.52 average fluorescent units and 29.78 ± 1.73 average fluorescent units vs. control 20.05 + 5.57 average fluorescent units and 24.77 ± 1.96 average fluorescent units, p=0.75) (Figure 2).
Fig 1. Paneth cell disruption with dithizone significantly decreases the small intestine microvascular perfusion.
(A) Representative confocal fluorescent microscopy whole mount ileal images from mice treated with dithizone compared to controls showing decreased intestinal microvascular perfusion following dithizone treatment. (B) Dithizone treated mice had significantly decreased green Dylight 488 fluorescence in the whole mount intestine compared to controls (p <0.01). (C) Representative confocal fluorescent microscopy images of the ileum in cross section from mice treated with dithizone compared to controls showing decreased perfusion of the individual intestinal villi compared to control. (D) Dithizone treated mice had significantly decreased green Dylight 488 fluorescence in the individual small intestinal villi compared to controls (p<0.01). Scale bar equals 100μm.
Fig 2. Paneth cell disruption with diphtheria toxin results in no significant change in the small intestine microvascular perfusion.
(A) Representative confocal fluorescent microscopy whole mount ileal images from mice treated with diphtheria toxin compared to controls showing similar intestinal perfusion. (B) The diphtheria toxin treated mice had no significant difference in the green Dylight 488 fluorescence in the whole mount intestine as controls (p=0.96). (C) Representative confocal fluorescent microscopy images of the ileum in cross section from mice treated with diphtheria toxin compared to controls again showing similar intestinal perfusion in the individual villi as the control. (D) The diphtheria toxin treated mice had no significant difference in the green Dylight 488 fluorescence in individual small intestinal villi compared to the controls (p=0.21). Scale bar equals 100μm.
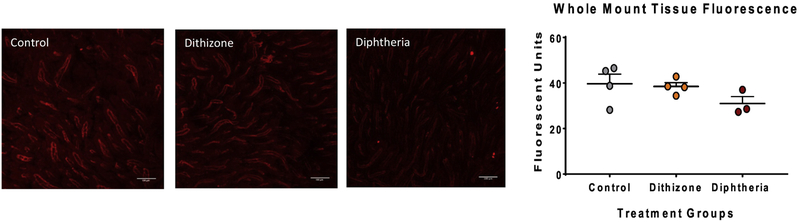
To examine if dithizone-induced decreased intestinal microvasculature perfusion was due to destruction or alteration of the intestinal microvasculature, intestinal samples from all treatment groups were stained for the endothelial marker CD31 (PECAM-1). Histologic examination showed similar CD31 staining in all treatment groups (Figure 3).
Fig 3. PECAM staining of the intestinal microvascular endothelium was similar among all treatment groups.
(A) Representative confocal fluorescent microscopy whole mount images of the intestine stained for CD31 (red fluorescence) from each treatment group (Control, Dithizone, and Diphtheria toxin) shows similar staining among all three groups. Scale bar equals 100μm. (B) There was no significant difference in the average fluorescence of CD31 among all treatment groups (p=0.27).
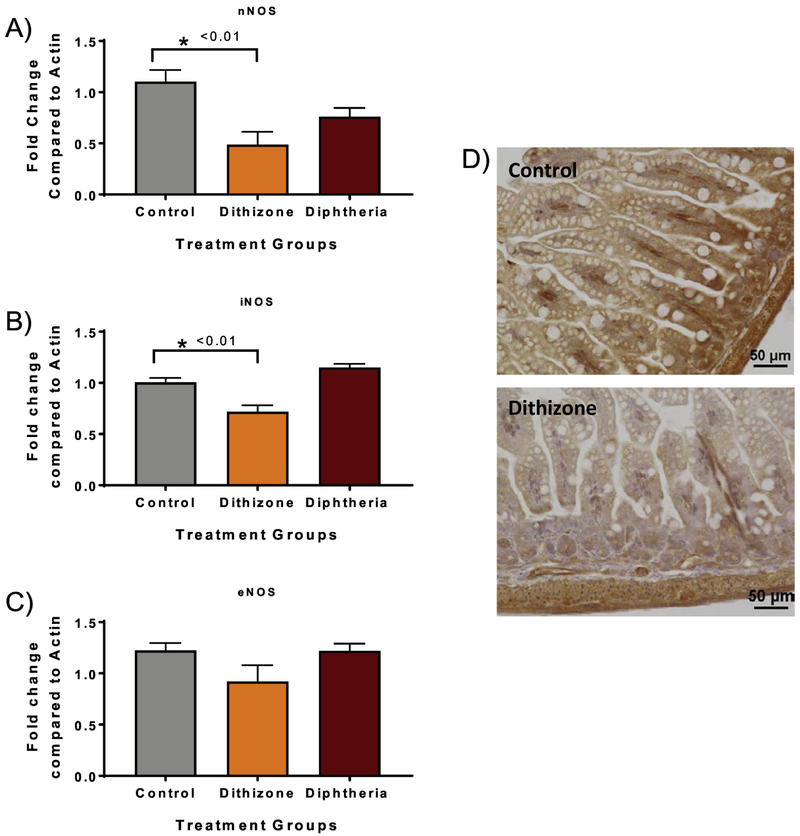
As there were no structural differences in the microvasculature due to dithizone treatment, we next examined small intestinal tissue NOS gene expression as nitric oxide is a potent vasodilator previously postulated to play a role in the pathogenesis of NEC [26]. Mice treated with dithizone had significantly decreased gene expression of nNOS (dithizone 0.49 vs. control 1.10, p=0.007) and iNOS compared to control (dithizone 0.72 vs. control 1.00, p=0.003). There was a trend towards decreased eNOS expression following dithizone compared to control (dithizone 0.92 vs. control 1.22, p=0.10, n=4). There was also no significant difference in any of the NOS gene expression in diphtheria toxin treated mice compared to controls (p>0.08) (Figure 4). To evaluate whether the decreased eNOS gene expression was associated with decreased intestinal tissue levels of eNOS, immunohistochemical staining for eNOS was performed on distal small intestines from control and dithizone treated mice. Histologic examination showed decreased eNOS staining in dithizone treated groups compared to controls (Figure 4).
Fig 4. Paneth cell disruption with dithizone results in a significant decrease in small intestine mRNA expression of NOS genes.
(A) Dithizone treated mice had significantly decreased mRNA expression of nNOS (p<0.01) and (B) significantly decreased mRNA expression of iNOS compared to controls (p<0.01). (C) Dithizone treated mice had a trend towards decreased eNOS expression but significance was not quite reached (p=0.10). Diphtheria toxin treated mice had no significant difference in any NOS gene expression compared to controls. (D) Immunohistochemical staining for eNOS (brown) in the small intestine from control and dithizone treated mice showed decreased eNOS staining in the dithizone treated group. Scale bar equals 50μm.
Discussion
NEC is the single most devastating intestinal disorder seen in premature infants. The bowel injury that occurs with NEC is believed to be due to microbial dysbiosis with bacterial translocation across the immature intestinal epithelial layer, regional hypoxia with free radical formation and tissue necrosis [10, 22, 30, 31]. Hypoperfusion of the intestinal microvasculature has been theorized to play a role in either the initial development or the progression of intestinal injury seen in NEC [1, 3, 7]. However, mechanisms linking hypoperfusion with the development of NEC remain poorly defined. Paneth cell dysfunction has been shown in adults to disrupt adult intestinal microvasculature [13], and studies from our group have shown that Paneth cell disruption in immature animals followed by enteral dysbiosis results in intestinal injury and inflammation that is comparable to other animal models (hypoxia-hypothermia model and LPS + PFA model) and human tissue affected by NEC [22, 25, 32].
To determine if Paneth cell dysfunction alone could induce disturbances in the intestinal microvasculature in the immature mice, we used two established complimentary methods of disrupting Paneth cell biology [22, 25, 32, 33] and measured the resulting effects on microvasculature perfusion. Our data demonstrate that Paneth cell disruption in immature mice can result in a significant decrease in intestinal perfusion compared to controls, but that these effects are method-dependent. This divergent outcome is likely due to the fact that our Paneth cell disruption models work through different mechanisms while still inducing similar phenotypic results [33]. These data support the emerging hypothesis that Paneth cells help to regulate not just the intestinal microbiome, but host physiology as well.
Experimentally, a commonly used methodology for Paneth cell disruption is through administration of the heavy metal chelator dithizone. Dithizone is thought to affect Paneth cells because of their high concentration of zinc [14, 22, 34]. Initial studies in rats showed that 5 minutes following dithizone treatment, zinc-dithizonate complexes are seen within the Paneth cells, and within 60 minutes, these cells degenerate, become necrotic, and are subsequently shed into the crypt lumen [34]. However, recent studies by our group showed that treatment with dithizone significantly altered several autophagy genes including Atg10, Atg4a, Atg12, and Ambra1 suggesting that dithizone may disrupt murine Paneth cells by induction of autophagy pathways [33]. Our lab has also shown that treatment of neonatal mice with dithizone significantly decreases Paneth cell counts by ~50% with no effect on the number of mucin-positive Goblet cells [22] and no effect on barrier function, serum zinc levels, serum glucose levels, or serum cytokine levels [25]. However, as dithizone is a non-specific chelator, it remains very possible that it may have unintended off-target effects on either the host or its associated microbial community.
To help mitigate these potential issues, we utilized a transgenic mouse with specific sensitivity to diphtheria toxin. C57Bl/6J mice are naturally resistant to diphtheria toxin as they lack a receptor that can bind to diphtheria toxin (DTR). However, transgenic PCDTR mice express human DTR on the cell surface of their Paneth cells. Following exposure, diphtheria toxin selectively binds to DTR inducing receptor-mediated endocytosis [35]. Upon entry into the cytoplasm, diphtheria toxin inactivates elongation factor 2 which inhibits protein synthesis and results in necrotic death of the Paneth cell [35, 36]. Our lab has previously shown that treatment of PC-DTR mice significantly decreases the quantity of granule-positive Paneth cells per crypt and the expression of both the Paneth cell markers cryptdin and lysozyme [25].
While both these methods can selectively disrupt and decrease the number of intestinal Paneth cells, the results of our study show that only dithizone reduces the perfusion of the intestinal microvasculature. This novel effect of dithizone on the mesenteric perfusion is not due to damage or disruption of the intestinal microvasculature as the immunohistochemical staining for platelet endothelial cell adhesion molecule-1 (PECAM-1) of the intestinal vasculature was similar in all treatment groups. However, our data suggests that dithizone may cause vasoconstriction through altered NOS gene expression as dithizone treatment results in a significant decrease in RNA expression of neuronal nitric oxide synthase (nNOS) and inducible nitric oxide synthase (iNOS) as well as a trend towards decreased endothelial nitric oxide synthase (eNOS).
Nitric oxide (NO) is a potent vasodilator and has been implicated in regulating vascular tone, inflammation, and tissue repair [37]. NO is produced by the conversion of arginine to citrulline by nitric oxide synthase (NOS) [37]. There are three isoforms of nitric oxide synthase (NOS), each encoded by a separate gene: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS) [38]. eNOs and nNOS are constitutively expressed at low levels whereas iNOS is not expressed under normal conditions but induced in pro-inflammatory states. Nitric oxide is the primary vasodilator in the newborn intestinal circulation [3]. Studies have shown that the concentration of NO in the mesenteric artery and the response of the mesenteric vasculature to various NO mediated vasodilators are greater in newborns than in adults [1, 3, 39]. This makes the neonatal intestine more sensitive to changes in NO levels where even subtle changes may result in significant alterations in the hemodynamic parameters of the intestine. This may conceivably place premature infants at risk for the development of intestinal ischemia. Our results suggest that dithizone results in a significant downregulation of nNOS and iNOS expression. In addition, it also causes decreased eNOS expression, although significance was night quite reached, likely related to the small sample size. The decreased eNOS gene expression was accompanied by a decreased amount of eNOS within the small intestinal as seen with immunohistochemical staining. Therefore, the decrease in various NOS isoforms within the distal small intestine, could subsequently lead to an overall decrease in the total nitric oxide levels, which could then result in increased vasoconstriction leading to decreased intestinal perfusion.
Interestingly, prior data from rodent models of NEC have shown a significant increase in inflammatory cytokines, iNOS mRNA expression, NO production, and enterocyte apoptosis following gavaging Cronobacter sakazakii to immature mice to induce significant intestinal injury consistent with NEC [40]. Similar results have also been reported with animal models of NEC that utilize formula feeding and hypoxia or LPS [41–43]. However, it is important to note that all of these animal models result in significant tissue injury and elevation of inflammatory cytokines which act as potent inducers of NO production. Talavera et al has shown that intestinal epithelial cells exposed to inflammatory cytokines resulted in a significant increase in iNOS mRNA expression and NO production greater than direct LPS exposure [42]. In addition, these same studies have shown that increased NO production results in significant enterocyte apoptosis and necrosis while inhibiting repair mechanisms leading to increased intestinal injury, intestinal barrier failure, and bacterial translocation [40–43]. While these studies attempted to understand the role of NO isoforms in NEC-like injury, our study was designed to understand the specific effect of Paneth cell disruption on the blood flow of the intestinal microvasculature and were not designed to induce tissue injury. Therefore, the results of our study likely differ from those previously published because Paneth cell disruption with dithizone or diphtheria toxin administration alone does not produce significant intestinal injury or inflammation that is required for the increased iNOS expression or NO production as previously reported.
In conclusion, our data shows that dithizone-induced Paneth cell disruption in immature mice results in a significant decrease in the intestinal perfusion compared to controls and a concurrent decrease in nNOS and iNOS gene expression and tissue eNOS levels. However, this effect is importantly method-dependent as Paneth cells necrosis using a transgenic diphtheria toxin model results in neither response. This study provides additional insight on Paneth cell biology and their role on regulating the intestinal microvasculature. This data also advances our understanding of the NOS signaling pathway and downstream effects which are critical due to the role they play in the pathogenesis of NEC.
Acknowledgements:
Funding: SJM is supported by the National Institutes of Health DK097335 and the Carver College of Medicine at the University of Iowa. MG is supported by K08DK101608, R03DK111473 and R01DK118568 from the National Institutes of Health, March of Dimes Foundation Grant No. 5-FY17–79, the Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital, and the Department of Pediatrics at Washington University School of Medicine, St. Louis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- [1].Zhang HY, Wang F, Feng JX. Intestinal microcirculatory dysfunction and neonatal necrotizing enterocolitis. Chin Med J (Engl) 2013;126(9):1771–8. [PubMed] [Google Scholar]
- [2].Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr 1990;117(1 Pt 2):S6–13. [DOI] [PubMed] [Google Scholar]
- [3].Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Seminars in perinatology 2008;32(2):83–91. [DOI] [PubMed] [Google Scholar]
- [4].Yu X, Radulescu A, Zorko N, Besner GE. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology 2009;137(1):221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ito Y, Doelle SM, Clark JA, Halpern MD, McCuskey RS, Dvorak B. Intestinal Microcirculatory Dysfunction During the Development of Experimental Necrotizing Enterocolitis. Pediatric research 2007;61(2):180–4. [DOI] [PubMed] [Google Scholar]
- [6].Barlow B, Santulli TV. Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery 1975;77(5):687–90. [PubMed] [Google Scholar]
- [7].Downard CD, Grant SN, Matheson PJ, Guillaume AW, Debski R, Fallat ME, et al. Altered intestinal microcirculation is the critical event in the development of necrotizing enterocolitis. Journal of Pediatric Surgery 2011;46(6):1023–8. [DOI] [PubMed] [Google Scholar]
- [8].Murdoch EM, Sinha AK, Shanmugalingam ST, Smith GC, Kempley ST. Doppler flow velocimetry in the superior mesenteric artery on the first day of life in preterm infants and the risk of neonatal necrotizing enterocolitis. Pediatrics 2006;118(5):1999–2003. [DOI] [PubMed] [Google Scholar]
- [9].Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annual review of physiology 2013;75:289–311. [DOI] [PubMed] [Google Scholar]
- [10].McElroy SJ, Underwood MA, Sherman MP. Paneth cells and necrotizing enterocolitis: a novel hypothesis for disease pathogenesis. Neonatology 2013;103(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mallow EB, Harris A, Salzman N, Russell JP, DeBerardinis RJ, Ruchelli E, et al. Human enteric defensins. Gene structure and developmental expression. J Biol Chem 1996;271(8):4038–45. [DOI] [PubMed] [Google Scholar]
- [12].Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nature reviews Microbiology 2011;9(5):356–68. [DOI] [PubMed] [Google Scholar]
- [13].Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proceedings of the National Academy of Sciences of the United States of America 2002;99(24):15451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sherman MP, Bennett SH, Hwang FF, Sherman J, Bevins CL. Paneth cells and antibacterial host defense in neonatal small intestine. Infection and immunity 2005;73(9):6143–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proceedings of the National Academy of Sciences of the United States of America 2008;105(52):20858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bel S, Pendse M, Wang Y, Li Y, Ruhn KA, Hassell B, et al. Paneth cells secrete lysozyme via secretory autophagy during bacterial infection of the intestine. Science 2017;357(6355):1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Delorme-Axford E, Klionsky DJ. Secretory autophagy holds the key to lysozyme secretion during bacterial infection of the intestine. Autophagy 2018:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bevins CL. The Paneth cell and the innate immune response. Current opinion in gastroenterology 2004;20(6):572–80. [DOI] [PubMed] [Google Scholar]
- [19].Almohazey D, Lo YH, Vossler CV, Simmons AJ, Hsieh JJ, Bucar EB, et al. The ErbB3 receptor tyrosine kinase negatively regulates Paneth cells by PI3K-dependent suppression of Atoh1. Cell death and differentiation 2017;24(5):855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Coutinho HB, da Mota HC, Coutinho VB, Robalinho TI, Furtado AF, Walker E, et al. Absence of lysozyme (muramidase) in the intestinal Paneth cells of newborn infants with necrotising enterocolitis. Journal of clinical pathology 1998;51(7):512–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McElroy SJ, Weitkamp JH. Innate Immunity in the Small Intestine of the Preterm Infant. NeoReviews 2011;12(9):e517–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhang C, Sherman MP, Prince LS, Bader D, Weitkamp JH, Slaughter JC, et al. Paneth cell ablation in the presence of Klebsiella pneumoniae induces necrotizing enterocolitis (NEC)-like injury in the small intestine of immature mice. Disease models & mechanisms 2012;5(4):522–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Seminars in immunology 2007;19(2):70–83. [DOI] [PubMed] [Google Scholar]
- [24].Underwood MA. Paneth cells and necrotizing enterocolitis. Gut microbes 2012;3(6):562–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].White JR, Gong H, Pope B, Schlievert P, McElroy SJ. Paneth-cell-disruption-induced necrotizing enterocolitis in mice requires live bacteria and occurs independently of TLR4 signaling. Disease models & mechanisms 2017;10(6):727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yazji I, Sodhi CP, Lee EK, Good M, Egan CE, Afrazi A, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proceedings of the National Academy of Sciences of the United States of America 2013;110(23):9451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Good M, Sodhi CP, Yamaguchi Y, Jia H, Lu P, Fulton WB, et al. The human milk oligosaccharide 2’-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. The British journal of nutrition 2016;116(7):1175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].McElroy SJ, Frey MR, Yan F, Edelblum KL, Goettel JA, John S, et al. Tumor necrosis factor inhibits ligand-stimulated EGF receptor activation through a TNF receptor 1-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 2008;295(2):G285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brown KS, Gong H, Frey MR, Pope B, Golden M, Martin K, et al. Tumor necrosis factor induces developmental stage-dependent structural changes in the immature small intestine. Mediators of inflammation 2014;2014:852378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proceedings of the National Academy of Sciences of the United States of America 2000;97(11):6043–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, et al. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol 2009;297(3):G442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].McElroy SJ, Castle SL, Bernard JK, Almohazey D, Hunter CJ, Bell BA, et al. The ErbB4 ligand neuregulin-4 protects against experimental necrotizing enterocolitis. The American journal of pathology 2014;184(10):2768–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lueschow SR, Stumphy J, Gong H, Kern SL, Elgin TG, Underwood MA, et al. Loss of murine Paneth cell function alters the immature intestinal microbiome and mimics changes seen in neonatal necrotizing enterocolitis. PLoS One 2018;13(10):e0204967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sawada M, Takahashi K, Sawada S, Midorikawa O. Selective killing of Paneth cells by intravenous administration of dithizone in rats. International journal of experimental pathology 1991;72(4):407–21. [PMC free article] [PubMed] [Google Scholar]
- [35].Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nature methods 2005;2(6):419–26. [DOI] [PubMed] [Google Scholar]
- [36].Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. The Journal of biological chemistry 1997;272(38):23729–40. [DOI] [PubMed] [Google Scholar]
- [37].Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg 1997;32(2):275–82. [DOI] [PubMed] [Google Scholar]
- [38].Hunter CJ, De Plaen IG. Inflammatory signaling in NEC: Role of NF-kappaB, cytokines and other inflammatory mediators. Pathophysiology : the official journal of the International Society for Pathophysiology 2014;21(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nankervis CA, Nowicki PT. Role of endothelin-1 in regulation of the postnatal intestinal circulation. Am J Physiol Gastrointest Liver Physiol 2000;278:G367–G75. [DOI] [PubMed] [Google Scholar]
- [40].Emami CN, Mittal R, Wang L, Ford HR, Prasadarao NV. Role of neutrophils and macrophages in the pathogenesis of necrotizing enterocolitis caused by Cronobacter sakazakii. J Surg Res 2012;172(1):18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 2000;92(1):71–7. [DOI] [PubMed] [Google Scholar]
- [42].Talavera MM, Nuthakki S, Cui H, Jin Y, Liu Y, Nelin LD. Immunostimulated Arginase II Expression in Intestinal Epithelial Cells Reduces Nitric Oxide Production and Apoptosis. Frontiers in cell and developmental biology 2017;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Whitehouse JS, Xu H, Shi Y, Noll L, Kaul S, Jones DW, et al. Mesenteric nitric oxide and superoxide production in experimental necrotizing enterocolitis. J Surg Res 2010;161(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]