Summary
Mesenchymal stem cells (MSCs) are one of the most easily accessible stem cells that can be obtained from various human tissues. They have raised considerable interests for their potential applications in tissue repair, anti‐cancer therapy, and inflammation suppression. Stem cell‐based therapy was first used to treat muscular dystrophies and has been studied intensively for its efficacy in various disease models, including myocardial infarction, kidney injuries, liver injuries, and cancers. In this review, we summarized the potential mechanisms underlying MSC‐derived EVs therapy as a drug delivery platform. Additionally, based on currently published data, we predicted a potential therapeutic role of cargo proteins shuttled by EVs from MSCs. These data may support the therapeutic strategy of using the MSC‐derived EVs to accelerate this strategy from bench to bedside. stem cells translational medicine 2019;8:880&886
Significance Statement.
The future of exosome therapeutics has great potential, but several challenges, as discussed in the present study, must be overcome before exosome‐based therapy will become an important option as a next‐generation drug delivery system.
Introduction
Mesenchymal stem cells (MSCs), multipotent adult stem cells, are one of the most easily accessible stem cells that can be obtained from various human tissues. They have been widely tested as therapeutics due to their accessibility, therapeutic efficacy in various diseases, and tissue damage regeneration, their easy accessibility, and their availability from ethically acceptable tissues such as bone marrow aspirates and fat tissues 1.
MSCs have raised considerable interests for their potential applications in tissue repair, anticancer therapy, and inflammation suppression. Stem cell‐based therapy was first used to treat muscular dystrophies 2 and has been studied intensively for its efficacy in various disease models including myocardial infarction 3, kidney injuries 4, liver injuries 5, and cancers 6.
Recent studies have suggested the possibility that the key therapeutic effects of MSCs in tissue repair are mediated mainly via paracrine mediators secreted from the MSCs and only partially from MSCs themselves. The “secretomes” of MSCs, including various secretory proteins such as growth factors, cytokines, and chemokines and extracellular vesicles (EVs) such as microvesicles (MVs; 100–1,000 nm diameter) and exosomes (40–150 nm diameter), have been shown to induce many of the therapeutic properties of MSCs. For example, in an acute kidney injury (AKI) model, systemically injected MSCs induced significant recovery from cisplatin‐induced kidney damage, despite the low permanent engraftment of MSCs within the kidney 7. Further studies demonstrated that the paracrine factors collected from MSCs were sufficient to induce MSCs‐mediated recovery from renal injury 8. The beneficial effects of MSCs can be replicated through secretomes of conditioned media from MSCs, especially by exosomes 9.
Even though the precise mechanism of their paracrine effect is not clearly understood, EVs have been recognized as potent therapeutic vehicles that can transfer various proteins and regulatory genes to the targets. EVs are considered nonimmunogenic nanovesicles, which can protect their cargoes from serum proteases and immune systems to transfer information and communicate with other cells 10.
In this review, we summarized the potential mechanisms underlying MSC‐derived EVs therapy as a drug delivery platform. Additionally, based on currently published data, we predicted a potential therapeutic role of cargo proteins shuttled by EVs from MSCs. These data may support the therapeutic strategy of using the MSC‐derived EVs to accelerate this strategy from bench to bedside.
Extracellular Vesicles
EVs were initially identified as a quality control system used by cells to dispose of unwanted content 11. After years of intensive research, the role of EVs as important cell‐to‐cell communication mediators, which deliver various bioactive molecules such as functional proteins, nucleic acids, and lipids, was identified 10, 12, 13, 14. Depending on their size, cells or tissue of origin, biogenesis mechanisms, or proposed functions, EVs are generally classified as MVs, exosomes, or apoptotic bodies.
MVs, also known as ectosomes, shedding vesicles, or microparticles, are 100‐ to 1,000‐nm vesicles released by budding from the plasma membrane. Exosomes, 40–150 nm in diameter, are generated from an inward budding of endosomes, forming intraluminal vesicles inside the endosomal compartment, which are called multivesicular bodies (MVBs). Exosomes are actively released from cells by membrane fusion of MVBs and the plasma membrane. Although the cargos of MVs are similar to the composition of parental cells due to simple diffusion, the cargos of exosomes differ from those of parental cells, suggesting selective cargo‐sorting mechanisms. Cargo loading of exosomes is well controlled and well regulated, although the mechanism is not fully understood. The endosomal‐sorting complex required for transport (ESCRT) family has been shown to play a key role in cargo loading and exosome biogenesis 15, but there are also ESCRT‐independent pathways in cargo sorting 12. Exosomes, unlike other synthetic nanoparticles such as liposomes, contain transmembrane and membrane‐anchored proteins that may enhance endocytosis to facilitate delivery of their cargos 13, 14. Even though the underlying mechanisms and pharmacokinetics are still not well understood, extensive studies have been directed toward utilizing exosomes as a therapeutic conveyor in various diseases, by using the exosome itself or engineering exosomes to increase stability, targetability, or loading efficiency of specific pharmaceutical cargos. Apoptotic bodies, which are larger than MVs or exosomes, are generally larger than 1 μm in diameter and are released as blebs from dying cells. They contain fragmented DNA and are further characterized by phosphatidylserine externalization 16, 17.
Therapeutic Potential and Manufacturing of MSC‐Derived EVs
Stem cells are currently the best candidate for treating intractable degenerative or genetic diseases because of their capacity to differentiate and produce new, healthy cells that can replace injured or diseased cells. In addition, nonhematopoietic tissue stem cells, such as the MSC, can similarly treat nonhematopoietic disorders by replacing diseased cells with newly generated cells 18, 19, 20.
Of the stem cells that are currently in clinical trials, the most extensively used cell type is the MSC. Interestingly, sufficient MSC research exists to support alternative proposals that MSC exerts its therapeutic effects through a secretion and not through a differentiation mechanism 21. MSC‐conditioned culture medium has been suggested to recapitulate the efficacy of MSCs in cardio‐protection 22, 23, renal tubular cell survival 8, and hepatic failure protection 24, 25, as well as relieve immune disease 26. As MSC‐conditioned culture medium is rich in EVs, a complex cargo of lipids, proteins, and RNAs in EVs is the most likely candidate of the therapeutic effects. In addition, MSC‐derived EVs also have been shown to elicit an immunosuppressive phenotype and the therapeutic benefits of their parental cells. Therefore, the therapeutic potential of MSCs‐derived EVs acting as a surrogate of parental cells has been rigorously investigated.
MSC‐derived EVs are known to have various therapeutic effects such as tissue regeneration, wound healing, antitumor action, and immunomodulation. Tested in various disease models, EVs derived from MSCs of different organs have demonstrated similar or better therapeutic capacity compared with their parental cells. MSC‐derived EVs have advantages in safety issues, because they are considered nonimmunogenic with a lower risk of allogenic immune rejection from the host 27. In addition, exosomes can bypass the blood‐brain barrier by transcytosis through the endothelial layers to deliver cargo biomolecules to the brain parenchyma 28 (Table 1).
Table 1.
Comparison of MSC therapy and MSC‐derived EVs therapy
| MSC therapy | MSC‐derived EVs therapy | |
|---|---|---|
| Therapeutic effects | Tissue regeneration, wound healing, antitumor effects, immunomodulation 29, 30 | Retains therapeutic effects of MSCs, and loading therapeutic cargo can increase their effects 2, 5, 25, 31, 32 |
| Homing to target tissue | Home to sites of injury and cancer, but less than 1% of MSCs result in engraftment 33 | Mostly homes to the liver and spleen, and surface engineering of exosomes can induce targeting ability 34, 35, 36
Can pass though the blood‐brain barrier 37 |
| Rejection | Can induce allogenic immune rejection 38, 39 | Considered to be nonimmunogenic |
Abbreviations: EV, extracellular vesicle; MSC, mesenchymal stem cell.
Exogenous MSC EVs in Tissue Regeneration
Much research has shown the beneficial effects of MSC‐derived EVs in healing a variety of stressed tissues (Fig. 1). Bruno et al. showed an improvement of recovery from glycerol‐induced AKI by treating it with MSC‐derived EVs, which contained mRNA associated with the mesenchymal phenotype 40. Similarly, He et al. showed protection against kidney damage in the subtotal nephrectomy murine model of renal regeneration by decreasing the levels of uric acid, creathinine, fibrosis, and lymphocyte infiltration by treating MSC‐derived EVs 31. In another murine model of myocardial ischemia‐reperfusion injury, MSC‐derived EVs enhanced the recovery by increasing phosphorylated Akt and phosphorylated glycogen synthase kinase‐3β and by decreasing phosphorylated mitogen‐activated protein kinase 8 and oxidative stress 41. Furthermore, the therapeutic potential of MSC‐derived EVs was also verified in a murine model of fibrotic liver induced by carbon tetrachloride 5 and in a rat skin burn model 42. Overall, these results indicate that MSC‐derived EVs mediate the therapeutic effects in multiple diseases through multiple mechanistic pathways and provide a novel approach for the treatment of degenerative and acute injury‐related diseases. However, the mechanism of action of MSC‐derived EVs is still not fully understood. Further extensive investigation into the mechanism of action is essential to establish MSC‐derived EVs as Food and Drug Administration‐approved therapeutics. Besides the MSC‐derived EVs, effects of neural stem cells (NSCs) and endothelial progenitor cells (EPCs) derived EVs were also studied. Grafted NSCs communicate with the host immune system via interferon gamma signaling mediated by EV‐associated IFN gamma/Interferon gamma receptor 1 complexes 43. Human EPCs‐derived EVs delivered mRNA and microRNA, which activated the endothelial cell proliferation to support revascularization of injured murine tissue 44. In addition, exosomes from human cardiac progenitor cells expanded ex vivo regenerated injured murine hearts by inhibiting apoptosis and increasing the proliferation of cardiomyocytes and endothelial cells 32.
Figure 1.
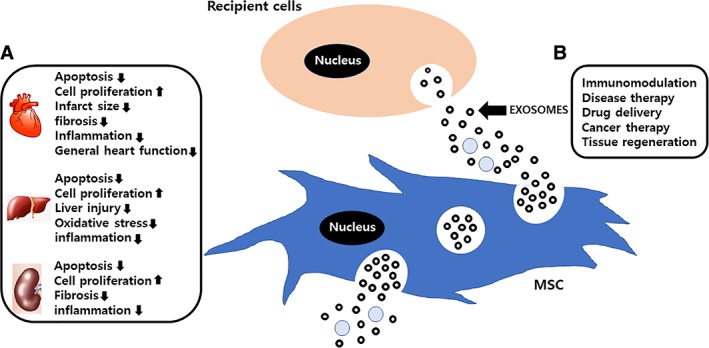
The characteristics of MSC‐derived extracellular vesicles (EVs). (A): The function of the MSC‐EV‐mediated therapeutic effect studied in animal model such as the kidney, liver, and heart. (B): Secreted MSC‐derived exosomes through fusion of multivesicular bodies with the cell membrane are mediated paracrine effects. Exosomes have the potential to exert various effects such as immunomodulation, disease therapy, and tissue repair in recipient cells. Abbreviation: EXPLOR, exosomes for protein loading via optically reversible protein‐protein interaction; MSC, mesenchymal stem cell.
Manufacturing Exosomes for Clinical Use
Most of exosomes are collected based on their size, and the most common way is to use differential centrifugation. However, centrifugation has a low recovery yield and low specificity due to nonexosomal or MV debris.
Unlike the strategies of isolating exosomes or MVs by size, the immunoaffinity‐based approach sorts them via detecting the expression pattern of specific proteins on their surface. This approach has the advantage of isolating specific subpopulations of exosomes and simultaneously reducing copurification of cell debris and protein aggregates. One example of immunoaffinity‐based sorting is the use of conventional magnetic‐activated cell‐sorting (MACS) columns 45. The Taylor and Cercel‐Taylor repurposed MACS to isolate exosomes from serum. In this study, exosomes with epithelial cell adhesion molecule (EpCAM) were incubated with anti‐EpCAM magnetic microbeads and then the microbeads were trapped using a conventional MACS Separator 45. However, applying this technique to clinical setting is doubtful because of difficulties in upscaling and automating such a process. It is necessary to develop an isolation method that can distinguish each type of exosome and facilitate a large‐scale production of exosomes.
MSC was easily expanded relative to the other isolation methods using conventional tissue flasks and bioreactors, but their growth capacity in culture is limited and their biological properties can be changed with repeated passage. Certain strategies such as MSC immortalization by natural selection or by genetic modification could be used to overcome this limitation, although this would raise safety issues 46, 47. Other approaches to scale up the amount of isolated exosomes could include using bioreactors to culture the MSCs 48. However, it is important to determine whether bioreactor culture conditions could change exosome protein and RNA content, which may affect therapeutic efficacy 49. There are many challenges associated with oxygen supply, shear stress, and pH balance by using bioreactor culture systems 50, 51. Also, the impacts of these parameters may vary depending on the different cell types. In conclusion, to facilitate the production of large‐scale MSC‐derived exosome, new batches of MSCs should be periodically derived through testing and validation.
Engineered Exosomes as Therapeutics
Due to the various therapeutic potential of exosomes, clinical trials are currently in progress. One clinical trial is testing the effects of naïve MSC‐derived exosomes in promoting healing of large and refractory macular holes 52. In contrast, the majority of other clinical trials currently in progress use engineered exosomes rather than naïve exosomes. For example, a clinical trial is in progress for promoting neurovascular remodeling and functional recovery after acute ischemic stroke using miR‐124‐loaded MSC‐derived exosomes 53. These engineered exosomes have a higher therapeutic potential when compared with naïve exosomes. There are mainly two different strategies that can improve the therapeutic potential of MSC‐derived exosomes: loading cargo into the exosomes and targeting via exosomes (Fig. 2).
Figure 2.
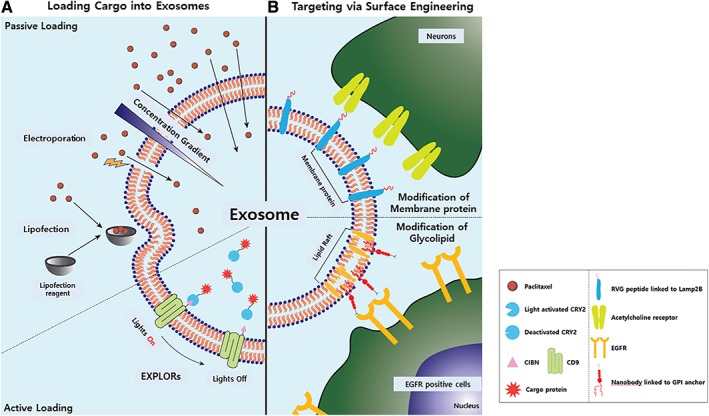
Engineering of exosomes. Exosomes are engineered to acquire therapeutic potential. Two different engineering methods are used. Exosomes are engineered (A) to load therapeutic cargos and (B) to acquire targeting abilities. Abbreviations: CIBN, truncated form of CIB(cryptochrome‐interacting basic‐helix‐loop‐helix); EGFR, epidermal growth factor receptor; RVG, rabies viral glycoprotein.
Loading Cargo into Exosomes
Passive Loading
To enhance the therapeutic potential of naïve exosomes, researchers have developed several methods to load exogenous molecules into exosomes, involving passive and active cargo loading. Passive loading uses the concentration gradient of the molecules. By incubating exosomes with paclitaxel at 37°C for 1 hour, a small amount of paclitaxel was loaded into exosomes 54. Cells were also incubated with paclitaxel to produce paclitaxel‐loaded exosomes 55. The loading capacity depended on the hydrophobic nature of the cargo molecules. Hydrophobic cargos bind to the lipid bilayer of exosomes and remain stable. However, the major downside of passive loading is the low loading capacity, even for hydrophobic cargos.
To compensate for the low loading capacity of concentration gradient‐based methods, various physical and chemical techniques that can directly modify the exosomal membrane have been developed. Electroporation, sonication, extrusion, and freeze‐thaw cycles are included in the physical methods, whereas lipofection, drug‐associated loading, and click chemistry are examples of chemical methods 56. However, these methods also have disadvantages, such as the aggregation of exosomes, compromise of exosomal membranes, toxicity to recipient cells, and excessive purification steps.
Active Loading
To overcome the low passive loading efficiency, researchers have targeted the membranes of exosomes during the exosome biogenesis processes. Fang et al. proposed a technology that uses plasma membrane anchors to allow highly oligomeric proteins to be targeted into exosomes because of the link between the cargo and anchor upon treatment to the recipient 57.
Exosomes are enriched in transmembrane proteins, such as tetraspanins including CD9, CD63, CD81, CD82 58, lactadherin 59, and lysosome‐associated membrane glycoprotein 2 (Lamp2B) 60. By fusing transmembrane proteins to cargo molecules, transmembrane proteins have the potential to directly load cargo molecules into the exosomes in a manner similar to Fang's approach. Yim et al. introduced a technology called exosomes for protein loading via optically reversible protein‐protein interaction, which allows the cytosolic localization of cargo proteins. Upon treatment with recipient cells, the cargo proteins were not just restricted to membranes, but were scattered in the cytosol 61.
In addition to protein cargos, methods to load RNA into exosomes have also been developed. Hung et al. introduced a technology called TAMEL (Targeted and Modular EV Loading), which used Lamp2B as a fusion target. Hung et al. transfected cells with a Lamb2B‐MS2 bacteriophage coat protein dimer (RNA binding domain) and MS2 stem loops fused to the cargo DNA. MS2 bacteriophage coat protein specifically recognizes the MS2 stem loop of RNA. Upon transcription, the RNA cargo is loaded inside exosomes due to recognition of the RNA loop 62.
Targeting via Surface Engineering
Since the discovery of exosomes, researchers have thoroughly studied their natural tropism. Many factors such as the cell source, route of administration, and dosage have effects on the biodistribution of EVs in vivo. Once administered exogenously, naïve exosomes are generally distributed to the liver, spleen, intestines, and lungs of mice, where the mononuclear phagocyte system (MPS) is active 63. In macrophage‐depleted mice, clearance of exosomes from the circulation was much slower compared with that in control mice, indicating the important role of macrophages in exosome biodistribution 64. Engineering exosomes with targeting ability increases the chances of exosomes reaching target cells/tissues before being taken up by the MPS. Eventually this will decrease the off‐target/side effects, leading to lower therapeutic dosages while maintaining therapeutic efficacy. In addition, surface modifications can be used to increase the delivery efficiency. Kim et al. introduced a membrane‐editing technology by which vascular stomatitis virus‐G protein‐engineered exosomes increased the cargo delivery via low‐density lipoprotein receptor targeting 65. Here, we describe two different techniques that engineer exosome biogenesis processes to equip exosomes with increased targeting ability.
Modification of Membrane Protein (Lamp2B)
Alvarez et al. modified the N‐terminus of Lamp2B with rabies viral glycoprotein (RVG). RVG specifically binds to acetylcholine receptors, which are rich in neuronal cells. By transfecting cells with Lamp2B fused to RVG, exosomes displaying RVG protein at the outer membrane were produced. Using electroporation, they loaded the RVG‐exosomes with siRNA against BACE1, a protease that has an important role in Alzheimer's disease pathogenesis by cleaving the amyloid precursor protein. Upon treatment, RVG‐exosomes specifically targeted neuronal cells compared with naïve exosomes, and successfully knocked down the BACE1 mRNA in wild‐type mice 66. However, targeting peptides fused to Lamp2B were vulnerable to degradation due to localization in the lumen of endosomes during exosome biogenesis 67.
Modification of Glycosylphosphatidylinositol (GPI)
Instead of modifying exosomal membrane proteins, Kooijman et al. modified a glycolipid that could be integrated into the exosomal membrane during exosome biogenesis. EVs are enriched in lipid raft‐associated lipids and proteins. The glycosylphosphatidylinositol (GPI)‐linked protein decay‐accelerating factor (DAF) is one of the EV‐rich proteins loaded during reticulocyte maturation. Kooijman et al. genetically engineered cells with DAF‐derived GPI‐linked peptides fused with nanobodies. They used specific nanobodies that targeted the epidermal growth factor receptor. Nanobodies were significantly enriched in exosomes compared with parent cells in fusion with GPI‐anchors. It is suggested that GPI‐anchoring could be used as a versatile tool to incorporate a variety of protein on exosomes, such as enzymes, antibodies, reporter proteins, and signaling molecules 68.
Conclusion
The future of exosome therapeutics has great potential, but additional challenges must be overcome. The following obstacles still must be adequately addressed: (a) exosome components and the mode of action must be fully understood. To be validated by the Federal Drug Administration as a drug, the safety and efficacy of exosomes should be thoroughly studied and the components of the preparations and modes of action must be validated. (b) A database of absorption, distribution, metabolism, and excretion (ADME) should be established. As previously mentioned, various parameters such as the cell source and the route of administration affect the ADME of exogenously administered exosomes. To reach the maximum therapeutic potential and establish the dosage, an ADME study is essential. (c) Exosome production efficiency must be increased. Even though MSCs are known to produce more exosomes than do other types of primary cells, it is necessary to develop culture methods that increase the production of exosomes or immortalized MSCs that produce validated exosomes for clinical applications. (d) Better targeting mechanisms should be developed. To reduce the off‐target/side effects and avoid clearance, screening for targeting molecules is key. If these challenges are sufficiently addressed, exosome‐based therapy will be an important option not only in the field of regenerative medicine but also as a next‐generation drug delivery system.
Disclosure of Potential Conflicts of Interest
C.C. declare leadership position, patent holder, and stock interest with Cellex Life Sciences Incorporated and grant funding from Cellex to KAIST. The other authors indicated no potential conflicts of interest.
Acknowledgment
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF‐2016M3A9B6945831), Korea.
Contributor Information
Hai‐Chon Lee, Email: hclee2018cls@iliasbio.com.
Chulhee Choi, Email: cchoi@kaist.ac.kr.
References
- 1. Yeo RW, Lai RC, Zhang B et al. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 2013;65:336–341. [DOI] [PubMed] [Google Scholar]
- 2. Ferrari G, Cusella‐De Angelis G, Coletta M et al. Muscle regeneration by bone marrow‐derived myogenic progenitors. Science 1998;279:1528–1530. [DOI] [PubMed] [Google Scholar]
- 3. Gnecchi M, He H, Noiseux N et al. Evidence supporting paracrine hypothesis for Akt‐modified mesenchymal stem cell‐mediated cardiac protection and functional improvement. FASEB J 2006;20:661–669. [DOI] [PubMed] [Google Scholar]
- 4. Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med 2008;59:311–325. [DOI] [PubMed] [Google Scholar]
- 5. Tan CY, Lai RC, Wong W et al. Mesenchymal stem cell‐derived exosomes promote hepatic regeneration in drug‐induced liver injury models. Stem Cell Res Ther 2014;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fonsato V, Collino F, Herrera MB et al. Human liver stem cell‐derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs. Stem Cells 2012;30:1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morigi M, Imberti B, Zoja C et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 2004;15:1794–1804. [DOI] [PubMed] [Google Scholar]
- 8. Bi B, Schmitt R, Israilova M et al. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 2007;18:2486–2496. [DOI] [PubMed] [Google Scholar]
- 9. Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol 2015;40:82–88. [DOI] [PubMed] [Google Scholar]
- 10. Ha D, Yang NN, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharmaceut Sinica B 2016;6:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983;33:967–978. [DOI] [PubMed] [Google Scholar]
- 12. Li SP, Lin ZX, Jiang XY et al. Exosomal cargo‐loading and synthetic exosome‐mimics as potential therapeutic tools. Acta Pharmacol Sin 2018;39:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnsen KB, Gudbergsson JM, Skov MN et al. A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta 2014;1846:75–87. [DOI] [PubMed] [Google Scholar]
- 14. Vader P, Mol EA, Pasterkamp G et al. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev 2016;106:148–156. [DOI] [PubMed] [Google Scholar]
- 15. Juan T, Furthauer M. Biogenesis and function of ESCRT‐dependent extracellular vesicles. Semin Cell Dev Biol 2018;74:66–77. [DOI] [PubMed] [Google Scholar]
- 16. Fadok VA, Voelker DR, Campbell PA et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 1992;148:2207–2216. [PubMed] [Google Scholar]
- 17. Martin SJ, Reutelingsperger CP, McGahon A et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl‐2 and Abl. J Exp Med 1995;182:1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wan W, Cao L, Kalionis B et al. Applications of induced pluripotent stem cells in studying the neurodegenerative diseases. Stem Cell Int 2015;2015:382530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griffin M, Greiser U, Barry F et al. Genetically modified mesenchymal stem cells and their clinical potential in acute cardiovascular disease. Discov Med 2010;9:219–223. [PubMed] [Google Scholar]
- 20. Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. J Neurol Sci 2013;333:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076–1084. [DOI] [PubMed] [Google Scholar]
- 22. Shabbir A, Zisa D, Suzuki G et al. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: A noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol 2009;296:H1888–H1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dai W, Hale SL, Kloner RA. Role of a paracrine action of mesenchymal stem cells in the improvement of left ventricular function after coronary artery occlusion in rats. Regen Med 2007;2:63–68. [DOI] [PubMed] [Google Scholar]
- 24. Parekkadan B, van Poll D, Suganuma K et al. Mesenchymal stem cell‐derived molecules reverse fulminant hepatic failure. PLoS One 2007;2:e941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Poll D, Parekkadan B, Cho CH et al. Mesenchymal stem cell‐derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008;47:1634–1643. [DOI] [PubMed] [Google Scholar]
- 26. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815–1822. [DOI] [PubMed] [Google Scholar]
- 27. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat Biotechnol 2014;32:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen CC, Liu L, Ma F et al. Elucidation of exosome migration across the blood‐brain barrier model in vitro. Cell Mol Bioeng 2016;9:509–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. DiMarino AM, Caplan AI, Bonfield TL. Mesencymal stem cells in tissue repair. Front Immunol 2013;4:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee DE, Ayoub N, Agrawal DK. Mesenchymal stem cells and cutaneous wound healing: Novel methods to increase cell delivery and therapeutic efficacy. Stem Cell Res Ther 2016;7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He J, Wang Y, Sun S et al. Bone marrow stem cells‐derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton) 2012;17:493–500. [DOI] [PubMed] [Google Scholar]
- 32. Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep 2014;2:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karp JM, Teo GSL. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell 2009;4:206–216. [DOI] [PubMed] [Google Scholar]
- 34. Cataldi M, Vigliotti C, Mosca T et al. Emerging role of the spleen in the pharmacokinetics of monoclonal antibodies, nanoparticles and exosomes. Int J Mol Sci 2017;18:1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vinas JL, Spence M, Gutsol A et al. Receptor‐ligand interaction mediates targeting of endothelial colony forming cell‐derived exosomes to the kidney after ischemic injury. Sci Rep 2018;8:16320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang QL, Zhuang XY, Sriwastva MK et al. Blood exosomes regulate the tissue distribution of grapefruit‐derived nanovector via CD36 and IGFR1 pathways. Theranostics 2018;8:4912–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manca S, Upadhyaya B, Mutai E et al. Milk exosomes are bioavailable and distinct microRNA cargos have unique tissue distribution patterns. Sci Rep 2018;8:11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Badillo AT, Beggs KJ, Javazon EH et al. Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol Blood Marrow Transplant 2007;13:412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Poncelet AJ, Vercruysse J, Saliez A et al. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation 2007;83:783–790. [DOI] [PubMed] [Google Scholar]
- 40. Bruno S, Grange C, Deregibus MC et al. Mesenchymal stem cell‐derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 2009;20:1053–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arslan F, Lai RC, Smeets MB et al. Mesenchymal stem cell‐derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 2013;10:301–312. [DOI] [PubMed] [Google Scholar]
- 42. Zhang B, Yin Y, Lai RC et al. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev 2014;23:1233–1244. [DOI] [PubMed] [Google Scholar]
- 43. Cossetti C, Iraci N, Mercer TR et al. Extracellular vesicles from neural stem cells transfer IFN‐gamma via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell 2014;56:193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cantaluppi V, Biancone L, Figliolini F et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant 2012;21:1305–1320. [DOI] [PubMed] [Google Scholar]
- 45. Taylor DD, Gercel‐Taylor C. MicroRNA signatures of tumor‐derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008;110:13–21. [DOI] [PubMed] [Google Scholar]
- 46. Pollock K, Stroemer P, Patel S et al. A conditionally immortal clonal stem cell line from human cortical neuroepithelium for the treatment of ischemic stroke. Exp Neurol 2006;199:143–155. [DOI] [PubMed] [Google Scholar]
- 47. Chen TS, Arslan F, Yin Y et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC‐derived MSCs. J Transl Med 2011;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hupfeld J, Gorr IH, Schwald C et al. Modulation of mesenchymal stromal cell characteristics by microcarrier culture in bioreactors. Biotechnol Bioeng 2014;111:2290–2302. [DOI] [PubMed] [Google Scholar]
- 49. de Jong OG, Verhaar MC, Chen Y et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell‐derived exosomes. J Extracell Vesicles 2012;1(1):18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. King JA, Miller WM. Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol 2007;11:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yeatts AB, Choquette DT, Fisher JP. Bioreactors to influence stem cell fate: Augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta 2013;1830:2470–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. MSC‐Exos Promote Healing of MHs (MSCs). Available at https://clinicaltrials.gov/ct2/show/NCT03437759. Accesses October 1, 2018.
- 53. Allogenic Mesenchymal Stem Cell Derived Exosome in Patients With Acute Ischemic Stroke. Available at https://clinicaltrials.gov/ct2/show/NCT03384433. Accessed September 28, 2018.
- 54. Kim MS, Haney MJ, Zhao Y et al. Development of exosome‐encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine 2016;12:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun DM, Zhuang X, Xiang X et al. A novel nanoparticle drug delivery system: The anti‐inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 2010;18:1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Luan X, Sansanaphongpricha K, Myers I et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 2017;38:754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fang Y, Wu N, Gan X et al. Higher‐order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS Biol 2007;5:e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thery C, Zitvogel L, Amigorena S. Exosomes: Composition, biogenesis and function. Nat Rev Immunol 2002;2:569–579. [DOI] [PubMed] [Google Scholar]
- 59. Delcayre A, Estelles A, Sperinde J et al. Exosome Display technology: Applications to the development of new diagnostics and therapeutics. Blood Cells Mol Dis 2005;35:158–168. [DOI] [PubMed] [Google Scholar]
- 60. Simhadri VR, Reiners KS, Hansen HP et al. Dendritic cells release HLA‐B‐associated transcript‐3 positive exosomes to regulate natural killer function. PLoS One 2008;3:e3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yim N, Ryu SW, Choi K et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein‐protein interaction module. Nat Commun 2016;7:12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hung ME, Leonard JN. A platform for actively loading cargo RNA to elucidate limiting steps in EV‐mediated delivery. J Extracell Vesicles 2016;5:31027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wiklander OPB, Nordin JZ, O'Loughlin A et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 2015;4:26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Imai T, Takahashi Y, Nishikawa M et al. Macrophage‐dependent clearance of systemically administered B16BL6‐derived exosomes from the blood circulation in mice. J Extracell Vesicles 2015;4:26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yang YS, Hong Y, Nam GH et al. Virus‐mimetic fusogenic exosomes for direct delivery of integral membrane proteins to target cell membranes. Adv Mater 2017;29:1605604. [DOI] [PubMed] [Google Scholar]
- 66. Alvarez‐Erviti L, Seow Y, Yin HF et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011;29:U341–U179. [DOI] [PubMed] [Google Scholar]
- 67. Hung ME, Leonard JN. Stabilization of exosome‐targeting peptides via engineered glycosylation. J Biol Chem 2015;290:8166–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kooijmans SAA, Aleza CG, Roffler SR et al. Display of GPI‐anchored anti‐EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles 2016;5:31053. [DOI] [PMC free article] [PubMed] [Google Scholar]


