Abstract
The positive effects of therapeutic human allogeneic cardiac stem/progenitor cells (hCPC) in terms of cardiac repair/regeneration are very likely mediated by paracrine effects. Our previous studies revealed the advantageous immune interactions of allogeneic hCPC and proposed them as part of the positive paracrine effects occurring upon their application postmyocardial infarction (MI). Currently, extracellular vesicles/exosomes (EV/Exs) released by stem/progenitor cells are also proposed as major mediators of paracrine effects of therapeutic cells. Along this line, we evaluated contribution of EV/Exs released by therapeutic hCPC to the benefit of their successful allogeneic clinical application. Through tailored allogeneic in vitro human assay models mimicking the clinical setting, we demonstrate that hCPC‐released EV/Exs were rapidly and efficiently up‐taken by chief cellular actors of cardiac repair/regeneration. This promoted MAPK/Erk1/2 activation, migration, and proliferation of human leukocyte antigens (HLA)‐mismatched hCPC, mimicking endogenous progenitor cells and cardiomyocytes, and enhanced endothelial cell migration, growth, and organization into tube‐like structures through activation of several signaling pathways. EV/Exs also acted as pro‐survival stimuli for HLA‐mismatched monocytes tuning their phenotype toward an intermediate anti‐inflammatory pro‐angiogenic phenotype. Thus, while positively impacting the intrinsic regenerative and angiogenic programs, EV/Exs released by therapeutic allogeneic hCPC can also actively contribute to shaping MI‐inflammatory environment, which could strengthen the benefits of hCPC allogeneic interactions. Collectively, our data might forecast the application of allogeneic hCPC followed by their cell‐free EV/Exs as a strategy that will not only elicit the cell‐contact mediated reparative/regenerative immune response but also have the desired long‐lasting effects through the EV/Exs. stem cells translational medicine 2019;8:911&924
Keywords: Human cardiac stem/progenitor cells, Extracellular vesicles, Exosomes, Allogeneic stem cell‐based therapy, Paracrine effect, Cardiac repair/regeneration
Significance Statement.
Extracellular vesicles/exosomes (EV/Exs) released from therapeutic cardiac stem/progenitor cells (hCPC) regulate the activity of chief cellular actors of cardiac repair/regeneration. Using a tailored in vitro human model mimicking the clinical allogeneic hCPC administration, this study provides human proof‐of‐concept of active contribution of EV/Exs to the benefit of therapeutic cells. In the context of advantageous immune interactions of allogeneic hCPC, the data propose administration of cells then their cell‐free EV/Exs to elicit the cell‐contact reparative/regenerative immune response and have the desired long‐lasting effects through the EV/Exs. Establishing the therapeutic value of hCPC‐EV/Exs might provide a new dimension for cardiac repair therapies.
Introduction
Myocardial infarction (MI) is a significant cause of morbidity and premature death for both men and women. Cardiac stem/progenitor cell‐based therapies are among the exciting options for repairing injured tissue to circumvent life‐threatening heart failure. The exact mechanisms of cardiac repair by transplanted cells are still largely unknown. The direct trans‐differentiation hypothesis was initially proposed. Yet currently, the release of molecules by the therapeutic stem/progenitor cells that can stimulate the endogenous cardiac repair/regeneration machinery appears as a more realistic hypothesis. Subsequently, the paracrine effect has been proposed 1 and further accentuated by the sustained wholesome effects of therapeutic cells administrated to prevent post‐MI heart failure 2, 3, 4.
Extracellular vesicles (EV) are naturally cell‐derived vesicles secreted by various cell types throughout the body for local and remote cell‐to‐cell communication. They contain a payload of specific lipids, small RNAs, and proteins that can be transferred from parent cells to target cells and regulate their activities 5. The visualization of exosome‐like vesicles released by cardiac progenitor cells in the mouse heart in vivo 6, 7, and the internalization of these vesicles by cells implicated in cardiac repair after injury, suggested EV as important players mediating the wholesome effects of therapeutic stem/progenitor cells 8, 9, 10.
Cardiac repair after MI is finely orchestrated by complex series of events. The initial intense sterile inflammation is rapidly followed by a reparative phase leading to resolution of inflammation, myofibroblasts proliferation and scar formation, and neovascularization 11. A robust postinfarction angiogenic response managed by vascular cells, in particular, endothelial cells, promotes inflammatory cell extravasation into the infarct tissue and starts cardiac repair. Early activation of inflammatory cells is necessary for the transition to reparative and proliferative programs. Both phases are driven by the recruited pro‐inflammatory monocytes, which would switch their phenotype to anti‐inflammatory macrophages during the reparative phase to promote healing 12, 13.
Human cardiac stem/progenitor cells (hCPC) are stem/progenitor cells with mixed phenotype, expressing stemness factors oct4, sox2, and nanog as well as early cardiac lineage transcription factors GATA‐4, MEF2C, Nkx2.5 14 (Supporting Information Fig. S1). They form cardiospheres and differentiate into principal cardiac lineages in vitro, and promote cardiac repair/regeneration in experimental MI rodent models 14. From an immunological standpoint, allogeneic hCPC are immunogenic immune‐modulator triggering beneficial rather than deleterious immune responses that might contribute to post‐MI cardiac repair 14, 15, 16, 17, 18. Within this promising preclinical background, allogeneic hCPC entered successful multicenter phase I/II clinical investigations in the double‐blind randomized and controlled CAREMI trial (https://clinicaltrials.gov/ct2/show/NCT02439398) 19, 20.
We previously projected, within tailored allogeneic in vitro human model systems mimicking clinical settings, the advantageous immune interactions of allogeneic hCPC as part of their positive paracrine effects occurring upon their clinical administration post‐MI. To the best of our knowledge, most of the studies investigating the cardio‐reparative effect of various human CPC‐derived EV were conducted within immune‐deficient experimental animal models. Although providing valuable insights, the ingrained differences between species and the fact that therapeutic cells are intended for immune‐competent hosts warrant a proof‐of‐concept within human settings. Therefore, in this study, we used our established allogeneic human model to look into how EV secreted by clinically investigated hCPC would contribute to their paracrine effects.
Materials and Methods
Detailed materials and experimental procedures are provided under Supporting Information.
Ethical Statement
Human cardiac biopsies were obtained from patients undergoing open‐chest surgery after signed informed consent in accordance with the Declaration of Helsinki. The ethical committees of “Hospital 12 de Octubre” and “Fundación Jiménez Díaz” (Madrid), Spain have approved the project. Blood donors signed an informed consent following human ethics committee “Comité consultatif pour la protection des personnes dans les recherches biomédicales” (Saint Louis Hospital, Paris, France) and all methods and experimental protocols were approved by the institution and were conducted in accordance with guidelines and regulation.
Isolation and Culture of hCPC
hCPC were isolated by immune‐selection of CD117 (c‐kit; Supporting Information Table S1), and maintained in a combination of Dulbecco's modified Eagle's medium (DMEM)/F12 and Neurobasal medium supplemented with 10% fetal bovine serum (FBS) embryonic stem cell qualified at 3% O2 atmosphere. hCPC have been fully characterized in our previous report 14 and genotyped for human leukocyte antigens (HLA; human major histocompatibility complex; Supporting Information Table S2). All assays were performed with passages 3–7 hCPC at 80%–90% confluence.
Cardiomyocytes, Endothelial Cells, and Human Monocytes
Human AC16 cardiomyocytes (MERCK, Fontenay sous Bois Île‐de‐France, France) were maintained in 10% FBS‐DMEM at 5% CO2. ATCC CRL‐3243 human microvascular endothelial cells (HMEC‐1; gift from N. Mooney, INSERM 1106, Paris, France) were maintained in 10% FBS‐DMEM at 5% CO2. ATCC CRL‐1730 human umbilical vein endothelial cells (HUVEC; gift from F. Mechta‐Grigoriou, INSERM U830, Curie Institute, Paris, France) were maintained in endothelial cell medium (ScienCell, CliniScience, Nanterre, France) at 5% CO2. CD14+CD16− monocytes were negatively selected from human peripheral blood mononuclear cells prepared from blood samples of HLA‐genotyped healthy donors (Supporting Information Table S2) using MACS magnetic beads (Miltenyi Biotec, Germany) to ≥95% purity.
Isolation, Characterization, and Internalization of EV Secreted by hCPC
As recommended in latest guidelines 21, EV secreted by hCPC were isolated using differential ultracentrifugation, then assessed by two different but complementary technologies nanoparticle tracking analysis (NTA; 3.0, Malvern) and transmission electron microscopy (TEM). hCPC released EV were also characterized for the expression of EV markers, the tetraspanins CD81 and CD63, HLA class I, and actin by Western blotting and flow cytometry 22. Carboxyfluorescein N‐succinimidyl ester (CFSE)‐labeled EV internalization by different cell types was assessed by immunofluorescence microscopy and by flow cytometry monitoring. Fluorescence images were processed using the Imaris program (Bitplane AG, Switzerland).
EV‐Induced Signaling
Activation of signaling pathways was determined by Western blotting and Human phospho‐MAPK Array Kit (ARY003; R&D Systems).
Migration, Proliferation, Cell Growth, and Tube Formation Assays
Migratory capacity of different cells was evaluated through wound healing assays. Proliferation was evaluated by either, Ki67 staining, BrdU incorporation, or cell counting. Cell growth was evaluated by Incucyte incubation and monitoring. For organization of endothelial cells in tube structures, cells were seeded on Matrigel‐coated 24‐well plates in serum‐free DMEM and then number of nodes and junctions was determined.
Monocytes Survival, Immune Phenotype, and Secretome
Survival of freshly isolated CD14+CD16− monocytes was evaluated by determining percentage of viable (7‐aminoactinomycin D [7‐AAD]‐negative) and dead (7‐AAD‐positive) cells by flow cytometry. Their immune‐phenotype was determined by flow cytometry using specific antibodies, whereas their chemokines and cytokines profiles were determined by Human Chemokine Array Kit (R&D Systems, ARY017, Lille, France) and Human Cytokines Array Kit (R&D Systems, ARY005B), respectively.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, Inc., San Diago, CA, USA). Data on the graphs represent the mean ± SEM from at least three independent experiments. Comparisons were performed using Mann–Whitney test for nonpaired groups, paired Student's t test for paired groups, and two‐way analysis of variance—Sidak's multiple comparisons test for multiple comparisons. A p‐value of less than .05 was considered statistically significant (*, p < .05; **, p < .01; ***, p < .001; ****, p < .0001).
Phospho‐array mean pixel intensity data were exported into an Excel file and analyzed using R studio interface with an in‐house R scripts. A heatmap was then generated.
Results
hCPCs Produce Functional EV
To confirm the release of EV, hCPC culture supernatant (SN) was subjected to differential centrifugation and processed for NTA and TEM. NTA demonstrated 95% enrichment in nanovesicles with typical small‐EV/exosomes (Exs) size range (30–150 nm) and a 5%‐minute fraction of medium/large‐EV (≥200 nm) and/or apoptotic bodies 21, 23. These results were consistent with TEM, which identified primarily particles of the size of small‐EV/Exs (30–100 nm). Furthermore, characterization of the EV fraction by Western blot and flow cytometry revealed a high enrichment the EV markers CD63, CD81, HLA I, and actin (Supporting Information Fig. S2A, S2B), and will be referred to hereafter as EV/Exs. In theory, the infused hCPC would have to operate within a post‐MI inflammatory environment highly enriched with an assortment of growth factors and pro‐inflammatory cytokines. To evaluate the impact of MI inflammatory environment on EV production, we next used IFNγ inured hCPC (IFNγ‐hCPC). Higher amounts of EV within the exosomal size range were produced by IFNγ‐inured hCPC (40% more) than steady state cells (Supporting Information Fig. S2C). NTA and Western blot analysis demonstrated that these EV displayed the same characteristics of EV/Exs (data not shown).
To mimic in situ clinical setting, we then used an in vitro allogeneic experimental model to investigate the effects of EV/Exs on resident cardiac stem/progenitor cells activity. We first examined the uptake of EV/Exs by HLA‐mismatched hCPC (mimicking resident cells). CFSE‐labeled EV/Exs were added to cultures of HLA‐mismatched hCPC and their uptake was visualized by fluorescence microscopy and monitored over 8 hours by flow cytometry (Fig. 1A). The uptake of hCPC‐EV/Exs by allogeneic hCPC was visualized as early as 15 minutes. Kinetic analysis showed a gradual increase reaching 14% within 1 hour and 77% by 8 hours.
Figure 1.
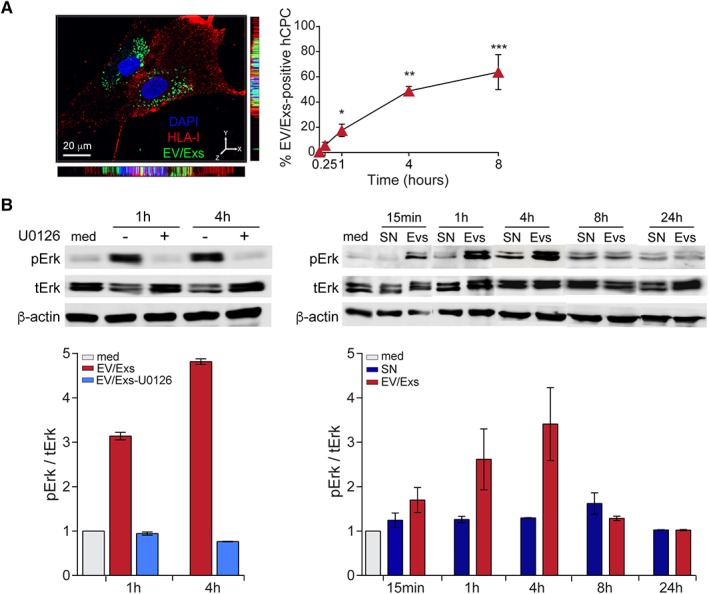
Internalization of extracellular vesicles/exosomes (EV/Exs) by human leukocyte antigens (HLA)‐mismatched activate MAPK/Erk1/2. (A): Uptake of carboxyfluorescein N‐succinimidyl ester (CFSE)‐labeled EV/Exs by HLA‐mismatched human allogeneic cardiac stem/progenitor cells (hCPC) was assessed by fluorescent microscopy (left panel) and flow cytometry at different time points as indicated (right panel). Representative Z‐stack projections of immunofluorescence image by Imaris program for CFSE‐labeled EV/Exs uptake by HLA‐mismatched hCPC showing a tendency of EV/Exs to accumulate within the perinuclear regions. Orthogonal views are shown with Y–Z and X–Z orientations. Histograms display the uptake of EV/Exs by HLA‐mismatched hCPC in function of time. Results are mean values ± SD from three independent experiments. (B): HLA‐mismatched hCPC were treated with EV/Exs or EV/Exs‐free medium (SN) in the absence (med) or presence of MAPK/Erk1/2 inhibitor U0126 for indicated time and the level of phosphorylated Erk1/2 (pErk1/2) was determined. Illustrated blots are representative of three independent experiments (upper panel). Quantification of the levels of phosphorylated Erk1/2 relative to total Erk1/2 in the presence or absence of U0126 and compared with medium presented as mean values ± SD from three independent experiments (lower panel). *, p < .05; **, p < .01; ***, p < .001 compared with medium.
We then verified the functionality of EV/Exs, by evaluating their capacity to activate the MAPK/Erk signaling pathway in hCPC since it is a major regulator of their cellular activities. HLA‐mismatched hCPC were stimulated with EV/Exs in the presence or absence of specific MAPK/Erk inhibitor U0126 for 1 and 4 hours and analyzed for Erk1/2 phosphorylation by immunoblotting. EV/Exs induced a significant increase in the level of phosphorylated Erk1/2 reaching twofold and fourfold after 1 and 4 hours, respectively, which was abolished in the presence of specific MEK1/2 inhibitor U0126 (Fig. 1B). Kinetics studies demonstrated that the EV/Exs‐induced activation of MAPK/Erk pathway in allogeneic hCPC by EV/Exs is initiated within 15 minutes to reach a maximum within 4 hours and decline thereafter to baseline (Fig. 1B).
Together, these results suggest that EV/Exs secreted by hCPC within allogeneic settings could be readily up‐taken by resident hCPC and have the capacity to activate signaling pathways that regulate cells activity.
EV/Exs Promote HLA‐Mismatched hCPC Recruitment and Proliferation
As functional readouts of MAPK/Erk activation in hCPC, we assessed the capacity of EV/Exs to recruit resident cardiac stem/progenitor and to induce their proliferation within the in vitro allogeneic experimental settings.
We used wound‐healing assay to monitor the migration of HLA‐mismatched hCPC in response to EV/Exs. Analysis of wounded area and wound closure demonstrated that HLA‐mismatched hCPC efficiently migrate into the scratched area in response to EV/Exs, resulting in the complete closure of the wounded area within 3 days (Fig. 2A). In contrast, in the presence of EV/Exs‐free SN or control medium cells did not migrate to the scratched area. Pretreatment of allogeneic hCPC with U0126 abolished their migration in response to EV/Exs indicating that the EV/Exs‐induced recruitment of hCPC is MAPK/Erk‐dependent (Fig. 2A).
Figure 2.
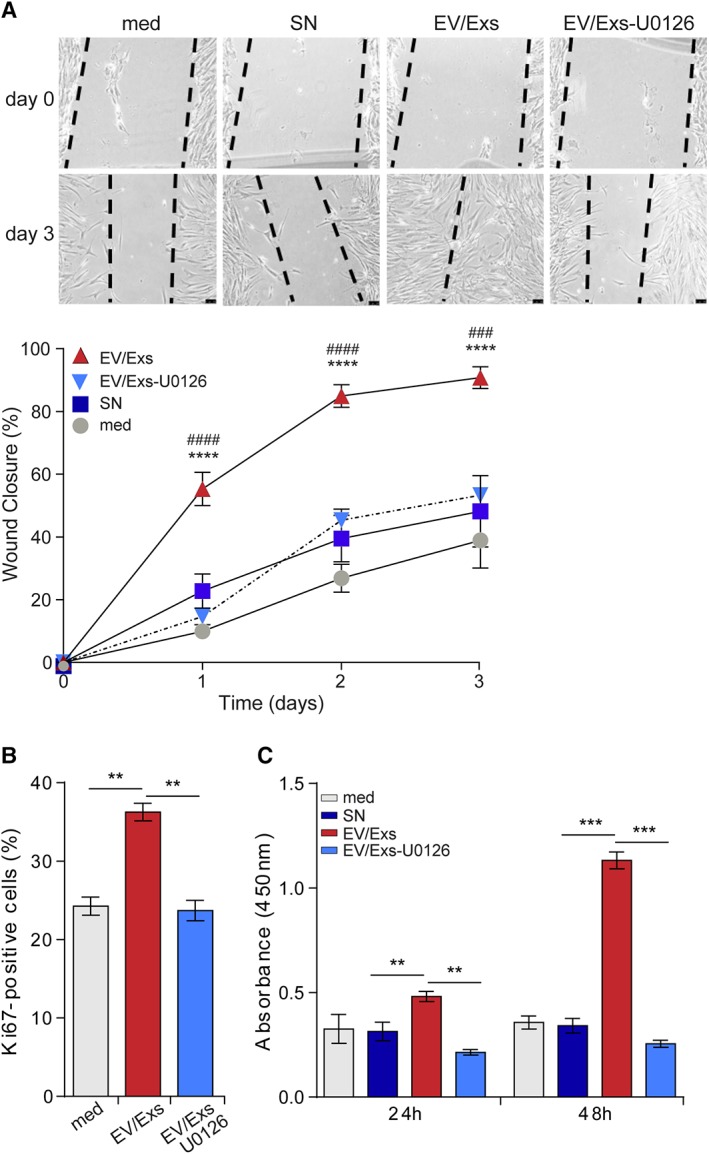
Extracellular vesicles/exosomes (EV/Exs) induce MAPK/Erk1/2‐dependent migration and proliferation of human leukocyte antigens (HLA)‐mismatched human allogeneic cardiac stem/progenitor cells (hCPC). (A): Migration of allogeneic hCPC in response to EV/Exs in the absence or the presence of U0126, in response to EV/Exs‐free medium (SN) or to medium alone (med). Representative images of five independent scratch wound assays at day 0 and day 3 (upper panel, scale bar: 1 mm) and percentage of wound closure (lower panel) as calculated by ImageJ software. Results are presented as mean values ± SD from five independent experiments conducted with two different EV/Exs preparations. (B): Proliferation of U0126‐pretreated or non‐HLA‐mismatched hCPC in response to EV/Exs as evaluated by Ki67 staining at 24 hours. Results are presented as mean% of Ki67‐positive cells ± SD from three independent experiments. (C): Proliferation of U0126‐pretreated or non‐HLA‐mismatched hCPC in response to EV/Exs, EV/Exs‐free medium (SN), or to medium alone (med) as evaluated by BrdU incorporation at 24 and 48 hours. Results as presented as mean absorbance values ± SD from three independent experiments. **, p < .01; ***, p < .001; ****, p < .0001 compared with medium; ###, p < .001; ####, p < .0001 compared with SN.
We then evaluated the capacity of EV/Exs to induce HLA‐mismatched hCPC proliferation. Cells were cultured in the absence or presence of EV/Exs for 24 hours then stained for Ki67 proliferation marker. The presence of EV/Exs increased the number of Ki67‐positive allogeneic hCPC by approximately 40%, which was also abolished when allogeneic hCPC were pretreated with U0126 for 1 hour (Fig. 2B). To further confirm this observation, HLA‐mismatched hCPC were either kept untreated or pretreated with U0126 then incubated in the absence or presence of EV/Exs for 24 and 48 hours and their proliferation was evaluated by BrdU incorporation assay. An approximate twofold and fourfold increase in the proliferation was observed when allogeneic hCPC were cultured with EV/Exs for 24 hours and 48 hours, respectively, but not in the presence of SN or control medium (Fig. 2C). The inhibition of Erk1/2 activation by U0126 abolished the effect of EV/Exs.
Thus, EV/Exs released by the infused allogeneic hCPC could promote the recruitment of resident cardiac stem/progenitors to the site of MI injury and enhance their proliferation in a MAPK/Erk‐dependent manner underscoring the ability of EV/Exs to regulate resident cardiac stem/progenitor cells activity.
hCPC‐EV/Exs Promote Migration, Survival, and Proliferation of Cardiomyocytes
Stimulation of the endogenous cardiomyocytes' proliferation is integral to cardiac repair and regeneration after injury 24. AC16 cardiomyocytes are proliferating cells derived from adult human ventricular heart tissues and frequently used to study cardiac functions 25. Accordingly, and based on extreme polymorphism of HLA system and extremely low probability of unrelated donor HLA‐matching, the AC16 cardiomyocytes were used as a model of cardiomyocytes to investigate the potential effects of EV/Exs on resident cardiomyocytes activity. Similar to hCPC, the uptake of CFSE‐labeled EV/Exs by AC16 cardiomyocytes was rapid and efficient reaching 60% and 80% within 4 and 8 hours, respectively (Fig. 3A).
Figure 3.
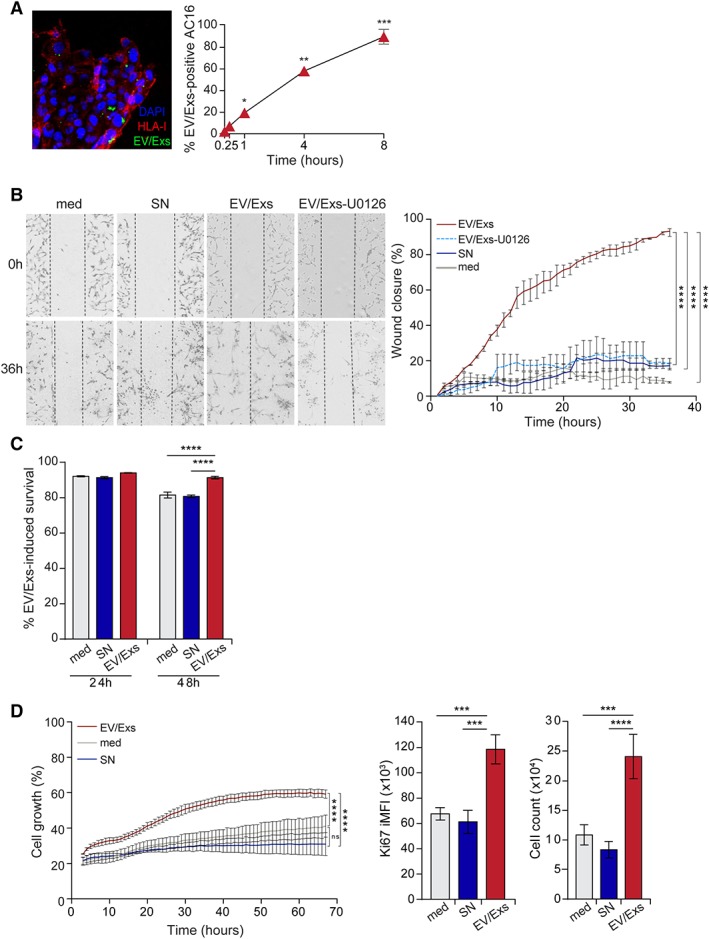
Internalization of extracellular vesicles/exosomes (EV/Exs) by AC16 cardiomyocytes promotes their migration, survival, and proliferation. (A): Uptake of carboxyfluorescein N‐succinimidyl ester‐labeled EV/Exs by AC16 cardiomyocytes as assessed by fluorescent microscopy (left panel, ×200 magnification) and flow cytometry at different time points as indicated (right panel). Results are mean values ± SD from three independent experiments. (B): EV/Exs and non‐EV/Exs‐free medium (SN) or medium alone (med) promote AC16 cardiomyocytes migration, which is blocked in the presence of U0126. Representative images (×10 magnification) of four independent scratch wound assays at 0 and 36 hours (left panel), and the %wound closure as monitored over time and calculated by ImageJ software. Results are presented as mean values ± SD from two independent experiments. (C): Survival of 12‐hours starved AC16 cardiomyocytes in fetal bovine serum (FBS)‐free medium in the absence (med) or the presence of EV/Exs or EV/Exs‐free medium (SN) as determined by cell viability dye at 24 and 48 hours. Results are presented as mean values ± SD from three independent experiments. (D): AC16 cardiomyocytes proliferation in FBS‐free medium in the absence (med) or presence of EV/Exs or EV/Exs‐free medium (SN) as evaluated by monitoring cell growth over 70 hours in Incucyte (left panel), and staining for Ki67 proliferation marker (middle panel) and cell counting using flow cytometry (right panel) at 24 hours. Results are presented as mean value ± SD of %cell growth, Ki67 integrated mean fluorescence intensity, and number of cells, respectively, from three independent experiments. *, p < .05; **, p < .01; ***, p < .001; ****, p < .0001 compared with med or SN.
We then monitored migration of AC16 cardiomyocytes in response to EV/Exs over 36 hours. Analysis of wounded area and wound closure demonstrated that AC16 cells efficiently migrate into the scratched area in response to EV/Exs, but not to SN or control medium (Fig. 3B). Similar to hCPC, pretreatment of AC16 cells with U0126 significantly decreased their capacity to migrate in response to EV/Exs (Fig. 3B) suggesting the implication of MAPK/Erk pathway in this EV/Exs‐induced activity.
Because cardiac injury generates a lot of stress on cardiomyocytes provoking their massive loss, we evaluated whether the presence of EV/Exs under stressful conditions could restraint cardiomyocyte loss. We assessed the survival of AC16 cardiomyocytes when cultured in serum‐deprived medium in the presence of EV/Exs, SN, or control medium. Neither the presence of EV/Exs nor SN showed any pronounced effect on cardiomyocytes survival in 24 hours‐cultures. Nonetheless, the presence of EV/Exs significantly sustained the viability of AC16 cardiomyocytes in 48 hours‐cultures (Fig. 3C). These results suggest that the release of EV/Exs by hCPC within postinjury stressful environment could potentially restraint cardiomyocytes loss.
Consequently, we investigated whether EV/Exs would promote cardiomyocytes growth and proliferation under similar conditions. AC16 cardiomyocytes were starved overnight then cultured in the presence of EV/Exs, SN, or control medium, and their growth was monitored in Incucyte live cell system over 65 hours. Within few hours of culture, EV/Exs were able to enhance the growth of AC16 cardiomyocytes, which reached a maximum of 60% in approximately 50 hours. The capacity of EV/Exs to promote AC16 proliferation was further confirmed by ki67 staining and direct cell count (Fig. 3D). Flow cytometry analysis of Ki67‐positive cardiomyocytes showed nearly twofold increase in the percentage and the geomean fluorescence intensity (MFI) in cardiomyocytes that were subjected to EV/Exs treatment for 24 hours (Supporting Information Fig. S3). We calculated the Ki67 integrated MFI (iMFI) by incorporating both the magnitude (the total frequency [%] of cells displaying a particular response) and the quality of a response (assessed by the MFI) as previously described 26, to reflect the total response of a population of cells (Fig. 3D). We found that iMFI of Ki67‐positive cardiomyocytes is significantly higher when cells are exposed to EV/Exs, which translated in a higher cell number compared with AC16 cultured in the presence of SN or control medium.
Together, these results forecast that the uptake of allogeneic hCPC EV/Exs by resident cardiomyocytes could restraint their loss and promote their recruitment, survival, and proliferation at the site of MI injury.
EV/Exs Promote Endothelial Cells Migration, Growth, and Organization in Tube‐Like Structures
Since endothelial cells play essential roles in cardiac repair/regeneration post‐MI injury, we investigated effects of EV/Exs on endothelial cells in the allogeneic settings. We used HLA‐A1/68, HLA‐B35/58, HLA‐Cw4/6, HLA‐DR18/12, HLA‐DQ4/5 HMEC, and HUVEC, which are also highly likely HLA‐mismatched. Both cell lines are actively proliferating cells, retain most of the angiogenic features of primary endothelial cells, and have good stability presenting therefore, common endothelial models for cardiac studies 27, 28, 29, 30.
HUVEC and HMEC efficiently internalized EV/Exs displaying, albeit slower capacity compared with hCPC and AC16 cardiomyocytes, nearly 55% and 65% EV/Exs uptake, respectively, within 8 hours of culture (Fig. 4A and Supporting Information Fig. S4A). EV/Exs treatment of endothelial cells substantially promotes cell migration in wound healing assay (Fig. 4B) but had only modest effect on their growth evaluated by Incucyte monitoring over 50 hours (Fig. 4C). In line, endothelial cells treated with EV/Exs displayed modest expression of Ki67 compared with untreated or SN‐treated cells, which translated to a meaningful increase in cell number at 48 hours (Supporting Information Fig. S4B).
Figure 4.
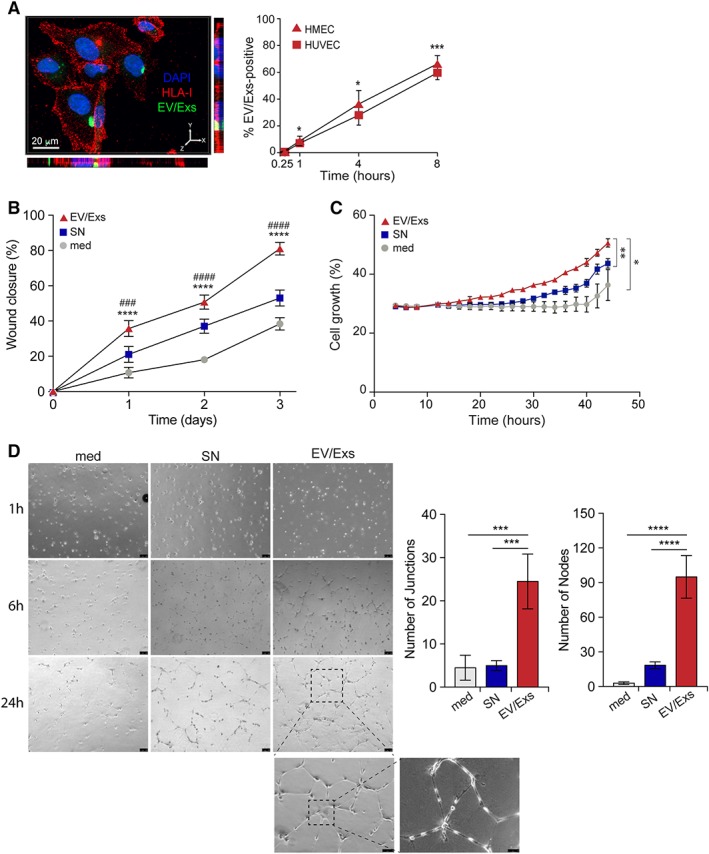
Internalization of extracellular vesicles/exosomes (EV/Exs) by human microvascular endothelial cells (HMEC) and human umbilical vein endothelial cells (HUVEC) promote migration, growth, and tube formation. (A): Representative Z‐stack projections of immunofluorescence image by Imaris program for carboxyfluorescein N‐succinimidyl ester (CFSE)‐labeled EV/Exs uptake by HUVEC. Orthogonal views are shown with Y–Z and X–Z orientations (left panel). In the right panel, the uptake of CFSE‐labeled EV/Exs by HUVEC and HMEC as assessed by flow cytometry at different time points as indicated. (B): EV/Exs and non‐EV/Exs‐free medium (SN) or medium alone (med) promote HUVEC migration. The %wound closure as monitored on over time as indicated and calculated by ImageJ software. Similar data were obtained with HMEC. (C): Serum‐starved HMEC growth in the absence (med) or presence of EV/Exs or EV/Exs‐free medium (SN) monitored over 48 hours in Incucyte and assessed by integrated software. Similar data were obtained with HUVEC. (D): Organization of HUVEC in tube structures in the absence (med) or presence of EV/Exs or EV/Exs‐free medium (SN) over 24 hours. Representative images of three independent experiments (left panel, ×40, ×100, and ×200 magnification) and histograms presenting number of nodes and junctions as determined by angiogenesis analyzer for ImageJ software (right panel). All results are presented as mean values ± SD from at least two independent experiments. *, p < .05; **, p < .01; ***, p < .001; ****, p < .0001 compared with medium; ###, p < .001; ####, p < .0001 compared with SN.
Because angiogenesis is integral to cardiac repair/regeneration, we then evaluated whether EV/Exs could induce angiogenic activity in allogeneic settings. The ability of endothelial cell models to organize into vascular‐like tubular structures was evaluated by the number of nodes and junctions per mm2. Both HUVEC (Fig. 4D) and HMEC (Supporting Information Fig. S4C) showed significantly higher number of nodes and junctions in the presence of EV/Exs compared with SN or control.
Collectively, these in vitro data show that EV/Exs within an allogeneic environment positively impact endothelial cells activity. By modulating the activity of endothelial cells and promoting angiogenic process, EV/Exs could facilitate neovascularization of the injured myocardium receiving allogeneic hCPC.
Effect of EV/Exs Secreted by hCPC on Kinase Signaling in Endothelial Cells
To provide some mechanistic insights into how EV/Exs modulate various activities of endothelial cells, we examined MAPK signaling pathway using a human phospho‐MAPK array. Untreated or EV/Exs‐treated for 1, 4, and 8 hours HUVEC were subjected to phospho‐array analysis. Heatmap analysis using an in‐house R script demonstrated that pixel intensity of a total of 26 MAPKs and serine/threonine kinases was remarkably increased in EV/Exs‐treated cells compared with medium control reaching a maximum for most kinases within 4 hours (Fig. 5A). Seven pronounced differences (≥1.5‐fold) in EV/Exs‐induced kinase phosphorylation were detected (Fig. 5B). Pixel intensity of phosphorylated MAPK/Erk1, MAPK/JNK, TOR, p70S6K, and CREB increased by almost 150% compared with control and those of GSK‐3β and p53 were much higher reaching almost 250% and 300% increase, respectively. Thus, EV/Exs produced by allogeneic hCPC can promote endothelial cell migration, proliferation and angiogenesis through the induction of kinases phosphorylation and activation of several downstream signaling pathways.
Figure 5.
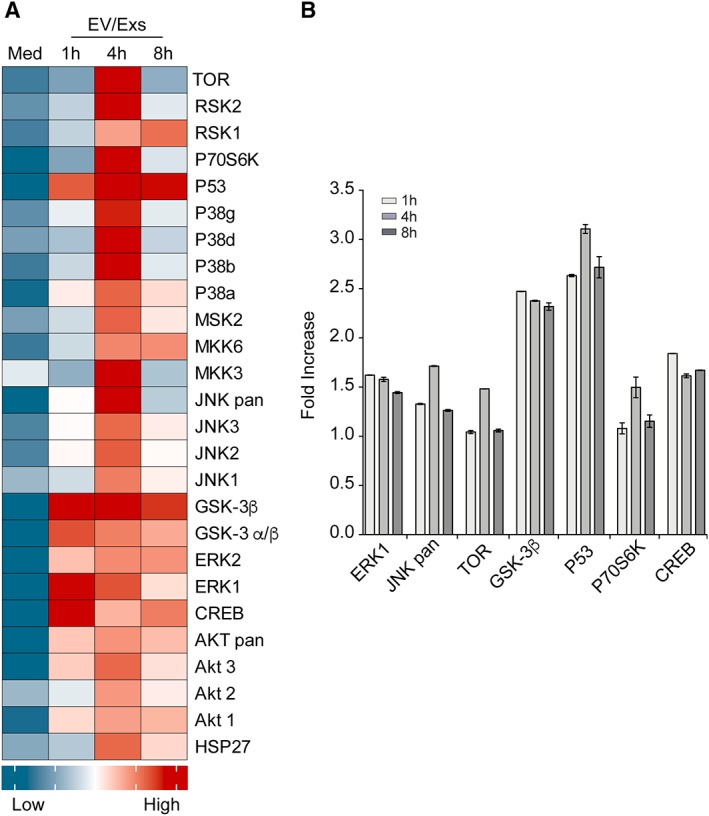
Extracellular vesicles/exosomes (EV/Exs) upregulate phosphorylation of various kinases in endothelial cells: a human phosphor‐kinase array was used to determine kinase phosphorylation in HUVEC upon their exposure to EV/Exs or medium alone (med) for indicated time. (A): Heatmap presenting quantification of signals from array as pixel intensities normalized to control signals. (B): Histograms presenting the seven more pronounced kinase activation induced by EV/Exs as fold increase, compared with medium, in pixel intensities (signals) from array at 1, 4, and 8 hours of treatment. Results are presented as mean values ± SD of duplicates.
Internalization of hCPC‐EV/Exs by CD14+CD16− Monocytes Protects from Spontaneous Cell Death
Because monocytes/macrophages are also critical actors of myocardium injury and repair, we evaluated the impact of EV/Exs on the behavior of primary HLA‐typed CD14+CD16− monocytes within the in vitro allogeneic experimental model. Compared with myocytes and endothelial cells, CFSE‐labeled EV/Exs were rapidly and more efficiently up‐taken by HLA‐mismatched CD14+CD16− monocytes. Nearly 16.5% of HLA‐mismatched monocytes were CFSE‐positive within 15 minutes and 94% of cells became CFSE‐positive by 8 hours (Fig. 6A).
Figure 6.
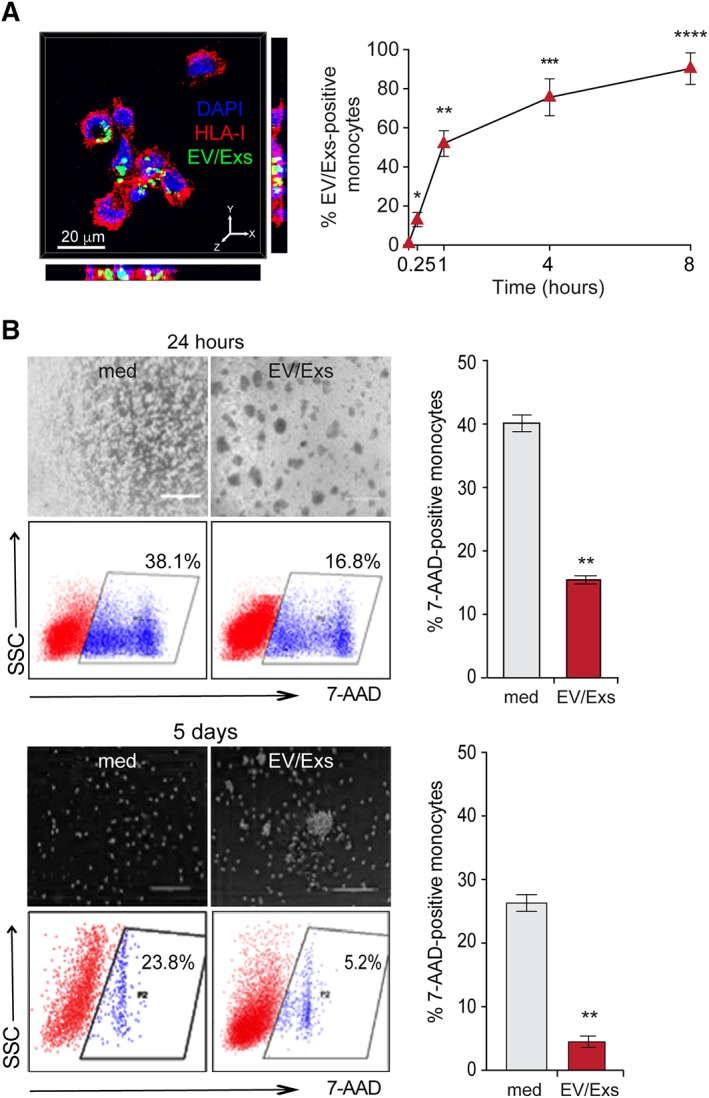
Internalization of extracellular vesicles/exosomes (EV/Exs) by human leukocyte antigens (HLA)‐mismatched CD14+CD16− monocytes protect from spontaneous cell death. (A): Representative Z‐stack projections of immunofluorescence image by Imaris program for carboxyfluorescein N‐succinimidyl ester (CFSE)‐labeled EV/Exs uptake by freshly isolated HLA‐mismatched CD14+CD16− monocytes. Orthogonal views are shown with Y–Z and X–Z orientations (left panel). In the right panel, the uptake of CFSE‐labeled EV/Exs by as assessed by flow cytometry at different time points as indicated. Results are mean values ± SD from four independent experiments. (B): Survival of CD14+CD16− monocytes in complete or in EV/Exs‐conditioned medium for 24 hours (upper panel, scale bar: 400 μm) or for 5 days (lower panel, scale bar: 200 μm) and their spontaneous death under each condition. Representative images and cytometry dot‐blots of three independent experiments are illustrated in the left panel, and histograms in the right panel presents mean% 7‐AAD‐positive cells ± SD. *, p < .05; **, p < .01; ***, p < .001; ****, p < .0001 compared with medium.
Monocytes are short lived cells with enhanced intrinsic susceptibility to constitutive apoptosis that can be reversed by growth factors and pro‐inflammatory stimuli 31. As a first‐line readout of EV/Exs capacity to regulate the activity of CD14+CD16− monocytes, we evaluated their impact on monocytes susceptibility to spontaneous apoptosis. HLA‐mismatched CD14+CD16− monocytes were seeded in their complete medium alone or in the presence of 50 μg/ml of EV/Exs for 24 hours. The presence of hCPC‐EV/Exs induced a twofold reduction in HLA‐mismatched monocytes death increasing their viability and their aggregation/adhesion (Fig. 6B). HLA‐mismatched monocytes cultured alone for 5 days were less dense, and displayed nearly 24% of 7‐AAD‐positive cells (dead cells) certainly due to massive cell loss in long‐term cultures. But again, the presence of EV/Exs during the 5‐days culture induced a nearly fivefold reduction of 7‐AAD‐positive cells increasing the viability and cellular aggregation (Fig. 6B). These data suggest that EV/Exs within allogeneic injured myocardium could act as pro‐survival stimuli for the recruited monocytes by promoting their activation.
hCPC‐EV/Exs Contribute to Tuning HLA‐Mismatched Monocyte Behavior
We then investigated the conduct of HLA‐mismatched CD14+CD16− monocytes within EV/Exs‐conditioned microenvironment. HLA‐mismatched CD14+CD16− monocytes cultured in complete medium for 24 hours displayed an intermediate monocytes profile 32 marked by bright expression of CD163 and major histocompatibility complex (MHC) molecule HLA‐DR (Fig. 7A and Supporting Information Fig. S5). These cells were also positive for CD16, the CD86 costimulatory molecule and Toll‐like receptors TLR‐1 and TLR‐2. Likewise, CD14+CD16− monocytes cultured for 5 days showed intermediate monocytes phenotype yet with a dimmer expression of most receptors except the CD163, which displayed brighter expression. When CD14+CD16− monocytes were cultured in EV/Exs‐conditioned medium, whether for 24 hours or for 5 days, they expressed the same set of receptors but at higher levels, evidenced either by an increase in the percentage of positive cells or in MFI (Fig. 7A and Supporting Information Fig. S5). The two main notable differences in EV/Exs effect were illustrated by a dimmer expression of CD163 and the induction of CD80 expression in 50% of the cells. However, both effects were lost after 5‐days of culture; CD14+CD16− monocytes lost their expression of CD80 whereas they increased the expression of CD163 (Fig. 7A and Supporting Information Fig. S5). Collectively, these profiles suggest that EV/Exs secreted by hCPC would primarily enhance a pro‐inflammatory monocyte phenotype but then would rather tune for a tissue‐reparative pro‐angiogenic CD14, CD16 intermediate monocyte phenotype characterized by high expression of CD163 and low expression of HLA‐DR intermediate Mo2 phenotype 32, 33.
Figure 7.
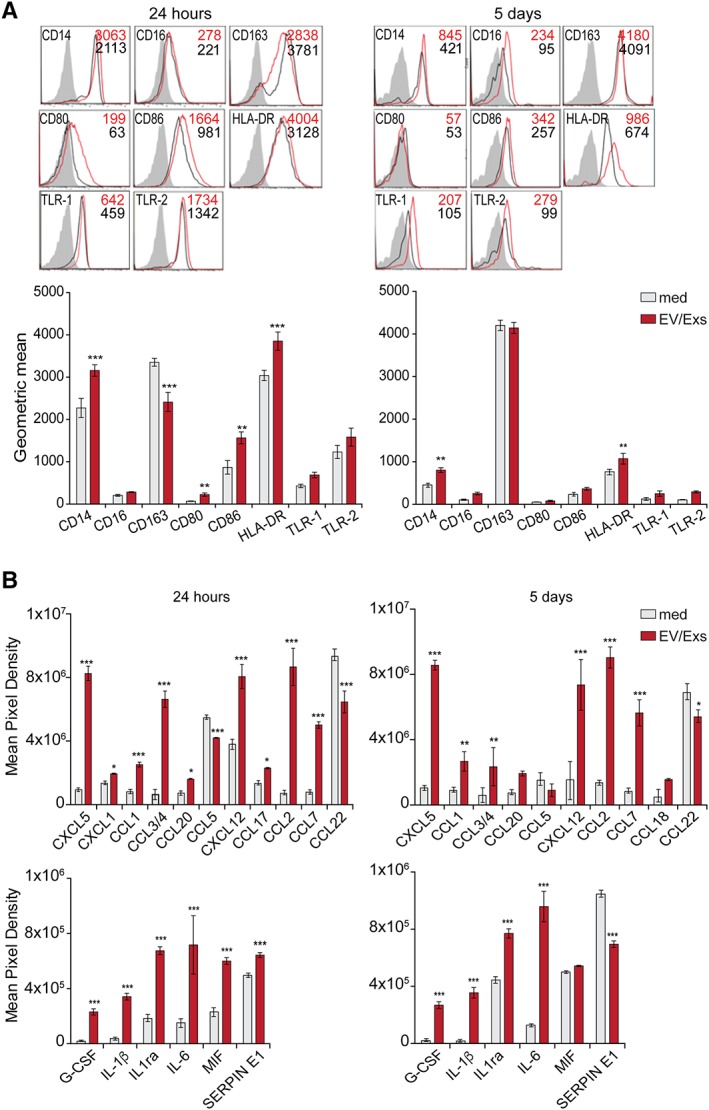
Extracellular vesicles/exosomes (EV/Exs) support intermediate CD163hiCD14hiCD16low monocytes/macrophages: freshly isolated human leukocyte antigens (HLA)‐mismatched CD14+CD16− monocytes were exposed or not (medium) to EV/Exs for 24 hours (left panel) or 5 days (right panel). (A): The expression of informative monocytes/macrophages lineage markers as determined by flow cytometry. Results are displayed as representative FACS histograms (upper panel) and as mean values of geometric mean obtained from three independent experiments (lower panel). (B): Chemokines (upper panel) and cytokines (lower panel) secreted by HLA‐mismatched CD14+CD16− maintained in complete (medium) or EV/Exs‐conditioned medium for 24 hours (left panel) and 5 days (right panel). Profilers were analyzed with CellQuest software and presented as mean pixel density of triplicates. *, p < .05; **, p < .01; ***, p < .001 compared with medium.
To provide meaningful insights into these findings, we determined the chemokines/cytokines secreted by CD14+CD16− monocytes under different culturing conditions using proteome profiling. EV/Exs induced substantial alterations to the secretome of HLA‐mismatched CD14+CD16− monocytes whether at 24 hours or 5 days. On one hand, the secretion of several pleiotropic chemokines, which are relevant in the context of infarcted myocardium damage and repair 34, was enhanced by at least 2 and up to 10‐folds; notably CXCL5 and CXCL12 from CXC chemokines and CCL3/4, CCL2, and CCL7 from CC‐class (Fig. 7B, upper panel). On the other hand, the secretion of both CCL5 and CCL22, CC‐class chemokines involved in effector T cells trafficking, was rather decreased (Fig. 7B, upper panel). Monocytes secretion of other chemokines, including lymphotactin, leukotaktin, CCL19, CXCL10, CXCL16, and Fractalkine that are also involved in leukocytes recruitment was not affected by the presence of EV/Exs (Supporting Information Fig. S6). CD14+CD16− monocytes cultured alone for 24 hours or 5 days produced a narrow panel of pleiotropic cytokines; IL‐1ra, IL‐6, macrophage inhibitory factor (MIF), and SERPIN E1. EV/Exs induced significant increases of IL1ra, IL‐6 secretion, and induced the secretion of G‐CSF and IL‐1β (Fig. 7B, lower panel) both at 24 hours or 5 days of coculture. Increases of MIF and SERPIN E1 production by hCPC‐EV/Exs‐conditioned CD14+CD16− monocytes were observed only at 24 hours. At later time, EV/Exs‐conditioned monocytes secreted similar levels of MIF to nonconditioned monocytes but considerably less SERPIN E1 (Fig. 7B, lower panel).
Thus, EV/Exs alter the secretome of HLA‐mismatched monocytes in different manners. The differential modulation of pleiotropic pro‐inflammatory chemokines and cytokines will promote early recruitment of other leukocytes. At a longer exposure, EV/Exs would rather promote the secretion of chemokines/cytokines that could contribute not only to resolving the initial inflammatory response (such as IL‐1ra 35) but also to recruiting and activating endogenous stem/progenitor cells, and promoting angiogenesis and neovascularization (such as G‐CSF, MIF, IL‐6 36, 37, 38).
Collectively, within allogeneic inflammatory MI environment, internalization of allogeneic hCPC‐EV/Exs by monocytes could tune their phenotypic and functional plasticity and promote injured heart neovascularization, healing, and repair.
Discussion
The beneficial immunogenicity/allogenicity of hCPC prompted their successful clinical investigation for cardiac repair post‐MI 16, 20. Herein, we report within a human tailored in vitro allogeneic model mimicking clinical situation the ample capacity of hCPC released EV/Exs to positively impact the activity of chief cellular actors of cardiac repair/regeneration. Collectively, our data are primary in linking, within a human model, the benefit of allogeneic stem/progenitor‐based therapy to the activity of EV/Exs secreted by therapeutic cells.
Resident cardiac stem/progenitor cells and cardiomyocytes are critical not only for tissue homeostasis and renewal of cardiomyocyte pool 39, but also for organ regeneration and/or repair after cardiac injury 40, 41, 42. The rapid internalization of EV/Exs by allogeneic hCPC and cardiomyocytes, which represented in situ resident myocytes in our in vitro model, promoted both migration and proliferation. Mechanistically, EV/Exs‐induced migration and proliferation of allogeneic hCPC relays on the activation of MAPK/Erk signaling pathway. This signaling pathway regulates multiple cellular activities and is substantially activated upon internalization of EV/Exs by hCPC. Therefore, it is conceivable that EV/Exs via activation of MAPK/Erk pathway would also promote the survival and differentiation of hCPC. In agreement, survival and migration of c‐kit+ cardiac progenitor cells as well as the differentiation of embryonic and adult stem cells are dependent on MAPK/Erk signaling pathway both in vivo and in vitro 43, 44, 45. Beside migration and proliferation, EV/Exs also preserved cardiomyocytes viability under growth factors/serum‐deprivation suggesting their potential ability to protect resident myocytes within an injured environment. Collectively, these results indicate that EV/Exs released by therapeutic allogeneic hCPC harbor potential to initiate the intrinsic cardiac reparative/regenerative program, which could be part of their benefit.
In stem cell transplantation, the primary beneficial effects are attributable to angiogenesis stimulation within the infarct and peri‐infarct regions of the heart. The efficient uptake of EV/Exs by two representative endothelial cell models and their capacity to modulate kinase signaling as well as other key endothelial cell activities underscore the potential of these allogeneic EV/Exs to promote reparative/regenerative angiogenesis. In this study, we show that MAPK/Erk1, MAPK/JNK, TOR, p70S6K, GSK‐3β, p53, and CREB among other serine/threonine kinases are highly phosphorylated as a consequence of EV/Exs internalization by endothelial cells. EV/Exs triggered the activation of the tumor suppressor p53 and inactivation of the migration relevant glycogen synthase kinase 3 β (GSK‐3β). Since both events regulate the migration of various cell models 46, their implication in EV/Exs‐induced migration of endothelial cells is conceivable. The activation of AKT/mTOR and MAPK/Erk signaling cascades by apelin phosphorylates p70S6K leads to HUVEC proliferation 47. Serine 133 phosphorylation of the cAMP responsive element binding protein (CREB) is at the endpoint of various signaling pathways, like growth factor signaling, and regulates vascular smooth muscle cell proliferation 48. That EV/Exs activated AKT and Erk cascade in HUVEC and phosphorylated the same residues of p70S6K and CREB, strongly suggest the implication of these cascades in EV/Exs‐induced proliferation of endothelial cells.
In the context of cardiac repair, Exs from various cellular origins including adult stem cells can stimulate angiogenesis to improve cardiac function 49. In experimental MI models, Exs released by hypoxia‐preconditioned MSC or cardiosphere‐derived cells foster increased cardioprotective effect 50, attributable at least in part to their increased capacity to promote tube formation and angiogenesis 51, 52. Therapeutic hCPC are grown within hypoxic atmosphere to mimic the pathophysiological conditions of their therapeutic administration and EV/Exs under investigation were generated under the same conditions. Within this preconditioning, our findings would thus suggest that allogeneic hCPC upon their administration to the injured myocardium would act as a potent positive regulator of angiogenesis at least in part through the secretion of EV/Exs.
Agents that affect monocyte survival, such as growth factors, interfere with their activation and functioning. The monocyte functional subsets are classified according to their effects as (a) pro‐inflammatory, (b) tissue reparative, and (c) inducers of humoral immunity based on the expression of scavenger receptor (CD163) and antigen presentation capacity (HLA‐DR), whereas different chemokines axis including CCL2 and CCL7 shape monocyte differentiation and polarization 53, 54, 55. Internalization of EV/Exs by allogeneic CD14+CD16− monocytes sustained their survival and promoted a pro‐inflammatory/reparative phenotype. After an initial increase in the expression of costimulatory molecules and antigen‐presenting molecule HLA‐DR, a considerable downregulation of the adaptive immune response machinery concomitant with enhanced expression of CD163 prompted the development of a rather tissue‐reparative pro‐angiogenic CD14, CD16 intermediate monocyte phenotype. In line, EV/Exs‐conditioned monocytes secreted a combination of pro‐inflammatory/immune‐stimulatory as well as anti‐inflammatory/reparative/regenerative chemokines/cytokines.
However, in the context of cardiac injury, the classification pro‐inflammatory versus anti‐inflammatory/regenerative does not always apply. Although excessive amounts of CCL2 and IL‐6 have been linked with many inflammatory diseases, within cardiac injuries their role goes beyond the simple recruitment/pro‐inflammatory effects toward fine and critical modulation of cardiac homeostasis, repair and regeneration after a detrimental assault 56, 57, 58. Therefore, the mediators released by EV/Exs‐conditioned monocytes would most likely act as a “working‐network” rather than one‐to‐one chemokine/cytokine‐function to potentially engender cardioprotective responses and pleiotropic cellular effects both on immune and myocardial resident cells. The fact that the anti‐inflammatory IL‐1ra (IL‐1 receptor antagonist, produced by intermediate monocytes/macrophages 54, is also more highly regulated in EV/Exs‐treated monocytes than inflammatory IL‐1β further supports this notion.
In rat and pig models, the protective effect of cardiosphere‐derived cells is mainly governed by their action on macrophages inflammatory/anti‐inflammatory functions via Exs 59. To the best of our knowledge, the herein study is the first evaluating the effect of hCPC‐derived EV/Exs on human monocytes. Our findings within human settings consolidate those reported in animal models and suggest that within clinical allogeneic settings the EV/Exs released by therapeutic hCPC could maintain/prolong their favorable beneficial interactions with monocytes/macrophages in injured myocardium.
Inflammation and recruitment/activation of immune cells are likely providers of initial pro‐regenerative cues and main sponsors of damaged tissues regeneration rather than barriers of intrinsic and stem cell‐based tissue regeneration/repair 60. Immunosuppressed neonatal mice cannot regenerate injured hearts 38 and immune modulation of stem cells is part of their reparative mechanisms 61. Our previous findings embodied these notions by linking the cardiac protective/reparative potential of allogeneic hCPC to their dual immunogenic immune‐modulator character that promotes cell‐contact‐dependent tuning of immune/inflammatory T and NK cells but also monocytes/macrophages toward immune/regulatory cells 14, 15, 16, 17, 18. Furthermore support is provided by studies showing the crucial role of adaptive T cell immune response for tissue regeneration 62, 63. Our findings within an in vitro human model support EV/Exs secreted by allogeneic hCPC as sponsors of their cardiac bioactivity and corroborate previous studies in experimental‐MI animal models 59, 64. Yet, from an immunological standpoint whether EV/Exs would recapitulate the essential wholesome cell‐dependent inflammatory/immunomodulatory interactions of therapeutic allogeneic hCPC with components of the immune system is not yet evidenced. Allogeneic hCPC recruit and fine‐tune the components of innate and adaptive immune response in a cell‐contact‐dependent fashion generating and expanding M2c‐like macrophages 18, regulatory IL‐10‐producing decidual NK‐like cells 15, and last but not least regulatory T cells 14. Despite potential advantages of EV/Exs, allogeneic hCPC might be better suited to harness MI injury than their solo Exs. Cells are natural release platforms for Exs, thus ideally allogeneic cardiac stem/progenitor in combination with EV/Exs might turn out to be more efficient durable therapeutic strategies.
Conclusion
Our tailored in vitro allogeneic human model systems demonstrated that by regulating the activity of chief cellular actors of cardiac repair/regeneration, EV/Exs released by therapeutic allogeneic hCPC could actively contribute to their wholesome reparative/regenerative benefit. Yet, EV/Exs as cell‐free therapeutics are still at a primitive stage with fundamental biological issues that are as yet unsolved 23. Cells are unique and time alone will tell whether allogeneic hCPC‐based therapy could be supplanted by their derived EV/Exs. Meanwhile, our previous and herein data might forecast the application of allogeneic hCPC followed by their cell‐free EV/Exs as a strategy that will not only elicit the cell‐contact mediated reparative/regenerative immune response but also have the desired long‐lasting effects through the EV/Exs. Establishing the therapeutic value of hCPC‐EV/Exs would probably provide a new dimension for cardiac repair post MI.
Author Contributions
H.R.H., S.B.: experimental design, collection, analysis and interpretation of data, manuscript drafting; Q.C.: confocal microscopy; J.G.: manuscript critical reading; M.J.S.R.: transmission electron microscopy; Y.J.F.: statistical analysis of data; I.P. and O.d.l.R.: provision of hCPC cells; E.L.: manuscript critical reading, final approval of the manuscript; A.B., D.C.: manuscript critical reading, financial support; N.J‐F.: manuscript drafting, final approval of the manuscript; R.A.‐D.: conception and design, data collection, analysis, and interpretation, financial support, manuscript drafting, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
I.P., O.d.l.R., and E.L. are full‐time employees of TiGenix. The other authors indicated no potential conflicts of interest.
Data Availability Statement
Data available on request due to privacy/ethical restrictions.
Supporting information
Appendix S1: Supplementary Information
Figure S1 Phenotype of isolated hCPC: Human biopsies from atrial appendage were minced up and digested the cell suspensions of CD45‐CD117+(c‐Kit) cells were selected and characterized as we previously described (Lauden L., et al Circ. Res., 2013, 112:451). A) Representative image of hCPC phenotype as determined by flow cytometry (upper panel) and expression of stemness and as well as early cardiac lineage transcription factors (lower panel, 40x magnification). B) Inflammatory conditions preserve morphology and stemness of hCPC but modulate their immunological profile. PBMC are negative control cells. The upper panel shows morphology of hCPC and IFNγ‐hCPC (20x magnification), and representative expression of pluripotency transcription factors by hCPC and IFNγ‐hCPC. The lower panel shows representative expression of immune relevant molecules by hCPC (black histograms) and IFNγ‐treated hCPC (IFNγ‐hCPC) (red histograms) against isotype controls (gray filled histograms). The percentages [%] of positive cells are indicated.
Figure S2 Characterization of hCPC‐derived‐extracellular vesicles: hCPC‐EV were isolated from 48 hours serum‐deprived hCPC culture media after the removal of cells and cell debris. A) Left panel, representative image of Nanoparticles Tracking Analysis (NTA) of hCPC‐EV populations' size distribution. Red line represents the mean of five videos acquired for a single biological sample. Right panel, Transmission Electron Microscopy (TEM) image of hCPC‐EV preparation negatively stained with 2% uracyl acetate. Cup‐shaped small‐EV/exosome structures (30–100 nm) are indicated by the arrowheads. Scale Bar 200 nm. B) Western blot analysis of EV markers (CD63, CD81, HLA I, and βactin) (left panel). The right panel displays representative flow cytometry histograms showing the expression of Ev surface markers CD63 and CD81 and HLA I on hCPC‐EV‐bound latex beads. C) The amount of hCPC‐EV released per cell increased substantially when hCPC where maintained under inflammatory conditions as determined by NTA. Results are presented as mean values from 3 independent EV/Exs preparations from two different cells. *P < .05.
Figure S3 Proliferation of AC16 cardiomyocytes in the presence of EV/Exs: AC16 cardiomyocytes proliferation as evaluated by Ki67 staining. Results are presented as mean values of geometric mean fluorescence intensity (MFI) and mean values of % Ki67‐positive cells from three independent experiments. *P < .05 compared to medium or SN; **P < .01 compared to SN.
Figure S4 Impact of EV/Exs on HLA‐mismatched endothelial cells: A) Uptake of CFSE‐labeled EV/Exs by HMEC as assessed by fluorescent microscopy (200x magnification). B) Representative images (scale bar 1 mm) of wound healing assay with HUVEC. Cells were exposed to 50 μg/ml CFSE‐labeled EV/Exs and their migration was monitored over indicated periods of time. C) EV/Exs‐induced HMEC growth as evaluated by direct cell counts and Ki67 expression. D) Representative images of tube formation assay with HMEC (40x magnification). Cells were treated or not (medium) with EV/Exs overnight and pictures were captured using EVOS XL microscope and analyzed for the number of nodes and junctions presented as mean ± SD from the three independent experiments. (lower panel). *P < .05; **P < .01; ***P < .001; ****p < .0001 compared to medium or SN.
Figure S5 Allogeneic EV/Exs favor intermediate CD163 hi CD14 hi CD16 low monocytes/macrophages: Freshly‐isolated HLA‐mismatched CD14+CD16− monocytes from 5 different healthy donors were exposed or not (med) to 50 μg/ml EV/Exs for 24 hours (upper panel) or 5 days (lower panel). The Expression of informative monocytes/macrophages lineage markers as determined by flow cytometry is displayed as mean values of %positive cells obtained from three independent experiments (lower panel). *P < .05; **P < .01; ***P < .001.
Figure S6 Internalization of EV/Exs does not affect the secretion of various chemokines by HLAmismatched monocytes: Freshly‐isolated HLA‐mismatched CD14+CD16− monocytes were exposed or not (med) to 50 μg EV/Exs for 24 hours (upper panel) or 5 days (lower panel). Displayed, the chemokines secreted by HLA‐mismatched CD14+CD16− maintained in complete medium (med) or EV/Exsconditioned medium (EV/Exs). Secretome Profiler was analyzed with CellQuest software and presented as mean pixel density of triplicates.
Table S1 Percentage of isolated hCPC among 4 different donors
Table S2: HLA haplotypes of hCPC
Table S3: HLA haplotypes of PBMC from which monocytes were isolated
Acknowledgments
We thank Amira Othman for technical assistance during her training in the laboratory. Special thanks to C. Théry (Institute Curie/INSERM U932) and the platform PICT‐IBiSA, member of the France‐BioImaging National Research Infrastructure supported by the CelTisPhyBio Labex (ANR‐10‐LBX‐0038) part of the IDEX PSL (ANR‐10‐IDEX‐0001‐02 PSL). This work was supported in part by the European grant “FP7‐HEALTH‐242038‐CAREMI” and by LABEX TRANSPLANTEX “ANR‐11‐LABX‐0070,” “HLA et Médicine” and INSERM grants for UMRS976. H.R.H. held a postdoctoral fellowship from “HLA et Médicine” France. H.R.H. is currently affiliated with the Institute Curie, Stress and Cancer Laboratory, INSERM U830, Paris, France.
References
- 1. Chimenti I, Smith RR, Li TS et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere‐derived cells transplanted into infarcted mice. Circ Res 2010;106:971–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang XL, Li Q, Rokosh G et al. Long‐term outcome of administration of c‐kit(POS) cardiac progenitor cells after acute myocardial infarction: Transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ Res 2016;118:1091–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crahes M, Bories MC, Viquin JT et al. Long‐term engraftment (16 years) of myoblasts in a human infarcted heart. Stem Cells Translational Medicine 2018;7:705–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gnecchi M. Cell therapy for heart regeneration: Learning from the past to build a brighter future. Stem Cells Translational Medicine 2018;7:702–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 2018;75:193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sahoo S, Klychko E, Thorne T et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 2011;109:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barile L, Gherghiceanu M, Popescu LM et al. Ultrastructural evidence of exosome secretion by progenitor cells in adult mouse myocardium and adult human cardiospheres. J Biomed Biotechnol 2012;2012:354605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan M, Nickoloff E, Abramova T et al. Embryonic stem cell‐derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 2015;117:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kishore R, Khan M. More than tiny sacks: Stem cell exosomes as cell‐free modality for cardiac repair. Circ Res 2016;118:330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maring JA, Beez CM, Falk V et al. Myocardial regeneration via progenitor cell‐derived exosomes. Stem Cells Int 2017;2017:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cahill TJ, Choudhury RP, Riley PR. Heart regeneration and repair after myocardial infarction: Translational opportunities for novel therapeutics. Nat Rev Drug Discov 2017;16:699–717. [DOI] [PubMed] [Google Scholar]
- 12. Epelman S, Liu PP, Mann DL. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 2015;15:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circ Res 2016;119:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lauden L, Boukouaci W, Borlado LR et al. Allogenicity of human cardiac stem/progenitor cells orchestrated by programmed death ligand 1. Circ Res 2013;112:451–464. [DOI] [PubMed] [Google Scholar]
- 15. Boukouaci W, Lauden L, Siewiera J et al. Natural killer cell crosstalk with allogeneic human cardiac‐derived stem/progenitor cells controls persistence. Cardiovasc Res 2014;104:290–302. [DOI] [PubMed] [Google Scholar]
- 16. Al‐Daccak R, Charron D. Allogenic benefit in stem cell therapy: Cardiac repair and regeneration. Tissue Antigens 2015;86:155–162. [DOI] [PubMed] [Google Scholar]
- 17. Hocine HR, Costa HE, Dam N et al. Minimizing the risk of allo‐sensitization to optimize the benefit of allogeneic cardiac‐derived stem/progenitor cells. Sci Rep 2017;7:41125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dam N, Hocine HR, Palacios I et al. Human cardiac‐derived stem/progenitor cells fine‐tune monocyte‐derived descendants activities toward cardiac repair. Front Immunol 2017;8:1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanz‐Ruiz R, Casado Plasencia A, Borlado LR et al. Rationale and design of a clinical trial to evaluate the safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with acute myocardial infarction and left ventricular dysfunction: The Randomized Multicenter Double‐Blind Controlled CAREMI Trial (cardiac stem cells in patients with acute myocardial infarction). Circ Res 2017;121:71–80. [DOI] [PubMed] [Google Scholar]
- 20. Fernandez‐Aviles F, Sanz‐Ruiz R, Bogaert J et al. Safety and efficacy of intracoronary infusion of allogeneic human cardiac stem cells in patients with ST‐segment elevation myocardial infarction and left ventricular dysfunction. Circ Res 2018;123:579–589. [DOI] [PubMed] [Google Scholar]
- 21. Thery C, Witwer KW, Aikawa E et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El‐Andaloussi S, Lee Y, Lakhal‐Littleton S et al. Exosome‐mediated delivery of siRNA in vitro and in vivo. Nat Protoc 2012;7:2112–2126. [DOI] [PubMed] [Google Scholar]
- 23. Davidson SM, Yellon DM. Exosomes and cardioprotection—A critical analysis. Mol Aspects Med 2018;60:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eschenhagen T, Bolli R, Braun T et al. Cardiomyocyte regeneration: A consensus statement. Circulation 2017;136:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davidson MM, Nesti C, Palenzuela L et al. Novel cell lines derived from adult human ventricular cardiomyocytes. J Mol Cell Cardiol 2005;39:133–147. [DOI] [PubMed] [Google Scholar]
- 26. Darrah PA, Patel DT, De Luca PM et al. Multifunctional TH1 cells define a correlate of vaccine‐mediated protection against Leishmania major. Nat Med 2007;13:843–850. [DOI] [PubMed] [Google Scholar]
- 27. Willam C, Koehne P, Jurgensen JS et al. Tie2 receptor expression is stimulated by hypoxia and proinflammatory cytokines in human endothelial cells. Circ Res 2000;87:370–377. [DOI] [PubMed] [Google Scholar]
- 28. Timmers L, Lim SK, Hoefer IE et al. Human mesenchymal stem cell‐conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res 2011;6:206–214. [DOI] [PubMed] [Google Scholar]
- 29. Mewhort HEM, Svystonyuk DA, Turnbull JD et al. Bioactive extracellular matrix scaffold promotes adaptive cardiac remodeling and repair. JACC Basic Transl Sci 2017;2:450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anderson JD, Johansson HJ, Graham CS et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor‐KappaB signaling. Stem Cells 2016;34:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voss OH, Kim S, Wewers MD et al. Regulation of monocyte apoptosis by the protein kinase Cdelta‐dependent phosphorylation of caspase‐3. J Biol Chem 2005;280:17371–17379. [DOI] [PubMed] [Google Scholar]
- 32. Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: Phenotypical vs. functional differentiation. Front Immunol 2014;5:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayer A, Hiebl B, Lendlein A et al. Support of HUVEC proliferation by pro‐angiogenic intermediate CD163+ monocytes/macrophages: A co‐culture experiment. Clin Hemorheol Microcirc 2011;49:423–430. [DOI] [PubMed] [Google Scholar]
- 34. Dusi V, Ghidoni A, Ravera A et al. Chemokines and heart disease: A network connecting cardiovascular biology to immune and autonomic nervous systems. Mediators Inflamm 2016;2016:5902947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bujak M, Dobaczewski M, Chatila K et al. Interleukin‐1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol 2008;173:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shim W, Mehta A, Lim SY et al. G‐CSF for stem cell therapy in acute myocardial infarction: Friend or foe? Cardiovasc Res 2011;89:20–30. [DOI] [PubMed] [Google Scholar]
- 37. Dayawansa NH, Gao XM, White DA et al. Role of MIF in myocardial ischaemia and infarction: Insight from recent clinical and experimental findings. Clin Sci 2014;127:149–161. [DOI] [PubMed] [Google Scholar]
- 38. Han C, Nie Y, Lian H et al. Acute inflammation stimulates a regenerative response in the neonatal mouse heart. Cell Res 2015;25:1137–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smart N, Bollini S, Dube KN et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011;474:640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ellison GM, Vicinanza C, Smith AJ et al. Adult c‐kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 2013;154:827–842. [DOI] [PubMed] [Google Scholar]
- 41. Senyo SE, Steinhauser ML, Pizzimenti CL et al. Mammalian heart renewal by pre‐existing cardiomyocytes. Nature 2013;493:433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Magadum A, Singh N, Kurian AA et al. Ablation of a single N‐glycosylation site in human FSTL 1 induces cardiomyocyte proliferation and cardiac regeneration. Mol Ther Nucleic Acids 2018;13:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hamilton WB, Brickman JM. Erk signaling suppresses embryonic stem cell self‐renewal to specify endoderm. Cell Rep 2014;9:2056–2070. [DOI] [PubMed] [Google Scholar]
- 44. Chan WS, Sideris A, Sutachan JJ et al. Differential regulation of proliferation and neuronal differentiation in adult rat spinal cord neural stem/progenitors by ERK1/2, Akt, and PLCgamma. Front Mol Neurosci 2013;6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jaiswal RK, Jaiswal N, Bruder SP et al. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by mitogen‐activated protein kinase. J Biol Chem 2000;275:9645–9652. [DOI] [PubMed] [Google Scholar]
- 46. Munnich N, Wernhart S, Hogstrand C et al. Expression of the zinc importer protein ZIP9/SLC39A9 in glioblastoma cells affects phosphorylation states of p53 and GSK‐3beta and causes increased cell migration. Biometals 2016;29:995–1004. [DOI] [PubMed] [Google Scholar]
- 47. Masri B, Morin N, Cornu M et al. Apelin (65‐77) activates p70 S6 kinase and is mitogenic for umbilical endothelial cells. FASEB J 2004;18:1909–1911. [DOI] [PubMed] [Google Scholar]
- 48. Hudson C, Kimura TE, Duggirala A et al. Dual role of CREB in the regulation of VSMC proliferation: Mode of activation determines pro‐ or anti‐mitogenic function. Sci Rep 2018;8:4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Todorova D, Simoncini S, Lacroix R et al. Extracellular vesicles in angiogenesis. Circ Res 2017;120:1658–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Agarwal U, George A, Bhutani S et al. Experimental, systems, and computational approaches to understanding the microRNA‐mediated reparative potential of cardiac progenitor cell‐derived exosomes from pediatric patients. Circ Res 2017;120:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gonzalez‐King H, Garcia NA, Ontoria‐Oviedo I et al. Hypoxia inducible factor‐1alpha potentiates Jagged 1‐mediated angiogenesis by mesenchymal stem cell‐derived exosomes. Stem Cells 2017;35:1747–1759. [DOI] [PubMed] [Google Scholar]
- 52. Namazi H, Mohit E, Namazi I et al. Exosomes secreted by hypoxic cardiosphere‐derived cells enhance tube formation and increase pro‐angiogenic miRNA. J Cell Biochem 2018;119:4150–4160. [DOI] [PubMed] [Google Scholar]
- 53. Nieto C, Bragado R, Municio C et al. The activin A‐peroxisome proliferator‐activated receptor gamma axis contributes to the transcriptome of GM‐CSF‐conditioned human macrophages. Front Immunol 2018;9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sierra‐Filardi E, Nieto C, Dominguez‐Soto A et al. CCL2 shapes macrophage polarization by GM‐CSF and M‐CSF: Identification of CCL2/CCR2‐dependent gene expression profile. J Immunol 2014;192:3858–3867. [DOI] [PubMed] [Google Scholar]
- 55. Xuan W, Qu Q, Zheng B et al. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukoc Biol 2015;97:61–69. [DOI] [PubMed] [Google Scholar]
- 56. Dewald O, Zymek P, Winkelmann K et al. CCL2/monocyte chemoattractant protein‐1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res 2005;96:881–889. [DOI] [PubMed] [Google Scholar]
- 57. Morimoto H, Takahashi M, Izawa A et al. Cardiac overexpression of monocyte chemoattractant protein‐1 in transgenic mice prevents cardiac dysfunction and remodeling after myocardial infarction. Circ Res 2006;99:891–899. [DOI] [PubMed] [Google Scholar]
- 58. Mayfield AE, Kanda P, Nantsios A et al. Interleukin‐6 mediates post‐infarct repair by cardiac explant‐derived stem cells. Theranostics 2017;7:4850–4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Couto G, Gallet R, Cambier L et al. Exosomal MicroRNA transfer into macrophages mediates cellular postconditioning. Circulation 2017;136:200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature 2016;529:307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Aurora AB, Olson EN. Immune modulation of stem cells and regeneration. Cell Stem Cell 2014;15:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hui SP, Sheng DZ, Sugimoto K et al. Zebrafish regulatory T cells mediate organ‐specific regenerative programs. Dev Cell 2017;43:659.e655–672.e655. [DOI] [PubMed] [Google Scholar]
- 63. Ali N, Zirak B, Rodriguez RS et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell 2017;169:1119.e1111–1129.e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. El Harane N, Kervadec A, Bellamy V et al. Acellular therapeutic approach for heart failure: in vitro production of extracellular vesicles from human cardiovascular progenitors. Eur Heart J 2018;39:1835–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Information
Figure S1 Phenotype of isolated hCPC: Human biopsies from atrial appendage were minced up and digested the cell suspensions of CD45‐CD117+(c‐Kit) cells were selected and characterized as we previously described (Lauden L., et al Circ. Res., 2013, 112:451). A) Representative image of hCPC phenotype as determined by flow cytometry (upper panel) and expression of stemness and as well as early cardiac lineage transcription factors (lower panel, 40x magnification). B) Inflammatory conditions preserve morphology and stemness of hCPC but modulate their immunological profile. PBMC are negative control cells. The upper panel shows morphology of hCPC and IFNγ‐hCPC (20x magnification), and representative expression of pluripotency transcription factors by hCPC and IFNγ‐hCPC. The lower panel shows representative expression of immune relevant molecules by hCPC (black histograms) and IFNγ‐treated hCPC (IFNγ‐hCPC) (red histograms) against isotype controls (gray filled histograms). The percentages [%] of positive cells are indicated.
Figure S2 Characterization of hCPC‐derived‐extracellular vesicles: hCPC‐EV were isolated from 48 hours serum‐deprived hCPC culture media after the removal of cells and cell debris. A) Left panel, representative image of Nanoparticles Tracking Analysis (NTA) of hCPC‐EV populations' size distribution. Red line represents the mean of five videos acquired for a single biological sample. Right panel, Transmission Electron Microscopy (TEM) image of hCPC‐EV preparation negatively stained with 2% uracyl acetate. Cup‐shaped small‐EV/exosome structures (30–100 nm) are indicated by the arrowheads. Scale Bar 200 nm. B) Western blot analysis of EV markers (CD63, CD81, HLA I, and βactin) (left panel). The right panel displays representative flow cytometry histograms showing the expression of Ev surface markers CD63 and CD81 and HLA I on hCPC‐EV‐bound latex beads. C) The amount of hCPC‐EV released per cell increased substantially when hCPC where maintained under inflammatory conditions as determined by NTA. Results are presented as mean values from 3 independent EV/Exs preparations from two different cells. *P < .05.
Figure S3 Proliferation of AC16 cardiomyocytes in the presence of EV/Exs: AC16 cardiomyocytes proliferation as evaluated by Ki67 staining. Results are presented as mean values of geometric mean fluorescence intensity (MFI) and mean values of % Ki67‐positive cells from three independent experiments. *P < .05 compared to medium or SN; **P < .01 compared to SN.
Figure S4 Impact of EV/Exs on HLA‐mismatched endothelial cells: A) Uptake of CFSE‐labeled EV/Exs by HMEC as assessed by fluorescent microscopy (200x magnification). B) Representative images (scale bar 1 mm) of wound healing assay with HUVEC. Cells were exposed to 50 μg/ml CFSE‐labeled EV/Exs and their migration was monitored over indicated periods of time. C) EV/Exs‐induced HMEC growth as evaluated by direct cell counts and Ki67 expression. D) Representative images of tube formation assay with HMEC (40x magnification). Cells were treated or not (medium) with EV/Exs overnight and pictures were captured using EVOS XL microscope and analyzed for the number of nodes and junctions presented as mean ± SD from the three independent experiments. (lower panel). *P < .05; **P < .01; ***P < .001; ****p < .0001 compared to medium or SN.
Figure S5 Allogeneic EV/Exs favor intermediate CD163 hi CD14 hi CD16 low monocytes/macrophages: Freshly‐isolated HLA‐mismatched CD14+CD16− monocytes from 5 different healthy donors were exposed or not (med) to 50 μg/ml EV/Exs for 24 hours (upper panel) or 5 days (lower panel). The Expression of informative monocytes/macrophages lineage markers as determined by flow cytometry is displayed as mean values of %positive cells obtained from three independent experiments (lower panel). *P < .05; **P < .01; ***P < .001.
Figure S6 Internalization of EV/Exs does not affect the secretion of various chemokines by HLAmismatched monocytes: Freshly‐isolated HLA‐mismatched CD14+CD16− monocytes were exposed or not (med) to 50 μg EV/Exs for 24 hours (upper panel) or 5 days (lower panel). Displayed, the chemokines secreted by HLA‐mismatched CD14+CD16− maintained in complete medium (med) or EV/Exsconditioned medium (EV/Exs). Secretome Profiler was analyzed with CellQuest software and presented as mean pixel density of triplicates.
Table S1 Percentage of isolated hCPC among 4 different donors
Table S2: HLA haplotypes of hCPC
Table S3: HLA haplotypes of PBMC from which monocytes were isolated
Data Availability Statement
Data available on request due to privacy/ethical restrictions.


