Ethanol is an important biologically active molecule produced by many bacteria and fungi. It has also been identified as a potential marker for disease state in cystic fibrosis. In line with previous data showing that ethanol promotes biofilm formation by Pseudomonas aeruginosa, here we report that ethanol reduces swimming motility using some of the same proteins involved in surface sensing. We propose that these data may provide insight into how microbes, via their metabolic byproducts, can influence P. aeruginosa colocalization in the context of infection and in other polymicrobial settings.
KEYWORDS: Ethanol, Pseudomonas aeruginosa, c-di-GMP, motility, stator
ABSTRACT
Pseudomonas aeruginosa frequently encounters microbes that produce ethanol. Low concentrations of ethanol reduced P. aeruginosa swim zone area by up to 45% in soft agar. The reduction of swimming by ethanol required the flagellar motor proteins MotAB and two PilZ domain proteins (FlgZ and PilZ). PilY1 and the type 4 pilus alignment complex (comprising PilMNOP) were previously implicated in MotAB regulation in surface-associated cells and were required for ethanol-dependent motility repression. As FlgZ requires the second messenger bis-(3′-5′)-cyclic dimeric GMP (c-di-GMP) to represses motility, we screened mutants lacking genes involved in c-di-GMP metabolism and found that mutants lacking diguanylate cyclases SadC and GcbA were less responsive to ethanol. The double mutant was resistant to its effects. As published previously, ethanol also represses swarming motility, and the same genes required for ethanol effects on swimming motility were required for its regulation of swarming. Microscopic analysis of single cells in soft agar revealed that ethanol effects on swim zone area correlated with ethanol effects on the portion of cells that paused or stopped during the time interval analyzed. Ethanol increased c-di-GMP in planktonic wild-type cells but not in ΔmotAB or ΔsadC ΔgcbA mutants, suggesting c-di-GMP plays a role in the response to ethanol in planktonic cells. We propose that ethanol produced by other microbes induces a regulated decrease in P. aeruginosa motility, thereby promoting P. aeruginosa colocalization with ethanol-producing microbes. Furthermore, some of the same factors involved in the response to surface contact are involved in the response to ethanol.
IMPORTANCE Ethanol is an important biologically active molecule produced by many bacteria and fungi. It has also been identified as a potential marker for disease state in cystic fibrosis. In line with previous data showing that ethanol promotes biofilm formation by Pseudomonas aeruginosa, here we report that ethanol reduces swimming motility using some of the same proteins involved in surface sensing. We propose that these data may provide insight into how microbes, via their metabolic byproducts, can influence P. aeruginosa colocalization in the context of infection and in other polymicrobial settings.
INTRODUCTION
Ethanol is a common microbial fermentation product that, in culture supernatants, can vary widely from 0.3% to 0.8% (∼45 to 140 mM) to much higher concentrations depending on growth conditions (1–4). For diverse microbes, ethanol can serve as a carbon source and a signaling molecule. For example, fungal gardens formed as part of a symbiosis between ambrosia beetles and their fungal symbionts, Ambrosiella and Raffaelea, are preferentially localized to sites with higher ethanol (5). In the parasite Toxoplasma gondii, low concentrations of ethanol (<200 mM, or 1.2%) facilitate an increase in the second messenger inositol 1,4,5-triphosphate, resulting in increased intracellular calcium and increased host colonization (6, 7). In Acinetobacter baumannii, a Gram-negative opportunistic pathogen, ethanol causes an increase in virulence and biofilm formation and repression of motility through many mechanisms, some not yet described (8–10). Oral streptococci have been shown to use ethanol as a carbon source, as a majority of these microbes usually express three alcohol dehydrogenases at a given time (11).
Ethanol is also known to be produced in the context of infections, such as in the lungs of individuals with cystic fibrosis (CF), a genetic disorder that results in an accumulation of thick mucus in the airways (12–16). In addition to Pseudomonas aeruginosa, CF lung infections often contain other microbes, many of which are capable of producing ethanol (17). Metabolomic and nuclear magnetic resonance (NMR) studies examining the bronchoalveolar lavage fluid (BALF) and exhaled breath condensate (EBC) of patients with CF indicate that volatiles such as ethanol are present in the CF lung (12, 13, 15, 16).
In P. aeruginosa, exogenous ethanol, such as that produced by the fungus Candida albicans, alters phenazine production and promotes biofilm formation on plastic and airway cells (18). Ethanol also leads to increased Pel matrix production and decreased surface motility, two factors that are necessary for biofilm formation and maturation (18–20). In this setting, the ethanol-dependent stimulation of the second messenger, cyclic di-GMP (c-di-GMP), via WspR, a diguanylate cyclase (DGC), caused increased Pel matrix production and motility repression (18). WspR is among the ∼40 enzymes in P. aeruginosa thought to metabolize c-di-GMP, including other DGCs (21), c-di-GMP-degrading phosphodiesterases (PDEs) (22–24), and proteins that possess both activities. In P. aeruginosa and other pseudomonads, c-di-GMP metabolic enzymes have additional domains (e.g., PAS, REC, HAMP, CAHCE, and GAF) that can sense external stimuli or promote protein-protein interactions in order to modulate enzyme activities at appropriate times (25–27) or in response to specific cues (26). In addition to the c-di-GMP metabolic enzymes, P. aeruginosa has eight effector proteins with PilZ domains that bind c-di-GMP at various affinities to affect many behaviors, including biofilm formation and motility (28, 29).
In pseudomonads and other bacteria, motility repression occurs in multiple ways, including (i) obstruction of the flagellum by exopolysaccharides (30, 31), (ii) transcriptional downregulation of flagellar gene expression (32, 33), (iii) loss of flagellar rotation by c-di-GMP-bound effector proteins and their interactions with flagellum motor components (32, 34, 35), (iv) sequestration of flagellar motor proteins by c-di-GMP-bound effectors (29, 36, 37), and (v) inhibition of flagellar rotation switching (clockwise versus counterclockwise) (38–40). In most Gram-negative bacteria, the flagellar motor is composed of two structures, the rotor (FliG, FliM, and FliN), which determines clockwise or counterclockwise rotation (the switch complex), and the stator (MotA and MotB), which generates torque for flagellar rotation powered by proton motive force (41–43). Pseudomonads have a second stator set (MotCD) that is incorporated into the stator complex to facilitate optimal motor function (29, 36, 44, 45). In P. aeruginosa, the two stator sets have distinct roles: MotAB is required to reduce swarming motility when c-di-GMP levels are high, while MotCD is critical for promoting swimming and swarming motility (36, 44).
In the present study, we outline a pathway by which low concentrations of ethanol repress swimming and swarming motility in P. aeruginosa. Genetic screens of mutant collections identified the MotAB flagellar motor proteins, two PilZ domain c-di-GMP effector proteins (FlgZ and PilZ), and two DGCs, SadC and GcbA, as components required for ethanol-dependent motility repression. In addition, PilY1 and the PilMNOP proteins, components of the type 4 pili (T4P) machinery that are involved in surface sensing, were also required for the ethanol responses in 0.3% (soft) agar and in swarming. Microscopic analysis of the wild type showed that ethanol increased the fraction of cells that were paused or nonmotile within the 8-s time interval analyzed. In contrast, the mutants that were resistant to the effects of ethanol in macroscopic assays did not show a reduction in the fraction of cells that were continuously swimming with ethanol, with the exception of the ΔsadC ΔgcbA strain. We also show that in planktonic cells, ethanol led to an increase in c-di-GMP that required SadC and GcbA. Taken together with previous studies (18, 37), we propose that ethanol, a common metabolite produced by microbes, acts as a signal to rapidly repress P. aeruginosa swimming and swarming motility and induces matrix production to promote biofilm initiation. The proteins involved in the response to ethanol overlap significantly with those involved in the response to surface contact.
RESULTS
Subinhibitory concentrations of ethanol repress P. aeruginosa PA14 swimming motility independent of changes in growth rate, extracellular matrix production, or catabolism.
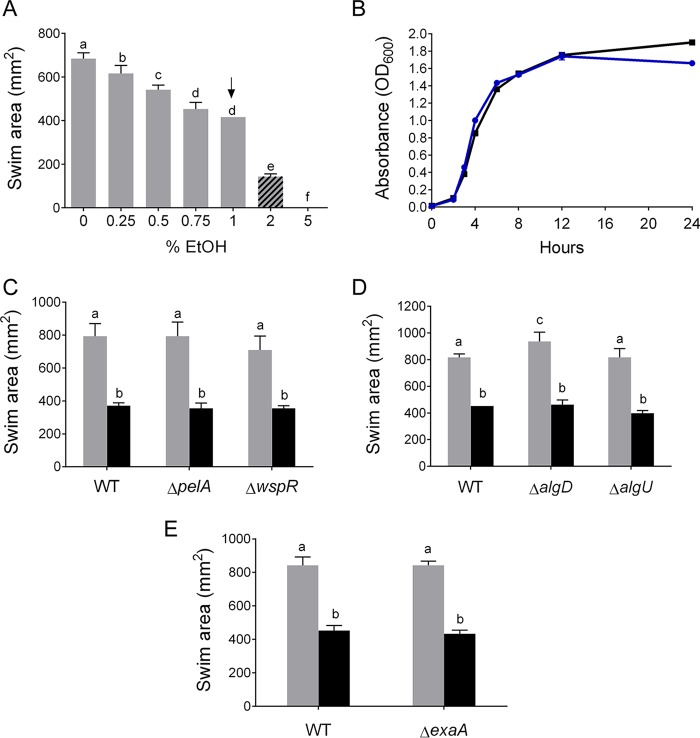
We previously showed that ethanol (1%) stimulates P. aeruginosa attachment to glass and plastic, microcolony formation on airway cells, and pellicle formation and reduces swarming motility across agar surfaces in part through the stimulation of Pel extracellular matrix production (18, 46). To further study how subinhibitory concentrations of ethanol enhance biofilm, we assessed the effects of ethanol on flagellar motility using a 0.3% (soft) agar motility assay. Ethanol led to dose-dependent decreases in swim zone size (Fig. 1A), with a 10% decrease in swim zone area at 0.25% ethanol, and a 39% reduction in swim zone area in medium with 1% ethanol (Fig. 1A). The effects of 1% ethanol on swim zone size were still evident when succinate replaced glucose as the major carbon source. Swim zone area in M63 plus succinate plus CAA was 1,018 ± 46 mm2 under control conditions and 521 ± 39 mm2 with 1% ethanol for an overall decrease in motility of 49% (P < 0.05 by t test, n = 4).
FIG 1.
Ethanol decreases swim zone area in a dose-dependent manner independent of changing growth, increasing matrix, or ethanol catabolism. (A) Swim zone area of wild-type P. aeruginosa PA14 in M63 medium solidified with 0.3% agar (soft agar) and supplemented with 0, 0.25, 0.5, 0.75, 1, 2, or 5% ethanol. Hashed bars (2% and 5% ethanol) depict ethanol concentration where growth was affected, and the arrow indicates the ethanol concentration used in this study. (B) Growth curves of wild-type P. aeruginosa PA14 in liquid M63 medium without (blue) and with (black) 1% ethanol grown for 24 h at 37°C with OD600 measured at the indicated time points. No significant differences were observed until the late 24-h time point, as measured by two-way ANOVA with multiple comparisons (P value of <0.05 is significant). (C) Swim zone area of wild-type P. aeruginosa PA14 (WT) and the ΔpelA (Pel defective mutant) and ΔwspR mutants in soft agar without (gray) and with (black) 1% ethanol. (D) Swim zone area of WT and the ΔalgD and ΔalgU mutants in soft agar without (gray) and with (black) 1% ethanol. (E) Swim zone area of WT and the ΔexaA mutant (ethanol catabolic mutant) in soft agar without (gray) and with (black) 1% ethanol. All swim zones were measured after 18 to 20 h. All error bars indicate standard deviations, n = 4 replicates. The same lowercase letters indicate samples that are not significantly different and different lowercase letters indicate significant differences (P < 0.05), as determined by two-way ANOVA with multiple comparisons.
To assess whether the decrease in motility observed in ethanol was attributable to differences in the rate of growth, we performed a 24-h growth kinetic experiment with wild-type P. aeruginosa PA14. We showed that at 1% ethanol, there was no difference in growth from that in control cultures (Fig. 1B). At 2% ethanol, growth was slightly inhibited, and at 5% ethanol, growth was completely inhibited (data not shown). We chose 1% ethanol as our concentration for all further experiments in this study.
Previously published work from our laboratory showed that P. aeruginosa increases Pel matrix in a WspR-dependent manner in response to 1% ethanol (18). Alginate and Pel matrix production in P. aeruginosa have both been implicated in biofilm formation and motility repression, possibly via steric hindrance of the flagellum (19, 20). Although ethanol activates WspR-dependent production of Pel polysaccharide matrix in cells on 1.5% agar or a hard surface (18), neither PelA nor WspR was required for the reduction in swim zone area (Fig. 1C). Alginate was also not required for reducing the swim zone area in the presence of ethanol, as two mutants altered in alginate production, ΔalgD and ΔalgU strains, showed similar levels of swim zone reduction (51% decrease in swim zone area for both, P < 0.05) as the wild type (Fig. 1D).
Although P. aeruginosa can catabolize ethanol (47, 48), ethanol catabolism is not required for the stimulation of biofilm formation or induction of Pel matrix (18). Similarly, we found that reduction in swim zone area by ethanol still occurred in a ΔexaA mutant, which cannot grow with ethanol as a carbon source (18). The ΔexaA strain still showed a 49% decrease in motility in soft agar with 1% ethanol (Fig. 1E). Overall, these data indicated that motility repression in medium with 1% ethanol was not a result of a change in growth rate or increased matrix production, nor did it require ethanol catabolism.
Ethanol-dependent motility repression is mediated by the MotAB stator.
In P. aeruginosa, the ability of the flagellum to rotate, and thereby move the cell, is dependent on two proton-driven stator sets, MotAB and MotCD. Both of these stators are capable of generating torque to turn the flagellum, though they are not equal (36, 44, 45, 49–51). Previous work showed that P. aeruginosa motility is regulated by the two stator sets via a stator exchange mechanism (29, 36, 37). MotAB incorporation into the flagellum was associated with repressed motility in surface-associated cells (29, 36, 37).
We found that the ΔmotAB mutant did not show reduced swim zone size upon inclusion of ethanol in the medium (Fig. 2). In fact, ethanol caused a modest but significant 13% increase in swim zone area in the ΔmotAB mutant (Fig. 2). The ΔmotCD mutant formed a swim zone that was ∼90% smaller than that of the wild type in the absence of ethanol (36) (Fig. 2), and like the ΔmotAB mutant, the ΔmotCD mutant formed a larger swim zone in the presence of ethanol. A ΔflgK mutant that lacks a flagellum and is nonmotile demonstrated the size of the swim zone of a strain lacking flagellar motility (Fig. 2). Overall, these data support the conclusion that the MotAB and MotCD stator sets are required for ethanol to affect swim zone area.
FIG 2.

Ethanol effects on swimming motility require the MotAB flagellar stator complex. Swim zone areas of wild-type P. aeruginosa PA14 (WT) and the ΔflgK, ΔmotAB, and ΔmotCD mutants in soft agar without (gray) and with (black) 1% ethanol after 18 to 20 h. Error bars indicate standard deviations, n = 4 replicates. Each sample was statistically compared to every other sample; the same lowercase letters indicate samples that are not significantly different and different lowercase letters indicate significant differences (P < 0.05) as determined by two-way ANOVA with multiple comparisons.
Ethanol-dependent motility repression requires two PilZ domain proteins, FlgZ and PilZ.
Stator complex composition in the flagellum is controlled by c-di-GMP through c-di-GMP effector proteins (PilZ domain proteins) that sequester stators from the motor (29, 36, 37). PilZ domain proteins such as FlgZ/YcgR mediate c-di-GMP-dependent decreases in motility through effects on flagellar stators in P. aeruginosa and other species (29, 31, 36, 37, 52–54). In P. aeruginosa, FlgZ, upon c-di-GMP binding, promotes binding of MotC and delocalization of MotCD from the flagellum. The delocalization of MotCD leads to increased incorporation of MotAB, which cannot support swimming in viscous environments such as in soft agar or on swarm agar (29, 36, 37). There are eight known or predicted PilZ domain proteins in P. aeruginosa that control cellular behaviors, which include motility and biofilm formation (29, 55). We therefore assessed whether the stator-dependent motility repression in the presence of ethanol was dependent on one or more PilZ domain proteins.
In the absence of ethanol, seven mutants had swim zones similar to those of the wild type with the exception of ΔPA14_25420, which had a smaller swim zone area (Fig. 3A). In the presence of ethanol, two mutants displayed slightly greater swimming motility than the wild-type strain, the ΔflgZ and ΔpilZ strains (Fig. 3A), and their increased resistance was significant (Fig. 3B). We constructed a ΔpilZ ΔflgZ double mutant and found that it did not show ethanol-dependent motility repression (Fig. 3B). Interestingly, both PilZ and FlgZ were previously shown to be involved in the repression of swarming motility on agar surfaces in a P. aeruginosa strain that had high levels of c-di-GMP due to the absence of a phosphodiesterase; furthermore, FlgZ regulates flagellar motility in other species (29, 30, 56, 57). Our data indicated that PilZ and FlgZ play either independent or partially functionally redundant roles in motility repression by ethanol.
FIG 3.
PilZ domain proteins, PilZ and FlgZ, are required for ethanol effects on swim zone area. (A) Swim zone areas for wild-type P. aeruginosa PA14 (WT) and the ΔflgZ, ΔPA14_00130, ΔPA14_60970, ΔPA14_27930, ΔPA14_56180, Δalg44, ΔPA14_25420, and ΔpilZ mutants in soft agar without and with 1% ethanol (EtOH) after 18 to 20 h. Floating bars represent the minimum, maximum, and mean values from 4 replicates. Shaded boxes represent the ethanol samples that are significantly different from their controls (P < 0.05) as determined by one-way ANOVA with multiple comparisons. Arrows indicate the candidate mutants that were analyzed further. (B) WT and the ΔpilZ, ΔflgZ, and ΔpilZ ΔflgZ mutants in soft agar without (gray) and with (black) 1% ethanol after 18 to 20 h. Error bars indicate standard deviations, n = 4 replicates. Each sample was statistically compared to every other sample; the same lowercase letters indicate samples that are not significantly different and different letters indicate significant differences (P < 0.05) as determined by two-way ANOVA with multiple comparisons.
Ethanol elicits an increase in c-di-GMP levels via a MotAB-dependent mechanism.
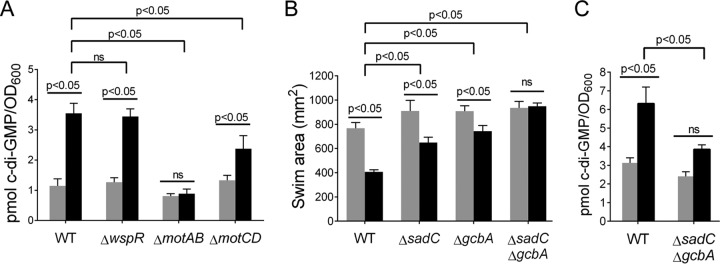
FlgZ is a c-di-GMP-binding effector protein that leads to decreased motility in response to c-di-GMP (29) and in the presence of ethanol (Fig. 3). Thus, we examined the effects of ethanol on c-di-GMP levels. We extracted c-di-GMP from cells grown planktonically in the same medium as the swim assay in order to capture the ethanol-mediated changes to c-di-GMP without any influences from the soft agar, which does impact bacterial movement (58). We found that wild-type cells grown for 16 h in liquid medium with 1% ethanol had 3.2-fold higher levels of c-di-GMP (P < 0.0001) than the control cultures (Fig. 4A). We previously showed that WspR is involved in c-di-GMP-dependent production of Pel matrix exopolysaccharide in surface-associated cells (18). However, in liquid, a ΔwspR mutant behaved like the wild type in that it had 2.6-fold higher levels of c-di-GMP than controls when grown with ethanol (P > 0.05) (Fig. 4A).
FIG 4.
Ethanol increases cyclic di-GMP levels. (A and C) Quantification of cyclic di-GMP levels in wild-type P. aeruginosa PA14 (WT) and the ΔwspR, ΔmotAB, and ΔmotCD mutants (A) and WT and the ΔsadC ΔgcbA mutant (C), exposed for 16 h to 1% ethanol (black) or medium with no ethanol (gray). Bars depict the averages of normalized values (n = 5 replicates). Error bars indicate standard deviations. (B) Swim zone areas of WT and the ΔsadC, ΔgcbA, and ΔsadC ΔgcbA mutants in soft agar without (gray) and with (black) 1% ethanol measured at 18 to 20 h. Error bars indicate standard deviations, n = 4 replicates. Each sample was statistically compared to every other sample by two-way ANOVA with multiple comparisons. P values are indicated. ns, not significantly different.
The c-di-GMP profiles of the stator mutants, shown as described above to lack ethanol-dependent motility repression (Fig. 2), were also assessed. The ΔmotAB mutant did not have higher c-di-GMP levels (37) when grown in ethanol, indicating the importance of MotAB in the c-di-GMP production observed in the wild type (Fig. 4A). We also noted that under control conditions, the ΔmotAB mutant had c-di-GMP levels that were 27% lower than those seen in the wild type (0.8 pmol/optical density at 600 nm [OD600] versus 1.1 pmol/OD600, respectively) (Fig. 4A) (37). The ΔmotCD mutant showed induction of c-di-GMP when grown with ethanol, but the levels were lower (1.8-fold compared to the 3.2-fold increase seen in the wild type in the same experiment) (Fig. 4A). These data suggest that the relationship between stator activity and c-di-GMP levels that is observed in the absence of ethanol is also true in the presence of ethanol (37).
A screen of proteins that contribute to c-di-GMP metabolism reveals multiple enzymes involved in the ethanol response.
We next screened the collection of the reported P. aeruginosa PA14 in-frame deletion mutant library containing mutants lacking each of the ∼40 known c-di-GMP-metabolizing enzymes (25) to identify the gene(s) involved in increasing c-di-GMP in response to ethanol and the ethanol-dependent motility repression. Our primary focus was on mutants that (i) had a swim zone area greater than or equal to that of the wild type under control conditions and (ii) showed less reduction in swim zone area when ethanol was present in the medium. Using these criteria, analysis of the data from three independent screens of the mutant collection identified SadC and GcbA as the most promising candidates (see Table S1 in the supplemental material); data for mutants with swim zone areas smaller than that of the wild type under control conditions are provided (see Table S2) but not pursued as part of these studies.
The ΔsadC and ΔgcbA single mutants had slightly larger swim zone areas than observed for the wild type (Fig. 4B). These modest phenotypes in the ΔsadC and ΔgcbA mutants were able to be complemented in trans with the wild-type sadC and gcbA genes, respectively (see Fig. S1A and B). The effects of SadC and GcbA on changes in motility in response to ethanol were additive, as the ΔsadC ΔgcbA double mutant showed no significant difference in swim zone area in medium without and with ethanol (Fig. 4B).
Both SadC (in surface-associated cells) and GcbA (in planktonic cells) have been reported to contribute to c-di-GMP levels (20, 21, 39). In planktonic cultures, we show that the ΔsadC ΔgcbA mutant had lower levels of c-di-GMP than the wild type under the same conditions, both in the absence and presence of ethanol (Fig. 4C). These data suggest that SadC and GcbA contribute to ethanol-dependent motility repression and that they are involved in the increase in c-di-GMP levels in cells grown with ethanol.
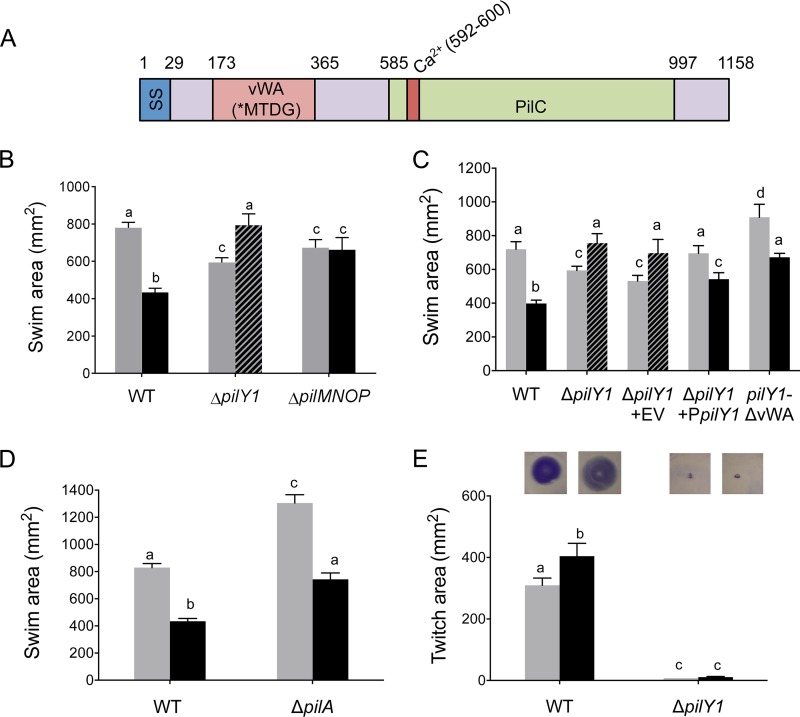
Ethanol-mediated motility repression requires PilY1, the PilMNOP T4P alignment complex, and the minor pilins PilVWX but is independent of T4P activity.
PilY1 is proposed to serve as a surface-sensing protein required for decreased motility in cells on 0.5% agar surfaces (swarming motility) and for the stimulation of biofilm formation (58, 59) (Fig. 5A). Previous studies indicated that for swarming in P. aeruginosa, PilY1, in conjunction with the type 4 pili (T4P) alignment complex, PilMNOP (58), appears to function upstream of SadC (59), FlgZ (29), and the MotAB stator (59) to control the production of and the response to c-di-GMP. Because the responses of SadC, FlgZ, PilZ, and MotAB to ethanol were altered in soft agar, we tested the roles of PilY1 and PilMNOP in ethanol-induced repression of swimming motility. In contrast to the wild-type strain, the ΔpilY1 mutant did not show decreased motility when ethanol was added to the medium (Fig. 5B). Instead, the ΔpilY1 mutant showed a reproducible and significant ∼35% increase in the swim zone area when ethanol was added to the medium (Fig. 5B), and a wild-type copy of the pilY1 gene complemented this phenotype (Fig. 5C). Moreover, a mutant lacking pilMNOP showed no difference in swimming motility when ethanol was added to the medium (Fig. 5B and Table 1). The single mutants, ΔpilM, ΔpilN, ΔpilO, and ΔpilP strains, were similarly resistant to ethanol-mediated repression of swimming motility (Table 1) (P > 0.05).
FIG 5.
PilY1 and PilMNOP, but not T4P activity, are necessary for ethanol effects on swim zone area. (A) Schematic of the PilY1 protein showing the amino acid positions of the signal sequence (SS), von Willebrand A factor domain (vWA), calcium-binding domain (red), and the PilC domain (green). (B) Swim zone areas of wild-type P. aeruginosa PA14 (WT) and the ΔpilY1 and ΔpilMNOP mutants in soft agar without (gray) and with (black) 1% ethanol measured after 18 to 20 h. Error bars indicate standard deviations, n = 4 replicates. The hashed bar indicates mutant that swam more in ethanol than in control cultures. (C) Swim zone areas of WT and the ΔpilY1, ΔpilY1 mutants with an empty vector (EV), with a plasmid-borne pilY1 (PpilY1), or pilY1 with the vWA domain deleted (pilY1-ΔvWA) and placed at the native pilY1 locus in soft agar without (gray) and with (black) 1% ethanol measured after growth for 18 to 20 h; 0.05% arabinose was added to the medium. Error bars indicate standard deviations, n = 4 replicates. Hashed bars indicate mutants that swam more in ethanol than in control cultures. (D) Swim zone areas of WT and the ΔpilA (pilus-deficient mutant) mutant in soft agar without (gray) and with (black) 1% ethanol after 18 to 20 h. Error bars indicate standard deviations, n = 4 replicates. (E) Twitch zone areas of WT and the ΔpilY1 (twitching deficient) mutant in medium without (gray) and with (black) 1% ethanol after 40 h. Error bars depict standard deviations, n = 4 replicates and repeated in more than four separate experiments. Pictures at the top show representative twitch zones from the indicated sample. Each sample was statistically compared to every other sample; the same lowercase letters indicate samples that are not significantly different and different lowercase letters indicate significant differences (P < 0.05) as determined by two-way ANOVA with multiple comparisons.
TABLE 1.
Swim zone area for P. aeruginosa strains in the absence and presence of ethanola
| PA14 strain or genotype | Swim zone area (mm2) |
% reduction (EtOH/control)b | P valuec | |
|---|---|---|---|---|
| No EtOH | 1% EtOH | |||
| WT | 780.4 | 490.9 | 37.1 | <0.0001 |
| ΔpilM | 617.8 | 605.0 | 2.1 | >0.05 |
| ΔpilN | 573.0 | 617.4 | −7.7 | >0.05 |
| ΔpilO | 605.8 | 616.6 | −1.8 | >0.05 |
| ΔpilP | 584.2 | 605.0 | −3.6 | >0.05 |
| ΔpilMNOP | 627.0 | 605.4 | 3.4 | >0.05 |
| ΔpilV | 672.2 | 731.3 | −8.8 | >0.05 |
| ΔpilW | 649.8 | 768.0 | −18.2 | <0.01 |
| ΔpilX | 551.8 | 683.8 | −23.9 | <0.01 |
| ΔpilVWX | 594.2 | 730.9 | −23.0 | <0.01 |
Representative data from three assays of swim zone area and percent reduction in the absence and presence of ethanol (EtOH) for T4P alignment complex mutants and minor pilin mutants.
Negative percentages indicate strains where motility in ethanol was greater than that of the no ethanol control.
P values of statistical analysis comparing control and ethanol samples using two-way ANOVA.
Finally, since PilY1 and the PilMNOP complex are necessary for T4P activity (60), we determined if PilY1- and PilMNOP-dependent reduction in flagellar motility by ethanol could be attributed to a change in T4P activity. A ΔpilA mutant (which lacks pili) showed the same level of responsiveness to ethanol as wild-type cells while maintaining hypermotility under control conditions (59, 61, 62) (Fig. 5D), indicating that pili are not required for the effects of ethanol on motility. In fact, ethanol did not reduce twitching motility in wild-type cells; rather, a significant 30% increase in twitch zone area was observed (Fig. 5E). In addition, with the previous observation that the PilVWX minor pilins, together with PilY1, control the virulence of P. aeruginosa to Caenorhabditis elegans, we assessed the role of minor pilins in motility repression. To this end, the ΔpilW and ΔpilX single mutants as well as the ΔpilVWX triple mutant all phenocopied the ΔpilY1 mutant, having significant 18.2%, 23.9%, and 23.0% increases in motility, respectively, in the presence of ethanol (P < 0.01), with ΔpilV trending the same with an 8.8% increase (P > 0.05) (Table 1). These data suggest that the ethanol-induced decreases in swimming motility require PilY1, PilMNOP, and PilVWX but occur independently of changes in T4P function.
Previously described elements involved in PilY1 activation were dispensable for the ethanol-dependent reduction in motility.
We sought to determine if known factors involved in PilY1 activation were involved in the ethanol response. Previous studies had shown that pilY1 transcription is regulated by PilJ, a component of the Pil-Chp pathway, in response to surface engagement (58) through stimulation of cAMP production (63). The cyaAB genes, which encode adenylate cyclases responsible for cAMP production by P. aeruginosa, were also implicated in PilY1 activation (58). We found that the ΔpilJ and ΔcyaAB mutants, although hypermotile in control cultures, both exhibited motility repression in response to ethanol (P < 0.0001) (see Fig. S2). These data indicated that the upstream cAMP signal previously shown to be required for pilY1 transcription, upon surface contact, was not required for the PilY1-dependent changes in motility in response to ethanol.
The von Willebrand factor A (vWA) domain of PilY1, depicted in the schematic in Fig. 5A, is necessary for surface-associated swarming motility repression (59). To probe whether the same domain of PilY1 required for surface-sensing was also necessary for ethanol responsiveness, we used a strain where a mutated pilY1 with the vWA domain deleted (pilY1-ΔvWA) was placed at the native pilY1 locus. The pilY1-ΔvWA strain was still responsive to ethanol-dependent motility repression (P < 0.0001) (Fig. 5C). These data indicate that the vWA domain of PilY1 is dispensable for ethanol-mediated swim repression.
Microscopic analysis of the effects of ethanol on swimming motility.
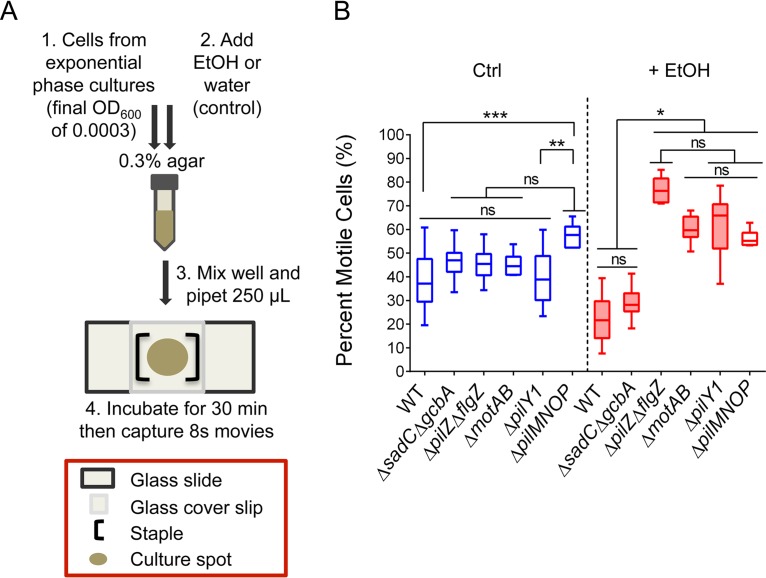
P. aeruginosa is a monotrichous flagellated bacterium whose flagellar motility is governed by a run-reverse pattern (38, 64) rather than the run-tumble pattern in organisms with peritrichous flagella such as Escherichia coli (38). P. aeruginosa directional movement is driven by the control of flagellar rotation (clockwise or counterclockwise), and this rotation change is called a “reversal.” The frequency of reversals can impact the area covered, since P. aeruginosa must slow its normal speed from 40 to 55 μm/s (45, 64, 65) to as low as 15 μm/s immediately before a reversal (64). While ethanol led to significant increases in the average reversal frequency, from 4.6 ± 4.1 to 22 ± 9.1 reversals/10 s, in wild-type cells in medium with 3% Ficoll (P < 0.0001) (see Fig. S3A), the effects of ethanol on reversal rates were similar in mutants that were resistant to the effects of ethanol in the macroscopic swim zone area assay (ΔsadC ΔgcbA, ΔpilY1, and ΔpilMNOP strains) (Fig. S3A and B), suggesting that changes in reversal frequency were not responsible for this phenotype.
To quantify the effect of ethanol on the motility of cells, the wild type and mutants from exponential-phase cultures were inoculated in soft agar without and with ethanol, incubated for 30 min, and then imaged for 8 s to assess motility behavior (scheme in Fig. 6A). Microscopic observation of single wild-type cells revealed that in the presence of ethanol, a larger fraction of cells paused or stopped during an 8-s time interval (38% ± 11% motile [control] and 22% ± 9% motile [ethanol]; P ≤ 0.05) (Fig. 6B). For most of the ethanol-resistant mutants (ΔmotAB, ΔpilZ ΔflgZ, ΔpilY1, and ΔpilMNOP strains), motility in the control samples was similar to the wild type (Fig. 6B), but cells were either unaffected by ethanol or there was an increase in the fraction of cells that were continuously moving during the assay in ethanol-containing medium (Fig. 6B). A ΔmotCD mutant, which lacks the stator set needed to drive motility under viscous conditions and on surfaces, had very fewer cells moving, but similar numbers of cells were motile in control and ethanol-treated samples (11% ± 4% and 8% ± 3%, respectively). Of the mutants that were resistant to the effects of ethanol in the macroscopic swim zone assay, only the ΔsadC ΔgcbA double mutant behaved like the wild-type strain (46% ± 6% motile [control] and 29% ± 6% motile [ethanol]) (Fig. 6B). These data suggest that after a 30-min exposure to ethanol, P. aeruginosa is less able to maintain motility in soft agar and that this response is dependent on MotAB, PilZ, FlgZ, PilY1, and PilMNOP proteins. We see a strong correspondence between the macroscopic and single-cell phenotypes for all but one of the mutants we have analyzed. These data also suggest that although SadC and GcbA are both necessary and sufficient for ethanol-mediated reduction of swimming in the macroscopic swim assay, these two diguanylate cyclases are not sufficient for such an effect in the single-cell assay. This point is further explored below.
FIG 6.
Ethanol decreases the number of continuously motile cells in soft agar, in a manner dependent on MotAB, FlgZ, PilZ, PilY1, and PilMNOP. (A) Schematic of agar motility assay treated with water (control) or 1% ethanol in soft agar. A sample was mixed well, 250 μl was pipetted onto a glass slide, and staples were used to create a chamber using a glass coverslip. The sample was then incubated at room temperature for 30 min and then imaged as outlined in Materials and Methods. (B) Agar motility assay of wild-type P. aeruginosa PA14 (WT) and the ΔsadC ΔgcbA, ΔpilZ ΔflgZ, ΔmotAB, ΔpilY1, and ΔpilMNOP mutants in soft agar without (Ctrl) and with (+ EtOH) 1% ethanol after 30 min. Three time-lapse (8 s) movies of the cells in the agar matrix, for each sample, were captured. Shown is a box and whiskers plot of the average population percentages of the continuously motile subpopulation in each movie. Error bars represent the minimum to maximum data points, n ≥ 6 replicate movies. Shaded boxes represent the ethanol samples that are significantly different from their controls. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant as determined by one-way ANOVA with multiple comparisons.
Ethanol inhibits swarming motility.
In addition to its effects on planktonic cells and motility in soft agar, ethanol also inhibits flagellum-dependent swarming motility on agar surfaces (Fig. 7A) (18). Swarming motility is also influenced by matrix production (66, 67). We found that wild-type swarming motility was strongly repressed in the presence of 1% ethanol (Fig. 7A) and that mutants lacking MotAB, the PilZ and FlgZ c-di-GMP effector proteins, PilY1, and the PilMNOP alignment complex were all more resistant to the effects on ethanol on swarming motility (Fig. 7A, B, D, and E). Furthermore, while the vWA domain of PilY1 has been shown to be important for surface sensing (59), we found that this domain was not required for ethanol-mediated repression of swarming motility (Fig. 7D). Consistent with our observation that the ΔsadC, ΔgcbA, and ΔsadC ΔgcbA mutants were resistant, to various degrees, to the effects of ethanol on motility in the soft agar macroscopic assay, these mutants were also less responsive to ethanol in the surface-associated swarming motility assay (Fig. 7C). Together, these data suggest that common factors are involved in the repression of flagellar motility by ethanol in cells grown under viscous conditions (0.3% agar) as well as in cells on a surface.
FIG 7.
Ethanol effects on swarming motility repression require the same components needed for motility repression in soft agar. Representative images of swarming motility assays of wild-type P. aeruginosa PA14 (WT) and ΔmotAB mutant (A), WT and the ΔflgZ, ΔpilZ, and ΔpilZ ΔflgZ mutants (B), WT and the ΔsadC, ΔgcbA, and ΔsadC ΔgcbA mutants (C), WT, the ΔpilY1 mutant, and the ΔpilY1 mutant with an empty vector (EV) or with a plasmid that enables arabinose-inducible expression pilY1 (PpilY1) or pilY1 without a vWA domain (pilY1-ΔvWA) (D), and WT and ΔpilMNOP mutant (E) on M8 medium with 0.5% agar (swarm agar) without and with 1% ethanol and grown for 16 h. Images are representative of observed phenotypes, n = 4 replicates per experiment, and each experiment was performed 3 to 5 times.
DISCUSSION
Here, we present a model (Fig. 8) in which ethanol leads to decreased flagellar motility in P. aeruginosa under conditions that promote both swimming and swarming. Previous work from our groups and the work of other labs showed that ethanol at low concentrations increases biofilm formation on abiotic and biotic surfaces (18, 46). We propose that this impacts multiple steps in the initial formation of polymicrobial communities formed by P. aeruginosa with ethanol-producing microbes. Our model proposes that stator occupancy, or ratio of the MotAB/MotCD stators, changes in response to ethanol. Based on our phenotypic analyses, we suggest that MotAB contributes to the delocalization of MotCD from the flagellar motor, resulting in a motor that is now less able to support full motility in soft agar, which is consistent with the model proposed in a previous report (37). We also propose that this delocalization of MotCD requires the PilZ domain protein FlgZ (29, 37) and/or PilZ (this report), which might indicate either that there are functional redundancies between these two proteins or that they participate in two distinct pathways. FlgZ, a homolog of E. coli YcgR, has been shown to regulate flagellar motility by directly interacting with the flagellar motor proteins, thereby behaving like a “brake” on flagellar rotation (29, 52, 68). We propose that c-di-GMP-bound PilZ likely functions in a similar fashion. A recent study showed that MotC interacts with the diguanylate cyclase SadC and stimulates SadC activity (37). Thus, delocalization of the MotCD stator from the flagellar motor would make MotC available to interact with and stimulate the activity of SadC, thereby increasing c-di-GMP levels. Increased c-di-GMP may have two nonexclusive roles, including increased c-di-GMP to cause the reduction in motility as well as induction of matrix production (18). Because the ΔsadC ΔgcbA mutant had a motility phenotype in the macroscopic assay but not in the short-term microscopic assay, we favor a model in which c-di-GMP may be downstream of the initial effects on stator function.
FIG 8.
Model for the effects of ethanol on Pseudomonas aeruginosa motility. We propose that in the absence of ethanol (No EtOH), P. aeruginosa remains motile with a bias toward a MotCD-dominant stator. As shown on the right, ethanol (depicted as red curvy lines) leads to decreased motility through a mechanism that requires the MotAB stator complex, PilY1, and PilVWX and PilMNOP protein complexes. We propose that PilZ and FlgZ, two PilZ domain proteins, may aid in MotCD delocalization from the motor. Two diguanylate cyclases, SadC and GcbA, also participate in ethanol-induced motility repression. As we show, ethanol may interact with the bacterial membrane, directly affect proteins, such as PilY1, or have other indirect effects such as the increase in c-di-GMP observed in this pathway.
The genes required for the response to ethanol in soft agar overlap with genes that were shown previously to play key roles in surface sensing, the regulation of swarming motility, and early biofilm formation by P. aeruginosa, including MotAB, PilZ, FlgZ, and the diguanylate cyclase SadC, as discussed above, as well as PilY1 and PilMNOP (29, 36, 58, 59). This observation suggests that ethanol may provide a direct or indirect signal that is analogous or complementary to surface engagement. That is, this chemical signal may contribute to P. aeruginosa reducing its motility in order to colocalize with ethanol-producing microbes.
Microscopic observations of cells in soft agar, a medium that is widely used to assess chemotaxis and swimming motility (25, 69–73), showed that ethanol decreased the fraction of cells that remained continuously motile in the 8-s time interval analyzed, and this phenotype likely contributes to the differences in swim zone size for most mutants. The “pausing” of single cells in the microscopic assay, performed at 30 min after exposure to ethanol, required all of the components of the pathway outlined above, with the exception of SadC and GcbA. We speculate that perhaps other DGCs may contribute to this pausing phenomenon and that SadC and GcbA may affect swimming and swarming motility at later time points.
A key question is how do the proteins PilY1, PilVWX, PilMNOP, and MotAB/MotCD, which are localized to the inner membrane and at the cell surface (59, 74), contribute to the repression of motility in the presence of ethanol? Alcohols such as ethanol have been implicated in changing membranes (75–79). For example, ethanol can intercalate into membranes with lipid compositions similar to those for P. aeruginosa and associate with the head groups of phospholipids (78). Ethanol will disrupt membrane structure and composition even at concentrations of 1% (80). The effects of ethanol on lipid bilayers include increased fluidity, changes to lateral pressure within the bilayer, and disruption of the structure of membrane anchored proteins such as the ones outlined in our pathway described above (78, 79). These effects on membrane structure could cause conformation changes in the membrane-localized/associated PilY1, PilVWX, and PilMNOP proteins and may lead to their activation as signaling molecules. Ethanol can also have other physiological impacts on other microorganisms. Cao et al. showed that in E. coli, 2.5% to 5% (428 to 855 mM) ethanol resulted in increased reactive oxygen species (ROS) stress, reduced peptidoglycan, and a decrease in the proton gradient that might be explained by increased membrane fluidity (76). Ethanol (<3% [vol/vol]) was also shown to increase proton influx across the plasma membrane and disrupt the proton motive force in Saccharomyces cerevisiae (81). Thus, if ethanol perturbs the membrane or disrupts the proton motive force in P. aeruginosa, such effects might impact proteins such as MotAB/MotCD, which use the proton gradient to generate the torque needed to rotate the flagellum. It is also possible that there are unknown regulators that are activated by ethanol to induce the activity of the pathway described above.
We also showed that the N-terminal vWA domain of PilY1 is dispensable for responding to ethanol, implicating the C-terminal “PilC” domain of this protein as key for the observed ethanol response. The C terminus of the PilY1 protein has a seven-bladed, modified β-propeller structure that shares structural similarity to the quinohemoprotein alcohol dehydrogenase from Comamonas testosteroni (60). Therefore, it is possible that PilY1 has the ability to bind ethanol, or the cofactor (PQQ) required for its catabolism, to cause the activation of downstream components required for motility repression. Future work will address the structural role of the PilY1 C-terminal domain in the ethanol motility response, but our findings suggest that PilY1 might be a central point to integrate nutritional and surface-sensing signals for this microbe.
To conclude, our findings indicate that ethanol triggers a complex response that modulates behaviors related to biofilm initiation in order to facilitate the transition from motility to a sessile lifestyle. Therefore, the effects of ethanol on microbes, at concentrations much lower than those used for the purpose of sterilization, is of interest in the context of biofuel production, microbial remediation of industrial waste, and the activity of naturally occurring communities in the environment and in association with humans. Future studies will determine if ethanol’s effects on P. aeruginosa motility contribute to the stimulation of biofilm formation and if the effects of ethanol on motility and biofilm formation in other Gram-negative species, such as Acinetobacter baumannii (8), occur through a common pathway.
MATERIALS AND METHODS
Strains and media.
Strains and plasmids used in this study are listed in Table S3 is the supplemental material. P. aeruginosa PA14 and E. coli strains were routinely cultured on lysogeny broth (LB) solidified with 1.5% agar, or in LB broth at 37°C with shaking. Gentamicin (Gm) was used at 60 μg/ml and carbenicillin (Cb) at 700 μg/ml for P. aeruginosa. Gm was used at 10 μg/ml for E. coli. For P. aeruginosa phenotypic assays, either M63 [22 mM KH2PO4, 40 mM K2HPO4, and 15 mM (NH4)2SO4] or M8 (42 mM Na2PO4, 22 mM KH2PO4, and 8.5 mM NaCl) minimal salts medium supplemented with MgSO4 (1 mM), glucose (0.2%), and Casamino Acids (CAA; 0.5%), was used. When stated, 1% (vol/vol) ethanol (200 proof, nondenatured; Koptec) was added to cooled medium (∼50°C), and an equivalent volume of water was added to control medium. For expression plasmids harboring the pBAD promoter, arabinose was added to the culture as needed (0.02% or 0.05%).
Growth curve of P. aeruginosa PA14 wild type in the presence of ethanol.
Growth curve analysis was performed by diluting overnight P. aeruginosa cultures to an OD600 of ∼0.01 in 6 ml M63 medium without and with 1% (vol/vol) ethanol and incubation at 37°C on a roller drum. OD600 was measured at specified time points using a Spectronic 20 spectrophotometer. Each sample type was analyzed in triplicates.
Molecular techniques.
Plasmids were made using previously described homologous recombination in Saccharomyces cerevisiae (82). Plasmids were then extracted from the yeast using the “smash and grab” method, electroporated into E. coli S-17 cells, and confirmed via colony PCR. E. coli with confirmed constructs were then conjugated with the various P. aeruginosa strains to generate in-frame deletion mutants using allelic replacement as previously described (82). Merodiploids were selected on solid LB using gentamicin and nalidixic acid followed by counterselection on 5% sucrose. PCR amplification and DNA sequencing, using primers that flanked the site of deletion, were used to confirm all resulting mutants.
For arabinose-inducible complementation, the gene being complemented was expressed on either pMQ80 (60 μg/ml gentamicin) or pDPM73 (700 μg/ml carbenicillin) plasmid backbones. Confirmed constructs were electroporated into the various P. aeruginosa strains, selecting for the appropriate antibiotic resistance marker. Arabinose (0.02% or 0.05%) was added to the medium, and complementation was confirmed via the indicated phenotypic assay.
Swimming motility assays.
Swim assays were performed as previously described (25). Briefly, M63 medium without and with 1% (vol/vol) ethanol and solidified with 0.3% agar (soft agar) was poured into petri plates and allowed to dry at room temperature (∼25°C) for ∼4 h prior to inoculation. Sterile toothpicks were used to inoculate bacteria into the center of the agar without touching the bottom of the plate; liquid cultures grown for 8 to 16 h were used as inoculum. No more than four strains were assayed per plate. Plates were incubated upright at 37°C in stacks of no more than four plates per stack for 18 to 20 h; the swim zone area was then measured. P. aeruginosa wild type was included in each experiment so that the change in motility between the conditions for each mutant could be assessed despite day-to-day variation in swim zone area for wild-type cultures. The wild-type swim zone areas for the control condition usually ranged from ∼750 to 850 mm2, with most being ∼800 mm2. Each strain was inoculated in four replicates, and replicate values were averaged to obtain a final swim zone area for each strain. All strains were assessed on at least three separate days.
Twitching motility assays.
Twitching motility assays were performed with T-agar medium (10 g tryptone, 5 g NaCl, and 15 g agar in 1 liter) without and with 1% ethanol in petri plates that were allowed to dry at room temperature for 24 h prior to inoculation. Sterile toothpicks were used to inoculate into the agar until the toothpick touched the bottom of the petri plate; liquid cultures grown for 16 h were used as inoculum. No more than four strains were analyzed per plate, and six replicate plates were included in each experiment. Plates were incubated in inverted stacks of four at 37°C for 40 h. To visualize the twitch zone, a spatula was used to gently ease the agar out of the petri plates, and 2 ml of 0.1% (wt/vol) crystal violet in water was added to each plate and allowed to stand for 10 min. The crystal violet was removed, and the plates were rinsed with water and allowed to air dry. Twitch zone area was measure and recorded. All strains were assessed on at least three separate days.
Swarming motility assays.
Swarm assays were performed as previously described (25). Briefly, M8 medium, without and with 1% ethanol, and with 0.5% agar (swarm agar) was poured into 60 mm by 15 mm plates and allowed to dry at room temperature for ∼4 h prior to inoculation. Each plate was inoculated with 0.5 μl of a liquid culture that was grown for 8 to 16 h, and the plates incubated face up at 37°C in stacks of no more than four for 16 h. Each strain was inoculated in four replicates and was assessed on at least three separate days. Images were captured using a Canon EOS Rebel T6i camera, and images were measured for ethanol-dependent swarm repression.
Reversal rate measurements.
To measure the frequency at which a motile cell changes its direction, we used a modified version of a method that was previously described (39, 67). Briefly, overnight liquid cultures were subcultured 1:100 in 5 ml M63 medium and incubated at 37°C for 2 h. Once cultures reached exponential phase, they were then diluted 1:1,000 in fresh M63 medium, and Ficoll was added to a final concentration of 3% to obtain higher viscosity conditions that slowed the swimming cells sufficiently to allow the monitoring of reversal rates and mimic swimming in soft agar. Cells were then exposed to either control medium or medium containing 1% (vol/vol) ethanol for 15 min. Two hundred fifty microliters of treated cell culture was next gently pipetted into a 35-mm glass bottom MatTek dish, and a glass coverslip was placed over the added culture. Four time-lapse movies per strain and condition were captured with dark field using the Nikon Eclipse Ti microscope (Nikon Instruments Inc., Melville, NY) equipped with a 10× lens objective, a Hamamatsu ORCA-Flash 4.0 camera, and Nikon NIS Elements AR 4.13.04 64 bit software. Time-lapse movies were 8 s in duration, with images captured at 20- to 25-ms intervals with RAM capture and 50 fps. Fiji ImageJ TrackMate (83) was used to process, analyze, and quantify the reversal rates of 40 cells per movie. Movies were advanced frame by frame, and individual cells were evaluated for the number of times they changed direction within the field of view; reversal rates were normalized and recorded as reversals per 10 s.
Microscopic agar motility assay.
The population percentages of cells that remained continuously motile were calculated in 0.3% agar. Overnight liquid cultures were subcultured 1:100 in 5 ml M63 medium and incubated at 37°C for 2 h. Once cultures reached exponential phase, the cells were visually examined under the microscope to ensure the starting culture was motile. Cultures were then diluted by 1:1,000 into freshly prepared M63 agar (0.3%) without and with 1% (vol/vol) ethanol cooled to ∼45°C. Two hundred fifty microliters of each agar mixture was pipetted into a chamber slide (Fig. 6A) and allowed to solidify and acclimate to treatment for 30 min. Three to four time-lapse movies per chamber slide, with two chamber slides per condition, were captured using the 40× lens objective on the Nikon Eclipse Ti microscope (Nikon Instruments Inc., Melville, NY) equipped with a Hamamatsu ORCA-Flash 4.0 camera and Nikon NIS Elements AR 4.13.04 64 bit software. Time-lapse movies were 8 s in duration, with images captured at 5-ms intervals with RAM capture and 100 fps. Fiji ImageJ TrackMate (83) was used to process, analyze, and quantify the percentages of cells that were continuously motile for the entire duration of each movie. Movies were advanced frame by frame, and individual cells were evaluated for movement. All strains were assessed on at least two separate days.
In vivo cyclic di-GMP quantification.
c-di-GMP was measured as previously described (18) with modifications. Overnight liquid cultures were diluted 1:1,000 in 6 ml M63 medium with either 1% ethanol or an equivalent volume of water and grown at 37°C for 16 h on a roller drum. Cultures were then adjusted to similar densities (OD600) and their densities recorded. Five milliliters of each culture was pelleted at 4,500 × g for 15 min at 4°C. c-di-GMP was extracted by vigorously suspending the pellet in 250 μl of ice-cold extraction buffer (40:40:20 methanol [MeOH]-acetonitrile-distilled water [dH2O] and 0.1 N Formic acid, stored at −20°C) and incubating at −20°C for 1 h with tubes positioned upright. Tubes were then centrifuged briefly prior to transfer of the entirety of each extraction mix to a preweighed ice-cold 1.5-ml Eppendorf tube. Cell debris was pelleted at 15,682 × g for five min at 4°C, 250 μl of the extracted nucleotide was recovered into a clean 1.5-ml ice-cold Eppendorf tube, and samples were each neutralized with 4 μl of 15% NH4HCO3 per 100 μl of sample. Pellets were dried on high for 1 h and the liquid samples on low overnight using the Savant Speed Vac SC110. The pellet weights were measured to get sample dry weight, and the dried liquid samples containing the extracted nucleotides were each suspended in 250 μl high-pressure liquid chromatography (HPLC)-grade water. Two hundred microliters of each sample was sent to the RTSF Mass Spectrometry and Metabolomics Core at Michigan State University for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Each strain and treatment condition were analyzed in five replicates and reported as picomoles c-di-GMP per OD600 unit.
Statistical analysis.
Unpaired Student’s t test, two-way analysis of variance (ANOVA) with multiple comparisons, and one-way ANOVA with multiple comparisons were performed pairwise between the wild type and each strain, as well as between ethanol and control conditions, using Prism 6 software (GraphPad, La Jolla, CA).
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by grants from the National Institutes of Health to D.A.H. (R01 GM108492) and to G.A.O. (R37 AI83256), a pilot project from STANTO19R0, and NSF 1458359 (D.A.H. and K.A.L.). Support for C.E.H. came in part from 5T32AI007519 and NIAID A1007519. Additional support was provided by the NCI Cancer Center support grant, 5P30 CA023108, through the Molecular Biology Shared Resource, and NIGMS P20GM113132 through the Molecular Interactions and Imaging Core (MIIC).
We thank. Karin Sauer for sharing reagents, Emily L. Dolben for assistance with early experiments, Alan J. Collins for designing the chamber slide setup used in the microscopic agar motility assays, Dae Gon Ha for making the gcbA complementation construct, and Zdenek Svindrych for assistance with microscopy analyses.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00285-19.
REFERENCES
- 1.Elshaghabee FM, Bockelmann W, Meske D, de Vrese M, Walte HG, Schrezenmeir J, Heller KJ. 2016. Ethanol production by selected intestinal microorganisms and lactic acid bacteria growing under different nutritional conditions. Front Microbiol 7:47. doi: 10.3389/fmicb.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morales DK, Grahl N, Okegbe C, Dietrich LE, Jacobs NJ, Hogan DA. 2013. Control of Candida albicans metabolism and biofilm formation by Pseudomonas aeruginosa phenazines. mBio 4:e00526-12. doi: 10.1128/mBio.00526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logan BK, Distefano S. 1998. Ethanol content of various foods and soft drinks and their potential for interference with a breath-alcohol test. J Anal Toxicol 22:181–183. doi: 10.1093/jat/22.3.181. [DOI] [PubMed] [Google Scholar]
- 4.Tanimura A, Kikukawa M, Yamaguchi S, Kishino S, Ogawa J, Shima J. 2015. Direct ethanol production from starch using a natural isolate, Scheffersomyces shehatae: toward consolidated bioprocessing. Sci Rep 5:9593. doi: 10.1038/srep09593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ranger CM, Biedermann PHW, Phuntumart V, Beligala GU, Ghosh S, Palmquist DE, Mueller R, Barnett J, Schultz PB, Reding ME, Benz JP. 2018. Symbiont selection via alcohol benefits fungus farming by ambrosia beetles. Proc Natl Acad Sci U S A 115:4447–4452. doi: 10.1073/pnas.1716852115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovett JL, Marchesini N, Moreno SN, Sibley LD. 2002. Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from inositol 1,4,5-triphosphate (IP(3)/ryanodine-sensitive stores. J Biol Chem 277:25870–25876. doi: 10.1074/jbc.M202553200. [DOI] [PubMed] [Google Scholar]
- 7.Carruthers VB, Moreno SN, Sibley LD. 1999. Ethanol and acetaldehyde elevate intracellular [Ca2+] and stimulate microneme discharge in Toxoplasma gondii. Biochem J 342:379–386. doi: 10.1042/0264-6021:3420379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nwugo CC, Arivett BA, Zimbler DL, Gaddy JA, Richards AM, Actis LA. 2012. Effect of ethanol on differential protein production and expression of potential virulence functions in the opportunistic pathogen Acinetobacter baumannii. PLoS One 7:e51936. doi: 10.1371/journal.pone.0051936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camarena L, Bruno V, Euskirchen G, Poggio S, Snyder M. 2010. Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog 6:e1000834. doi: 10.1371/journal.ppat.1000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith MG, Gianoulis TA, Pukatzki S, Mekalanos JJ, Ornston LN, Gerstein M, Snyder M. 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev 21:601–614. doi: 10.1101/gad.1510307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavlova SI, Jin L, Gasparovich SR, Tao L. 2013. Multiple alcohol dehydrogenases but no functional acetaldehyde dehydrogenase causing excessive acetaldehyde production from ethanol by oral Streptococci. Microbiology 159:1437–1446. doi: 10.1099/mic.0.066258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montuschi P, Paris D, Melck D, Lucidi V, Ciabattoni G, Raia V, Calabrese C, Bush A, Barnes PJ, Motta A. 2012. NMR spectroscopy metabolomic profiling of exhaled breath condensate in patients with stable and unstable cystic fibrosis. Thorax 67:222–228. doi: 10.1136/thoraxjnl-2011-200072. [DOI] [PubMed] [Google Scholar]
- 13.Montuschi P, Paris D, Montella S, Melck D, Mirra V, Santini G, Mores N, Montemitro E, Majo F, Lucidi V, Bush A, Motta A, Santamaria F. 2014. Nuclear magnetic resonance-based metabolomics discriminates primary ciliary dyskinesia from cystic fibrosis. Am J Respir Crit Care Med 190:229–233. doi: 10.1164/rccm.201402-0249LE. [DOI] [PubMed] [Google Scholar]
- 14.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Doring G. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest 109:317–325. doi: 10.1172/JCI200213870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sofia M, Maniscalco M, de Laurentiis G, Paris D, Melck D, Motta A. 2011. Exploring airway diseases by NMR-based metabonomics: a review of application to exhaled breath condensate. J Biomed Biotechnol 2011:403260. doi: 10.1155/2011/403260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolak JE, Esther CR, O’Connell TM. 2009. Metabolomic analysis of bronchoalveolar lavage fluid from cystic fibrosis patients. Biomarkers 14:55–60. doi: 10.1080/13547500802688194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bos LD, Meinardi S, Blake D, Whiteson K. 2016. Bacteria in the airways of patients with cystic fibrosis are genetically capable of producing VOCs in breath. J Breath Res 10:047103. doi: 10.1088/1752-7163/10/4/047103. [DOI] [PubMed] [Google Scholar]
- 18.Chen AI, Dolben EF, Okegbe C, Harty CE, Golub Y, Thao S, Ha DG, Willger SD, O'Toole GA, Harwood CS, Dietrich LE, Hogan DA. 2014. Candida albicans ethanol stimulates Pseudomonas aeruginosa WspR-controlled biofilm formation as part of a cyclic relationship involving phenazines. PLoS Pathog 10:e1004480. doi: 10.1371/journal.ppat.1004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merighi M, Lee VT, Hyodo M, Hayakawa Y, Lory S. 2007. The second messenger bis-(3'-5')-cyclic-GMP and its PilZ domain-containing receptor Alg44 are required for alginate biosynthesis in Pseudomonas aeruginosa. Mol Microbiol 65:876–895. doi: 10.1111/j.1365-2958.2007.05817.x. [DOI] [PubMed] [Google Scholar]
- 20.Merritt JH, Brothers KM, Kuchma SL, O'Toole GA. 2007. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. J Bacteriol 189:8154–8164. doi: 10.1128/JB.00585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merritt JH, Ha DG, Cowles KN, Lu W, Morales DK, Rabinowitz J, Gitai Z, O'Toole GA. 2010. Specific control of Pseudomonas aeruginosa surface-associated behaviors by two c-di-GMP diguanylate cyclases. mBio 1:e00183-10. doi: 10.1128/mBio.00183-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stelitano V, Giardina G, Paiardini A, Castiglione N, Cutruzzola F, Rinaldo S. 2013. c-di-GMP hydrolysis by Pseudomonas aeruginosa HD-GYP phosphodiesterases: analysis of the reaction mechanism and novel roles for pGpG. PLoS One 8:e74920. doi: 10.1371/journal.pone.0074920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuchma SL, Brothers KM, Merritt JH, Liberati NT, Ausubel FM, O'Toole GA. 2007. BifA, a cyclic-Di-GMP phosphodiesterase, inversely regulates biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol 189:8165–8178. doi: 10.1128/JB.00586-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazmierczak BI, Lebron MB, Murray TS. 2006. Analysis of FimX, a phosphodiesterase that governs twitching motility in Pseudomonas aeruginosa. Mol Microbiol 60:1026–1043. doi: 10.1111/j.1365-2958.2006.05156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha DG, Richman ME, O'Toole GA. 2014. Deletion mutant library for investigation of functional outputs of cyclic diguanylate metabolism in Pseudomonas aeruginosa PA14. Appl Environ Microbiol 80:3384–3393. doi: 10.1128/AEM.00299-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giacalone D, Smith TJ, Collins AJ, Sondermann H, Koziol LJ, O'Toole GA. 2018. Ligand-mediated biofilm formation via enhanced physical interaction between a diguanylate cyclase and its receptor. mBio 9:e01254-18. doi: 10.1128/mBio.01254-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei J, Grishin NV. 2001. GGDEF domain is homologous to adenylyl cyclase. Proteins 42:210–216. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Valentini M, Filloux A. 2016. Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from Pseudomonas aeruginosa and other bacteria. J Biol Chem 291:12547–12555. doi: 10.1074/jbc.R115.711507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker AE, Diepold A, Kuchma SL, Scott JE, Ha DG, Orazi G, Armitage JP, O'Toole GA. 2016. PilZ domain protein FlgZ mediates cyclic di-GMP-dependent swarming motility control in Pseudomonas aeruginosa. J Bacteriol 198:1837–1846. doi: 10.1128/JB.00196-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waters CM. 2013. Bacterial wheel locks: extracellular polysaccharide inhibits flagellar rotation. J Bacteriol 195:409–410. doi: 10.1128/JB.02147-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zorraquino V, Garcia B, Latasa C, Echeverz M, Toledo-Arana A, Valle J, Lasa I, Solano C. 2013. Coordinated cyclic-di-GMP repression of Salmonella motility through YcgR and cellulose. J Bacteriol 195:417–428. doi: 10.1128/JB.01789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfe AJ, Visick KL. 2008. Get the message out: cyclic-Di-GMP regulates multiple levels of flagellum-based motility. J Bacteriol 190:463–475. doi: 10.1128/JB.01418-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choy WK, Zhou L, Syn CK, Zhang LH, Swarup S. 2004. MorA defines a new class of regulators affecting flagellar development and biofilm formation in diverse Pseudomonas species. J Bacteriol 186:7221–7228. doi: 10.1128/JB.186.21.7221-7228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko M, Park C. 2000. Two novel flagellar components and H-NS are involved in the motor function of Escherichia coli. J Mol Biol 303:371–382. doi: 10.1006/jmbi.2000.4147. [DOI] [PubMed] [Google Scholar]
- 35.Blair KM, Turner L, Winkelman JT, Berg HC, Kearns DB. 2008. A molecular clutch disables flagella in the Bacillus subtilis biofilm. Science 320:1636–1638. doi: 10.1126/science.1157877. [DOI] [PubMed] [Google Scholar]
- 36.Kuchma SL, Delalez NJ, Filkins LM, Snavely EA, Armitage JP, O'Toole GA. 2015. Cyclic di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa PA14 requires the MotAB stator. J Bacteriol 197:420–430. doi: 10.1128/JB.02130-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker AE, Webster SS, Diepold A, Kuchma SL, Bordeleau E, Armitage JP, O'Toole GA. 14 January 2019. Flagellar stators stimulate c-di-GMP production by Pseudomonas aeruginosa. J Bacteriol doi: 10.1128/JB.00741-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian C, Wong CC, Swarup S, Chiam KH. 2013. Bacterial tethering analysis reveals a “run-reverse-turn” mechanism for Pseudomonas species motility. Appl Environ Microbiol 79:4734–4743. doi: 10.1128/AEM.01027-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrova OE, Cherny KE, Sauer K. 2014. The Pseudomonas aeruginosa diguanylate cyclase GcbA, a homolog of P. fluorescens GcbA, promotes initial attachment to surfaces, but not biofilm formation, via regulation of motility. J Bacteriol 196:2827–2841. doi: 10.1128/JB.01628-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM. 2010. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol Cell 38:128–139. doi: 10.1016/j.molcel.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kojima S, Blair DF. 2001. Conformational change in the stator of the bacterial flagellar motor. Biochemistry 40:13041–13050. doi: 10.1021/bi011263o. [DOI] [PubMed] [Google Scholar]
- 42.Braun TF, Blair DF. 2001. Targeted disulfide cross-linking of the MotB protein of Escherichia coli: evidence for two H+ channels in the stator complex. Biochemistry 40:13051–13059. doi: 10.1021/bi011264g. [DOI] [PubMed] [Google Scholar]
- 43.Blair DF. 2003. Flagellar movement driven by proton translocation. FEBS Lett 545:86–95. doi: 10.1016/S0014-5793(03)00397-1. [DOI] [PubMed] [Google Scholar]
- 44.Toutain CM, Caizza NC, Zegans ME, O'Toole GA. 2007. Roles for flagellar stators in biofilm formation by Pseudomonas aeruginosa. Res Microbiol 158:471–477. doi: 10.1016/j.resmic.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Doyle TB, Hawkins AC, McCarter LL. 2004. The complex flagellar torque generator of Pseudomonas aeruginosa. J Bacteriol 186:6341–6350. doi: 10.1128/JB.186.19.6341-6350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tashiro Y, Inagaki A, Ono K, Inaba T, Yawata Y, Uchiyama H, Nomura N. 2014. Low concentrations of ethanol stimulate biofilm and pellicle formation in Pseudomonas aeruginosa. Biosci Biotechnol Biochem 78:178–181. doi: 10.1080/09168451.2014.877828. [DOI] [PubMed] [Google Scholar]
- 47.Gorisch H. 2003. The ethanol oxidation system and its regulation in Pseudomonas aeruginosa. Biochim Biophys Acta 1647:98–102. doi: 10.1016/S1570-9639(03)00066-9. [DOI] [PubMed] [Google Scholar]
- 48.Mern DS, Ha SW, Khodaverdi V, Gliese N, Gorisch H. 2010. A complex regulatory network controls aerobic ethanol oxidation in Pseudomonas aeruginosa: indication of four levels of sensor kinases and response regulators. Microbiology 156:1505–1516. doi: 10.1099/mic.0.032847-0. [DOI] [PubMed] [Google Scholar]
- 49.Baker AE, O'Toole GA. 2017. Bacteria, rev your engines: stator dynamics regulate flagellar motility. J Bacteriol 199:00088-17. doi: 10.1128/JB.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanda E, Tatsuta T, Suzuki T, Taguchi F, Naito K, Inagaki Y, Toyoda K, Shiraishi T, Ichinose Y. 2011. Two flagellar stators and their roles in motility and virulence in Pseudomonas syringae pv. tabaci 6605. Mol Genet Genomics 285:163–174. doi: 10.1007/s00438-010-0594-8. [DOI] [PubMed] [Google Scholar]
- 51.Thormann KM, Paulick A. 2010. Tuning the flagellar motor. Microbiology 156:1275–1283. doi: 10.1099/mic.0.029595-0. [DOI] [PubMed] [Google Scholar]
- 52.Ryjenkov DA, Simm R, Romling U, Gomelsky M. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J Biol Chem 281:30310–30314. doi: 10.1074/jbc.C600179200. [DOI] [PubMed] [Google Scholar]
- 53.Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U. 2010. Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141:107–116. doi: 10.1016/j.cell.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 54.McCarter LL, Gomelsky M. 2015. Fifty ways to inhibit motility via cyclic di-GMP: the emerging Pseudomonas aeruginosa swarming story. J Bacteriol 197:406–409. doi: 10.1128/JB.02483-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol 7:263–273. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- 56.Pratt JT, Tamayo R, Tischler AD, Camilli A. 2007. PilZ domain proteins bind cyclic diguanylate and regulate diverse processes in Vibrio cholerae. J Biol Chem 282:12860–12870. doi: 10.1074/jbc.M611593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christen M, Christen B, Allan MG, Folcher M, Jeno P, Grzesiek S, Jenal U. 2007. DgrA is a member of a new family of cyclic diguanosine monophosphate receptors and controls flagellar motor function in Caulobacter crescentus. Proc Natl Acad Sci U S A 104:4112–4117. doi: 10.1073/pnas.0607738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luo Y, Zhao K, Baker AE, Kuchma SL, Coggan KA, Wolfgang MC, Wong GC, O'Toole GA. 2015. A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. mBio 6:e02456-14. doi: 10.1128/mBio.02456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuchma SL, Ballok AE, Merritt JH, Hammond JH, Lu W, Rabinowitz JD, O'Toole GA. 2010. Cyclic-di-GMP-mediated repression of swarming motility by Pseudomonas aeruginosa: the pilY1 gene and its impact on surface-associated behaviors. J Bacteriol 192:2950–2964. doi: 10.1128/JB.01642-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orans J, Johnson MD, Coggan KA, Sperlazza JR, Heiniger RW, Wolfgang MC, Redinbo MR. 2010. Crystal structure analysis reveals Pseudomonas PilY1 as an essential calcium-dependent regulator of bacterial surface motility. Proc Natl Acad Sci U S A 107:1065–1070. doi: 10.1073/pnas.0911616107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anyan ME, Amiri A, Harvey CW, Tierra G, Morales-Soto N, Driscoll CM, Alber MS, Shrout JD. 2014. Type IV pili interactions promote intercellular association and moderate swarming of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 111:18013–18018. doi: 10.1073/pnas.1414661111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang A, Tang WS, Si T, Tang JX. 2017. Influence of physical effects on the swarming motility of Pseudomonas aeruginosa. Biophys J 112:1462–1471. doi: 10.1016/j.bpj.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belete B, Lu H, Wozniak DJ. 2008. Pseudomonas aeruginosa AlgR regulates type IV pilus biosynthesis by activating transcription of the fimU-pilVWXY1Y2E operon. J Bacteriol 190:2023–2030. doi: 10.1128/JB.01623-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cai Q, Li Z, Ouyang Q, Luo C, Gordon VD. 2016. Singly flagellated Pseudomonas aeruginosa chemotaxes efficiently by unbiased motor regulation. mBio 7:e00013-16. doi: 10.1128/mBio.00013-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toutain CM, Zegans ME, O'Toole GA. 2005. Evidence for two flagellar stators and their role in the motility of Pseudomonas aeruginosa. J Bacteriol 187:771–777. doi: 10.1128/JB.187.2.771-777.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang S, Yu S, Zhang Z, Wei Q, Yan L, Ai G, Liu H, Ma LZ. 2014. Coordination of swarming motility, biosurfactant synthesis, and biofilm matrix exopolysaccharide production in Pseudomonas aeruginosa. Appl Environ Microbiol 80:6724–6732. doi: 10.1128/AEM.01237-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caiazza NC, Merritt JH, Brothers KM, O'Toole GA. 2007. Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol 189:3603–3612. doi: 10.1128/JB.01685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Granero F, Navazo A, Barahona E, Redondo-Nieto M, Gonzalez de Heredia E, Baena I, Martin-Martin I, Rivilla R, Martin M. 2014. Identification of flgZ as a flagellar gene encoding a PilZ domain protein that regulates swimming motility and biofilm formation in Pseudomonas. PLoS One 9:e87608. doi: 10.1371/journal.pone.0087608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vater SM, Weiße S, Maleschlijski S, Lotz C, Koschitzki F, Schwartz T, Obst U, Rosenhahn A. 2014. Swimming behavior of Pseudomonas aeruginosa studied by holographic 3D tracking. PLoS One 9:e87765. doi: 10.1371/journal.pone.0087765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deziel E, Comeau Y, Villemur R. 2001. Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J Bacteriol 183:1195–1204. doi: 10.1128/JB.183.4.1195-1204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ha DG, Kuchma SL, O'Toole GA. 2014. Plate-based assay for swimming motility in Pseudomonas aeruginosa. Methods Mol Biol 1149:59–65. doi: 10.1007/978-1-4939-0473-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Croze OA, Ferguson GP, Cates ME, Poon WC. 2011. Migration of chemotactic bacteria in soft agar: role of gel concentration. Biophys J 101:525–534. doi: 10.1016/j.bpj.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Espeso DR, Martinez-Garcia E, de Lorenzo V, Goni-Moreno A. 2016. Physical forces shape group identity of swimming Pseudomonas putida cells. Front Microbiol 7:1437. doi: 10.3389/fmicb.2016.01437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuchma SL, Griffin EF, O'Toole GA. 2012. Minor pilins of the type IV pilus system participate in the negative regulation of swarming motility. J Bacteriol 194:5388–5403. doi: 10.1128/JB.00899-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huffer S, Clark ME, Ning JC, Blanch HW, Clark DS. 2011. Role of alcohols in growth, lipid composition, and membrane fluidity of yeasts, bacteria, and archaea. Appl Environ Microbiol 77:6400–6408. doi: 10.1128/AEM.00694-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao H, Wei D, Yang Y, Shang Y, Li G, Zhou Y, Ma Q, Xu Y. 2017. Systems-level understanding of ethanol-induced stresses and adaptation in E. coli. Sci Rep 7:44150. doi: 10.1038/srep44150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soufi B, Krug K, Harst A, Macek B. 2015. Characterization of the E. coli proteome and its modifications during growth and ethanol stress. Front Microbiol 6:103. doi: 10.3389/fmicb.2015.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patra M, Salonen E, Terama E, Vattulainen I, Faller R, Lee BW, Holopainen J, Karttunen M. 2006. Under the influence of alcohol: the effect of ethanol and methanol on lipid bilayers. Biophys J 90:1121–1135. doi: 10.1529/biophysj.105.062364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Terama E, Ollila OH, Salonen E, Rowat AC, Trandum C, Westh P, Patra M, Karttunen M, Vattulainen I. 2008. Influence of ethanol on lipid membranes: from lateral pressure profiles to dynamics and partitioning. J Phys Chem B 112:4131–4139. doi: 10.1021/jp0750811. [DOI] [PubMed] [Google Scholar]
- 80.Gurtovenko AA, Anwar J. 2009. Interaction of ethanol with biological membranes: the formation of non-bilayer structures within the membrane interior and their significance. J Phys Chem B 113:1983–1992. doi: 10.1021/jp808041z. [DOI] [PubMed] [Google Scholar]
- 81.Cartwright CP, Juroszek JR, Beavan MJ, Ruby FMS, Demorais SMF, Rose AH. 1986. Ethanol dissipates the proton-motive force across the plasma-membrane of Saccharomyces cerevisiae. Microbiology 132:369–377. doi: 10.1099/00221287-132-2-369. [DOI] [Google Scholar]
- 82.Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O'Toole GA. 2006. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from Gram-negative bacteria. Appl Environ Microbiol 72:5027–5036. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.