Most bacterial species synthesize surface-exposed appendages that are important for environmental interactions and survival under diverse conditions. It is often assumed that these appendages act independently of each other and that mutations in either system can be used to assess functionality in specific processes. However, we show that mutations in flagellar genes can impact the production of type IV pili, as well as alter general RNA transcriptional profiles compared to a wild-type strain. These data demonstrate that seemingly simple mutations can broadly affect cell-regulatory networks.
KEYWORDS: Caulobacter crescentus, flagella, gene regulation, type IV pili
ABSTRACT
Surface appendages, such as flagella and type IV pili, mediate a broad range of bacterial behaviors, including motility, attachment, and surface sensing. While many species harbor both flagella and type IV pili, little is known about how or if their syntheses are coupled. Here, we show that deletions of genes encoding different flagellum machinery components result in a reduction of pilus synthesis in Caulobacter crescentus. First, we show that different flagellar mutants exhibit different levels of sensitivity to a pilus-dependent phage and that fewer cells within populations of flagellar mutants make pili. Furthermore, we find that single cells within flagellar mutant populations produce fewer pili per cell. We demonstrate that these gene deletions result in reduced transcription of pilus-associated genes and have a slight but significant effect on general transcription profiles. Finally, we show that the decrease in pilus production is due to a reduction in the pool of pilin subunits that are polymerized into pilus fibers. These data demonstrate that mutations in flagellar gene components not only affect motility but also can have considerable and unexpected consequences for other aspects of cell biology.
IMPORTANCE Most bacterial species synthesize surface-exposed appendages that are important for environmental interactions and survival under diverse conditions. It is often assumed that these appendages act independently of each other and that mutations in either system can be used to assess functionality in specific processes. However, we show that mutations in flagellar genes can impact the production of type IV pili, as well as alter general RNA transcriptional profiles compared to a wild-type strain. These data demonstrate that seemingly simple mutations can broadly affect cell-regulatory networks.
INTRODUCTION
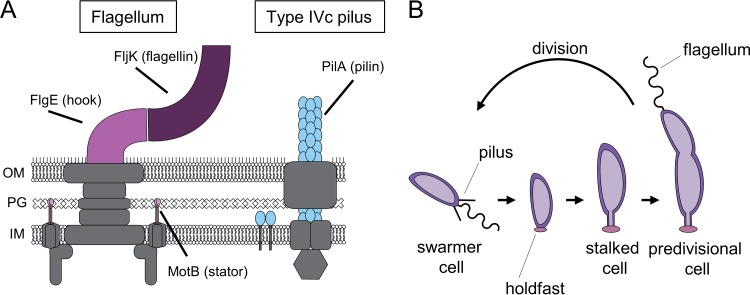
Bacterial surface appendages, such as flagella and type IV pili, play critical roles in bacterial life cycles in diverse environments. Flagella are motorized rotary filaments comprised of flagellin subunits that drive swimming motility and are often important for attachment (1, 2). Type IV pili are dynamic fibers that are extended and retracted through pilin subunit polymerization and depolymerization, respectively (3). Type IV pili participate in processes ranging from surface sensing and biofilm formation to infection and horizontal gene transfer (4–10). Both flagellar and pilus machineries span from the cytoplasmic membrane of the cell to the extracellular environment, allowing cells to both interact with and respond to their surroundings (Fig. 1A). Often, the production of both flagella and type IV pili is temporally coupled, as is the case for Caulobacter crescentus. C. crescentus exhibits a dimorphic life cycle (Fig. 1B) in which newborn, nonreplicative swarmer cells harbor a single flagellum and multiple, dynamic type IVc tad pili at the same pole. Upon differentiation into a reproductive cell type, the cells secrete an adhesive polysaccharide called the holdfast, and the flagellum and pili are replaced by a cell envelope extension called the stalk. While it is known that the expression of both pili and the flagellum is coordinated by the activity of the master transcriptional regulator CtrA in a cell cycle-dependent manner (11), whether their syntheses are interconnected remains unclear.
FIG 1.
Schematic of extracellular appendages and the C. crescentus life cycle. (A) Schematic of flagellum and pilus machinery present in C. crescentus. Flagellum components deleted in this study are shown in pink and magenta. Pilin subunits are shown in blue. The gray structures are additional machinery components of either apparatus. OM, outer membrane; PG, peptidoglycan; IM, inner membrane. (B) Diagram of the C. crescentus life cycle showing that newborn swarmer cells lose their pili and flagella before differentiating into replicative stalked cells.
Many bacteriophages use extracellular appendages such as pili as receptors during infection (12–14). It is thought that phage particles bind to pili, which retract and pull the phage to the cell body, where infection occurs. Previous studies have shown that flagellum mutations confer some resistance to the C. crescentus pilus-dependent phage ΦCbK (15). It has been shown that ΦCbK phage can directly bind to the flagellum filament, and it has been hypothesized that this binding is important for infection (13). Interestingly, however, different levels of phage resistance are conferred by different flagellum mutants (16). For example, while hook and flagellin mutants both lack the ability to produce flagellar filaments, they exhibit different fitness values upon infection with phage particles, suggesting a role for component assembly feedback in pilus synthesis. We recently showed that flagellum assembly can play a regulatory role in the production of the permanent adhesive holdfast in C. crescentus, and we thus hypothesized that a similar mechanism of regulatory feedback may exist for type IV pilus synthesis (17).
In this study, we investigate the role of flagellum assembly in pilus synthesis. We compare the abilities of mutants lacking different components involved in flagellum function and assembly to make pili. We reconfirm that different flagellum mutations exhibit different levels of ΦCbK phage sensitivity. We find that the ΔfljK flagellin mutant is the most severely affected for pilus biosynthesis and that this correlates with its level of ΦCbK phage resistance. Furthermore, we find that the reduction in pilus synthesis is due to a decrease in pilin subunits in mutant populations. RNA sequencing data on different flagellar mutants show not only a decrease in transcript levels of pilus-associated genes but also significant, subtle changes in transcription profiles, highlighting a role for flagellum assembly feedback in numerous cellular processes.
RESULTS
Assessment of phage sensitivity in different flagellar mutants.
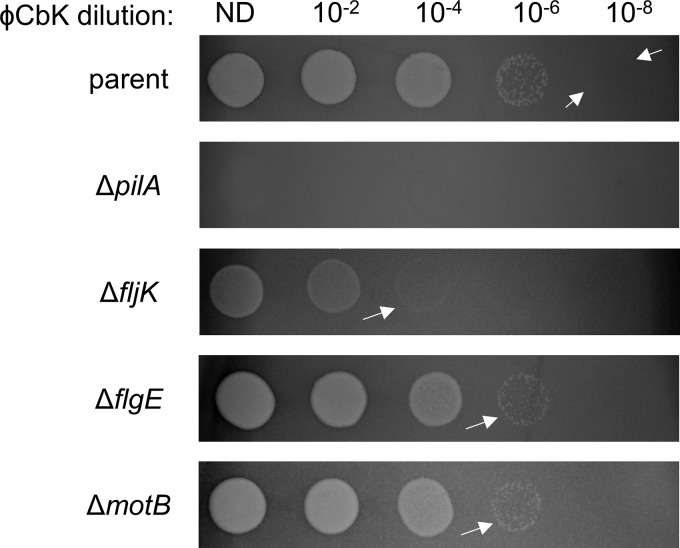
A previous study performed transposon sequencing (Tn-seq) on libraries infected with different quantities of the pilus-dependent phage ΦCbK to assess factors important for infection (16). Mutants deficient in phage adsorption, including those lacking pilus and flagellum machinery biosynthesis components, provided the greatest fitness advantage in the presence of phage. Surprisingly, mutations in different components of the flagellum machinery conferred different levels of phage resistance, despite most mutations ultimately resulting in a lack of filament synthesis. To confirm this result, we first performed ΦCbK phage sensitivity assays in mutants lacking different flagellum components using a phage-resistant pilin mutant (ΔpilA) as a control (Fig. 1A and 2). To test for phage sensitivity, we performed plaque assays in which we spotted different dilutions of ΦCbK phage onto lawns of C. crescentus strains and measured the formation of clear spots, or plaques, indicative of phage-induced cell death. We treated flagellar filament mutants lacking either the major flagellin subunit (ΔfljK) (18) or the hook (ΔflgE). We also assessed phage sensitivity in a mutant lacking the stator (ΔmotB), which possesses a paralyzed flagellar filament (Fig. 2). While pilin mutants were completely resistant to phage infection at all phage concentrations, as described previously, the parent strain exhibited plaques out to a 10−8 phage dilution. All the mutants exhibited more resistance to ΦCbK phage and formed fewer plaques at higher phage concentrations than the parent strain. The fljK mutant had the most severe decrease in phage sensitivity, exhibiting plaques out to only a 10−4 phage dilution. Interestingly, the flgE and motB mutants had the same level of phage resistance, both exhibiting cloudier plaques than the parent out to a 10−6 phage dilution.
FIG 2.
Flagellar mutants exhibit different levels of sensitivity to pilus-dependent ΦCbK phage. Shown are ΦCbK phage sensitivity assays on the indicated strains. The arrows indicate the lowest dilution with visible plaques for each strain, with the parent strain arrows indicating individual plaques. ND, no dilution.
Flagellar mutants make fewer pili.
It was previously shown that ΦCbK phage particles can bind the flagellum, leading to the hypothesis that phage resistance in flagellar mutants is due to reduced efficiency of phage to bind cells effectively (13). However, if the sole role of the flagellum in phage infection is adsorption for phage particles, different filament mutants should be equally deficient in phage infection. Since different filament mutations confer varied levels of phage resistance, we reasoned that the flagellum must play an additional role in processes that affect the efficiency of phage infection.
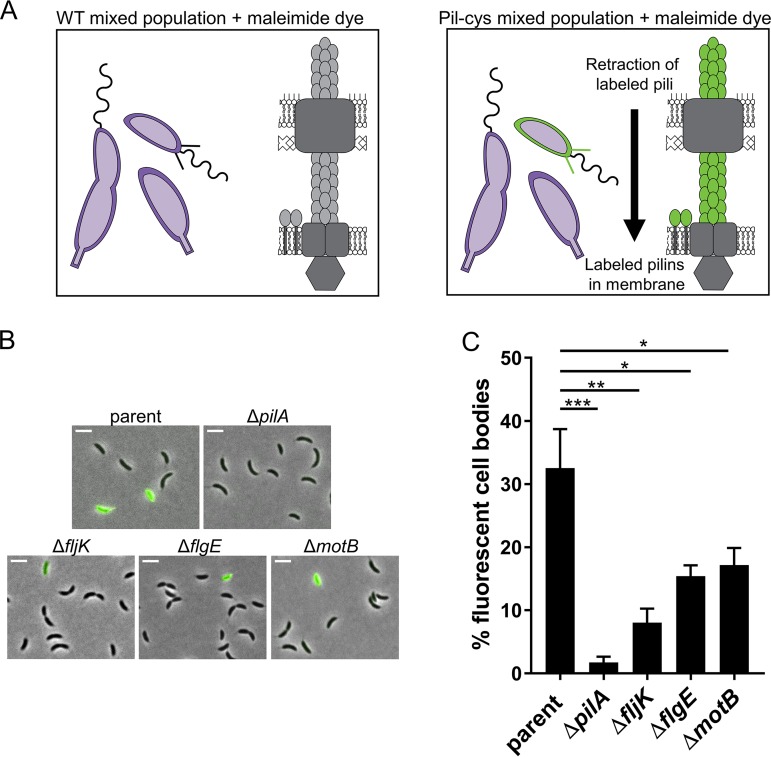
While the presence of a flagellum is not essential for ΦCbK phage infection, pilus biosynthesis is critical, as shown above (Fig. 2). Tn-seq analysis comparing ΦCbK phage-infected and uninfected transposon libraries showed that mutations in flagellar genes conferred resistance to phage infection that was similar to resistance conferred by mutations in the pilus machinery (16), and we thus hypothesized that flagellar mutations may affect pilus synthesis. To test this hypothesis, we used a previously described method for labeling pili (9, 10, 37). The method relies on a cysteine substitution within the PilA pilin subunit, resulting in a “pil-cys” strain that can be subsequently labeled with thiol-reactive maleimide dyes (Fig. 3A). In C. crescentus, the retraction of externally labeled pilin subunits results in fluorescent cell bodies, because fluorescently labeled pilins are disseminated into an inner membrane pilin pool. Consequently, cells that do not make pili, including stalked and predivisional cells, do not exhibit cell body fluorescence in populations containing all cell types (Fig. 3B) (9). We previously demonstrated that the pil-cys mutation has no effect on pilus function or phage sensitivity (9), and we therefore used cell body fluorescence in pil-cys versions of flagellar mutants as a metric for whether pilus synthesis was altered. Around 30% of the cells within mixed populations of the parent strain were fluorescent upon labeling with maleimide dye, consistent with previous reports (9). Less than 10% of cells within fljK mutant populations exhibited cell body fluorescence, while both flgE and motB mutant populations contained ∼15% fluorescent cells (Fig. 3B and C), consistent with phage resistance phenotypes (Fig. 2).
FIG 3.
Fewer cells make pili in populations of flagellar mutants. (A) Schematic of pilus labeling. Upon addition of fluorescent maleimide dyes, external PilA subunits of the pil-cys strain become labeled. Retraction of labeled pilins disseminates fluorescent pilins throughout the inner membrane, resulting in cell body fluorescence. (B) Microscopy images of pil-cys cells labeled with AF488-mal dye (green) that binds to cysteine residues within the pilin. Scale bars, 3 μm. (C) Quantification of the percentages of fluorescent cells in populations of different mutants. The bars indicate the means and standard errors of the mean (SEM) of the results of 3 or 4 independent biological replicates. At least 500 cells were analyzed per replicate. Statistical comparisons were made using Sidak’s multiple-comparison test. *, P < 0.05; **, P < 0.005; ***, P < 0.0001.
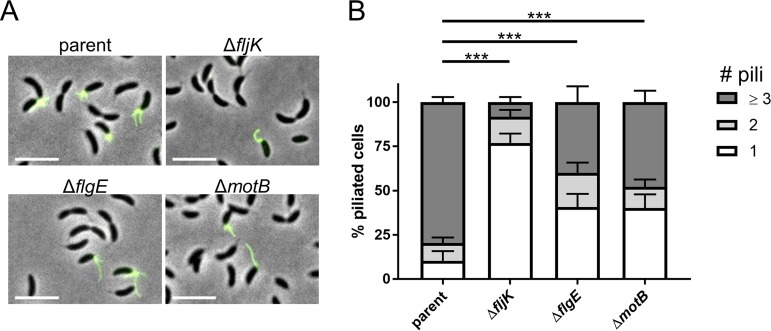
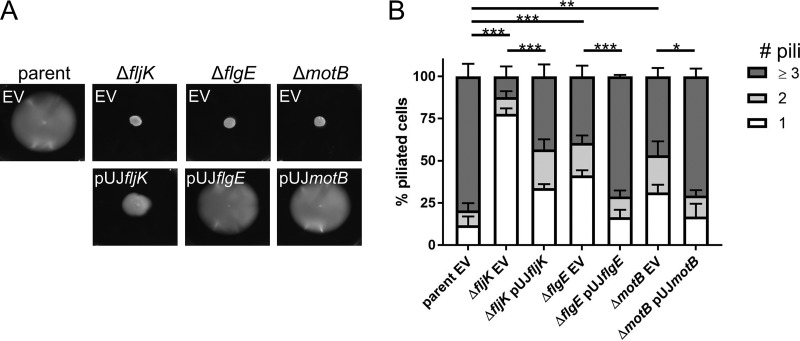
While these data show that fewer cells make pili within mixed populations of flagellum mutants, we previously demonstrated that flagellum mutants exhibit normal cell cycles (17), indicating that the decrease in the number of piliated cells was not due to a decrease in the number of swarmer cells or an alteration in the timing of cell differentiation. We therefore hypothesized that the number of pili that individual piliated swarmer cells produce may also be affected. To measure the total number of pili made per piliated cell, we used a method for blocking pilus retraction that involves the coincubation of cells with both maleimide dye and a bulky maleimide compound, polyethylene glycol-maleimide (PEG-mal). The pilin-bound PEG-mal blocks retraction, as it is presumably too large to penetrate the outer membrane pilus pore. This results in a hyperpiliation phenotype and lack of cell body fluorescence (Fig. 4A) (9). We found that ∼80% of blocked piliated cells within the parent population made three or more pili per cell, while flagellar mutant cells produced significantly fewer pili (Fig. 4B). Piliated fljK mutants largely made only one pilus per cell, while ∼45% and ∼50% of flgE and motB mutants, respectively, made less than three pili per cell. Importantly, complementation of flagellar genes expressed from a leaky xylose-inducible promoter on a high-copy-number plasmid restored both swimming motility and the number of pili made per cell (Fig. 5). Notably, fljK complementation only partially restored swimming motility (Fig. 5A), and this low level of complementation likely explains the partially complemented pilus phenotype, where only ∼45% of piliated cells made three or more pili (Fig. 5B).
FIG 4.
Flagellar mutants make fewer pili per cell. (A) Microscopy images of pil-cys strains with pilus retraction obstructed with PEG-mal and labeled with AF488-mal. Scale bars, 5 μm. (B) Quantification of the percentages of piliated cells within populations of flagellar mutants that make one, two, or three or more pili. The bars indicate the mean and SEM of the results of 4 independent biological replicates. At least 20 cells were analyzed per replicate. Statistical comparisons were made using Sidak’s multiple-comparison test between the numbers of pili per cell for each strain. ***, P < 0.0005.
FIG 5.
Complementation of flagellar genes restores defects in pilus production. (A) Low-percentage motility agar plates of strains containing either an empty-vector (EV) control or a complementation of the flagellar gene deletion. (B) Quantification of the percentages of piliated cells within populations of flagellar mutants that make one, two, or three or more pili in strains containing either an empty-vector control or a complementation of the flagellar gene deletion. The bars indicate the means and SEM of the results of 3 independent biological replicates. At least 20 cells were analyzed per replicate. Statistical comparisons were made using Sidak’s multiple-comparison test between the average numbers of pili per cell for each strain. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Flagellar mutants have reduced pilin levels.
To determine the cause of reduced pilus synthesis in flagellar mutants, we performed RNA sequencing on flgE and motB mutants to assess the transcriptional profiles of the mutant populations. We found a statistically significant reduction in the expression of pilus-associated genes, including pilA, which was reduced by ∼1.3-fold in both mutants (Table 1). Interestingly, the expression of genes encoding known pilus machinery components was unaffected, though expression of an uncharacterized gene within the pilus locus, CCNA_03034, was reduced ∼1.8-fold in both mutants. Additionally, expression of cpaL, which encodes a protein that is important for ΦCbK phage infection in C. crescentus (16), was reduced in flgE mutants, though its function in pilus biology remains unclear. In addition to altered expression of pilus components, we found that both flgE and motB mutants had subtle but significant alterations in their transcriptional profiles compared to the wild-type strain, suggesting feedback on multiple regulatory networks (see Table S1 in the supplemental material).
TABLE 1.
RNA profiles of flagellar mutants reveal reduction in expression of pilus-associated genes
| Mutation | Gene affecteda | Fold change compared to WTb |
|---|---|---|
| ΔflgE | pilA | −1.289833398 |
| cpaL | −1.267913697 | |
| CCNA_03034 | −1.824813033 | |
| ΔmotB | pilA | −1.323230512 |
| cpaL | No significant change | |
| CCNA_03034 | −1.883660437 |
No other pilus genes were significantly altered in expression in the mutants.
WT, wild type.
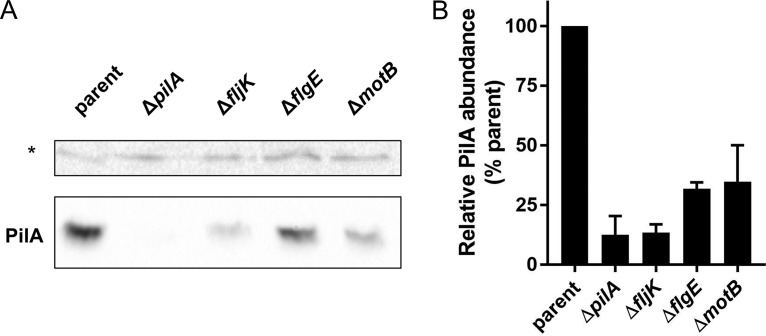
Because flagellar mutants exhibit decreased expression of pilA transcripts, we sought to measure the levels of PilA protein in different mutant strains. Using a PilA antibody, we probed flagellar mutants for PilA production and found that fljK mutants produced ∼12% of the amount of pilin produced by the parent strain, while flgE and motB mutants both produced ∼30% (Fig. 6). Together, these results suggest that reduced pilin levels are responsible for the deficiency in pilus synthesis observed in flagellum mutants and that flagellum assembly feedback plays a role in pilin production.
FIG 6.
Flagellar mutants produce fewer pilins. (A) Representative Western blot showing reduced pilin expression in flagellar mutants. PilA levels are shown in the bottom gel. The asterisk indicates a nonspecific background band that runs at approximately 50 kDa used as a loading control for normalization for the quantification of expression shown in panel B. (B) Quantification of pilin produced in different flagellar mutants relative to parental levels of expression. The bars indicate the means and SEM of the results of 3 independent biological replicates.
DISCUSSION
In this work, we investigated the role of flagellum assembly in the regulation of type IV pilus production. We show that flagellar mutant populations have fewer cells that make pili and that piliated cells within those populations make fewer pili per individual cell. This reduction in pilus synthesis is due to decreased pilin levels, and furthermore, we found that flagellar mutations lead to modest yet significant alterations in transcription profiles.
In some species, it is thought that pili and flagella play a direct role in providing sensory input from the environment to the cell in order to regulate specific behaviors, including permanent attachment in C. crescentus. Mutations in either component are often used under the assumption that flagellar mutants are solely deficient in flagellum synthesis and pilus mutants are solely deficient in pilus synthesis. However, a recent study demonstrated that there is feedback regulation between pilus components and flagellar machinery through the PilSR two-component system in Pseudomonas (19). Additionally, our data indicate that surface appendage mutations are not always independent, making it difficult to tease apart the roles of these structures in different processes. For example, recent studies using mutants in both pilus and flagellar machinery components inferred a role for either pili or flagella in C. crescentus surface sensing (9, 20). Interestingly, we found that, under static conditions, flagellar components, including the FlgE hook and MotB motor components, are not required for surface sensing (9, 17) and that obstruction of flagellum rotation did not stimulate holdfast synthesis, as was previously hypothesized (8). However, under those conditions, obstruction of pilus retraction stimulated holdfast synthesis independently of surface contact, suggesting a role for the tension on retracting surface-bound pili in bacterial surface sensing. Under flow conditions, however, it was shown that the flagellum motor plays an important role in surface colonization, as cells lacking the MotB stator component were deficient in undergoing permanent attachment (20). In light of the results presented here, where different flagellar mutations could affect pilus synthesis and the expression of diverse genes, the inability of motB mutant cells to sense surfaces under high-flow conditions may be the result of pilus synthesis deficiency or other flagellum assembly feedback regulation rather than a direct role of MotB in surface sensing. Though motB mutants can produce dynamic pili, it is not yet clear how many pili are required to trigger pilus-mediated holdfast synthesis, and it is unclear whether flow conditions may alter this parameter, leaving this a possibility to be tested.
In C. crescentus, flagellum synthesis is tightly linked with the cell cycle. Flagellum assembly is hierarchical, and genes are grouped into four classes based on this hierarchical profile, with the completion of flagellar synthesis occurring prior to division (21). Early class II components (type III secretion and regulators) must be expressed and assembled prior to class III gene components (hook and basal body), which likewise must be assembled prior to class IV gene (flagellins) expression. In line with this, RNA sequencing data revealed reduced expression of several flagellar genes within the class III flgE mutant but not in the motB mutant, which falls outside of the regulatory hierarchy (see Table S1 in the supplemental material). Interestingly, flagellin genes, which encode class IV flagellar components classified into α or β subunits, exhibited different changes in expression in the flgE mutant. There are six flagellins produced by C. crescentus (18), and while the β-flagellin genes fljM, fljN, and fljO all experienced at least a 3-fold decrease in expression, expression of the α-flagellin genes was variable, with fljJ experiencing no change, fljK experiencing a 14-fold reduction, and fljL experiencing a 5-fold increase in expression. Even more surprising, while fljK expression was severely reduced in the flgE mutant, the reduction of pilus synthesis in flgE mutants does not match pilus phenotypes of the fljK mutant, which makes significantly fewer pili. These results suggest that different mechanisms exist for the regulation of pilus synthesis and that different flagellar mutations may distinctively affect the regulatory pathways for pilus production.
Synthesis of flagella and pili is temporally and spatially coupled in C. crescentus, though flagellar assembly components are produced and assembled prior to pilin production. Interestingly, we report that pilA transcript levels exhibited only a moderate ∼1.3-fold decrease, while PilA levels from the same mutants were more severely reduced, with at least a 3-fold reduction in pilin concentration compared to the parent strain. These results suggest additional posttranscriptional regulation that is dependent on flagellar assembly feedback. Late-stage assembly of the class III assembly components is sensed by FlbT, which negatively controls translation of the class IV flagellin proteins (22). FlbT has been suggested to bind fljK mRNA to physically block translation from occurring, and it is possible that a similar regulatory mechanism exists to control pilin synthesis. Alternatively, C. crescentus type IV pilins are degraded upon cell differentiation by the activity of an unknown protease, and perhaps the regulation of this degradation is altered in flagellar mutants. It is unclear what the overall effect of reduced pilin levels means for the cell. As pilins reside in an inner membrane pilin pool, it remains to be determined how changes in total pilin abundance may alter this pilin pool and pilin availability for pilus extension machinery.
The majority of transcriptional changes we report are highly significant yet result in a less than 2-fold change in expression. Though it is unclear if all the transcriptional alterations observed are biologically relevant, it raises the point that mutations may have unanticipated effects on other aspects of cell biology. These subtle transcript alterations could be due to changes in gene regulation as a result of feedback from the flagellar synthesis transcriptional hierarchy.
In conclusion, we have observed that the production of type IV pili and that of flagella are linked in C. crescentus. We show that different classes of flagellar mutants are significantly deficient in pilus synthesis, and our data suggest that different stages of flagellum assembly act as checkpoints for the regulation of pilus expression.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains used in this study are listed in Table 2. C. crescentus strains were grown at 30°C in peptone-yeast extract (PYE) medium (23) supplemented with 5 μg/ml kanamycin (Kan) or 0.5 μg/ml chloramphenicol (Cm) where appropriate. Escherichia coli DH5α (Bioline) was used for cloning and grown at 37°C in lysogeny broth (LB) medium supplemented with 25 μg/ml kanamycin or 20 μg/ml chloramphenicol where appropriate.
TABLE 2.
Strains, plasmids, and primers used in this study
| Strain, plasmid, or primer | Description or construction | Source or reference | Sequencea |
|---|---|---|---|
| Strains | |||
| E. coli | |||
| YB4026 | S17-1/pNPTS138ΔflgE | 17 | |
| YB8286 | α-Select/pNPTS139PilAT36C | 9 | |
| YB3087 | S17-1/pLW98 | 33 | |
| YB9221 | α-Select/pUJfljK | This study | |
| YB428 | α-Select/pUJflgE | This study | |
| YB9222 | α-Select/pUJmotB | This study | |
| Caulobacter | |||
| YB8288 (parent) | NA1000 PilAT36C | 9 | |
| LS3118 (ΔpilA) | NA1000 ΔpilA | 34 | |
| YB8306 (ΔfljK) | NA1000 PilAT36C ΔfljK (lysate made from YB8287 transduced into TPA2234) | This study | |
| YB8307 (ΔflgE) | NA1000 PilAT36C ΔflgE (plasmid from strain YB4026 electroporated into strain YB8288) | This study | |
| YB8528 (ΔmotB) | NA1000::pLW98 PilAT36C ΔmotB (lysate from strain YB8524 transduced into strain YB8288) | This study | |
| FC764 | NA1000 hfsA+ | 35 | |
| YB6375 | NA1000 hfsA+ ΔflgE | 17 | |
| TPA2234 | NA1000 ΔfljK | 18 | |
| YB7377 | NA1000 hfsA+ ΔmotB | 17 | |
| YB8287 | NA1000::pNPTS139PilAT36C hfsA+ PilAT36C (electroporated strain YB8220 with plasmid from strain YB8286) | This study | |
| YB8524 | NA1000::pLW98 ΔmotB (strain YB3087 mated with YB7377) | This study | |
| YB9228 (parent EV) | NA1000 PilAT36C pUJ142 (electroporated strain YB8288 with pUJ142) | This study | |
| YB9229 (ΔpilA EV) | NA1000 ΔpilA pUJ142 (electroporated strain LS3118 with pUJ142) | This study | |
| YB9237 (ΔflK EV) | NA1000 PilAT36C ΔfljK pUJ142 (electroporated strain YB8306 with pUJ142) | This study | |
| YB9225 (ΔflK pUJfljK) | NA1000 PilAT36C ΔfljK pUJfljK (electroporated strain YB8306 with plasmid from strain YB9221) | This study | |
| YB9238 (ΔflgE EV) | NA1000 PilAT36C ΔflgE pUJ142 (electroporated strain YB8307 with pUJ142) | This study | |
| YB9230 (ΔflgE pUJflgE) | NA1000 PilAT36C ΔflgE pUJflgE (electroporated strain YB8307 with plasmid from strain YB428) | This study | |
| YB9239 (ΔmotB EV) | NA1000::pLW98 PilAT36C ΔmotB pUJ142 (electroporated strain YB8528 with pUJ142) | This study | |
| YB9231 (ΔmotB pUJmotB) | NA1000::pLW98 PilAT36C ΔmotB pUJmotB (electroporated strain YB8528 with plasmid from strain YB9222) | This study | |
| Plasmids | |||
| pNPTS138 | Litmus 38 derivative; oriT sacB Kanr | M. R. K. Alley | |
| pNPTS139 | Litmus 39 derivative; oriT sacB Kanr | M. R. K. Alley | |
| pUJ142 | Xylose-inducible promotor; Cmr | 36 | |
| pNPTS138ΔflgE | pNPTS138 containing 480-bp fragments upstream and downstream of flgE | 17 | |
| pLW98 | Suicide vector containing marker CMS18; Kanr | 33 | |
| pNPTS139PilAT36C | pNPTS139 containing 500-bp fragments upstream and downstream of PilAT36C point mutation | 9 | |
| pUJfljK | pUJ142 containing fljK gene under control of xylose-inducible promotor | This study | |
| pUJflgE | pUJ142 containing flgE gene under control of xylose-inducible promotor | This study | |
| pUJmotB | pUJ142 containing motB gene under control of xylose-inducible promotor | This study | |
| Primers | |||
| pUJfljKF | Construction of pUJfljK | TAATAA/AAGCTTGAGCAAAATGCTCCCGGCAGCCA | |
| pUJfljKR | Construction of pUJfljK | TAATAA/GGTACCCCAGACGACTCGCCCCCGTCCC | |
| 3flgEHindIIIF | Construction of pUJflgE | TAATAA/AAGCTTAGCGCTGAACCCTCGTCCCCC | |
| 3flgEKpnIR | Construction of pUJflgE | TAATAA/GGTACCGCCCTATGATTAGCGCTTAATATTCAAGAGTTCCTCA | |
| pUJmotBF | Construction of pUJmotB | TAATAA/GAATTCCTTCATGCCGCTGCTCCTAAAAAGAAGGC | |
| pUJmotBR | Construction of pUJmotB | TAATAA/AAGCTTCACAAGAGGCCACGCGCGGAGA | |
Boldface indicates the 5′ tail/restriction site.
Plasmids were transferred to C. crescentus by electroporation, transduction with ΦCr30 phage lysates, or conjugation with S-17 E. coli strains as described previously (24). In-frame deletion strains were made by double homologous recombination using pNPTS-derived plasmids as previously described (25). Briefly, plasmids were introduced into C. crescentus, and then two-step recombination was performed using sucrose and kanamycin resistance or sensitivity as a selection for each step. Complementation constructs were made using the high-copy-number vector pUJ142 with genes under the control of the xylose-inducible promoter. Because expression from this promoter is leaky, no xylose was used for complementation experiments. For construction of the fljK, flgE, and motB complementation plasmids, genomic C. crescentus DNA was PCR amplified using primers containing restriction sites (Table 2), and the amplified DNA was purified (QIAquick). The fljK and flgE PCR fragments were digested using HindIII and KpnI, and the digested product was purified and ligated into plasmid pUJ142 digested by the same enzymes. The motB PCR fragment was treated in the same manner but using EcoRI and KpnI enzymes.
Phage sensitivity assays.
Phage sensitivity assays were performed by spotting dilutions of ΦCbK phage onto lawns of growing C. crescentus strains. To make the lawns, 200 μl of stationary-phase cultures was mixed with 3 ml of top agar (0.5% agar in PYE) and spread over 1.5% PYE agar plates. After the top agar solidified, 5 μl of phage diluted in PYE was spotted on top. The plates were grown for 2 days at 30°C before imaging.
Quantification of fluorescent cells and quantification of pili on cells blocked for pilus retraction.
Pili were labeled as described previously (9). Briefly, bacterial cultures were grown to an optical density at 600 nm (OD600) of 0.2 to 0.4, and 100 μl was labeled with 25 μg/ml Alexa Fluor 488 C5 maleimide dye (AF488-mal) (ThermoFisher) for 5 min at room temperature. To block pilus retraction, cells were incubated simultaneously with AF488-mal and 500 μM methoxypolyethylene glycol maleimide (PEG5000-mal) (Sigma) for 5 min at room temperature. The cells were centrifuged at 5,200 × g for 1 min to pellet them and then washed with 100 μl of PYE and centrifuged again. The supernatant was removed, and the cells were concentrated in 5 to 7 μl of PYE. One microliter of washed, labeled cells was spotted onto a 60- by 22-mm glass coverslip and covered with a 1% agarose (SeaKem) PYE pad before imaging. Imaging was performed on a Nikon Ti-2 microscope using a Plan Apo 60× objective, a green fluorescent protein (GFP) filter cube, a Hamamatsu OrcaFlash 4.0 camera, and Nikon NIS Elements imaging software. Quantification of fluorescent cells was performed using MicrobeJ (26). Determination of the number of pili per cell was performed manually using ImageJ software (27).
Motility assays.
Motility assays were performed as described previously (17). Briefly, a toothpick containing bacterial cells of each strain was stabbed into 0.3% agar in PYE medium in six-well polystyrene plates supplemented with chloramphenicol. The plates were then stored in a humid chamber for 4 days at 30°C before imaging and analysis.
RNA extraction, sequencing, and analysis.
Total RNA from three independent biological replicates of strains FC764, YB6375, and YB7377 grown to an OD600 of 0.2 to 0.3 was extracted using an RNA Isolate II kit (Bioline). To remove DNA from samples, the extracted RNA was treated with DNase I (Ambion Turbo DNA-free kit). The DNase-treated RNA was then purified using an RNeasy MinElute cleanup kit (Qiagen).
The total extracted RNA was sent to the Center for Genomics and Bioinformatics core facility, Indiana University, Bloomington, IN, for library preparation and next-generation sequencing analysis. The purified total RNA was first treated with a Ribo-Zero rRNA (bacteria) removal kit (Illumina; catalog no. MRZB12424) and then subjected to library preparation using a TruSeq Stranded mRNA HT sample preparation kit (Illumina; catalog no. RS-122-2103) according to the manufacturer’s protocols. Multiple dual-indexed adapters of 8 nucleotides (nt) each (D701 to D712 and D501 to D508) were added to the libraries during construction for multiplexing. The barcoded libraries were cleaned by double-side bead cutting with AMPure XP beads (Beckman Coulter; catalog no. A63882), verified using a Qubit 3 fluorometer (ThermoFisher) and a 2200 TapeStation bioanalyzer (Agilent Technologies), and then pooled. The pool was sequenced on a NextSeq 500 (Illumina) with a NextSeq 75 high-output v2 kit (Illumina; catalog no. FC-404-2005). Single-end 73-bp read sequences were generated. The read sequences were demultiplexed using bcl2fastq software v2.20.0.422.
Trimmomatic (28) (version 0.33; nondefault parameters, ILLUMINACLIP<adapter_file>:2:20:6 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:40) was used to trim reads of adapter and low-quality bases. The reads were mapped to the C. crescentus NA1000 (GenBank accession no. NC_011916.1) with bowtie2 using default parameters (29). Read counts were assigned to genomic features, including genes and custom-specified intergenic regions 100 bp in length, using a custom PERL script. Appropriate differential expression comparisons of all features with 5 or more reads (total across all samples) were carried out with DESeq2 (R package version 3.4.0) (30), along with the IHW package to adjust for multiple testing procedures (31). Only transcript levels with adjusted P values of less than 0.2 and IHW P values of less than 0.01 were included in Table 1 and Table S1 in the supplemental material.
Western analysis.
To measure the levels of PilA proteins produced by different strains, approximately 109 cells from exponentially growing cultures were centrifuged for 3 min at 16,100 × g. The supernatant was removed, and the cell pellets were resuspended in phosphate-buffered saline (PBS) and a final concentration of 1× SDS loading buffer (62.5 mM Tris-HCl [pH 6.8], 10% [vol/vol] glycerol, 2% [wt/vol] SDS, 0.05% [vol/vol] β-mercaptoethanol, 0.0025% [wt/vol] bromophenol blue). Samples were boiled for 6 min and then separated on SDS-18% polyacrylamide gels. The samples were transferred from the gels to nitrocellulose membranes and probed with anti-PilA antibody (32) at 1:5,000 dilution in 5% nonfat milk powder resuspended in 1× TTBS (20 mM Tris-HCl [pH 7.6], 130 mM NaCl, 0.05% Tween 20) for 16 to 20 h. The membrane was then washed three times with 1× TTBS and probed with horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (Bio-Rad) at 1:20,000 dilution in 5% nonfat milk solution for 1 h. The membrane was developed using SuperSignal West Dura substrate (ThermoFisher) and imaged on a Bio-Rad ChemiDoc imaging system. For quantification, three blots containing independent biological replicates of each strain were probed as described above. The PilA signal from each strain was measured using ImageJ (27) and normalized to the signal measured from a nonspecific band used as a loading control. The normalized parent strain signal for each blot was set to 100% for comparisons of relative PilA levels for all mutant strains.
Data availability.
The RNA sequencing data reported in this study can be accessed by GEO accession no. GSE126478.
Supplementary Material
ACKNOWLEDGMENTS
We thank P. Caccamo and C. Berne for critical comments on the manuscript. We also thank the Center for Genomics and Bioinformatics at Indiana University for RNA-sequencing work.
This study was supported by grant R35GM122556 from the National Institutes of Health, by a Canada 150 Research Chair in Bacterial Cell Biology to Y.V.B., and by National Science Foundation fellowship 1342962 to C.K.E.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00031-19.
REFERENCES
- 1.Belas R. 2014. Biofilms, flagella, and mechanosensing of surfaces by bacteria. Trends Microbiol 22:517–527. doi: 10.1016/j.tim.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Li G, Tang JX. 2006. Low flagellar motor torque and high swimming efficiency of Caulobacter crescentus swarmer cells. Biophys J 91:2726–2734. doi: 10.1529/biophysj.106.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burrows LL. 2012. Twitching motility: type iv pili in action. Annu Rev Microbiol 66:493–520. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 4.Bodenmiller D, Toh E, Brun YV. 2004. Development of surface adhesion in Caulobacter crescentus. J Bacteriol 186:1438–1447. doi: 10.1128/JB.186.5.1438-1447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Persat A, Inclan YF, Engel JN, Stone HA, Gitai Z. 2015. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 112:7563–7568. doi: 10.1073/pnas.1502025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Lim MS, Li S, Brock M, Pique ME, Woods VL, Craig L. 2008. Vibrio cholerae toxin-coregulated pilus structure analyzed by hydrogen/deuterium exchange mass spectrometry. Structure 16:137–148. doi: 10.1016/j.str.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig L, Li J, Egelman E, Leslie A. 2008. Type IV pili: paradoxes in form and function. Curr Opin Struct Biol 18:267–277. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li G, Brown PJB, Tang JX, Xu J, Quardokus EM, Fuqua C, Brun YV. 2012. Surface contact stimulates the just-in-time deployment of bacterial adhesins. Mol Microbiol 83:41–51. doi: 10.1111/j.1365-2958.2011.07909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellison CK, Kan J, Dillard RS, Kysela DT, Ducret A, Berne C, Hampton CM, Ke Z, Wright ER, Biais N, Dalia AB, Brun YV. 2017. Obstruction of pilus retraction stimulates bacterial surface sensing. Science 358:535–538. doi: 10.1126/science.aan5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellison CK, Dalia TN, Vidal Ceballos A, Wang JC-Y, Biais N, Brun YV, Dalia AB. 2018. Retraction of DNA-bound type IV competence pili initiates DNA uptake during natural transformation in Vibrio cholerae. Nat Microbiol 3:773–780. doi: 10.1038/s41564-018-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laub MT, Chen SL, Shapiro L, McAdams HH. 2002. Genes directly controlled by CtrA, a master regulator of the Caulobacter cell cycle. Proc Natl Acad Sci U S A 99:4632–4637. doi: 10.1073/pnas.062065699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotz W, Pfister H. 1975. Attachment of a long-tailed Rhizobium bacteriophage to the pili of its host. J Virol 16:725–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrero-Ferreira RC, Viollier PH, Ely B, Poindexter JS, Georgieva M, Jensen GJ, Wright ER. 2011. Alternative mechanism for bacteriophage adsorption to the motile bacterium Caulobacter crescentus. Proc Natl Acad Sci U S A 108:9963–9968. doi: 10.1073/pnas.1012388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harvey H, Bondy-Denomy J, Marquis H, Sztanko KM, Davidson AR, Burrows LL. 2018. Pseudomonas aeruginosa defends against phages through type IV pilus glycosylation. Nat Microbiol 3:47–52. doi: 10.1038/s41564-017-0061-y. [DOI] [PubMed] [Google Scholar]
- 15.Bender RA, Refson CM, O'Neill EA. 1989. Role of the flagellum in cell-cycle-dependent expression of bacteriophage receptor activity in Caulobacter crescentus. J Bacteriol 171:1035–1040. doi: 10.1128/jb.171.2.1035-1040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christen M, Beusch C, Bösch Y, Cerletti D, Flores-Tinoco CE, Del Medico L, Tschan F, Christen B. 2016. Quantitative selection analysis of bacteriophage ΦCbK Susceptibility in Caulobacter crescentus. J Mol Biol 428:419–430. doi: 10.1016/j.jmb.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 17.Berne C, Ellison CK, Agarwal R, Severin GB, Fiebig A, Morton RI, Waters CM, Brun YV. 2018. Feedback regulation of Caulobacter crescentus holdfast synthesis by flagellum assembly via the holdfast inhibitor HfiA. Mol Microbiol 110:219–238. doi: 10.1111/mmi.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faulds-Pain A, Birchall C, Aldridge C, Smith WD, Grimaldi G, Nakamura S, Miyata T, Gray J, Li G, Tang JX, Namba K, Minamino T, Aldridge PD. 2011. Flagellin redundancy in Caulobacter crescentus and its implications for flagellar filament assembly. J Bacteriol 193:2695–2707. doi: 10.1128/JB.01172-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilmury SLN, Burrows LL. 2018. The Pseudomonas aeruginosa PilSR two-component system regulates both twitching and swimming motilities. mBio 9:e01310-18. doi: 10.1128/mBio.01310-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hug I, Deshpande S, Sprecher KS, Pfohl T, Jenal U. 2017. Second messenger-mediated tactile response by a bacterial rotary motor. Science 358:531–534. doi: 10.1126/science.aan5353. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Newton A. 1997. Regulation of the Caulobacter flagellar gene hierarchy; not just for motility. Mol Microbiol 24:233–239. doi: 10.1046/j.1365-2958.1997.3281691.x. [DOI] [PubMed] [Google Scholar]
- 22.Brown PJ, Hardy GG, Trimble MJ, Brun YV. 2009. Complex regulatory pathways coordinate cell-cycle progression and development in Caulobacter crescentus. Adv Microb Physiol2008/10/22 54:1–101. doi: 10.1016/S0065-2911(08)00001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poindexter JS. 1964. Biological properties and classification of the Caulobacter group. Bacteriol Rev 28:231–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ely B. 1991. [17] Genetics of Caulobacter crescentus. Methods Enzymol 204:372–384. doi: 10.1016/0076-6879(91)04019-K. [DOI] [PubMed] [Google Scholar]
- 25.Ried JL, Collmer A. 1987. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57:239–246. doi: 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 26.Ducret A, Quardokus EM, Brun YV. 2016. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. Nat Microbiol 1:16077. doi: 10.1038/nmicrobiol.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ignatiadis N, Klaus B, Zaugg JB, Huber W. 2016. Data-driven hypothesis weighting increases detection power in genome-scale multiple testing. Nat Methods 13:577–580. doi: 10.1038/nmeth.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viollier PH, Sternheim N, Shapiro L. 2002. A dynamically localized histidine kinase controls the asymmetric distribution of polar pili proteins. EMBO J 21:4420–4428. doi: 10.1093/emboj/cdf454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.West L, Yang D, Stephens C. 2002. Use of the Caulobacter crescentus genome sequence to develop a method for systematic genetic mapping. J Bacteriol 184:2155–2166. doi: 10.1128/JB.184.8.2155-2166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skerker JM, Shapiro L. 2000. Identification and cell cycle control of a novel pilus system in Caulobacter crescentus. EMBO J 19:3223–3234. doi: 10.1093/emboj/19.13.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marks ME, Castro-Rojas CM, Teiling C, Du L, Kapatral V, Walunas TL, Crosson S. 2010. The genetic basis of laboratory adaptation in Caulobacter crescentus. J Bacteriol 192:3678–3688. doi: 10.1128/JB.00255-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meisenzahl AC, Shapiro L, Jenal U. 1997. Isolation and characterization of a xylose-dependent promoter from Caulobacter crescentus. J Bacteriol 179:592–600. doi: 10.1128/jb.179.3.592-600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellison CK, Dalia TN, Dalia AB, Brun YV. 2019. Real-time microscopy and physical perturbation of bacterial pili using maleimide-conjugated molecules. Nat Protocols 14:1803–1819. doi: 10.1038/s41596-019-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequencing data reported in this study can be accessed by GEO accession no. GSE126478.