Abstract
Post-thoracotomy pain is one of the most severe forms of post-operative pain. Anaesthetists usually manage post-thoracotomy pain with an epidural or paravertebral block. However, both of these techniques have their limitations. Ultrasound-guided interfascial plane block like serratus anterior plane block is a new concept and is proposed to provide analgesia to the hemithorax. We report our experience with 10 thoracotomy cases where this block was used as a post-operative analgesic technique. Patients undergoing pulmonary metastasectomy or lobectomy received ultrasound-guided serratus anterior plane block between the serratus anterior and the external intercostal muscles with 0.25% ropivacaine, and a catheter was inserted. Post-operatively, 0.125% ropivacaine with fentanyl (1 mcg/ml) was given as infusion at 5–7 ml/h. Other analgesics were paracetamol and diclofenac. Fentanyl infusion at 0.25 mcg/kg/h was the rescue analgesic if pain persisted. Four out of 10 patients required fentanyl infusion. Uncontrolled pain in two of these patients was at the intercostal drain site; in the third patient, two ribs were resected; and in the 4th patient, there was poor drug spread and the catheter could not be placed in the desired plane due to poor muscle mass. The catheter was kept in situ for a minimum of 48 h to a maximum of 6 days after surgery. Serratus anterior block could be an attractive option for post-thoracotomy analgesia. Further studies can take the help of the surgeon for catheter placement in the desired plane at the time of wound closure to ensure adequate drug spread.
Keywords: Post-thoracotomy pain, Interfascial blocks, Serratus anterior plane block
Introduction
Pain after thoracotomy is one of the most severe forms of post-operative pains [1]. Proper management of post-thoracotomy pain is essential to improve surgical outcome. Inadequately managed post-thoracotomy pain may lead to impaired respiratory mechanics and complications like atelectasis, pooled secretions, hypoxemia, etc. [1]. Traditionally, both pharmacological and regional analgesia techniques have been used to manage acute post-thoracotomy pain [2] and regional techniques like thoracic epidural anaesthesia (TEA) or paravertebral block (PVB) are recommended as standard approaches for analgesia after thoracic surgical procedures [3]. TEA is considered the gold standard among regional techniques for post-thoracotomy pain [4]. However, regional techniques like TEA, PVB or multimodal analgesia with opioids and other drugs may not be suitable or feasible for every patient. Chest wall is innervated anteriorly by the medial and lateral pectoral nerves and posterolaterally by the branches of spinal nerves. Serratus anterior plane (SAP) block is proposed to anaesthetise the hemithorax by blocking branches of intercostal nerves [5]. This block may be tried as an alternative technique to alleviate post-thoracotomy pain.
We report our experience of 10 cases where SAP block was used to manage post-thoracotomy pain with a catheter in situ and continuous local anaesthetic infusion.
Methodology
Written informed consent was taken from the patients. Patients aged 18–65 years, American Society of Anaesthesia physical score (ASA PS) 1–3, undergoing pulmonary metastasectomy or lobectomy were included in the study.
Patients having chest wall deformities or known allergy to local anaesthetics were excluded from the study. The technique of general anaesthesia and post-operative pain management were standardised in all patients.
Intrathecal morphine (5 mcg/kg) was given prior to induction. Anaesthesia was induced with intravenous fentanyl (2mcg/kg), propofol (1–2 mg/kg) and vecuronium (0.1 mg/kg), followed by insertion of double lumen tube or other lung separation technique. Paracetamol (15 mg/kg) intravenous (IV) was given before incision. Anaesthesia was maintained with oxygen, air and desflurane (0.8–1 MAC). Ventilation was maintained using volume-controlled ventilator and adjusted to achieve an end tidal carbon dioxide of 35–45 mmHg.
Intraoperative vecuronium boluses were administered as required. IV fentanyl (0.5 mcg/kg) was given on the discretion of the anaesthetist if HR or SBP increased by more than 20% of the baseline values.
Sap Block
After surgical wound closure, before surgical dressing, ultrasound-guided SAP block and catheter placement was done with the patient in the lateral decubitus position, as positioned for surgery.
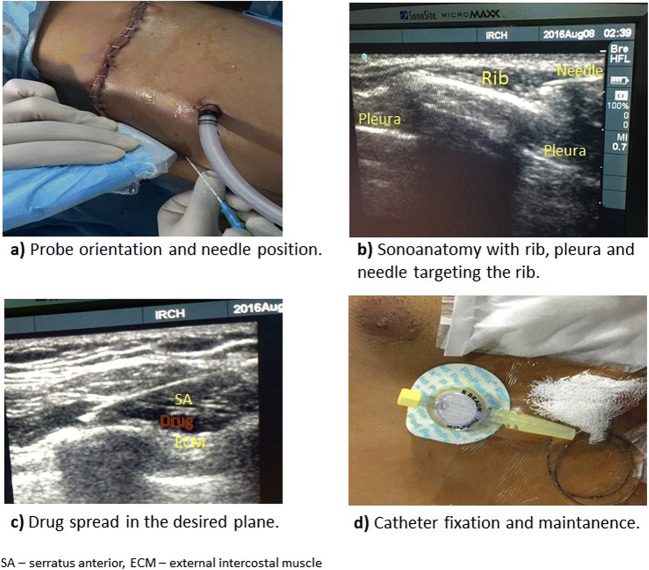
Linear high-frequency ultrasound (USG) probe (SonoSite*Micromaxx*, Inc., Bothell, WA, USA, C6oe/6–13 MHz) was placed in the sagittal plane in the thoracic cage and the fifth rib was identified. Continuous peripheral nerve block catheter set (20G, 400 mm (Contiplex®, B.Braun Melsungen AG, Germany)) was used for the block. The needle was introduced ‘in plane’ with the ultrasound probe targeting the interfascial plane between the serratus anterior and external intercostal muscles (Fig. 1a, b).
Fig. 1.
The block technique, and catheter fixation
Under ultrasound guidance, a bolus dose of 0.25% ropivacaine (0.4 ml/kg) was given. The spread of drug in the desired plane was confirmed with ultrasound and a catheter was inserted (Fig. 1c). The catheter was threaded 1 to 2 cm inside the interfascial plane and was secured with subcutaneous tunnelling (Fig. 1d); the patient was turned supine, reversed and extubated.
Multimodal analgesic regime was given in the post-operative period with IV paracetamol (15 mg/kg) 6th hourly, IV diclofenac (1.5 mg/kg) 8th hourly and ropivacaine 0.125% with fentanyl (1 microgram/ml at 5–7 ml/h) via SAP catheter. Numeric rating scores (NRS) for pain were recorded at 1, 2, 6, 12, 18, 24, 30, 36, 42 and 48 h post-operatively. Rescue analgesia was given with IV fentanyl infusion at 0.25 mcg/kg/h—if NRS was > 4 and the time of starting rescue analgesic was noted.
Results
A total of 10 patients were included and analysed. The diagnosis, surgical and analgesic details are summarised in Table 1.
Table 1.
Details of the surgery and analgesia in the patients
| Diagnosis | Surgery + surgical approach | Rib removal | Incision at ICS | SAP catheter at ICS | NRS* | NRS^ | Rescue analgesia | SAP catheter removed on POD | Special point |
|---|---|---|---|---|---|---|---|---|---|
| Osteosarcoma femur with lung metastasis | Left metastasectomy (open MSPLT) | No | 4th | 6th | 2.5 (1) | 2–3 | No | 3 | – |
| Osteosarcoma tibia with lung metastasis | Right metastasectomy + middle lobectomy (VATS assisted) | No | 4th, 6th, 8th | 5th | 1 (0.25) | 1–2 | No | 3 | – |
| Osteosarcoma femur with lung metastasis | Left metastasectomy (VATS assisted) | No | 5th, 7th, 8th | 6th | 2 (1) | 1–3 | No | 2 | – |
| Osteosarcoma tibia with lung metastasis | Left upper lobectomy (VATS assisted) | No | 4th, 7th, 8th | 6th | 2.5 (1.25) | 1–6 | Yes, at 24 h post-op | 3 | Pain at ICD (8th ICS) |
| Carcinoma lung | Right lower lobectomy (VATS to open MSPLT) | Yes | 5th | 6th | 2.5 (1.25) | 2–7 | Yes, at 18 h post-op | 2 | Two ribs removed |
| Carcinoma lung | Right upper lobectomy (open MSPLT) | No | 4th | 5th | 2.5 (1.25) | 2–7 | Yes, at 24 h post-op | 2 | Pain at ICD site (7th ICS) |
| Carcinoma lung | Left upper lobectomy (VATS to open MSPLT) | No | 4th, 7th, 8th | 6th | 2 (1.25) | 1–6 | Yes, at 1 h post-op | 0 | Poor spread of drug |
| Carcinoma lung | Left lower lobectomy (VATS to open MSPLT) | No | 4th, 7th, 8th | 6th | 2 (1) | 2–3 | No | 2 | – |
| Neuroendocrine tumour of lung | Left lower lobectomy (open MSPLT) | No | 5th | 7th | 2 (1) | 2–3 | No | 6 | – |
| Posterior mediastinal nerve sheath tumour | Mass excision (VATS to open MSPLT) | No | 4th, 7th | 6th | 2 (1.25) | 1–3 | No | 4 | – |
ICS intercostal space, POD post-operative day, MSPLT muscle-sparing posterolateral thoracotomy, VATS video-assisted thoracoscopy, NRS* numeric rating scores for pain expressed as median with interquartile range, NRS^ range of NRS
NRS for each patient are reported as median with interquartile range. Range of NRS, i.e. the maximum and minimum pain scores for each patient, are also shown (Table 1).
All patients were extubated immediately after surgery and were shifted to post-operative ICU for further observation and analgesic management. Four out of 10 patients required rescue analgesic in the form of fentanyl infusion. No technique-related adverse effect was seen in any patient.
Discussion
Analgesia for acute post-thoracotomy pain is a unique challenge for the anaesthetist as the pain is multifactorial in nature and adequate pain control plays an important part in the overall success of surgery and post-operative recovery.
Pain after thoracotomy may be due to rib injury or rib resection, injury to intercostal nerves due to prolonged resection and dislocation of costochondral joints. Pain after video-assisted thoracoscopy (VATS) may be less but is still a major concern in post-operative recovery [6]. Both TEA and PVB used as analgesia techniques in the majority of post-thoracotomy cases have their own adverse effects and limitations. TEA is known to have adverse effects like sympathetic block, hypotension, cardiovascular instability, urinary retention, etc. [7]. PVB, although considered to have lesser hemodynamic variations as compared to TEA, still has a risk of pleural puncture, pneumothorax, intrathecal or epidural spread of the drug; also, this technique may have a longer learning curve and may require multiple injections to obtain the desired analgesic effect [8].
Thus, the search for an effective regional analgesia technique as an alternative to TEA or PVB to alleviate post-thoracotomy pain continues. Recent literature shows that ultrasound-guided interfascial plane blocks like SAP block may be safer and a minimally invasive alternative technique to provide analgesia to the hemithorax [5].
SAP block was first described in 2013 by Blanco as an analgesic technique to block the ventral cutaneous rami of intercostal nerves to anaesthetise the hemithorax. The local anaesthetic injection was described in two planes: the ‘superficial plane’, i.e. the plane between the latissimus dorsi and serratus anterior; and the ‘deep plane’, i.e. the plane between the serratus anterior and external intercostal muscles [5]. This block is proposed to block thoracic intercostal nerves and cover dermatomal areas T2 to T9. This block is proposed to be safe and easy to perform. Pleural puncture and local anaesthetic systemic toxicity may be considered as potential complications with this block.
Blanco in his initial description of the block described better drug spread covering a wider area and giving a longer duration of analgesia in the superficial plane as compared to the deep plane [5]. However, recent literature points out that drug deposition in the superficial plane may be equally efficacious as compared to the deep plane for analgesia of hemithorax [9]. We chose the deeper plane, i.e. between the serratus anterior and external intercostals, as we found this plane easier to identify and inject the drug.
SAP block has been successfully used in randomised control trials to provide analgesia after breast surgeries [9]. However, its use as an analgesic technique after thoracotomies is limited to case reports [10]. We conducted this prospective case series to assess the efficacy of SAP block as a post-thoracotomy analgesic technique and to fine-tune the technique of SAP catheter insertion and its maintenance in the post-operative period.
Out of our 10 cases, we could easily thread in the catheter after giving a bolus dose in 9 of our patients. In one patient, we had difficulty in threading the catheter; this was a case of VATS-assisted left upper lobectomy converted to open thoracotomy. The patient was cachexic and had poor muscle mass. In this patient, we were unable to demarcate the muscle layers under ultrasound, the patient complained of pain and the drug mostly spread in the subcutaneous plane. The patient was shifted to IV fentanyl 1 h after surgery and the catheter was removed.
Overall, 4 patients required rescue analgesia. Out of these, 2 patients required fentanyl infusion at 24 h, one patient at 18 h and one at 1 h post-operatively. We hypothesise that intrathecal morphine used before induction of anaesthesia helped in intraoperative and immediate post-operative analgesia in our patients. Once the effect of intrathecal morphine wore off, SAP infusion acted as analgesic. Three out of 4 patients requiring rescue analgesia reported increased NRS at around 18–24 h after surgery which corresponds to the duration of action of single-dose intrathecal morphine thus indicating SAP block failure.
Two patients had pain at the intercostal drain (ICD) site at the 7th and 8th intercostal space, respectively. This could be attributed to continuous irritation if the intercostal nerves due to the ICD in situ. In the third patient, 2 ribs were excised and removed when VATS was converted into open thoracotomy for right lower lobectomy. In the 4th patient, drug spread was poor owing to poor muscle mass as described above.
We could maintain the SAP catheter post-operatively until post-operative day (POD) 2 in 4 patients, until POD 3 in 3 patients, until POD 4 in 1 patient and maximum until POD 6 in 1 patient. The catheter was removed on the day of surgery (POD 0) in the patient with poor muscle mass and drug spread into the subcutaneous plane. We attribute the successful maintenance of the catheter without dislodgement to the subcutaneous tunnelling approach employed to stabilise the catheter.
Overall, we found SAP block with continuous local anaesthetic infusion as a satisfactory technique of analgesia for post-thoracotomy pain. The added advantage with interfascial blocks like SAP block is that we can take the help of a surgeon at the time of thoracotomy closure to place a catheter in the superficial or deep SAP plane and use the catheter for post-operative analgesia. This can be done in centres where ultrasound is not available or the anaesthetist is not well versed with the technique of block. Thus, the surgeon may also play an important role in post-operative pain management after thoracotomy and improve their patient outcomes.
With this study, we can come to a conclusion that a SAP catheter can easily be maintained in the post-operative period and used for analgesia of the chest wall. However, future studies should work on comparing different doses of local anaesthetics and the addition of adjuvants like dexamethasone, ketamine, clonidine, etc., to see its effect on the duration and efficacy of analgesia in thoracotomy patients. Also, this technique should be compared to traditionally advocated regional techniques like TEA or PVB in well-designed prospective randomised trials.
Compliance with Ethical Standards
Written informed consent was taken from the patients.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gerner P. Post-thoracotomy pain management problems. Anesthesiol Clin. 2008;26(2):355–vii. doi: 10.1016/j.anclin.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenk M, Schug SA. Perioperative pain management after thoracotomy. Curr Opin Anaesthesiol. 2011;24(1):8–12. doi: 10.1097/ACO.0b013e3283414175. [DOI] [PubMed] [Google Scholar]
- 3.Joshi GP, Bonnet F, Shah R, Wilkinson RC, Camu F, Fischer B, Neugebauer EAM, Rawal N, Schug SA, Simanski C, Kehlet H. A systematic review of randomized trials evaluating regional techniques for postthoracotomy analgesia. Anesth Analg. 2008;107(3):1026–1040. doi: 10.1213/01.ane.0000333274.63501.ff. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Aldrete D, Candiotti KA, Janakiraman R, Rodriguez-Blanco YF. Trends and new evidence in the management of acute and chronic post-thoracotomy pain-an overview of the literature from 2005 to 2015. J Cardiothorac Vasc Anesth. 2016;30(3):762–772. doi: 10.1053/j.jvca.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 5.Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68(11):1107–1113. doi: 10.1111/anae.12344. [DOI] [PubMed] [Google Scholar]
- 6.Furrer M, Rechsteiner R, Eigenmann V, Signer C, Althaus U, Ris HB. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg. 1997;12(1):82–87. doi: 10.1016/S1010-7940(97)00105-X. [DOI] [PubMed] [Google Scholar]
- 7.Ng A, Swanevelder J. Pain relief after thoracotomy: is epidural analgesia the optimal technique? Br J Anaesth. 2007;98(2):159–162. doi: 10.1093/bja/ael360. [DOI] [PubMed] [Google Scholar]
- 8.Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade. Incidence of failed block and complications. Anaesthesia. 2001;56(12):1184–1188. doi: 10.1046/j.1365-2044.2001.02084-2.x. [DOI] [PubMed] [Google Scholar]
- 9.Abdallah, Faraj W., et al. Too deep or not too deep?: a propensity-matched comparison of the analgesic effects of a superficial versus deep serratus fascial plane block for ambulatory breast cancer surgery. (2018):480-487 [DOI] [PubMed]
- 10.Madabushi R, Tewari S, Gautam SK, Agarwal A, Agarwal A. Serratus anterior plane block: a new analgesic technique for post-thoracotomy pain. Pain Physician. 2015;18(3):E421–E424. [PubMed] [Google Scholar]