Abstract
Four decades of preclinical research demonstrating survival, functional integration, and behavioral effects of transplanted stem cells in experimental stroke models have provided ample scientific basis for initiating limited clinical trials of stem cell therapy in stroke patients. Although safety of the grafted cells has been overwhelmingly documented, efficacy has not been forthcoming. Two recently concluded stroke clinical trials on mesenchymal stem cells (MSCs) highlight the importance of strict adherence to the basic science findings of optimal transplant regimen of cell dose, timing, and route of delivery in enhancing the functional outcomes of cell therapy. Echoing the Stem Cell Therapeutics as an Emerging Paradigm for Stroke and Stroke Treatment Academic Industry Roundtable call for an NIH‐guided collaborative consortium of multiple laboratories in testing the safety and efficacy of stem cells and their derivatives, not just as stand‐alone but preferably in combination with approved thrombolytic or thrombectomy, may further increase the likelihood of successful fruition of translating stem cell therapy for stroke clinical application. The laboratory and clinical experience with MSC therapy for stroke may guide the future translational research on stem cell‐based regenerative medicine in neurological disorders. stem cells translational medicine 2019;8:983&988
Keywords: Cerebral ischemia, Stem cell transplantation, Regenerative medicine, Basic science, Translation, Clinical
Significance Statement.
Almost 4 decades of laboratory research have shown safety and efficacy of stem cells in stroke animals. Yet, this cell‐based regenerative medicine remains designated as “experimental” in the clinic. Equally disappointing, two recently concluded clinical trials indicated stem cells are safe but not effective in stroke patients. These failed clinical trials may be due to a loss in translation of optimal laboratory stem cell transplantation protocols to clinical trial designs. A concerted effort between basic scientists and clinicians, with NIH and Food and Drug Administration guidance, is key to realizing the safe and effective translation of stem cell therapy for stroke.
Stem Cell Therapy for Stroke Has Reached Clinical Trials. The Long Wait Is Over! Or Is the Wait Still On?
In the late 1980s, Sharp and colleagues ushered one of the pioneering laboratory investigations in cell therapy for stroke, demonstrating the survival of rat fetal neocortical grafts in ischemic adult rat cortex 1, 2. Subsequent studies showed that these grafted fetal cells integrated with the ischemic brain received afferent fibers and vascularization from the host intact tissue 3, 4 and responded to contralateral sensory stimulation with increased metabolic activity 5. Equally promising are the observations that stroke animals transplanted with fetal striatal cells into the ischemic striatum displayed some improvements in a simple cognitive task of passive avoidance 6, as well as in a more complex water maze learning test 7.
Over the next 4 decades of preclinical research, additional evidence of graft survival, migration, differentiation, and functional integration in the ischemic brain, modest anatomical reconstruction, and remodeling of brain circuitry, neurochemical, physiological, and behavioral recovery have been documented 2, 8. Several mechanisms have also been postulated to mediate the therapeutic effects of cell transplants in stroke; although initially designed as a cell replacement for dead or ischemic cells, the current view puts robust by‐stander effects of the grafted cells to secrete therapeutic substances 9, 10, 11, 12. The initial studies on human neuroteratocarcinoma cells were to convert these cells into postmitotic neuron‐like cells 13. Subsequent studies on embryonic stem cells 14, genetically engineered mesenchymal stem cells (MSCs; Sanbio, Mountain View, CA) 15, and fetal‐derived stem cells (by Reneuron, Bridgend, UK) 16, and even with the present modification of induced pluripotent stem cells (iPSCs) for stroke indication, still maintain the need to generate an ample amount of neuron‐like cells based on the notion that functional recovery can be achieved by repairing the neuronal synaptic circuitry via replenishing infarcted cells and ischemic cells with neuronal cells. The recognition that stroke not only affects neurons but also other neural cell types, especially vascular cells, prompted the search for alternative regenerative processes that rescue in tandem neural and vascular cells, under the theme of attenuating the impaired neurovascular unit 17. Toward stimulating these non‐neuronal repair processes, the stem cells' by‐stander effects have been proposed, including the grafted cells' ability to secrete substances that promote neurogenesis, angiogenesis, vasculogenesis, anti‐inflammation, among other therapeutic substances. Over the last 5 years, additional novel stem cell component‐based mechanisms have been demonstrated to accompany stem cell therapy, such as the transfer of stem cell‐derived mitochondria, exosomes, microvesicles, and micro‐RNAs into the ischemic area 18, 19, 20, 21, 22. Additionally, although stroke is traditionally considered a brain disorder, the role of peripheral organs, such as the spleen and the gut, has been implicated in the disease pathology, and that sequestration of their aberrant inflammatory and microbiome response has been deemed therapeutic, which can be achieved with stem cell transplantation 19, 23. With these regenerative mechanisms ascribed to stem cells, developing them into stem cell release criteria would enhance the quality control screening of viable and transplantable cells that would ensure therapeutic potency following their transplantation into the stroke brain. One could also envision using these potency assays as indices of stem cell functional status during the poststroke transplantation period, in that one can monitor the levels of growth factors, inflammation, mitochondrial function, splenic function, and gut microbiota both centrally and peripherally. Thus, in addition to phenotypic markers of stemness and the optimal transplant regimen (i.e., dose, timing, and route of delivery), adding these potency assays will likely improve the clinical outcomes of stem cell therapy for stroke.
These important basic science experiments have laid the groundwork for advancing cell therapy for stroke to the clinic. Recognizing that same species allogeneic transplantation (i.e., from rat‐to‐rat cell‐donor‐transplant recipient to human‐to‐human cell‐donor‐transplant recipient) may provide safe and effective treatment for the envisioned clinical trial, the search for transplantable human cells became paramount to realize translation of cell therapy from the laboratory to the clinic. The use of fetal cells poses ethical and logistical challenges. A moratorium in the 1990s on the use of federal funds for embryonic stem cell research, and with iPSCs still not yet available at that time, nullified pursuing these tissue sources for clinical cell therapy. The first human clinical trial using human neuroteratocarcinoma cells transformed into postmitotic neurons transplanted into stroke patients was performed in 1998 24 based on our earlier preclinical work 13. Financial strain to the company sponsor of these neuroteratocarcinoma cells contributed to abandoning this clinical trial, prompting the search for alternative transplantable cells. Adult tissue‐derived stem cells, including those harvested from bone marrow, umbilical cord, and adipose, eventually took center stage and to date remain as the front‐runner cell source for cell therapy for stroke 25, 26, 27.
Primarily because of the long‐track record of solid safety profile, the bone marrow has emerged as the widely used adult tissue stem cell source 26, 28, 29, 30, 31, 32. Bone marrow‐derived cell populations as well as engineered stem/progenitor cells have been characterized, including but not limited to MSCs, mononuclear cells (MNCs), endothelial progenitor cells, SB623, multipotent adult progenitor cells (MAPCs), and multilineage‐differentiating stress‐enduring cells (Muse) 33, 34, 35, 36, 37. These bone marrow‐derived stem cells have been widely examined in preclinical stroke models, revealing the cells' ability to display multipotent cell properties in vitro 38, 39, 40, 41, 42, 43 and to reduce behavioral and histological deficits after transplantation in vivo 44, providing solid basis for clinical trials. Autologous bone marrow MSCs when intravenously transplanted in patients at 4 weeks after stroke onset displayed no adverse effects and even improved neurological outcomes, but such efficacy declined by 12 months post‐transplantation 45. An open‐labeled trial of autologous transplantation of bone marrow MNCs intravenously delivered in patients within 24–72 hours poststroke was also safe and exerted better functional recovery that lasted for over 6 month post‐transplantation 46. However, a subsequent phase II, multicenter, parallel group, randomized, and blinded trial demonstrated that autologous bone marrow MNCs intravenously delivered at median of 18.5 days after stroke onset was again safe but did not result in functional improvement 47. Interestingly, another open‐labeled trial employing an intra‐arterial deliver of a subset of CD34+ bone marrow MNCs within 7 days of stroke onset was also shown to be safe with improved functional outcomes during the 6‐month post‐transplantation period 48. More recent clinical trials have reported mixed outcomes, in that while overwhelmingly safe, demonstrating the efficacy of stem cell therapy for stroke has failed in both the intravenous transplantation of MAPCs in acute stroke patients 49 and the intracerebral transplantation of SB623 in chronic stroke patients 50, 51, 52.
Based on these interim clinical trials, transplantation of bone marrow stem cell derivatives, primarily MSCs and MNCs, is deemed safe for stroke, but their efficacy remains elusive. Conclusive interpretations of the clinical data are hindered by small number of enrolled patients and the open‐labeled approach in some of these trials. Moreover, vis‐a‐vis comparisons between these trials will be difficult because of different donor cells and varied clinical transplant protocols (Table 1). Phenotypic markers for MSCs include SH‐2 and SH‐4 45; specific flow cytometric antibodies (CD3, CD14, CD16, CD19, CD20, CD34, CD45, CD56, Lin 1, CD133‐2) 46; or limited to CD34 and CD45 47; or magnetic cell isolation procedures focused on CD34+ cells 48; MAPCs are defined as c‐Kit+, CD9+, CD13+, CD31+, CD44−, MHC‐I‐, CD45−, Thy1‐ 49, 53, while SB623 are Notched‐induced MSCs 15, 50, 51, 54, 55. Additionally, the therapeutic windows spanned from acute to chronic stroke stages, as well as routes of administration varied across trials 45, 46, 47, 48, 49, 50, 51.
Table 1.
Stem cell transplantation protocols for stroke therapy
| Reports | Stem cell type | Route | Dose | Timing |
|---|---|---|---|---|
| Laboratory studies | ||||
| Mays et al. 59 | MAPCs | i.c. | 0.4 million | 7 days |
| Mays et al. 59 | MAPCs | i.v. | 4 million | 1–7 days |
| Acosta et al. 89 | MSCs | i.v. | 4 million | 60 days |
| Borlongan et al. 90 | MSCs | i.c. | 0.2 million | 3 hours |
| Clinical trials | ||||
| Bang et al. 45 | MSCs | i.v. | 100 million | 4 weeks |
| Savitz et al. 46 | MNCs | i.v. | 100 million | 1–3 days |
| Prasad et al. 47 | MNCs | i.v. | 280.75 million | 18.5 days |
| Banerjee et al. 48 | HSCs | i.a. | 100 million | 7 days |
| Hess et al. 49 | MAPC | i.v. | 400–1,200 million | 24–48 hours |
| Steinberg et al. 50 | SB623 | i.c. | 2.5–10 million | 6–60 months |
| Hess et al. 60 | MAPC | i.v. | 1,200 million | 18–36 hours |
| Kalladka et al. 91 | CTX‐DP | i.c. | 2–20 million | 6–60 months |
| Lee et al. 92 | MSCs | i.v. | 50 million | 4 weeks |
Type of cells, route of administration, doses, and timing are listed.
Abbreviations: HSCs, hematopoietic stem cells; i.c., intracerebral; MAPCs, multipotent adult progenitor cells; MNCs, mononuclear cells; MSCs, mesenchymal stem cells.
A major contributor to the failure of clinical trials to reach efficacy endpoints is the translational discrepancy between the laboratory and clinical stem cell transplant protocols (Table 1). Efficacy readouts in the laboratory, which were achieved under strict cell dose and timing of transplantation windows, are not strictly followed in the clinic. The preclinical effective intravenous dose is around 4 million cells in a stroke rat weighing 250 g, which translates to approximately 840 million cells in a stroke patient weighing 75 kg 56, yet most clinical trials use doses well below this efficacious dose 45, 47, 48. Of note, stroke patients who received a dose that adhered to this preclinical cell dose displayed clinical improvements 46. In the same token, the intracerebral dose of 200,000 cells is efficacious in the stroke rat, which is equivalent to approximately 56 million cells in the stroke patient, but again the clinical doses (2.5 and 5 million cells) used were at least 10‐fold below this dose, which may explain the lack of efficacy 50, 51. The cell dose found effective in the stroke rat was correctly gated in the MAPC trial (400 to 1,200 million cells) 49 but still did not reach efficacy 57. Post hoc analysis, however, revealed that those patients who received the MAPC transplants less than 36 hours exhibited functional improvements 58, which were predicted in the preclinical study 59 and now the targeted therapeutic window in a subsequent MAPC trial 60. Strict adherence to the preclinical outcomes of optimal cell dose, timing, and route is a must if efficacy is to be achieved in the clinic.
Lab‐to‐clinic translation and the subsequent clinical trial design of stem cell therapy have emphasized the logistics and technical aspects of the transplant regimen and may have neglected the basic science discoveries that defined stemness properties and mechanisms of stem cells. The rule of thumb when contemplating any envisioned stem cell clinical product must consider a well‐defined set of phenotypic markers of the stem cells and a solid insight into their mode of action. Access to clinical‐grade stem cells should preclude a set of product release criteria of either homogenous population of cells or a consistent and reproducible generation of the same stem cell population. The clinical transplant regimen should also build upon the lessons learned from the laboratory about postulated mechanisms, including cell replacement, growth factor secretion, and promotion of endogenous brain repair processes 61, 62, 63, 64, 65, which may synergistically work together to combat the multipronged cell death pathways associated with stroke 66, 67, 68. In view of this intricate stroke‐induced cell death cascade, stem cell therapy may be optimized not as a stand‐alone treatment but as a combination therapy with tissue Plasminogen Activator (tPA), other neuroprotective drugs, biomaterials 69, or thrombectomy (see below), as well as with standard stroke care management involving rehabilitation 70, 71.
Although efficacy in preclinical stroke models is paramount toward moving the stem cell product to the clinic, equally important is establishing the safety profile of the stem cells, such as their proliferative, tumorgeneic or ectopic tissue formation capacity, persistence specifically tissue or organ deposition, and cell fate following transplantation into the stroke brain. The differentiation of stem cells into a phenotype when lodged in a particular organ may elicit deleterious immune response or inflammatory reaction that can be toxic or can elicit tumorigenesis. Ample consideration should also be given when using genetically engineered stem cells, such as SB623 or immortalized cells such as CTX03 26, 30. With the notion that stem cells may confer therapeutic effects via secreted substances, there is also a translational push favoring decellularized over cellularized compositions, and such pharmacologic‐like or cell‐free treatments may benefit from the translational Stroke Treatment Academic Industry Roundtable (STAIR) criteria toward achieving the neuroprotective drug's safety profile 72. Specific safety deliberations are necessary when using freshly harvested, cultured, or cryopreserved cells, naked, encapsulated, or extracellular matrix‐loaded cells, and novel delivery devices such as sustained, controlled, and highly regulated nanoparticles, exosomes, extracellular vesicles, microRNAs, mitochondria, and other cellular components 18, 20, 73, 74, 75, 76. The once cell‐directed transplantation approach has been replaced with much sophisticated cell components that have expanded toward emerging therapies using innovative cell‐derivative and even cell‐free compositions that will require a unique set of safety outcome evaluations.
If clinical entry of stem cell products is the desired goal, then the use of clinical‐grade stem cells from the get‐go would allow a more efficient entry of stem cells to the clinic. The rigid regulatory translational path of stem cell from the laboratory to the clinic provides no allowance for modification of the stem cell product. Developing clinical‐grade lines would require many changes to the original protocols, because the standards for manufacturing clinical biological agents are stricter than the standards for research‐grade cell lines, necessitating the need to find alternatives to the laboratory animal‐derived reagents that most likely are not allowed for clinical use 77. In the end, the Good Manufacturing Practice (GMP)‐manufactured stem cells are likely different from the laboratory‐grade stem cells, in that the phenotype and biological properties originally designed to treat a specific disease in the laboratory may now have a different disease indication in the clinic. Similarly, other cell manufacturing technical aspects may change the eventual stem cell product. Key cell product release criteria to the commercial and clinical application of stem cells are suitable cryopreservation protocols for long‐term storage, which may prove refractory for some stem cells 78. The expansion time and amplification process, including the matrices and reagents (e.g., serum or platelet lysate) under clinical GMP may generate MSCs that differ in their immunomodulatory properties 79, thereby affecting the cells' therapeutic effects. Finally, because human stem cells are tested in animal models, this cross‐species paradigm likely will present with different safety and efficacy scenario in the same‐species clinical application. This would indicate that the risk profile of stem cell‐based products should consider the envisioned clinical product of autologous or allogeneic stem cells, including their differentiation status and proliferation capacity, the route of administration, the intended location, and long‐term survival of engrafted cells which will affect the clinical risks of tumor formation, unwanted immune responses, and the transmission of adventitious agents 80, and the clinical efficacy readouts. In the end, the theoretical or potential application of stem cells observed in animal studies should closely approximate the applied setting in the clinic if GMP‐certified stem cells were to replicate the safety and efficacy profile of the lab‐grade stem cells.
To this end, in support of allowing the basic science‐generated efficacy and safety readouts of stem cell transplantation in preclinical stroke to dictate the entry of this therapy to the clinic, a set of guidelines for translational research and clinical trial design has been recommended by a consortium of basic scientists, clinicians, industry partners, and NIH and Food and Drug Administration regulators under the consortium of Stem cell Therapeutics as an Emerging Paradigm for Stroke (STEPS). A collaborative effort among these stakeholders may provide an expeditious clinical entry of stem cell therapeutics. The overarching thesis of STEPS is that basic science should dictate the translation and eventual clinical application of stem cell therapy for stroke. Unfortunately, a careful examination of the literature reveals few studies adhering to these STEPS lab‐to‐clinic translational guidelines 81. The investigation of stem cell therapy in a clinically relevant setting, in particular assessing its efficacy and safety in direct comparison to rehabilitation, has not been fully examined 81.
In an effort to enhance successful translation of stroke therapeutics, a new NIH NINDS initiative called Stroke Preclinical Assessment Network (SPAN) program 82 has solicited projects designed to evaluate the potential of neuroprotective drugs in improving functional outcomes of currently approved stroke treatments, specifically thrombectomy. Approximately 87% of strokes are ischemic in nature and occur due to an arterial occlusion 83. The most common approach to endovascular revascularization of large vessel occlusion (LVO) is stent retriever thrombectomy 84. Once a stent is deployed at the occlusion site, the clot becomes engaged and entrapped within the struts of the device, allowing subsequent withdrawal of both the stent and clot as a single unit. Stent retriever thrombectomy is currently recommended in patients with acute ischemic stroke from LVO 85. However, the risk of excessive bleeding is inherent with thrombectomy, necessitating the need for adjunctive treatments to minimize such adverse effect. The NIH SPAN program acknowledges the transient middle cerebral artery occlusion (tMCAO) as the standardized and validated model of stroke. By subsequently adding aging and other comorbidities to this tMCAO model and by enlisting multiple established laboratories (with solid track record of publications on the use of this MCAO model), a much more stringent platform will be in place to test therapeutics for translation into clinical application. The anticipated six SPAN sites will be identified and tasked to work closely together under the guidance of a coordinating center consisting of key thought leaders in stroke and neuroprotection, epitomizing the collaborative spirit of STEPS and STAIR consortia. Not too long ago, big pharmaceutical companies have remained on the sidelines from supporting stroke therapeutics, primarily due to the bleak outlook that the disease is treatable with neuroprotective drugs beyond thrombolytic agent tPA or mechanical thrombectomy. Now we have reached the crossroad of identifying a neuroprotective drug, not as a stand‐alone but as an adjunct to thrombectomy. One can envision stem cells as biologics 86 operating as pharmacologic‐like agents as noted above, which may similarly be combined with thrombectomy. As noted above, stem cells may secrete a cocktail of therapeutic growth factors that collectively can induce regenerative processes 87, 88 which may aid the neurovascular unit to respond better to thrombectomy.
So are we there yet? The recent failures of stem cell transplantation to reach efficacy in stroke clinical trials (MAPC and SB623) appear to set the bar much higher before we can replace the current designation of “experimental treatment” to “treatment option” for stem cell therapy (Figure 1). At the very least, a redesign of ongoing trials is needed to reconcile the disconnect between the laboratory and the clinical stem cell transplant protocols. A much narrower treatment window (i.e., transplantation within 36 hours of stroke onset) has now been indicated for the second MAPC clinical trial in acute ischemic stroke patients 60. A cell dosage closer to the preclinical dose‐approximated 56 million cells will likely need to be pursued with a new SB623 clinical trial for chronic stroke patients. Unfortunately, the long wait is not yet over. A collaborative effort and a commitment to basic science represent critical lab‐to‐clinic translational enabling factors toward a safe and effective stem cell therapy for stroke.
Figure 1.
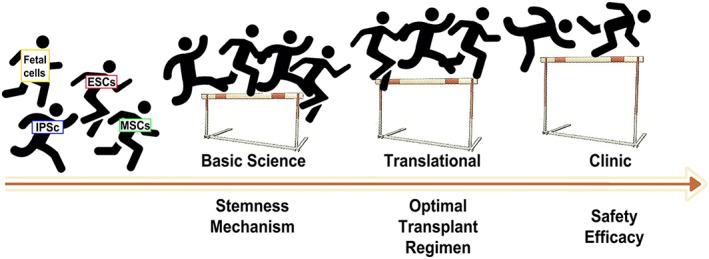
Lab‐to‐clinic translational hurdles in stem cell therapy for stroke. Several factors enable the ascent of stem cell therapy at the basic science, translational, and clinical levels. Tissue sources for stem cell transplantation include fetal cells, iPSCs, ESCs, and adult tissue‐derived cells such as bone marrow MSCs. At the basic science level, stemness and underlying mechanisms of stem cells need to be probed. At the translational level, the transplant regimen needs to be optimized. At the clinical level, the safety and efficacy of the transplanted cells must be ensured, with the efficacy readouts remaining elusive in recent clinical trials. Abbreviations: ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells; MSCs, mesenchymal stem cells.
Disclosure of Potential Conflicts of Interest
C.V.B. has patents and patent applications on stem cell biology and its applications.
Acknowledgment
The author extends his sincere gratitude to Dr. Jea‐Young Lee, Julian P. Tuazon, and Vanessa Castelli for their excellent technical assistance during the preparation of this manuscript. C.V.B. was funded by National Institutes of Health, National Institute of Neurological Disorders and Stroke (1R01NS090962, 1R01NS102395, and 1R21NS109575).
References
- 1. Mampalam TJ, Gonzalez MF, Weinstein P et al. Neuronal changes in fetal cortex transplanted to ischemic adult rat cortex. J Neurosurg 1988;69:904–912. [DOI] [PubMed] [Google Scholar]
- 2. Sharp FR. Transplants for stroke patients? Ann Neurol 1993;34:322–323. [DOI] [PubMed] [Google Scholar]
- 3. Grabowski M, Brundin P, Johansson BB. Fetal neocortical grafts implanted in adult hypertensive rats with cortical infarcts following a middle cerebral artery occlusion: Ingrowth of afferent fibers from the host brain. Exp Neurol 1992;116:105–121. [DOI] [PubMed] [Google Scholar]
- 4. Grabowski M, Christofferson RH, Brundin P et al. Vascularization of fetal neocortical grafts implanted in brain infarcts in spontaneously hypertensive rats. Neuroscience 1992;51:673–682. [DOI] [PubMed] [Google Scholar]
- 5. Grabowski M, Brundin P, Johansson BB. Functional integration of cortical grafts placed in brain infarcts of rats. Ann Neurol 1993;34:362–368. [DOI] [PubMed] [Google Scholar]
- 6. Nishino H, Aihara N, Czurko A et al. Reconstruction of GABAergic transmission and behavior by striatal cell grafts in rats with ischemic infarcts in the middle cerebral artery. J Neural Transplant Plast 1993;4:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aihara N, Mizukawa K, Koide K et al. Striatal grafts in infarct striatopallidum increase GABA release, reorganize GABAA receptor and improve water‐maze learning in the rat. Brain Res Bull 1994;33:483–488. [DOI] [PubMed] [Google Scholar]
- 8. Borlongan CV, Koutouzis TK, Jorden JR et al. Neural transplantation as an experimental treatment modality for cerebral ischemia. Neurosci Biobehav Rev 1997;21:79–90. [DOI] [PubMed] [Google Scholar]
- 9. Bliss T, Guzman R, Daadi M et al. Cell transplantation therapy for stroke. Stroke 2007;38:817–826. [DOI] [PubMed] [Google Scholar]
- 10. Liu X, Ye R, Yan T et al. Cell based therapies for ischemic stroke: From basic science to bedside. Prog Neurobiol 2014;115:92–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang R, Zhang Z, Chopp M. Function of neural stem cells in ischemic brain repair processes. J Cereb Blood Flow Metab 2016;36:2034–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stonesifer C, Corey S, Ghanekar S et al. Stem cell therapy for abrogating stroke‐induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol 2017;158:94–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borlongan CV, Tajima Y, Trojanowski JQ et al. Transplantation of cryopreserved human embryonal carcinoma‐derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol 1998;149:310–321. [DOI] [PubMed] [Google Scholar]
- 14. Ikeda R, Kurokawa MS, Chiba S et al. Transplantation of neural cells derived from retinoic acid‐treated cynomolgus monkey embryonic stem cells successfully improved motor function of hemiplegic mice with experimental brain injury. Neurobiol Dis 2005;20:38–48. [DOI] [PubMed] [Google Scholar]
- 15. Dezawa M, Kanno H, Hoshino M et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest 2004;113:1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Virley D, Ridley RM, Sinden JD et al. Primary CA1 and conditionally immortal MHP36 cell grafts restore conditional discrimination learning and recall in marmosets after excitotoxic lesions of the hippocampal CA1 field. Brain 1999;122:2321–2335. [DOI] [PubMed] [Google Scholar]
- 17. Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke 2004;35:354–356. [DOI] [PubMed] [Google Scholar]
- 18. Venkat P, Chopp M, Chen J. Cell‐based and exosome therapy in diabetic stroke. Stem Cells Translational Medicine 2018;7:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khoshnam SE, Winlow W, Farzaneh M et al. Pathogenic mechanisms following ischemic stroke. Neurol Sci 2017;38:1167–1186. [DOI] [PubMed] [Google Scholar]
- 20. Borlongan CV, Nguyen H, Lippert T et al. May the force be with you: Transfer of healthy mitochondria from stem cells to stroke cells. J Cereb Blood Flow Metab 2019;39:367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cui C, Ye X, Chopp M et al. miR‐145 regulates diabetes‐bone marrow stromal cell‐induced neurorestorative effects in diabetes stroke rats. Stem Cells Translational Medicine 2016;5:1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doeppner TR, Herz J, Gorgens A et al. Extracellular vesicles improve post‐stroke neuroregeneration and prevent postischemic immunosuppression. Stem Cells Translational Medicine 2015;4:1131–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu K, Lee JY, Kaneko Y et al. Human stem cells transplanted into the rat stroke brain migrate to spleen via lymphatic and inflammation pathways. Haematologica 2018;104:1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kondziolka D, Wechsler L, Goldstein S et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology 2000;55:565–569. [DOI] [PubMed] [Google Scholar]
- 25. Bateman ME, Strong AL, Gimble JM et al. Concise review: Using fat to fight disease: A systematic review of nonhomologous adipose‐derived stromal/stem cell therapies. Stem Cells 2018;36:1311–1328. [DOI] [PubMed] [Google Scholar]
- 26. Napoli E, Borlongan CV. Stem cell recipes of bone marrow and fish: Just what the stroke doctors ordered. Stem Cell Rev 2017;13:192–197. [DOI] [PubMed] [Google Scholar]
- 27. Sun JM, Kurtzberg J. Cord blood for brain injury. Cytotherapy 2015;17:775–785. [DOI] [PubMed] [Google Scholar]
- 28. Napoli E, Lippert T, Borlongan CV. Stem cell therapy: Repurposing cell‐based regenerative medicine beyond cell replacement. Adv Exp Med Biol 2018;1079:87–91. [DOI] [PubMed] [Google Scholar]
- 29. Napoli E, Borlongan CV. Recent advances in stem cell‐based therapeutics for stroke. Transl Stroke Res 2016;7:452–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borlongan CV. Age of PISCES: Stem‐cell clinical trials in stroke. Lancet 2016;388:736–738. [DOI] [PubMed] [Google Scholar]
- 31. Borlongan CV. Preliminary reports of stereotaxic stem cell transplants in chronic stroke patients. Mol Ther 2016;24:1710–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tang Y, Yasuhara T, Hara K et al. Transplantation of bone marrow‐derived stem cells: A promising therapy for stroke. Cell Transplant 2007;16:159–169. [PubMed] [Google Scholar]
- 33. Gnecchi M, Melo LG. Bone marrow‐derived mesenchymal stem cells: Isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol 2009;482:281–294. [DOI] [PubMed] [Google Scholar]
- 34. Li YF, Ren LN, Guo G et al. Endothelial progenitor cells in ischemic stroke: An exploration from hypothesis to therapy. J Hematol Oncol 2015;8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yasuhara T, Matsukawa N, Hara K et al. Notch‐induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev 2009;18:1501–1514. [DOI] [PubMed] [Google Scholar]
- 36. Yasuhara T, Hara K, Maki M et al. Intravenous grafts recapitulate the neurorestoration afforded by intracerebrally delivered multipotent adult progenitor cells in neonatal hypoxic‐ischemic rats. J Cereb Blood Flow Metab 2008;28:1804–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uchida H, Morita T, Niizuma K et al. Transplantation of unique subpopulation of fibroblasts, muse cells, ameliorates experimental stroke possibly via robust neuronal differentiation. Stem Cells 2016;34:160–173. [DOI] [PubMed] [Google Scholar]
- 38. Borlongan CV, Glover LE, Tajiri N et al. The great migration of bone marrow‐derived stem cells toward the ischemic brain: Therapeutic implications for stroke and other neurological disorders. Prog Neurobiol 2011;95:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Borlongan CV. Bone marrow stem cell mobilization in stroke: A 'bonehead' may be good after all! Leukemia 2011;25:1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rowart P, Erpicum P, Detry O et al. Mesenchymal stromal cell therapy in ischemia/reperfusion injury. J Immunol Res 2015;2015:602597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eckert MA, Vu Q, Xie K et al. Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. J Cereb Blood Flow Metab 2013;33:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kocsis JD, Honmou O. Bone marrow stem cells in experimental stroke. Prog Brain Res 2012;201:79–98. [DOI] [PubMed] [Google Scholar]
- 43. Joyce N, Annett G, Wirthlin L et al. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 2010;5:933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Velthoven CT, Gonzalez F, Vexler ZS et al. Stem cells for neonatal stroke‐ the future is here. Front Cell Neurosci 2014;8:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bang OY, Lee JS, Lee PH et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 2005;57:874–882. [DOI] [PubMed] [Google Scholar]
- 46. Savitz SI, Misra V, Kasam M et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol 2011;70:59–69. [DOI] [PubMed] [Google Scholar]
- 47. Prasad K, Sharma A, Garg A et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: A multicentric, randomized trial. Stroke 2014;45:3618–3624. [DOI] [PubMed] [Google Scholar]
- 48. Banerjee S, Bentley P, Hamady M et al. Intra‐arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Translational Medicine 2014;3:1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hess DC, Wechsler LR, Clark WM et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): A randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Neurol 2017;16:360–368. [DOI] [PubMed] [Google Scholar]
- 50. Steinberg GK, Kondziolka D, Wechsler LR et al. Two‐year safety and clinical outcomes in chronic ischemic stroke patients after implantation of modified bone marrow‐derived mesenchymal stem cells (SB623): A phase 1/2a study. J Neurosurg 2018;1:1–11. [DOI] [PubMed] [Google Scholar]
- 51. Steinberg GK, Kondziolka D, Wechsler LR et al. Clinical outcomes of transplanted modified bone marrow‐derived mesenchymal stem cells in stroke: A phase 1/2a study. Stroke 2016;47:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. SanBio Co. Ltd . SanBio and Sumitomo Dainippon Pharma Announce Topline Results from a Phase 2b Study in the U.S. Evaluating SB623, a Regenerative Cell Medicine for the Treatment of Patients with Chronic Stroke. Available at https://www.ds‐pharma.com/ir/news/pdf/ene20190129.1.pdf. Accessed March 11, 2019.
- 53. Sohni A, Verfaillie CM. Multipotent adult progenitor cells. Best Pract Res Clin Haematol 2011;24:3–11. [DOI] [PubMed] [Google Scholar]
- 54. Tajiri N, Kaneko Y, Shinozuka K et al. Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site. PLoS One 2013;8:e74857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tajiri N, Duncan K, Antoine A et al. Stem cell‐paved biobridge facilitates neural repair in traumatic brain injury. Front Syst Neurosci 2014;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Diamandis T, Borlongan CV. One, two, three steps toward cell therapy for stroke. Stroke 2015;46:588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kenmuir CL, Wechsler LR. Update on cell therapy for stroke. Stroke Vasc Neurol 2017;2:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Athersys Inc. Neurological (Stroke Phase II). Available at http://www.athersys.com/clinical‐programs/neurological. Accessed March 11, 2019.
- 59. Mays RW, Borlongan CV, Yasuhara T et al. Development of an allogeneic adherent stem cell therapy for treatment of ischemic stroke. J Exp Stroke Transl Med 2010;3:34–46. [Google Scholar]
- 60. U.S. National Library of Medicine . MultiStem® Administration for Stroke Treatment and Enhanced Recovery Study (MASTERS‐2). Available at https://clinicaltrials.gov/ct2/show/NCT03545607. Accessed March 11, 2019.
- 61. Doeppner TR, Hermann DM. Mesenchymal stem cells in the treatment of ischemic stroke: Progress and possibilities. Stem Cells Cloning 2010;3:157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Anderson JD, Johansson HJ, Graham CS et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor‐kappab signaling. Stem Cells 2016;34:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Duffy GP, Ahsan T, O'Brien T et al. Bone marrow‐derived mesenchymal stem cells promote angiogenic processes in a time‐ and dose‐dependent manner in vitro. Tissue Eng Part A 2009;15:2459–2470. [DOI] [PubMed] [Google Scholar]
- 64. Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]
- 65. Ishikawa H, Tajiri N, Shinozuka K et al. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke 2013;44:3473–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Schweizer S, Meisel A, Marschenz S. Epigenetic mechanisms in cerebral ischemia. J Cereb Blood Flow Metab 2013;33:1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Puyal J, Ginet V, Clarke PG. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: A challenge for neuroprotection. Prog Neurobiol 2013;105:24–48. [DOI] [PubMed] [Google Scholar]
- 68. Sozmen EG, Hinman JD, Carmichael ST. Models that matter: White matter stroke models. Neurotherapeutics 2012;9:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Incontri Abraham D, Gonzales M, Ibarra A et al. Stand alone or join forces? Stem cell therapy for stroke. Expert Opin Biol Ther 2018;19:25–33. [DOI] [PubMed] [Google Scholar]
- 70. Polgar S, Borlongan CV, Koutouzis TK et al. Implications of neurological rehabilitation for advancing intracerebral transplantation. Brain Res Bull 1997;44:229–232. [DOI] [PubMed] [Google Scholar]
- 71. Borlongan CV, Jolkkonen J, Detante O. The future of stem cell therapy for stroke rehabilitation. Future Neurol 2015;10:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liebeskind DS, Derdeyn CP, Wechsler LR et al. Stair X. Stroke 2018;49:2241–2247. [DOI] [PubMed] [Google Scholar]
- 73. Nguyen H, Zarriello S, Rajani M et al. Understanding the role of dysfunctional and healthy mitochondria in stroke pathology and its treatment. Int J Mol Sci 2018;19:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Labriola NR, Azagury A, Gutierrez R et al. Concise Review: Fabrication, customization, and application of cell mimicking microparticles in stem cell science. Stem Cells Translational Medicine 2018;7:232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lamanna JJ, Gutierrez J, Urquia LN et al. Ferumoxytol labeling of human neural progenitor cells for diagnostic cellular tracking in the porcine spinal cord with magnetic resonance imaging. Stem Cells Translational Medicine 2017;6:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen B, Li Q, Zhao B et al. Stem cell‐derived extracellular vesicles as a novel potential therapeutic tool for tissue repair. Stem Cells Translational Medicine 2017;6:1753–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Crow D. From cell stem cell to clinical trials: The biotech journey of two papers. Cell Stem Cell 2017;20:746–748. [Google Scholar]
- 78. Hunt CJ. Cryopreservation of human stem cells for clinical application: A review. Transfus Med Hemother 2011;38:107–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Menard C, Pacelli L, Bassi G et al. Clinical‐grade mesenchymal stromal cells produced under various good manufacturing practice processes differ in their immunomodulatory properties: Standardization of immune quality controls. Stem Cells Dev 2013;22:1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med 2011;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Savitz SI, Cramer SC, Wechsler L et al. Stem cells as an emerging paradigm in stroke 3: Enhancing the development of clinical trials. Stroke 2014;45:634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. U.S. National Institutes of Health . Stroke Preclinical Assessment Network (SPAN) to Support Translational Studies for Acute Neuroprotection (U01 Clinical Trial Not Allowed). Available at https://grants.nih.gov/grants/guide/rfa-files/rfa-ns-18-033.html. Accessed March 11, 2019.
- 83. Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart disease and stroke statistics‐2017 update: A report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Campbell BC, Hill MD, Rubiera M et al. Safety and efficacy of solitaire stent thrombectomy: Individual patient data meta‐analysis of randomized trials. Stroke 2016;47:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Powers WJ, Derdeyn CP, Biller J et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:3020–3035. [DOI] [PubMed] [Google Scholar]
- 86. Crowley MG, Liska MG, Borlongan CV. Stem cell therapy for sequestering neuroinflammation in traumatic brain injury: An update on exosome‐targeting to the spleen. J Neurosurg Sci 2017;61:291–302. [DOI] [PubMed] [Google Scholar]
- 87. Kin K, Yasuhara T, Kameda M et al. Cell encapsulation enhances antidepressant effect of the mesenchymal stem cells and counteracts depressive‐like behavior of treatment‐resistant depressed rats. Mol Psychiatry 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 88. Nguyen H, Aum D, Mashkouri S et al. Growth factor therapy sequesters inflammation in affording neuroprotection in cerebrovascular diseases. Expert Rev Neurother 2016;16:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Acosta SA, Tajiri N, Hoover J et al. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke 2015;46:2616–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Borlongan CV, Lind JG, Dillon‐Carter O et al. Intracerebral xenografts of mouse bone marrow cells in adult rats facilitate restoration of cerebral blood flow and blood‐brain barrier. Brain Res 2004;1009:26–33. [DOI] [PubMed] [Google Scholar]
- 91. Kalladka D, Sinden J, Pollock K et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first‐in‐man study. Lancet 2016;388:787–796. [DOI] [PubMed] [Google Scholar]
- 92. Lee JS, Hong JM, Moon GJ et al. A long‐term follow‐up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010;28:1099–1106. [DOI] [PubMed] [Google Scholar]


