Abstract
Purpose
This study was undertaken to compare semen quality, hormonal status, and social factors in transgender women seeking fertility preservation with those of fertile cisgender men. Long-range goals are to establish standard practice measures ensuring optimum semen quality for cryopreservation and fertility preservation in transgender women.
Methods
This is a case-control study carried out at an academic medical center. Cases are transgender women seeking fertility preservation prior to initiation of hormone therapy. Controls are cisgender men recently fathering a child. All participants completed the Depression Anxiety Stress Scales 21 survey and additional survey questions related to personal behaviors. Complete semen analysis was carried out in a clinical andrology laboratory according to WHO guidelines, 5th edition. Serum follicle stimulating hormone, estradiol, and testosterone were measured at the time of semen analysis.
Results
Sperm concentration, total sperm per ejaculate, total motile sperm, volume, and normal sperm morphology were significantly lower in transgender females compared with fertile cisgender men. Other measures of semen parameters and hormone concentrations were not different between groups. Survey results indicated transgender women were more likely to have symptoms of depression, anxiety, and stress and utilize tucking and tight undergarments, compared with controls; however, both groups reported similar numbers of ejaculations per week.
Conclusions
Although semen parameters were low, cryopreservation of sperm prior to hormone therapy is a viable fertility preservation option for most transgender women. The etiology of the differences in semen parameters is not known. Enhanced education related to personal behaviors or treatment to reduce effects of stressors prior to cryopreservation may improve future fertility potential.
Keywords: Transgender, Semen analysis, Fertility preservation
Introduction
In 2011, the Institute of Medicine published a report concluding that little is known regarding the health needs of transgender (LGBT) persons in the USA and launched a strategic plan to advance research on the health of sexual and gender minorities [1]. Transgender is an umbrella term for those individuals whose gender identity or expression is different than that typically associated with their assigned sex at birth. Gender dysphoria refers to the distress that is caused by this discrepancy and the associated gender role and sex characteristics [2]. Treatment is available to assist people with such distress to explore their gender identity and find a gender role that is comfortable for them. Some transgender people may use cross-sex hormones and/or undergo surgery to remove or alter their reproductive organs. These medical therapies limit transgender individuals’ fertility options. According to the World Professional Association of Transgender Health (WPATH), the American Society for Reproductive Medicine and the Endocrine Society appropriate care of transgender individuals involves a discussion about reproductive options, including fertility preservation, before the initiation of medical and or surgical treatment for gender dysphoria [3, 4] (https://www.endocrine.org/-/media/endosociety/files/advocacy-and-outreach/position-statements/2017/position_statement_transgender_health.pdf?la=en). Reproductive options include cryopreservation of gametes for future use as a means of fertility preservation. Transgender women (male-to-female transgender) have the option to cryopreserve spermatozoa before undergoing gender-affirming treatment. However, little data is currently available defining the semen characteristics in transgender women and thus, the potential for successful cryopreservation is poorly understood.
The present study was undertaken to assess semen quality in transgender women seeking fertility preservation before hormone treatment and compare that with semen quality in cisgender men who had recently fathered a child. In addition, hormonal status and social factors were compared across the two study groups. The long-range goals of these studies are to better understand spermatogenesis in transgender women and to ultimately establish standard practice measures to ensure optimum semen quality for cryopreservation and fertility preservation in transgender women.
Materials and methods
Study design and population
This pilot was designed as a case-control study. Controls consisted of cisgender men 18 years of age or older and fathering a child within the preceding 24 months by self-report, fertile cisgender men. Control inclusion parameters were chosen with the expectation that the semen characteristics of this group would be representative of reference values in accordance with methods defined by the WHO [5]. A goal of this work is to insure optimum fertility preservation options for transgender women. Any intervention that might be subsequently designed would include an ideal goal of reaching semen parameters near those defined by WHO as reference ranges and as observed in fertile men. Thus, cisgender men recently fathering a child was chosen as the control group for comparison of semen parameters. Transgender cases consisted of individuals with natal male sex diagnosed with gender dysphoria as defined in the DSM V by a qualified mental health professional as defined by World Professional Association for Transgender Health standards of care version 7 [3, 4] (https://www.endocrine.org/-/media/endosociety/files/advocacy-and-outreach/position-statements/2017/position_statement_transgender_health.pdf?la=en) and had not initiated hormone or gender-affirming therapy. Cases were presented to the clinic for initiation of feminizing hormones and were 18 years of age or older. Sample size was selected to detect a 10% decrease in semen parameters of transgender women as compared with fertile cisgender men calculated based on the mean semen parameters of recent parents as reported in the Study for Future families [6]. This analysis indicated a minimum required enrollment of 11 controls and 11 cases. The present study population consisted of 17 fertile cisgender men (controls) and 22 transgender women (cases) and thus is powered to detect a difference in semen parameters.
Ethical approval
All participants gave informed consent prior to entry into the study. This study was approved by the Institutional Review Board at the University of Kansas Medical Center, Kansas City, Kansas USA (#140407).
Questionnaire
All study participants completed a questionnaire via a secure online portal including the DASS-21 and additional questions related to personal behavior. The Depression, Anxiety and Stress Scale (DASS-21) is a short form of DASS composed of three subscales: depression, anxiety, and stress and which has been validated in large populations [7]. Self-reported responses on each item range from 0 (did not apply to me at all) to 3 (applied to me very much). The intensity of any of the three conditions is determined by the sum scores of responses to its 7-item subscale. The alpha reliability coefficients for the DASS-21 subscales have been examined in clinical and nonclinical samples and reported as 0.94 for DASS-D, 0.87 for DASS-A, and 0.91 for DASS-S [8–10]. Cutoff points have been suggested for each subscale reflecting the severity within each category (Table 1); however, it is suggested that interpretation should be cautious and used in conjunction to other clinical and observational data.
Table 1.
DASS-21 score interpretation
| Stress | Anxiety | Depression | |||
|---|---|---|---|---|---|
| Normal | 0–10 | Normal | 0–6 | Normal | 0–9 |
| Mild | 11–18 | Mild | 7–9 | Mild | 10–12 |
| Moderate | 19–26 | Moderate | 10–14 | Moderate | 13–20 |
| Severe | 27–34 | Severe | 15–19 | Severe | 21–27 |
| Extremely severe | 35–42 | Extremely severe | 20–42 | Extremely severe | 28–42 |
Additional questions related to personal behaviors included questions related to smoking, use of medications, drugs or alcohol, wearing tight undergarments or tucking testes, shaving or the use of creams for hair removal, frequency of ejaculation, exposure to heat, previous chemotherapy or radiation, and the use of hormones or other medications, education, race, income, and children. Self-reported responses for each item were as yes/no, or as a range of frequency per day or week.
Semen analysis
Semen analysis was carried out in a CLIA-certified clinical andrology laboratory according to the WHO laboratory manual for the examination and processing of human semen 5th edition using manual methods [11]. In addition, methods compare with those in the Bjorndahl guidelines [12] while using MicroCell chamber slides. Study participants were instructed to abstain from sexual activity for two to seven days prior to collection of the specimen for analysis. Complete semen analysis included assessment of color, liquefaction, viscosity, volume, pH, sperm concentration and motility, presence of round cells and leukocytes if necessary, and morphology by strict parameters.
Hormone measurement
A single peripheral blood sample was collected on the day of semen analysis between 10 am and 2:30 pm by standard venipuncture using red-top tubes. Following clotting of 30 min, serum was collected by centrifugation, aliquoted, and stored frozen until analysis of all samples as a batch. FSH, total testosterone, and estradiol were analyzed at Quest Diagnostics using electrochemiluminescence immunoassay (FSH) or LC/MS/MS (testosterone and estradiol).
Statistical analysis
Data were analyzed for normality using the D’Agostino & Pearson normality test. If the data were normal, controls and cases were compared by Student’s t test. Nonparametric data were compared by the Mann-Whitney test. Differences of p < 0.05 were considered significant. Correlation analysis between stress, anxiety, and depression within a group and between personal behaviors and semen parameters was determined using Spearman’s rank correlation coefficient (GraphPad Prism7). Spearman’s correlation (rs) = 1.0–0.8 very strong, 0.79–0.6 strong, 0.59–0.4 moderate, 0.39–0.2 weak, and 0.19–0 very weak correlation.
Results
Control fertile cisgender men (n = 17) were recruited from an obstetrics clinic and consisted of men recently fathering a child. Transgender women (n = 22) were recruited from a transgender clinic at the time of consultation and prior to the initiation of hormone treatment. General demographics of the study groups are presented in Table 2. Transgender women were younger and smaller in stature and weight than control fertile cisgender men.
Table 2.
Demographics
| Cisgender Mena | Transgender Womenb | |||
|---|---|---|---|---|
| Median | Mean ± sem | Median | Mean ± sem | |
| Age (years) | 31.0 | 32.0 ± 1.04 | 25.0 | 26.70 ± 1.85 f |
| Height (in.) | 71.0 | 71.41 ± 0.54 | 69.0 | 69.20 ± 0.55 g |
| Weight (lbs) | 210.0 | 207.8 ± 9.49 | 177.0 | 173.7 ± 10.47 h |
| BMI | 28.59 | 28.80 ± 1.16 | 26.65 | 25.49 ± 1.56 i |
| Tuck testesc | 1.0 | 1.0 ± 0 | 1.0 | 1.68 ± 0.25 j |
| Tight undergarmentsc | 1.0 | 1.18 ± 0.10 | 2.0 | 2.37 ± 0.29k |
| Frequency of ejaculationd | 3.0 | 3.06 ± 0.26 | 3.0 | 3.00 ± 0.24 |
| Hormones past 18 monthse | 0/17 | 0/22 | ||
aCisgender men are fertile men recently fathering a child.
bTransgender women have not initiated medical or surgical gender-affirming treatment
c1 = never, 2 = 1–2 days/week, 3 = 3–4 days/week, 4 = 5 or more days/week
d1 = rare, 2 = 1–2 days/week, 3 = 3–4 days/week, 4 = 5–7 days/week, 5 = multiple times per day
eNumber yes/total number
f, p = 0.0001, g, p = 0.023; h, p = 0.032; i, p = 0.047; j, p = 0.008; k, p = 0.002
Complete semen analysis revealed differences in semen parameters between transgender women and fertile cisgender men. The concentration of sperm was lower in transgender women compared with fertile cisgender men (31.89/31.66 ± 5.58 vs 54.60/67.42 ± 17.95, median/mean ± sem, p = 0.0014). Semen from 3 of the 22 transgender women was azoospermic, contained no sperm. In addition to sperm concentration, total sperm per ejaculate (44.49/74.14 ± 17.10 vs 183.10/226.30 ± 48.46, median/mean ± sem, p = 0.0001) and total motile sperm per ejaculate (27.63/40.71 ± 9.65 vs 106.60/111.7 ± 20.10, median/mean ± sem, p = 0.0007) were lower in transgender women compared with fertile cisgender men, respectively. Analysis of sperm morphology by strict criteria revealed the percent normal forms was lower in transgender women compared with fertile cisgender men (3.0/2.97 ± 0.41 vs 4.5/4.38 ± 0.51, median/mean ± sem; respectively, p = 0.034). Semen volume was also lower in transgender women compared with fertile cisgender men (2.4/2.63 ± 0.34 vs 3.6/3.72 ± 0.38, median/mean ± sem, p = 0.044). Other semen parameters including motility were not different between groups (Table 3).
Table 3.
Semen parameters
| Cisgender men1 | Transgender women2 | General population3 | Fertile men4 | |||
|---|---|---|---|---|---|---|
| Median | Mean ± sem | Median | Mean ± sem | Range of medians [11, 13–26] | Median [11] | |
| Abstinence, days | 2.5 | 2.5 ± 0.25 | 2.0 | 2.33 ± 0.19 | ||
| Volume, ml | 3.6 | 3.72 ± 0.38 | 2.4 | 2.63 ± 0.34 a | 2.3–3.8 | 3.7 |
| pH | 7.6 | 7.4 ± 0.05 | 7.4 | 7.4 ± 0.04 | ||
| Concentration, × 106/ml | 54.6 | 67.42 ± 17.95 | 31.89 | 31.66 ± 5.58 b | 41–69.9 | 73 |
| Oligospermia, no. of total (%) | 0 of 17 (0%) | 6 of 22 (27.3%) | ||||
| Motility | ||||||
| % progressive | 45.0 | 44.88 ± 2.65 | 44.0 | 39.68 ± 3.97 | 46–69.9 | 55.0 |
| % nonprogressive | 5.0 | 6.41 ± 1.63 | 6.3 | 7.26 ± 0.76 | ||
| % immotile | 49.0 | 50.0 ± 2.63 | 51.3 | 53.12 ± 4.27 | ||
| Asthenospermia, no. of total (%) | 2 of 17 (11.8%) | 5 of 19 (26.3%) | ||||
| Total sperm per ejaculate, × 106/ml | 183.1 | 226.30 ± 48.46 | 44.49 | 74.14 ± 17.10 c | 111.6–208 | 255 |
| Total motile sperm, × 106/ml | 106.6 | 111.7 ± 20.10 | 27.63 | 40.71 ± 9.65 d | ||
| Morphology, % normal | 4.5 | 4.38 ± 0.51 | 3.0 | 2.97 ± 0.41 e | 5–15.8 | 15 |
| Teratospermia, no. of total (%) | 5 of 17 (29.4%) | 14 of 19 (73.7%) | ||||
1Cisgender men are fertile men recently fathering a child
2Transgender women have not initiated medical or surgical gender-affirming treatment
3A range of medians from recent literature, mixed populations of men of unknown fertility assumed to be representative of the general population (references 11, 13–26)
4Fertile men: fathers with a partner with time-to-pregnancy of equal to or less than 12 months, WHO (Reference 11)
a, p = 0.044; b, p = 0.014; c, p = 0.0001; d, p = 0.0007; e, p = 0.034
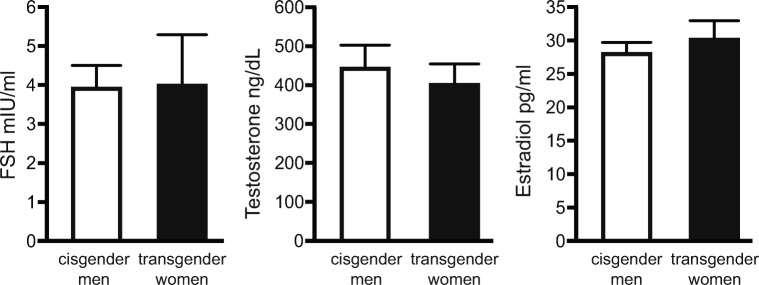
Serum concentrations of FSH, testosterone, and estradiol were determined at the time of semen analysis. Concentrations of these hormones were not different between the groups (Fig. 1).
Fig. 1.
Serum concentrations of hormones at the time of semen analysis. Serum concentration of follicle stimulating hormone (FSH), testosterone, and estradiol was measured in cisgender men and transgender women. No differences in hormone concentrations were observed between the two groups. Cisgender men: fertile men recently fathering a child. Transgender women: transgender women prior to the initiation of medical or surgical gender-affirming surgery.
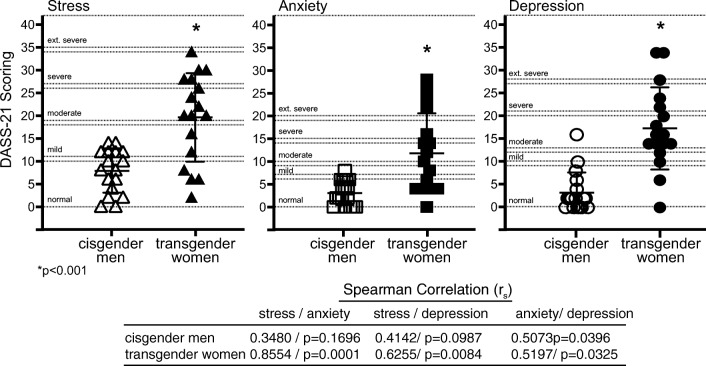
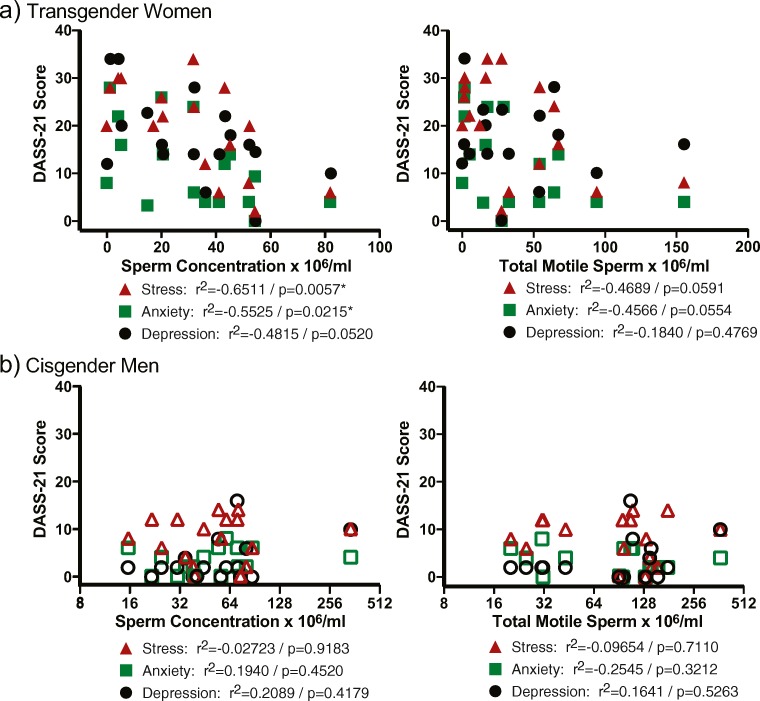
All study participants completed an online survey consisting of the Depression Anxiety Stress Scales 21 (DASS-21) survey and additional questions related to personal behavior. The DASS-21 survey revealed transgender women exhibited greater stress, anxiety, and depression compared with cisgender men (Fig. 2). In addition, stress, anxiety, and depression were moderately to very strongly correlated within the transgender group where moderate to weak correlations between the DASS-21 measures were observed in the cisgender men group (Fig. 2). Analyses of correlations between depression, anxiety, and stress and sperm concentration and total motile sperm in transgender women and fertile cisgender men were examined. A significant negative correlation was observed in the transgender women group between anxiety and stress and sperm concentration, while similar trends were noted for stress and anxiety and total motile sperm (Fig. 3).
Fig. 2.
DASS-21 survey results. Cisgender men and transgender women self-reported results of the DASS-21 survey. Transgender women exhibited higher levels of stress, anxiety, and depression compared with cisgender men. The presence of stress, anxiety, and depression was highly correlated within the transgender group. *p < 0.001 transgender women vs cisgender men. Spearman’s correlation (rs) = 1.0–0.8 very strong, 0.79–0.6 strong, 0.59–0.4 moderate, 0.39–0.2 weak, 0.19–0 very weak correlation; correlation and associated p value are indicated for each correlation. Cisgender men: fertile men recently fathering a child. Transgender women: transgender women prior to the initiation of medical or surgical gender-affirming surgery.
Fig. 3.
Correlation of psychosocial stress and sperm concentration and total motile sperm counts. Spearman’s correlation analysis between depression, anxiety, and stress and sperm concentration and total motile sperm was determined in the transgender women group (A) and cisgender men group (B). Spearman’s correlation (rs) = 1.0–0.8 very strong, 0.79–0.6 strong, 0.59–0.4 moderate, 0.39–0.2 weak, 0.19–0 very weak; correlation and associated p value are indicated for each correlation. Cisgender men: fertile men recently fathering a child. Transgender women: transgender women prior to the initiation of medical or surgical gender-affirming surgery.
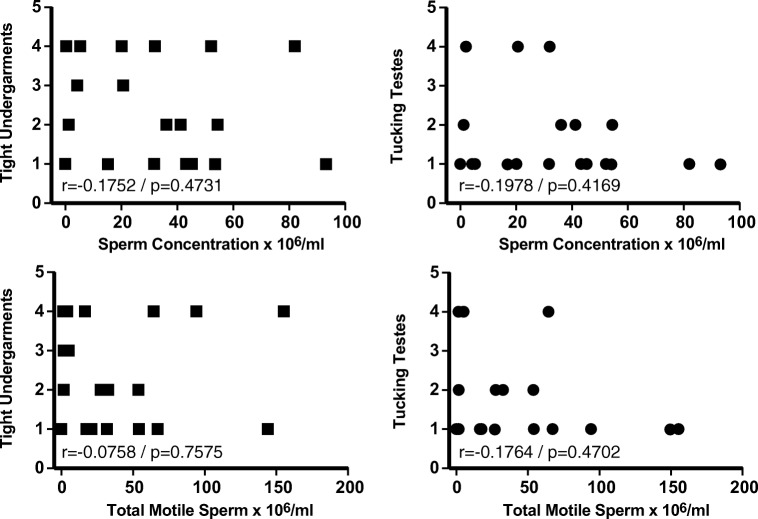
Additional questions surveyed personal behavior and included questions related to smoking, use of medications, drugs or alcohol, wearing tight undergarments or tucking testes, frequency of ejaculation, exposure to heat, previous chemotherapy or radiation, and the use of hormones. The significance of many of the survey questions is limited due to the relatively small sample size; for example, only one individual in both study groups combined smoked tobacco. However, several questions were informative. The use of tight undergarments and the practice of tucking was significantly greater in the transgender group (Table 2); however, there was no correlation between the use of tight undergarments or tucking and either sperm concentration or total motile sperm (Fig. 4). In addition, the frequency of ejaculation was not different between transgender women and cisgender men (Table 2).
Fig. 4.
Analysis of correlation between tight undergarments and tucking and semen parameters in transgender women. Correlations between the use of tight undergarments or the practice of tucking testes and sperm concentration and total motile sperm were assessed by Spearman’s correlation analysis. Use of tight undergarments or testicular tucking was scored as follows: 1 = never, 2 = 1–2 days/week, 3 = 3–4 days/week, 4 = 5 or more days per week. Only weak to very weak correlation was observed in all instances. Spearman’s correlation (rs) = 1.0–0.8 very strong, 0.79–0.6 strong, 0.59–0.4 moderate, 0.39–0.2 weak, 0.19–0 very weak; correlation and associated p value are indicated for each correlation. Transgender women: transgender women prior to the initiation of medical or surgical gender-affirming surgery.
Discussion
The present pilot study is the first case-control analysis to demonstrate differences in the semen parameters in transgender women as compared with cisgender men recently fathering a child. Several semen parameters were significantly lower in transgender women compared with the fertile cisgender men control group including sperm concentration, total sperm per ejaculate, total motile sperm, and morphology. Two previous reports describe semen characteristics in transgender women [27, 28]. The first is a case report of 29 transgender women that described a high incidence of oligospermia (27.58%), asthenospermia (31%), and teratospermia (31%), when compared with the WHO references values [27]. When the present data are categorized based on reference limits defined by the WHO [11], similar results are observed where, within the transgender group, 27.3% were oligospermic, 26.3% were asthenospermic, and 73.7% exhibited teratospermia. More recently, a retrospective analysis of semen samples stored at a cryogenic center for fertility preservation compared semen characteristics of samples stored from healthy cisgender men and transgender women. In that study, sperm concentration, sperm count, and total motile sperm were lower in the transgender group compared with the cisgender healthy men group [28]. Overall, the present case-control study demonstrates similar observations whereby sperm concentration, total sperm per ejaculate, total motile sperm, and percent normal morphology were found to be statistically different between groups. It is also of interest to note that, in the present series of 29 transgender women, 3 (10.3%) were azoospermic. The incidence of azoospermia in the general population is 1%, and approximately 10 to 20% of infertile men have azoospermia [29].
The control population in the present study included cisgender man recently fathering a child with the expectation that the semen characteristics of this group would be representative of reference values in accordance with methods defined by the WHO [5]. One goal of this work is to insure optimum fertility preservation options for transgender women. Any intervention that might be subsequently designed would include an ideal goal of reaching semen parameters near those defined by WHO as reference ranges and as observed in fertile men. It is generally recognized that semen characteristics of men with recent fertility as a group differ from those of a cross-section of men in the general population (Table 3) [5]. A survey of recent published studies describing semen characteristics in general populations of men reveals a range of semen values [13–26]. Some general observations can be made; for example, the median sperm concentration in fertile men as described by the WHO (73 x 106 sperm/ml, [5, 11]) is higher than the range of medians reported for cross-sectional general populations of men (41 to 69.9 × 106 sperm/ml [13–26]) which is still higher than the median found in the group of transgender women in the current study (31.89 × 106 sperm/ml, Table 3). Similar trends can be observed when comparing median total ejaculated sperm in fertile men (255 × 106 [5, 11]), in a series of studies of general populations of men (111.6 × 106 to 208 × 106 [13–26]) and the group of transgender women in the current study (44.49 × 106, Table 3). Thus, although a comparison only, it appears sperm numbers in the group of transgender women in the current study are lower than those reported in several studies representing the general population (Table 3). It is further recognized that semen parameters vary across racial, social, and demographic parameters including across regions of the USA [6, 14, 30–33]. The current study was undertaken in the US Midwest where previous reports indicated overall sperm concentrations were lower compared with other regions of the USA [30]. Regional difference may account for the somewhat lower medians observed in the current group of fertile cisgender men and medians for fertile men reported by the WHO (Table 3) [5, 11]. In continuing studies, the present series will be extended to include a cross-sectional population of men from the same region of the US Midwest to determine if the differences in semen parameters observed in the present study between transgender women and fertile cisgender men are also observed when compared with a general population of reproductive-aged cisgender men from the same geographic region.
Semen characteristics have been shown to vary dependent on the duration between ejaculations [34, 35]. The days of abstinence prior to collection of the specimen analyzed (Table 3) and the self-reported frequency of ejaculation per week (Table 2) did not differ between the transgender women and cisgender fertile men. Thus, it is unlikely differences observed in semen parameters are related to the period of abstinence or ejaculatory frequency.
A primary finding of the current study is lower numbers of sperm in the transgender women group. One possible explanation for this finding is increased scrotal temperature due to the use of tight undergarments and or tucking. Studies experimentally elevating scrotal temperature in men have demonstrated reductions in sperm concentration and motility with increased scrotal temperature [36, 37]. A study monitoring scrotal temperature over a 2-year period found increased scrotal temperature in obese men and men with varicocele correlated with reduced total motile sperm [38]. Several studies have shown wearing tight underwear is associated with poor semen characteristics, reduced sperm concentration, and reduced sperm motility [39, 40], although other studies question the strength of any link [41, 42]. In studies where experimental elevation in scrotal temperature resulted in poor semen characteristics, the effects were transient and semen parameters returned to normal after heat treatment was stopped [36, 37]. All participants in the current study answered a series of questions related to personal behavior. The use of tight undergarments was significantly greater by transgender women compared with cisgender men (Table 2). In addition, tucking testicles was reported by 7 of 22 transgender women (Table 2). Although not measured in the present study, the use of tight undergarments and testicular tucking might be expected to result in an elevation of scrotal and testicular temperature which in turn may further affect spermatogenesis and semen characteristics. The current data indicated no correlation between the use of tight undergarments or testicular tucking and sperm concentration or total motile sperm (Fig. 4). The lack of correlation observed here may indicate no interaction exists; however, it is also possible the ability to detect a correlation is limited in part by variation in personal behavior, such as hours of tucking per day, and or the number of study participants. Ongoing studies are directly addressing the possibility that altered semen characteristics observed in transgender women are related to elevated scrotal temperature due to personal behaviors.
Testicular function and spermatogenesis are primarily regulated by the gonadotropins FSH and LH and the steroid hormone testosterone [43]. In the present series, no differences were found in the serum concentrations of FSH or testosterone when comparing the transgender women and cisgender men groups (Fig. 1). This indicates scrotal temperature may not be a factor in the decreased sperm concentrations observed in the transgender group. Previous studies have demonstrated that, with intrinsic testicular hyperthermia, or increased scrotal temperature associated with varicocele or obesity, decreased spermatogenesis is associated with increased FSH and sometimes although not consistently increased testosterone [38, 43, 44]. The absence of changes in FSH or testosterone suggests reduced sperm concentrations in transgender women are not a consequence of low testosterone levels. In addition, no difference was found in serum estradiol levels between the two groups. This confirms the transgender women were not currently taking estrogenic hormone therapy that would be recognized in the estradiol assay as well as cause a hypogonadotropic hypogonadal state. This further suggests decreased sperm concentrations observed in the transgender group were not a result of elevated estrogens.
A statistical difference in age was observed in comparing the transgender women (mean age 27.4) and the cisgender fertile men (mean age 32.2) in this study (Table 2). At these ages, there would be no anticipated difference in semen parameters due to age alone. Changes in semen characteristics associated with aging including decreased sperm concentration are typically reported to occur in the fourth decade [45–48]. Although the transgender women group exhibited lower sperm concentration and total motile sperm, they were younger than the cisgender men group and thus, we anticipate differences observed in semen parameters are not related to age.
Results of the DASS-21 questionnaire indicated transgender women exhibited higher levels of stress, anxiety, and depression compared with the cisgender men group and the presence of increased stress, anxiety, and depression was correlated within the transgender women group (Fig. 2). The potential role of psychosocial state on parameters of semen quality has been addressed in several studies. In a meta-analysis of 46 studies, stress exhibited a negative impact on sperm concentration, motility, and the incidence of abnormal morphology [49]. A second meta-analysis of 74 reports further indicated psychological stress including anxiety and depression was associated with poor semen quality including reduced sperm concentration [50]. In another recent cross-sectional study of 1215 men, poorer semen quality was observed when stress was measured to be at an intermediate level or above [51]. Similarly, in an examination of 744 fertile men in the Study for Future Families [6, 31], men reporting two or more recent stressful life events had reduced sperm concentration [52]. Depression has also been associated with reduced parameters of semen quality. In a study of 587 college students, higher scores of depression based on the Zung self-rated depression scale correlated with lower sperm concentration and total sperm count [53]. Examination of the prevalence of stress, anxiety, and or depression in men undergoing IVF treatments also indicates that increased stress in that population is associated with reduced semen quality [54–57]. However, whether reduced semen quality is a result of psychosocial state or whether reduced semen quality resulting for the need of IVF treatment increases depression, anxiety, and stress is not clear [58–60]. Increased levels of superoxide dismutase and nitric oxide in seminal fluid during periods of stress have been demonstrated and provide some insight toward potential mechanisms of stress-induced reduction of semen quality [55, 61]. Importantly, the negative impacts of stress-related factors on semen quality are considered to be largely reversible with treatment or removal of the stressors [50, 52, 55, 61, 62].
Treatment of depression with selective serotonin reuptake inhibitors (SSRIs) is associated with poor semen quality that can be reversed after discontinuing use [63, 64]. In the present study group, four transgender women and 2 fertile cisgender men were currently taking SSRIs. Although the sperm concentration and total motile sperm values for those taking SSRIs did not cluster toward the lower fraction for their respective groups, conclusions cannot be made based on the limited number of participants taking SSRIs in the present study.
In conclusion, our results show that transgender women have lower sperm concentrations, total sperm, total motile sperm, and percent sperm with normal morphology as compared with fertile cisgender men. Although semen parameters were lower, cryopreservation of sperm prior to hormone therapy is a viable fertility preservation option for most transgender women. We have also demonstrated an increase in the rates of psychosocial stress in transgender women as compared with fertile cisgender men. Enhanced education related to personal behaviors or treatment to reduce effects of stressors prior to cryopreservation may improve future fertility potential. The etiology of the differences in semen parameters is not known. Future research should be directed toward elucidating what factors are responsible for differences in semen quality in transgender women.
Acknowledgments
The authors thank the participants for contributing to this study.
Funding information
This study was funded in part by a clinical and multidisciplinary pilot grant from the University of Kansas Medical Center Research Institute.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Courtney Marsh, Phone: 913-588-2229, Email: cmarsh2@kumc.edu.
Katherine F. Roby, Phone: 913-588-7426, Email: kroby@kumc.edu
References
- 1.Institute of Medicine Committee on Lesbian GB, Transgender Health I, Research, Gaps Opportunities,. The National Academies Collection: Reports funded by National Institutes of Health. National Academies Press (US) National Academy of Sciences. 2011.
- 2.Fisk NM. Editorial: Gender dysphoria syndrome--the conceptualization that liberalizes indications for total gender reorientation and implies a broadly based multi-dimensional rehabilitative regimen. West J Med. 1974;120:386–391. [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman E, Bockting M, Botzer P. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgender. 2012;13:165–232. doi: 10.1080/15532739.2011.700873. [DOI] [Google Scholar]
- 4.Medicine ECotASfR Access to fertility services by transgender persons: an Ethics Committee opinion. Fertil Steril. 2015;104:1111–1115. doi: 10.1016/j.fertnstert.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo MT, Vogelsong KM. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–245. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 6.Redmon JB, Thomas W, Ma W, Drobnis EZ, Sparks A, Wang C, Brazil C, Overstreet JW, Liu F, Swan SH. Semen parameters in fertile US men: the Study for Future Families. Andrology. 2013;1:806–814. doi: 10.1111/j.2047-2927.2013.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-U. [DOI] [PubMed] [Google Scholar]
- 8.Sinclair SJ, Siefert CJ, Slavin-Mulford JM, Stein MB, Renna M, Blais MA. Psychometric evaluation and normative data for the depression, anxiety, and stress scales-21 (DASS-21) in a nonclinical sample of U.S. adults. Eval Health Prof. 2012;35:259–279. doi: 10.1177/0163278711424282. [DOI] [PubMed] [Google Scholar]
- 9.Antony MM, Bieling PJ, Cox BJ, Enns MW, Swinson RP. Psychometric properties of the 42- item and 21-item versions of the Depression Anxiety Stress Scales in clinical groups and a community sample. Psychol Assess. 1998;10:176–181. doi: 10.1037/1040-3590.10.2.176. [DOI] [Google Scholar]
- 10.Henry JD, Crawford JR. The short-form version of the Depression Anxiety Stress Scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br J Clin Psychol. 2005;44:227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 11.Organization WH. WHO laboratory manual for the examination and processing of human semen. 5th ed: WHO Press; 2010.
- 12.Bjorndahl L, Barratt CL, Mortimer D, Jouannet P‘. How to count sperm properly’: checklist for acceptability of studies based on human semen analysis. Hum Reprod. 2016;31:227–232. doi: 10.1093/humrep/dev305. [DOI] [PubMed] [Google Scholar]
- 13.de Neergaard R, Nielsen JE, Jorgensen A, Toft BG, Goetze JP, Jorgensen N. Positive association between cholesterol in human seminal plasma and sperm counts: results from a cross-sectional cohort study and immunohistochemical investigations. Andrology. 2018;6:817–828. doi: 10.1111/andr.12532. [DOI] [PubMed] [Google Scholar]
- 14.Erenpreiss J, Punab M, Zilaitiene B, Hlevicka S, Zayakin P, Matulevicius V, Tomas Preiksa R, Jorgensen N. Semen quality of young men from the general population in Baltic countries. Hum Reprod. 2017;32:1334–1340. doi: 10.1093/humrep/dex062. [DOI] [PubMed] [Google Scholar]
- 15.Priskorn L, Bang AK, Nordkap L, Krause M, Mendiola J, Jensen TK, Juul A, Skakkebaek NE, Swan SH, Jorgensen N. Anogenital distance is associated with semen quality but not reproductive hormones in 1106 young men from the general population. Hum Reprod. 2019;34:12–24. doi: 10.1093/humrep/dey326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart RJ, Doherty DA, McLachlan RI, Walls ML, Keelan JA, Dickinson JE, Skakkebaek NE, Norman RJ, Handelsman DJ. Testicular function in a birth cohort of young men. Hum Reprod. 2015;30:2713–2724. doi: 10.1093/humrep/dev244. [DOI] [PubMed] [Google Scholar]
- 17.Jensen TK, Swan S, Jorgensen N, Toppari J, Redmon B, Punab M, Drobnis EZ, Haugen TB, Zilaitiene B, Sparks AE, Irvine DS, Wang C, Jouannet P, Brazil C, Paasch U, Salzbrunn A, Skakkebaek NE, Andersson AM. Alcohol and male reproductive health: a cross-sectional study of 8344 healthy men from Europe and the USA. Hum Reprod. 2014;29:1801–1809. doi: 10.1093/humrep/deu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C, Li B, Xu K, Liu D, Hu J, Yang Y, Nie H, Fan L, Zhu W. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil Steril. 2017;107:83–88.e2. doi: 10.1016/j.fertnstert.2016.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Hammoud AO, Meikle AW, Peterson CM, Stanford J, Gibson M, Carrell DT. Association of 25-hydroxy-vitamin D levels with semen and hormonal parameters. Asian J Androl. 2012;14:855–859. doi: 10.1038/aja.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23:646–659. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez MF, Duran I, Olea N, Avivar C, Vierula M, Toppari J, Skakkebaek NE, Jorgensen N. Semen quality and reproductive hormone levels in men from Southern Spain. Int J Androl. 2012;35:1–10. doi: 10.1111/j.1365-2605.2010.01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, Olesen IA, Juul A, Andersson AM, Carlsen E, Petersen JH, Toppari J, Skakkebaek NE. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2:e000990. doi: 10.1136/bmjopen-2012-000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorgensen N, Vierula M, Jacobsen R, Pukkala E, Perheentupa A, Virtanen HE, Skakkebaek NE, Toppari J. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. Int J Androl. 2011;34:e37–e48. doi: 10.1111/j.1365-2605.2010.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Virtanen HE, Jorgensen N, Toppari J. Semen quality in the 21(st) century. Nat Rev Urol. 2017;14:120–130. doi: 10.1038/nrurol.2016.261. [DOI] [PubMed] [Google Scholar]
- 25.Richthoff J, Rylander L, Hagmar L, Malm J, Giwercman A. Higher sperm counts in Southern Sweden compared with Denmark. Hum Reprod. 2002;17:2468–2473. doi: 10.1093/humrep/17.9.2468. [DOI] [PubMed] [Google Scholar]
- 26.Axelsson J, Rylander L, Rignell-Hydbom A, Giwercman A. No secular trend over the last decade in sperm counts among Swedish men from the general population. Hum Reprod. 2011;26:1012–1016. doi: 10.1093/humrep/der045. [DOI] [PubMed] [Google Scholar]
- 27.Hamada A, Kingsberg S, Wierckx K, T’Sjoen G, De Sutter P, Knudson G, Agarwal A. Semen characteristics of transwomen referred for sperm banking before sex transition: a case series. Andrologia. 2015;47:832–838. doi: 10.1111/and.12330. [DOI] [PubMed] [Google Scholar]
- 28.Li K, Rodriguez D, Gabrielsen JS, Centola GM, Tanrikut C. Sperm cryopreservation of transgender individuals: trends and findings in the past decade. Andrology. 2018;6:860–864. doi: 10.1111/andr.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142:62–65. doi: 10.1016/S0022-5347(17)38662-7. [DOI] [PubMed] [Google Scholar]
- 30.Glazer CH, Li S, Zhang CA, Giwercman A, Bonde JP, Eisenberg ML. Racial and sociodemographic differences of semen parameters among US men undergoing a semen analysis. Urology. 2019;123:126–132. doi: 10.1016/j.urology.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 31.Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, Hatch M, Redmon JB, Wang C, Overstreet JW. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414–420. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, Barad DH. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996;65:1009–1014. doi: 10.1016/S0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- 33.Nordkap L, Joensen UN, Blomberg Jensen M, Jorgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355:221–230. doi: 10.1016/j.mce.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal A, Gupta S, Du Plessis S, Sharma R, Esteves SC, Cirenza C, Eliwa J, Al-Najjar W, Kumaresan D, Haroun N, Philby S, Sabanegh E. Abstinence time and its impact on basic and advanced semen parameters. Urology. 2016;94:102–110. doi: 10.1016/j.urology.2016.03.059. [DOI] [PubMed] [Google Scholar]
- 35.Ayad BM, Horst GV, Plessis SSD. Revisiting the relationship between the ejaculatory abstinence period and semen characteristics. Int J Fertil Steril. 2018;11:238–246. doi: 10.22074/ijfs.2018.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao M, Xia W, Yang J, Hu LX, Hu SF, Lei H, Wu YQ, Zhu CH. Transient scrotal hyperthermia affects human sperm DNA integrity, sperm apoptosis, and sperm protein expression. Andrology. 2016;4:1054–1063. doi: 10.1111/andr.12228. [DOI] [PubMed] [Google Scholar]
- 37.Rao M, Zhao XL, Yang J, Hu SF, Lei H, Xia W, Zhu CH. Effect of transient scrotal hyperthermia on sperm parameters, seminal plasma biochemical markers, and oxidative stress in men. Asian J Androl. 2015;17:668–675. doi: 10.4103/1008-682X.146967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garolla A, Torino M, Miola P, Caretta N, Pizzol D, Menegazzo M, Bertoldo A, Foresta C. Twenty-four-hour monitoring of scrotal temperature in obese men and men with a varicocele as a mirror of spermatogenic function. Hum Reprod. 2015;30:1006–1013. doi: 10.1093/humrep/dev057. [DOI] [PubMed] [Google Scholar]
- 39.Jurewicz J, Radwan M, Sobala W, Radwan P, Jakubowski L, Hawula W, Ulanska A, Hanke W. Lifestyle factors and sperm aneuploidy. Reprod Biol. 2014;14:190–199. doi: 10.1016/j.repbio.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Sanger WG, Friman PC. Fit of underwear and male spermatogenesis: a pilot investigation. Reprod Toxicol. 1990;4:229–232. doi: 10.1016/0890-6238(90)90063-2. [DOI] [PubMed] [Google Scholar]
- 41.Jung A, Schuppe HC. Influence of genital heat stress on semen quality in humans. Andrologia. 2007;39:203–215. doi: 10.1111/j.1439-0272.2007.00794.x. [DOI] [PubMed] [Google Scholar]
- 42.Munkelwitz R, Gilbert BR. Are boxer shorts really better? A critical analysis of the role of underwear type in male subfertility. J Urol. 1998;160:1329–1333. doi: 10.1016/S0022-5347(01)62528-X. [DOI] [PubMed] [Google Scholar]
- 43.Shiraishi K, Matsuyama H. Gonadotoropin actions on spermatogenesis and hormonal therapies for spermatogenic disorders [Review] Endocr J. 2017;64:123–131. doi: 10.1507/endocrj.EJ17-0001. [DOI] [PubMed] [Google Scholar]
- 44.Mieusset R, Bujan L, Plantavid M, Grandjean H. Increased levels of serum follicle-stimulating hormone and luteinizing hormone associated with intrinsic testicular hyperthermia in oligospermic infertile men. J Clin Endocrinol Metab. 1989;68:419–425. doi: 10.1210/jcem-68-2-419. [DOI] [PubMed] [Google Scholar]
- 45.Cardona Maya W, Berdugo J, Cadavid Jaramillo A. The effects of male age on semen parameters: analysis of 1364 men attending an andrology center. Aging Male. 2009;12:100–103. doi: 10.3109/13685530903322841. [DOI] [PubMed] [Google Scholar]
- 46.Levitas E, Lunenfeld E, Weisz N, Friger M, Potashnik G. Relationship between age and semen parameters in men with normal sperm concentration: analysis of 6022 semen samples. Andrologia. 2007;39:45–50. doi: 10.1111/j.1439-0272.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 47.Stone BA, Alex A, Werlin LB, Marrs RP. Age thresholds for changes in semen parameters in men. Fertil Steril. 2013;100:952–958. doi: 10.1016/j.fertnstert.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 48.Veron GL, Tissera AD, Bello R, Beltramone F, Estofan G, Molina RI, Vazquez-Levin MH. Impact of age, clinical conditions, and lifestyle on routine semen parameters and sperm kinematics. Fertil Steril. 2018;110:68–75. doi: 10.1016/j.fertnstert.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95:116–123. doi: 10.1016/j.fertnstert.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 50.Durairajanayagam D. Lifestyle causes of male infertility. Arab J Urol. 2018;16:10–20. doi: 10.1016/j.aju.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nordkap L, Jensen TK, Hansen AM, Lassen TH, Bang AK, Joensen UN, Blomberg Jensen M, Skakkebaek NE, Jorgensen N. Psychological stress and testicular function: a cross-sectional study of 1,215 Danish men. Fertil Steril. 2016;105:174–87.e1-2. doi: 10.1016/j.fertnstert.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 52.Gollenberg AL, Liu F, Brazil C, Drobnis EZ, Guzick D, Overstreet JW, Redmon JB, Sparks A, Wang C, Swan SH. Semen quality in fertile men in relation to psychosocial stress. Fertil Steril. 2010;93:1104–1111. doi: 10.1016/j.fertnstert.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 53.Zou P, Wang X, Sun L, Chen Q, Yang H, Zhou N, Chen H, Zhang G, Ling X, Wang Z, Gao J, Mo M, Huang L, Peng K, Chen S, Cui Z, Liu J, Ao L, Cao J. Semen quality in Chinese college students: associations with depression and physical activity in a cross-sectional study. Psychosom Med. 2018;80:564–572. doi: 10.1097/PSY.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 54.Bartolo A, Reis S, Monteiro S, Leite R, Montenegro N. Psychological adjustment of infertile men undergoing fertility treatments: an association with sperm parameters. Arch Psychiatr Nurs. 2016;30:521–526. doi: 10.1016/j.apnu.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Eskiocak S, Gozen AS, Taskiran A, Kilic AS, Eskiocak M, Gulen S. Effect of psychological stress on the L-arginine-nitric oxide pathway and semen quality. Braz J Med Biol Res. 2006;39:581–588. doi: 10.1590/S0100-879X2006000500003. [DOI] [PubMed] [Google Scholar]
- 56.Vellani E, Colasante A, Mamazza L, Minasi MG, Greco E, Bevilacqua A. Association of state and trait anxiety to semen quality of in vitro fertilization patients: a controlled study. Fertil Steril. 2013;99:1565–1572. doi: 10.1016/j.fertnstert.2013.01.098. [DOI] [PubMed] [Google Scholar]
- 57.Volgsten H, Ekselius L, Poromaa IS, Svanberg AS. Personality traits associated with depressive and anxiety disorders in infertile women and men undergoing in vitro fertilization treatment. Acta Obstet Gynecol Scand. 2010;89:27–34. doi: 10.3109/00016340903447396. [DOI] [PubMed] [Google Scholar]
- 58.Wdowiak A, Bien A, Iwanowicz-Palus G, Makara-Studzinska M, Bojar I. Impact of emotional disorders on semen quality in men treated for infertility. Neuro Endocrinol Lett. 2017;38:50–58. [PubMed] [Google Scholar]
- 59.Bhongade MB, Prasad S, Jiloha RC, Ray PC, Mohapatra S, Koner BC. Effect of psychological stress on fertility hormones and seminal quality in male partners of infertile couples. Andrologia. 2015;47:336–342. doi: 10.1111/and.12268. [DOI] [PubMed] [Google Scholar]
- 60.Clarke RN, Klock SC, Geoghegan A, Travassos DE. Relationship between psychological stress and semen quality among in-vitro fertilization patients. Hum Reprod. 1999;14:753–758. doi: 10.1093/humrep/14.3.753. [DOI] [PubMed] [Google Scholar]
- 61.Eskiocak S, Gozen AS, Kilic AS, Molla S. Association between mental stress & some antioxidant enzymes of seminal plasma. Indian J Med Res. 2005;122:491–496. [PubMed] [Google Scholar]
- 62.Lampiao F. Variation of semen parameters in healthy medical students due to exam stress. Malawi Med J. 2009;21:166–167. doi: 10.4314/mmj.v21i4.49635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drobnis EZ, Nangia AK. Psychotropics and male reproduction. Adv Exp Med Biol. 2017;1034:63–101. doi: 10.1007/978-3-319-69535-8_8. [DOI] [PubMed] [Google Scholar]
- 64.Norr L, Bennedsen B, Fedder J, Larsen ER. Use of selective serotonin reuptake inhibitors reduces fertility in men. Andrology. 2016;4:389–394. doi: 10.1111/andr.12184. [DOI] [PubMed] [Google Scholar]