Abstract
Objectives
Management options for PCOS, as the most prevalent endocrine disorder in women of reproductive age, using natural supplements have a high priority for physicians, especially based on the etiological pathways. Therefore, this study was conducted to describe the effect of resveratrol on the angiogenesis pathway, for management of PCOS through assessing VEGF, HIF1 gene expression, and laboratory parameters.
Methods
In this triple-blind RCT, PCOS was confirmed in ICSI candidates based on the Rotterdam criteria. Sixty-two patients that met the inclusion criteria were randomly assigned to two groups. All patients took resveratrol 800 mg/day or placebo for 40 days orally from the beginning of their previous menstruation cycle until the oocyte retrieval day. The serum levels of different hormones were measured, and the expression of HIF1 & VEGF genes was quantified by real-time PCR.
Results
As for the laboratory hormone assay in 61 PCOS patients, a significant mean difference was seen in the FSH, LH, TSH, and testosterone between the two groups (P < 0.05). The results showed a reduction in the expression of VEGF & HIF1 genes under the effect of resveratrol in the granulosa cells (P = 0.0001). The number of mature oocytes, cleavage rate, fertilization rate, and fertility rate were not significantly different between the two groups (P > 0.05), but the high-quality oocyte rate and high-quality embryo rate were higher in the resveratrol group (P < 0.05).
Conclusions
Based on the results, resveratrol may improve some outcomes of PCOS patients, probably through changing the serum levels of some sex hormones and expression of VEGF & HIF1 genes in the angiogenesis pathway of granulosa cells.
Electronic supplementary material
The online version of this article (10.1007/s10815-019-01461-6) contains supplementary material, which is available to authorized users.
Keywords: Resveratrol, Polycystic ovary syndrome, Intracytoplasmic sperm injection, Hormones, VEGF gene, HIF1 gene
Introduction
Polycystic ovary syndrome (PCOS) is an important condition in women characterized by hyperandrogenism, oligo/anovulation (ovulation abnormality), and polycystic ovaries on ultrasound [1]. PCOS affects the quality of life of the patients and is one of the most important causes of infertility and the most common cause of ovarian factor infertility [2, 3]. Its prevalence is about 5–7% in women of reproductive age [4, 5]. A wide range of symptoms like obesity, hirsutism, hyperlipidemia, and cardiovascular disorders (as part of metabolic syndrome) can be detected in these patients [2].
Nowadays, studies in the field of reproductive medicine mostly address PCOS management as the most prevalent cause of female infertility. The management options for this disease have a high priority for physicians [6]. Although no specific pathogenesis is defined for this syndrome, many studies have shown angiogenic irregularities in the patients’ ovaries due to hyperandrogenism and hormone impairment [7, 8]. The most important finding of PCOS research in recent years is angiogenesis irregularities, especially in the VEGF involved pathway.
VEGF is an important angiogenic factor and its role in physiologic and pathologic angiogenesis, especially in PCOS, is well known [9]. There are two main sources of VEGF in the female reproductive system: granulosa cells and macrophages [10]. Some studies have shown a high VEGF expression level in PCOS women’s hyperechoic ovaries [11]. High levels of this factor in the serum and follicular fluid of PCOS patients have been confirmed in many studies [9, 12]. VEGF expression is affected by many factors such as hyperandrogenism, and since hyperandrogenism is the main feature of PCOS, VEGF expression due to androgen induction in the ovaries is the main known cause of VEGF overexpression in PCOS patients [13, 14]. HIF1 is an intermediate factor in this process. It is a transcription factor whose main target is the VEGF gene [15]. Alterations in the VEGF concentration in PCOS ovaries have a negative impact on oocyte maturity [16].
Any treatment option that reduces the level of VEGF in PCOS could have a direct effect on reducing OHSS (ovarian hyperstimulation syndrome) as the most important and life-threatening side effect of the ovulation induction process. Recognition of VEGF mechanisms in the OHSS process can provide many diagnostic and treatment options for this dangerous iatrogenic syndrome [10]. For these reasons, we selected resveratrol as a treatment option for PCOS.
Resveratrol (trans-3,5,4′-trihydroxystilbene) is an herbal supplement (phytoalexin) that affects the oocyte quality and maturation because of its main properties like anti-inflammatory, antioxidant, anti-cancer, and anti-aging effects [17–19]. Recent studies have confirmed the effect of resveratrol as a natural antioxidant, anti-carcinogenic, and anti-inflammatory supplement in various diseases and as a female hormonal modulator [20–30]. Due to the significant effect of resveratrol on the reduction of VEGF in rats and the reduction of HIF1 and VEGF in human patients [29, 31–36], we performed a clinical trial to determine the effect of resveratrol on the expression level of the VEGF and HIF1 genes in granulosa cells in angiogenesis pathway of PCOS patients.
Methods
Study design
This interventional, triple-blind, randomized clinical trial (RCT) was conducted in documented infertile PCOS patients aged 18–40 years old (ICD-10 code; E28.2) who fulfilled the Rotterdam criteria [1]. The patients had no endocrinopathy except PCOS, and all of them were candidates for ICSI (intracytoplasmic sperm injection) due to six cycles of ovarian induction (like Clomid and/or letrozole) with timed intercourse and three other cycles of IUI that all failed to result in pregnancy (as relative indication) [37, 38]. There was no history of male factor infertility.
The patients with the following conditions were excluded from the study:
FSH > 10 mg/ml
Severe endometriosis (stages III and IV based on the revised AFS-rAFS classification of endometriosis)
Cushing’s disease
Hyperprolactinemia
Thyroid disease
Ovary tumors
Severe male factor infertility (especially non-obstructive azoospermia)
Other female infertility factors (except tubal and cervical factors)
History of using drugs affecting ovarian function like the use of steroids and OCP in the past 3 months
Any autoimmune disease like SLE (systemic lupus erythematosus)
Malnutrition and severe obesity (BMI over 35)
Systemic disorders like metabolic syndrome, hyperlipidemia, diabetes, and cardiovascular disease
As exclusion criteria, patients with FSH above 10 mg/dl were excluded because it indicated the presence of central factors of female infertility. Moreover, severe endometriosis, autoimmune factors, systemic disorders, and malnutrition/obesity could affect the results and outcomes as confounding factors. Some diseases like Cushing’s disease, hyperprolactinemia, thyroid disease, and ovarian tumors may cause PCOS or PCO-like features for which other treatment and management protocols have been defined. Severe male factor infertility and other female factors of infertility were excluded because of their confounding effects on all fertility outcomes, except tubal and cervical factors that could be bypassed by the ICSI.
All patients were referred from Omid Clinic from July 2016 to December 2017 and were candidates for the ICSI protocol. The project was approved by the Ethics Committee of Tehran University of Medical Sciences (Ethics committee reference number IR.TUMS.REC.1394.1690 dated January 19, 2016). The study was registered in the Iranian Registry of Clinical Trials (IRCT-ID: IRCT2016030126860N1, date: March 15, 2016, available at www.IRCT.ir). Informed consent was obtained from all participants.
Randomization
Sixty-two women were initially included in the study. Using blocked randomization, the participants were randomly assigned to intervention (resveratrol) and control (placebo) groups. Figure 1 shows the study map.
Fig. 1.
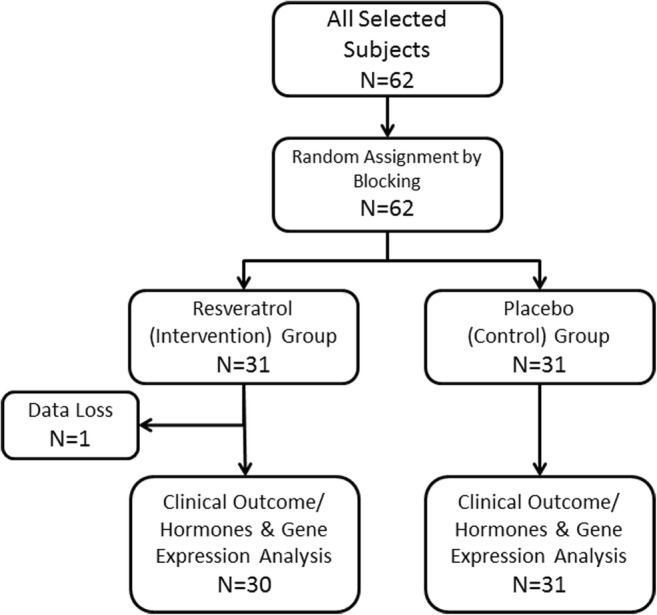
The study flow diagram as a randomized clinical trial
Blinding
The investigator, patients, and statistical analyzer were blind to grouping.
Intervention and ovulation stimulation protocol
The patients in the intervention (resveratrol) group received resveratrol 800 mg/day (2 × 400 mg capsules) orally (Mega resveratrol, 99% pure trans-resveratrol, Southampton, UK) for 40 days (from the beginning of the previous cycle before ovarian stimulation until oocyte pick-up). Patients in the placebo group received oral placebo capsules that were identical in shape with resveratrol and provided by the same company at the same time. The dose of resveratrol (800 mg/day orally) was selected based on previous studies of the effective dose of resveratrol for achieving anti-inflammatory and anti-oxidant effects in other medical conditions [20–23, 27, 39–44]. All patients in both groups underwent the same ovulation induction protocol (flexible antagonist regime). All patients received oral contraception (OCP-Ovocept LD®, Abureihan, Iran) for 21 days in the cycle before ovarian stimulation. On the second day of LD discontinuation, a pituitary gland desensitization drug (150–225 IU, Gonal-F®, Merck Serono SA, Switzerland) was started and continued until HCG administration. A GnRH antagonist (cetrorelix acetate Cetrotide 0.25 mg, Merck Serono SA, Switzerland) was administered when at least two follicles reached 13–14 mm in size and continued until HCG administration. In both groups, HCG (10,000 IU) was prescribed for patients who had at least two follicles ≥ 18 mm or endometrial thickness ≥ 8 mm on ultrasound. The follicular puncture was done 36 h after HCG administration. Embryos were transferred in the cleavage state and luteal phase support was provided by intramuscular progesterone (50 mg/ml, Iran Hormone, Iran) 1 day after oocyte pick-up. All participants underwent the standard ICSI protocol.
Laboratory tests for serum hormone levels
Blood samples were taken from patients twice: the first one was before OCP administration and the second sample was taken on the oocyte retrieval day. The levels of serum AMH (anti-Mullerian hormone), estradiol, progesterone, testosterone, FSH, LH, TSH, and prolactin were measured using the electrochemiluminescent immunoassay (ECLIA) method.
Clinical data and patient outcome records
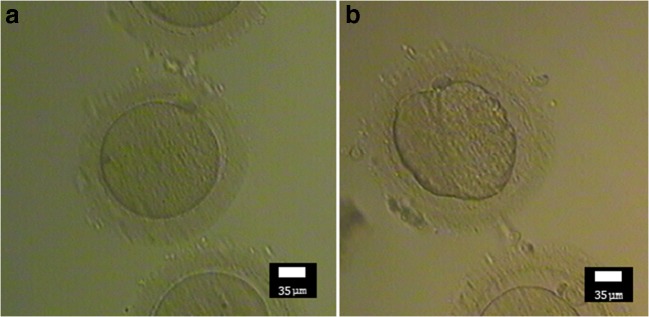
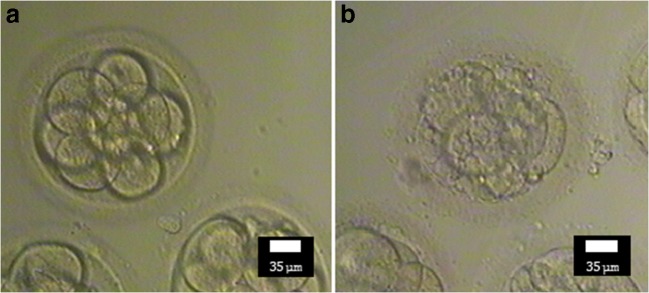
Demographic and physical characteristics, past medical history, drug history, menstrual history, and sperm quality of the husband were recorded in a questionnaire on the first day. On the puncture day, the oocyte data such as the number of retrieved oocytes, number of M-II (mature) oocytes, quality of oocytes, and the rate of high-quality oocytes were recorded. The high-quality oocyte rate was defined as the number of good quality oocytes divided by the total number of retrieved oocytes (within a subject) × 100. A high-quality oocyte (Fig. 2a) was defined as a round, normal-sized oocyte with one regular polar body in the perivitelline space (neither too big nor too small), homogeneous ooplasm without irregularities, and appropriate thickness of the zona pellucida [45, 46] (Fig. 2b shows a comparison with low/bad quality oocyte). Within 16–18 h after ICSI, oocyte fertilization was evaluated to calculate the fertilization rate. Two to three days after ICSI, data related to the embryo development like the percentage of embryos, quality of embryos, number of cleavage embryos (cleavage rate), and high-quality embryo rate were recorded. The high-quality embryo rate was defined as the number of high-quality embryos divided by the number of successful fertilizations (within a subject) × 100 [47]. Grade A and B cleavage embryos were defined as high-quality embryos based on the ASEBIR criteria (Fig. 3a) including the number of cells (blastomeres), percentage of embryo fragmentation, cell symmetry, and presence of multi-nucleation, vacuoles, and pitting [48] (compare it with low-quality embryo, Fig. 3b).
Fig. 2.
Morphological comparison of a high-quality oocyte (a) with low-quality oocyte (b)—(Nikon, × 200). Note the shape and size of the oocyte, regularity of polar body, perivitelline space, and regularity of ooplasm
Fig. 3.
Morphological comparison of high quality (a) and low-quality embryo (day 3) (b)—(Nikon, × 200). Note the blastomere number, percentage of embryo fragmentation, cell symmetry, multi-nucleation, vacuole, and pitting
In the follow-up of the patients, the chemical (using the β-HCG test) and clinical pregnancy rate (by visualization of the gestational sac on ultrasound) were calculated.
Cellular and molecular processes
The punctured oocytes denudated with hyaluronidase and the remaining granulosa cells around the oocyte were collected from the dissect dish and centrifuged at 2500 rpm for 5 min. The cell plaques were washed with RBC lysis buffer (SinaClon®) and centrifugation was repeated (2500 rpm, 5 min), if they were contaminated with blood. The obtained plaques were completely dissolved in 500 to 700 μl TRIzol® Reagent (Ambion - Life Technologies) and kept at – 20 °C until RNA extraction.
RNA extraction and cDNA synthesis
The RNA of granulosa cells was extracted by the phenol-chloroform extraction method using the TRIzol® Reagent protocol. Then, RNA extraction control was done by Agarose gel electrophoresis and Colibri Microvolume Spectrometer (Titertek® Berthold) to make sure of the quality and quantity (purity and concentration) of the extracted RNA. cDNA was synthesized in the RT-PCR process using the QuantiTect® Reverse Transcription Kit (QIAGEN). It should be noted that a mixture of Oligo(dt) and random hexamer was used for total RNA cDNA synthesis. The product of this process was used as a template for real-time PCR.
Real-time PCR and primer design
The real-time protocol for relative quantification of the expression of two target genes (VEGF and HIF1 with internal control of GAPDH as a housekeeping gene) was applied to a final volume of 10 μl, containing real-time SYBR Green master mix Ampliqon® (5 μl), primer mix (including forward and reverse primer) (0.3 μl), target cDNA (1 μl, 80–100 ng/μl concentration), and D.D. water (3.7 μl). The primers used in this study are shown in Table 1. Using the reference databases (Genatlas, Genecard & AceView), the primers were designed based on the expressed variant of the target organ (ovary). All primers were designed from exon-exon junctions for specific and unique target amplification.
Table 1.
Primer sequences for HIF1 & VEGF genes and their target lengths
| Gene (target) | Forward oligo sequence 5′-- > 3′ | Reverse oligo sequence 5′-- > 3′ | Amplicon length (bp) |
|---|---|---|---|
| VEGF | GCACCCATGGCAGAAGG | CTCGATTGGATGGCAGTAGCT | 90 |
| HIF1 | CATCAGCTATTTGCGTGTGAGGA | AGCAATTCATCTGTGCTTTCATGTC | 89 |
| GAPDH | AGAAGGCTGGCGCTCATT | CCCATTGCTGATGATCTTGA | 134 |
The running PCR method for all genes included the following:
- Pre-amplification/holding stage
- 95 °C for 15 min
- Amplification/cycling stage (for 40 cycles)
- Denaturation 95 °C for 15 s
- Annealing 60.5 °C for 30 s
- Extension 72 °C for 30 s
- Melt curve stage
- From 60 to 95 °C (fluorescent emission detection at + 0.3 °C increments)
Real-time PCR was done by the Real-Time ABI® Step One thermocycler. All genes were designed and set up at 60.5 °C as the annealing temperature. In the real-time setup for the genes, a standard curve was achieved in the appropriate efficiency percentage range (90–110%) with controlling the template size without any non-specific amplification template or dimer primer formation in the melting curve view. The amplification curves, standard curves, and melting curves of the target genes and housekeeping gene and their efficiency data are presented in Figs. 4, 5, and 6 as a supplement file.
Statistical analysis
The student t test, paired t test, chi-square test, and Fisher’s exact test were applied for analysis. The data were analyzed with the SPSS software version 23, and the graphs were designed by the GraphPad Prism version 6.
Results
The data of 30 PCOS patients in the resveratrol group (intervention) and 31 patients in the placebo group (control) were analyzed in this study (the data of one subject in the placebo group were not included in the analysis due to missing data and follow-up error, Fig. 1). Table 2 shows demographic, personal, clinical, and laboratory data of the subjects in the resveratrol and placebo groups before intervention. Among 61 subjects, 35 (57.4%) were over 30 years old. Thirty-three (54.1%) subjects were from Tehran, and others were from other cities of Iran. Thirty-three patients (54.1%) had a BMI below 24.9, 18 (29.5%) were overweight (BMI = 25–29.9), and 10 (16.4%) were obese (BMI 30–34.9). The frequency of the BMI class below 24.9, 25–29.9, and 30–34.5 was 15, 10, and 5 subjects in the intervention group and 18, 8, and 5 subjects in the control group, respectively (chi-square test, P value = 0.7871).
Table 2.
Comparison of baseline parameters between resveratrol and placebo groups
| Variable | Total patients mean (SD) | Case (intervention) mean (SD) | Control (placebo) mean (SD) | Statistical test characteristics (t test) | P value (intervention vs placebo) |
|---|---|---|---|---|---|
| Age (year) | 30.08 (3.95) | 29.30 (4.44) | 30.84 (3.30) | t = − 1.540, df = 59 | 0.129 |
| BMI (kg/m2) | 25.56 (4.23) | 25.92 (4.22) | 25.21 (4.28) | t = 0.653, df = 59 | 0.516 |
| Infertility duration (year) | 4.88 (2.58) | 4.38 (2.49) | 5.35 (2.61) | t = − 1.485, df = 59 | 0.143 |
| Mean menstruation duration (day) | 6.77 (2.41) | 6.43 (2.03) | 7.10 (2.72) | t = −1.076, df = 59 | 0.286 |
| Mean menstrual cycle duration (day) | 44.21 (11.86) | 45.80 (12.26) | 42.68 (11.46) | t = 1.028, df = 59 | 0.308 |
| AMH (ng/mL-baseline) | 8.76 (4.50) | 9.09 (4.03) | 8.44 (4.95) | t = 0.562, df = 59 | 0.576 |
|
Estradiol or E2 (pg/mL-baseline) |
63.12 (51.71) | 63.76 (35.98) | 62.50 (63.99) | t = 0.94, df = 59 | 0.925 |
| Progesterone (ng/mL-baseline) | 0.99 (0.57) | 1.01 (0.61) | 0.98 (0.53) | t = 0.217, df = 59 | 0.829 |
| Total testosterone (ng/mL-baseline) | 1.08 (0.44) | 1.02 (0.39) | 1.13 (0.49) | t = −0.977, df = 59 | 0.333 |
| FSH (mIU/mL-baseline) | 4.15 (1.53) | 3.89 (1.55) | 4.41 (1.49) | t = −1.318, df = 59 | 0.193 |
| LH (mIU/mL-baseline) | 10.06 (4.35) | 10.65 (4.05) | 9.49 (4.62) | t = 1.042, df = 59 | 0.302 |
| Prolactin (ng/mL-baseline) | 12.65 (5.03) | 11.61 (3.61) | 13.64 (5.99) | t = −1.611, df = 49.58 | 0.114 |
| TSH (mIU/mL-baseline) | 2.08 (1.15) | 2.04 (1.15) | 2.13 (1.17) | t = −0.295, df = 59 | 0.769 |
SD standard deviation, df degree of freedom
Twenty-five subjects (41%) had a history of at least one previous ICSI, 32 (52.5%) had hirsutism, 45 (73.8%) had irregular menstruation cycles, and 19 (31.1%) suffered from dysmenorrhea. Twenty-one patients (34.4%) had a normal menstruation, 33 (54.1%) had oligomenorrhea, and 7 (11.5%) had hypomenorrhea. All of the patients had primary infertility. There was no history of alcohol use, smoking, chronic diseases, and the use of specific drugs in the participants. All of the husbands had normal spermograms. None of the patients had galactorrhea. All of the patients underwent the flexible antagonist ovarian stimulation protocol.
Table 3 presents the laboratory parameters before and after administration of resveratrol/placebo and stimulation protocol. There was a significant change in all parameters in the resveratrol group after the intervention compared to before the intervention. However, despite other parameters, the serum levels of total testosterone and TSH did not change significantly in the control group (paired t test). Comparison of the second laboratory evaluation (after treatment) in the study groups showed that serum levels of total testosterone and LH were significantly lower, and the serum levels of FSH and TSH were significantly higher in the resveratrol group compared to the control group.
Table 3.
Comparison of laboratory tests intragroup (before and after treatment) and intergroup (after treatment between resveratrol and placebo groups)
| Laboratory parameter | Intervention mean (SD) | Before vs after resveratrol; paired t test P value |
Placebo mean (SD) | Before vs after placebo; paired t test P value |
Resveratrol compare with placebo after treatment (t test) P value | ||
|---|---|---|---|---|---|---|---|
| Before (baseline) | After | Before (baseline) | After | ||||
| AMH (ng/mL) | 9.09 (4.03) | 5.27 (3.01) | 0.0001 | 8.44 (4.95) | 6.31 (3.53) | 0.004 |
(t = − 1.243, df = 59) 0.219 |
| Estradiol (pg/mL) | 63.76 (35.98) | 2381.70 (938.46) | 0.0001 | 62.50 (63.99) | 2452.61 (876.08) | 0.0001 |
(t = − 0.305, df = 59) 0.761 |
| Progesterone (ng/mL) | 1.01 (0.61) | 1.70 (1.00) | 0.001 | 0.98 (0.53) | 1.69 (1.13) | 0.003 |
(t = 0.048, df = 59) 0.962 |
| Total testosterone (nmol/L) | 1.02 (0.39) | 0.77 (0.39) | 0.011 | 1.13 (0.49) | 1.11 (0.50) | 0.695 |
(t = − 3.003, df = 59) 0.004 |
| FSH (mIU/mL) | 3.89 (1.55) | 9.73 (2.36) | 0.0001 | 4.41 (1.49) | 8.33 (3.00) | 0.0001 |
(t = 2.02, df = 59) 0.048 |
| LH (mIU/mL) | 10.65 (4.05) | 2.78 (2.21) | 0.0001 | 9.49 (4.62) | 4.33 (2.15) | 0.0001 |
(t = − 2.793, df = 59) 0.007 |
| Prolactin (ng/mL) | 11.61 (3.61) | 26.83 (16.36) | 0.0001 | 13.64 (5.99) | 23.61 (12.98) | 0.001 |
(t = 0.853, df = 59) 0.397 |
| TSH (mIU/mL) | 2.04 (1.15) | 3.15 (2.73) | 0.030 | 2.13 (1.17) | 2.02 (1.51) | 0.517 |
(t = 2.099, df = 38.739) 0.042 |
Table 4 shows the difference in the hormonal profile of the patients in both groups. Testosterone, FSH, LH, and TSH difference levels significantly changed in the resveratrol group compared to the placebo group (levels of testosterone and LH decreased and the levels of FSH and TSH increased—see Table 4).
Table 4.
Mean difference comparison of laboratory parameters between two groups (change of parameters after treatment compared with baseline values)
| Laboratory parameter | Mean difference (SD) (after minus before) | Statistical test characteristics (t test) |
P value | |
|---|---|---|---|---|
| Resveratrol | Placebo | |||
| AMH (Dif.) | − 3.82 (3.42) | − 2.12 (3.83) | t = − 1.823, df = 59 | 0.073 |
| Estradiol (Dif.) | 2317.94 (935.94) | 2390.11 (865.35) | t = − 0.313, df = 59 | 0.755 |
| Progesterone (Dif.) | 0.694 (1.05) | 0.71 (1.21) | t = 0.065, df = 59 | 0.949 |
| Total testosterone (Dif.) | − 0.26 (0.53) | − 0.003 (0.36) | t = − 2.054, df = 59 | 0.044 |
| FSH (Dif.) | 5.84 (2.30) | 3.93 (3.00) | t = 2.786, df = 59 | 0.007 |
| LH (Dif.) | − 7.87 (3.82) | − 5.15 (4.56) | t = − 2.518, df = 59 | 0.015 |
| Prolactin (Dif.) | 15.22 (16.56) | 9.97 (14.25) | t = 1.329, df = 59 | 0.189 |
| TSH (Dif.) | 1.11 (2.66) | − 0.11 (0.91) | t = 2.409, df = 59 | 0.019 |
Dif. difference value
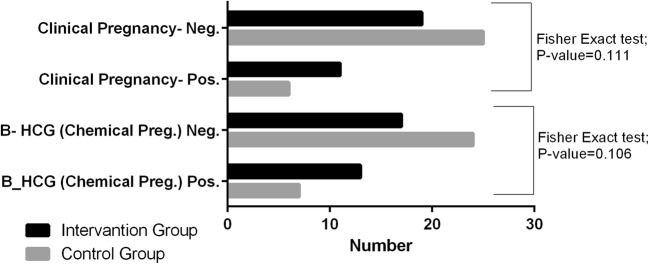
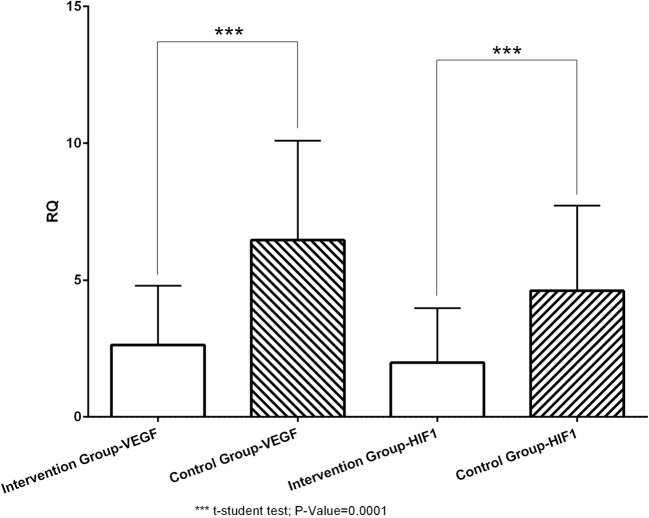
The ovulation induction outcomes (number of retrieved follicles, number of M-II (mature) oocytes, number of high-quality oocytes, and high-quality oocyte rate), ICSI, and fertility indices (number of embryos, fertilization rate, number of cleavage embryos, cleavage rate, number of high-quality embryos, and high-quality embryo rate) were compared between the two groups. Two indices (high-quality oocyte rate and high-quality embryo rate) were significantly different between the two groups (P = 0.002 and 0.024, respectively) (Table 5). It should be noted that only 17.4% of all patients had below 10 follicles retrieved (6.5% ≤ 5 follicles). The embryo transfer was canceled in 11 subjects (4 (13.3%) in resveratrol and 7 (22.58%) in control group) because of the excessive number of oocytes achieved resulting in OHSS symptoms like abdominal distension and apparent pelvic fluid (as moderate to severe OHSS based on Rizk and Aboulghar OHSS classification) [49]. Frozen embryo transfer was performed immediately in the next cycle in canceled cycles. As the final outcomes of the study (Fig. 7), the chemical pregnancy rate (chemical pregnancy per ET or embryo transfer) was 22.58% (7/31) in the placebo group and 43.3% (13/30) in the resveratrol group (Fisher’s exact test; one-sided P = 0.073, two-sided P = 0.106). Moreover, the clinical pregnancy/ET rate was 19.35% (6/31) in the placebo group and 36.67% (11/30) in the resveratrol group (Fisher’s exact test, one-sided P = 0.161, two-sided P = 0.111—see Fig. 7). Relative quantification (RQ) of the expression of VEGF & HIF1 genes in the granulosa cells in two groups are given in Fig. 8 and Table 6. According to Fig. 8 and Table 6, the expression of both VEGF and HIF1 genes was significantly lower in the resveratrol (intervention) group compared to the placebo (control) group (P value = 0.0001 for both genes).
Table 5.
Comparison of clinical outcomes between resveratrol and placebo groups
| Variable | Total patients’ mean (SD) | Intervention mean (SD) | Placebo mean (SD) | Statistical test characteristics | P value (intervention vs placebo) |
|---|---|---|---|---|---|
| Number of retrieved follicles | 19.33 (9.83) | 19.10 (9.66) | 19.55 (10.13) | t = −0.177, df = 59 | 0.860 |
| Number of M-II (mature) oocytes | 15.36 (8.09) | 15.73 (7.46) | 15.00 (8.76) | t = 0.351, df = 59 | 0.726 |
| Number of high-quality oocytes | 14.29 (7.24) | 15.47 (7.44) | 13.16 (6.98) | t = 1.248, df = 59 | 0.217 |
| High quality oocyte rate | 75.43 (16.53) | 81.93 (10.81) | 69.13 (18.71) | t = 3.257, df = 59 | 0.002 |
| Number of embryos | 12.08 (7.18) | 13.00 (7.00) | 11.19 (7.35) | t = 0.982, df = 59 | 0.330 |
| Fertilization rate | 81.11 (22.93) | 83.15 (16.97) | 79.11 (27.80) | t = 0.671, df = 47.986 | 0.505 |
| Number of cleavage embryos | 10.64 (6.34) | 11.93 (6.55) | 9.39 (5.96) | t = 1.588, df = 59 | 0.118 |
| Cleavage rate | 88.06 (15.75) | 91.74 (11.26) | 84.37 (18.70) | t = 1.851, df = 47.599 | 0.070 |
| Number of high-quality embryos | 10.20 (6.37) | 11.63 (6.44) | 8.81 (6.08) | t = 1.763, df = 59 | 0.083 |
| High quality embryo rate | 84.32 (18.89) | 89.80 (11.53) | 78.83 (23.04) | t = 2.333, df = 42.680 | 0.024 |
Fig. 7.
Comparison of pregnancy occurrence (chemical/clinical) between two groups
Fig. 8.
Expression levels of VEGF & HIF1 in the intervention compared with the control group
Table 6.
Mean (SD) of gene expression between two groups
| Gene expression | Intervention (resveratrol) RQ mean (SD) | Control (placebo) RQ mean (SD) | Statistical test characteristics (t test) | P value |
|---|---|---|---|---|
| VEGF | 2.63 (2.17) | 6.46 (3.63) | t = − 4.939, df = 47.587 | 0.0001 |
| HIF1 | 1.98 (1.99) | 4.61 (3.11) | t = − 3.874, df = 49.516 | 0.0001 |
RQ relative quantification
Discussion
It is already known that ovarian angiogenesis irregularity is one of the main pathogeneses of PCOS. As mentioned earlier, the main purpose of this study was to discover whether resveratrol can affect ovarian angiogenesis in PCOS by its effects on the expression of the main targeted genes (VEGF & HIF1) in the granulosa cells. Therefore, we tried to answer this question in a triple blind RCT and the following analyses and interpretations.
Due to the blocked randomization method, the demographic and baseline parameters of the subjects were distributed equally (without significant statistical differences) in both groups as shown in Table 2.
In the intra-group analysis presented in Table 3, the results were obtained as the effect of ovarian induction intervention plus supplement/placebo administration. Statistical tests showed a significant difference in laboratory parameters before and after the intervention. Serum estradiol levels increased not only in the intervention group, but also in the placebo group after the intervention because of the direct effect of ovulation induction on increasing the number of the grown follicles. In the ovulation induction cycle, administration of GnRH antagonist led to hypophyseal suppression, resulting in decreased levels of FSH and LH. Increased levels of FSH on the oocyte retrieval day were logical, due to exogenous FSH administration [50].
Although the personal, demographical, clinical, and laboratory characteristics did not show any differences between the two groups at the beginning of the study as a result of randomization (Table 2), some parameters, such as the hormonal profile (testosterone, FSH, LH, and TSH), were significantly different in the Resveratrol group compared to the placebo group after the intervention (Tables 3 and 4). Our findings of the serum level of testosterone were consistent with the results of studies by Banaszewska et al. [27] and Duleba et al. [51], in which resveratrol caused a significant decrease in the testosterone level. The decrease in the androgen level may be due to the lower levels of LH secretion in the resveratrol group that is concordant with the feedback control mechanism of the ovarian source androgen by LH. It should be noted that Kjaer et al. did not report any changes of the serum testosterone level in men in the resveratrol group [52], which could be due to the mechanism of action of resveratrol in different hormonal conditions (PCOS women versus men).
The serum level of AMH did not show a significant difference between the two groups, which was in contrast to the results of a study by Ergenoglu et al. [53] in which PCOS was induced by dihydrotestosterone in a rat model and the authors concluded that AMH reduced because of the antioxidant effect of resveratrol. The reason for the difference between these two studies could be the non-etiologically full-matched rat model compared to PCOS women.
The results of a study by Banaszewska et al. are not consistent with our finding regarding the significant difference in the FSH and LH serum levels [27]. However, decreased levels of LH in the resveratrol group were in agreement with the results of a study by Furat Rencber at al. [20]. Since LH hypersecretion is one of the main hallmarks of PCOS and considering the decreased levels of LH in the resveratrol group in a study by Farut Rencber and our study, it can be concluded that resveratrol can be used to control high levels of LH in PCOS patients. Even though the level of LH decreased in both groups because of the GnRH antagonist ovulation induction protocol, the decrease was more prominent in the resveratrol group than in the placebo group. This difference may be due to the effect of Resveratrol as a modulator of the LH level in PCOS women. The greater reduction of the serum LH level in our study may be due to the central effect of resveratrol on the hypothalamus by reducing the GnRH secretion pulse frequency and/or the peripheral effect through the negative feedback of LH secretion. However, it is required to design other studies to evaluate the effect of resveratrol on the LH level in PCOS women that have not received ovulation induction agents.
In our study, the level of FSH was significantly higher in the resveratrol group compared to the control group, which it could be explained by the effect of resveratrol on the reduction of serum androgen level, because hyperandrogenism is one of the reasons for the lower level of FSH in PCOS patients by higher peripheral transformation of androgens into steroids. Therefore, resveratrol may be effective in increasing the level of FSH through reducing the level of steroids derived from androgens. The secondary effect of these mechanisms is improved oocyte quality and better pregnancy outcomes, because FSH is a major factor in follicular maturity and growth.
In this study, the mean difference of serum TSH level was significantly higher in the intervention group compared to the control group. This finding also confirms the results of previous studies by Duntas and Giuliani et al. that showed anti-thyroid and goitrogenic effects of resveratrol in their studies [54, 55]. L. H. Duntas explained that resveratrol increased TSH secretion by activation of sirtuins and the phosphatidylinositol-4-phosphate 5 kinase γ (PIP5Kγ) pathway [55]. As mentioned earlier, a safe dose of resveratrol without significant side effects was selected for this study. Increased levels of TSH in the resveratrol group compared to the control group were an incidental finding during a hormonal profile assessment in our study. Elevated levels of TSH in most of the patients were in the normal range, and confirmation with serum T3 and T4 levels and clinical symptoms is necessary for a diagnosis of goiter. Moreover, the probability of the goitrogenic effect of resveratrol should be considered in future studies of resveratrol.
Regarding the ART (ICSI) outcomes, the mean number of M-II oocytes as an oocyte maturation index and the mean number of the high-quality oocytes as an index of overall oocyte quality were minimally higher than the placebo group, but the difference was not significant (P > 0.05). Moreover, the cleavage rate and the mean number of high-quality embryos were not statistically different between the two groups (P = 0.07 and 0.083, respectively). The results showed a significant difference in two indices between the two groups: The “high-quality oocyte rate” (as an oocyte quality index) and the “high-quality embryo rate” (as an embryo quality index). These indices are important for the overall effect of resveratrol on the ICSI protocol quality improvement. Considering the small sample size of this study and the formula used to discuss the high-quality oocyte rate and the high-quality embryo rate, it can be discussed that the high-quality oocyte rate is a more sensitive index compared to the “mean number of high-quality oocyte” for the oocyte quality assessment, because high-quality oocyte rate can change easily following a little change in the numerator and/or denominator of the fraction. In our case, the number of high-quality oocytes (in the numerator of the fraction) was a little larger and the total number of retrieved oocytes (in the denominator of the fraction) was a little smaller in the resveratrol group compare to the control group. This also applies to the “high-quality embryo rate” compared to the “mean number of the high-quality embryos” (both as embryo quality indices). Another point is the larger difference in high-quality oocyte rate compare to high-quality embryo rate. This shows that, resveratrol may have an effect on the promotion of the oocyte function and its potential for better embryo production. In other words, the better the embryo quality on day 3 might be the result of the better oocyte quality under the effect of resveratrol. The fertility rate, as a critical index of oocyte quality, was not significantly higher in the resveratrol group compared to the placebo group (P = 0.073). The chemical pregnancy rate increased from 22.58% in the placebo group to 43.3% in the resveratrol group. Similarly, the clinical pregnancy rate also improved from 19.35% in the placebo group to 36.67% in the resveratrol group. The pregnancy rate, as the main IVF outcome, which is an index of the quality control of all the processes and an exclusive index of uterine receptivity, did not improved significantly following resveratrol administration in the interventional group versus the placebo group (P > 0.05, Fisher’s exact test), probably because of the small sample size for fertility rate calculation. We did not find any studies evaluating fertility and ICSI outcomes of resveratrol administration in humans. However, it has been shown that resveratrol improves the overall fertility and in vitro maturation of the oocytes (as medium culture supplement) in rat and mice [18, 56, 57]. The differences between our finding and the results of these studies could arise from resveratrol metabolism and its pharmacokinetics in the body, resveratrol dosage, exposure duration, and exposure phase (in the follicle or denuded oocyte).
It should be noted that the critical molecular pathway in the pathogenesis of PCOS selected in this study was angiogenesis. Angiogenesis is one of the important findings of recent studies in PCO research as an etiological pathway. The main molecule in this pathway that plays a basic role is VEGF or vascular endothelial growth factor. As mentioned in the GeneCards® (human genes database), this growth factor induces proliferation and migration of vascular endothelial cells, and is essential to both physiological and pathological angiogenesis, especially under hypoxic conditions, by induction of other genes like HIF1 [58].
One of the most important sources of VEGF secretion in the ovary is granulosa cells, and LH is an important factor in the stimulation of VEGF expression in ovarian cells [10]. In PCOS patients, the LH secretion level is higher than normal, so it leads to higher VEGF expression in granulosa and theca cells around the follicles [9]. High levels of VEGF increase vascular permeability and shift fluids from the vessels to the extravascular space. Increased ovarian stroma blood flow can result in the loss of the ovarian auto-regulatory mechanism causing uni-follicular ovulation in the normal ovary. Irregularity in the ovarian environment leads to disruption of follicle maturation, which has negative effects on the oocyte nutritional conditions and maturation and decreases fertility outcomes [9]. Intense vascularization may lead to abnormal growth of the theca interna in PCOS ovaries (which is one of the main sites of androgen steroidogenesis). This hyperandrogenism condition is another mechanism of infertility in PCOS [9]. The findings of some studies indicate that elevated serum and follicular VEGF levels on the oocyte pick-up day could be considered a predictive marker to identify patients at risk for OHSS [10].
Based on our findings, resveratrol can decrease (modify) the expression of the VEGF gene in the granulosa cells of the follicles after 40 days of administration (P = 0.0001). This finding is supported by the results of an in vitro experimental study conducted by Ortega et al. in rat granulosa cells in which resveratrol decreased the VEGF expression, too [29]. The findings of these studies are similar despite differences in the study setting (in vitro vs. in vivo), condition (normal vs. PCOS), and organism (rats vs. humans). According to these results, resveratrol acts as a modulator of the gene expression and optimizes the overall condition of the cells and tissues in a system biology manner in a network of interactions. Based on this effect of resveratrol, it can be used not only to improve the infertility problems of PCOS women, but also to decrease the chance of OHSS in ovarian induction cycles Table 6.
Since hyperandrogenism is the most prevalent sign of PCOS, high androgen levels may be the most important reason for increased VEGF levels in these women. Androgen activates hypoxia-inducible factor (HIF1), a known activator of VEGF. HIF1 increases the expression of VEGF in steroid-processing cells [14]. In our study, the expression of the HIF1 gene decreased significantly in granulosa cells following the use of resveratrol as a supplement therapy for PCOS patients (P = 0.0001). This effect of resveratrol on the HIF1 level is supported by other studies in other human body organs. No similar studies were found in PCOS patients; however, it has been shown that resveratrol affects the hypoxia-inducible factor in some other diseases [33, 35, 59, 60]. For example, resveratrol has suppressive effects on HIF1a in ischemic liver cells in rats [60]. Decreased HIF1 expression is consistent with decreased expression of VEGF (in the resveratrol group) and confirms the role of the androgen-induced pathway in controlling the expression of VEGF, as also shown by previous studies [14].
Conclusion
Based on our results, it can be concluded that resveratrol, as a natural supplement with anti-inflammatory and anti-oxidant effects, can modify the molecular pathways of angiogenesis by reducing the expression of VEGF and HIF1 genes in the granulosa cells of follicles and can improve the high-quality oocyte rate and the high-quality embryo rate as the clinical outcomes. Resveratrol may decrease the serum total testosterone and LH levels and increase the TSH and FSH levels. Therefore, PCOS patients may benefit from resveratrol administration; however, attention should be paid to possible goitrogenic effects of resveratrol, especially in long-term administration.
Electronic supplementary material
(PDF 483 kb)
Acknowledgements
This work was financially supported by the Research Deputy of Tehran University of Medical Sciences (Grant Number 94-02-30-29469). The biological material was kindly provided by Omid IVF Center.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rotterdam EA-SPcwg. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004;19(1):41–47. [DOI] [PubMed]
- 2.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, Piltonen T, Norman RJ, International PCOS Network. Andersen M, Azziz R, Balen A, Baye E, Boyle J, Brennan L, Broekmans F, Dabadghao P, Devoto L, Dewailly D, Downes L, Fauser B, Franks S, Garad RM, Gibson-Helm M, Harrison C, Hart R, Hawkes R, Hirschberg A, Hoeger K, Hohmann F, Hutchison S, Joham A, Johnson L, Jordan C, Kulkarni J, Legro RS, Li R, Lujan M, Malhotra J, Mansfield D, Marsh K, McAllister V, Mocanu E, Mol BW, Ng E, Oberfield S, Ottey S, Peña A, Qiao J, Redman L, Rodgers R, Rombauts L, Romualdi D, Shah D, Speight J, Spritzer PM, Stener-Victorin E, Stepto N, Tapanainen JS, Tassone EC, Thangaratinam S, Thondan M, Tzeng CR, van der Spuy Z, Vanky E, Vogiatzi M, Wan A, Wijeyaratne C, Witchel S, Woolcock J, Yildiz BO. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602–1618. doi: 10.1093/humrep/dey256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7(4):219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril. 2016;106(1):6–15. doi: 10.1016/j.fertnstert.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, Koebnick C. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril. 2013;100(2):470–477. doi: 10.1016/j.fertnstert.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tannus S, Tan J, Son WY, Dahan MH. Prevalence, clinical characteristics, and reproductive outcomes of polycystic ovary syndrome in older women referred for tertiary fertility care. Arch Gynecol Obstet. 2018;297(4):1037–1042. doi: 10.1007/s00404-017-4642-z. [DOI] [PubMed] [Google Scholar]
- 7.Alexiou E, Hatziagelaki E, Pergialiotis V, Chrelias C, Kassanos D, Siristatidis C, Kyrkou G, Kreatsa M, Trakakis E. Hyperandrogenemia in women with polycystic ovary syndrome: prevalence, characteristics and association with body mass index. Horm Mol Biol Clin Invest. 2017;29(3):105–111. doi: 10.1515/hmbci-2016-0047. [DOI] [PubMed] [Google Scholar]
- 8.Di Pietro M, Pascuali N, Parborell F, Abramovich D. Ovarian angiogenesis in polycystic ovary syndrome. Reproduction. 2018;155(5):R199–R209. doi: 10.1530/REP-17-0597. [DOI] [PubMed] [Google Scholar]
- 9.Peitsidis P, Agrawal R. Role of vascular endothelial growth factor in women with PCO and PCOS: a systematic review. Reprod BioMed Online. 2010;20(4):444–452. doi: 10.1016/j.rbmo.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Artini PG, Monti M, Cristello F, Matteucci C, Bruno S, Valentino V, et al. Vascular endothelial growth factor in females of reproductive age. Gynecol Endocrinol. 2003;17(6):477–492. doi: 10.1080/09513590312331290418. [DOI] [PubMed] [Google Scholar]
- 11.Ben Salem A, Megdich F, Kacem O, Souayeh M, Hachani Ben Ali F, Hizem S, et al. Vascular endothelial growth factor (VEGFA) gene variation in polycystic ovary syndrome in a Tunisian women population. BMC Genomics. 2016;17(Suppl 9):748. doi: 10.1186/s12864-016-3092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudsy M, Alhalabi M, Al-Quobaili F. Follicular fluid vascular endothelial growth factor (VEGF) could be a predictor for pregnancy outcome in normo-responders and polycystic ovary syndrome women undergoing IVF/ICSI treatment cycles. Middle East Fertil Soc J. 2016;21(1):52–56. [Google Scholar]
- 13.Meek CL, Bravis V, Don A, Kaplan F. Polycystic ovary syndrome and the differential diagnosis of hyperandrogenism. Obstet Gynaecol. 2013;15(3):171–176. [Google Scholar]
- 14.Lebbe M, Woodruff TK. Involvement of androgens in ovarian health and disease. Mol Hum Reprod. 2013;19(12):828–837. doi: 10.1093/molehr/gat065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Leo V, Musacchio MC, Cappelli V, Massaro MG, Morgante G, Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol. 2016;14(1):38. doi: 10.1186/s12958-016-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, Tian X, Zhang L, He C, Ji P, Li Y, et al. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil Steril. 2014;101(2):577–86.e1. doi: 10.1016/j.fertnstert.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 18.Liu MJ, Sun AG, Zhao SG, Liu H, Ma SY, Li M, Huai YX, Zhao H, Liu HB. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil Steril. 2018;109(5):900–907. doi: 10.1016/j.fertnstert.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Kwak SS, Cheong SA, Jeon Y, Lee E, Choi KC, Jeung EB, Hyun SH. The effects of resveratrol on porcine oocyte in vitro maturation and subsequent embryonic development after parthenogenetic activation and in vitro fertilization. Theriogenology. 2012;78(1):86–101. doi: 10.1016/j.theriogenology.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Furat Rencber S, Kurnaz Ozbek S, Eraldemir C, Sezer Z, Kum T, Ceylan S, et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res. 2018;11(1):55. doi: 10.1186/s13048-018-0427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asghari S, Rafraf M, Farzin L, Asghari-Jafarabadi M, Ghavami SM, Somi MH. Effects of pharmacologic dose of resveratrol supplementation on oxidative/antioxidative status biomarkers in nonalcoholic fatty liver disease patients: a randomized, double-blind, placebo-controlled trial. Adv Pharm Bull. 2018;8(2):307–317. doi: 10.15171/apb.2018.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimoh A, Tanko Y, Ahmed A, Mohammed A, Ayo JO. Resveratrol prevents high-fat diet-induced obesity and oxidative stress in rabbits. Pathophysiology. 2018;25:359–364. doi: 10.1016/j.pathophys.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Seyyedebrahimi S, Khodabandehloo H, Nasli Esfahani E, Meshkani R. Correction to: the effects of resveratrol on markers of oxidative stress in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. Acta Diabetol. 2018. 10.1007/s00592-018-1160-9. [DOI] [PubMed]
- 24.Ghowsi M, Khazali H, Sisakhtnezhad S. The effect of resveratrol on oxidative stress in the liver and serum of a rat model of polycystic ovary syndrome: an experimental study. Int J Reprod Biomed (Yazd) 2018;16(3):149–158. [PMC free article] [PubMed] [Google Scholar]
- 25.Oh WY, Shahidi F. Antioxidant activity of resveratrol ester derivatives in food and biological model systems. Food Chem. 2018;261:267–273. doi: 10.1016/j.foodchem.2018.03.085. [DOI] [PubMed] [Google Scholar]
- 26.Khojah HM, Ahmed S, Abdel-Rahman MS, Elhakeim EH. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: a clinical study. Clin Rheumatol. 2018;37(8):2035–2042. doi: 10.1007/s10067-018-4080-8. [DOI] [PubMed] [Google Scholar]
- 27.Banaszewska B, Wrotynska-Barczynska J, Spaczynski RZ, Pawelczyk L, Duleba AJ. Effects of resveratrol on polycystic ovary syndrome: a double-blind, randomize,Placebo-controlled Trial. J Clin Endocrinol Metab. 2016;101(11):4322–4328. doi: 10.1210/jc.2016-1858. [DOI] [PubMed] [Google Scholar]
- 28.Aquino CI, Nori SL. Complementary therapy in polycystic ovary syndrome. Transl Med UniSa. 2014;9:56–65. [PMC free article] [PubMed] [Google Scholar]
- 29.Ortega I, Wong DH, Villanueva JA, Cress AB, Sokalska A, Stanley SD, Duleba AJ. Effects of resveratrol on growth and function of rat ovarian granulosa cells. Fertil Steril. 2012;98(6):1563–1573. doi: 10.1016/j.fertnstert.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palsamy P, Subramanian S. Ameliorative potential of resveratrol on proinflammatory cytokines, hyperglycemia mediated oxidative stress, and pancreatic beta-cell dysfunction in streptozotocin-nicotinamide-induced diabetic rats. J Cell Physiol. 2010;224(2):423–432. doi: 10.1002/jcp.22138. [DOI] [PubMed] [Google Scholar]
- 31.Yan F, Sun X, Xu C. Protective effects of resveratrol improve cardiovascular function in rats with diabetes. Exp Ther Med. 2018;15(2):1728–1734. doi: 10.3892/etm.2017.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu HB, Zhang HF, Zhang X, Li DY, Xue HZ, Pan CE, et al. Resveratrol inhibits VEGF expression of human hepatocellular carcinoma cells through a NF-kappa B-mediated mechanism. Hepatogastroenterology. 2010;57(102–103):1241–1246. [PubMed] [Google Scholar]
- 33.Cao Z, Fang J, Xia C, Shi X, Jiang BH. Trans-3,4,5′-trihydroxystibene inhibits hypoxia-inducible factor 1alpha and vascular endothelial growth factor expression in human ovarian cancer cells. Clin Cancer Res. 2004;10(15):5253–5263. doi: 10.1158/1078-0432.CCR-03-0588. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee A, Ronghe A, Padhye SB, Spade DA, Bhat NK, Bhat HK. Antioxidant activities of novel resveratrol analogs in breast cancer. J Biochem Mol Toxicol. 2018;32(1). 10.1002/jbt.21925. [DOI] [PubMed]
- 35.Li Y, Liu Y, Lu Y, Zhao B. Inhibitory effects of 17beta-estradiol or a resveratrol dimer on hypoxia-inducible factor-1alpha in genioglossus myoblasts: involvement of ERalpha and its downstream p38 MAPK pathways. Int J Mol Med. 2017;40(5):1347–1356. doi: 10.3892/ijmm.2017.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106(13):1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 37.Mokhtar S, Sadeghi MR, Akhondi MM, Zafardoust S, Badenush B, Fatemi F, et al. ART outcomes in GnRH antagonist protocol (flexible) and long GnRH agonist protocol during early follicular phase in patients with polycystic ovary syndrome: a randomized clinical trial. J Reprod Infertil. 2015;16(3):148–154. [PMC free article] [PubMed] [Google Scholar]
- 38.Rajashekar L, Krishna D, Patil M. Polycystic ovaries and infertility: our experience. J Hum Reprod Sci. 2008;1(2):65–72. doi: 10.4103/0974-1208.44113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sattarinezhad A, Roozbeh J, Shirazi Yeganeh B, Omrani GR, Shams M. Resveratrol reduces albuminuria in diabetic nephropathy: a randomized double-blind placebo-controlled clinical trial. Diabetes Metab. 2019;45:53–59. doi: 10.1016/j.diabet.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Ozgur S, Oktem M, Altinkaya SO, Oktem EO, Cenksoy C, Erdem O, Elbeg S, Helvaci A, Erdem A, Erdem M. The effects of resveratrol on ovarian hyperstimulation syndrome in a rat model. Taiwan J Obstet Gynecol. 2018;57(3):383–388. doi: 10.1016/j.tjog.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: a review. Crit Rev Food Sci Nutr. 2018;58(9):1428–1447. doi: 10.1080/10408398.2016.1263597. [DOI] [PubMed] [Google Scholar]
- 42.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, et al. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007;80(11):1033–1039. doi: 10.1016/j.lfs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 43.Labinskyy N, Csiszar A, Veress G, Stef G, Pacher P, Oroszi G, et al. Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem. 2006;13(9):989–996. doi: 10.2174/092986706776360987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahal HS, Mukherjee T. Scavenging of reactive oxygen radicals by resveratrol: antioxidant effect. Res Chem Intermed. 2006;32(1):59–71. [Google Scholar]
- 45.Lazzaroni-Tealdi E, Barad DH, Albertini DF, Yu Y, Kushnir VA, Russell H, Wu YG, Gleicher N. Oocyte scoring enhances embryo-scoring in predicting pregnancy chances with IVF where it counts Most. PLoS One. 2015;10(12):e0143632. doi: 10.1371/journal.pone.0143632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rienzi L, Balaban B, Ebner T, Mandelbaum J. The oocyte. Hum Reprod. 2012;27(suppl_1):i2–i21. doi: 10.1093/humrep/des200. [DOI] [PubMed] [Google Scholar]
- 47.Hershko-Klement A, Rovner E, Yekutieli D, Ghetler Y, Gonen O, Cohen I, et al. Embryo quality and implantation rates are not influenced by total motile count values in an ICSI programme: a novel point of view. Int J Mol Epidemiol Genet. 2012;3(3):205–212. [PMC free article] [PubMed] [Google Scholar]
- 48.Alpha Scientists in Reproductive M, Embryology ESIGo The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod. 2011;26(6):1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 49.Rizk B, Aboulghar MA. Classification, pathophysiology and management of ovarian hyperstimulation syndrome. In: Pe B, editor. In-vitro fertilization and assisted reproduction. New York, London: The Parthenon Publishing Group; 1999. pp. 131–155. [Google Scholar]
- 50.Ron-El R, Raziel A, Schachter M, Strassburger D, Kasterstein E, Friedler S. Induction of ovulation after gnRH antagonists. Hum Reprod Update. 2000;6(4):318–321. doi: 10.1093/humupd/6.4.318. [DOI] [PubMed] [Google Scholar]
- 51.Duleba A, Spaczynski RZ, Pawelczyk L. Effects of resveratrol on polycystic ovary syndrome. Fertil Steril. 2016;106,3(Supplement):e36. [Google Scholar]
- 52.Kjaer TN, Ornstrup MJ, Poulsen MM, Jorgensen JO, Hougaard DM, Cohen AS, et al. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate. 2015;75(12):1255–1263. doi: 10.1002/pros.23006. [DOI] [PubMed] [Google Scholar]
- 53.Ergenoglu M, Yildirim N, Yildirim AG, Yeniel O, Erbas O, Yavasoglu A, et al. Effects of resveratrol on ovarian morphology, plasma anti-Mullerian hormone, IGF-1 levels, and oxidative stress parameters in a rat model of polycystic ovary syndrome. Reprod Sci. 2015;22(8):942–947. doi: 10.1177/1933719115570900. [DOI] [PubMed] [Google Scholar]
- 54.Giuliani C, Iezzi M, Ciolli L, Hysi A, Bucci I, Di Santo S, et al. Resveratrol has anti-thyroid effects both in vitro and in vivo. Food Chem Toxicol. 2017;107(Pt A):237–247. doi: 10.1016/j.fct.2017.06.044. [DOI] [PubMed] [Google Scholar]
- 55.Duntas LH. Resveratrol and its impact on aging and thyroid function. J Endocrinol Investig. 2011;34(10):788–792. doi: 10.3275/7926. [DOI] [PubMed] [Google Scholar]
- 56.Wong DH, Villanueva JA, Cress AB, Sokalska A, Ortega I, Duleba AJ. Resveratrol inhibits the mevalonate pathway and potentiates the antiproliferative effects of simvastatin in rat theca-interstitial cells. Fertil Steril. 2011;96(5):1252–1258. doi: 10.1016/j.fertnstert.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Kyselova V, Peknicova J, Buckiova D, Boubelik M. Effects of p-nonylphenol and resveratrol on body and organ weight and in vivo fertility of outbred CD-1 mice. Reprod Biol Endocrinol. 2003;1:30. doi: 10.1186/1477-7827-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54(1 30):1–1 3. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 59.Mitani T, Harada N, Tanimori S, Nakano Y, Inui H, Yamaji R. Resveratrol inhibits hypoxia-inducible factor-1alpha-mediated androgen receptor signaling and represses tumor progression in castration-resistant prostate cancer. J Nutr Sci Vitaminol (Tokyo) 2014;60(4):276–282. [PubMed] [Google Scholar]
- 60.Zhang M, Li W, Yu L, Wu S. The suppressive effect of resveratrol on HIF-1alpha and VEGF expression after warm ischemia and reperfusion in rat liver. PLoS One. 2014;9(10):e109589. doi: 10.1371/journal.pone.0109589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 483 kb)