Abstract
This retrospective study compared the immediate post-operative short-term outcomes of Lateral Approach-Video Endoscopic Inguinal Lymphadenectomy (L-VEIL) and open surgery approach in patients with TNM stage N0 and N1 tumors. Inguinal lymphadenectomies performed for various TNM stage N0 and N1 cancers between January 2011 and December 2015 at a single center were analyzed by collecting data from operation theater records and case files. Mean blood loss, operative time, drain output, nodal yield, nodal positivity, and complications were analyzed as post-procedural outcomes. Among the 116 surgeries performed, 92 were open surgery and 24 were L-VEIL. Compared with open surgery, L-VEIL led to significantly lower blood loss (64.8 mL vs. 23.3 mL; p = 0.002), mean nodal yield (11.04 vs. 8.38; p = 0.001), and mean hospital stay (3.08 vs. 8 days; p < 0.001). However, the operative time was similar for both the groups (94.5 vs. 68.1 min; p = 0.08). Complications that were significantly low in L-VEIL were flap necrosis [RR 1.29; 95% CI (1.03–1.72); p < 0.001], wound dehiscence [RR 1.25; 95% CI (1.19–1.51); p = 0.005), wound infection [RR 1.34; 95% CI (1.19–1.51); p = 0.003], readmission [RR 1.3; 95% CI (1.17–1.44); p = 0.005], and re-surgery [p = 0.014]. Occurrence of complications such as lymphocele [RR 1.25; 95% CI (0.33–4.78); p = 0.5], lymphorrhea [RR 1.27; 95% CI (1.15–1.40); p = 0.5], and pedal edema [p = 0.2] were similar for both the approaches. L-VEIL was effective and safe compared with open inguinal block dissection in treatment of various TNM stage N0 and N1 urogenital and skin cancers.
Electronic supplementary material
The online version of this article (10.1007/s13193-019-00951-4) contains supplementary material, which is available to authorized users.
Keywords: Lateral approach-video endoscopic inguinal lymphadenectomy (L-VEIL), Inguinal block dissection, Vulvar cancer, Penile cancer, Melanoma
Introduction
Vulvar and penile cancer are rare diseases that account for < 5% of all female genital malignancies and ≤ 10% of male malignancies in developed countries. However, these cancers are very common in coastal areas of developing countries [1–3]. On the other hand, 132,000 melanoma skin cancers occur globally each year [4]. Involvement of inguinal lymph node is a prognostic factor in penile and vulvar cancers and in melanoma [5–7]. Therefore, surgical management of the inguinal lymph node and regional lymph node metastases of genitourinary and other cutaneous malignancies via inguinal lymphadenectomy [8, 9] has an accepted prognostic and therapeutic value [10]. With respect to urogenital cancers, inguinal lymphadenectomy is advised based on image evidences due to multiple reasons which include appearance of palpable lymph nodes due to failure of antibiotic therapy during post-operative follow-up, and risk factors for inguinal metastasis [10, 11]. Inguinal lymphadenectomy of N0 genitourinary tumors involves tissue dissection superficial to the deep fascia of the thigh and those surrounding the saphenofemoral junction in the femoral triangle, when it is performed electively. For N1 cases of melanoma and genital cancers, all tissue deep to the fossa ovalis and medial to the femoral vein, extending superiorly to the femoral canal, are resected [12]. N0 melanomas undergo sentinel node biopsy.
Conventional inguinal block dissection is associated with complications such as wound breakdown, wound infection (abscess, cellulitis, infected hematoma, sepsis), and large seromas which may need drainage and wound packing [13–15]. On the other hand, minimally invasive video endoscopic inguinal lymphadenectomy (VEIL) offers better results, reduces complications and morbidity, and improves cosmesis [10, 16–19]. Martin et al. reported similar overall survival between the open-surgery approach and VEIL but reduced morbidity with VEIL (47.5% vs. 80.0%; p = 0.002) in patients with metastatic melanoma [7]. Wang et al. demonstrated that operative time, lymph node harvest, and hospital stay did not differ significantly between the groups, however, operative blood loss, drainage tube removal time, incidence of complications, and suture removal time were significantly reduced by utilizing VEIL (p < 0.05) [20].
Inguinal lymphadenectomy is associated with wound-related complications and fails to maintain proper nodal clearance. To overcome these drawbacks, VEIL appears to be a promising technique. We performed VEIL using a lateral approach and assessed its efficacy and safety by retrospectively comparing the surgical outcomes and the complications associated with lateral approach video endoscopic inguinal lymphadenectomy (L-VEIL) and open inguinal block dissection in patients with various primary tumors.
Patients and Methods
Patients
This 4-year, single-center retrospective study was conducted from January 2011 to December 2015. Data were collected from operation theater records and case files of patients who underwent prophylactic and therapeutic inguinal lymphadenectomies for various cancers. Oncologic outcomes after L-VEIL or open inguinal block dissection were compared.
Patients were included in the study if they had: (i) tumor with clinical stage N0 and N1 (smaller than 1 cm size); (ii) any operable primary tumor of the penis, vulva, melanoma, and others including squamous cell carcinoma (SCC) of the leg, malignancy of unknown primary (MUO) or gluteal SCC. Patients were excluded if they had clinically large (> 1 cm) lymph node, skin involvement by node, or distant metastatic tumors.
The study was approved by the ethics committee of Kidwai Memorial Institute of Oncology, Bangalore. The study was conducted in accordance with the national guidelines and the Declaration of Helsinki.
Surgical Procedure
Open surgery was performed according to the standard techniques with horizontal and S-shaped open inguinal block dissection carried out as described previously [13–15, 21]. Briefly, the superficial and deep nodes within the triangle bounded by the inguinal ligament superiorly, the sartorius muscle laterally, and the adductor longus muscle medially are removed, while skin flaps contain all the tissue present above the Scarpa fascia.
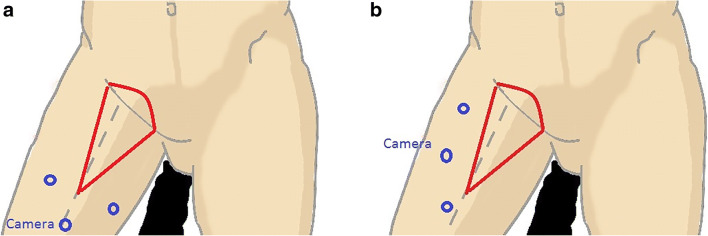
L-VEIL is a modification of the VEIL described by other authors which is performed with an axial camera placement (Fig. 1a) [17–21]. In L-VEIL, all the ports were placed on the lateral aspect of the thigh (Fig. 1b). The procedure involved marking of the femoral triangle on the thigh and placing the video monitor contralaterally to the operated side near the patient’s waist. The extent of surgery was similar to the open procedure. After administering general anesthesia, a 15-mm vertical incision was made 2 cm lateral to the lateral boundary of the femoral triangle, and deepened up to the facia lata. Space was developed on either side with a blunt and sharp dissection and two 5-mm ports were inserted on either side of the primary incision, parallel to the lateral border of the femoral triangle. A 10- or 12-mm port was placed in the primary incision and secured with silk suture to attain air lock. This was followed by expansion of the working space with gas insufflation (pressure 10 mmHg and flow of 10 l/min), separation of the skin flap between the fascia scarpa and camper. The dissection of the tissue was performed using ultrasonic dissector or bipolar tissue sealers (based on availability) starting from lateral to medial approach by cutting the facia over sartorius muscle and then rolling the tissue medially. The long saphenous vein at the apex of the femoral triangle is secured with clips, and the long saphenofemoral junction is secured. The surgical specimen was then removed through the 15-mm incision using specimen retrieval bag and vacuum drained through the 5-mm orifice. The large incisions were sutured to complete the procedure.
Fig. 1.
a Standard video endoscopic inguinal lymphadenectomy port placement, b Lateral approach-video endoscopic inguinal lymphadenectomy port placement
Study Outcomes
The primary clinical outcomes that were evaluated were mean blood loss, operative time, drain output, nodal yield, and nodal positivity. The secondary outcomes included post-surgical complications in patients after open surgery or L-VEIL.
Statistical Analysis
Statistical analysis was performed using the Epi Info7 software for Windows (Center for Disease Control and Prevention (CDC); Atlanta, GA, USA). Mean and median values were used to define numerical variables. Bivariate statistical testing was performed using two-sided chi-square tests while non-parametric statistical associations were examined by the Mann-Whitney test. Bartlett’s test and Wilcoxon Two-Sample Test (Kruskal-Wallis test for two groups) were used for inequality of population variances and p < 0.05 was considered significant.
Results
Baseline Characteristics of the Patients
Demographic characteristics of the patients are described in Table 1. A total of 84 patients with various primary tumors were identified (mean age 53.44 ± 14.31 years). Of the 84, 43 patients had history of tobacco consumption while 31 suffered from comorbidities such as type 2 diabetes mellitus (n = 18) and hypertension (n = 13). Majority of the patients included had ECOG grade 0 (70.69%). From the 84 patients, 32 needed bilateral inguinal block dissections and the rest unilateral inguinal block dissections. For one patient with penile cancer, open surgery was performed on right side for large lymph nodes involving skin and L-VEIL on the left side. For other patients undergoing bilateral dissection, the procedure followed was same for both sides. Thus, the total number of inguinal block dissections performed was 116. Further analysis of the data was performed based on the 116 instances of block dissections and not the number of patients (Supplementary Fig. S1).
Table 1.
Demographic and baseline details of patients with primary tumor
| Characteristics | N = 84 |
|---|---|
| Age (years) | |
| Mean ± SD | 53.44 ± 14.31 |
| Median (range) | 55 (28–85) |
| Male:Female | 50:66 (3:4) |
| Tobacco consumption, n (%) | |
| No | 73 (62.93) |
| Yes | 43 (37.07) |
| ECOG PSa, n (%) | |
| Grade 0 | 82 (70.69) |
| Grade 1 | 29 (25.00) |
| Grade 2 | 05 (04.31) |
| Hb levelsb (mg/dL), n (%) | |
| 9–11 | 7 (14) males, 14 (21.21) females |
| 11–13 | 19 (38) males, 30 (45.45) females |
| > 13 | 24 (48) males, 22, (33.33) females |
| Comorbidities only with open casesc, n (%) | |
| T2DM | 18 (15.52) |
| Hypertension (HTN) | 13 (10.54) |
aECOG PS: Eastern Cooperative Oncology Group scale of Performance status
bHB levels: Hemoglobin levels
cNo DM and HTN was found in patients undergoing VEIL
The distribution of primary tumors (Table 2) showed 39, 46, 27, 2, and 2 cases of penile, vulvar, melanoma, SCC leg, and MUO or gluteal SCC, respectively. In the 116 block dissections, 92 (79.4%) were treated with open surgical approach whereas 24 (20.69%) underwent L-VEIL. Median follow-up was 6 and 9 months after open surgery and L-VEIL, respectively.
Table 2.
Distribution of the primary tumor and surgery
| Distribution Parameter | na = 116 |
|---|---|
| Primary tumor for inguinal dissection, n (%) | |
| Penis | 39 (33.62) |
| Melanoma | 27 (23.27) |
| Vulva | 46 (39.66) |
| SCC leg | 02 (1.72) |
| Others (MUOc & Gluteal SCCb) | 02 (1.72) |
| Surgical approach, n (%) | |
| Open | 92 (79.4%) |
| L-VEIL | 24 (20.69) |
| Intent of surgery, n (%) | |
| Elective | 34 (37%) open case, 19 (79.2%) L-VEILd |
| Therapeutic | 58 (63%) open case, 5 (20.8%) L-VEIL |
| Incision in open therapy, n (%) | |
| Horizontal | 60 (65.21) |
| S-shaped | 32 (34.78) |
an = total number of inguinal lymphadenectomies
bSCC: Squamous cell carcinoma
cMUO: Malignancy of unknown origin
dL-VEIL: Lateral video endoscopic inguinal lymphadenectomy
Peri-operative and Pathological Outcomes
Peri-operative/surgical outcome was evaluated in terms of blood loss which was significantly lower in the L-VEIL group compared with open surgery group (23.3 mL vs. 64.8 mL; p = 0.002). However, the operative time was non-significantly longer in the L-VEIL group (94.5 min vs. 68.1 min; p = 0.08). Pathological evaluation showed that mean nodal yield and nodal positivity were significantly better in the L-VEIL group compared with the open surgery group (p = 0.001 and < 0.001, respectively). Mean duration of hospital stay was significantly longer in the open surgery group compared with the L-VEIL group (8 days vs. 3.08 days; p < 0.001) (Table 3). There was no conversion of the decided procedure (L-VEIL or open surgery) during the surgery, i.e., conversion rate was 0%.
Table 3.
Operative outcomes and pathological results
| Parameters | Open | L-VEIL | p |
|---|---|---|---|
| Blood loss (mL), mean (range) | 64.8 (30–150) | 23.3 (10–55) | 0.002 |
| Operative time (min), mean (range) | 68.1 (50–95) | 94.5 (60–125) | 0.08 |
| Nodal yield, mean ± SD | 8.38 ± 3.24 | 11.04 ± 4.17 | 0.001 |
| Nodal positivity, mean ± SD | 1.69 ± 2.04 | 0.13 ± 0.34 | < 0.001 |
| Hospital stay (days), mean | 8 ± 6.28 | 3.08 ± 0.775 | < 0.001 |
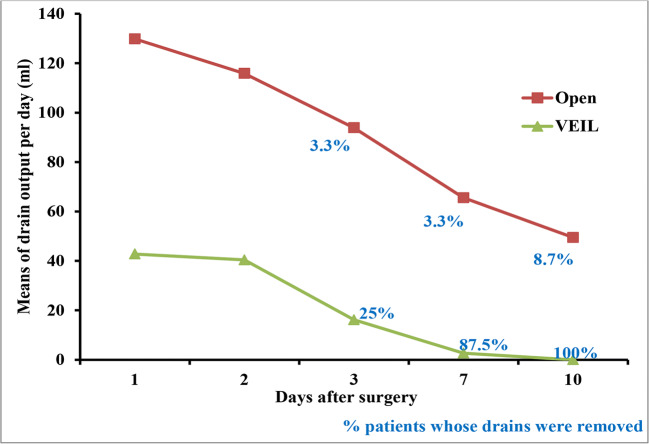
Post-operative drain output in patients who underwent open surgery was significantly higher on all the days when compared with the VEIL cases (p < 0.001). The percentage of patients whose drains were removed was higher in the L-VEIL group than the open surgery group on the 3rd (25% vs. 3.3%), 7th (87.5% vs. 3.3%), and 10th days (100% vs. 8.7%) (Fig. 2).
Fig. 2.
Post-operative drain output (after 10 days of surgery) % represent the percentage of patients whose drains were removed by respective days. None of the drains were removed among both the groups on postoperative days 1 and 2. p < 0.0001 at all points for drain output
Post-surgical Complications
Risk of all the post-procedural complications was higher in the open surgery group compared with the L-VEIL group (Supplementary Table S1). The open surgery group had significantly higher complications of flap necrosis [wound grade 1; 43 vs. 1; RR 1.29; 95% CI (1.03–1.72), p = <0.001], wound dehiscence (wound grade 2), and wound infection (wound grade 3) [22 vs. 0; RR 1.34; 95% CI (1.19–1.51), p = 0.005 and p = 0.003) respectively] when compared with L-VEIL. Lymphocele, lymphorrhea, and pedal edema were insignificant for both open and L-VEIL cases. Readmissions for associated complications were significantly higher in the open surgery group than in the L-VEIL group [12 vs. 0; RR 1.3; 95% CI (1.17–1.44) p = 0.005]. Survival analysis was not performed as the groups had heterogenous primaries with different survival patterns and would not have had any scientific value.
Discussion
Lymphadenectomy has therapeutic potential and aims to completely remove all lymph nodes associated with the drainage basin especially those in the inguinal region as they are considered as first-line regional nodes [21]. Management of lymph nodes in penile cancer, vulvar cancer, and melanoma involves: (1) lymphadenectomy, (2) lymphatic drainage of clinically palpable inguinal nodes, (3) removal of superficial and deep inguinal nodes and positive sentinel lymph node, (4) pelvic lymphadenectomy, (5) chemotherapy, (6) optimizing the time of lymphadenectomy depending on the TNM (tumor, node, metastasis) stage, and (7) radiotherapy [22–25]. Many a times simultaneous bilateral endoscopic inguinal lymphadenectomy is performed as a technically feasible and efficient surgical approach to minimize the overall anesthetic and operative time [26]. Previous studies reporting efficacy of VEIL have not utilized statistically robust method for measuring its efficacy and safety [2, 11, 27, 28]. Also, none of these studies has presented a direct comparison of outcomes in patients undergoing VEIL and those receiving open surgery. To the best of our knowledge, ours is the first study that evaluated efficacy and safety of VEIL over open surgery with statistical measure as risk ratio (for safety).
The standard VEIL described by other authors involves placement of the camera port axillary along the long axis of the thigh, and the two operating ports on either side of the camera port [17–20]. This port position has two major problems with respect to ergonomics. Firstly, the camera movement is restricted by the thigh itself and secondly, the operating surgeon has to extend the hand over the thigh to reach and operate the contralaterally placed instrument. In this study, a modification of VEIL was used for surgery where all the ports were shifted to the lateral aspect of the thigh for ergonomic reasons. The port positions for L-VEIL avoid both of these problems as all the ports have ample movement and the operating surgeon’s position is ergonomic.
This retrospective study demonstrated significantly increased blood loss, mean nodal positivity, and mean hospital stay in patients who underwent open inguinal block dissection as compared to VEIL as an elective or therapeutic intent to surgery for treatment of various cancers. The open procedure showed lower mean nodal yield and higher drain output than L-VEIL. Operative time was higher with L-VEIL, albeit non-significantly. These results coincide with similar studies by Martin et al., Wang et al., and Tobias-Machado et al. [7, 19, 20]. Operative time reported in these studies was significantly higher with L-VEIL than for the open procedure (181.3 vs. 155.9 min, p = 0.008) [7]. In other studies as well, the operative time was higher with VEIL and ranged from 80 to 110 min in open case, 90–130 min or 90–180 min in VEIL cases [16, 19, 27, 29]. However, the operative time required in this study with L-VEIL was lesser than the operative time reported with conventional central VEIL in previous studies (Tobias-Machado et al. 120 min; Dhangar et al.: range of 200–240 min; Pahwa et al.: range of 120–180 min) [16, 27, 30]. Nevertheless, it is not clear if L-VEIL requires lesser operative time than robotic-assisted VEIL (R-VEIL) due to varied results reported by previous studies (R-VEIL mean operative time: Waigankar S., et al.110 min; Jain et al. 69.3 min) [31, 32].
Mean blood loss was significantly reduced with L-VEIL in this study which was similar to that reported with R-VEIL by Jain et al. (23.3 ml [range10–55] vs. 30 ml [range 50–250 ml]), but lower than the blood loss reported by Wang et al. who used central VEIL (68.44 ± 42.19 ml, p < 0.05) [20]. L-VEIL minimizes blood loss and chances of severe infection as it enables the surgeon to make incision (port site) away from the femoral blood vessel, whereas in open surgery, in the incision lies in close proximity of the femoral vessels. Because of this, L-VEIL procedure does not require a sartorius flap that is usually needed with the open procedure. Nonetheless, there are operative concerns with both VEIL and open surgery in order to minimize pre and post-operative complications and morbidity, and maximize the benefits of minimally invasive technique namely initial blunt dissection of the correct plane (under Scarpa’s fascia) of dissection [33], by maintaining some fat under the skin [34]. Moreover, an experienced hand is a requirement that contributes to the increased success of VEIL over open surgery. Emphasis is laid on three more steps in VEIL: distal lymphatic tissue ligation at the femoral triangle vertex, proximal control of visualized lymphatics, and ligation of the proximal portion of the lymphatic tissue at the deep portion of the femoral channel using clips or tissue sealers [34]. The authors believe that the bipolar tissue sealers used in VEIL contribute to the reduced drain and early removal of drains [35, 36].
Drains are usually removed to discharge the patient earlier after the surgery, especially when the drainage is < 50 mL/day—as was observed with L-VEIL [21]. We reported a drainage output of < 50 mL from day 1 for L-VEIL, which was similar to another study [16]. Although VEIL requires higher operative time, it also coincides with higher mean nodal yield than open surgery, as observed in this and other studies [16, 18]. With an operative time of 130 min for VEIL and nearly 90 min for open surgery, the nodal yield was 7 for open and 8 nodes for VEIL [18] and in another study it was 9.7 for open and 10.8 for VEIL (p = 0.4) [16]. In contrast, Yadav et al. reported mean numbers of lymph nodes removed as 7.6 with open surgery (operative time 92.35 min) and 8.3 with central VEIL (operative time 7.6 min) [37]. Though it is unclear whether L-VEIL can provide better nodal yield than central VEIL as the results reported in previous studies ranged from 7.1 to 16, central VEIL led to lower nodal yield in studies by Sotelo et al. (mean 9; range 4–15) [38] and Schwentner et al. (7.1 ± 2.9), [39] but higher nodal yield in those by Mathevet et al. [40] and Xu et al. [41] in comparison to that observed with L-VEIL (11.04 ± 4.17). Observations made in other studies utilizing central VEIL (range 10.5–12.3) are comparable to our findings [9, 12, 16, 42].
Mean nodal yield of L-VEIL of our study is similar to the findings demonstrated by Martin et al. where in a prospective evaluation of oncological adequacy of robotic-assisted VEIL in eight patients, the median blood loss was 100 mL in patients with pN0 disease and the number of lymph nodes removed were ranging from 5 to 21 on the left and 6–17 on the right side [33]. Inguinal lymphadenectomy includes inguinofemoral, superficial groin, and groin dissections [29]. In a retrospective study by Li et al., in 21 patients with gynecological malignancies who underwent endoscopic groin lymphadenectomy, the median operative time was 210 min (including the dissection of both groins and other procedures), the median blood loss was 200 mL, and the median number of retrieved lymph nodes was 13 (range, 8–26), and all of these were negative to metastasis [43]. A decrease in length of hospital stay along with equivalent or superior lymph node retrieval was demonstrated with VEIL over open cases in a review involving 67 patients undergoing 94 procedures for metastatic melanoma, cutaneous malignancies of the genitourinary area, and lower extremities [29].
Lymphocele is a common complication of axillary and inguinal lymphadenectomy which can be treated by lymph collections by excision, open or percutaneous drainage, and sclerosing agents [44]. The complication of lymphocele, lymphorrhea, and pedal edema was insignificant with both open surgery and L-VEIL in this study whereas readmission and re-surgery for wound-related problems was required only in open cases. There was a significant increase in wound grades 1, 2, and 3 (flap necrosis, wound dehiscence, and wound infection, respectively) with the open inguinal block dissection. The extent of these complications is comparable to other comparative studies [16, 18–20, 45] which have reported a reduction or absence of the complications with VEIL. Tobias-machado et al. reported insignificant complications such as hematoma and lymphatic complications (VEIL 10% vs. Open 20%; p = 0.58) and significant complications such as skin-related events (VEIL 5% vs. Open 50%, p = 0.009) [16]. The same authors also reported that patients undergoing VEIL experienced only lymphorrhea and hematoma whereas patients who underwent open-surgery experienced cutaneous necrosis, lymphedema, epidermolysis, lymphocele, cellulitis, and incision infection [19]. In a case report of bilateral VEIL, there were no intraoperative complications, however, on skin necrosis was observed only on the left side [18]. Wang et al. reported zero cases of wound grade 3 and 4 complications in both the types of surgery whereas wound grade 1 was 15.79% for VEIL vs. 38.1% for open (p = 0.12) and wound grade 2 was 5.26% for VEIL and 23.81% for open cases (p = 0.10) [20]. Kumar et al. reported no intraoperative complications in either surgical approach. The wound complication rate was significantly lower in the VEIL group than in the open group (6% vs. 68%) with reduced length of stay after VEIL (4.8 days, p = 0.001). However, lymphocele rates were similar in both the cases [45].
The strength of this study is the statistical evaluation of complications in terms of risk ratio. The limitations include: (i) evaluation of the endpoints immediately after the surgery with a short follow-up period of 6–9 months and (ii) small patient size.
In conclusion, this study confirmed the effectiveness and safety of L-VEIL for primary penile, vulvar tumors, melanoma, and other urogenital malignancies over open inguinal block dissection. However, more confirmatory trials with larger population size and longer duration of follow-up are warranted for confirming the findings. Future studies comparing results obtained with L-VEIL with that of conventional central VEIL are warranted to confirm if L-VEIL approach can provide better surgical outcomes.
Electronic supplementary material
Strobe flow chart (PNG 82 kb)
(DOCX 14 kb)
Acknowledgments
The authors declare that they have no conflict of interest.
Author Contributions
Study conceptualization and design: NSP, PH, GJ; data acquisition and analysis: NSP, PH, GJ, DK, CS, VM; manuscript writing and reviewing: NSP, PH, GJ; guarantor: NSP, PH, GJ, DK, CS, VM
Footnotes
Key Messages
This study for statistically compared effectiveness and safety of L-VEIL with open inguinal block dissection for treatment of TNM stage N0 and N1 urogenital and skin cancers and reported L-VEIL to be the better approach.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang H, Li L, Yao D, Li F, Zhang J, Yang Z. Preliminary experience of performing a video endoscopic inguinal lymphadenectomy using a hypogastric subcutaneous approach in patients with vulvar cancer. Oncol Lett. 2015;9:752–756. doi: 10.3892/ol.2014.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudhir R, Krishnappa RS, Khanna S, Sekon R, Koul R. Video endoscopic inguinal lymphadenectomy (VEIL): minimally invasive radical inguinal lymphadenectomy technique. Indian J Surg Oncol. 2012;3:257–261. doi: 10.1007/s13193-012-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romanelli P, Nishimoto R, Suarez R, Decia R, Abreu D, Machado M, Arroyo C, Campolo H, Campos E, Carlos AS, Tobias-Machado M. Video endoscopic inguinal lymphadenectomy: surgical and oncological results. Actas Urol Esp. 2013;37:305–310. doi: 10.1016/j.acuro.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Skin cancers. https://www.who.int/uv/faq/skincancer/en/index1.html. Accessed 10 Jun 2019
- 5.Carlos AS, Romanelli P, Nishimoto R, Montoya LM, Juliano CAB, da Costa RMM, Pompeo ACL, Tobias-Machado M. Expanded criteria for video endoscopic inguinal lymphadenectomy (VEIL) in penile cancer: palpable lymph nodes. Int Braz J Urol. 2013;39:893–894. doi: 10.1590/S1677-5538.IBJU.2013.06.17. [DOI] [PubMed] [Google Scholar]
- 6.Wu Q, Gong Z, Zhao Y, Sun Z, Shao H, Dai Z, Qu J, Xu H. Video endoscopic inguinal lymphadenectomy via 3-incision lateral approach for vulvar cancers: our preliminary outcome of 37 cases. Int J Gynecol Cancer. 2016;26:1706–1711. doi: 10.1097/IGC.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 7.Martin BM, Etra JW, Russell MC, Rizzo M, Kooby DA, Staley CA, Master VA, Delman KA. Oncologic outcomes of patients undergoing videoscopic inguinal lymphadenectomy for metastatic melanoma. J Am Coll Surg. 2014;218:620–626. doi: 10.1016/j.jamcollsurg.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Sommariva A, Pasquali S, Rossi CR. Video endoscopic inguinal lymphadenectomy for lymph node metastasis from solid tumors. Eur J Surg Oncol. 2015;41:274–281. doi: 10.1016/j.ejso.2014.10.064. [DOI] [PubMed] [Google Scholar]
- 9.Abbott AM, Grotz TE, Rueth NM, Hernandez Irizarry RC, Tuttle TM, Jakub JW. Minimally invasive inguinal lymph node dissection (MILND) for melanoma: experience from two academic centers. Ann Surg Oncol. 2013;20:340–345. doi: 10.1245/s10434-012-2545-6. [DOI] [PubMed] [Google Scholar]
- 10.Tobias-Machado M, Tavares A, Molina WR, Forseto PH, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy (VEIL): minimally invasive resection of inguinal lymph nodes. Int Braz J Urol. 2006;32:316–321. doi: 10.1590/s1677-55382006000300012. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Lu Y, Yao D-S. Feasibility and safety of video endoscopic inguinal lymphadenectomy in vulvar cancer: a systematic review. PLoS One. 2015;10:e0140873. doi: 10.1371/journal.pone.0140873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delman KA, Kooby DA, Rizzo M, Ogan K, Master V. Initial experience with videoscopic inguinal lymphadenectomy. Ann Surg Oncol. 2011;18:977–982. doi: 10.1245/s10434-010-1490-5. [DOI] [PubMed] [Google Scholar]
- 13.Koifman L, Hampl D, Koifman N, Vides AJ, Ornellas AA. Radical open inguinal lymphadenectomy for penile carcinoma: surgical technique, early complications and late outcomes. J Urol. 2013;190:2086–2092. doi: 10.1016/j.juro.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Vordermark JS, Jones BM, Harrison DH. Surgical approaches to block dissection of the inguinal lymph nodes. Br J Plast Surg. 1985;38:321–325. doi: 10.1016/0007-1226(85)90235-8. [DOI] [PubMed] [Google Scholar]
- 15.Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: the M.D. Anderson Cancer Center experience. J Urol. 2002;167:1638–1642. [PubMed] [Google Scholar]
- 16.Tobias-Machado M, Tavares A, Silva MNR, Molina WR, Forseto PH, Juliano RV, Wroclawski ER. Can video endoscopic inguinal lymphadenectomy achieve a lower morbidity than open lymph node dissection in penile cancer patients? J Endourol. 2008;22:1687–1691. doi: 10.1089/end.2007.0386. [DOI] [PubMed] [Google Scholar]
- 17.Tobias-Machado M, Correa WF, Reis LO, Starling ES, de Castro Neves O, Juliano RV, Pompeo ACL. Single-site video endoscopic inguinal lymphadenectomy: initial report. J Endourol. 2011;25:607–610. doi: 10.1089/end.2010.0269. [DOI] [PubMed] [Google Scholar]
- 18.Tobias-Machado M, Tavares A, Molina WR, Zambon JP, Medina JA, Forseto PH, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy (VEIL): initial case report and comparison with open radical procedure. Arch Esp Urol. 2006;59:849–852. doi: 10.4321/s0004-06142006000800020. [DOI] [PubMed] [Google Scholar]
- 19.Tobias-Machado M, Tavares A, Ornellas AA, Molina WR, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy: a new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J Urol. 2007;177:953–957. doi: 10.1016/j.juro.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Du P, Tang X, An C, Zhang N, Yang Y. Comparison of efficiency of video endoscopy and open inguinal lymph node dissection. Anticancer Res. 2017;37:4623–4628. doi: 10.21873/anticanres.11863. [DOI] [PubMed] [Google Scholar]
- 21.Horenblas S. Lymphadenectomy for squamous cell carcinoma of the penis. Part 2: the role and technique of lymph node dissection. BJU Int. 2001;88:473–483. doi: 10.1046/j.1464-410x.2001.00379.x. [DOI] [PubMed] [Google Scholar]
- 22.Leveridge M, Siemens DR, Morash C. What next? Managing lymph nodes in men with penile cancer. Can Urol Assoc J. 2008;2:525–531. doi: 10.5489/cuaj.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark PE, Spiess PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, Inman BA, Kuban DA, Kuzel TM, Lele SM, Michalski J, Pagliaro L, Pal SK, Patterson A, Plimack ER, Pohar KS, Porter MP, Richie JP, Sexton WJ, Shipley WU, Small EJ, Trump DL, Wile G, Wilson TG, Dwyer M, Ho M, National Comprehensive Cancer Network Penile cancer: clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2013;11:594–615. doi: 10.6004/jnccn.2013.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoffels I, Dissemond J, Schulz A, Hillen U, Schadendorf D, Klode J. Reliability and cost-effectiveness of complete lymph node dissection under tumescent local anaesthesia vs. general anaesthesia: a retrospective analysis in patients with malignant melanoma AJCC stage III. J Eur Acad Dermatol Venereol. 2012;26:200–206. doi: 10.1111/j.1468-3083.2011.04036.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim HS, Lee M. Video endoscopic inguinal lymphadenectomy (VEIL) for vulvar cancer. Gynecol Oncol. 2017;144:225–226. doi: 10.1016/j.ygyno.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 26.Herrel LA, Butterworth RM, Jafri SM, Ying C, Delman KA, Kooby DA, Ogan KE, Canter DJ, Master VA. Bilateral endoscopic inguinofemoral lymphadenectomy using simultaneous carbon dioxide insufflation: an initial report of a novel approach. Can J Urol. 2012;19:6306–6309. [PubMed] [Google Scholar]
- 27.Pahwa HS, Misra S, Kumar A, Kumar V, Agarwal A, Srivastava R. Video endoscopic inguinal lymphadenectomy (VEIL)--a prospective critical perioperative assessment of feasibility and morbidity with points of technique in penile carcinoma. World J Surg Oncol. 2013;11:42. doi: 10.1186/1477-7819-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhari R, Khant S, Patel D. Video endoscopic inguinal lymphadenectomy for radical management of inguinal nodes in patients with penile squamous cell carcinoma. Urol Ann. 2016;8:281–285. doi: 10.4103/0974-7796.184883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin BM, Master VA, Delman KA. Videoscopic inguinal lymphadenectomy for metastatic melanoma. Cancer Control. 2013;20:255–260. doi: 10.1177/107327481302000403. [DOI] [PubMed] [Google Scholar]
- 30.Dhangar SP, Kothawala I, Kumar A, et al (2016) Video Endoscopic Inguinal Lymphadenectomy (Veil): Experience from A Single Institute Study. Paripex-Indian J Res 5:4
- 31.Waigankar S, Yuvaraja T, Pednekar A, Wagaskar V. Surgical and oncological outcomes after robotic-video endoscopic inguinal lymphadenectomy in cN0/cN1 groins: single institute series. Eur Urol Suppl. 2017;16:e2439. [Google Scholar]
- 32.Jain V, Sekhon R, Giri S, Hassan N, Batra K, Shah SH, Rawal S. Robotic-assisted video endoscopic inguinal lymphadenectomy in carcinoma vulva: our experiences and intermediate results. Int J Gynecol Cancer. 2017;27:159–165. doi: 10.1097/IGC.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 33.Matin SF, Cormier JN, Ward JF, Pisters LL, Wood CG, Dinney CPN, Royal RE, Huang X, Pettaway CA. Phase 1 prospective evaluation of the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy in patients with penile carcinoma. BJU Int. 2013;111:1068–1074. doi: 10.1111/j.1464-410X.2012.11729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stievano Carlos A, Reis LO, Tobias-Machado M. Re: video-assisted left inguinal lymphadenectomy for penile cancer. Int Braz J Urol. 2012;38:567–568. doi: 10.1590/s1677-55382012000400020. [DOI] [PubMed] [Google Scholar]
- 35.Longo F, Crucitti P, Quintarelli F, Rocco R, Mangiameli G, Rocco G. Bipolar sealing devices in video-assisted thoracic surgery. J Vis Surg. 2017;3:13. doi: 10.21037/jovs.2017.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santini M, Vicidomini G, Baldi A, Gallo G, Laperuta P, Busiello L, Di Marino MP, Pastore V. Use of an electrothermal bipolar tissue sealing system in lung surgery. Eur J Cardio-Thorac Surg. 2006;29:226–230. doi: 10.1016/j.ejcts.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 37.Yadav SS, Tomar V, Bhattar R, Jha AK, Priyadarshi S. Video endoscopic inguinal lymphadenectomy vs open inguinal lymphadenectomy for carcinoma penis: expanding role and comparison of outcomes. Urology. 2018;113:79–84. doi: 10.1016/j.urology.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Sotelo R, Sanchez-Salas R, Clavijo R. Endoscopic inguinal lymph node dissection for penile carcinoma: the developing of a novel technique. World J Urol. 2009;27:213–219. doi: 10.1007/s00345-009-0372-6. [DOI] [PubMed] [Google Scholar]
- 39.Schwentner C, Todenhöfer T, Seibold J, Alloussi SH, Mischinger J, Aufderklamm S, Stenzl A, Gakis G. Endoscopic inguinofemoral lymphadenectomy--extended follow-up. J Endourol. 2013;27:497–503. doi: 10.1089/end.2012.0489. [DOI] [PubMed] [Google Scholar]
- 40.Mathevet P, Roy M, Dargent D. Inguinoscopy or video-endoscopic inguinal lymph node dissection.http://www.thetrocar.net. Accessed 10 Jun 2019
- 41.Xu H, Wang D, Wang Y, Li Y, Chen Y, Liang Z. Endoscopic inguinal lymphadenectomy with a novel abdominal approach to vulvar cancer: description of technique and surgical outcome. J Minim Invasive Gynecol. 2011;18:644–650. doi: 10.1016/j.jmig.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Zhou X-L, Zhang J-F, Zhang J-F, Zhou S-J, Yuan X-Q. Endoscopic inguinal lymphadenectomy for penile carcinoma and genital malignancy: a preliminary report. J Endourol. 2013;27:657–661. doi: 10.1089/end.2012.0437. [DOI] [PubMed] [Google Scholar]
- 43.Li M, Wang S, Guo S, Zhang Z. Endoscopic groin lymphadenectomy with a thigh approach to gynecologic malignancies: a retrospective study with 5-year experience. Int J Gynecol Cancer. 2015;25:325–330. doi: 10.1097/IGC.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 44.Crabtree JH, Fishman A. Videoscopic argon beam coagulator treatment of large persistent lymphocele. J Laparoendosc Adv Surg Tech A. 2000;10:63–65. doi: 10.1089/lap.2000.10.63. [DOI] [PubMed] [Google Scholar]
- 45.Kumar V, Sethia KK. Prospective study comparing video-endoscopic radical inguinal lymph node dissection (VEILND) with open radical ILND (OILND) for penile cancer over an 8-year period. BJU Int. 2017;119:530–534. doi: 10.1111/bju.13660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strobe flow chart (PNG 82 kb)
(DOCX 14 kb)