Abstract
Ionizing radiation, commonly used in the treatment of solid tumors, has unintended but deleterious effects on overlying skin and is associated with chronic nonhealing wounds. Skin‐derived mesenchymal stromal cells (SMSCs) are a pluripotent population of cells that are critically involved in skin homeostasis and wound healing. The aim of this study was to isolate and functionally characterize SMSCs from human skin that was previously irradiated as part of neoadjuvant or adjuvant cancer therapy. To this end, SMSCs were isolated from paired irradiated and nonirradiated human skin samples. Irradiated SMSCs expressed characteristic SMSC markers at lower levels, had disorganized cytoskeletal structure, and had disordered morphology. Functionally, these cells had diminished proliferative capacity and substantial defects in colony‐forming capacity and differentiation in vitro. These changes were associated with significant differential expression of genes known to be involved in skin physiology and wound healing. Conditioned media obtained from irradiated SMSCs affected fibroblast but not endothelial cell proliferation and migration. These results suggest that in situ damage to SMSCs during neoadjuvant or adjuvant radiation may play a critical role in the pathogenesis of slow or nonhealing radiation wounds. stem cells translational medicine 2019;8:925&934
Keywords: Stem cells, Ionizing radiation, Radiotherapy, Human, Skin, Proliferation, Differentiation, Migration, Mesenchymal, Stromal cell, Wound healing
Significance Statement.
To the authors’ knowledge, the present study is the first to identify key changes in the function of human mesenchymal stem cells (MSCs) as a direct result of in vivo radiotherapy, including diminished capacity for proliferation, differentiation, and alterations in paracrine secretion. MSCs are known to be critically involved in skin homeostasis and wound healing. The findings of the study elucidate a mechanism by which ionizing radiation may contribute to chronic nonhealing wounds. Furthermore, potential therapeutic targets for the prevention and/or treatment of these wounds are identified.
Introduction
Although effective in the treatment of solid tumors, ionizing radiation's unintended but deleterious effects on wound healing are well documented 1, 2. The underlying mechanism of pathogenesis, however, is complex and incompletely understood. Many studies have suggested microvascular tissue damage with subsequent local tissue hypoxia as a primary etiology 1, 2, 3. Evaluation of the ultrastructure of irradiated human skin, however, demonstrates largely normal microvasculature, and patients with history of extensive radiotherapy have normal transcutaneous oxygen pressures 4, 5. Cellular depletion and/or alterations in cell function, then, may account for chronic radiation‐induced skin injury.
Mesenchymal stromal cells (MSCs) are a heterogeneous population of multipotent cells with the capacity for differentiation along multiple lineages 6. These cells can be found in a variety of tissues and home to sites of injury, including irradiated skin 7, 8. Dermal stem cells in irradiated wounds demonstrate persistent depletion and altered function 9. Application of skin‐derived MSCs (SMSCs) to wounds, conversely, improves healing, suggesting that these cells may be involved in radiation‐induced skin injury 10, 11. In vitro studies evaluating the effect of irradiation on SMSCs are inconclusive and fail to recapitulate the complex microenvironment of irradiated human skin 12, 13. Additionally, SMSCs are tissue specific, with each population contributing to wound healing differently 14, 15. Unfortunately, to date, there has been no characterization of SMSCs that have been irradiated in vivo.
Given their potential involvement in maintenance of the chronic radiation‐induced skin injury phenotype, the aim of this study was to evaluate the effect of ionizing radiation on SMSCs isolated from human skin irradiated in situ, which incidentally occurs as part of neoadjuvant or adjuvant treatment for solid tumors. To that end, we isolated these cells from paired irradiated and nonirradiated tissue samples from patients undergoing postoncologic reconstruction and performed detailed cellular and functional analyses. We found that SMSCs from irradiated skin are defective in proliferation, differentiation, and colony formation capacity. Conditioned media from MSCs from irradiated skin is also associated with decreased paracrine stimulation of fibroblast migration. Moreover, these defects are associated with a distinct pattern of altered gene expression. The investigation of these differentially expressed genes may aid in the future development of targeted therapies to mitigate damaged pathways in nonhealing radiation wounds.
Materials and Methods
Procurement of Human Skin Samples
All tissue samples were obtained from patients with informed consent under an approved protocol with the University of Southern California Institutional Review Board and in accordance with all national and international guidelines for clinical research. Criteria for inclusion in the study were previous oncologic resection, history of neoadjuvant or adjuvant radiotherapy, and admission to the hospital for reconstruction. Irradiated skin was obtained directly from the irradiated field. Nonirradiated (normal) skin was obtained from the same patient from a distant location on the body (i.e., from the donor harvest site in the case of microvascular free flap tissue transfer). Paired samples were retrieved from the operating room and processed for SMSC extraction immediately after harvest.
MSC Isolation and Culture
Fresh tissue was processed for SMSC extraction according to standard protocol (Fig. 2) 16, 17. Briefly, all subcutaneous tissue was removed from the skin using sharp dissection, resulting in isolated dermis and epidermis. The skin samples were then minced to 1 mm2 and placed in a digestive solution composed of dispase and collagenase overnight. The solution was filtered through a 40 μm cell strainer, spun down in a centrifuge, and resuspended in media. All SMSCs were maintained in culture using minimum essential media with 15% fetal bovine serum at 37°C with 5% CO2.
Figure 2.
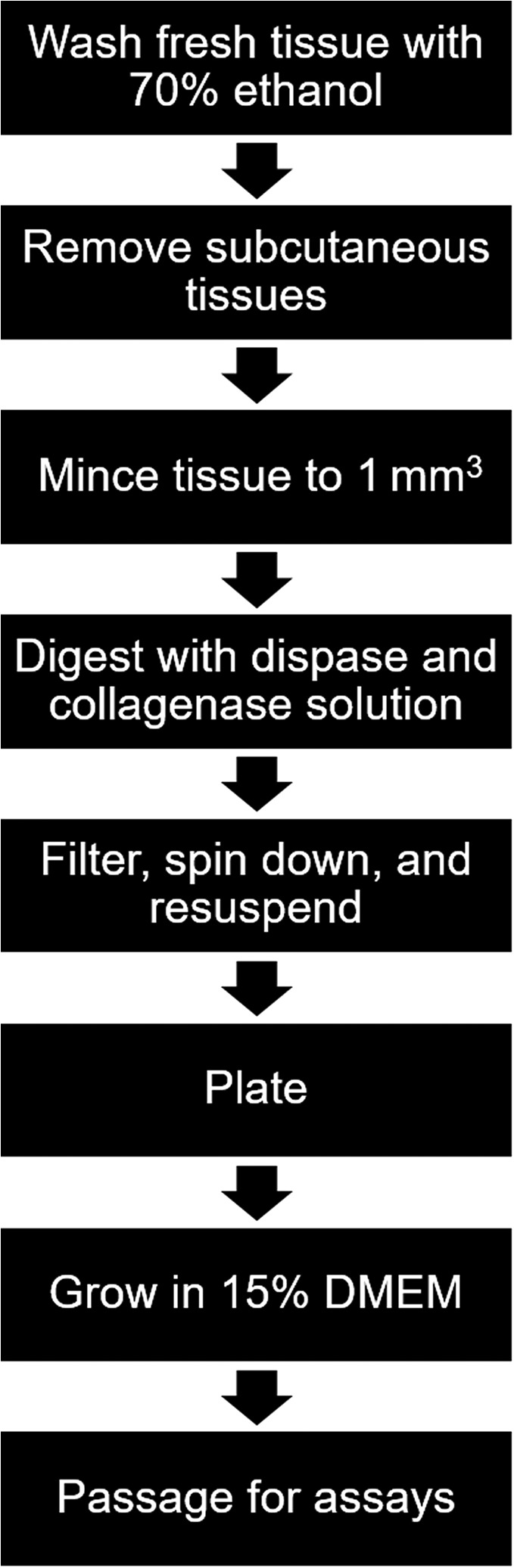
Flow diagram for isolation and culture of skin‐derived mesenchymal stromal cells used in this experiment. Abbreviation: DMEM, Dulbecco's modified Eagle's medium.
MSC Marker Characterization
In order to confirm the identity of cells isolated and cultured by the aforementioned method, immunofluorescence using a panel of cell markers for SMSCs was performed. Positive and negative markers were selected based on a literature review 18. Cells were plated in four‐chamber slides and allowed to achieve 60% confluence. The cells were then fixed, permeabilized, and blocked with 4% paraformaldehyde (VWR, Radnor, PA), 0.1% Triton X‐100 (Sigma‐Aldrich, St. Louis, MO), and 1% bovine serum albumin (Sigma‐Aldrich). Cells were incubated overnight with primary antibodies against CD31 (Abcam, Cambridge, UK), CD44, CD45, CD90, CD105, CD106, and STRO‐1 (R&D Systems, Minneapolis, MN), followed by incubation with a secondary antibody (Alexa Fluor 488, ThermoFisher Scientific, Waltham, MA). In order to more specifically confirm the identity of these cells, costaining was performed using a similar protocol with antibodies against CD90 (R&D Systems) and CD44 (Abcam). Secondary antibodies for costaining were Alexa Fluor 488 and 594, respectively (ThermoFisher Scientific). All images were obtained using a Keyence BZ‐X700 (Keyence, Osaka, Japan).
Histological Assessment of Whole Tissue and Isolated SMSCs
Subcutaneous tissue was removed from the paired skin samples using sharp dissection and then prepared for paraffin embedding and sectioning according to standard protocol. Pairs of whole skin samples were stained with H&E and picrosirius red. Isolated SMSCs were stained with phalloidin and 4′,6‐diamidino‐2‐phenylindole. Quantification of nuclear and total cell area was completed with ImageJ (U.S. NIH, Bethesda, MD). Brightfield imaging of isolated SMSCs was also completed. All images were obtained using a Keyence BZ‐X700 (Keyence).
Cellular Proliferation and Colony‐Forming Unit‐Fibroblast Assays
MSCs were cultured to 80% confluence prior to passaging for all assays. WST‐1 (Roche Life Science, Basel, Switzerland) was used to assess and compare cellular proliferation of pairs of isolated SMSCs according to manufacturer protocol. A colony‐forming unit‐fibroblast (CFU‐F) assay was used to evaluate SMSCs function after extraction. Cells were plated at a concentration of 150 cells per milliliter and allowed to proliferate in culture for 14 days, after which they were stained with 1% crystal violet in methanol. Visible colonies were counted.
Evaluation of Multipotency with Lineage Differentiation Assays
In order to determine that the extracted SMSCs were multipotent, early‐passage cells were subjected to osteogenic and adipogenic differentiation using the StemPro Adipogenic and Osteogenic Differentiation Kits (ThermoFisher Scientific). Cells were placed in a six‐well plate at a seeding density of 1 × 104 cells per centimeter square and cultured in the appropriate differentiation media. For adipogenic differentiation, cells were stained with Oil Red O (Sigma‐Aldrich) after 14 days in culture. For osteogenic differentiation, cells were stained with Alizarin Red S (Sigma‐Aldrich) after 21 days in culture. Capacity for differentiation of both irradiated and normal SMSCs was quantified using ImageJ.
Conditioned Media
Conditioned media was generated by culturing SMSCs in serum‐free media for 24 hours. WST‐1 (Roche Life Science) was used to evaluate the effect of this conditioned media on the proliferation of fibroblasts, lymphatic endothelial cells, and blood endothelial cells (BECs). A gold salt migration assay was used to evaluate the effect of the conditioned media on wound healing in vitro 19. Briefly, glass coverslips were coated with colloidal gold salt in an eight‐well plate then coated with fibronectin. Fibroblasts were plated at a density of 5 × 103 cells per well and cultured in the conditioned media for 24 hours. The cells were fixed and visualized using a Keyence BZ‐X700 microscope (Keyence). Migration was quantified by calculating the migration index with ImageJ (U.S. NIH) 19.
Evaluation of Differential Gene Transcription in SMSC Pairs Using RNA Sequencing
MSCs were cultured to 80% confluence, followed by RNA extraction using the PureLink RNA Mini Kit (ThermoFisher Scientific) according to manufacturer protocol. The standard protocol for library construction began with 1 μg of total RNA. As the protocol used was based on polyA capture, RNA was visualized on a BioRad Experion (Hercules, CA) to ensure that RNA Quality Index values were greater than 8. For library construction, the Illumina TruSeq v2 mRNA kit (San Diego, CA) was used. The protocol was followed according to manufacturer's instructions except the final number of polymerase chain reaction (PCR) cycles was 12 and not 15. Following construction, libraries were visualized by Bioanalyzer (Agilent, Santa Clara, CA) using the High Sensitivity Chip and quantified for pooling and sequencing using the Kapa Biosystems quantitative PCR quantitation kit (Wilmington, MA) according to manufacturer protocol. For sequencing, libraries were diluted to 16 pM then applied to a V3 flowcell using the Illumina cBot according to manufacturer protocol. Sequencing was carried out on the Hi‐Seq 2000 using HSCS v 1.5.15.1. Image analysis and basecalling were carried out using RTA 1.13.48.0, and deconvolution and fastq file production were performed with CASAVA 1.8.2 (Illumina). A literature search was completed on the 10 most differentially upregulated and downregulated genes identified with RNA sequencing (RNA‐Seq), and disheveled‐binding‐antagonist of beta‐catenin 1 (DACT1), FMN1, and IL32 were selected for their known involvement in skin homeostasis, skin pathology, and/or wound healing. The directionality of the differential expression of these genes was confirmed using real‐time PCR (RT‐PCR) according to standard protocol. The primers used for these genes are presented in Table 1.
Table 1.
Primers used for real‐time polymerase chain reaction
| Primer | Forward | Reverse |
|---|---|---|
| DACT1 | CTTGTCAAGCTCTTGCAACTG | TCTTGGAGGAGAACATCTTGC |
| FMN1 | TCTTTGTCTCCACTTTCTTCTCTG | ATGGTGTGGTATGAGTTCTGC |
| IL32 | CAGCTTCTTCATGTCATCAGAGA | TTGTGCCAGGAAGACTGC |
Statistical Analysis
Data were analyzed using IBM SPSS (North Castle, NY). Pairwise comparisons for all assays were made using paired t‐tests with α < 0.05.
Results
SMSC Isolation and Culture from Radiation‐Damaged Human Skin
Although many reports have previously described the isolation of MSCs from normal skin, it was unclear to us if MSCs could be isolated from radiated skin 16, 17. Thus, in order to assess the effects of in vivo irradiation on SMSCs, we harvested paired irradiated and normal skin samples from patients who had a history of radiation for cancer treatment that were undergoing postoncologic reconstructive surgery. To definitively localize prior sites of external beam radiation, we used tattoo landmarks that are typically placed by radiation oncologists. In all cases, the radiated skin used in this study had hallmark features of radiation damage, including hyperpigmentation and skin contraction/fibrosis (Fig. 1A). Paired whole skin samples were stained with H&E and Sirius Red and demonstrated classic findings of chronic radiation injury, including fibrosis, polymorphic fibroblasts, and epidermal atrophy (Fig. 1B–1E).
Figure 1.
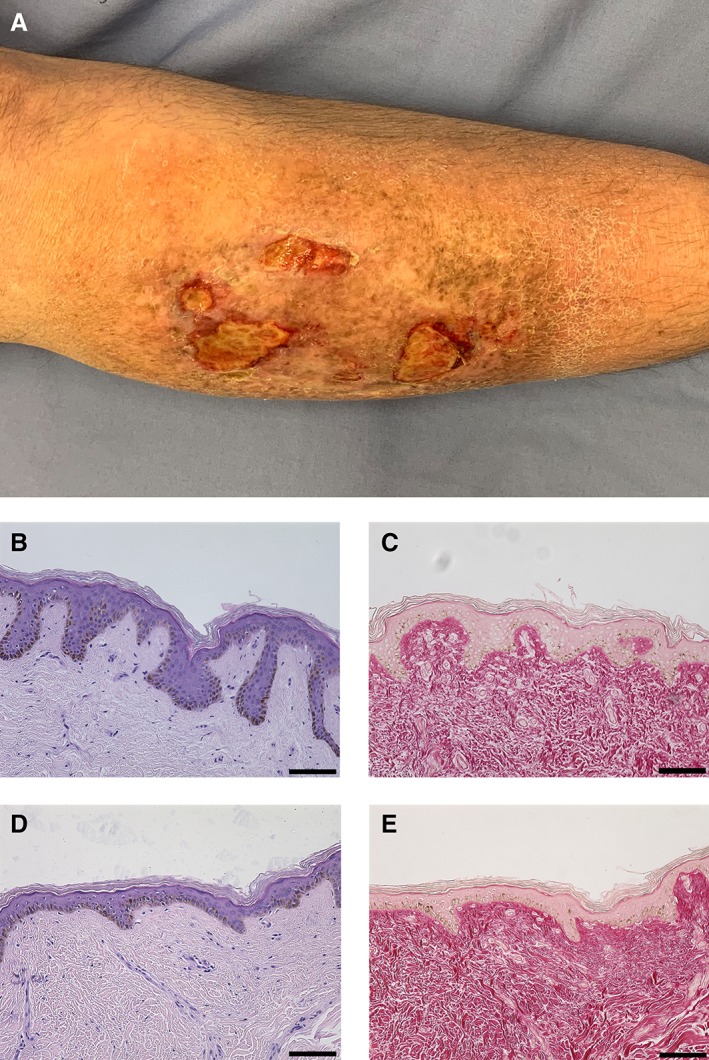
(A): Photograph of a chronic radiation skin and a chronic nonhealing wound. (B): H&E stain of normal skin. (C): Picrosirius red stain of normal skin. (D): H&E stain of irradiated human skin demonstrating classic histologic findings of radiation skin injury. (E): Picrosirius red stain of irradiated human skin. Note the disordered and abnormal dermal collagen. Scale bars represent 100 μm.
SMSCs were isolated from these paired skin samples according to established techniques (Fig. 2) 16, 17. Both cell populations were adherent to tissue culture plastic and expressed appropriate cell markers including CD40, CD90, CD105, STRO‐1, and CD106 but not CD31 or CD45 (Fig. 3A). Costaining was performed to further confirm SMSC identity, which demonstrated that these cells could be characterized as CD90+/CD44+/CD31−/CD45− (Fig. 3A, 3B). SMSCs derived from radiated skin (referred to from now on as rSMSCs) proved to be substantially more difficult to subculture and expressed markers at weaker levels than their nonirradiated counterparts.
Figure 3.
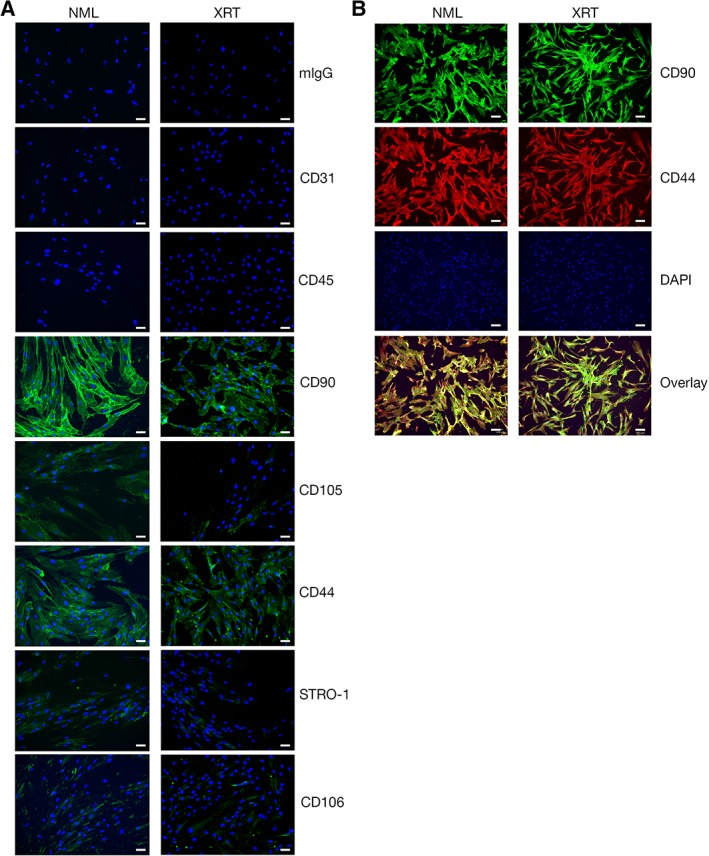
(A): Immunofluorescence characterization of skin‐derived mesenchymal stromal cells (SMSCs) isolated from NML and XRT human skin pairs. As expected, no positive (green) signal is observed for mouse immunoglobulin (control), CD31, and CD45 in either population. Both normal and irradiated human SMSCs are positive for CD90, CD105, CD44, STRO‐1, and CD106. Irradiated SMSCs exhibit qualitatively less intense staining for CD105, STRO‐1, and CD106 but, this is not statistically significant. (B): Costaining of SMSC pairs for CD90 (green) and CD44 (red). Note: DAPI is blue in all frames. Scale bars represent 100 μm. Abbreviations: DAPI, 4′,6‐diamidino‐2‐phenylindole; NML, normal; XRT, irradiated.
SMSCs from Irradiated Skin Have Abnormal Morphology
Brightfield imaging revealed substantial morphological heterogeneity in rSMSCs (Fig. 4A). Phalloidin staining demonstrated that rSMSCs were polygonal in shape, had poorly developed actin cytoskeletons that tended to be oriented linearly, and often overlapped in culture even at low plate density. In contrast, SMSCs from normal skin had characteristic spindle morphology, randomly oriented actin cytoskeletons, and never overlapped. Quantification of these images demonstrated that, although the mean cell area between pairs was comparable, rSMSCs had greater heterogeneity in cell size and had significantly large nuclei (p < .05; Fig. 4B, 4C). In addition to differences in morphology, a WST1 assay revealed that rSMSCs proliferated more poorly than normal SMSCs in culture (p < .05; Fig. 4D).
Figure 4.
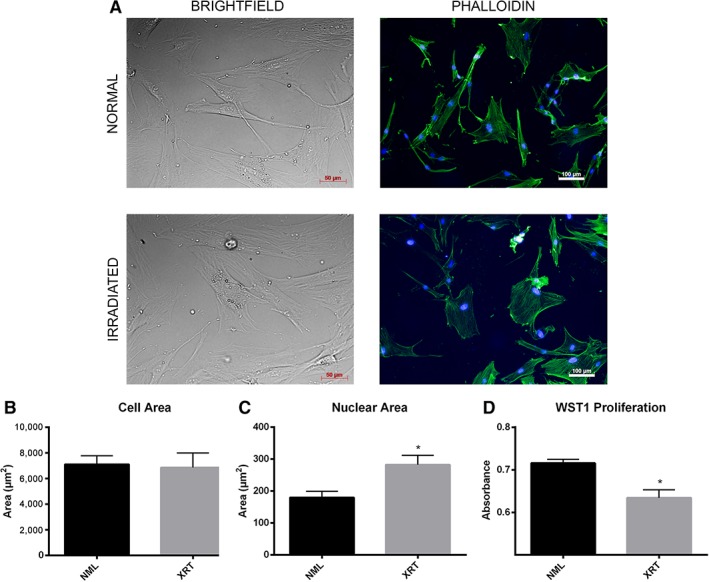
(A): Brightfield and phalloidin stains of paired irradiated and nonirradiated skin‐derived mesenchymal stromal cells (SMSCs), demonstrating dissimilar morphology and cytoskeletal arrangement. Brightfield scale bars represent 50 μm. Phalloidin stain scale bars represent 100 μm. Comparisons of cell area (B) and nuclear area (C) across SMSC pairs. (D): WST1 proliferation assay demonstrating significant reduction in irradiated SMSC proliferation in vitro. All asterisks denote significance at p < .05. Abbreviations: NML, normal; XRT, irradiated.
Characterization of Functional Defects of rSMSCs In Vitro
Given the stark difference in cell morphology and performance in culture, we next sought to evaluate rSMSC function. MSCs are multipotent and contribute to wound healing by differentiating directly into terminal cells 11. In order to confirm multipotency, we chose to differentiate our SMSCs along adipogenic and osteogenic pathways. Both cell lines differentiated appropriately (Fig. 5A). Irradiated SMSCs, however, possessed a significantly reduced capacity to differentiate to these lineages (p < .05; Fig. 5B). A CFU‐F assay, another measure of SMSC function, demonstrated that rSMSCs have a 5.6‐fold reduction in the ability to form colonies in vitro (p < .05; Fig. 5C, 5D) 20.
Figure 5.
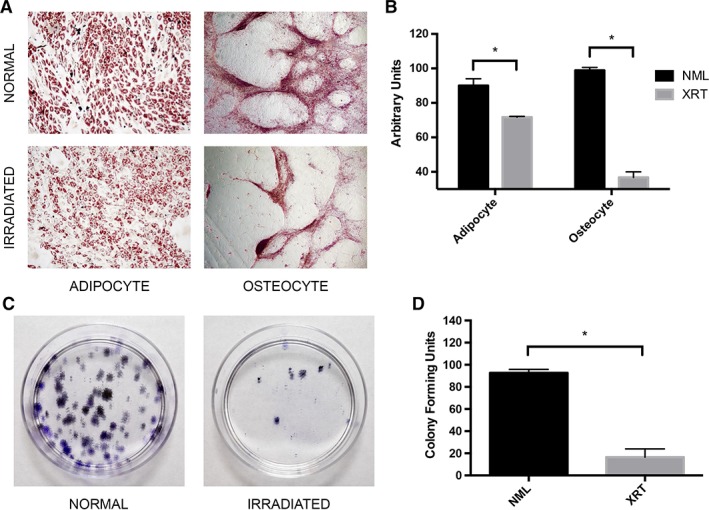
(A): Adipogenic and osteogenic lineage differentiation of skin‐derived mesenchymal stromal cell (SMSC) pairs. Images captured at ×10 and ×4 magnification, respectively. (B): Quantitative comparison of differentiation potential across SMSC pairs. (C): Representative colony‐forming unit‐fibroblast assay for an SMSC pair. (D): Quantitative analysis of colony‐forming capacity across SMSC pairs. All asterisks denote significance at p < .05. Abbreviations: NML, normal; XRT, irradiated.
Another way in which SMSCs exert their effect on wound healing is via the production of growth factors 10, 11. In order to evaluate radiation‐induced changes in paracrine signaling, we generated serum‐free conditioned media with rSMSC and SMSC pairs. Measures of cellular migration are highly relevant in vitro assays for wound healing, so we performed a gold salt migration assay with human fibroblasts using the conditioned media. Fibroblasts cultured in standard Dulbecco's modified Eagle's medium demonstrated equivalent migration to fibroblasts cultured in conditioned media from normal SMSCs. Fibroblasts cultured in media from rSMSCs, however, migrated significantly less than the other two groups (p < .05; Fig. 6A, 6B). Interestingly, conditioned media from rSMSCs also resulted in a significant reduction in fibroblast proliferation (p < .05) but did not substantially effect lymphatic or BEC proliferation (Fig. 6C).
Figure 6.
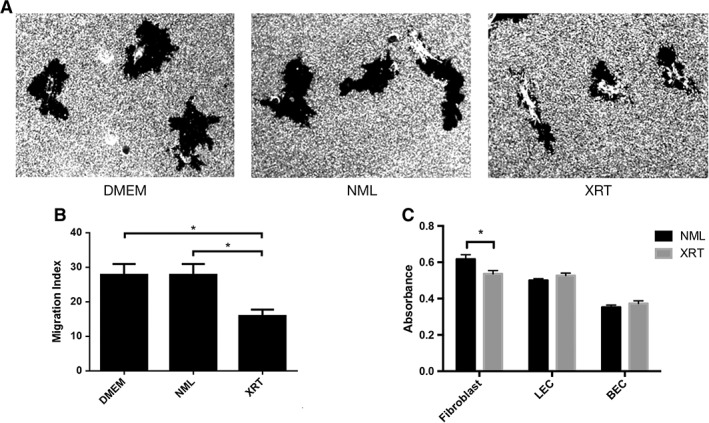
(A): Gold salt migration assay of human fibroblasts cultured in standard media and conditioned media obtained from irradiated and nonirradiated skin‐derived mesenchymal stromal cells (SMSCs). Images captured at ×20. (B): Quantitative analysis of fibroblast migration cultured in these media. (C): Proliferation assay of fibroblasts, LEC, and BEC cultured in conditioned media obtained from irradiated and nonirradiated SMSCs. All asterisks denote significance at p < .05. Abbreviations: BEC, blood endothelial cell; DMEM, Dulbecco's modified Eagle's medium; LEC, lymphatic endothelial cell; NML, normal; XRT, irradiated.
Gene Expression Profiles Are Altered in rSMSCs
Finally, in order to investigate potential cellular mechanisms for these morphological and functional changes, we performed RNA sequencing to determine genome‐wide radiation‐induced changes in SMSC gene transcription. Initial principal component analysis (PCA) of the samples demonstrated that each sample clustered with its pair (Fig. 7A). Using criteria of read per kilobase of transcript per million mapped reads > 0.1, fold‐change > 1.5, and p < 0.05, we identified over 100 differentially expressed genes (Table 2). The 10 genes with greatest upregulation and downregulation are presented in Table 3. PCA and hierarchical clustering using these criteria demonstrated clear clustering of samples on the basis of exposure to radiation (Fig. 7B, 7C). Three candidate genes—DACT1, FMN1, and IL32—were selected from Table 3 based on their involvement in skin homeostasis, skin pathology, and/or wound healing. In order to validate the RNA‐Seq data, the direction of differential expression of these genes was confirmed using RT‐PCR (Fig. 7D–7F).
Figure 7.
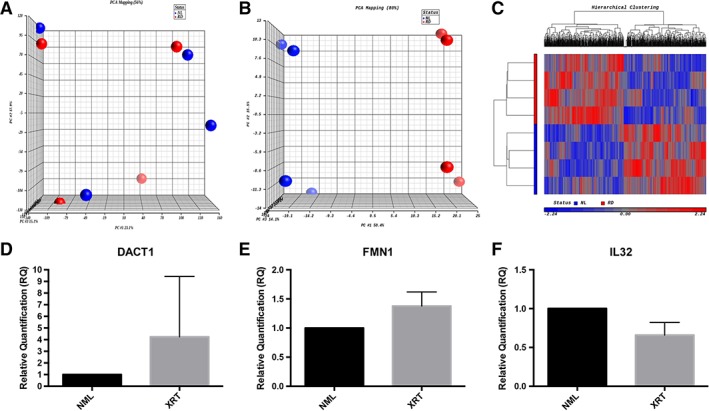
(A): Unfiltered principal component analysis of RNA sequencing (RNA‐Seq) data from paired irradiated and nonirradiated skin samples. (B): Principal component analysis of RNA‐Seq data from paired skin samples using selective criteria of read per kilobase of transcript per million mapped reads >0.1, fold‐change >1.5, and p < .05. (C): Hierarchical clustering of this data using the same criteria. (D–F): Quantitative real‐time polymerase chain reaction of differentially regulated genes known to be involved in skin homeostasis or wound healing. Abbreviations: NML, normal; XRT, irradiated.
Table 2.
Summary of differentially expressed genes in RNA sequencing analysis between paired irradiated and nonirradiated mesenchymal stromal cells
| Cut‐off criteria | AceView | NCBI | Ensembl | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fold change | p | Up | Down | Total | Up | Down | Total | Up | Down | Total |
| >1.2 | <.05 | 158 | 211 | 369 | 116 | 138 | 254 | 62 | 78 | 140 |
Table 3.
Fold change (FC) values for the Top differentially expressed genes in RNA sequencing analysis between paired irradiated and nonirradiated mesenchymal stromal cells
| Gene symbol | FC | p |
|---|---|---|
| DACT1 | 2.2 | <.05 |
| LIMCH1 | 1.7 | <.05 |
| FMN1 | 1.6 | <.05 |
| NPTXR | 1.6 | <.05 |
| TRPV2 | −1.7 | <.05 |
| IL32 | −1.7 | <.05 |
| BAALC | −1.9 | <.05 |
| LSAMP | −2.0 | <.05 |
All values are based on a read per kilobase of transcript per million mapped reads >0.1.
Discussion
Although effective in the treatment of solid tumors, radiotherapy produces a number of off target effects in the overlying skin. Acutely, changes include erythema, tenderness, desquamation, and mucositis, all of which are generally reversible. Chronic injury, conversely, is permanent and includes tissue atrophy, vascular damage, and chronic ulceration 21. These changes result in skin that responds poorly to wounding by trauma or surgery, predisposing to the development of chronic wounds. Unfortunately, current treatments focus on general wound care, with limited evidence for their effectiveness 22, 23.
Microvascular damage and subsequent local tissue hypoxia have been widely regarded as the primary mechanism by which ionizing radiation causes tissue damage. In response to irradiation, BECs undergo apoptosis without subsequent proliferation to restore vascular integrity 24. This results in increased vessel permeability, producing local edema that diminishes gas and metabolite exchange 25. Although some studies report intimal proliferation that results in obliterative endarteritis and permanent hypoxia, there is evidence to suggest that microvascular damage and hypoxia are transient 4, 5, 26. This suggests that ionizing radiation may produce its chronic effects in skin via alternative mechanisms.
Cellular depletion and alterations in cellular function are also implicated in the pathogenesis of radiation skin injury. Numerous studies have shown that ionizing radiation results in upregulation of mediators of apoptosis and cell death, most prominent in tissue undergoing high cell turnover like skin and coinciding with the formation of granulation tissue in wound beds 27, 28, 29, 30. Irradiation also has a substantial impact on skin cell function, mediating altered collagen expression in fibroblasts, decreased contractility of myofibroblasts, and the induction of proinflammatory cytokines 31, 32, 33. Importantly, some of these changes can be reversed by implanting nonirradiated cells 34, 35, 36. In short, radiation‐mediated cell death and alterations in cell function are considerable mediators of the irradiated skin phenotype and related poor wound healing.
MSCs are a heterogeneous population of multipotent cells found in a wide variety of tissues, including bone marrow, adipose, and the dermis. These cells are increasingly popular in regenerative medicine given their ease of isolation, multipotency, and paracrine secretion of numerous growth factors 37. MSCs have been shown to promote healing in wounds and radiation injury 7, 38, 39. Although there is some evidence to suggest that irradiation causes a persistent reduction in number and function of dermal stem cells, little is known about the effect of irradiation on SMSCs in situ 9.
To that end, we sought to isolate and characterize SMSCs after they were exposed to ionizing radiation in vivo and compare these rSMSCs to SMSCs from normal skin. A primary difficulty in isolating and culturing SMSCs in vitro is confirming cell identity. Three criteria are frequently used for identification: (a) adherence to tissue culture plastic, (b) the expression of specific cell markers, and (c) multipotency as demonstrated by differentiation in vitro 6, 15, 18, 40. Additionally, we sought to evaluate early‐passage SMSCs given the substantial alterations in cell function and gene expression that occurs with long‐term culture, making assays requiring substantial cell number unfeasible. As a result, we chose to confirm the identity of our SMSCs with the use of a well‐established and validated protocol for isolation, adherence to plastic, and differentiation into both osteocytes and adipocytes 16. Cells isolated from our paired skin samples met all three criteria (Figs. 3A, 5A, 5B). Immunohistochemistry demonstrated that these cells could be characterized as CD90+/CD44+/CD31−/CD45−. Interestingly, rSMSCs expressed characteristic cell markers at much lower levels, suggesting that ionizing radiation may alter an SMSC's stemness (Fig. 3A).
By selecting patients with severe radiation wounds (Fig. 1A), we wished to maximize detectable differences between isolated SMSCs. Histological evaluation of the paired skin samples obtained from these patients confirmed substantial and chronic skin injury (Fig. 1B–1D). Significant differences in cell morphology, nuclear size, cytoskeletal arrangement, and proliferation in culture suggested the presence of a distinct irradiated SMSC phenotype (Fig. 4A–4D). Large nuclei, in particular, are associated with abnormal cellular behavior and used as prognostic indicators of disease severity in a number of cancers 41. This phenotype was consistent across all isolated pairs, demonstrating a clear and consistent effect of ionizing radiation on rSMSC morphology.
One mechanism by which SMSCs contribute to wound healing is via terminal differentiation into skin stromal cells 11. rSMSCs demonstrated significant impairments in differentiation into both adipogenic and osteogenic lineages (Fig. 5A, 5B). Similarly, CFU‐F assays demonstrated a significant 5.6‐fold reduction in colony‐forming capacity in rSMSCs, further underscoring diminished function in response to radiation (Fig. 5C, 5D). This suggests that defective cellular machinery for differentiation and self‐renewal may underlie some of the findings in chronic radiation skin injury and associated poor wound healing.
MSCs also exert potent effects on their microenvironment via paracrine signaling. Many studies have characterized the expression of a number of progrowth and immunomodulatory factors by SMSCs, in some instances having a profound impact on wound healing 10, 11, 42. To assess changes in the expression of these signaling molecules, we generated conditioned media. An in vitro assay for wound healing demonstrated that fibroblasts exposed to cultured media from rSMSCs migrated poorly, paralleling radiation‐induced poor wound healing in vivo (Fig. 6A, 6B). Proliferation assays revealed that the same conditioned media resulted in a cell type‐specific reduction in fibroblast proliferation without an equivalent effect in lymphatic or BECs (Fig. 6C). This specificity may be due to alterations of lineage‐specific signaling molecules, and although it is beyond the scope of this work, it is a research question that warrants further investigation.
As previously mentioned, there are few effective treatments for chronic radiation skin injury in clinical practice. There are also no true preventive therapies. This is due, in large part, to the paucity of available molecular targets that might account for the previously described derangements in SMSC function after radiation injury. To that end, we used RNA‐Seq and quantitative RT‐PCR to evaluate genome‐wide differential expression between rSMSCs and SMSCs from nonirradiated skin, resulting in the identification of over 100 differentially expressed genes (Fig. 7; Tables 2, 3). Many of these genes were implicated in skin homeostasis, skin pathology, and wound healing. DACT1, upregulated 2.2‐fold in irradiated SMSCs, was of particular interest given its role as an inhibitor of the Wnt signaling pathway. Wnt signaling is triggered by injury to the skin and promotes activation of stem cells and wound healing 43, 44, 45. Upregulation of DACT1 and subsequent inhibition of Wnt in irradiated SMSCs and the microenvironment, then, may be one mechanism by which these cells contribute to radiation skin injury and poor wound healing.
Conclusion
Overall, despite patient and sample heterogeneity, SMSCs isolated from irradiated human skin exhibit a characteristic phenotype that is associated with substantial derangements of cellular function. Importantly, these rSMSCs exhibit both a significant impairment in capacity for differentiation and altered paracrine signaling, affecting cells known to be critically involved in wound healing. These changes are associated with a distinct pattern of differential gene expression. Modulation of these differentially expressed genes may aid in the identification of targeted therapies aimed at preventing or correcting radiation skin injury.
Author Contributions
M.B.J.: collection and/or assembly of data, data analysis and interpretation, manuscript writing; S.N.‐B., V.S., X.X., B.P., E.J., S.Y.P.: collection and/or assembly of data; D.J.G.: data analysis and interpretation, manuscript writing; C.W., X.C.: data analysis and interpretation; R.Y.B.: provision of study material or patients; M.C., W.L.: collection and/or assembly of data, data analysis and interpretation; Y.‐K.H.: administrative support, provision of study material or patients, data analysis and interpretation; A.K.W.: conception and design, financial support, administrative support, provision of study material or patients, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
Acknowledgment
This work was supported in part by a grant from the Robert E. and May R. Wright Foundation.
References
- 1. Gu Q, Wang D, Cui C et al. Effects of radiation on wound healing. J Environ Pathol Toxicol Oncol 1998;17:117–123. [PubMed] [Google Scholar]
- 2. Dormand EL, Banwell PE, Goodacre TE. Radiotherapy and wound healing. Int Wound J 2005;2:112–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vegesna V, McBride WH, Withers HR. Postoperative irradiation impairs or enhances wound strength depending on time of administration. Radiat Res 1995;143:224–228. [PubMed] [Google Scholar]
- 4. Rudolph R, Arganese T, Woodward M. The ultrastructure and etiology of chronic radiotherapy damage in human skin. Ann Plast Surg 1982;9:282–292. [DOI] [PubMed] [Google Scholar]
- 5. Rudolph R, Tripuraneni P, Koziol JA et al. Normal transcutaneous oxygen pressure in skin after radiation therapy for cancer. Cancer 1994;74:3063–3070. [DOI] [PubMed] [Google Scholar]
- 6. Dominici M, Le Blanc K, Mueller I et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- 7. Chapel A, Bertho JM, Bensidhoum M et al. Mesenchymal stem cells home to injured tissues when co‐infused with hematopoietic cells to treat a radiation‐induced multi‐organ failure syndrome. J Gene Med 2003;5:1028–1038. [DOI] [PubMed] [Google Scholar]
- 8. Francois S, Bensidhoum M, Mouiseddine M et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: A study of their quantitative distribution after irradiation damage. Stem Cells 2006;24:1020–1029. [DOI] [PubMed] [Google Scholar]
- 9. Ahmed EA, Agay D, Schrock G et al. Persistent DNA damage after high dose in vivo gamma exposure of minipig skin. PLoS One 2012;7:e39521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen L, Tredget EE, Wu PY et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu Y, Chen L, Scott PG et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007;25:2648–2659. [DOI] [PubMed] [Google Scholar]
- 12. Chen MF, Lin CT, Chen WC et al. The sensitivity of human mesenchymal stem cells to ionizing radiation. Int J Radiat Oncol Biol Phys 2006;66:244–253. [DOI] [PubMed] [Google Scholar]
- 13. Li J, Kwong DL, Chan GC. The effects of various irradiation doses on the growth and differentiation of marrow‐derived human mesenchymal stromal cells. Pediatr Transplant 2007;11:379–387. [DOI] [PubMed] [Google Scholar]
- 14. Boink MA, van den Broek LJ, Roffel S et al. Different wound healing properties of dermis, adipose, and gingiva mesenchymal stromal cells. Wound Repair Regen 2016;24:100–109. [DOI] [PubMed] [Google Scholar]
- 15. Vaculik C, Schuster C, Bauer W et al. Human dermis harbors distinct mesenchymal stromal cell subsets. J Invest Dermatol 2012;132:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orciani M, Di Primio R. Skin‐derived mesenchymal stem cells: Isolation, culture, and characterization. Methods Mol Biol 2013;989:275–283. [DOI] [PubMed] [Google Scholar]
- 17. Toma JG, Akhavan M, Fernandes KJ et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 2001;3:778–784. [DOI] [PubMed] [Google Scholar]
- 18. Maleki M, Ghanbarvand F, Reza Behvarz M et al. Comparison of mesenchymal stem cell markers in multiple human adult stem cells. Int J Stem Cells 2014;7:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cogan J, Weinstein J, Wang X et al. Aminoglycosides restore full‐length type VII collagen by overcoming premature termination codons: Therapeutic implications for dystrophic epidermolysis bullosa. Mol Ther 2014;22:1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: Revisiting history, concepts, and assays. Cell Stem Cell 2008;2:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stone HB, Coleman CN, Anscher MS et al. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol 2003;4:529–536. [DOI] [PubMed] [Google Scholar]
- 22. Chan RJ, Webster J, Chung B et al. Prevention and treatment of acute radiation‐induced skin reactions: A systematic review and meta‐analysis of randomized controlled trials. BMC Cancer 2014;14:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wong RK, Bensadoun RJ, Boers‐Doets CB et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer 2013;21:2933–2948. [DOI] [PubMed] [Google Scholar]
- 24. Miller SH, Rudolph R. Healing in the irradiated wound. Clin Plast Surg 1990;17:503–508. [PubMed] [Google Scholar]
- 25. Baker DG, Krochak RJ. The response of the microvascular system to radiation: A review. Cancer Invest 1989;7:287–294. [DOI] [PubMed] [Google Scholar]
- 26. Aitasalo K, Aro H. Irradiation‐induced hypoxia in bones and soft tissues: An experimental study. Plast Reconstr Surg 1986;77:256–267. [DOI] [PubMed] [Google Scholar]
- 27. Devalia HL, Mansfield L. Radiotherapy and wound healing. Int Wound J 2008;5:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang J, Boerma M, Fu Q et al. Radiation responses in skin and connective tissues: Effect on wound healing and surgical outcome. Hernia 2006;10:502–506. [DOI] [PubMed] [Google Scholar]
- 29. Kolesnick R, Fuks Z. Radiation and ceramide‐induced apoptosis. Oncogene 2003;22:5897–5906. [DOI] [PubMed] [Google Scholar]
- 30. Liu X, Liu JZ, Zhang E et al. Impaired wound healing after local soft x‐ray irradiation in rat skin: Time course study of pathology, proliferation, cell cycle, and apoptosis. J Trauma 2005;59:682–690. [PubMed] [Google Scholar]
- 31. Bernstein EF, Harisiadis L, Salomon GD et al. Healing impairment of open wounds by skin irradiation. J Dermatol Surg Oncol 1994;20:757–760. [DOI] [PubMed] [Google Scholar]
- 32. Rodemann HP, Bamberg M. Cellular basis of radiation‐induced fibrosis. Radiother Oncol 1995;35:83–90. [DOI] [PubMed] [Google Scholar]
- 33. Schaffer M, Weimer W, Wider S et al. Differential expression of inflammatory mediators in radiation‐impaired wound healing. J Surg Res 2002;107:93–100. [PubMed] [Google Scholar]
- 34. Dantzer D, Ferguson P, Hill RP et al. Effect of radiation and cell implantation on wound healing in a rat model. J Surg Oncol 2003;83:185–190. [DOI] [PubMed] [Google Scholar]
- 35. Ferguson PC, Boynton EL, Wunder JS et al. Intradermal injection of autologous dermal fibroblasts improves wound healing in irradiated skin. J Surg Res 1999;85:331–338. [DOI] [PubMed] [Google Scholar]
- 36. Illsley MC, Peacock JH, McAnulty RJ et al. Increased collagen production in fibroblasts cultured from irradiated skin and effect of TGF beta(1)—Clinical study. Br J Cancer 2000;83:650–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 2007;213:341–347. [DOI] [PubMed] [Google Scholar]
- 38. Bey E, Prat M, Duhamel P et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow‐derived stem cell administrations. Wound Repair Regen 2010;18:50–58. [DOI] [PubMed] [Google Scholar]
- 39. Francois S, Mouiseddine M, Mathieu N et al. Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Ann Hematol 2007;86:1–8. [DOI] [PubMed] [Google Scholar]
- 40. Riekstina U, Cakstina I, Parfejevs V et al. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev 2009;5:378–386. [DOI] [PubMed] [Google Scholar]
- 41. Wolberg WH, Street WN, Mangasarian OL. Importance of nuclear morphology in breast cancer prognosis. Clin Cancer Res 1999;5:3542–3548. [PubMed] [Google Scholar]
- 42. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood 2007;110:3499–3506. [DOI] [PubMed] [Google Scholar]
- 43. De Boer J, Wang HJ, Van Blitterswijk C. Effects of Wnt signaling on proliferation and differentiation of human mesenchymal stem cells. Tissue Eng 2004;10:393–401. [DOI] [PubMed] [Google Scholar]
- 44. Fathke C, Wilson L, Shah K et al. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol 2006;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Whyte JL, Smith AA, Helms JA. Wnt signaling and injury repair. Cold Spring Harb Perspect Biol 2012;4:a008078. [DOI] [PMC free article] [PubMed] [Google Scholar]


