Abstract
Objectives:
Quality of Life (QoL) at baseline is frequently found to be a prognostic factor in cancer studies. However, little is known about the relationship of the trajectory of QoL and survival in patients with advanced cancer. This study evaluates the effects of both level and change of QoL on survival to explore the potential of utilizing longitudinal information of QoL for prognosis.
Methods:
A series of joint models were used in a sample (N=512) of patients diagnosed with advanced cancer (sample consisted of nine different cancer sites) with assessments of QoL across six time points and with survival information recorded up to 28 months after diagnosis. We used FACT-G as the QoL measure, and we evaluated the effects of change in QoL controlling for the time-dependent effects of chemotherapy and radiation.
Results:
The median survival for patients was 14.2 months, and 10% of the sample had survived beyond 28 months after the diagnosis of advanced cancer. The effect of change of QoL on survival was significant (hazard ratio = 0.98; p < 0.001) controlling for time-dependent treatment effects. Also, the slope of the trajectory in QoL was found to be a significant predictor of survival (hazard ratio = 0.18; p < 0.001).
Conclusion:
preliminary findings suggest that the patient’s longitudinal experience in QoL may be a significant prognostic factor of survival, a novel finding with potentially important implications in medical decision making. Longitudinal information on QoL can be used for updating the patient’s prognosis of survival.
Introduction
In the last two decades, we have witnessed an evolution of end-of-life care and treatment models in cancer focusing on the integration of life-extending treatments with palliation and quality of life (QoL) considerations [1–6]. Epitomized by recent studies, QoL is increasingly viewed not merely as an end in itself but as a predictor of survival [6–10]. This prognostic function of QoL on survival has been shown in the literature to hold for a variety of cancer sites [7,11–14] and QoL measures [8] even after adjusting for known prognostic clinical variables [6,8,9]. Therefore, the adoption of QoL measures as prognostic tools in estimating survival in cancer patients has been consistently encouraged [6–8].
Undoubtedly, the illness experience of cancer patients is a highly complex time-dependent process. However, it has been shown that QoL is modestly associated with past clinical history of the disease [5] and strongly associated with the patient’s perception of the disease, including how they make sense of an advanced-stage cancer diagnosis, how they perceive the physical symptoms of the disease, and how they evaluate their functional and psychosocial well-being [15,16]. Understanding that subjective QoL evaluation in time reflects real clinical information which is crucial in providing care to patients with advanced stage cancer. This subjective evaluation may provide insight into the illness itself and how it progresses and can be utilized to improve treatment decisions. In addition, if the clinician observed divergence of QoL evaluations and disease history, steps may be taken to align perceptual understanding with disease severity and improve adherence to treatment and other illness behaviors [17].
This plethora of studies concerning the link of QoL and survival, even if they all emphasize the predictive significance of QoL, do not explicate the whole relationship in a single picture. There are several reasons for this. First, the great majority of these studies employ only baseline measures of QoL as predictors of survival, assuming implicitly that the effects are constant, and overlooking the potential dynamic relationship of QoL and survival. Second, studies [18–21] that do employ longitudinal measures do not take full advantage of individual-level methods of analysis, such as random effects models, to estimate patient-specific trajectories and the effects of these trajectories on survival. Third, QoL has been conceptually understood and analytically employed as a predictor of survival without acknowledging the endogenous nature of the relationship between QoL and survival (exogeneity in this case is the assumption that the level of QoL at any given time is independent of the probability of death, apparently a non-valid assumption in this context).
The primary aim of this study is to unmask the relationship of QoL with the probability of death as they co-evolve in time. Knowledge of this relationship can be used to make dynamic patient-specific predictions of survival based both on the patient’s level of QoL and on patient’s change in QoL after diagnosis and at any point in the patient’s illness history. Moving beyond traditional logistic and Cox regression models, we employ a joint modeling approach, as suggested by Ibrahim et al., [22] to improve our understanding of the dynamic relationship between QoL and survival.
Data and methods
Patients
All participants were enrolled in a randomized controlled trial to test the effects of a coping and communication support intervention for advanced cancer patients [23,24]. Participants received their care at the MetroHealth Medical Center or Veterans Affairs Hospital in Cleveland, Ohio and they were followed for approximately 28 months after enrollment in the study. Approximately 71% of eligible patients were enrolled in the study, with recruitment occurring on average 9 to 10 weeks following the diagnosis of advanced cancer. Inclusion criteria were: age > 40 years, recent diagnosis of advanced stage cancer, not yet referred to hospice, cognitively intact, and able to speak English. The original sample consisted of 576 patients diagnosed with advanced stage cancer. Fifty of them had missing data in QoL at baseline and were not included in the analysis. In addition, 14 patients were also excluded from the analysis because, although they died within the study period, their exact time of death was not known. The final analytical sample consisted of 512 participants.
Measurements
We used measures from baseline interviews on patients’ age, gender, race, whether the patient was living alone, whether the specific cancer diagnosis was a recurrence, education in years, hospital site, and receipt of chemotherapy and radiation. In all our models, we adjusted for intervention status and time since diagnosis. Receipt of radiation and chemotherapy was also assessed and used in the analysis throughout the following five waves of data collection (3 months, 6 months, 12 months, 18 months, and 24 months).
We employed the Functional Assessment of Cancer Therapy-General (FACT-G) scale to assess QoL though telephone interviews at all six waves. The FACT-G [25] is a well-validated, 28-item, QoL measure for cancer patients with any tumor type. Each item is scored from 0 to 4 (anchored from ‘not at all’ to ‘very much’). FACT-G is a sum of four sub-scales: Functional Well-Being (7 items), Physical Well-Being (7 items), Social Well-Being (7 items), and Emotional Well-Being (6 items). FACT-G can range from 0 to 108 with higher scores in this scale indicating higher Quality of Life (QoL).
Statistical analysis
We fitted a series of models at two stages. In the first stage we fitted (a) a traditional Cox proportional hazard model where all covariates were assumed to be time independent, (b) an extended Cox model where QoL, receipt of chemotherapy, and receipt of radiation are treated as time-dependent covariates, and (c) a linear random effects model to estimate the patient specific trajectory of QoL to be used as a predictor in a survival model. At the second stage, we fitted three joint models combining the longitudinal submodel of QoL having a random intercept and a random slope with a survival submodel using three different param-eterizations. The longitudinal model included the same set of covariates as the Cox models. Joint models are designed to capture the simultaneous parallel relationship of two processes, in this case QoL and survival. A joint model produces a parameter which indicates the strength of the association of the two processes at any point in time.
We applied the joint modeling framework [26,27] because (a) QoL contains temporal variation between measurements which is not captured by survival models with time-dependent covariates, and (b) because QoL is not exogenous to the probability of death (the value of QoL is related to the probability of death), an assumption of standard survival models. To overcome these shortcomings of the standard survival models that may substantially bias the results [28,29], we estimate the true individual trajectory of QoL through a random effects model and then use this ‘true’ trajectory as a time-dependent covariate in a Cox model instead of the observed QoL measurement. In the first joint model we estimated the association parameter of the ‘true’ QoL level at time t and the risk of death at the same point in time. The second joint model is a modification of the first, and it involves estimating a parameter of the change (instead of level) in QoL of an individual and the risk for death. The final model extends the second by including both the ‘true’ level and change in QoL, and by handling the two types of treatment (chemotherapy and radiation) as time-dependent variables. We used a spline approximation of the log baseline risk function with five knots placed at equally spaced percentiles of the observed event times for all three joint models and a linear random effects model to estimate the QoL trajectory. We used R 3.0.1 (R foundation for Statistical Computing, Vienna, Austria) for both data management and data analysis. We used the ‘survival package’ for the survival models, the ‘nlme’ for the random effects models, and the ‘JM package’ for fitting the joint models [30].
Results
Sample characteristics
Demographic variables are presented for the analytical sample in Table 1. The majority of the participants were male, non-black, were living with someone, and for most of the participants, this diagnosis was not considered a recurrence. All participants had a median life expectancy of 14.2 months or less. There were multiple types of cancer represented, including lung 35.5%; gastrointestinal 23.2%; genitourinary 12.5%; head and neck 6.6%; breast 6.8%; and hematologic 2.9%.
Table 1.
Patient characteristics at baseline and median survival
| Nominal | Levels | n | % |
|---|---|---|---|
| Female | No | 345 | 67.4 |
| Yes | 167 | 32.6 | |
| Black | No | 334 | 65.2 |
| Yes | 178 | 34.8 | |
| Living alone | No | 360 | 70.3 |
| Yes | 152 | 29.7 | |
| Hospital | MH | 286 | 55.9 |
| VA | 226 | 44.1 | |
| Intervention | No | 253 | 49.4 |
| Yes | 259 | 50.6 | |
| Recurrence | No | 435 | 85.0 |
| Yes | 77 | 15.0 | |
| Chemotherapy | No | 191 | 37.3 |
| Yes | 321 | 62.7 | |
| Radiatior | No | 388 | 75.8 |
| Yes | 124 | 24.2 | |
| Cancer site | |||
| Brain | 8 | 1.6 | |
| Breast | 35 | 6.8 | |
| Connective tissue | 12 | 2.3 | |
| Gastrointestinal | 119 | 23.2 | |
| Genitourinary | 64 | 12.5 | |
| Gynecologic | 28 | 5.5 | |
| Head and neck | 34 | 6.6 | |
| Hematologic | 15 | 2.9 | |
| Lung | 182 | 35.5 | |
| Unknown | 15 | 2.9 | |
| Continuous | Mean | SD | |
| Age | 61.1 | 10.2 | |
| Education | 12.8 | 2.3 | |
| Months since diagnosis | 3.1 | 3 | |
| Median survival 14.2 months | |||
| N | 512 | ||
Over the 28-month period of study, approximately 10% (54) had survived and are treated as censored in the survival models (Table 2). Chemotherapy was more frequently used in all waves as compared to radiation, and its prescription declined throughout the study from 63% of the sample receiving chemotherapy at baseline to 35% of the sample at wave 6 (Table 2).
Table 2.
Descriptive statistics for chemotherapy and radiation, and QoL for each wave
| Levels | Baseline | 3 months | 6 months | 12 months | 18 months | 24 months | |
|---|---|---|---|---|---|---|---|
| Categorical variables | % (count) | ||||||
| Chemotherapy | No | 37 (191) | 43 (170) | 50 (151) | 62 (105) | 68 (89) | 65 (40) |
| Yes | 63 (321) | 57 (227) | 50 (151) | 38 (64) | 32 (42) | 35 (22) | |
| Radiatior | No | 76 (388) | 79 (315) | 91 (274) | 89 (151) | 88 (115) | 85 (53) |
| Yes | 24 (124) | 21 (82) | 9 (28) | 11 (18) | 12 (16) | 15 (9) | |
| Longitudinal process | Mean (SD) | ||||||
| QoL | 68.1 (16.5) | 66.7 (16.6) | 68.9 (17.3) | 70.4 (16.6) | 71.4 (15.8) | 74.3 (14.8) | |
| N | 512 | 397 | 302 | 169 | 131 | 62 | |
Survival analysis
At the initial stage of the analysis, we fitted two relative risks models: First, a Cox regression model with all covariates constant, including quality of life, measured at baseline only; and second, a Cox regression model with QoL exerting time-dependent effects on the hazard of dying, and controlling for the time-dependent effects of chemotherapy and radiation (Note: hazard ratios described in the text are the reciprocal exponentiated log coefficients shown in the tables). Table 3 presents the results of the two models. In the conventional Cox regression we observed that receipt of chemotherapy at baseline was associated with an increase in the risk of death by about 30% (hazard ratio = 1.29). Interestingly, QoL did not have a significant effect on the hazard of dying in the first Cox model. In contrast, in the model specified with time-dependent treatment and QoL effects, we observed that at any point in time, receipt of chemotherapy was positively related to the risk of dying by 43% (hazard ratio = 1.43) and receipt of radiation by 44% (hazard ratio= 1.44), and for each unit increase in QoL the risk of dying was decreased by almost 2% (hazard ratio = 0.98).
Table 3.
Results of the two survival models: with time-independent and time-dependent covariates
|
Cox PH |
Cox PH with TD Cov. |
|||||
|---|---|---|---|---|---|---|
| Estimate | Std. err |
p-Value | Estimate | Std. err |
p-Value | |
| Age | 0.01 | 0.01 | 0.083 | 0.01 | 0.01 | 0.010 |
| Female | −0.16 | 0.13 | 0.223 | −0.22 | 0.14 | 0.132 |
| Black | −0.08 | 0.11 | 0.463 | −0.05 | 0.11 | 0.634 |
| Educatlor | −0.01 | 0.02 | 0.597 | 0.00 | 0.02 | 0.908 |
| Living alore | 0.21 | 0.11 | 0.060 | 0.07 | 0.12 | 0.544 |
| Wks. from diagnosis | −0.02 | 0.01 | 0.003 | −0.02 | 0.01 | 0.001 |
| Site (VA) | −0.18 | 0.14 | 0.198 | −0.19 | 0.14 | 0.174 |
| Intervention | −0.03 | 0.10 | 0.768 | 0.01 | 0.10 | 0.911 |
| Recurrent cancer | 0.08 | 0.16 | 0.595 | 0.07 | 0.16 | 0.675 |
| Chemotherapy | 0.26 | 0.12 | 0.022 | 0.36 | 0.11 | 0.001 |
| Radiation | −0.09 | 0.12 | 0.492 | 0.37 | 0.13 | 0.002 |
| QoL | 0.00 | 0.00 | 0.231 | −0.02 | 0.00 | <0.001 |
Joint models of survival and longitudinal processes
At this stage of the analysis, we estimated three different joint models of QoL and survival: first, a joint model assessing the degree of dependence: (a) of the risk of death at a point in time t with QoL at the same time point; (b) between change in QoL, captured by a random effects model, and the risk of death; and (c) between both the level and change of QoL on the risk of death. Table 4 presents the results of the three models in the same sequence enumerated above. Receipt of chemotherapy had a significant effect on survival in all models. The highest dependency was noted in the third model where receipt of chemotherapy increased the risk of dying by 53% (hazard ratio = 1.53).
Table 4.
Parameter estimates, standard errors, and p-values for three joint models
| Joint Model 1 |
Joint Model 2 |
Joint Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. err | p-Value | Estimate | Std. err | p-Value | Estimate | Std. err | p-Value | |
| Age | 0.01 | 0.01 | 0.015 | 0.01 | 0.01 | 0.341 | 0.02 | 0.01 | 0.020 |
| Female | −0.17 | 0.13 | 0.208 | −0.12 | 0.17 | 0.497 | −0.14 | 0.18 | 0.433 |
| Black | −0.08 | 0.11 | 0.483 | −0.07 | 0.14 | 0.611 | −0.10 | 0.15 | 0.511 |
| Educatlor | 0.00 | 0.02 | 0.963 | 0.00 | 0.03 | 0.873 | 0.02 | 0.03 | 0.570 |
| Living alore | 0.19 | 0.11 | 0.086 | 0.32 | 0.14 | 0.028 | 0.37 | 0.16 | 0.017 |
| Wks from diagnosis | −0.02 | 0.01 | 0.004 | −0.03 | 0.01 | 0.000 | −0.03 | 0.01 | 0.000 |
| Site (VA) | −0.20 | 0.14 | 0.143 | −0.15 | 0.18 | 0.378 | −0.24 | 0.19 | 0.193 |
| Control | −0.02 | 0.10 | 0.830 | −0.03 | 0.13 | 0.799 | −0.04 | 0.14 | 0.772 |
| Recurrent cancer | 0.05 | 0.16 | 0.727 | 0.21 | 0.20 | 0.298 | 0.18 | 0.21 | 0.406 |
| Chemotherapy | 0.26 | 0.12 | 0.023 | 0.34 | 0.15 | 0.021 | 0.43 | 0.16 | 0.008 |
| Radiation | −0.13 | 0.13 | 0.318 | −0.11 | 0.16 | 0.501 | −0.24 | 0.17 | 0.166 |
| QoL level | −0.02 | 0.00 | 0.001 | −0.03 | 0.01 | 0.002 | |||
| Qol change | −1.86 | 0.34 | <0.001 | −1.68 | 0.34 | <0.001 | |||
| AIC | 16,143.53 | 16,118.25 | 16,108.32 | ||||||
| BIC | 16,296.67 | 16,271.38 | 16,265.71 | ||||||
| −Log.lik | −8035.764 | −8023.123 | −8017.16 | ||||||
QoL was associated with the risk of death both in terms of level of QoL presented in models 1 and 3, and in terms of change of QoL presented in models 2 and 3. According to the model fit criteria AIC (Akaike Information Criterion) and BIC (Bayesian Information Criterion) model 3 had the best fit (lower values of both AIC and BIC indicate better model fit). Looking at the third joint model, each one unit increase in QoL decreased the risk of death by 3% (hazard ratio = 0.97). More noticeable was the significant effect of the change of QoL on the risk of death controlling for the level of QoL. For each unit increase in the trajectory of QoL the risk of death decreased by about 82% (hazard ratio = 0.18).
Figure 1 (supplement) presents the joint relationship captures in our models. The survival probabilities of a randomly selected patient are dynamically updated (right side of each plot) because of new measurements in QoL that affect the overall trajectory in the patient’s QoL.
Figure 1.
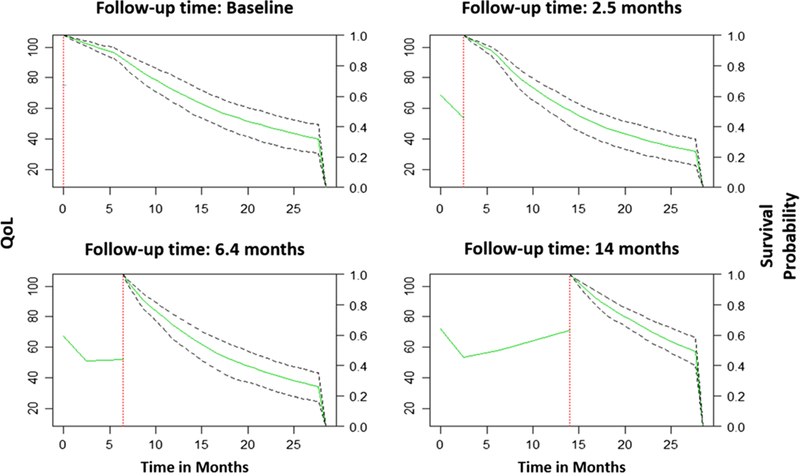
Conditional survival probabilities at each time point for a randomly selected patient based on the best joint model. The vertical dotted line represents the time of the last QoL measurement. On the left of this line is the longitudinal trajectory of QoL up to this point, and on the right the solid line represents the median survival estimation of this specific patient and the dashed lines the 95% confidence interval
Discussion
We studied the relationship between QoL and survival dynamically and in greater detail that has been previously reported in the literature. We have conducted an advanced statistical analysis of the relationship of QoL and time to death relying on parameters estimated from a series of joint models, and from which less biased inferences can be drawn [22,31]. We estimated the relationship of baseline QoL on survival, the relationship between QoL at any point in time and survival, and through a joint modeling framework the relationship between change in QoL and survival. The consistent significant results of this relationship across models in this study strongly suggest that change in QoL of advanced stage cancer patients is a predictor of survival even after conditioning on the level of QoL. We found that, it is not whether patients with advanced-stage cancer have high or low QoL at baseline, but change in QoL that is associated with survival. Understanding the dynamic relationship of QoL and survival may aid in providing care that extends life while maintaining the QoL of the patient.
The finding that the specific patient QoL trajectory (whether increasing or decreasing) is strongly associated with survival independent of the time-specific level of QoL is a novel finding with potentially important implications in medical decision making in terms of refining and adapting interventions and treatment strategies for cancer patients. Our main contribution is that medical decision making can be improved by updating predictions of survival. Our main goal in this paper was to explore if changes in QoL can assist clinicians to make dynamic estimates of survival, at the individual level, and potentially modify treatment based on these updated estimates. QoL measures at diagnosis are already applied by clinicians to make survival predictions. In this paper we suggest that updating survival predictions based on longitudinal changes in QoL has two benefits: (a) dynamically updated predictions of survival, and (b) adaptation of disease management to reflect the updated predictions of survival. Combining medical expertise with the survival probability of a patient that takes into account the patient’s longitudinal trajectory in QoL would enable care providers to make better informed decisions and dynamic patient-specific predictions, and thus improve clinical outcomes. This can be achieved by updating the prognostic estimate for survival of a patient based on the totality of the patients QoL experience (level and change) and adapting treatment strategies to reflect this experience. Patients not only experience different levels of QoL but also their QoL changes in different ways in their disease trajectory. Explicating the nature of QoL experience and integrating it into an individualized treatment plan that addresses the course of the illness in a continuum may improve patient outcomes in terms of both QoL and survival. Even when the profile of the patient’s history does not present a continuous disease progression, as it may be the case when treated with multiple lines, knowledge of QoL change can be useful to identify the optimal treatment plan, which will benefit the patient most and at the same time being sensitive to patient preferences. This is especially critical in the care for advanced-stage cancer patients where the decision for tumor-shrinkage or tumor-stabilization therapy, maintenance, or palliation therapy is not easily reached [32].
Although not the central focus of this study, we should also note the finding of a downward significant trend in QoL after diagnosis derived from the joint model specification. The estimate of change in QoL in the joint models, which models the dropout through a shared parameter model [33], indicates that there is a significant decline in QoL in patients as they were experiencing their illness. This finding suggests that dropout because of death is not missing at random (NMAR), and it needs to be modeled [34]. The consequences of ignoring dropout because of death when investigating QoL issues may be detrimental to inference and prediction of survival [26], as well as, in QoL studies in general because, at least in this study, ignoring dropout biases the estimate of the QoL trajectory upward.
Our analyses have several important limitations. The generalizability of results is a consideration, because the patients with advanced cancer in this study were part of a randomized controlled trial that was testing a coping and communication support intervention over time. The great majority of patients were economically disadvantaged, and approximately 35% were African Americans. Whether these results would apply to other patient groups is not clear. Future analyses of this type would benefit from larger sample sizes and extended follow-up with patients who are living with advanced cancer. Replication and validation of the model are needed in different populations and clinical settings and using a variety of QoL measures. In this study we examined the QoL and survival relationship without stratifying by cancer type but at an aggregated level assuming homogeneity of effects on the basis that all patients were diagnosed with advanced stage cancer. It is possible that the longitudinal relationship between QoL and survival differs among cancer types, as it has been shown to be the case with QoL measured at baseline [7], and future work should investigate this. We also did not have available in our dataset a performance status measure, which has been shown to be related to survival. It is possible that some of the effects of QoL on survival may be accounted by performance status, although it has been shown that patient-reported QoL has unique contribution as a prognostic measure independent of performance status and other biomedical indicators [9]. In addition, although we conditioned on receipt of radiation and treatment longitudinally in the analysis, we did not explicitly test for the potential moderating or mediating effects of treatment in the QoL and survival relationship. Finally, the patients’ reports of QoL may not reflect real changes in their QoL but rather their perception of their QoL. Their perception may shift based on expectations, values and other internal to the patient processes (an adaptation named ‘response shift’) [35]. This a complex phenomenon, and we have not addressed it here.
The results of this study are consistent with the results of a limited number of studies that examine the relationship of change in QoL and survival [18,20,21,36]. However, the present study is the only one to our knowledge that addresses the endogenous relationship of QoL and survival through a joint modeling framework, and estimates the patients’ probability of survival based on the totality (level and change) of QoL experience. It has been suggested that integrating patient-reported outcomes (PRO) in the routine assessments of clinical symptoms and survival in clinical trials can aid in the understanding of clinical outcomes [37]. It is our hope that the modeling approach presented in this paper will enhance the understanding and interpretation of quality of life in longitudinal datasets, and generate findings that will be useful in both clinical trials and patient care. This is especially important in advanced cancer where QoL may become a priority for the patients over extending life [38], or in the realization that their illness is terminal [39].
References
- 1.Patel JD, Krilov L, Adams S et al. Clinical cancer advances: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol 2013; 53:129–160. [DOI] [PubMed] [Google Scholar]
- 2.Lipscomb J, Gotay CC, Snyder C. Outcomes Assessment in Cancer, Cambridge University Press: Cambridge, Mass, USA, 2005. [Google Scholar]
- 3.Kelley AS, Meier DE. Palliative care—a shifting paradigm. N Engl J Med 2010;363(8): 781–782. [DOI] [PubMed] [Google Scholar]
- 4.Efficace F, Osoba D, Gotay C, Sprangers M, Coens C, Bottomley A. Has the quality of health-related quality of life reporting in cancer clinical trials improved over time? Towards bridging the gap with clinical decision making. Ann Oncol 2007;18(4): 775–781. [DOI] [PubMed] [Google Scholar]
- 5.Temel JS et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363(8):733–742. [DOI] [PubMed] [Google Scholar]
- 6.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol 2008;26(8):1355–1363. [DOI] [PubMed] [Google Scholar]
- 7.Quinten C et al. A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 2013;120(2): 302–311. [DOI] [PubMed] [Google Scholar]
- 8.Montazeri A QoL data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes 2009;7(102):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sloan JA, Zhao X, Novotny PJ et al. Relationship between deficits in overall QoL and non-small-cell lung cancer survival. J Clin Oncol 2012;30(13):1498–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganz PA, Jack Lee J, Siau J. QoL assessment. An independent prognostic variable for survival in lung cancer. Cancer 1991;67(12): 3131–3135. [DOI] [PubMed] [Google Scholar]
- 11.Oskam IM, Irma M, Leeuw V-d et al. Prospective evaluation of health-related quality of life in long-term oral and oropharyngeal cancer survivors and the perceived need for supportive care. Oral Oncol 2013;49(5): 443–448. [DOI] [PubMed] [Google Scholar]
- 12.Shields LB, Choucair A, Ali K. Quality of life measures as a preliminary clinical indicator in patients with primary brain tumors. Surg Neurol Int 2013;4:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Østhus AA, Aarstad AKH, Olofsson J, Aarstad HJ. Prediction of survival by pretreatment health-related quality-of-life scores in a prospective cohort of patients with head and neck squamous cell carcinoma survival and HRQOL in HNSCC patients. JAMA Otolaryngol Head Neck Surg 2013;139(1):14–20. [DOI] [PubMed] [Google Scholar]
- 14.Tan A, Novotny P, Kaur J et al. A patient-level meta-analytic investigation of the prognostic significance of baseline quality of life (QOL) for overall survival among 3,704 patients participating in 24 North Central Cancer Treatment Group and Mayo Clinic Cancer Center oncology clinical trials. J Clin Oncol 2008;26(20 suppl):9515. [Google Scholar]
- 15.Leventhal H, Brissette I, Leventhal EA. In The Self-regulation of Health and Illness Behaviour, Cameron LD, Leventhal H (eds.), The common-sense model of self-regulation of health and illness. Psychology Press, 2003. [Google Scholar]
- 16.Buick DL. Illness representations and breast cancer: coping with radiation and chemotherapy. Percept Health IHn 1997;379–409. [Google Scholar]
- 17.Croom AR, Hamann HA, Kehoe SM, Paulk E, Wiebe DH. Illness perceptions matter: understanding quality of life and advanced illness behaviors in female patients with late-stage-cancer. J Support Oncol 2013;11(4):165–173. [DOI] [PubMed] [Google Scholar]
- 18.Eton DT, Fairclough DL, Cella D, Yount SE, Bonomi P, Johnson DH. Early change in patient-reported health during lung cancer chemotherapy predicts clinical outcomes beyond those predicted by baseline report: results from Eastern Cooperative Oncology Group Study 5592. J Clin Oncol 2003;21(8): 1536–1543. [DOI] [PubMed] [Google Scholar]
- 19.Gupta D, Braun DP, Staren ED. Prognostic value of changes in quality of life scores in prostate cancer. BMC Urol 2013;13(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun DP, Gupta D, Grutsch JF, Staren ED. Can changes in health related quality of life scores predict survival in stages III and IV colorectal cancer? Health Qual Life Outcomes 2011;9(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Digant G, Braun DP, Staren ED, Markman M. Longitudinal health-related quality of life assessment: implications for prognosis in ovarian cancer. J Ovarian Res 2013;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol 2010;28: 2796–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rose JH, Radziewicz R, Bowman KF, O’Toole EE. A coping and communication support intervention tailored to older patients diagnosed with late stage cancer. Clin Interv Aging 2008;3:77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radziewicz R, Rose JH, Bowman KF, Berila R, Spuckler A, O’Toole EE. Establishing treatment fidelity in a coping and communication support telephone intervention for aging advanced cancer patients and their family caregivers. Cancer Nurs 2009;32:193–202. [DOI] [PubMed] [Google Scholar]
- 25.Cella DF, Tulsky DS, Gray G et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570–579. [DOI] [PubMed] [Google Scholar]
- 26.Tsiatis A, Davidian M. Joint modeling of longitudinal and time to event data: an overview. Stat Sin 2004;14:809–834. [Google Scholar]
- 27.Rizopoulos D Dynamic predictions and prospective accuracy in joint models for longitudinal and time-to-event data. Biometrics 2011;67:819–829. [DOI] [PubMed] [Google Scholar]
- 28.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data (2nd ed.), John Wiley: New York, 2002. [Google Scholar]
- 29.Sweeting MJ, Thompson SG. Joint modelling of longitudinal and time-to-event data with application to predicting abdominal aortic aneurysm growth and rupture. Biom J 2011; 53(5):750–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizopoulos D JM: an R package for the joint modelling of longitudinal and time-to-event data. J Stat Softw 2010;35:11–33. [Google Scholar]
- 31.Prentice RL. Covariate measurement errors and parameter estimates in a failure time regression model. Biometrika 1982;69:331–342. [Google Scholar]
- 32.Fallowfield LJ, Fleissig A. The value of progression-free survival to patients with advanced-stage cancer. Nat Rev Clin Oncol 2011;9(1):41–47. [DOI] [PubMed] [Google Scholar]
- 33.Follmann D, Wu M. An approximate generalized linear model with random effects for informative missing data. Biometrics 1995;51(1): 151–168. [PubMed] [Google Scholar]
- 34.Little R, Rubin D. Statistical Analysis with Missing Data (2nd ed.), Wiley: New York, 2002. [Google Scholar]
- 35.Sprangers MAG, Sloan JA, Barsevick A et al. Scientific imperatives, clinical implications, and theoretical underpinnings for the investigation of the relationship between genetic variables and patient-reported quality-of-life outcomes. Qual Life Res 2010;19(10): 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernhard J, Dietrich D, Glimelius B et al. Estimating prognosis and palliation based on tumor marker CA 19–9 and quality of life indicators in patients with advanced pancreatic cancer receiving chemotherapy. Br J Cancer 2010;103(9):1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quinten C, Coens C, Mauer M et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol 2009;10(9):865–871. [DOI] [PubMed] [Google Scholar]
- 38.Meropol NJ, Egleston BL, Buzaglo JS et al. Cancer patient preferences for quality and length of life. Cancer 2008;113(12): 3459–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mack JW, Weeks JC, Wright AA, Block SD, Prigerson HG. End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 2010;28(7):1203–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]


