Abstract
Hepcidin, a cationic cysteine-rich antimicrobial peptide (AMP) acts in hormone regulation and iron homeostasis in the host body. However, the biological property of hepcidin in immune reaction remains unexplored. In aquatic milieu, environmental and pathogenic stressors cause detrimental infections, which are defended by various immunological cells and antimicrobial peptides. In this study, hepcidin gene has been cloned from freshwater carp, Catla catla. The partially cloned hepcidin consists of 200 bp nucleotide sequence encoding 66 amino acids. Nucleotide sequence showed 97% and 91% similarity with Labeo rohita and Cyprinus carpio, respectively. Expression profile revealed significant up-regulation (P ≤ 0.0001) in liver as compared to other tissues in different conditions. In Aeromonas hydrophila challenged C. catla, liver showed higher expression level of hepcidin at 72 h as compared to other tissues. In skin, hepcidin expression showed significant upraise during 24 h in Streptococcus uberis infection. In Argulus sp. infected fishes, up-regulation of hepcidin expression was noted in liver, intestine and skin. The inactivated viral antigen-stimulated fishes, a substantial rise in liver was observed implying hepcidin as an important molecule in combating the pathogenic infections in freshwater carp, C. catla. Fishes stimulated with pathogen-associated molecular patterns (PAMPs) triggered the increased expression of hepcidin mRNA in liver, kidney and skin. This study indicates the presence of hepcidin as antimicrobial peptide in neutralizing the pathogenic infection in fishes.
Keywords: Antimicrobial peptide, Hepcidin, Catla catla, Pathogen challenge, PAMPs, Transcriptomic expression
Introduction
Antimicrobial peptides (AMPs) are gene encoded important component of the host immune system against various pathogens (Schmitt et al. 2012). These AMPs have broad-spectrum antimicrobial activity against bacteria, virus and fungi to protect the host from dreadful diseases (Diamond et al. 2009). In last 20 years, misgurnin, parasin, pardaxin, chrysophin, oncorhyncin, oncorhyncin III, moronecidin, defensin, piscidin, NK-lysin, BPI, cathelicidin, hipposin, pleurocidin, and hepcidin were discovered as AMPs in distinct vertebrates in the whole evolution (Pridgeon et al. 2012). Among the above-mentioned AMPs, hepcidin (HAMP or LEAP-1) was first isolated from human liver (Krause et al. 2000). Later, HAMP was also identified in other vertebrates including amphibians indicating its role as endogenous antimicrobial agents in various piscine families (Shi and Camus 2006). These studies thrived to a high limit, considering hepcidin as the multifunctional peptides consisting of eight cysteine rich residues and four intramolecular disulfide bonds for regulation of iron metabolism and inflammatory responses. The conserved amino acid sequences of hepcidin across multiple species at specific positions signify an importance of disulfide bond in its antibacterial activity (Hunter et al. 2002; Melino et al. 2005). Three isoforms of hepcidin comprising 20, 22 and 25 amino acids, respectively, are known to exhibit antibacterial activities as reported in humans (Chi et al. 2015). This socialized hepcidin in host body distresses the absorption of iron from intestine through interaction with ferroportin, an iron exporter mediating the iron retention in macrophages (Collins et al. 2008).
In teleosts, hepcidin recognizes the surface membrane of both Gram-positive and Gram-negative pathogenic bacteria (Hu et al. 2007; Chi et al. 2015; Das et al. 2015). In addition, AMPs have been reported to disrupt membrane of the pathogens by altering the morphology of fungi, virus, cancerous cells and mycobacterium (Semreen et al. 2018). It is noteworthy that transcriptomic expression of hepcidin increases dramatically in the infected teleost to create an iron restriction milieu for disruption of bacteria (Michels et al. 2015). In poikilothermic species, hepcidin was first identified in the hybrid striped bass (Morone chrysops × Micrococcus saxatilis) followed by Danio rerio, Salmo salar, Ctenopharyngodon idella, Chrysophrys major, Paralichthys olivaceus, Puntius sarana and Japanese flounder (Hirono et al. 2005; Kim et al. 2005). It was reported that fish possess seven copies of HAMP gene, whereas higher vertebrates including humans and mice have similar AMPs with one or two copies, playing a crucial role in iron regulation and defense mechanism (Shike et al. 2002; Zhang et al. 2004). Fishes are considered to be the major component of aquatic fauna, which continuously secrete different AMPs to trigger the activation of defense system against detrimental pathogens. Jiang et al. (2017) showed an increase in hepcidin by CpG motifs of bacterial stimulation along with CpG-deoxynulclotides (CpG-ODN) which induces toll-like receptor-9 (TLR9) signaling pathways. This mechanism had been widely used as efficient adjuvants in vaccination (Bode et al. 2011). Sustained infection might lead to overproduction of hepcidin which can cause anemia of inflammation and hypoferremia. Hence, from the host defense perspective, this mechanism can be considered as an adaptive defense system against pathogenic threats by favouring innate defense mechanism of host against bacterial invasion. Based on this concept, infectious stimulants/inflammatory signals (CpG-ODN) have been considered as strong boosters for iron-mediated antimicrobial defense network and TLR9 signaling blockade leading to the clearance of inflammatory signals. These signals may have a great clinical impact for the prevention of anemia of inflammation and hypoferremia (Roy and Andrews 2005). Therefore, AMPs in pathogen-stimulated host body had been found as the alternative to therapeutic agents to augment the aquaculture production.
Aquaculture industry is a flourishing worldwide industry which continuously gets affected with the myriad of harmful pathogens. Various cultured freshwater carps along with other piscine species are continuously exposed to different pathogens leading to infections. These outrageous infections affect the reduction in carp survivability and production. Till date, no such report is available regarding the identification and the presence of hepcidin in Indian major carps (IMCs) C. catla. Therefore, the presence of hepcidin in C. catla and its gene expression in basal level and infected group of fishes have been studied to understand the production of alternative immunotherapeutic agents to diminish the mortality rate in poikilothermic species.
Materials and methods
Experimental fish
Healthy C. catla (50 g) fingerlings were obtained from ICAR-Central Institute of Freshwater Aquaculture (CIFA), Bhubaneswar, India and reared in 500 L fiber tank (FBL) with continuous aeration to maintain the level of oxygen. Catla fishes were acclimatized for 4 weeks by daily exchange of three-fourth water from the tank to remove the waste products excreted from the reared fish. The fishes were fed with the commercial carp diet (ad libitum) once in a day depending on their body weight. The ambient temperature and pH of the water in tank were maintained at 28 ± 2 °C and 7.0−7.5, respectively, throughout the wet laboratory experiment following Banerjee et al. (2017).
Isolation of RNA and cDNA synthesis
Healthy catla fishes were grouped into control, Gram-positive bacterium (Streptococcus uberis)-infected fish and Gram-negative bacterium (Aeromonas hydrophila)-infected fish. The total RNA of immunologically relevant tissues (liver, spleen, kidney, intestine, gill, skin and blood) from the anaesthetized fishes (control and infected groups) was extracted with TRIzol reagent (Invitrogen, USA) following the manufacturer’s protocol. The isolated RNA was quantified in Nano-drop (Eppendorf, Germany) and 2 µg RNA from each sample was used for cDNA synthesis. To minimize the genomic DNA contamination, DNase treatment was performed by providing 1 µl of DNaseI (Sigma-Aldrich, USA). DNase activity was deactivated subsequently by adding stop solution and incubating the solution at 70 °C for 10 min. After the DNase treatment, RNA was then transcribed for the cDNA with the RevertAid cDNA synthesis kit (Thermo Scientific, USA). Finally, β-actin gene (housekeeping gene) was used for PCR amplification for the confirmation of cDNA synthesis. The PCR product was checked and confirmed by 1.5% agarose gel electrophoresis.
Cloning and partial sequencing of hepcidin from Catla catla
Different sets of primers were designed from the submitted sequence of Labeo rohita (GenBank: KC934745.1), Ctenopharyngodon idella (GenBank: JQ246442.1) and Danio rerio (GenBank: AY130989.1). 25 µL of total PCR was set for standardisation of the designed primer using 1 µL of cDNA as a template. The PCR conditions were carried out by maintaining the following parameters: initial denaturation at 94 °C for 5 min followed by 45 cycles at 94 °C for 2 min, 58 °C for 30 s, 72 °C for 1 min and final extension at 72 °C for 10 min. The amplified PCR product was checked in 1.5% agarose gel electrophoresis using GelDoc (Bio-Rad, USA), and the desired band was excised from the gel. The DNA was purified from the agarose gel with the gel extraction kit (Roche, Germany). The purified DNA was used for blue−white screening using pGEM-T Easy Vector (Promega, Madison, USA), and plasmid DNA was isolated from the random white colonies and was sequenced with ABI prism 3000 (ABI, USA) with T7 and Sp6 primers (Table 1).
Table 1.
Designed primers for cloning and expression studies
| Sl no. | Primers for cloning | Base pair | Tm (°C) | References |
|---|---|---|---|---|
| 1 |
Fw: AGATCACAGCCGTTCCCTTC Rv: CAGCAGTATCCGCAGCCTTT |
205 | 57 | Present study |
| 2 |
Fw: AACGTGTTTCTGGCTGCTGT Rv: CTGCACAGGGAAAGGTGACT |
209 | 55 | Present study |
| 3 |
Fw: CAGATCACAGCCGTTCCCTT Rv: CTGCACAGGGAAAGGTGACT |
161 | 55 | Present study |
| Sl no. | Primers for qRT-PCR | Base pair | Tm (°C) | References |
|---|---|---|---|---|
| 4 |
Fw: AACGTGTTTCTGGCTGCTGT Rv: CTGCACAGGGAAAGGTGACT |
209 | 57 | Present study |
| β-actin |
Fw: AGACCACCTTCAACTCCATCATG Rv: TCCGATCCAGACAGAGTATTTACG |
200 | 55 | Banerjee et al. (2017) |
Expression of hepcidin gene in various tissues following pathogenic assault and PAMPs stimulation
Bacterial challenge and sampling
Aeromonas hydrophila (ATCC 35654) and Streptococcus uberis (ATCC 700407) were cultured in Luria–Bertani (LB) broth (Himedia, India) with constant shaking at 37 °C for overnight. To obtain the fresh bacterial culture, the harvested culture was subjected to centrifugation at 3000 rpm for 5 min at 4 °C. Pellet was washed twice with phosphate buffer saline (PBS) (pH 7.5) and further re-suspended in PBS. For experimental study, colony-forming unit (CFU) was determined along with the 10-fold serial dilution in the nutrient agar (Himedia, India) plate. Fishes were challenged with Aeromonas hydrophila (4 × 106 CFU/50 g fish) and Streptococcus uberis (1 × 106 CFU/50 g fish) by intra-peritoneal (i.p) injection and were observed for specific time duration (24 h, 48 h and 72 h) (Banerjee et al. 2017).
Parasitic challenge
Healthy catla fishes (~ 50 g) were cultured in 500 L tank along with the population of ecto-parasite Argulus sp. The eggs were deposited along with fish in the tank for hatching at 28 °C. The fishes were fed once daily with the floating carp feed along with the ambient atmosphere (Kar et al. 2015). The development of the argulosis helped in determination of the metanauplii and it was routinely inspected. The degree of infection was analyzed on the 15th day in the infected groups and subsequently moderate and high dose was determined. The argulus-infected fishes were aseptically sacrificed using tricaine methanesulfonate (Sigma-Aldrich, USA) and dissected for isolation of seven immunological relevant tissues (liver, spleen, kidney, intestine, gill, skin and blood) and were processed for the transcriptomic study.
Virus antigenic stimulation
Catla catla fingerlings were treated with inactivated rhabdoviral antigen which was commercially acquired as Rabipur (GlaxoSmithKline, UK) following our previous studies (Banerjee et al. 2017). Viral antigen of 100 µl was intra-muscularly (i.m) injected in the fishes keeping aside the control group by injecting 100 µl PBS. The fishes were incubated for specific time intervals (24 h, 48 h, 72 h, 96 h and 120 h) in water, after which the tissues were isolated for examining and processing for transcriptomic study.
PAMPs stimulation
Healthy C. catla was stimulated with various pathogen-associated molecular patterns (PAMPs) for the transcriptomic study. The fishes were treated with polyinosinic: polycytidylic acid (poly I:C), peptidoglycan (PGN), lipopolysaccharide (LPS) and flagellin (FLA) following Patel et al. (2016). The untreated fishes were injected with 100 µl PBS (control) and the stock solutions of the ligands were prepared as reported previously (Basu et al. 2012; Swain et al. 2012; Samanta et al. 2012). Poly I:C (Sigma-Aldrich, USA) was mixed in DEPC water and 300 µg/fish was injected intravenously (IV). The other group was challenged with PGN which was purified from Staphylococcus aureus (Sigma-Aldrich, USA) and was injected in fish at 50 µg/fish intravenously. Simultaneously, FLA (Invitrogen, USA) were injected in fishes with the dosage of 1.25 µg/fish and the last group of fishes were intravenously injected with LPS which was isolated from Escherichia coli (Sigma-Aldrich, USA) at the dosage of 20 µg/fish. The untreated group of fishes and treated group (four groups of ligand stimulated) of fishes were sacrificed with the time interval of 4 h and 8 h for the gene expression analysis.
Bioinformatic analysis of hepcidin gene
The submitted nucleotide sequence of C. catla hepcidin (Cchepcidin) was analyzed by BLASTp software to check the similarity with the homologous sequences of vertebrates submitted in databases. The stretch of sequenced nucleotides of Cchepcidin was further used for deducing the encoding amino acids using BioEdit software. The encoded amino acids were aligned with the homologous sequences of different vertebrates which indicated the conserved cysteine residues (*). The predicted partial sequence of Cchepcidin was aligned for comparing the hepcidin sequences of varied species of the primordial species along with the higher vertebrates using Clustal Omega (Sievers and Higgins 2014). The tertiary structure was predicted with the bioinformatic resource viz., SMART and SWISS MODEL.
Construction of phylogenetic tree of hepcidin gene
The cloned sequence of hepcidin in C. catla was analyzed with the other reported hepcidin sequences of poikilothermic and higher vertebrates. The collection of the protein sequences was incorporated in the MEGA 6.0 software (Tamura et al. 2013). Further, the phylogenetic tree was constructed with the neighbor-joining (NJ) method using 1000 bootstrap replications in Clustal W program.
Transcriptomic analysis of hepcidin in Catla catla
Immunologically relevant tissues isolated from the control and infected groups of fishes were processed for isolation of total RNA followed by cDNA synthesis using manufacturer’s protocol. Analysis of hepcidin expression in the tissues of control and infected groups was done using quantitative real-time PCR (qRT-PCR) (CFX96, Bio-Rad, USA). The schematic program used for amplification consisted of 2.5 U of 2X SYBR (iTaq™ Universal SYBR®) Green Supermix (Bio-Rad, USA), 0.25 µM of forward and reverse primer each and 1 µL of template (cDNA). Final volume of 5 µL was made with autoclaved Milli-Q water. The cycling parameter maintained for qRT-PCR was: pre-incubation in 95 °C for 2 min, followed by 45 cycles of denaturation at 95 °C for 15 s, annealing at 57 °C for 30 s and extension at 72 °C for 45 s proceeding with melting curve analysis (Patel et al. 2016; Banerjee et al. 2017). The reaction was carried out with positive control (β-actin gene) to normalize the target gene irrespective of various conditions. The relative expression of Cchepcidin transcriptome was determined using Ct values of the samples deducted from the Ct value of housekeeping gene providing the δCt value. Further, δCt of the sample was subtracted from the δCt of the calibrator (control samples) to obtain the δδCt. Finally, fold change was acquired by calculating 2−δδCt (Pfaffl 2001).
Statistical analysis
Fold change of the respective samples from qRT-PCR experiments was analyzed statistically in GraphPad Prism software (version 5.01). One-way ANOVA was used to determine the basal expression of hepcidin in C. catla. The significant differences in the mean expression levels were determined by two-way ANOVA between the infected group at various time points and across the seven immunologically relevant tissues. P ≤ 0.05 was considered as statistically significant.
Result and discussion
cDNA identification and phylogenetic relationship of C. catla hepcidin (Cchepcidin)
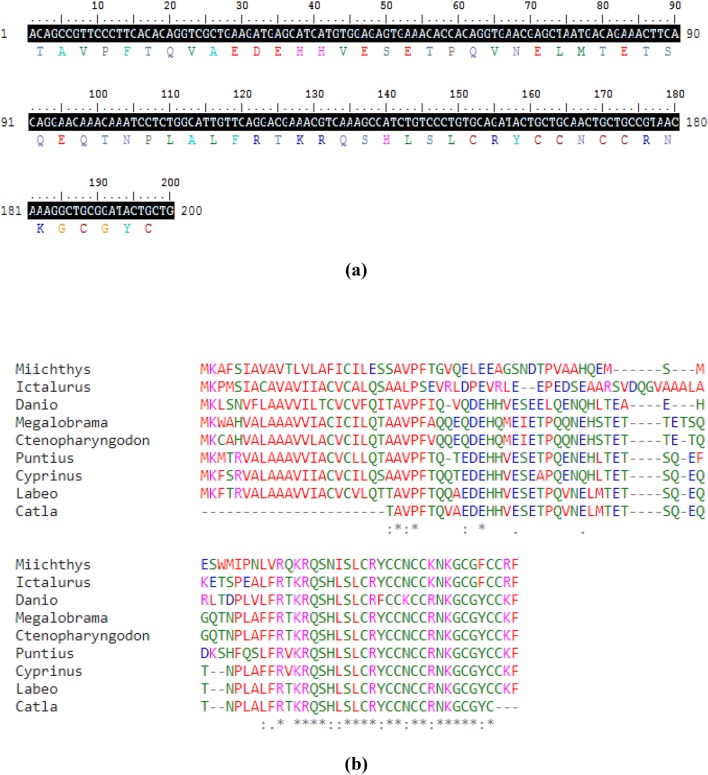
BLAST analysis confirmed the cDNA sequence of Cchepcidin containing a stretch of 200 bp nucleotide which was submitted in GenBank database (Accession Number MH025838). The nucleotide BLAST search showed 97% similarity with Labeo rohita (KC934745.1), followed by 91% and 82% resemblance with Cyprinus carpio (KC795559.1) and Ctenopharyngodon idella (JQ246442.1), respectively. The submitted nucleotide sequence was deduced as amino acid sequence using an ExPASY tool as depicted in Fig. 1a. Clustal Omega was used for analyzing the multiple sequence alignment along with other submitted hepcidin sequences from the carp species as shown in Fig. 1b.
Fig. 1.
Sequence analysis of cloned Cchepcidin. a Amino acid deduced from the partial nucleotide sequences of Catla catla mRNA transcript. The protein sequence is shown below the nucleotide sequence (shaded). b Multiple protein sequence alignment of Catla catla hepcidin along with other known submitted sequences are shown using Clustal Omega. The asterisks (*) indicate the identical residues and dots and colons indicate the similar amino acids
Bioinformatic analysis of Cchepcidin
The submitted sequence of Cchepcidin was predicted in SMART analysis software which revealed the presence of hepcidin domain and its function toward the immune system as revealed in Fig. 2.
Fig. 2.
Bioinformatic analysis: SMART analysis of the derived hepcidin protein sequence
Phylogeny study of Cchepcidin
The evolutionary relationship of Cchepcidin along with submitted hepcidin sequences of other poikilothermic species showed higher similarity with Labeo rohita (KC934745.1) followed by Megalobrama amblycephala (AFO8476.1), Ctenopharyngodon idella (AEZ5185.1), Labeo rohita (AGS17144.1), Systomus sarana (CAZ68137.1), Danio rerio (NP_001276723.1) and Ictalurus punctatus (AAX39714.1) which were placed in a single clad showing close relationship with Cyprinidae family. Miichthys miiuy (AEK98543.1), Larimichthys crocea (ABC18307.1) Dicentrarchus labrax (AAZ85124.1), Pagrus major (AAV34605.1) and Lateolabrax japonicas (ACK57557.1) had similarity in hepcidin sequences among themselves leading to form a lineage branch with regard to C. catla (Fig. 3). Similar report was found in the phylogenetic relationship of Cyprinus carpio at amino acid level which showed cluster of two different groups of vertebrates. The first group comprised fish species and the second group included other species involving avian, mammalian, amphibian and reptilia. The phylogenetic tree coincidently showed the pattern with the evolutionary sequence of species (Li et al. 2013). Thus, phylogenetic analysis comprehends the fact that evolutionary pressure along with gene duplication and modification had led to the grouping of multiple copies of HAMP gene playing its function in innate defense (Xu et al. 2012; Hilton and Lambert 2013).
Fig. 3.
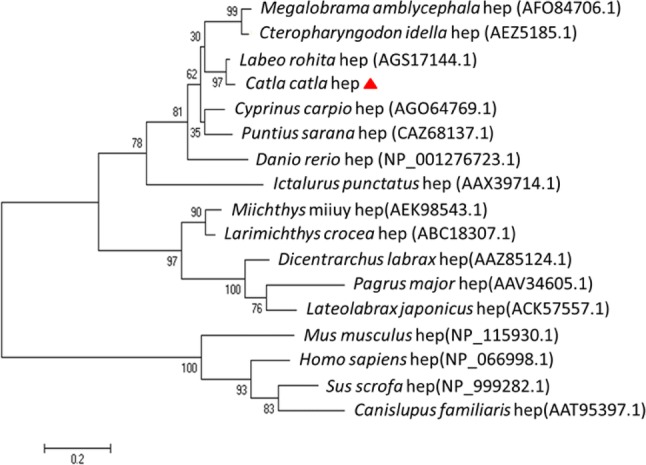
Unrooted phylogenetic tree showing the functional relationship between hepcidin constant region sequences of different fish species and the protein sequences were aligned using Clustal Omega. The tree was constructed using the neighbor-joining (NJ) method along with 1000 bootstrap replications using MEGA 6 program. The divergence time is indicated with the help of scale bar
Expression of Cchepcidin in immunologically relevant tissues
Basal level expression of hepcidin in Catla catla
mRNA from the untreated group was used for analyzing the basal level expression of Cchepcidin. Gill had been used as a calibrator for analyzing the expression of other tissues. Spleen (~ 3-fold), liver (~ 12-fold) and skin (~ 25-fold) showed a very low expression as compared to kidney, intestine and blood. Blood (~ 130-fold) had significant higher expression in comparison to intestine (~ 75-fold) and kidney (~ 93-fold) (Fig. 4). The hepcidin expression in C. catla was found to be similar to that of higher vertebrates ensuring the role of innate immunity (Robertson 2009; Hilton and Lambert 2013). Contrary to carps, hepcidin expression in humans was upregulated due to iron load and the inflammation was further decreased during hypoxia or anemia similar to fishes (D’Angelo 2013).
Fig. 4.
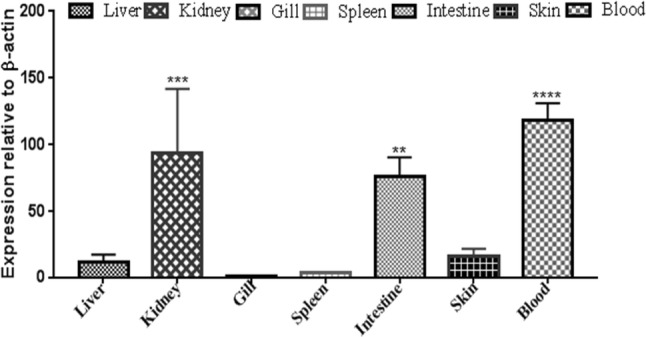
Basal expression level of Cchepcidin in varied immunologically relevant tissues. The expression level was represented as a ratio relative to β-actin (internal control) level in the sample. The fold change (2−δδCt) was calculated, where Ct (target) – Ct (internal control) = δCt and δδCt = δCt(target) – δCt(calibrator). β-actin was taken as an internal control. Gill tissue is used as a calibrator. The results were expressed as mean ± standard deviation from three samples (n = 3). Significant differences were determined with one-way ANOVA using PRISM 5 software
Expression of Cchepcidin gene in bacterial infection
To study the hepcidin gene transcript in tissues, mRNA was quantified from sacrificed C. catla. The qRT-PCR data demonstrated that Cchepcidin was expressed in different tissues ubiquitously. Aeromonas hydrophila-infected fishes showed significant higher expression of Cchepcidin in liver (~ 258-fold, 72 h) followed by mild expression in other tissues. It was observed that with gradual increase in time interval, the expression of Cchepcidin was increased in liver followed by skin, whereas there was a down-regulation in other tissues such as kidney, spleen and gill with increase in time interval (Fig. 5a). Similar result was also found in winter flounder (Pseudopleuronectes americanus) and atlantic salmon (Salmo salar) when infected with A. salmonicida (Douglas et al. 2003), due to higher iron content in liver as compared to other tissues. A. hydrophila challenged fish also showed similar result at all time points as found in blotched snakehead (Channa maculate) (Gong et al. 2014). Puntius sarana infected with A. hydrophila revealed an intensive higher expression of HAMP gene in liver when compared to skin, brain, intestine and muscle. Expression profile of HAMP gene in A. hydrophila-infected P. sarana showed an up-regulation during 1 h, 3 h, 6 h and 12 h followed by significant down-regulation during 24 h (Das et al. 2015). Channel catfish when challenged with Edwardsiella ictaluri showed high level of hepcidin expression in liver at different time intervals such as 4 h, 24 h and 48 h (Hu et al. 2007). Similarly, V. anguillarum-infected sea bass showed significantly high HAMP expression in liver and intestine, proving an immediate response of host (Neves et al. 2017).
Fig. 5.

Transcriptomic Cchepcidin expression on bacterial infection in Catla catla. a Aeromonas hydrophila, and b Streptococcus uberis. The immunologically relevant tissues were processed for qRT-PCR analysis after 24 h, 48 h and 72 h of infection. β-actin was used as an endogenous control. Normalized untreated samples were used as a calibrator to evaluate relative Cchepcidin expression in all tissues at various treatment conditions in terms of fold changes. The results were expressed as mean ± standard deviation from three samples (n = 3). Significant differences among treatment conditions were evaluated by two-way ANOVA using PRISM 5 with ***P < 0.001 and **P < 0.01 as significant levels
Apart from infecting the fingerlings with A. hydrophila, there was a group of fishes infected with Streptococcus uberis. Fishes were sacrificed at different time intervals (24 h, 48 h and 72 h) for the transcriptomic study of Cchepcidin. It was observed that with the escalation in time, there was a gradual increase of Cchepcidin level in blood followed by skin and liver. Other tissues had shown down-regulation with increase in the time interval. During 24 h post-infection, skin had shown a significant increase (~ 85-fold) in Cchepcidin expression, whereas with the increase in time the level of expression reduced to 4-fold at post-72 h (Fig. 5b). Moderate expression was seen in liver and kidney followed by intestine, but significantly higher expression (P < 0.001) was observed for other tissues such as gill and spleen. Striped bass (Morone chrysops × Morone saxatilis) fingerlings when infected with Streptococcus iniae exhibited higher expression in liver with increase in time till 48 h (Lauth et al. 2005), whereas a relatively consistent expression profile was reported in mice and humans (Nemeth et al. 2004). In the present study, S. uberis-infected C. catla had shown similar hepcidin expression when compared with S. parauberis-infected and Lactococcus garvieae-infected Dicentrarchus labrax. Both the bacterial infection showed downregulation of hepcidin in liver and intestine with increase in time interval (Neves et al. 2015, 2017).
The hepcidin expression observed in various tissues helps in prediction of iron metabolism. Iron absorption in fishes is mainly found in the mid and posterior regions of intestine, indicating the major site for iron metabolism (Neves et al. 2017). Gill is known to have small amount of iron absorption from water particularly during the early stages of development as compared to the adults (Bury and Grosell 2003a, b; Bury et al. 2003). Liver being a crucial immunological tissue acts as major organ for maintaining the iron homeostasis and acts as the sensor of changes in systemic iron requirement (Neves et al. 2015). Further exploration related to the role of hepcidin can provide better understanding of iron homeostasis and also the mechanism of hepcidin as iron-regulatory hormone. It is noteworthy that fishes upon bacterial infection initiate various signaling pathways along with complement system (classical, lectin and alternative pathway). PAMPs on the surface of bacteria induce lectin and alternative pathway during the bacterial infection, while the absorbed antibodies on the bacterial cell wall activate classical pathway (Wooster et al. 2006). This activated pathways lead to the formation of membrane attack complex which is crucial for bacterial lysis. However, bacterium escapes from this complex by expressing outer membrane protein (OmpA) on their surfaces binding to the complement molecules to inactivate the pathway (Smith et al. 2007). OmpA is strongly correlated with iron content, suggesting that iron is not only solely responsible for bacterial growth but also have regulatory role in bacterial defense activity against the host defense mechanism.
On the other hand, teleosts respond differently to various pathogens. The presence of additional outer membrane in Gram-negative bacteria protects peptidoglycan from the effect of lysozyme (Wang 2014). Hence, a remarkable difference in the expression of hepcidin in the live pathogen-infected C. catla was noticed. The physiological role of hepcidin has been widely studied in teleosts for the destruction of bacterial membranes (Katzenback 2015). Therefore, the upsurge of hepcidin expression in carps is significant with various pathogenic infections and inflammations. This creates an iron restriction milieu to the bacteria leading to an anti-infection condition. Thus, the dual function of hepcidin (bactericidal and iron regulation) may improve the antimicrobial activity by influencing the iron-regulatory pathway (Jiang et al. 2017).
Expression of Cchepcidin in parasitic infection
Parasite-infected fishes die due to organ disruption and this affects the aquaculture industry. Fishes were grouped into two, comprising moderate dosed infection and heavily dosed infection. Significantly higher expression of hepcidin was observed in liver in both the groups as compared to other immunologically relevant tissues (Fig. 6). There was a significant upsurge in skin and intestine in high dosed post-infection, whereas relatively low expression was observed in moderately infected fishes. Similar results were observed in Perca fluviatilis and Pseudosciaena crocea when infected with Acanthocephalus lucii and Cryptocaryon irritans, respectively, showing significant expression in liver and skin as compared to other tissues during the first day (Dezfuli et al. 2013; Niu et al. 2013). Thus, the present study demonstrates the AMPs of the hosts bonding with pathogens leading to the opsonisation for enhancing the activity of immune system for combating the dreadful disease.
Fig. 6.

Transcriptomic Cchepcidin expression on Argulus infection in Catla catla. The immunologically relevant tissues were processed for qRT-PCR analysis after moderate and heavy infection. β-actin was used as an endogenous control. Normalized untreated samples were used as a calibrator to evaluate relative Cchepcidin expression in all tissues at various treatment conditions in terms of fold changes. The results were expressed as mean ± standard deviation from three samples (n = 3). Significant differences among treatment conditions were evaluated by two-way ANOVA using PRISM 5 with *P < 0.05 as significant levels
Transcriptomic tissue expression of Cchepcidin in inactivated rhabdoviral treatment
Hepcidin level was studied in immunologically relevant tissues of inactivated rhabdoviral-treated fish at different time intervals, viz., 24 h, 48 h, 72 h, 96 h and 120 h to understand its role in innate defense. The hepcidin expression was found significantly higher in liver (~ 900-fold, 96 h) in all the time intervals compared to other tissues. It was observed that skin, blood and kidney had a variation of Cchepcidin expression level in all the time intervals (Fig. 7). Spleen had high expression during 120 h irrespective of mild expressions in other time intervals, whereas gill showed low expression in all the time durations indicating low level of iron content in gills. Liver showed significant higher expression in all time points in rhabdoviral-treated C. catla which corroborates with the study done in Sparus aurata after the viral hemorrhagic septicemia virus infection depicting higher expression of hepcidin in liver, head-kidney, skin and peritoneal leukocytes, confirming the role of hepcidin in immunity (Cuesta et al. 2008). In human, chronic viral infections showed down-regulation in liver, stating that inflammatory cytokines play a role in enhancing transferrin receptor 1(TfR1). TfR1, a protein encoded by TFRC gene, arbitrates the uptake of iron in hepatocytes (Kobune et al. 1994). Therefore, transcriptomic result states that viruses have the capacity to immunomodulate the body of the host for immunosuppressive environment for viral replication leading to the regulation of AMPs (Adamek et al. 2013). Thus, it can be concluded that viral pathogenic antigens had triggered a significant hepcidin expression in liver at different time intervals. AMPs present in fishes have immunomodulatory activity providing a wide range of application for well-being of fishes.
Fig. 7.

Transcriptomic Cchepcidin expression on inactivated rhabdovirus antigenic stimulation in Catla catla. The immunologically relevant tissues were processed for qRT-PCR analysis after 24 h, 48 h and 72 h of infection. β-actin was used as endogenous control. Normalized untreated samples were used as calibrator to evaluate relative Cchepcidin expression in all tissues at various treatment conditions in terms of fold changes. The results were expressed as mean ± standard deviation from three samples (n = 3). Significant differences among treatment conditions were evaluated by two-way ANOVA using PRISM 5 with ***P < 0.001 and **P < 0.01 as significant levels
Transcriptomic tissue expression of Cchepcidin in PAMPs stimulation
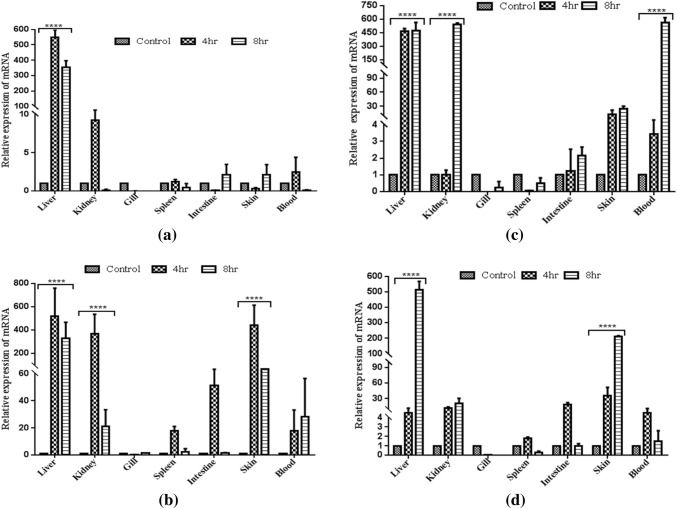
Various ligands also induce AMP expression for protecting the host. PGN-infected fishes had shown a significant up-regulation (~ 550-fold) in liver post-4 h, whereas during 8 h it was down-regulated to ~ 350-fold. Other tissues, such as kidney (~ 9.8-fold) and blood (~ threefold), had a moderate expression post-4 h but showed down-regulation with the time interval. Mild expressions were exhibited in intestine followed by skin and spleen post-8 h but gill had no such variations in its expression level with respect to the time intervals (Fig. 8a). This PGN-stimulated carp had exhibited liver to have significant expression in all time intervals as compared to other infected tissues, asserting its primary role in immune mechanism and metabolism as reported in Salmo salar (Martin et al. 2010). Liver was stated to be an important tissue playing essential roles, especially for the acute phase response protein.
Fig. 8.
qRT-PCR analysis of Cchepcidin transcript expression after PAMPs stimulation in various tissues. a Peptidoglycan, b polyinosinic: polycytidylic (poly I:C), c lipopolysaccharide, and d flagellin. RNA extraction of the treated tissues was performed after 4 h and 8 h of stimulation and processed for qRT-PCR analysis. β-actin was used as an endogenous control. Normalized untreated samples were used as a calibrator to evaluate relative Cchepcidin expression in all tissues at various treatment conditions in terms of fold changes. The results were expressed as mean ± standard deviation from three samples (n = 3). Two-way ANOVA was used for determination of the significant differences using PRISM 5 with ***P < 0.001, **P < 0.01 and *P < 0.05 as significant levels
In case of poly I:C stimulation, liver (~ 520-fold) had shown significant higher expression followed by skin (~ 420-fold) and kidney (~ 360-fold) post-4 h compared to other tissues which exhibited moderate expression. However, gill did not show expression with the increase in time (Fig. 8b). LPS-stimulated fishes were sacrificed for the similar transcriptomic study which exhibited a significant expression post-4 h in liver and skin followed by blood, intestine and kidney. However, spleen and gill showed mild hepcidin expression during 8 h as compared to 4 h (Fig. 8c). The synthetic mimic of dsRNA was studied in different fish species and found significantly higher expression in liver, kidney and spleen in post-poly I:C-infected rock bream and gilthead seabream (Cuesta et al. 2008; Cho et al. 2009). Tilapia comprising three different hepcidins, namely TH1-5, TH2-2 and TH2-3, showed no expression of TH2-2 after the treatment with poly I:C (Huang et al. 2007). However, C. catla showed expression of hepcidin in liver, skin and kidney by stimulation with poly I:C. Interestingly, Chiou et al. (2007) performed stimulation of poly I:C in rainbow trout macrophages for hepcidin expression and found the significant expression in spleen compared to liver. Yellow croaker with poly I:C treated also had an up-regulation in spleen (Zheng et al. 2006).
Catla fingerlings were challenged with LPS at different time intervals for speculating the hepcidin level. Thus, qRT-PCR analyses were performed which showed higher expression in kidney, liver and blood at all time points corroborating with olive founder, yellow croaker and tilapia (Wang et al. 2009). Huang et al. (2007) also showed the increasing peak of hepcidin expression in liver after 3 h of LPS stimulation in tilapia. Hepcidin also gets affected when fishes are exposed to various contaminants (Robertson 2009). Rapid expression of hepcidin at independent time intervals against infection in mammals defines hepcidin as the crucial mediator of inflammation (Nemeth et al. 2004; Ganz 2005).
Flagellin, a pathogen-derived TLR agonist when stimulated in healthy catla exhibited expression in liver, kidney and intestine in different time duration. However, down-regulation of Cchepcidin was observed in blood (~ 1.5-fold) and intestine (~ 1-fold) followed by spleen and gill (Fig. 8d). Similar results were observed in in vivo study of hepcidin expression of rainbow trout, indicating its role in activation of the down-stream signaling cascade for the activation of cytokines and chemokines (Tsujita et al. 2004; Raida and Buchmann 2009). Hence, it has been envisioned that liver plays a significant role in the Cchepcidin expression in comparison to other tissues in all the different time intervals in response to the PAMPs treatment.
Conclusion
The present study demonstrates the hepcidin gene expression in healthy and pathogen-infected freshwater carp, C. catla. The naïve fishes exposed to direct ligands had shown a significant increase in hepcidin expression as compared to the live pathogen load. The magnitude of infection in both the cases was ascertained by increase in the expression of hepcidin which is involved in encoding the innate immune system as defense mechanism. It is obvious that hepcidin gene is predominantly expressed in liver similar to that of mammals. This is the first report on identification of HAMP gene in freshwater carp C. catla. This study will pave the way for uncovering the role of AMP in the innate immune system to prevent fishes from the bacterial diseases caused due to hazardous pathogens in aquaculture industry.
Acknowledgements
The work was partly supported financially by the National Agricultural Science Fund (NASF), Indian Council of Agricultural Research (ICAR), Govt. of India (project code: NASF/BS-4003). The authors would like to acknowledge the Director of NIT, Rourkela and CIFA, Bhubaneswar for extending necessary facilities for conducting this work.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- Adamek M, Syakuri H, Harris S, Rakus KŁ, Brogden G, Matras M, Steinhagen D. Cyprinid herpesvirus 3 infection disrupts the skin barrier of common carp (Cyprinus carpio L.) Vet Microbiol. 2013;162(2−4):456–470. doi: 10.1016/j.vetmic.2012.10.033. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Patel B, Basu M, Lenka SS, Paicha M, Samanta M, Das S. Molecular cloning, characterization and expression of immunoglobulin D on pathogen challenge and pathogen associated molecular patterns stimulation in freshwater carp, Catla catla. Microbiol Immunol. 2017;61(10):452–458. doi: 10.1111/1348-0421.12534. [DOI] [PubMed] [Google Scholar]
- Basu M, Swain B, Maiti NK, Routray P, Samanta M. Inductive expression of toll-like receptor 5 (TLR5) and associated downstream signaling molecules following ligand exposure and bacterial infection in the Indian major carp, mrigal (Cirrhinus mrigala) Fish Shellfish Immunol. 2012;32(1):121–131. doi: 10.1016/j.fsi.2011.10.031. [DOI] [PubMed] [Google Scholar]
- Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10(4):499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury N, Grosell M. Iron acquisition by teleost fish. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135(2):97–105. doi: 10.1016/S1532-0456(03)00021-8. [DOI] [PubMed] [Google Scholar]
- Bury NR, Grosell M. Waterborne iron acquisition by a freshwater teleost fish, zebrafish Danio rerio. J Exp Biol. 2003;206(19):3529–3535. doi: 10.1242/jeb.00584. [DOI] [PubMed] [Google Scholar]
- Bury NR, Walker PA, Glover CN. Nutritive metal uptake in teleost fish. J Exp Biol. 2003;206(1):11–23. doi: 10.1242/jeb.00068. [DOI] [PubMed] [Google Scholar]
- Chi JR, Liao LS, Wang RG, Jhu CS, Wu JL, Hu SY. Molecular cloning and functional characterization of the hepcidin gene from the convict cichlid (Amatitlania nigrofasciata) and its expression pattern in response to lipopolysaccharide challenge. Fish Physiol Biochem. 2015;41(2):449–461. doi: 10.1007/s10695-014-9996-6. [DOI] [PubMed] [Google Scholar]
- Chiou PP, Lin CM, Bols NC, Chen TT. Characterization of virus/double-stranded RNA-dependent induction of antimicrobial peptide hepcidin in trout macrophages. Dev Comp Immunol. 2007;31(12):1297–1309. doi: 10.1016/j.dci.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Cho YS, Lee SY, Kim KH, Kim SK, Kim DS, Nam YK. Gene structure and differential modulation of multiple rockbream (Oplegnathus fasciatus) hepcidin isoforms resulting from different biological stimulations. Dev Comp Immunol. 2009;33(1):46–58. doi: 10.1016/j.dci.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Collins JF, Wessling-Resnick M, Knutson MD. Hepcidin regulation of iron transport. J Nutr. 2008;138(11):2284–2288. doi: 10.3945/jn.108.096347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta A, Meseguer J, Esteban MA. The antimicrobial peptide hepcidin exerts an important role in the innate immunity against bacteria in the bony fish gilthead seabream. Mol Immunol. 2008;45(8):2333–2342. doi: 10.1016/j.molimm.2007.11.007. [DOI] [PubMed] [Google Scholar]
- D’Angelo G. Role of hepcidin in the pathophysiology and diagnosis of anemia. Blood Res. 2013;48(1):10–15. doi: 10.5045/br.2013.48.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Mohapatra A, Sahoo PK. Cloning and characterization of antimicrobial peptide, hepcidin in medium carp, Puntius sarana. Int J Pept Res Ther. 2015;21(1):139–147. doi: 10.1007/s10989-014-9438-4. [DOI] [Google Scholar]
- Dezfuli BS, Lui A, Giari L, Pironi F, Manera M, Lorenzoni M, Noga EJ. Piscidins in the intestine of European perch, Perca fluviatilis, naturally infected with an enteric worm. Fish Shellfish Immunol. 2013;35(5):1539–1546. doi: 10.1016/j.fsi.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Diamond G, Beckloff N, Weinberg A, Kisich KO. The roles of antimicrobial peptides in innate host defense. Curr Pharm Des. 2009;15(21):2377–2392. doi: 10.2174/138161209788682325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SE, Gallant JW, Liebscher RS, Dacanay A, Tsoi SC. Identification and expression analysis of hepcidin-like antimicrobial peptides in bony fish. Dev Comp Immunol. 2003;27(6):589–601. doi: 10.1016/S0145-305X(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Ganz T. Hepcidin—a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18(2):171–182. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Gong LC, Wang H, Deng L. Molecular characterization, phylogeny and expression of a hepcidin gene in the blotched snakehead Channa maculate. Dev Comp Immunol. 2014;44(1):1–11. doi: 10.1016/j.dci.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Hilton KB, Lambert LA. Molecular evolution and characterization of hepcidin gene products in vertebrates. Gene. 2013;415(1):40–48. doi: 10.1016/j.gene.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Hirono I, Hwang JY, Ono Y, Kurobe T, Ohira T, Nozaki R, Aoki T. Two different types of hepcidins from the Japanese flounder Paralichthys olivaceus. FEBS J. 2005;272(20):5257–5264. doi: 10.1111/j.1742-4658.2005.04922.x. [DOI] [PubMed] [Google Scholar]
- Hu X, Camus AC, Aono S, Morrison EE, Dennis J, Nusbaum KE, Shi J. Channel catfish hepcidin expression in infection and anemia. Comp Immunol Microbiol Infect Dis. 2007;30(1):55–69. doi: 10.1016/j.cimid.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Huang PH, Chen JY, Kuo CM. Three different hepcidins from tilapia, Oreochromis mossambicus: analysis of their expressions and biological functions. Mol Immunol. 2007;44(8):1922–1934. doi: 10.1016/j.molimm.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Hunter HN, Fulton DB, Ganz T, Vogel HJ. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J Biol Chem. 2002;277(40):37597–37603. doi: 10.1074/jbc.M205305200. [DOI] [PubMed] [Google Scholar]
- Jiang XF, Liu ZF, Lin AF, Xiang LX, Shao JZ. Coordination of bactericidal and iron regulatory functions of hepcidin in innate antimicrobial immunity in a zebrafish model. Sci Rep. 2017;7(1):4265. doi: 10.1038/s41598-017-04069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar B, Mohapatra A, Mohanty J, Sahoo PK. Transcriptional changes in three immunoglobulin isotypes of rohu, Labeo rohita in response to Argulus siamensis infection. Fish Shellfish Immunol. 2015;47(1):28–33. doi: 10.1016/j.fsi.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Katzenback B. Antimicrobial peptides as mediators of innate immunity in teleosts. Biology. 2015;4(4):607–639. doi: 10.3390/biology4040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YO, Hong S, Nam BH, Lee JH, Kim KK, Lee SJ. Molecular cloning and expression analysis of two hepcidin genes from olive flounder Paralichthys olivaceus. Biosci Biotechnol Biochem. 2005;69(7):1411–1414. doi: 10.1271/bbb.69.1411. [DOI] [PubMed] [Google Scholar]
- Kobune M, Kohgo Y, Kato J, Miyazaki E, Niitsu Y. Interleukin-6 enhances hepatic transferrin uptake and ferritin expression in rats. J Hepatol. 1994;19(6):1468–1475. doi: 10.1002/hep.1840190623. [DOI] [PubMed] [Google Scholar]
- Krause A, Neitz S, Mägert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480(2–3):147–150. doi: 10.1016/S0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- Lauth X, Babon JJ, Stannard JA, Singh S, Nizet V, Carlberg JM, Westerman ME. Bass hepcidin synthesis, solution structure, antimicrobial activities and synergism, and in vivo hepatic response to bacterial infections. J Biol Chem. 2005;280(10):9272–9282. doi: 10.1074/jbc.M411154200. [DOI] [PubMed] [Google Scholar]
- Li H, Zhang F, Guo H, Zhu Y, Yuan J, Yang G, An L. Molecular characterization of hepcidin gene in common carp (Cyprinus carpio L.) and its expression pattern responding to bacterial challenge. Fish Shellfish Immunol. 2013;35(3):1030–1038. doi: 10.1016/j.fsi.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Martin SA, Douglas A, Houlihan DF, Secombes CJ. Starvation alters the liver transcriptome of the innate immune response in Atlantic salmon (Salmo salar) BMC Genomics. 2010;11(1):418. doi: 10.1186/1471-2164-11-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melino S, Garlando L, Patamia M, Paci M, Petruzzelli R. A metal-binding site is present in the amino terminal region of the bioactive iron regulator hepcidin-25. J Pept Res. 2005;66(S1):65–71. doi: 10.1111/j.1747-0285.2006.00328.x. [DOI] [Google Scholar]
- Michels K, Nemeth E, Ganz T, Mehrad B. Hepcidin and host defense against infectious diseases. PLoS Pathog. 2015;11(8):e1004998. doi: 10.1371/journal.ppat.1004998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Investig. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves JV, Caldas C, Vieira I, Ramos MF, Rodrigues PN. Multiple hepcidins in a teleost fish, Dicentrarchus labrax: different hepcidins for different roles. J Immunol. 2015;195(6):2696–2709. doi: 10.4049/jimmunol.1501153. [DOI] [PubMed] [Google Scholar]
- Neves JV, Ramos MF, Moreira AC, Silva T, Gomes MS, Rodrigues PN. Hamp1 but not Hamp2 regulates ferroportin in fish with two functionally distinct hepcidin types. Sci Rep. 2017;7(1):14793. doi: 10.1038/s41598-017-14933-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu SF, Jin Y, Xu X, Qiao Y, Wu Y, Mao Y, Wang J. Characterization of a novel piscidin-like antimicrobial peptide from Pseudosciaena crocea and its immune response to Cryptocaryon irritans. Fish Shellfish Immunol. 2013;35(2):513–524. doi: 10.1016/j.fsi.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Patel B, Banerjee R, Basu M, Lenka S, Samanta M, Das S. Molecular cloning of IgZ heavy chain isotype in Catla catla and comparative expression profile of IgZ and IgM following pathogenic infection. Microbiol Immunol. 2016;60(8):561–567. doi: 10.1111/1348-0421.1239. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgeon JW, Mu X, Klesius PH. Expression profiles of seven channel catfish antimicrobial peptides in response to Edwardsiella ictaluri infection. J Fish Dis. 2012;35(3):227–237. doi: 10.1111/j.1365-2761.2011.01343.x. [DOI] [PubMed] [Google Scholar]
- Raida MK, Buchmann K. Innate immune response in rainbow trout (Oncorhynchus mykiss) against primary and secondary infections with Yersinia ruckeri O1. Dev Comp Immunol. 2009;33(1):35–45. doi: 10.1016/j.dci.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Robertson LS (2009) Expression in fish of hepcidin, a putative antimicrobial peptide and iron regulatory hormone. In: Proceedings of The Third Bilateral Conference Between the United States and Russia: Aquatic Animal Health: Sheperdstown, WV pp 284–292
- Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005;12(2):107–111. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- Samanta M, Swain B, Basu M, Panda P, Mohapatra GB, Sahoo BR, Maiti NK. Molecular characterization of toll-like receptor 2 (TLR2), analysis of its inductive expression and associated down-stream signaling molecules following ligands exposure and bacterial infection in the Indian major carp, rohu (Labeo rohita) Fish Shellfish Immunol. 2012;32(3):411–425. doi: 10.1016/j.fsi.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Schmitt P, Rosa RD, Duperthuy M, Jde Lorgeril, Bachère E, Destoumieux-Garzón D. The antimicrobial defense of the Pacific oyster, Crassostrea gigas. How diversity may compensate for scarcity in the regulation of resident/pathogenic microflora. Front Microbiol. 2012;3:160. doi: 10.3389/fmicb.2012.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semreen MH, El-Gamal MI, Abdin S, Alkhazraji H, Kamal L, Hammad S, Kourbaj L. Recent updates of marine antimicrobial peptides. Saudi Pharm J. 2018;26(3):396–409. doi: 10.1016/j.jsps.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Camus AC. Hepcidins in amphibians and fishes: antimicrobial peptides or iron-regulatory hormones? Dev Comp Immunol. 2006;30(9):746–755. doi: 10.1016/j.dci.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Shike H, Lauth X, Westerman ME, Ostland VE, Carlberg JM, Van Olst JC, Burns JC. Bass hepcidin is a novel antimicrobial peptide induced by bacterial challenge. FEBS J. 2002;269(8):2232–2237. doi: 10.1046/j.1432-1033.2002.02881.x. [DOI] [PubMed] [Google Scholar]
- Sievers F, Higgins DG. Clustal omega. Curr Protoc Bioinform. 2014;48(1):3–13. doi: 10.1002/0471250953.bi0313s48. [DOI] [PubMed] [Google Scholar]
- Smith SGJ, Mahon V, Lambert MA, Fagan RPA. Molecular swiss army knife: OmpA structure, function and expression. FEMS Microbiol Lett. 2007;273(1):1–11. doi: 10.1111/j.1574-6968.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- Swain B, Basu M, Samanta M. Molecular cloning and characterization of nucleotide binding and oligomerization domain-1 (NOD1) receptor in the Indian major carp, rohu (Labeo rohita), and analysis of its inductive expression and down-stream signaling molecules following ligands exposure and gram-negative bacterial infections. Fish Shellfish Immunol. 2012;32(5):899–908. doi: 10.1016/j.fsi.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujita T, Tsukada H, Nakao M, Oshiumi H, Matsumoto M, Seya T. Sensing bacterial flagellin by membrane and soluble orthologs of toll-like receptor 5 in rainbow trout (Onchorhynchus mykiss) J Biol Chem. 2004;279(47):48588–48597. doi: 10.1074/jbc.M407634200. [DOI] [PubMed] [Google Scholar]
- Wang G. Human antimicrobial peptides and proteins. Pharmaceuticals. 2014;7(5):545–594. doi: 10.3390/ph7050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KJ, Cai JJ, Cai L, Qu HD, Yang M, Zhang M. Cloning and expression of a hepcidin gene from a marine fish (Pseudosciaena crocea) and the antimicrobial activity of its synthetic peptide. Peptides. 2009;30(4):638–646. doi: 10.1016/j.peptides.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Wooster DG, Maruvada R, Blom AM, Prasadarao NV. Logarithmic phase Escherichia coli k1 efficiently avoids serum killing by promoting c4bp-mediated c3b and c4b degradation. Immunology. 2006;117:482–493. doi: 10.1111/j.1365-2567.2006.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Sun Y, Shi G, Wang R. Miiuy croaker hepcidin gene and comparative analyses reveal evidence for positive selection. PLoS One. 2012;7(4):e35449. doi: 10.1371/journal.pone.0035449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YA, Zou J, Chang CI, Secombes CJ. Discovery and characterization of two types of liver-expressed antimicrobial peptide 2 (LEAP-2) genes in rainbow trout. Vet Immunol Immunopathol. 2004;101(3–4):259–269. doi: 10.1016/j.vetimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Zheng W, Liu G, Ao J, Chen X. Expression analysis of immune-relevant genes in the spleen of large yellow croaker (Pseudosciaena crocea) stimulated with poly I:C. Fish Shellfish Immunol. 2006;21(4):414–430. doi: 10.1016/j.fsi.2006.01.006. [DOI] [PubMed] [Google Scholar]