Abstract
Objective
To determine whether younger oocyte donor age is associated with better outcomes after in vitro fertilization (IVF) compared with older oocyte donor age.
Design
A retrospective cohort study.
Setting
Large academically affiliated infertility treatment center.
Patients
We included all women ≥ 18 years who started their first fresh cycle using donor oocytes at our center from January 2002 through October 2017; only the first oocyte recipient cycle was analyzed.
Intervention
Log-binomial regression was used to compare the incidence of clinical pregnancy and live birth among the following donor age groups: < 25 years, 25 to < 30 years, and 30 to <35 years.
Main outcome measure
Incidence of clinical pregnancy and live birth among donor age groups.
Results
We included 774 donor cycles; 269 (34.8%) used donors < 25 years, 399 (51.6%) used donors 25 to < 30 years, and 106 (13.7%) used donors 30 to < 35 years. Median donor age was 26 years (range 18–34.5), and median recipient age and partner age were both 42 years. Per cycle start, after adjusting for recipient age, cycles using donors < 25 years were not associated with a higher incidence of clinical pregnancy (RR 0.90; 95% CI 0.77–1.06) or live birth (RR 0.87; 95% CI 0.72–1.04) compared with donors age 25–< 30 years.
Conclusions
Donor age < 25 was not associated with better outcomes after IVF. Under the age of 30, the prioritization of <25 year old donors may not be recommended given the lack of evidence for superior pregnancy or live birth outcomes.
Keywords: IVF, Oocyte donation, Live birth, Assisted reproductive technology, Age
Introduction
Selection of an oocyte donor is an important step for couples who are undergoing in vitro fertilization (IVF) using donated oocytes. Younger donors usually are preferred to older ones based on the simple fact that female fertility declines with age. Oocyte donation offers a probability of success that is generally more consistent with the age of the donor than the age of the recipient, with the incidence of live birth as high as 50% per cycle or a cumulative incidence of live birth of 60–80% after up to 6 cycles for recipients in their 40s and 50s [1–4]. For this reason, the age of the donor has long been considered the most important determinant of outcomes after oocyte donation [5–8].
More than a dozen studies have evaluated the influence of donor age on pregnancy and/or live birth in recipients, and relatively few have found a significant association [8–12]. Most were published more than 10 years ago, were limited by small samples, and had inconsistent age thresholds [8–20]. Additionally, no studies published within the last 10 years have reported the specific incidence of live birth among donors less than 25 years. Studies that have stratified by donor age often categorize donors less than 30 into a single age group, thus obscuring potential differences in outcomes among younger donors.
Furthermore, there is reason to question the belief that younger women produce better treatment outcomes and higher-quality oocytes. A large study by Wang et al. in Australia and New Zealand compared four donor age groups (< 30, 30–34, 35–39, and ≥ 40 years) and found that the cycles utilizing donors 30 to 34 years had a higher incidence of live birth than cycles with donors under 30 years, as well as donors over 34 [12]. With regard to autologous (non-donor) IVF, Humm et al. analyzed 16,792 cycles and found that women less than 25 years had a lower cumulative incidence of live birth in up to six IVF cycles than women age 25 to < 30 or 30 to < 35 years, though these findings did not reach statistical significance [21]. A similar trend has been seen in 2009 and 2014 national registry data from Australia and New Zealand, in which infertile women under age 25 undergoing IVF had a lower cumulative incidence of live birth than their counterparts age 25 to 30 [22, 23]. Other studies have also shown paradoxically high rates of aneuploidy and miscarriage among women undergoing IVF in their early 20s [24, 25].
Women may benefit from selecting an oocyte donor with a higher likelihood of success. As the importance of donor age as a predictor of IVF success remains unclear, we reviewed the outcomes of women undergoing their first donor-oocyte cycles to determine the incidence of clinical pregnancy and live birth according to finer gradations of donor age. Based on our own work in autologous cycles, we hypothesized that cycles using donors under age 25 would not have better pregnancy and live birth outcomes compared with cycles using older donors.
Materials and methods
We performed a retrospective cohort study of all women ≥ 18 years who started their first fresh cycle using donor oocytes at our center from January 2002 through September 2016. The institutional review board at Beth Israel Deaconess Medical Center approved this study. Only the first oocyte donation cycle was retained in the analysis. We obtained demographic information, cycle characteristics, and cycle outcomes from a prospective clinical database and supplemented this with chart review. Oocyte donors were all volunteers with no prior infertility diagnosis, and they were primarily anonymous. Donors underwent evaluation followed by standardized treatment protocols for ovarian stimulation, monitoring, and oocyte retrieval [26]. Briefly, this includes an age range of 21–30 years with known/directed egg donors allowed to range between 21 and 35 years; AMH must be at least 2 ng/ml and AFC at least 12. We do not typically test for FSH.
The number of embryos transferred was consistent with national guidelines (ASRM Practice Committee Document). Patients received luteal phase support until 10 weeks of gestation [26]. Intracytoplasmic sperm injection (ICSI) was performed when indicated.
Fertilization success is the proportion of all retrieved oocytes that developed into embryos with two pronuclei on the day after fertilization; mature fertilization success was fertilization success among mature oocytes. All patients with positive serum β-hCG underwent early ultrasound. Implantation success was defined as the number of fetal heartbeats or sacs seen on ultrasound divided by the number of embryos transferred. The primary outcome of live birth was defined as the birth of one or more live infants at ≥ 20 weeks of gestation. Clinical pregnancy was defined as the presence of at least one intrauterine gestational sac with fetal heartbeat identified on ultrasound in the first trimester. Spontaneous abortion was defined as pregnancy loss < 20 weeks of gestation.
Cycles were divided into the following three groups based on donor age: < 25, 25 to < 30, and 30 to < 35 years. Descriptive data are presented as median and interquartile range (IQR) or frequency and proportion. The Mann-Whitney U test was used to compare medians between groups. Log-binomial regression was used to calculate crude and adjusted risk ratios (RR) and 95% confidence intervals (CI). In the regression model, we accounted for correlation between cycles for different recipients who used the same donor. Although not confounders because they are not a common cause of both the exposure and the outcome, there often is concern about the influence of variables such as donor and recipient body mass index (BMI), number of oocytes retrieved, use of ICSI, number of embryos transferred, and day of embryo transfer on the effect estimate [27]. Thus, we included these variables in regression models and evaluated their influence on the risk ratio. We included recipient age in all models due to its biological relevance. Otherwise, confounders were only included in the final model if they altered the crude RR by 10% or more, acknowledging that not all factors can be accounted for. P < 0.05 was considered statistically significant. All statistical analyses were conducted with Stata/SE 12.0 (StataCorp LLC, College Station, TX) and SAS 9.4 (SAS Institute, Cary, NC).
Results
We identified 784 first oocyte recipient cycles during the study period. Ten cycles (1.3%) were excluded because they used an oocyte donor ≥ 35 years. Of the remaining 774 oocyte recipients, 269 (34.8%) were paired with oocyte donors < 25 years, 399 (51.6%) were paired with donors 25 to < 30 years, and 106 (13.7%) were paired with donors ≥ 30 years (Table 1). There were 607 unique donors contributing to these 774 cycles; 82.0% of donors contributed to one recipient cycle, 12.9% contributed to two recipient cycles, 3.1% contributed to three recipient cycles, and 2.1% contributed to ≥ 4 recipient cycles. Donor age ranged from 18 to 34.5 years, with 98.8% of donors falling within the age range recommended by the ASRM of 21 to 34 years. Most recipients had been pregnant at least once, yet 75% remained nulliparous at the start of their first oocyte donation cycle. Recipients had undergone a median of two prior autologous IVF cycles (95% IQR 1.0–5.0). Nearly two-thirds had diminished ovarian reserve as their primary indication for oocyte donation, while others had ovulatory dysfunction, primary ovarian insufficiency, and repeated unexplained IVF failure. Donor and recipient characteristics are presented in Table 1.
Table 1.
Recipient and donor characteristics at the start of the first oocyte donation cycle
| Donor age (years) | ||||
|---|---|---|---|---|
| All cycles N = 774 |
< 25 n = 269 |
25–< 30 n = 399 |
30–< 35 n = 106 |
|
| Age (years) | ||||
| Donor age | 26.0 (24.0–28.0) | 23.0 (22.3–24.0) | 27.0 (26.0–28.0) | 31.0 (30.1–32.2) |
| Recipient age | 42.0 (38.4–44.9) | 42.1 (38.9–44.9) | 42.1 (38.5–44.9) | 41.3 (37.1–44.8) |
| Partner age* | 42.1 (37.5–46.1) | 42.2 (37.7–46.1) | 42.3 (37.7–46.2) | 41.3 (36.0–45.6) |
| Body mass index (kg/m2) | ||||
| Donor | 23.0 (21.0–25.0) | 22.0 (21.0–24.5) | 23.0 (21.0–25.0) | 23.2 (21.6–27.3) |
| Recipient | 24.0 (22.0–27.1) | 23.3 (21.6–27.1) | 24.0 (21.9–27.1) | 24.0 (22.4–27.3) |
| Recipient characteristics | ||||
| Nulligravid | 331 (43.6) | 117 (44.2) | 173 (44.3) | 41 (39.4) |
| Nulliparous | 579 (75.8) | 210 (78.7) | 289 (73.4) | 80 (77.7) |
| Number of prior cycles | 2.0 (1.0–5.0) | 2.0 (1.0–5.0) | 2.0 (1.0–5.0) | 2.0 (1.0–5.0) |
| Primary indication | ||||
| Diminished ovarian reserve | 500 (64.6) | 180 (66.9) | 266 (66.7) | 54 (50.9) |
| Ovulatory dysfunction | 39 (5.0) | 11 (4.1) | 19 (4.8) | 9 (8.5) |
| POI/POF | 36 (4.7) | 14 (5.2) | 17 (4.3) | 5 (4.7) |
| Unexplained failed in vitro fertilization | 48 (6.2) | 19 (7.1) | 20 (5.0) | 9 (8.5) |
| Other† | 102 (13.2) | 33 (12.3) | 55 (13.8) | 14 (13.2) |
| Missing | 49 (6.3) | 12 (4.5) | 22 (5.5) | 15 (14.2) |
Data are presented as median (interquartile range) or n (%)
POI/POF primary ovarian insufficiency/premature ovarian failure
*Partner age was excluded for couples using donor sperm
†e.g., polycystic ovarian syndrome, preimplantation genetic screening, endometriosis
The three donor age groups were similar with respect to recipient age, partner age, recipient BMI, donor BMI, recipient gravidity and parity, and cycle number (Table 1). Donors 30 to < 35 years had significantly fewer total oocytes retrieved than donors age 25 to < 30 years (P = 0.003), as well as fewer mature oocytes retrieved (P = 0.002) (Table 2). There were no significant differences between donors < 25 years and those 25 to < 30 years (all P ≥ 0.18).
Table 2.
Cycle characteristics of first oocyte donation cycle patients according to age of oocyte donor
| Donor age (years) | ||||||
|---|---|---|---|---|---|---|
| All cycles N = 774 |
< 25 n = 269 |
25–< 30 n = 399 |
P * | 30–< 35 n = 106 |
P † | |
| All cycles | ||||||
| Oocytes retrieved | 18.0 (13.0–25.0) | 19.0 (14.0–26.0) | 18.0 (13.0–25.0) | 0.18 | 16.0 (10.0–21.0) | 0.003 |
| Mature oocytes retrieved | 15.0 (11.0–21.0) | 16.0 (12.0–22.0) | 15.0 (11.0–21.0) | 0.62 | 13.5 (9.0–18.0) | 0.002 |
| Donor sperm | 51 (6.6) | 17 (6.3) | 28 (7.0) | 0.72 | 6 (5.7) | 0.81 |
| Intracytoplasmic sperm injection | 336 (43.4) | 121 (45.0) | 175 (43.9) | 0.77 | 40 (37.7) | 0.26 |
| Fertilization success | 70.0 (54.6–80.0) | 68.2 (52.9–77.8) | 70.0 (55.6–79.0) | 0.25 | 73.5 (58.0–85.7) | 0.07 |
| Mature fertilization success | 80.0 (66.7–90.0) | 79.5 (66.7–90.0) | 80.0 (66.7–88.9) | 0.82 | 83.3 (70.3–94.1) | 0.12 |
| Preimplantation genetic testing for aneuploidy | 3 (0.4) | 0 (0.0) | 1 (0.3) | 0.60 | 2 (1.9) | 0.08 |
| Canceled cycles | 28 (3.6) | 13 (4.8) | 15 (3.8) | 0.50 | 0 (0.0) | 0.049 |
| Embryo transfer cycles | ||||||
| Embryos transferred | 2.0 (2.0–2.0) | 2.0 (2.0–2.0) | 2.0 (2.0–2.0) | 0.68 | 2.0 (2.0–2.0) | 0.06 |
| Day of transfer | 0.49 | 0.34 | ||||
| Day 3 | 482 (70.4) | 165 (69.9) | 243 (69.4) | 74 (74.8) | ||
| Day 5/6 | 197 (28.8) | 70 (29.7) | 102 (29.1) | 25 (25.3) | ||
| Other | 6 (0.9) | 1 (0.4) | 5 (1.4) | 0 (0.0) | ||
Data are presented as median (interquartile range) or n (%)
*P is comparing 25–< 30 years to < 25 years
†P is comparing 25–< 30 years to 30–< 35 years
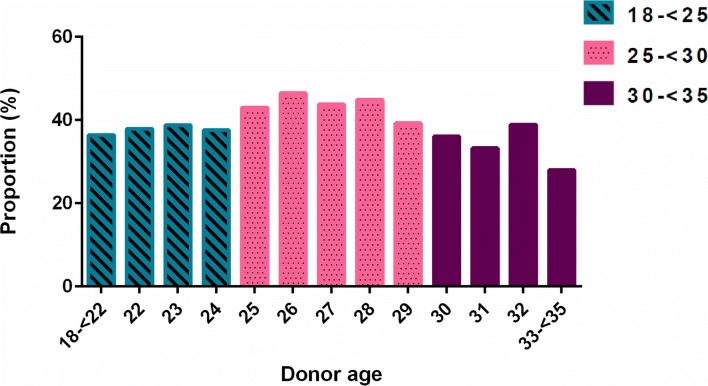
Recipients of oocytes from the youngest donor age group did not have superior IVF outcomes and indeed had less favorable IVF outcomes, though this was not statistically significant (Table 3). Cycles with donors < 25 years and those with donors 30 to < 35 years resulted in similar incidences of live birth of 37.9% and 34.0%, respectively, while cycles involving donors 25 to < 30 had an incidence of live birth of 43.9% (Table 3). Per cycle start, after adjusting for recipient age, cycles using donors < 25 years were less likely to result in a clinical pregnancy (RR 0.90; 95% CI 0.77–1.06) or live birth (RR 0.87; 95% CI 0.72–1.04), although these findings did not reach statistical significance. Results were similar for cycles using donors 30 to < 35 years. Approximately 4% of initiated cycles in our dataset were canceled prior to embryo transfer, and thus, the analysis of outcomes per embryo transfer produced similar results (Table 3). Implantation success did not differ between cycles using donors < 25 (0.44 ± 0.43) and those using donors 25 to < 30 (0.46 ± 0.41; P = 0.69); similarly, these was no difference between cycles using donors < 25 and those using donors 30 to < 35 (0.48 ± 0.44; P = 0.48). A graph of the incidences of clinical pregnancy and live birth per cycle according to donor age year shows higher incidence of live birth among cycles using donors 25 to < 30 years compared with cycles using younger and older donors (Fig. 1).
Table 3.
Outcomes per cycle start and per transfer for donors 25 to < 30 compared with donors < 25 and 30–< 35 years
| Donor age (years) | |||
|---|---|---|---|
| <25 n (%) RR* (95% CI) |
25–< 30 n (%) RR* (95% CI) |
30–< 35 n (%) RR* (95% CI) |
|
| Positive β-hCG | |||
| Per cycle start |
157 (58.4) 0.97 (0.85–1.10) |
241 (60.4) Ref |
55 (51.9) 0.86 (0.70–1.05) |
| Per transfer |
157 (66.5) 0.97 (0.87–1.09) |
241 (68.7) Ref |
55 (55.6) 0.81 (0.67–0.98) |
| Spontaneous abortion | |||
| Per cycle start |
19 (7.1) 1.17 (0.66–2.08) |
24 (6.0) Ref |
6 (5.7) 0.97 (0.41–2.27) |
| Per transfer |
19 (8.1) 1.17 (0.66–2.06) |
24 (6.8) Ref |
6 (6.1) 0.91 (0.39–2.11) |
| Clinical pregnancy | |||
| Per cycle start |
121 (45.0) 0.90 (0.77–1.06) |
199 (49.9) Ref |
42 (39.6) 0.79 (0.61–1.02) |
| Per transfer |
121 (51.3) 0.90 (0.77–1.06) |
199 (56.7) Ref |
42 (42.4) 0.90 (0.77–1.06) |
| Live birth | |||
| Per cycle start |
102 (37.9) 0.87 (0.72–1.04) |
175 (43.9) Ref |
36 (34.0) 0.76 (0.58–1.01) |
| Per transfer | 102 (43.2) | 175 (49.9) | 36 (36.4) |
| 0.87 (0.73–1.04) | Ref | 0.75 (0.58–0.95) | |
| Singleton |
69 (67.7) 0.83 (0.65–1.06) |
123 (70.3) Ref |
21 (58.3) 0.64 (0.43–0.94) |
| Twins |
32 (31.4) 0.98 (0.64–1.49) |
49 (28.0) Ref |
15 (41.7) 1.12 (0.66–1.89) |
| Triplets | 0 (0.0) | 2 (1.1) | 0 (0.0) |
| Unknown | 1 (1.0) | 1 (0.6) | 0 (0.0) |
For cycles using donors < 25 years, there were 269 cycle starts and 236 transfers; for cycles using donors 25–< 30 years, there were 399 cycle starts and 351 transfers; and for cycles using donors 30–< 35 years, there were 106 cycle starts and 99 transfers
*Adjusted for recipient age
Fig. 1.
Incidence of live birth per cycle start by year of donor age
Discussion
The results of this study contradict the paradigm that “younger is better,” revealing that younger donor age does not necessarily correlate with greater treatment success in oocyte donation cycles. Though not statistically significant, this study shows that cycles using donors < 25 years were less likely to result in clinical pregnancy and live birth compared with cycles using donors 25 to < 30 years, independent of recipient age and despite similar numbers of oocytes retrieved and similar fertilization success.
These findings may be explained by the presence of increased aneuploidy and poor embryo quality among donors in the youngest age group. Franasiak et al. reported aneuploidy in over 40% of embryos derived from women ages 22 and 23 compared with 20–27% of embryos from women ages 26 to 30. Aneuploidy involving multiple chromosomes was more commonly seen in embryos from women age ≤ 25 compared with those from women ages 26 to 30. Other studies of pre-preimplantation genetic screening have confirmed that even donors younger than 35 can have incidence of embryo aneuploidy of more than 50% [28–30]. Our graphs of the incidence of clinical pregnancy and live birth by donor age year reflect the inverse of the bimodal curves described by Franasiak et al. for aneuploidy in autologous IVF cycles [24]. More frequent aneuploidy could translate into worse outcomes, such as increased incidence of spontaneous abortion. Nazemian et al. found that donors age < 25 had fewer cleaved embryos and lower day 3 embryo quality, as well as higher incidence of miscarriage, compared with infertile women age 30 to 35 years [25].
Though not mutually exclusive, an alternative explanation implicates follicular atresia as a process that affects younger and older donors disproportionately. Breakdown of ovarian follicles during early life is accelerated and dynamic until first ovulation, after which atresia occurs at a rate of about 1000 follicles per month until menopause. As proposed by Casper and colleagues, it is possible that oocytes from the youngest donors, who are closer to the onset of puberty, may be undergoing programmed cell death at a higher rate than counterparts in their late 20s [25]. This could result in poorer embryo development and less frequent fertilization and pregnancy, even if by morphological or chromosomal analyses the oocytes and embryos appear to be normal.
Our study has some important limitations. Although we assessed the effect of donor BMI on our model, we did not have data on other donor-related factors, such as donor gravidity, parity, anti-Müllerian hormone level, history of miscarriage, history of donor IVF success, or race or ethnicity. There is some evidence that increasing donor parity is associated with improved pregnancy and live birth outcomes after IVF; however, Cohen et al. showed that after controlling for donor age, the effect of donor parity was not significant [8, 18]. Therefore, the association between donor parity and cycle outcomes actually may be driven by variations in donor age, in that older donors are more likely to have a history of live birth. Furthermore, despite attempts to adjust for confounding variables, there may be some element of selection bias still present. For example, it could be that poorer prognosis patients were more likely to select or be matched with a younger donor in an effort to maximize success, thereby explaining the seemingly poorer outcomes among donors < 25 years. A final limitation is the long duration of the study, 2002 to 2016. Although this provides a larger cohort of patients, it is also complicated by the changing clinical practice over this time range. The largest changes in practice are of course improvement of cryopreservation by the introduction of vitrification and also the shift in embryo transfer practice from predominantly 2–3 embryos on day 3 in the earlier stages to single blastocyst transfer in later years.
Quantifying IVF outcomes by donor age is highly relevant to clinical practice and will become increasingly important as oocyte donation expands. In the USA, the number of annual donor egg cycles more than doubled from 1997 to 2010 [31]. Outcomes after oocyte donation also have improved greatly over the last 10 years, such that the success of oocyte donation now consistently exceeds that of conventional IVF [1, 2, 32, 33]. Another rapidly expanding group is women who choose social egg freezing, and this study may help them choose a more appropriate time to freeze their eggs. This study shows that donor age < 25 years was not associated with better outcomes after IVF. Specifically, the incidence of live birth among cycles using donors < 25 years was 13% lower than the incidence among those using donors age 25 to < 30 years. While not statistically significant, this lower incidence may be clinically meaningful. More research investigating the implications of donor characteristics, specifically donor age, may improve donor selection and further optimize the care of patients undergoing oocyte donation.
Funding information
This work was conducted with financial support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award 1UL1 TR001102-01) and financial contributions from Harvard University and its affiliated academic health care centers.
Compliance with ethical standards
The institutional review board at Beth Israel Deaconess Medical Center approved this study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Budak E, Garrido N, Soares SR, Melo MA, Meseguer M, Pellicer A, et al. Improvements achieved in an oocyte donation program over a 10-year period: sequential increase in implantation and pregnancy rates and decrease in high-order multiple pregnancies. Fertil Steril. 2007;88(2):342–349. doi: 10.1016/j.fertnstert.2006.11.118. [DOI] [PubMed] [Google Scholar]
- 2.Yeh JS, Steward RG, Dude AM, Shah AA, Goldfarb JM, Muasher SJ. Pregnancy outcomes decline in recipients over age 44: an analysis of 27,959 fresh donor oocyte in vitro fertilization cycles from the Society for Assisted Reproductive Technology. Fertil Steril. 2014;101(5):1331–1336. doi: 10.1016/j.fertnstert.2014.01.056. [DOI] [PubMed] [Google Scholar]
- 3.Luke B, Brown MB, Wantman E, Lederman A, Gibbons W, Schattman GL, Lobo RA, Leach RE, Stern JE. Cumulative birth rates with linked assisted reproductive technology cycles. N Engl J Med. 2012;366(26):2483–2491. doi: 10.1056/NEJMoa1110238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paulson RJ, Hatch IE, Lobo RA, Sauer MV. Cumulative conception and live birth rates after oocyte donation: implications regarding endometrial receptivity. Hum Reprod. 1997;12(4):835–839. doi: 10.1093/humrep/12.4.835. [DOI] [PubMed] [Google Scholar]
- 5.Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, et al. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet. 1991;337(8754):1375–1377. doi: 10.1016/0140-6736(91)93060-M. [DOI] [PubMed] [Google Scholar]
- 6.Abdalla HI, Wren ME, Thomas A, Korea L. Age of the uterus does not affect pregnancy or implantation rates; a study of egg donation in women of different ages sharing oocytes from the same donor. Hum Reprod. 1997;12(4):827–829. doi: 10.1093/humrep/12.4.827. [DOI] [PubMed] [Google Scholar]
- 7.Faddy M, Gosden R, Ahuja K, Elder K. Egg sharing for assisted conception: a window on oocyte quality. Reprod BioMed Online. 2011;22(1):88–93. doi: 10.1016/j.rbmo.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MA, Lindheim SR, Sauer MV. Donor age is paramount to success in oocyte donation. Hum Reprod. 1999;14(11):2755–2758. doi: 10.1093/humrep/14.11.2755. [DOI] [PubMed] [Google Scholar]
- 9.Balmaceda JP, Bernardini L, Ciuffardi I, Felix C, Ord T, Sueldo CE, Asch RH. Oocyte donation in humans: a model to study the effect of age on embryo implantation rate. Hum Reprod. 1994;9(11):2160–2163. doi: 10.1093/oxfordjournals.humrep.a138410. [DOI] [PubMed] [Google Scholar]
- 10.Faber BM, Mercan R, Hamacher P, Muasher SJ, Toner JP. The impact of an egg donor’s age and her prior fertility on recipient pregnancy outcome. Fertil Steril. 1997;68(2):370–372. doi: 10.1016/S0015-0282(97)81532-4. [DOI] [PubMed] [Google Scholar]
- 11.Moomjy M, Cholst I, Mangieri R, Rosenwaks Z. Oocyte donation: insights into implantation. Fertil Steril. 1999;71(1):15–21. doi: 10.1016/S0015-0282(98)00420-8. [DOI] [PubMed] [Google Scholar]
- 12.Wang YA, Farquhar C, Sullivan EA. Donor age is a major determinant of success of oocyte donation/recipient programme. Hum Reprod. 2012;27(1):118–125. doi: 10.1093/humrep/der359. [DOI] [PubMed] [Google Scholar]
- 13.Abdalla HI, Baber R, Kirkland A, Leonard T, Power M, Studd JW. A report on 100 cycles of oocyte donation; factors affecting the outcome. Hum Reprod. 1990;5(8):1018–1022. doi: 10.1093/oxfordjournals.humrep.a137209. [DOI] [PubMed] [Google Scholar]
- 14.Rotsztejn DA, Ord T, Balmaceda JP, Asch RH. Variables which influence the selection of an egg donor. Hum Reprod. 1992;7(1):59–62. doi: 10.1093/oxfordjournals.humrep.a137558. [DOI] [PubMed] [Google Scholar]
- 15.Wong IL, Legro RS, Lindheim SR, Paulson RJ, Sauer MV. Efficacy of oocytes donated by older women in an oocyte donation programme. Hum Reprod. 1996;11(4):820–823. doi: 10.1093/oxfordjournals.humrep.a019260. [DOI] [PubMed] [Google Scholar]
- 16.Stolwijk AM, Zielhuis GA, Sauer MV, Hamilton CJ, Paulson RJ. The impact of the woman’s age on the success of standard and donor in vitro fertilization. Fertil Steril. 1997;67(4):702–710. doi: 10.1016/S0015-0282(97)81370-2. [DOI] [PubMed] [Google Scholar]
- 17.Shulman A, Frenkel Y, Dor J, Levran D, Shiff E, Maschiach S. The best donor. Hum Reprod. 1999;14(10):2493–2496. doi: 10.1093/humrep/14.10.2493. [DOI] [PubMed] [Google Scholar]
- 18.Harris SE, Faddy M, Levett S, Sharma V, Gosden R. Analysis of donor heterogeneity as a factor affecting the clinical outcome of oocyte donation. Hum Fertil (Camb) 2002;5(4):193–198. doi: 10.1080/1464727022000199112. [DOI] [PubMed] [Google Scholar]
- 19.Mirkin S, Gimeno TG, Bovea C, Stadtmauer L, Gibbons WE, Oehninger S. Factors associated with an optimal pregnancy outcome in an oocyte donation program. J Assist Reprod Genet. 2003;20(10):400–408. doi: 10.1023/A:1026236726568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barton SE, Missmer SA, Ashby RK, Ginsburg ES. Multivariate analysis of the association between oocyte donor characteristics, including basal follicle stimulating hormone (FSH) and age, and IVF cycle outcomes. Fertil Steril. 2010;94(4):1292–1295. doi: 10.1016/j.fertnstert.2009.07.1672. [DOI] [PubMed] [Google Scholar]
- 21.Humm KC, Dodge LE, Wu LH, Penzias AS, Malizia BA, Sakkas D, Hacker MR. In vitro fertilization in women under 35: counseling should differ by age. J Assist Reprod Genet. 2015;32(10):1449–1457. doi: 10.1007/s10815-015-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris KFO, Paul RC, Macaldowie A, Lee E, Chambers GM. Assisted reproductive technology in Australia and New Zealand 2014. Sydney: National Perinatal Epidemiology and Statistics Unit, the University of New South Wales; 2016. [Google Scholar]
- 23.Wang YA MA, Hayward I, Chambers GM, Sullivan EA. Assisted reproductive technology in Australia and New Zealand 2009. Australian Institute of Health and Welfare 2011;Assisted reproduction technology series no. 15.
- 24.Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, Scott RT., Jr The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656–663 e1. doi: 10.1016/j.fertnstert.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Nazemian Z, Esfandiari N, Javed M, Casper RF. The effect of age on in vitro fertilization outcome: is too young possible? J Assist Reprod Genet. 2011;28(2):101–106. doi: 10.1007/s10815-010-9499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eaton JL, Hacker MR, Harris D, Thornton KL, Penzias AS. Assessment of day-3 morphology and euploidy for individual chromosomes in embryos that develop to the blastocyst stage. Fertil Steril. 2009;91(6):2432–2436. doi: 10.1016/j.fertnstert.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Hernan MA, Hernandez-Diaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155(2):176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 28.Barri PN, Coroleu B, Clua E, Tur R, Boada M, Rodriguez I. Investigations into implantation failure in oocyte-donation recipients. Reprod BioMed Online. 2014;28(1):99–105. doi: 10.1016/j.rbmo.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 29.Reis Soares S, Rubio C, Rodrigo L, Simon C, Remohi J, Pellicer A. High frequency of chromosomal abnormalities in embryos obtained from oocyte donation cycles. Fertil Steril. 2003;80(3):656–657. doi: 10.1016/S0015-0282(03)00787-8. [DOI] [PubMed] [Google Scholar]
- 30.Munne S, Ary J, Zouves C, Escudero T, Barnes F, Cinioglu C, et al. Wide range of chromosome abnormalities in the embryos of young egg donors. Reprod BioMed Online. 2006;12(3):340–346. doi: 10.1016/S1472-6483(10)61007-3. [DOI] [PubMed] [Google Scholar]
- 31.Gleicher N, Kushnir VA, Weghofer A, Barad DH. The “graying” of infertility services: an impending revolution nobody is ready for. Reprod Biol Endocrinol. 2014;12:63. doi: 10.1186/1477-7827-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broekmans FJ, Knauff EA, te Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab. 2007;18(2):58–65. doi: 10.1016/j.tem.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Soares SR, Velasco JA, Fernandez M, Bosch E, Remohi J, Pellicer A, et al. Clinical factors affecting endometrial receptiveness in oocyte donation cycles. Fertil Steril. 2008;89(3):491–501. doi: 10.1016/j.fertnstert.2008.01.080. [DOI] [PubMed] [Google Scholar]