Abstract
Purpose
Preimplantation genetic testing for aneuploidy (PGT-A) has become increasingly controversial since normal euploid births have been reported following transfer of embryos diagnosed as “abnormal.” There is an increasing trend in transferring “abnormal” embryos; but it is still unknown how many IVF centers transfer “abnormal” embryos and with what efficiency.
Methods
We performed a worldwide web-survey of IVF centers to elucidate PGT-A related practice patterns including transfer of human embryos found “abnormal” by PGT-A. Participating centers reflected in vitro fertilization (IVF) cycles in the USA, Canada, Europe, Asia, South America, and Africa.
Results
One hundred fifty-one IVF centers completed the survey; 125 (83%) reported utilization of PGT-A. Europe had the highest utilization (32.3%), followed by the USA and Canada combined at 29.1%. The leading indications for PGT-A were advanced maternal age (77%), followed by recurrent implantation failure (70%), unexplained pregnancy loss (65%), and sex determination (25%); 14% of respondents used PGT-A for all of their IVF cycles; 20% of IVF units reported transfers of chromosomally “abnormal” embryos, and 56% of these took place in the USA, followed by Asia in 20%. Remarkably, 106 (49.3%) cycles resulted in ongoing pregnancies (n = 50) or live births (n = 56). Miscarriages were rare (n = 20; 9.3%).
Conclusions
The transfers of “abnormal” embryos by PGT-A offered robust pregnancy and live birth chances with low miscarriage rates. These data further strengthen the argument that PGT-A cannot reliably determine which embryos should or should not be transferred and leads to disposal of many normal embryos with excellent pregnancy potential.
Keywords: Pre-implantation genetic testing, Aneuploidy, Embryo mosaicism, IVF
Introduction
In July 2016, the International Preimplantation Diagnosis International Society (PGDIS) modified reporting guidelines for what until then was called preimplantation genetic screening (PGS), and for the first time allowed selective transfers of embryos that the society defined as “mosaic”[1, 2]. On that occasion, PGS was also renamed preimplantation genetic testing for aneuploidy (PGT-A). These changes, at least in part, were made in response to two published reports in 2015, for the first time demonstrating healthy live births after transfer of embryos previously diagnosed as chromosomally “abnormal” [3, 4]. Both reports convincingly demonstrated that the bimodal reporting system of euploid/aneuploid, up to that point in use worldwide, was no longer sustainable.
The new guidelines were all-encompassing in that they included recommendations for diagnostic laboratory practices and clinical reporting of results, as well as recommendations for physicians how to interpret the new reporting system in clinical practice. Because only next generation sequencing (NGS) was able to identify a second (i.e., mosaic) cell line in a single trophectoderm biopsy (TEB), the new guidelines recommended the exclusive use of NGS for defining ploidy of trophectoderm biopsies. Most importantly, however, the reporting system switched in describing embryos from a bi-modal system of euploid and aneuploid, to a tri-modal system of euploid, mosaic, and aneuploid. The latter represented a radical change because it, suddenly, allowed for the conditional transfer of at least some embryos which, up to that point, unequivocally had been disposed of as “abnormal.”
To define the new category of mosaic embryos, the PGDIS guidelines introduced the new concept of DNA “thresholds” which, based on percentage of aneuploid DNA load in a single TEB, defined whether an embryo was normal, mosaic, or aneuploid. Cut offs were, however, determined arbitrarily. Up to 20% aneuploid DNA in the TEB sample differentiated between a normal and mosaic embryo, and 80% between mosaic and aneuploidy [5]. Within the large mosaic range of 21–79% aneuploid DNA load, 40% was alleged to differentiate between better and poorer pregnancy chances if such embryos were to be transferred [6], a claim immediately refuted by Kushnir at al. [7] in a reanalysis of the original data set of Munné et al. [6]. The confusion created by the new PGDIS guidelines was further enhanced by a publication suggesting that approximately 40% of embryos, reported as either mosaic or aneuploid, were likely false-positive diagnoses [8].
These observations increased concerns that large numbers of potentially viable embryos may have been mistakenly discarded after PGS/PGT-A, as first suggested by the two initial reports demonstrating healthy live births after transfer of allegedly “abnormal” embryos by Gleicher et al. in the USA and Greco et al. in Italy [3, 4]. Since then, at least 200 healthy births have been reported from around the world in women who after PGS/PGT-A had no euploid embryos left for transfer and, therefore, were transferred either mosaic or aneuploid “abnormal” embryos [3, 4, 6, 9–13]. Those reports, however, with great likelihood represent only a fraction of pregnancies established in this way since 2015. While many IVF centers have started to transfer “abnormal” embryos (mostly those defined as mosaic), many others still refuse to perform such transfers and encourage women to undergo additional IVF cycles. How many centers currently offer such transfers is, indeed, unknown. It is also unknown how successful such transfers are in centers beyond those who published initial results. To answer these questions, we designed a web-based survey with a questionnaire accessible at the URL of IVF-Worldwide (http://www.ivf-worldwide.com/survey/are-you-transferring-aneuploid-mosaicembryo.html). The objectives of this survey were (i) to assess utilization and indications for PGS/PGT-A and the type of genetic diagnostic platforms used, (ii) to evaluate the extent and patterns of transferring “abnormal” embryos after PGS/PGT-A, and (iii) to report rate of ongoing pregnancies, live births, and miscarriages after transfer of “abnormal” embryos in daily clinical practice.
Methods
The same methodology described in multiple prior surveys conducted via the www.ivf-worldwide.com registry was utilized in this study. A 17-item questionnaire (Appendix) with multiple choice answers was developed. All but two questions allowed only one answer. The web-based survey was titled, “Are you transferring aneuploid or mosaic embryos?” It was posted on IVF-Worldwide.com on September 20, 2018 and was kept open for data entry until November 8, 2018. All registered members of IVFWorldwide.com, representing a majority of IVF centers worldwide, were invited by several e-mail messages to participate.
To avoid duplicate reporting, three parameters were compared before including submitted data with existing unit registrations on IVF-Worldwide.com: Those included name of unit, country, and email address [1].
Statistical analyses
The analyses were based on total number of IVF cycles in each center and, among those, cycles in which “abnormal” embryos were transferred. They were not based on numbers of IVF units in the survey. The relative proportion of answers, thus, reflects the total proportion of IVF cycles, or cycles receiving “abnormal” embryos, rather than the proportion of individual respondents to the survey questions. In order to limit the influence of large centers, we set the maximum number of annual IVF cycles for one center at 4500 cycles, which represents only three percent of total annual IVF cycles reported.
To calculate the number “abnormal” transferred cycles, we asked, “in how many cycles have you ever transferred “abnormal” embryos?” Possible answers were 1–5, 6–10, 11–15, 16–25, and more than 26. Midpoint values (3, 8, 13, 20, and 30, respectively) were then assigned to each of the answers, with number of respondents in each segment then multiplied by the midpoint value. For example, if ten centers selected the answer 1–5, we multiplied 10 by the midpoint value of 3, obtaining in that segment the number 30. Values calculated for each segment were then totaled. Survey results were calculated by using the formulas described in previously reported research from the IVF-Worldwide network [14]. For example, for a question with four possible answers (a, b, c, d), the following results were calculated:
Results
A total of 151 IVF centers, in aggregate performing 146,400 IVF cycles annually, completed the survey; the average clinic size was 1000 ± 50 annual IVF cycles, and among units who do transfer “abnormal” embryos the average of transferred embryos per unit was 9. It was difficult to estimate the real response rate since it was impossible to know how many units really opened the e-mails, and furthermore, web-based surveys are prone to recall and report bias. A recently published survey [1] descried 386 units (of the 3643 registered in www.IVF-worldwide.com) offering PGS/PGT-A (10.5%). Therefore, by applying the same metrics, our estimated survey response rate of centers offering PGS/PGT-A was 125/3643 (3.4%). However, if we use as a denominator the same number of units (n = 386) that recently answered as centers offering PGS/PGT-A, then the response rate was 32.3% (125/386). As “abnormal” transfer become more common, surveys will have larger cohorts, enabling us to subdivide the results, for example the term “abnormal” varies between units, a subdivision by level of mosaicism, or continents should be done in future research.
The 125 (83%) that reported routine utilization of PGS/PGT-A in total aggregate, they performed 135,800 IVF cycles (Table 1). Europe reported the highest utilization at 32.3% of all IVF cycles, followed by the USA and Canada (combined) at 29.1% and Asia at 21.1%, followed by South America and Australia/New Zealand, while Africa demonstrated the lowest utilization at 1.8%. Percentagewise, US IVF centers, however, utilized PGS/PGT-A most, followed by Europe and Asia.
Table 1.
Demographics and statistics of IVF units utilizing PGS/PGT-A
| Continent | Estimated annual % of all estimated IVF cycles annual IVF cycles | No. of IVF units | % of all no. of IVF units | |
|---|---|---|---|---|
| USA and Canada | 39,500 | 29.1 | 40 | 32 |
| South America | 9,400 | 6.9 | 16 | 12.8 |
| Australia and New Zealand | 12,100 | 8.9 | 7 | 5.6 |
| Asia | 28,600 | 21.1 | 28 | 22.4 |
| Europe | 43,800 | 32.3 | 32 | 25.6 |
| Africa | 2,400 | 1.8 | 2 | 1.6 |
| Total | 135,800 | 100 | 125 | 100 |
A great majority of centers offering PGS/PGT-A (90%), utilized NGS as their genetic screening platform. Allowing multiple answers, we then inquired about indications for PGS/PGT-A. The leading indications were advanced maternal age (77%), followed by recurrent implantation failure (70%), unexplained pregnancy loss (65%), and sex determination of embryo (25%). Remarkably, a full 14% of respondents used PGS/PGT-A for all of the center’s IVF cycles.
In our survey, of the 125 centers offering PGT/A, 25 (20%) performed transfers of abnormal embryos and 100 did not (Table 2). This number is, however, somewhat misleading because utilization of “abnormal” embryo transfers differed profoundly between regions of the world: In the USA, 56% of participating IVF centers reported such transfers, with Asia being second but with only 20%. Remarkably, Europe with 7% matched Africa, and Australia/New Zealand at only 2.3% almost matched South America at 4.7%. Interestingly, 135 of the 215 cycles in which abnormal embryos were transferred took place in the USA, 10 in Canada and 70 in the remaining geographical areas or the world.
Table 2.
Demographics and statistics of units that have transferred “abnormal embryos”
| Continent | Estimated annual IVF cycles | % of all estimated annual IVF cycles | No. IVF units | % of all no. of IVF units | Estimated of cycles transferring “Abnormal Embryos” | % of all estimated of cycles transferring “Abnormal Embryos” |
|---|---|---|---|---|---|---|
| USA and Canada | 10,700 | 45.1 | 14 | 56 | 145 | 67.4 |
| South America | 800 | 3.4 | 2 | 8 | 10 | 4.7 |
| Australia and New Zealand | 3,000 | 12.7 | 1 | 4 | 5 | 2.3 |
| Asia | 5,700 | 24.1 | 5 | 20 | 25 | 11.6 |
| Europe | 3,100 | 13.1 | 2 | 8 | 15 | 7 |
| Africa | 400 | 1.7 | 1 | 4 | 15 | 7 |
| 23,700 | 100 | 25 | 100 | 215 | 100 |
Remarkably, 106 (49.3%) of these cycles resulted either in ongoing pregnancies (n = 50) or live births n = 56). In contrast, the miscarriage rate (n = 20; 9.3%) was unexpectedly low, with 19 (95.0%) occurring in the first and 1 in the second trimester. Of ongoing pregnancies, 10 were already in the third trimester at time of reporting, 14 in the second and 26 in the first trimester. Among live births, 45 (80.4%) were at term, 9 (16.0%) were pre-term and 2 were very premature (< 28 weeks; 3.6%).
Almost three-quarter of pregnancies (72%) underwent early genetic screening, while in 28% no chromosome testing was performed. Among tested pregnancies, 93% had chromosomally normal fetuses/newborns. The remaining 7% with abnormal karyotypes, were all first trimester miscarriages. There were no chromosomally abnormal births or induced abortions reported.
Discussion
So far published data suggest close to 200 live births worldwide through transfers of embryos reported as “abnormal-aneuploid” or “mosaic,” by PGS/PGT-A [3, 4, 6, 9–13]. Here, presented survey data suggest that, especially in the USA, the number of live births after transfers of “abnormal “embryos may be substantially higher and that worldwide they are likely approaching at least 400.
Among reporting IVF centers to this survey, the largest number was from the USA (n = 40), followed by Europe (n = 32) and Asia (n = 28). In the same order, the USA also led world regions, with 32.0% of IVF units performing PGS/PGT-A, followed by Europe with 25.6% and Asia with 22.4%. Current utilization of PGS/PGT-A as of this point, therefore, appears most popular in the USA, with approximately a third of IVF units offering the test. With only 1.6%, the lowest utilization occurred in Africa.
In accordance with current PGDIS recommendations [2], 90% of units utilized NGS platforms for PGS/PGT-A. The leading reported indications for testing of embryos (multiple indications were allowed) were advanced maternal age (77%), repeat implantation failure (RIF, 70%), a history of repeat unexplained miscarriages (65%) and sex selection of embryos (25%). This ranking is of great interest because advanced female age is by many considered a contraindication to utilization of PGS/PGT-A since older women in most cases produce only few embryos. They, therefore, can least afford spurious losses of healthy embryos as a consequence of high false-positive rate of PGS/PGT-A [8]. RIF also represents a problematic indication for PGT-A since, to this day, there is no uniformly accepted definition for this condition [15] nor is there any evidence in the literature to suggest that RIF is in any way beneficially affected by PGS/PGT-A.
And, while some proponents of the procedure are still claiming that PGS/PGT-A reduces miscarriages [16], there has not been such a claim made for women with repeated miscarriages. A single study investigating PGS/PGT-A in such a population, indeed, did not reveal outcome advantages for PGS/PGT-A [17]. Further discussed below, the low miscarriage rate observed in this survey in women who underwent transfers with “abnormal” embryos also argue against beneficial effects of PGS/PGT-A on miscarriages. Remarkably, this leaves only sex selection of embryos, listed in 25% of cases, as a clinically relevant indication for PGS-PGT-A, Yet, 14% of IVF responding IVF centers indicated that they performed PGS/PGT-A on all of their IVF cycles.
Overall, only 20% of the IVF centers that utilize PGS/PGT-A reported transfers of chromosomally “abnormal” embryos, whether mosaic or aneuploid. However, this number is somewhat misleading since the USA alone represented 56.0% of the units that reported such transfers. Utilization of “abnormal” embryos in US IVF centers was, therefore, more than double that of the second-best region, Asia, where 20.0% of IVF centers transferred “abnormal” embryos. Considering the high utilization of PGS/PGT-A in Europe, the continent’s low (7.0%) transfer rate of “abnormal” embryos is noteworthy and was equal to Africa’s rate. Combined, the USA and Canada transferred 67.4% of all PGS/PGT-A cycles that utilized “abnormal” embryos reported to the survey, with Asia following in the second place with 11.6% of transfers.
The most interesting findings of this survey is, however, the remarkably high ongoing pregnancy and live birth rates (combined 49.3%) after transfers of allegedly chromosomally “abnormal” embryos and the equally impressive low miscarriage rate of only 9.3%. To a degree, both of these outcomes were unexpected since patients who undergo transfers with “abnormal” embryos usually do so because PGS/PGT-A left them without transferrable euploid embryos. They frequently also are women who produce few embryos and, therefore, relative poor-prognosis patients. Poor prognosis patients also have the highest miscarriage rates among IVF patients. A recent study, for example, reported a 19.2% miscarriage rate in young women with low ovarian reserve and of 33.9% in older women [18]. Both numbers very well reflect how remarkably low the here presented miscarriage rate really is.
These outcomes, however, matched well earlier reports, since healthy normal births after transfers of “abnormal” embryos were first almost simultaneously reported in 2015 by Gleicher et al. [3] from the USA and Greco et al. [4] from Italy. Both groups reported live birth rates in the mid-40s and mid-30s, respectively. Munné et al, who, except for this survey, so far published the largest patient cohort receiving mosaic embryos, reported 50% ongoing pregnancy rates with transfers of single monosomies and single trisomies. In that study, even embryos with complex multiple chromosomal abnormalities still produced 10% ongoing pregnancy rates [6]. Neither of these three studies reported miscarriages. The first to report a miscarriage rate were Morales et al. from Spain, and their miscarriages were also remarkably low [10].
The uniformity of outcomes between our survey results and the previously reported cohort studies is, therefore, noteworthy. It supports the argument that these results are reflective of unpublished clinical PGS/PGT-A experiences around the world and suggest many more additional chromosomally normal pregnancies delivered after the transfer of “abnormal” embryos. These survey results also confirm that pregnancy outcomes following transfer of embryos by PGS/PGT-A diagnosed as mosaic, indeed, are unexpectedly high and miscarriage rates unexpectedly low due to the false positive diagnoses. These data, therefore, cast further doubts on the original hypothesis of PGS/PGT-A that IVF outcomes would be improved by selecting only euploid embryos for transfer and that miscarriage risks would be reduced.
Hundreds of chromosomally normal offspring following transfers of embryos initially by PGS/PGT-A described as “abnormal,” now, however, must raise further concerns that the procedure may indeed cause potential harm to at least some patient populations with small embryo numbers (i.e., older women and/or younger women with low ovarian reserve) by reducing their pregnancy and live birth chances thus confirming what was recently published in a modeled study [8].
Yet, as this study demonstrated, advanced female age is the most common cited indication for utilization of PGS/PGT-A, raising serious questions about patient selection biases in studies that have claimed to demonstrate that women above the age of 38 years may be the biggest beneficiaries from PGT-A [19]. Our study also demonstrates that ca. 14% of IVF centers perform the test routinely on all of their IVF patients. This is, in itself, a remarkable observation, raising questions about rational and motivations for such a practice.
In this paper, we cannot address differences in laboratory practices between centers, nor the type of alleged chromosomal errors diagnosed by PGS/PGT-A, beyond the question which diagnostic platform was employed for PGT-A diagnoses. As one would expect since other platforms do not have the capability of diagnosing more than one cell line in a biopsy specimen, a large majority of respondent centers reported using NGS (90%). Here presented study, therefore, reflects similar patient cohorts as previous publications, where laboratory practices were discussed in more detail [1]. By conducting this survey, we wanted to inform ourselves about current worldwide practice patterns in association with the utilization of PGS/PGT-A and, especially, regarding transfers of presumed “aneuploid” embryos. By harnessing the knowledge and experience of the physician community linked to IVF-worldwide.com, we, furthermore, attempted to harness “the wisdom of the crowd.”
Survey studies are, by definition, limited in scope and do not offer the same level of evidence experimental clinical studies provide. They also are characterized by recall biases and are exposed to reporting biases. They, however, are well suited to assess practice patterns, especially if, as in this case, they offer relatively large numbers of study participants representative of different regions of the world.
This study added another 105 live births to already published cohorts of pregnancies and deliveries following transfers of chromosomal “abnormal” embryos, raising the number of delivered healthy euploid newborns to over 300 and, likely, approaching 400. These deliveries, ultimately, represent the strongest evidence for the inability of PGS/PGTA—to reliably determine which embryo should or should not be transferred. Maybe, cell-free DNA analysis of culture media, currently proposed as a potential fourth generation of PGS/PGT-A [20] will do better.
Considering that until July 2016, when the most recent PGDIS guidelines were published [2], all “abnormal” embryos were consistently disposed of the introduction of mosaicism into the diagnostic armamentarium of PGS/PGT-A at least established an opportunity for transfer of mosaic embryos. As this study demonstrates, this opportunity is, however, only chosen by relatively few IVF centers worldwide. Large numbers of embryos with excellent potential for pregnancy and live births are, therefore, still routinely discarded or are withheld from transfers.
The latter point is especially difficult to understand, as in mosaic range between 21 and 79% aneuploid DNA load percentages do not seem to affect pregnancy outcomes [7]. In addition, as recently described, the 80% cut off under PGDIS guidelines, differentiating between mosaic and aneuploid abnormal designations for embryos, has no basis in evidence and has never been clinically validated [21].
As large as this survey was in numbers of respondents, it was still too limited to allow for the extraction of data points, like degree of mosaicism in each embryo and specific chromosomal abnormalities. As transfers of “abnormal” embryos become more common, surveys will have even larger cohorts, enabling more specific predictions of outcomes, based on chromosomal abnormalities detected in each embryo. One recent study suggested that embryos with single monosomies or trisomies have a ca. 50% ongoing clinical pregnancy and, therefore, likely live birth rate. Even complex chromosomal abnormalities in embryos in that study still resulted in 10% ongoing clinical pregnancies [6]. Here presented data are fully compatible with those numbers.
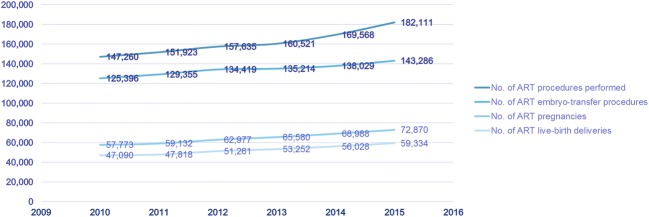
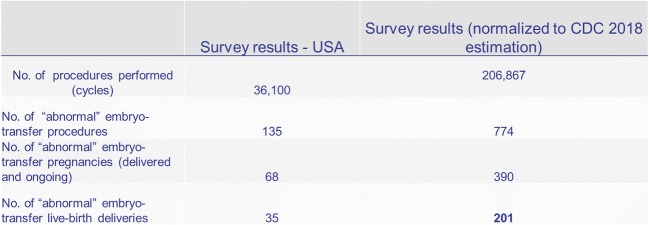
As a final consideration, using annual total IVF cycles reported to the Center for Disease Control and Prevention (CDC) (Fig. 1) and estimating a compounded annual growth rate (CAGR) of 4.3%, ca. 206,867 IVF cycles should have been completed in the USA in the year 2018. By normalizing this estimate with the IVF-worldwide web-based survey using a linear ratio specifically focusing in the USA and using the same number of IVF centers that answered the current survey, we calculated an additional 201 live births annually if only these few US IVF units continued to transfer abnormal embryos (Fig.2). The total number of births following transfer of “abnormal” embryos may, however, be even much larger, as our survey, likely, under- rather over-reports such transfers. If more units were to utilize PGS/PGTA-derived “abnormal” embryos, numbers would, of course, further increase.
Fig. 1.
Total IVF cycles as per CDC database
Fig. 2.
Estimates of additional live births in the USA from the transfer of abnormal embryos using a linear ratio
Appendix (Questionnaire)
Unit name, Country, Email
- Estimated the number of total IVF cycles performed by the unit annually
- Less than 100
- 100-200
- 201-300
- 301-400
- 401-500
- 501-600
- 601-700
- 701-800
- 801-900
- 901-1000
- 1001-1200
- 1201-1400
- 1401-1600
- 1601-1800
- 1801-2000
- 2001-2500
- 2501-3000
- 3001-3500
- 3501-4000
- More than 4000
-
My center utilizes PGS/PGT-A
- Yes
- No*
* If the answer is No, this is the end of your survey
- What is the percent of IVF cycles in which you utilize PGS/PGT-A?
- < 10%
- 11-20 %
- 11-20 %
- 31-40%
- 41-50%
- >50%
- What is the main reason for offering PGS/PGT-A? (multiple answers allowed)
- Advanced maternal Age
- Recurrent implantation failure
- Unexplained Recurrent pregnancy loss
- Sex determination
- Offered as a routine
-
My center has transferred embryos, by PGS/PGT-A found to be “abnormal”
- Yes
- No*
*If the answer is No, this is the end of your survey
- Do you know the testing platform used for your embryos? (multiple answers allowed)
- NGS
- aCGH
- qPCR
- No, I do not know the testing platform
- In how many cycles have you transferred “abnormal” embryos?
- 1-5 cycles
- 6-10 cycles
- 11-15 cycles
- 16-25 cycles
- >26 cycles
- Among those transfer cycles, how many have delivered at term (38 weeks and above)?
- 0 cycles
- 1-2 cycles
- 3-4 cycles
- 5-6 cycles
- 7-10 cycles
- 11-20 cycles
- >21 cycles
- Among those transfer cycles, how many have delivered premature (28-37 weeks)?
- 0 cycles
- 1-2 cycles
- 3-4 cycles
- 5-6 cycles
- 7-10 cycles
- 11-20 cycles
- >21 cycles
- Among those transfer cycles, how many have delivered very premature (< 28 weeks)?
- 0 cycles
- 1-2 cycles
- 3-4 cycles
- 5-6 cycles
- 7-10 cycles
- 11-20 cycles
- >21 cycles
- Among those transfer cycles, how many are ongoing in 1st trimester?
- 0 cycles
- 1-2 cycles
- 3-4 cycles
- 5-6 cycles
- 7-10 cycles
- 11-20 cycles
- >21 cycles
- Among those transfer cycles, how many are ongoing in 2nd trimester?
- 0 cycles
- 1-2 cycles
- 3-4 cycles
- 5-6 cycles
- 7-10 cycles
- 11-20 cycles
- >21 cycles
- Among those transfer cycles, how many are ongoing In 3rd trimester?
- 0 cycles
- 1-2 cycles
- 3-4 cycles
- 5-6 cycles
- 7-10 cycles
- 11-20 cycles
- >21 cycles
- Among those transfer cycles, how many have miscarried in 1st trimester?
- 0 cycles
- 1-2 cycles
- 3-4 cycles
- 5-6 cycles
- 7-10 cycles
- 11-20 cycles
- >21 cycles
- Among those transfer cycles, how many have miscarried in 2nd trimester?
- 0 cycles
- 1-2 cycles
- 3-4 cycles
- 5-6 cycles
- 7-10 cycles
- 11-20 cycles
- >21 cycles
- Have any of these transfer cycles resulted in a chromosomally abnormal pregnancy.
- Yes
- No
- Have all other pregnancies been confirmed as euploid?
- Yes
- No
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weissman A, Shoham G, Shoham Z, Fishel S, Leong M, Yaron Y. Chromosomal mosaicism detected during preimplantation genetic screening: results of a worldwide Web-based survey. Fertil Steril. 2017;107:1092–1097. doi: 10.1016/j.fertnstert.2017.02.119. [DOI] [PubMed] [Google Scholar]
- 2.Position statement on chromosome mosaicism and preimplantation aneuploidy testing at the blastocyst stage [Internet]. 2016 [cited 2019 Apr 26]. Available from: http://www.pgdis.org/docs/newsletter_071816.html. Accessed 3 Jan 2019
- 3.Gleicher N, Vidali A, Braverman J, Kushnir VA, Albertini DF, Barad DH. Further evidence against use of PGS in poor prognosis patients: report of normal births after transfer of embryos reported as aneuploid. Fertil Steril. 2015;104:e59. doi: 10.1016/j.fertnstert.2015.07.180. [DOI] [Google Scholar]
- 4.Greco E, Minasi MG, Fiorentino F. Healthy babies after intrauterine transfer of mosaic aneuploid blastocysts. N Engl J Med. 2015;373:2089–2090. doi: 10.1056/NEJMc1500421. [DOI] [PubMed] [Google Scholar]
- 5.Gleicher N, Orvieto R. Is the hypothesis of preimplantation genetic screening (PGS) still supportable? J Ovarian Res. 2017;10(1):21–8. Available from: https://ovarianresearch.biomedcentral.com/articles/10.1186/s13048-017-0318-3. Accessed 26 Apr 2019 [DOI] [PMC free article] [PubMed]
- 6.Munné S, Blazek J, Large M, Martinez-Ortiz PA, Nisson H, Liu E, Tarozzi N, Borini A, Becker A, Zhang J, Maxwell S, Grifo J, Babariya D, Wells D, Fragouli E. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;108:62–71.e8. doi: 10.1016/j.fertnstert.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Kushnir VA, Darmon SK, Barad DH, Gleicher N. Degree of mosaicism in trophectoderm does not predict pregnancy potential: a corrected analysis of pregnancy outcomes following transfer of mosaic embryos. Reprod Biol Endocrinol. 2018;16:6. doi: 10.1186/s12958-018-0322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulson RJ. Preimplantation genetic screening: what is the clinical efficiency? Fertil Steril. 2017;108:228–230. doi: 10.1016/j.fertnstert.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Gleicher N, Vidali A, Braverman J, Kushnir VA, Barad DH, Hudson C, et al. Accuracy of preimplantation genetic screening (PGS) is compromised by degree of mosaicism of human embryos. Reprod Biol Endocrinol. 2016;14:54. doi: 10.1186/s12958-016-0193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales R, Lledó B, Ortiz JA, Ten J, Lláce J, Bernabeu R. Embryos showing mosaicism in trophectoderm cells can achieve good pregnancy rates. Hum Reprod. 2016;1:0–030. [Google Scholar]
- 11.Lledo' B, Morales R, Ortiz JA, Blanca H, Ten J, Llacer J, Bernabeu R. Implantation potential of mosaic embryos. Syst Biol Reprod Med 2017;63(3):206–8. [DOI] [PubMed]
- 12.Spinella F, Fiorentino F, Biricik A, Bono S, Ruberti A, Cotroneo E, Baldi M, Cursio E, Minasi MG, Greco E. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil Steril. 2018;109:77–83. doi: 10.1016/j.fertnstert.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Zore T, Kroener LL, Wang C, Liu L, Buyalos R, Hubert G, Shamonki M. Fertil Steril. 2019;111(1):69–76. [DOI] [PubMed]
- 14.Vaisbuch E, Leong M, Shoham Z. RBMO. 2012;25(2):139–45. [DOI] [PubMed]
- 15.Bashiri A, Halper KI, Orvieto R. Recurrent Implantation Failure-update overview on etiology, diagnosis, treatment and future directions. Reprod Biol Endocrinol. 2018;16:121. doi: 10.1186/s12958-018-0414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neal SA, Morin SJ, Franasiak JM, Goodman LR, Juneau CR, Forman EJ, Werner MD, Scott RT., Jr Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertil Steril. 2018;110:896–904. doi: 10.1016/j.fertnstert.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Murugappan G, Shahine LK, Perfetto CO, Hickok LR, Lathi RB. Hum Reprod. 2016;31(8):1668–74. [DOI] [PubMed]
- 18.Chang Y, Li J, Li X, Liu H, Liang X. Egg quality and pregnancy outcome in young infertile women with diminished ovarian reserve. Med Sci Monit. 2018;24:7279–7284. doi: 10.12659/MSM.910410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy LA, Seidler EA, Vaughan DA, Resetkova N, Penzias AS, Toth TL, Thornton KL, Sakkas D. To test or not to test? A framework for counselling patients on preimplantation genetic testing for aneuploidy (PGT-A) Hum Reprod. 2019;34:268–275. doi: 10.1093/humrep/dey346. [DOI] [PubMed] [Google Scholar]
- 20.Santiago M. Forty years of IVF - evolution of preimplantation genetic screening. Fertil Steril. 2018;110:185–324. doi: 10.1016/j.fertnstert.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Gleicher N, Kushnir VA, Barad DH. How PGS/PGT-A laboratories succeeded in losing all credibility. Reprod BioMed Online. 2018;37:242–245. doi: 10.1016/j.rbmo.2018.06.019. [DOI] [PubMed] [Google Scholar]