Abstract
Purpose
To investigate the stability of osmolality in non-humidified and humidified incubators for assisted reproductive technologies (ART).
Methods
Drops of three single-step culture media (media A, B, and C) were incubated for 5 or 6 days covered with four different mineral oils (oils A, B, C, and D) in non-humidified incubator A, non-humidified incubator B, or humidified incubator C to investigate the effects of incubator environment (humidification), drop volume, culture media, and mineral oil on the stability of osmolality in microdrops.
Results
A significant and linear increase was shown in the osmolality of 50-μL and 200-μL microdrops covered with mineral oil during 5 days incubation in non-humidified benchtop incubators. The maximum increase was 20 mOsm/kg, and the extent of the increase was affected by microdrop volume and possibly by the type of mineral oil used to cover the drops. In contrast, the osmolality of 50-μL and 200-μL microdrops did not change during 5 days incubation in a humidified benchtop incubator.
Conclusions
Mineral oil alone may not adequately prevent gradual changes in the osmolality of low-volume microdrops during extended in vitro culture of human embryos in non-humidified incubators. As a result, the osmolality may increase to high enough levels to stress some human embryos and adversely affect clinical outcomes. We therefore recommend that the stability of osmolality should be given more consideration to ensure optimal culture conditions for ART.
Keywords: Osmolality, Embryo culture, Microdrop, Mineral oil, Non-humidified incubator
Introduction
A critical requirement for assisted reproductive technologies (ART) is the maintenance of stable and optimal conditions for the in vitro culture of human gametes, zygotes, and embryos. Culture media have developed from simple salt solutions designed for animal embryo culture [1, 2] to media designed for human embryo culture [3] and, in recent years, to more complex sequential and single-step culture media that better address the changing metabolic needs of the embryo and enable culture of human embryos to the blastocyst stage with minimal handling or changes of culture medium [4, 5]. The osmolality of embryo culture medium is important, and with the evolution of embryo culture media from simple salt solutions to more complex solutions containing amino acids, the sole reliance on inorganic salts for maintaining osmolality and embryo health has changed [6] and the osmolality of some culture media has decreased from 280–290 to 255–265 mOsm/kg.
A microdrop culture system [7] is frequently used to enable autocrine and paracrine factors to promote embryonic development [8]. In order to prevent evaporation, maintain optimal temperature, pH, and osmolality, and stabilize their shape, microdrops are covered by a layer of mineral oil and kept in humidified incubators. It has also been reported that microdrop preparation procedures and conditions, such as the duration from making drops to covering them with mineral oil, room temperature and humidity, and clean bench settings (airflow and heater ON/OFF), can significantly affect the osmolality of microdrops [9].
In recent years, non-humidified benchtop incubators have been used for embryo culture due to their small size, fast gas replenishment rate, and lower risk of fungal contamination. This is predicated on the basis that the layer of mineral oil will prevent evaporation and changes in osmolality, and while it has been shown that mineral oil can help maintain suitable conditions for extended microdrop culture in a humidified environment [7], the effect of a non-humidified environment on the osmolality of microdrops has not been thoroughly evaluated. Furthermore, it was recently reported that human embryos cultivated ex vivo in a dry incubator had statistically and significantly decreased implantation rates and ongoing clinical pregnancy rates [10]. This may have been caused by osmolality changes in the culture environment.
In this study, therefore, we investigated the effects of humidification, microdrop volume, culture medium, and mineral oil on the stability of osmolality in microdrops cultured for extended periods of 5 or 6 days under mineral oil in non-humidified and humidified benchtop incubators.
Materials and methods
Benchtop incubators
Two non-humidified benchtop incubators were used: incubator A (Compact Dry Incubator, Nakamedical, Japan); incubator B (G-185, K-system, Denmark). Both these incubators have circulating airflow and use N2/CO2 gas balanced with air to adjust the environment to 6% CO2 and 5% O2.
One humidified benchtop incubator was used: incubator C (K-MINC-1000, Cook, USA). This does not have circulating airflow and uses premixed high purity gas (6% CO2, 5% O2, and 89% N2).
All incubators were maintained at 37.0 °C. The consistency of temperature in each chamber in each incubator was assessed using 35-mm culture dishes containing 1 mL of Naka Onestep medium (OS020, Nakamedical, Japan) covered by mineral oil (WO100, Nakamedical, Japan). Dishes were placed in the incubators, and the temperature of each drop was measured daily for 1 week using a thermometer (Model BAT-12, physitemp, USA). All the incubator chambers used in these experiments maintained a temperature of 37.0 ± 0.2 °C over the 1-week period.
Culture media and mineral oil
Three readily available single-step culture media were used: medium A (OS020, Naka ONESTEP Medium®, Nakamedical, Japan); medium B (LGGT-100, global® total®, LifeGlobal, USA); medium C (90165, Continuous Single Culture® Complete with HSA, Irvine Scientific, USA). The osmolality specification ± range provided by the manufacturers were as follows: medium A, 265 ± 10 mOsm/kg; medium B, 265 ± 5 mOsm/kg; medium C, 265 ± 5 mOsm/kg.
Four readily available light and heavy mineral oils were used: oil A (WO100, Washed Oil, Nakamedical, Japan); oil B (LO150, Light Oil, Nakamedical, Japan); oil C (HO150, Heavy Oil, Nakamedical, Japan); oil D (MOHV-50, Heavy Oil, Kitazato, Japan). Unless otherwise specified, mineral oil was taken directly from the bottle and used without any further preparation or pre-equilibration.
Preparation of microdrops
Microdrops were prepared in 35-mm plastic culture dishes (150255, ThermoFisher Science, Denmark), one dish at a time. The drops were completely covered with 3 mL of mineral oil immediately after each dish had been prepared. A clean bench was used with the shield down, but the airflow and heater were turned off. Dishes contained either 6 × 50-μL drops or 2 × 200-μL drops. We prepared one dish for each osmolality data point, so for a 5-day experiment with daily measurements, we prepared 5 dishes and one dish was used for replicate osmolality measurements each day. The dishes used for measurements were discarded every day.
Room temperature and humidity were maintained at 24–26 °C and 40–50%, respectively, throughout the experiments.
Experimental design
First, we investigated the effect of environment (humidification) and drop volume on the stability of osmolality. Although 10–20-μL drops are often used for human embryo culture, we used larger drops to ensure consistent osmolality sampling. Drops (50 μL or 200 μL) of medium A were prepared, covered with oil A, and then incubated for 5 days in (i) non-humidified incubator A, (ii) non-humidified incubator B, and (iii) humidified incubator C. The osmolality of the drops was measured daily.
Second, we investigated the effect of culture medium on the stability of osmolality. Three single-step culture media (A, B, and C) were used. Drops (50 μL or 200 μL) of each medium were prepared, covered with oil A, and incubated for 5 days in non-humidified incubator A. The osmolality of the drops was measured daily.
Next, we investigated the effect of mineral oil on the stability of osmolality. Four different mineral oils (A, B, C, and D) were used. This included light and heavy mineral oils, as well as washed and unwashed light oils. Drops (50 μL) of medium A were prepared, covered with mineral oil, and incubated in non-humidified incubator B for 6 days. The osmolality of the drops was measured daily.
Finally, to determine if pre-equilibration (humidification) of mineral oil could mitigate the observed osmolality increase, 10 mL of oil B was placed in a 15-mL culture tube (2001, Falcon, USA) and equilibrated overnight at 37 °C in a humidified water jacket incubator (SCI-165D, ASTEC, Japan). Drops (50 μL) of medium A were prepared, covered with equilibrated oil B or non-equilibrated oil B, and incubated in non-humidified incubator B for 5 days. The osmolality of the drops was measured daily.
Measurement of osmolality
For each test sample, one dish was taken out of the incubator each day to measure osmolality. This was done at the same time of the day throughout the experimental period. The other dishes remained in the incubator. Dishes to be measured on the same day were placed in the same chamber of the incubator, so chambers were not opened until the day of measurement.
For the 6 × 50-μL drop dishes, one aliquot (20 μL) was taken from each of 5 drops. The sixth drop was reserved as a spare and not used unless one of the other measurements failed. For the 2 × 200-μL drop dishes, an aliquot (20 μL) was taken two or three times from each drop to provide the five replicates. Aliquots were placed in osmometer sample tubes (110825, Advanced Instruments, USA) which were immediately tested one by one. The osmolality of each aliquot was measured using a microsample osmometer (Fiske® 210, Advanced Instruments, USA) according to the manufacturer’s instructions. The osmometer was calibrated before starting one series of experiments using 50 and 850 mOsm standards supplied by the manufacturer. Furthermore, 290-mOsm standard solution was measured before use to confirm the calibration according to the manufacturer’s instructions.
Measurement of mineral oil
In order to investigate some of the physical properties of mineral oil that might affect the results of these experiments, we conducted four types of measurements on the different mineral oils used in this study. Dynamic viscosity was measured once for each oil at 37 °C using a vibro viscometer (SV-10, A&D, Japan). We were unable to make replicate measurements due to limited availability of the equipment. Density was measured five times for each oil at 24 °C using a portable density/specific gravity meter (DA-130, Kyoto Electronics, Japan). Absolute water content and water activity value were measured five times for each oil using a HUMICAP® MM70 sensor (Vaisala, Finland). Water activity is a value of the water saturation level of oil (1.0 is full saturation). Replicate measurements of each type were all conducted on the same day.
Statistical analysis
We placed a 50-μL drop dish and a 200-μL drop dish in the same chamber of the same incubator to compare the change of osmolality, so only the volume of media drops was different. So, data are presented as mean ± SD and were evaluated using Student’s t test.
Results
Comparison of the osmolality of microdrops in humidified and non-humidified incubators
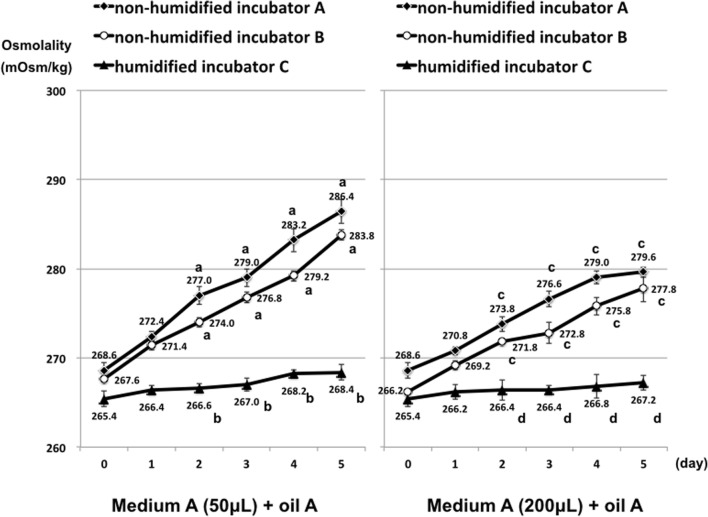
In both non-humidified incubators (A, B), the osmolality of microdrops increased during the culture period (Fig. 1). For example, in medium A covered with oil A in incubator A, the osmolality of 50-μL drops increased significantly by 17.8 mOsm/kg after 5 days. In comparison, the osmolality of microdrops in the humidified incubator (C) increased only by 4.5 mOsm/kg after 5 days, and there were no significant differences in the osmolality of 50-μL and 200-μL drops. Furthermore, the osmolality of microdrops in non-humidified incubators was significantly higher than that in the humidified incubator after only 2 days culture (P < 0.01) regardless of the microdrop volume. These results indicated that the osmolality of microdrops was less stable in non-humidified incubators compared with the humidified incubator.
Fig. 1.
Osmolality of microdrops cultured in different incubators. a vs. b P < 0.01, c vs. d P < 0.01 according to Student’s t test
Effect of culture medium on osmolality
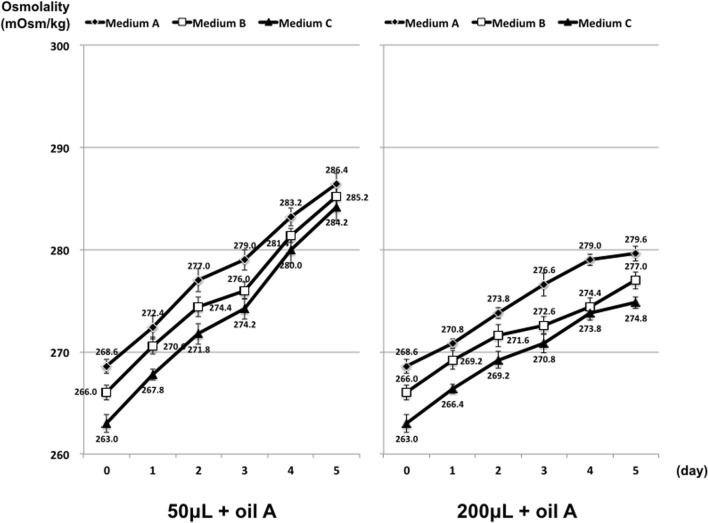
The osmolality results for the three single-step culture media are presented in Fig. 2. There were no significant differences in osmolality rise between the three different culture media. In addition, there were significant increases in osmolality over 5 days, and this was more pronounced in 50-μL drops compared with 200-μL drops (P < 0.01).
Fig. 2.
Osmolality of different culture media incubated in non-humidified incubator A. No significant difference according to Student’s t test
Effect of mineral oil on osmolality
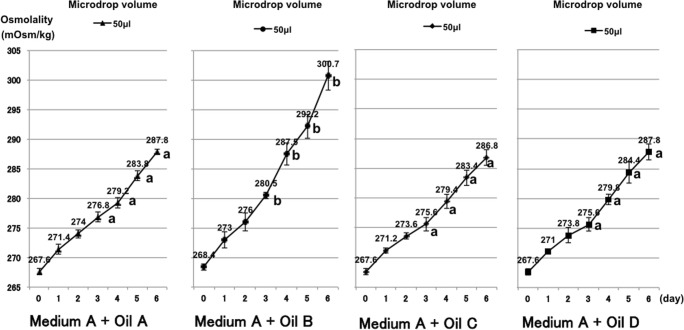
For each oil tested, the osmolality increased as described above (Fig. 3). The increases in osmolality were similar for oils A, C, and D, but the rate of increase was significantly higher for oil B (P < 0.01). The osmolality for oil B reached 292 mOsm/kg on day 5 (300 mOsm/kg on day 6) compared with ≤ 285 mOsm/kg on day 5 (≤ 288 mOsm/kg on day 6) for oils A, C, and D.
Fig. 3.
Osmolality of microdrops covered with four types of mineral oil and incubated in non-humidified incubator A. a vs. b P < 0.01 according to Student’s t test
To determine if the physical properties and water content of the mineral oils might have affected the rate of osmolality increase and caused the different result for oil B, we measured the dynamic viscosity, density, water content, and water activity of each of the four oils (Table 1). As expected, the viscosity and density of the two heavy mineral oils (C, D) were higher than the two light oils (A, B). The water content and water activity of oil B were significantly lower than those of the other oils (P < 0.01), but there were no differences between the other three oils.
Table 1.
The physical properties and water content of mineral oils
| Oil A | Oil B | Oil C | Oil D | |
|---|---|---|---|---|
| Viscosity at 37 °C (mPa·s) | 10.4 | 10.8 | 36.5 | 26.9 |
| Dynamic density at 24 °C (g/cm3) | 0.847 ± 0.0004a | 0.846 ± 0.0004a | 0.862 ± 0.0004b | 0.856 ± 0.0006c |
| Water content (ppm) | 26.2 ± 0.8d | 24.8 ± 0.5e | 26.0 ± 0.7d | 26.8 ± 1.1f |
| Water activity value | 0.41 ± 0.01g | 0.37 ± 0.01h | 0.40 ± 0.02g | 0.41 ± 0.01g |
a vs. b P < 0.01, a vs. c P < 0.01, b vs. c P < 0.01 according to Student’s t test
d vs. e P < 0.05, d vs. f P < 0.05, e vs. f P < 0.01 according to Student’s t test
g vs. h P < 0.01 according to Student’s t test
Effect of humidified mineral oil
Pre-equilibration (humidification) of mineral oil resulted in a small but significant (P < 0.05) reduction in the osmolality increase on day 5 of incubation. After 5 days, the osmolality of 50-μL drops of medium A covered with non-equilibrated mineral oil B increased from 270 to 298.0 ± 1.4 mOsm/kg, whereas drops covered with pre-equilibrated mineral oil B increased to only 292.3 ± 1.0 mOsm/kg (Fig. 4). The absolute water content of oil B increased significantly after overnight pre-equilibration from 24.8 ± 0.5 to 60.6 ± 0.5 ppm (P < 0.01), and the water activity value also increased significantly from 0.37 ± 0.01 to 0.80 ± 0.01 (P < 0.01).
Fig. 4.
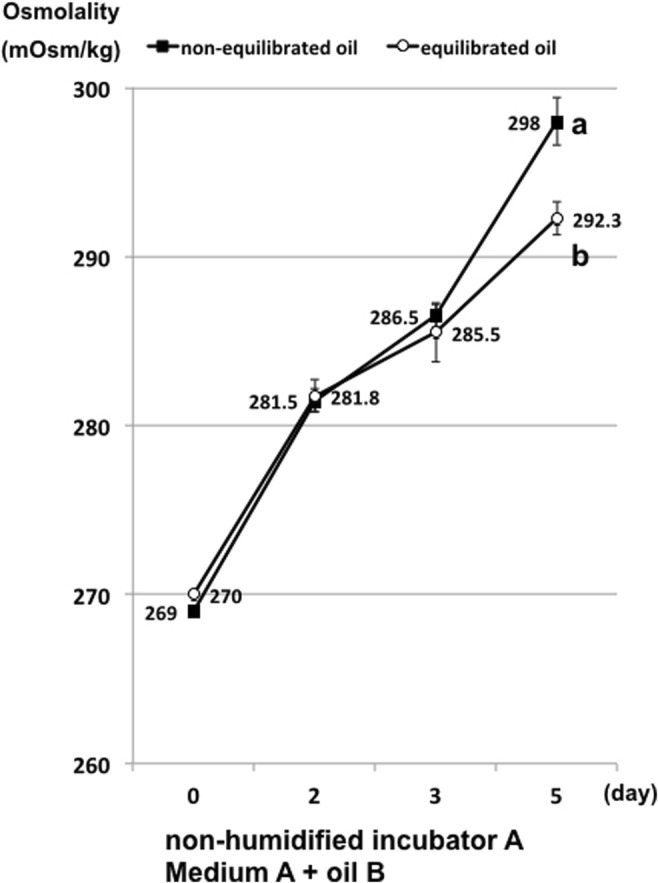
Osmolality of microdrops covered with non-equilibrated or equilibrated (humidified) oil and then incubated in non-humidified incubator A. a vs. b P < 0.05 according to Student’s t test
Discussion
The osmolality of 50-μL drops incubated under a layer of mineral oil was stable during extended culture in a humidified environment but increased significantly and linearly in a non-humidified environment over the same period. The increase was less pronounced in larger (200 μL) microdrops. This phenomenon did not seem to be related to the type of non-humidified incubator or the culture medium, but we found evidence that the type of mineral oil and the water content of mineral oil may affect the rate of osmolality increase. It is not surprising that similar results were obtained in all three culture media because they all had the same starting osmolality.
Since mineral oil directly contacts culture medium, the osmolality increase may result from (i) evaporation of water through the oil and into the atmosphere and/or (ii) absorption and sequestration of water by the oil. Furthermore, this may be affected by the following: (i) the volume and shape of the microdrops, which will affect the surface area of the medium-oil interface; (ii) the amount of oil between the top of the microdrop and the atmosphere; (iii) the water content and water saturation level of the oil. We did not evaluate (i) or (ii) in this study, but we did obtain some preliminary evidence that the water content and water saturation level of mineral oil may affect the rate of osmolality increase. Overnight pre-equilibration (humidification) of mineral oil in a humidified incubator significantly increased the water content and water activity (saturation) value of the oil, and while this did not prevent an osmolality increase during incubation in a non-humidified incubator, the rate of increase was significantly lower.
It was previously reported that the osmolality increase in microdrops was minimized by a greater volume of oil [11–13]. These authors also reported that the shape of the dish was critical, and a smaller contact area between medium and oil resulted in a reduced increase in osmolality. If mineral oil absorbs the water, a larger volume of oil should absorb more water, and the osmolality increase in microdrops should be higher, but these authors reported the opposite and stated that the oil did not absorb water from the microdrops. However, osmolality still linearly increased in their studies, so the water either evaporated through the oil into the atmosphere or was absorbed into the oil but perhaps at a slower rate due to the larger volume of oil which contained a greater total amount of water. This is difficult to test given the small size of microdrops and the resulting small changes in the water content of the microdrops and mineral oil.
The negative effect of high osmolality on the development of animal embryos has been well established. For example, in the hamster, the rate of blastocyst development significantly decreased under high osmolality (> 325 mOsm/kg) compared with the optimal osmolality of 275 mOsm/kg (Susan et al. 1990). In the mouse, high osmolality resulted in developmental arrest at the 2-cell stage as well as altered gene expression, suggesting that high osmolality might cause epigenetic modifications [14]. Furthermore, when dishes were prepared on a heated stage, the osmolality of microdrops increased significantly, and this adversely affected the development of mouse embryos [10]. Much less is known about the impact of osmolality on human embryo development, although it is generally considered to be an important aspect of embryo culture that must be adequately controlled within a relatively narrow range depending on the culture medium composition [6, 15].
The current study shows that covering microdrops with a layer of mineral oil did not adequately prevent osmolality changes during extended culture in non-humidified incubators. We recorded osmolality increases of ~ 4.3% in 200-μL drops and ~ 7.5% in 50-μL drops when oils A, C, and D were used. To enable consistent sampling for osmolality measurement, we used 50-μL drops rather than the 10–20-μL drops that are commonly used for human embryo culture. Nevertheless, we can consider the likely impact of our results on smaller microdrops. The curved surface area of a hemispheric drop is inversely proportional to the volume, so as the volume decreases, there will be relatively more contact area between the medium and oil for potential transfer of water from the culture medium to the oil. The curved surface area to volume ratios for 200-μL, 50-μL, and 10-μL hemispheric drops were calculated to be 0.6563, 1.0419, and 1.7816, respectively, so we might expect ~ 13% osmolality increase in 10-μL drops after 5 days incubation, from 265 to about 300 mOsm/kg. The increase for oil B would be even greater (~ 15%), and the osmolality would increase to about 305 mOsm/kg. Furthermore, the rate of osmolality increase was linear, so the increases should be even greater if longer (6–7 days) incubation periods were required to generate blastocysts. If verified experimentally, this suggests that the osmolality in small (10–20-μL) microdrops cultured continuously in non-humidified incubators may increase to high enough levels to stress some human embryos and adversely affect clinical outcomes.
Since the optimal osmolality for human embryo culture remains undefined [15], the effect of gradually increasing osmolality on human embryo development is also unclear. This may depend on a number of factors including culture medium composition [6], and it may affect some embryos more than others depending on the physiological competence of individual embryos. There may also be longer term clinical and epigenetic effects [15]. These results may also be relevant to the extended culture of human embryos in non-humidified time-lapse incubators, where the benefits of a closed and controlled system may be offset by any negative impact of osmolality increases.
Conclusions
We have shown that the osmolality of microdrops under a layer of mineral oil can increase significantly during extended culture in non-humidified benchtop incubators. The rate of osmolality increase was linear and was not related to the type of single-step culture medium but was related to the incubator environment (humidification), microdrop volume, water saturation level of mineral oil, and possibly the type of mineral oil. A significant increase in osmolality during extended culture may stress human embryos and could compromise their development potential, so it is important that ART laboratories carefully monitor the osmolality of culture media, especially if small microdrops and non-humidified incubators are used. Additionally, since we showed that pre-equilibrated oil could limit the osmolality rise, this might help provide more stable culture conditions in both non-humidified and humidified incubators.
Acknowledgments
This work was supported by all staffs in the Reproductive Centre, Mio Fertility Clinic, Japan.
Authors’ roles
K.Y., M.S., J.Y., and M.N. conducted the experiments. K.Y., M.T., T.S., S.F., K.I., and Y.M. developed the concept and prepared the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whitten WK, Biggers JD. Complete development in vitro of the pre-implantation stages of the mouse in a simple chemically defined medium. J Reprod Fertil. 1968;17(2):399–401. doi: 10.1530/jrf.0.0170399. [DOI] [PubMed] [Google Scholar]
- 2.Biggers JD, Whitten WK, Whittingham DG. In: The culture of mouse embryos in vitro. Methods in mammalian embryology. Daniel JD, editor. San Francisco: W.H.Freeman & Co; 1971. pp. 88–116. [Google Scholar]
- 3.Quinn P, Kerin JF, Warnes GM. Improved pregnancy rate in human in vitro fertilization with the use of a medium based on the composition of human tubal fluid. Fertil Steril. 1985;44(4):493–498. doi: 10.1016/S0015-0282(16)48918-1. [DOI] [PubMed] [Google Scholar]
- 4.Gardner DK, Lane M. Culture and selection of viable blastocysts: a feasible proposition for human IVF? Hum Reprod Update. 1997;3(4):367–382. doi: 10.1093/humupd/3.4.367. [DOI] [PubMed] [Google Scholar]
- 5.Gardner DK, Lane M, Schoolcraft WB. Physiology and culture of the human blastocyst. J Reprod Immunol. 2002;55(1–2):85–100. doi: 10.1016/S0165-0378(01)00136-X. [DOI] [PubMed] [Google Scholar]
- 6.Baltz JM. Media composition: salts and osmolality. Methods Mol Biol. 2012;912:61–80. doi: 10.1007/978-1-61779-971-6_5. [DOI] [PubMed] [Google Scholar]
- 7.Brinster RL. A method for in vitro cultivation of mouse ova from two-cell to blastocyst. Exp Cell Res. 1963;32:205–208. doi: 10.1016/0014-4827(63)90093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawamura K, Chen Y, Shu Y, Cheng Y, Qiao J, Behr B, Pera RA, Hsueh AJ. Promotion of human early embryonic development and blastocyst outgrowth in vitro using autocrine/paracrine growth factors. PLoS One. 2012;7(11):e49328. doi: 10.1371/journal.pone.0049328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swain JE, Cabrera L, Xu X, Smith GD. Microdrop preparation factors influence culture-media osmolality, which can impair mouse embryo preimplantation development. Reprod BioMed Online. 2012;24(2):142–147. doi: 10.1016/j.rbmo.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Fawzy M, AbdelRahman MY, Zidan MH, Abdel Hafez FF, Abdelghafar H, Al-Inany H, Bedaiwy MA. Humid versus dry incubator: a prospective, randomized, controlled trial. Fertil Steril. 2017;108(2):277–283. doi: 10.1016/j.fertnstert.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Swain JE. Controversies in ART: considerations and risks for uninterrupted embryo culture. Reprod BioMed Online. 2019;S1472-6483(19):30232–30239. doi: 10.1016/j.rbmo.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter G, Hammond E, Peek J, Morbeck D. The impact of dry incubation on osmolality of media in time lapse culture dishes. Hum Reprod. 2018;33:i61. [Google Scholar]
- 13.Olds S, Stemm K, Wachter K, Wiemer K. Analysis of embryo culture media pH changes during incubator use and media evaporation under oil using a continous pH monitoring system. Fertil Steril. 2015;104:e318–e319. doi: 10.1016/j.fertnstert.2015.07.997. [DOI] [Google Scholar]
- 14.Wang F, Kooistra M, Lee M, Liu L, Baltz JM. Mouse embryos stressed by physiological levels of osmolarity become arrested in the late 2-cell stage before entry into M phase. Biol Reprod. 2011;85(4):702–713. doi: 10.1095/biolreprod.111.090910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunde A, Brison D, Dumoulin J, Harper J, Lundin K, Magli MC, et al. Time to take human embryo culture seriously. Hum Reprod. 2016;31(10):2174–2182. doi: 10.1093/humrep/dew157. [DOI] [PubMed] [Google Scholar]