Abstract
Objectives
This study aimed to assess the repair of bone defects with or without grafts using biphasic synthetic micro-granular hyaluronic acid (HA) with β-TCP and bovine type I collagen (Osteon II Collagen) with or without either hyaluronic acid or a collagen membrane in surgically created bone defects in the tibia of rabbits.
Methods
Fifteen male rabbits were divided into 3 groups, each with 5 rabbits. Two bone defects were made in each tibia. In the right tibia, the defects were either filled with clot as a control or grafted with Osteon II Collagen and hyaluronic acid. In the left tibia, the other two defects were filled with Osteon II Collagen alone or with Osteon II Collagen and a collagen membrane. The specimens were observed one, two, and four weeks after surgery. Histological examinations were used to evaluate the degree of healing according to the amount of newly formed bone.
Results
The combination of the bone grafting biomaterial with hyaluronic acid was found to develop into the most advanced stages of the bone repair process at the second and fourth week only (p ≤ 0.05), compared to the biomaterial with a collagen membrane, as well as the other groups. On the other hand, the biomaterial in combination with a collagen membrane showed significantly more bone formation than the biomaterial alone or the control group by the fourth week.
Conclusions
The local application of hyaluronic acid and collagen membranes made a greater contribution to the bone repair process in the tibia of rabbits than the bone graft substitute (Osteon II Collagen) alone.
Keywords: Bone regeneration, Collagen membrane, Hyaluronic acid, Osteon II collagen
الملخص
أهداف البحث
يهدف هذا البحث إلى تقييم إصلاح عيوب العظام مع أو بدون تطعيمات باستخدام ثنائي الطور الاصطناعي الحبيبي-الصغير β-TCP +HA + النوع الأول من الكولاجين البقري (اوستيون ٢ كولاجين) منفردا أو غير منفرد مع حمض الهيالورونيك أو غشاء الكولاجين في خلل عظمي استحدث جراحيا في عظم الظنبوب لدى الأرانب.
طرق البحث
تم تقسيم ١٥ ذكرا من الأرانب إلى ٣ مجموعات، كل مجموعة من ٥ أرانب. كما تم استحداث عيبين عظميين في كل ظنبوب. ملئت العيوب بجلطة دموية أو تم تطعيمها بثنائي الطور الاصطناعي الحبيبي - الصغير β-TCP + HA (اوستيون ٢ كولاجين) مع حمض الهيالورونيك في ظنبوب الساق الأيمن والعيبين الآخرين المستحدثين في ظنبوب الساق الأيسر ملئت باوستيون ٢ كولاجين فقط أو اوستيون ٢ كولاجين مرتبطا مع غشاء الكولاجين. تم جمع العينات في الأسبوع الأول، والثاني والرابع بعد الجراحة. وتم استخدام الفحوصات النسيجية لتقييم درجة التئام العيوب العظمية اعتمادا على كمية العظم الجديد المتكون.
النتائج
أظهرت النتائج أن مزيج المواد الحيوية مع حمض الهيالورونيك شارك في مرحلة متقدمة في عمليات إصلاح العظام في الأسبوع الثاني والرابع فقط بالمقارنة بالمواد الحيوية مع غشاء الكولاجين والمجموعات الأخرى. من ناحية أخرى، أظهرت المواد الحيوية مع غشاء الكولاجين تشكيلا كبيرا للعظام من المواد الحيوية فقط والمجموعات الضابطة في نهاية التجربة.
الاستنتاجات
الإضافة الموضعية لحمض الهيالورونيك وغشاء الكولاجين كان لها تأثير أكبر على عملية إصلاح عظام الساق لدى الأرانب من الطعم العظمي البديل (اوستيون ٢ كولاجين).
الكلمات المفتاحية: تجديد العظام, غشاء الكولاجين, حمض الهيالورونيك, اوستيون٢
Introduction
Bone healing is a complex process involving a number of cellular functions and mineralisation followed by an eventual remodelling of the defect site to recover the original structure.1 Recently, dental studies have been focused on bone grafting therapy; patients are becoming more aware of grafting as a treatment modality and expect better predictability, fit, function, and aesthetics. Currently, with the introduction of advanced bone grafting techniques and the use of sophisticated bone replacement graft materials, it is possible to increase the volume, width, and height of bone in deficient areas to regenerate the supporting tissues.2 An ideal synthetic bone substitute should be tolerated by the host tissue, promote bone formation, have the appropriate mechanical strength, and be resorbed after it has completed its function. The search for a synthetic biomaterial that is able to successfully replace normal tissue involves identifying a material that mimics one or more properties of natural bone.3, 4
Synthetic bone graft (Osteon™ II) + bovine type I collagen is a combination of 70% hyaluronic acid (HA) and 30% β-tricalcium phosphate (β-TCP) and is an alloplastic material that shares similarities with human bone in terms of its major mineral components. The size of the particles and pores is 0.2–0.5 mm and 250 μm, respectively, with a porosity of 70%. It possesses osteoconductive properties that allows it to acts as a bone growth scaffold. Its appearance is similar to that of human cancellous bone thanks to the interconnected porosity of its structure.5 The addition of collagen to bone void filler (Osteon II Collagen) allows for the slow absorption of the latter over several weeks in addition to helping with the initial shaping. A resorbable collagen membrane (RCMs) is used as the scaffold for bone deposition in guided bone regeneration (GBR) and guided tissue regeneration (GTR) procedures.6, 7 Collagen membranes are a resorbable, cell occlusive barrier used for dental implants, bone defects, and ridge augmentation.8 This membrane is manufactured from allogeneic or xenogeneic sources.9 The placement of collagen membranes in the bone defect area prevents the intrusion of non-osteogenic cells into the defective region, while simultaneously creating a space that allows for the protraction and growth of osteoblasts. Collagen membranes also promote platelet aggregation, stabilise clots, attract fibroblasts, and facilitate wound healing.6, 7, 8 On the other hand, hyaluronic acid (HA) has osteoconductive potential; it accelerates bone regeneration by means of chemotaxis, proliferation, and successive differentiation of mesenchyme cells. HA may act as a biomaterial scaffold for other molecules, including bone morphogenic protein-2 (BMP-2) and transformation growth factor-β (TGF-β), used in guided bone regeneration techniques and tissue engineering studies.10 Rabbits were used as the animal model in this study due to their short developmental period and the faster bone turnover. The fast skeletal development and bone turnover (significant intracortical, Haversian remodelling) of rabbits allows them to achieve skeletal maturity shortly after reaching sexual maturity at approximately 6 months.11 Moreover, similarities have been reported between the bone composition and density of humans and rabbits.12 The objective of this work was to histologically assess and investigate the effectiveness of a new injectable bone graft substitute, Osteon II Collagen (biphasic synthetic micro-granular HA + β-TCP), on the bone healing process in surgically created defects, either alone or in combination with hyaluronic acid or a collagen membrane. Despite successful reports on the sole application of biomaterials in many different fields for the repair of bone defects, grafted defects combined with hyaluronic acid or collagen membranes still requires further study. According to our knowledge, this is the first study to compare these two materials (hyaluronic acid and collagen membrane) in combination with biomaterials (Osteon II Collagen) in grafted bone defects.
Materials and Methods
Animal model, housing, and sampling
Fifteen male local rabbits (∼8–10 months old, average weight 2000–3000 g) were used as the experimental animals. Animals were housed under a natural light cycle: 12 h light 12 h darkness, at 22 °C at the laboratory of Animal Experimentation of Surgical Department/Veterinary Collage of Duhok University throughout the experimental period. The animals were fed a fresh greenery diet and tap water three times a day. The heath of the rabbits was continuously monitored during the experiment by a veterinarian. The animals were randomly distributed into three groups (5 rabbits/group) according to the period before animal sacrifice (1, 2, or 4 weeks).13 For each animal, four bone defects were made as follows (Table 1): (i) Defect I (D1, control) in right tibia. Here, the bone defect was left empty so as to be covered by only by blood clots; (ii) Defect II (D2). The bone defects were covered with Osteon II Collagen and hyaluronic acid; (iii) Defect III (D3) in the left tibia. Osteon II Collagen with an absorbable collagen membrane was used to fill the bone defect; (iv) Defect IV (D4). The bone defect was filled with Osteon II Collagen alone. Animals were quarantined 7 days prior to the surgical procedure to ensure the animals were free of any infectious disease before operating.
Table 1.
Animal groups.
| Group no. | No. of animals | Treatment of bone defect |
|||
|---|---|---|---|---|---|
| D I | D II | D III | D IV | ||
| I | 5 | Control | Osteon II Collagen with Hyaluronic acid | Osteon II Collagen with collagen membrane | Osteon II Collagen alone |
Surgical procedure
Prior to intramuscular general anaesthesia, the left and right legs of the animals were washed using soap and water, shaved on the operation side, and disinfected with 10% povidone-iodine. The animals were sedated by first administering 2 mg/kg of atropine solution subcutaneously (Gracure Pharmaceuticals Ltd., Bhwadi, India) followed 15 min later by the administration of a general anaesthesia mixture containing 10 mg/kg ketamine hydrochloride (Gracure Pharmaceuticals Ltd., Bhwadi, India) and 2 mg/kg xylazine sedative (Interchemie, Holland). Complete anaesthesia was obtained within 10 min and the dose was high enough to anaesthetise the rabbits for about half hour. Then, a local anaesthetic of 2% lidocaine hydrochloride with epinephrine (1:80,000) was administered via infiltration at the surgical site prior to making any incision in order to achieve hemostasis. The rabbits were then placed in a lateral position for the duration of the procedure. A 5 cm-long incision was made on both the right and left tibia along the longitudinal axis of the side of each tibia using a #15 scalpel blade. The skin and subcutaneous tissues were dissected down to the periosteum, which was gently sectioned to expose the bone. Two monocortical holes (cylindrical in shape) were made on each tibia down to the bone marrow using a trephine drill (Dentium Company, Korea) under constant normal saline irrigation with a width of 3 mm and depth of 4 mm. The drill was mounted on a slow speed surgical hand piece at 1500 rpm to prevent thermal bone necrosis (SAESHIN X-Cube Implant surgery motor, South Korea). A distance of about 1 cm was left between each hole. This was followed by careful irrigation using saline to wash the surgical site and to eliminate any bone debris before filling the defects with biomaterials. For the defects on right tibia, the first was filled only with the blood clot to serve as a control, whereas the second was filled with 0.2 ml of hyaluronic acid gel (BioPolymer, Germany) and biomaterial (Osteon II Collagen; Dentium Company, Seoul, Korea). Before loading, the mixture was left for 5 min to achieve homogeneity. The mixture was then loaded into each defect and pressed gently. For the defects in the left tibia, the first was filled with Osteon II Collagen alone, whereas the second one was filled with Osteon II Collagen with a collagen membrane (resorbable membrane, made in Korea) (Figure 1). All wounds were sutured using 3-0 non-resorbable black silk suture (AM Instruments, China) which would be removed 10 days post-operation. The animals received a single 50 mg/kg dose of antibiotics (oxytetracycline hydrochloride injectable solution; Chongqing Fangtong Animal Pharmaceutical, China).
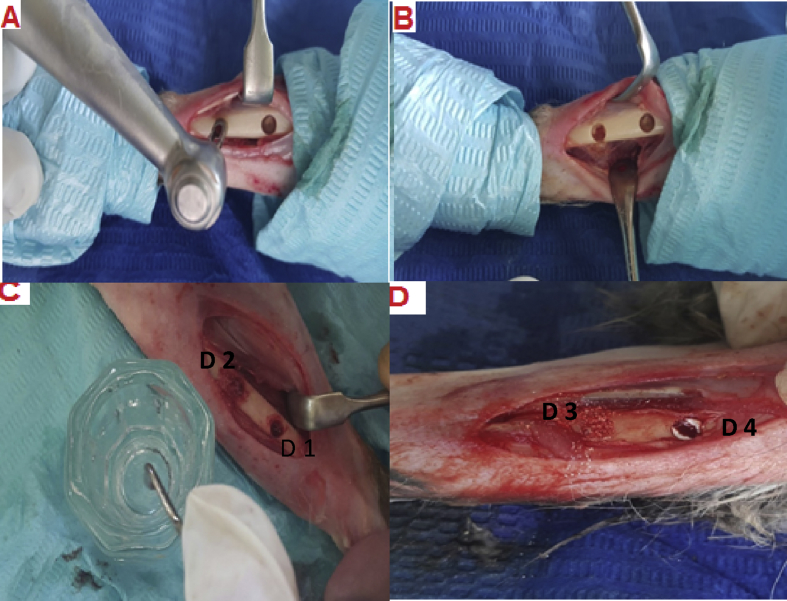
Figure 1.
Surgical procedure. A and B: Creation of bone defects using trephine drill in two rabbit tibias. C: Application of the biomaterials into the two bone defects created in the right tibia. One defect was left empty (control group, D1), and the other defect was filled with Osteon II Collagen and hyaluronic acid (D2). D: Another two defects were made in the left tibia. One was filled with Osteon II Collagen with a collagen membrane (D3) and the other was filled with Osteon II Collagen alone (D4).
Animal sacrifice and sample manipulation
Following animal sacrifice, the operated tibias were dissected subperiosteal to allow for the direct observation of newly formed bone. The samples were then fixed in buffered 10% formalin solution (pH 7.3) for 2 weeks. After fixation, the specimens were placed in 10% nitric acid for about four days for decalcification, then dehydrated through graded series of ethanol and xylene (70–99%) before being embedded in paraffin wax, sectioned using a microtome (5 μm thickness), and finally stained with hematoxylin and eosin (H&E).
Histopathological score
The histological scoring system followed the criteria of Lucaciu et al.14 with some modifications by a pathologist specialist. The scoring system includes: (i) new bone formation: Score 0, absent; Score 1, present at the periphery (Mild); Score 2, present centrally (Moderate); Score 3, present centrally and at the periphery (sever); (ii) inflammation: 1, low; 2, high; (iii) new blood vessels and granulation tissue: 1, low; 2, high. (iv) osteoblasts: Score 0, absent; Score 1, present at the periphery; Score 2, present centrally; Score 3, present centrally and at the periphery. (v) osteoclasts: Score 0, absent; Score 1, present at the periphery; Score 2, present centrally; Score 3, present centrally and at the periphery.
Histopathological examination was performed by specialist pathologist and examiners. These findings were scored semi-quantitatively and ordered scores were assigned for: absence (0), mild presence present at the periphery (1), moderate, present centrally (2), and severe presence centrally and at the periphery (3). All the slides were evaluated using a light microscope (Mottic, China) under a ×10 magnification low power field (LPF) to observe the presence of blood vessels, granulation tissue, and osteoid tissue, then under ×40 magnification to estimate the amount of the inflammatory, osteoblast, and osteoclast cells. Histological analysis of the bone defects included all possible phases of bone formation, from the periphery of the defect to the centre of the specimen. Statistical analysis was carried out on all the data using the Kruskal–Wallis and Mann–Whitney tests for comparison between groups with the help of the Statistical Package for Social Sciences (version 12; SPSS Inc., Chicago, IL, USA). A P-value ≤ 0.05 was considered significant.
Results
The histological analysis of the bone defects presented all the phases of bone formation, extending from the periphery of the defect to the centre of the specimen.
Histopathological analysis of specimens one week post-operation (Figure 2, Table 2)
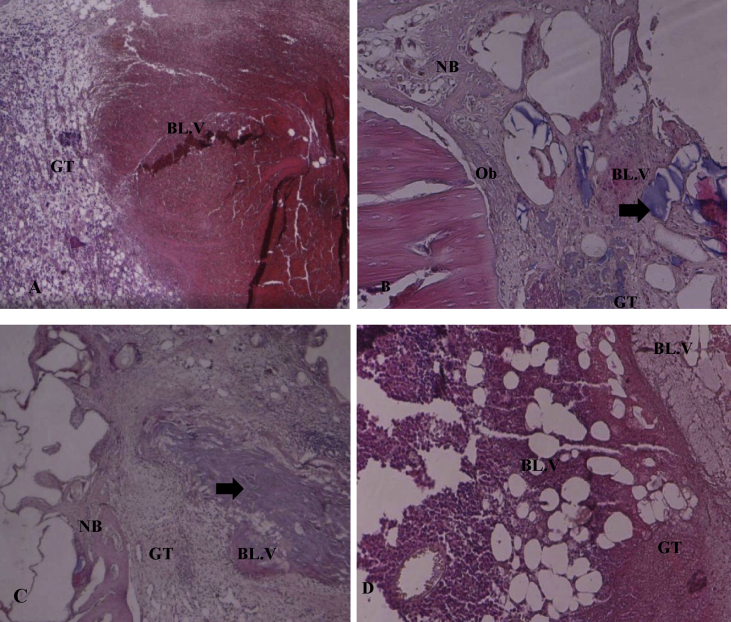
Figure 2.
Photomicrographs of specimens one week post-operation in H&E-stained sections (×40). A: The D1/control group consists of mature granulation tissue (GT), fatty tissue, blood vessels (BL.V), and inflammatory cells scattered throughout the bone defect. B: The D2/Osteon II Collagen with hyaluronic acid group showed new bone formation (NB) found at the defect border with osteoblast cells (Ob), granulation tissue (GT), and blood vessels (BL.V). The black arrow denotes the Osteon II Collagen particles. C: The D3/Osteon II Collagen with collagen membrane group consists of mature granulation tissue (GT) with blood vessels (BL.V) and lower levels of Osteon II Collagen material (black arrow) as well as new bone formation (NB). D: The D4/Osteon II Collagen alone group showed an abundance of inflammatory granulation with newly formed blood vessels.
Table 2.
Histopathological healing score analysis for bone regeneration one week post-operation (osteoblasts, osteoclast cells, bone formation, blood vessels).
|
Score |
Osteoblast D1/control |
Osteoblast D2/Osteon II Collagen with HA |
Mann–Whitney test |
| Frequency |
Frequency |
||
| 0 | 1 | 0 | U = 4.000 |
| 1 | 4 | 2 | Z = −2.008 |
| 2 | 0 | 1 | |
| 3 | 0 | 2 | |
| Total | 5 | 5 | P-value = 0.045* |
| D1 vs D3 U = 6.000, Z = −1.678, P = 0.093 D1 vs D4 U = 12.500, Z = 0.000, P = 1.000 D2 vs D4 U = 4.000, Z = −2.008, P = 0.045* D2 vs D3 U = 8.000, Z = −1.021, P = 0.307 D3 vs D4 U = 6.000, Z = −1.678, P = 0.093 | |||
|
Score |
Osteoclast cells D1/control |
Osteoclast cells D2/Osteon II Collagen with HA |
Mann–Whitney test |
| 0 | 0 | 0 | U = 2.000 |
| 1 | 0 | 1 | Z = −2.425 |
| 2 | 1 | 4 | |
| 3 | 4 | 0 | |
| Total | 5 | 5 | P-value = 0.015* |
| D1 vs D3 U = 1.000, Z = −2.545, P = 0.011* D1 vs D4 U = 4.000, Z = −1.928, P = 0.054* D2 vs D3 U = 7.500, Z = −1.225, P = 0.221 D2 vs D4 U = 12.000, Z = −0.120, P = 0.905 D3 vs D4 U = 9.000, Z = −0.808, P = 0.419 | |||
| Kruskal–Wallis test – bone formation | Chi-square = 2.787 | P-value = 0.426 | |
| Kruskal–Wallis test – blood vessels | Chi-square = 2.111 | P-value = 0.550 | |
One week after the operation, there were no statistically significant differences between the four groups in terms of the amount of granulation tissue and osteoid formation (P > 0.05). The experimental cavity area was observed to contain loose connective tissue with an irregular appearance. Blood vessels and capillaries were easily observed microscopically (P = 0.550). The amount of new bone formation was insignificant between all groups (P = 0.426). Osteoblast cell formation was significant in the D2 group compared to the D4 and D1 groups (P = 0.045), while osteoclast formation was significant only in the D1 group (P = 0.015). According to the amount of inflammatory cells, a minimal amount of acute inflammation was observed in all the groups.
D1: control group
The control group (D1) consisted of immature fibrous tissue, high levels of newly formed blood vessels and inflammatory cells infiltration, as well as more osteoclast cells than osteoblast cells (P = 0.045).
D2: experimental group treated with Osteon II Collagen and hyaluronic acid
The defects of the D2 group showed a moderate amount of granulation tissue with new blood vessel formation. New bone was found at the border of the attached to the original bone and scattered throughout the defect tissue. Osteoblast cells were less abundant than osteoclast cells. No inflammation was found in this group.
D3: experimental group treated with Osteon II Collagen and collagen membrane
New bone was found at the defect border of the D3 group, attached to the original bone and scattered throughout the defect tissue. Osteoclast cells were also less abundant than osteoblast cells, and an area of mature fibrous tissue was observed between the new bone with granulation tissue and new blood vessels. No inflammation was found in this group.
D4: experimental group treated with Osteon II Collagen alone
Light microscopic examination of the D4 group defects showed inflammation in all specimens, granulation tissue, and a moderate amount of blood vessels, as well as few osteoid cells.
Histological analysis of specimens two weeks post-operation (Figure 3, Table 3)
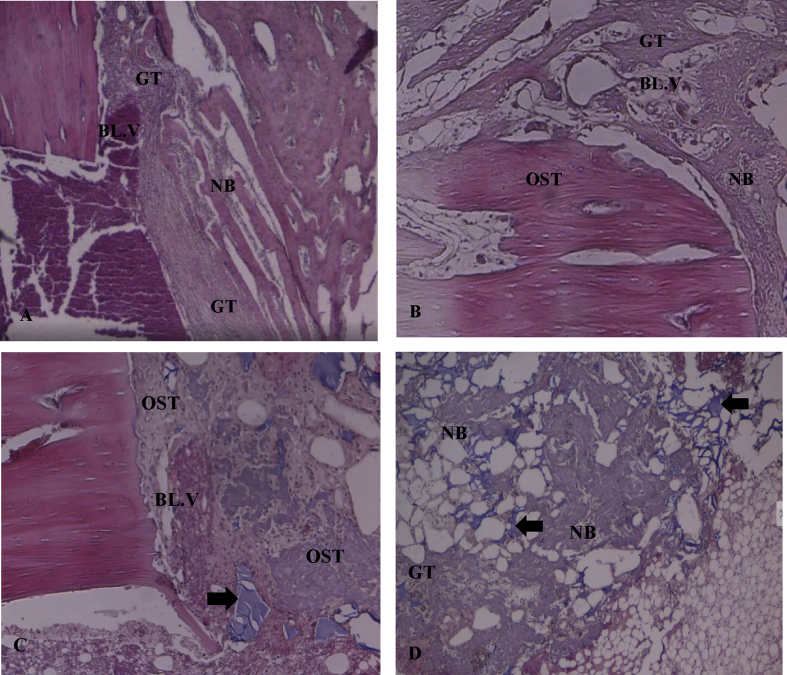
Figure 3.
Photomicrographs of specimens two weeks post-operation in H&E-stained sections (×40). A: The D1/control group showed a minimal amount of new bone which was thinner than that of the other groups. Chronic inflammatory cell infiltrates and blood vessels with granulation tissue were present in this group. B: The D2/Osteon II Collagen with hyaluronic acid group showed nearly complete bridging of the bone defect with mature new osteoid tissue (OST) and small amounts of fibrous tissue between the new bone. C: The D3/Osteon II Collagen with collagen membrane group showed nearly complete bridging of the bone defect with an abundance of mature new osteoid tissue and a small amount of fibrous tissue between the new bone. The black arrows represent the remaining particles of Osteon II Collagen material. D: The D4/Osteon II Collagen group showed newly formed bone scattered throughout the defect with newly formed blood vessels, inflammatory cells, and granulation tissue. Osteon II Collagen particles were also observed in this group (black arrows).
Table 3.
Histopathological healing score analysis for bone regeneration two weeks post-operation (osteoblasts, osteoclast cells, bone formation, granulation tissue, blood vessels).
|
Score |
New bone formation D1/control |
New bone formation D2/Osteon II Collagen with HA |
Mann–Whitney test |
| Frequency |
Frequency |
||
| 0 | 0 | 0 | U = 1.500 |
| 1 | 1 | 0 | Z = 0.241 |
| 2 | 3 | 1 | |
| 3 | 1 | 4 | |
| Total | 5 | 5 | P-value = 0.016* |
| D1 vs D3 U = 2.000, Z = −2373, P = 0.032* D1 vs D4 U = 8.500, Z = −0.956, P = 0.421 D2 vs D3 U = 10.000, Z = −655, P = 0.690 D2 vs D4 U = 3.000, Z = −2.154, P = 0.056 D3 vs D4 U = 4.000, Z = −2.032, P = 0.095 | |||
|
Score |
Osteoblast cells D1/Control |
Osteoblast cells D2/Osteon II Collagen with HA |
Mann–Whitney test |
| 0 | 0 | 0 | U = 4.000 |
| 1 | 1 | 0 | Z = −2.032 |
| 2 | 4 | 2 | |
| 3 | 0 | 3 | |
| Total | 5 | 5 | P-value = 0.045* |
| D1 vs D3 U = 6.000, Z = −1.678, P = 0.093 D1 vs D4 U = 9.500, Z = −0.693, P = 0.488 D2 vs D3 U = 10.000, Z = −0.600, P = 0.549 D2 vs D4 U = 4.500, Z = −1.771, P = 0.077 D3 vs D4 U = 5.500, Z = −1.549, P = 0.121 | |||
|
Score |
Blood vessels and granulation tissue |
Mann–Whitney test |
|
| D1/Control |
D2/Osteon II Collagen with HA |
||
| 1 | 0 | 5 | U = 0.000 |
| 2 | 5 | 0 | Z = −3.000 |
| Total | 5 | 5 | P-value = 0.003* |
| D1 vs D3 U = 4.000, Z = −0.000, P = 0.008* D1 vs D4 U = 7.500, Z = 1.500, P = 0.310 D2 vs D3 U = 12.500, Z = 0.000, P = 1.000 D2 vs D4 U = 5.000, Z = −0.964, P = 0.050* D3 vs D4 U = 5.000, Z = 1.964, P = 0.050* | |||
| Kruskal–Wallis test – osteoclast cells | Chi-square = 1.362 | P-value = 0.715 | |
At the end of two weeks, statistically significant differences were observed between the four groups in terms of the amount of new blood vessels and granulation tissue (P = 0.003). Osteoid formation was significantly increased in both the D2 (Osteon II with hyaluronic acid) and D3 (Osteon II with collagen membrane) groups (P = 0.016).
The amount of osteoblast cells was significantly increased in the D2 group (P = 0.045) and the amount of osteoclast cells was non-significantly decreased in all the groups (P = 0.715). Inflammatory cells were present in the control and Osteon II Collagen alone groups but not in the D2 or D3 groups.
D1: control group
The bone defects of the D1 group two-week post-operation showed a minimal amount of new bone formation, however this was less mature than in the D2 (Osteon II Collagen with hyaluronic acid), D3 (Osteon II Collagen with collagen membrane), or D4 (Osteon II Collagen alone) groups. Chronic inflammatory infiltration and granulation tissue were found in greater amounts in the control group compared to the other groups.
D2: experimental group treated with Osteon II Collagen and hyaluronic acid
This group showed superior bone formation throughout the defect compared to the other groups, except for the defect of the D3 group (Osteon II Collagen with collagen membrane) in which greater bone maturity was observed. This group contained a lesser amount of fibrous connective tissue in between new bone cells. Fibroblasts, microphages, blood vessels, capillaries, and thin collagen fibres were easily observed in this group in terms of their orientation and alignment. The beginning of osteoplastic activity was also observed, which was distinguished by the presence of partially calcified thin osteoid tissue at 2 weeks post-operation.
D3: experimental group treated with Osteon II Collagen and collagen membrane
This group showed greater bone formation throughout the bone defect area compared to the other groups. As with the Osteon II Collagen and HA group, an approximate bridging of the defect wall was observed. Bone maturity was greater than in this group than the Osteon II with hyaluronic acid group, however with less fibrous connective tissue in between the new bone cells (Table 3).
The experimental cavity area contained mature connective tissue of an regular appearance. The formation of fibroblasts, microphages, blood vessels, capillaries, and thin collagen fibres was also observed in this group, with a specific orientation and alignment. After two weeks the beginnings of osteoplastic activity were also observed, indicated by the presence of partially calcified thin osteoid tissue.
D4: experimental group treated with Osteon II Collagen alone
A moderate amount of new bone was scattered throughout the lesion between the mature granulation tissue. Collagen fibre and newly formed blood vessels were observed in the mature granulation tissues. Inflammation decreased to very low levels.
Histological analysis of specimens four weeks post-operation (Figure 4, Table 4)
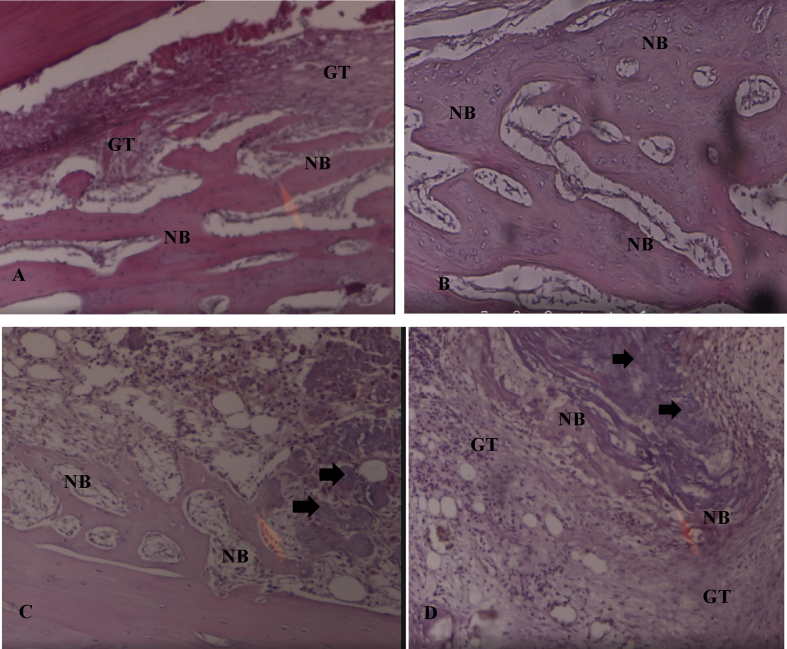
Figure 4.
Photomicrographs of specimens four weeks post-operation in H&E-stained sections (×40). A: The D1/control group showed small amounts of granulation tissue throughout the newly formed bone which filled the defect. B: The D2/Osteon II Collagen with hyaluronic acid group showed the highest amount of mature new bone formation with complete closure of the defect. C: The D3/Osteon II Collagen with collagen membrane group showed a large amount of new bone formation with the defect nearly fully closed. Osteon II Collagen material was observed between the tissues (black arrows). D: The D4/Osteon II Collagen alone group showed new bone formation which filled the entire defect and small amounts of granulation tissue scattered between the new bone cells. The black arrows denote the remaining particles of Osteon II Collagen.
Table 4.
Histopathological healing score analysis for bone regeneration four weeks post-operation period (osteoblasts, osteoclast cells, bone formation, blood vessels).
|
Score |
New bone formation D1/control |
New bone formation D2/Osteon II Collagen with HA |
Mann–Whitney test |
| Frequency |
Frequency |
||
| 0 | 0 | 0 | U = 0.000 |
| 1 | 1 | 0 | Z = 2.835 |
| 2 | 4 | 0 | |
| 3 | 0 | 5 | |
| Total | 5 | 5 | P-value = 0.008* |
| D1 vs D3 U = 3.000, Z = −2.154, P = 0.056 D1 vs D4 U = 12.500, Z = 0.000, P = 1.000 D2 vs D3 U = 7.500, Z = −1.500, P = 0.310 D2 vs D4 U = 0.000, Z = −2.835, P = 0.008* D3 vs D4 U = 3.000, Z = −2.154, P = 0.056 | |||
|
Score |
Osteoblast cells D1/Control |
Osteoblast cells D2/Osteon II Collagen with HA |
Mann–Whitney test |
| 0 | 0 | 0 | U = 2.000 |
| 1 | 1 | 0 | Z = −2.425 |
| 2 | 4 | 1 | |
| 3 | 0 | 4 | |
| Total | 5 | 5 | P-value = 0.015* |
| D1 vs D3 U = 4.000, Z = −2.032, P = 0.042* D1 vs D4 U = 9.500, Z = −0.693, P = 0.488 D2 vs D3 U = 10.000, Z = −0.655, P = 0.513 D2 vs D4 U = 3.500, Z = −2.041, P = 0.041* D3 vs D4 U = 4.500, Z = −0.771, P = 0.077 | |||
| Kruskal–Wallis test – osteoclast cells | Chi-square = 4.328 | P-value = 0.228 | |
| Kruskal–Wallis test – blood vessels | Chi-square = 3.000 | P-value = 0.392 | |
At the end of the fourth week, the amount of blood vessels and granulation tissue showed insignificant differences between all the groups (P = 0.392). The amount osteoclast cells was also insignificant among the groups (P = 0.228), however, the amount of osteoblast cells showed a significantly statistical difference, particularly in D2 (P = 0.015) and D3 when compared with the other groups. The amount of new bone formation was significantly increased in D2 (P = 0.008). No inflammatory response was found in any of the groups.
D1: control group
The bone defects in the control group showed little granulation tissue throughout the newly formed bone which filled the defect. No osteoclast cells were observed, however, but the amount of mature osteoblast cells was greater than osteocyte formation of new bone. No inflammation was found in this group at the fourth week.
D2: experimental group treated with Osteon II Collagen and hyaluronic acid
This group showed less granulation tissue than the other three groups and nearly complete bridging of the defect walls with new bone formation. The osteoblast cells were mature but were found in lower amounts, however, there was an abundance of osteocyte cells.
D3: experimental group treated with Osteon II Collagen and collagen membrane
This group showed nearly complete bridging with new bone, and the granulation tissue here was less than that of the other groups. There was a statistically significant difference between the Osteon II Collagen with hyaluronic acid and Osteon II Collagen with collagen membrane groups compared to the control and Osteon II Collagen alone group. All three treatments groups showed a significantly superior new bone formation compared to the control group (P = 0.003).
D4: experimental group treated with Osteon II Collagen alone
The newly formed bone filled the entire defect with low levels of granulation tissue scattered throughout the new bone. The blue particles appearing in the H&E-stained section of this group at week 4 represent the remaining Osteon II Collagen particles (Figure 4D).
Discussion
Bone grafting is a commonly used procedure to treat large bone defects, including segmental or large cortical defects, created by trauma, infection, tumour resection, aseptic loosening around implants, or skeletal abnormalities.15 The purpose of bone grafts is to regenerate bone defects and restore the original condition.16 Oral and maxillofacial surgery research aims to improve current bone grafting materials and provide a faster and more effective way to regenerate bone.17
This study used a newly developed alloplastic bone graft material, Osteon II Collagen, which contains 70% HA and 30% β-TCP, two biomaterials closely related to the major mineral components of human bone.5 The purpose of using graft material impregnated with collagen is to increase the osteoconductivity of Osteon II Collagen.18 In addition, this biomaterial is granular, porous, and contains both Ca and P which favour the early phases of bone repair. The application of biomaterials to bone defects should allow for osteoblasts to build bridges between granular cells and integrate with other osteoblasts to provide support for both the proliferation and differentiation of cells at the early phases of bone repair. This subsequently results in the intrinsic stimulation of new bone formation.19 The development of post-operative infections was avoided by carrying out a sterile surgical procedure and via the systematic administration of antibiotics during the post-operative care period. Moreover, the use of high concentration hyaluronic acid combined with biomaterials provided the greatest bacteriostatic effect against Aggregatibacter actinomycetemcomitans, Prevotella, and Staphylococcus aureus, three bacterial strains commonly found in oral gingival lesions and periodontal wounds.20 The combination of materials was hypothesised to provide a superior modulation of the repair process of the bone defects. As such, the clinical application of HA gels during surgery have a tendency to reduce the bacterial contamination of the surgical wound site, thereby reducing the risk of post-surgical infection and promoting a more predictable bone generation.
Resorbable collagen membranes are commercially available and can be used alone or in combination with other materials, such as BMP.21 In the present study, collagen membranes were used to act as a scaffold to prevent the apical migration of the epithelium and support new connective tissue attachment and tissue regeneration.22 The application of a collagen membrane with bone substitute materials to fill bone defects was evaluated with the aim of mimicking or potentially accelerating the normal process of bone formation to facilitate wound healing.23 This is the first study to assess and compare the effectiveness of combining hyaluronic acid and a collagen membrane with Osteon II Collagen for the repair of bone defects.
Histopathological analysis showed that the Osteon II Collagen with hyaluronic acid group had greater osteogenic potency than the Osteon II Collagen with collagen membrane group; however, these two groups both had greater osteogenic potency than the Osteon II Collagen alone and control groups. These results are correlate with those of Aslan et al., which demonstrated that Osteon II Collagen with hyaluronic acid has a histologically superior bone healing capacity.24
After the first week post-operation, no significant differences were observed between the four experimental groups, although the Osteon II Collagen with hyaluronic acid group and the Osteon II Collagen with collagen membrane group showed enhanced bone formation associated with increased bone healing. At the end of the second post-operative week, we observed significant increases in bone formation in all the experimental groups. At the end of the fourth week, the Osteon II Collagen with hyaluronic acid group showed the greatest amount of bone formation compared to the other experimental groups. These results correlated with those of Kim et al., which demonstrated the importance of osteoconductive and osteoinduction processes. Hyaluronic acid and grafting significantly enhanced the healing process when used both alone or together due to increased morphogenesis and tissue healing during bone regeneration.13 When hyaluronic acid comes into contact with bone it participates in bone morphogenesis and plays an important part in the early events of the osteogenic process,24 modifying the effects of several growth factors and cytokines. Moreover, HA also promotes bone formation in a way similar to osteogenic substrates, such as bone morphogenic protein and calcitonin. Additionally, it binds to proteins including fibrinogen, fibrin, fibronectin, and collagen, which are essential for wound healing.25
Aslan et al.24 compared the effects of autologous bone grafting with or without HA in a rabbit tibia defect model and reported that HA requires an osteoconductive scaffold to be effective. Our results are in agreement with this and other reports which show that the application of hyaluronic acid significantly accelerates the healing process, where HA was associated with superior early radiological bone healing compared with the bone grafting alone group, especially in the short term.26 Similarly, in 2016, Kim et al.27 found out that HA could increase bone formation and accelerate wound healing when applied to fresh extraction sockets that had been previously infected. On the other hand, in 2018, Osman et al. investigated the use of high molecular weight hyaluronic acid combined with xenografts in rabbit calvarial bone defects to determine bone healing capacity and found that it does not appear to have a significant effect on the structure of the bone trabeculae when compared to bone defects filled with xenograft alone. Regardless of these results, they concluded that HA had a slightly superior effect on the quality of the trabecular network in newly formed bone.28 The results obtained in this study support our hypothesis that HA can positively affect bone healing and result in new bone formation in a short period of time.
Regarding the effect of the collagen membrane, the resulting greater bone formation in the Osteon II Collagen with collagen membrane group at the end of four weeks could be explained as a result of the action of the collagen membrane in preventing non-osteogenic cell proliferation at the site of bone formation.29 Resorbable collagen membranes are frequently used as wound dressings because they act as a scaffold, promote platelet aggregation, stabilise clots, and attract fibroblasts, allowing for a faster wound healing; therefore, these membranes are often used for GBR.6 In a previous study, a collagen membrane was used as a scaffold for bone deposition in guided bone regeneration (GBR) to facilitate wound healing.29 Thus, the improved bone formation in this experimental group is likely to be closely linked with the increased number of osteoblasts and the increased deposition of collagen, considered an important precursor of mineral matrix deposition. The histological results of this study confirmed previous radiological analysis studies investigating the local application of HA and collagen membranes with a bone substitute material (Osteon II Collagen) and showed enhanced bone regeneration via clear radiographical findings of increased bone formation and bone density compared to the control and Osteon II Collagen alone groups.30 The results obtained for the HA and collagen membrane groups after 2 and 4 weeks post-operation support our hypothesis that HA and collagen membranes are able to induce new bone formation, most likely by increasing the osteoinductive effect of Osteon II Collagen.
Conclusion
Our findings suggest that the use of hyaluronic acid and a collagen membrane in a grafted bone defect with biphasic synthetic micro-granular HA + β-TCP + bovine type I collagen (Osteon II Collagen) was more effective in positively modulating the healing process in the tibia of rabbits than grafted bone defects without biomaterials. The results of bone defects grafted with a combination of hyaluronic acid and biomaterials showed the best outcome, with a more rapid bone formation of higher quality bone than the other experimental groups.
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
The animal protocol used in this study was applied and evaluated according to the principles of the Guide for the Care and Use of Laboratory Animals approved by Veterinary Collage of Duhok University Institutional Laboratory Animal Care and Utilization Committee, Iraq.
Authors' contributions
The study design, animal laboratory experiments, and the writing of the manuscript, as well as the critical revision of this article, were carried out at the Institutional Committee of Veterinary Collage of Duhok University. The interpretation of statistical analysis was conducted by SS. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
The authors express their great appreciation to Duhok University (formerly Veterinary Collage) for providing the opportunity to complete this research. The authors express their sincere thanks to all histopathological professional staff in the General Central Laboratory and Duhok Health Department who participated in this study and for their assessment of our results.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Oda T., Kinshita, Ueda M. Effect of cortical bone perforation on periosteal destruction. J Maxillofac Surg. 2009;67(7):1478–1485. doi: 10.1016/j.joms.2008.06.085. [DOI] [PubMed] [Google Scholar]
- 2.Hoexter D.L. Bone regeneration graft materials. J Oral Implantol. 2002;28(6):290–294. doi: 10.1563/1548-1336(2002)028<0290:BRGM>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Salgado A.J., Coutinho O.P., Reis R.L. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 4.Habibovic P., Gbureckc U., Doillond C.J., Bassetta D.C., Blitterswijkb C.A., Barrale J.E. Osteoconduction and osteoinduction of low-temperature 3D printed bioceramic implants. Biomaterials. 2008;29:944–953. doi: 10.1016/j.biomaterials.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Kim Y.K., Yun P.Y., Lim S.C., Kim S.G., Lee H.J., Ong J.L. Clinical evaluation of Osteon as anew alloplastic material in sinus bome grafting and its effect on bone healing. J Biomed Mater Res B Appl Biomater. 2008;86:270–277. doi: 10.1002/jbm.b.31015. [DOI] [PubMed] [Google Scholar]
- 6.Luitaud C., Laflamme C., Semlali A. Development of an engineering autologous palatal mucosa-like tissue for potential clinical applications. J Biomed Mater Res B Appl Biomater. 2007;83:554–561. doi: 10.1002/jbm.b.30828. [DOI] [PubMed] [Google Scholar]
- 7.Yamada M., Kubo K., Ueno T., Iwasa F., Att W., Hori N., Ogawa T. Alleviation of commercia collagen sponge- and membrane-induced apoptosis and dysfunction in cultured osteoblasts by an amino acid derivative. Int J Oral Maxillofac Implants. 2010;25:939–946. [PubMed] [Google Scholar]
- 8.Zhang Y.G., Yang Z., Guo X., Xu P. A new method for inducing bone tissue regeneration: negative pressure membrane technology. Med Hypotheses. 2009;73:906–909. doi: 10.1016/j.mehy.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 9.Taschieri S., Corbella S., Tsesis I., Bortolin M., Del Fabbro M. Effect of guided tissue regeneration on the outcome of surgical endodontic treatment of through-and-through lesions: a retrospective study at 4-year follow-up. J Oral Maxillofac Surg. 2011;15:153–159. doi: 10.1007/s10006-011-0272-y. [DOI] [PubMed] [Google Scholar]
- 10.Bansal J., Kedige S.D., Anand S. Hyaluronic acid: a promising mediator for periodontal regeneration. Indian J Dent Res. 2010;21(4):575–578. doi: 10.4103/0970-9290.74232. [DOI] [PubMed] [Google Scholar]
- 11.Gilsanz V., Roe T.F., Gibbens D.T., Schulz E.E., Carlson M.E., Gonzalez, Boechat M.I. Effect of sex steroid peak bone density of growing rabbits. Am J Physiol. 1988;255(4 Pt 1):E416–E421. doi: 10.1152/ajpendo.1988.255.4.E416. [DOI] [PubMed] [Google Scholar]
- 12.Wang X., Mabrey J.D., Agrawal C.M. An interspecies comparison of bone fracture properties. Bio Med Mater Eng. 1998;8(1):1–9. [PubMed] [Google Scholar]
- 13.Kim J.M., Kim M.H., Kang S.S., Kim G., Choi S.H. Comparable bone healing capacity of different bone graft matrices in a rabbit segmental defect model. J Vet Sci. 2013;15(2):289–295. doi: 10.4142/jvs.2014.15.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucaciu O., Gheban D., Soriţau O., Băciuţ M., Câmpian R.S., Băciuţ G. Comparative assessment of bone regeneration by histometry and a histological scoring system. Revista Română de Medicină de Laborator. 2015;23(1):31–45. [Google Scholar]
- 15.Dimitriou R., Jones E., McGonagle D., Giannoudis P.V. Bone regeneration: current concept and future directions. BMC Med. 2011;9:66. doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahata M., Ito M., Abe Y., Abumi K., Minami A. The effect of anti-resorptive therapies on bone graft healing in an ovariectomized rat spinal arthrodesis model. Bone. 2008;43:1057–1066. doi: 10.1016/j.bone.2008.08.124. [DOI] [PubMed] [Google Scholar]
- 17.Shayesteh Y.S., Khorsand A., Motahhary P., Dehghan M., Ardestani M.S. Evaluation of platelet-rich plasma in combination with deproteinized bovine bone mineral in the rabbit cranium; a pilot study. J Dent. 2005;2(4):127–134. [Google Scholar]
- 18.Keating J.F., McQueen M.M. Substitutes for autologous bone graft in orthopedic trauma. J Bone Joint Surg Br. 2001;83(1):3–8. doi: 10.1302/0301-620x.83b1.11952. [DOI] [PubMed] [Google Scholar]
- 19.Soares L.G.P., Marques A.M.C., Guarda M.G., Aciole J.M.S., Santos J.N., Pinheiro A.L.B. Influence of the λ780 nm laser light on the repair of surgical bone defects grafted or not with biphasic synthetic micro-granular hydroxylapatite + beta-calcium triphosphate. J Photochem Photobiol B Biol. 2014;131:16–23. doi: 10.1016/j.jphotobiol.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Pirnazar P., Wolinsky L., Nachnani S., Haake S., Pilloni A., Bernard G.W. Bacteriostatic effects of hyaluronic acid. J periodontal. 1999;70(4):370–374. doi: 10.1902/jop.1999.70.4.370. [DOI] [PubMed] [Google Scholar]
- 21.Spagnoli D., Choi C. Extraction socket grafting and buccal wall regeneration with recombinant human bone morphogenetic protein-2 and acellular collagen sponge. Atlas Oral Maxillofac Surg Clin North Am. 2013;21:175–183. doi: 10.1016/j.cxom.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Gazdag A.R., Lane J.M., Glaser D., Forster R.A. Alternatives to autogenous bone graft: efficacy and indications. J Am Acad Orthop Surg. 1995;3(1):1–8. doi: 10.5435/00124635-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Dimitriou R., Mataliotakis G.I., Calori G.M., Giannoudis P.V. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: current experimental and clinical evidence. BMC Med. 2012;10:81. doi: 10.1186/1741-7015-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aslan M., Simsek G., Dayi E. The effect of hyaluronic acid-supplemented bone graft in bone healing: experimental study in rabbits. J Biomater Appl. 2006;20(3):209–220. doi: 10.1177/0885328206051047. [DOI] [PubMed] [Google Scholar]
- 25.Stern M., Schmidt B., Dodson T.B., Stern R., Kaban L.B. Fetal cleft lip repair in rabbits: histology and role of hyaluronic acid. J Oral Maxillofac Surg. 1992;50:263–269. doi: 10.1016/0278-2391(92)90323-r. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes N.P., Hunt J.A., Longinotti C., Pavesio A. In vivo characterization of Hyalonect, a novel biodegradable surgical mesh. J Surg Res. 2011;168(1):e31–e38. doi: 10.1016/j.jss.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.J., Song H.Y., Ben Amara H., Kyung-Rim K., Koo K.T. Hyaluronic acid improves bone formation in extraction sockets with chronic pathology: a pilot study in dogs. J Periodontol. 2016;87(7):790–795. doi: 10.1902/jop.2016.150707. [DOI] [PubMed] [Google Scholar]
- 28.Osman F.A., İbrahim D., Ahmet A., Ufuk T., Ahmet G. To what extent does hyaluronic acid affect healing of xenografts? A histomorphometric study in a rabbit model. J Appl Oral Sci. 2018;26:e20170004. doi: 10.1590/1678-7757-2017-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunyaratavej P., Wang H.L. Collagen membranes: a review. J Periodontol. 2001;72(2):215–229. doi: 10.1902/jop.2001.72.2.215. [DOI] [PubMed] [Google Scholar]
- 30.AL Mukhtar Yusra H., Abid Wafaa K. Effect of osteon II collagen with hyaluronic acid and collagen membrane on bone Healing process in rabbits: a radiograghical study. International Journal of Enhanced Research in Science, Technology & Engineering. 2016;5(8):36–46. [Google Scholar]