Abstract
Background
Hyperornithinemia–hyperammonemia–homocitrullinuria (HHH) syndrome is a rare disorder of urea cycle characterized by progressive pyramidal and cerebellar dysfunction, whose pathophysiology is not yet fully understood. Here we describe the spectrum of the long fibers involvement in HHH syndrome, attempting a correlation between clinical, electrophysiological and neuro-radiological data.
Methods
Nine HHH patients were longitudinally evaluated by clinical examination, neurophysiological assessment including motor (MEPs), somato-sensory evoked potentials (PESS) and nerve conduction velocity (NCV), brain and spinal cord MRI
Results
All patients had pyramidal dysfunction and 3/9 an overt spastic paraplegia. Mild to moderate cerebellar signs were found in 7/9, intellectual disability in 8/9. At lower limbs, MEPs resulted abnormal in 7/8 patients and PESS in 2/8; peripheral sensory-motor neuropathy was found in 1/9. MRI documented atrophic changes in supra-tentorial brain regions in 6/9 patients, cerebellum in 6/9, spinal cord in 3/7.
Conclusions
A predominant corticospinal dysfunction is evident in HHH syndrome, along with milder cerebellar signs, intellectual disability of variable degree and rare peripheral neuropathy. Phenotypical similarities with other disorders affecting the urea cycle (argininemia and pyrroline-5-carboxylate synthetase deficiency) suggest possible common mechanisms contributing in the maintenance of the corticospinal tract integrity. HHH syndrome phenotype largely overlaps with complex Hereditary Spastic Paraplegias (HSPs), in the list of which it should be included, emphasizing the importance to screen all the unsolved cases of HSPs for metabolic biomarkers.
Keywords: HHH syndrome, Hereditary spastic paraplegia, Urea cycle defects, Ornithine
Background
Hyperornithinemia–hyperammonemia–homocitrullinuria (HHH) syndrome (OMIM # 238970), caused by mutations in ORNT1 (SLC25A15) gene, is a rare autosomal recessive disorder of the urea cycle [1, 2]. The metabolic triad of hyperammonemia, hyperornithinemia and homocitrullinuria establishes the diagnosis. Clinical manifestations are widely heterogeneous, ranging from neonatal life-threatening hyperammonemic crises to milder forms with onset at variable ages. Once the treatment is started, the clinical course is usually stable, but a progressive lower limb spasticity and a cerebellar dysfunction appear in most cases, leading to mild gait disturbance up to loss of deambulation [3–7]. Intellectual disability of variable degree and, more rarely, focal dystonia and myoclonus may also occur [3–6]. Pathophysiology of pyramidal and cerebellar dysfunction remains to be elucidated. To date, neurophysiological and neuroimaging studies have been conducted only in few patients. Somato-sensory evoked potentials (SSEPs) and peripheral nerve conduction velocity (NCV) were occasionally found to be abnormal [6]. Motor evoked potentials (MEPs) have been rarely performed and, despite pyramidal dysfunction is presumed to be progressive, no longitudinal studies have been reported so far [4, 5]. Similarly, available neuroimaging data are mostly based on anecdotal descriptions [5, 8–12]. Herein we describe neurological, neurophysiological and neuroradiological findings, collected over a 15-year follow-up, in 9 HHH patients. We aimed to correlate long motor and sensitive tracts and peripheral nervous system involvement with neuroimaging and neuro-functional outcome data.
Patients and methods
We report on 9 patients (5 female, 4 male; mean age at last examination 24.4 ± 16.3 years; range 7–52.6), with genetically confirmed HHH syndrome, regularly followed at the Division of Metabolism of the Bambino Gesù Children’s Hospital in Rome. The early course and molecular abnormalities of 8/9 patients have been reported in previous studies [3, 5, 12–15]. Clinical features are summarized in Table 1. Follow-up had a mean duration of 15.9 ± 12.6 years (range 3–34 years) and encompassed clinical, neurological, neurophysiological and neuro-radiological data, retrospectively reviewed and prospectively integrated whenever necessary.
Table 1.
Main clinical and genetic features in nine patients with HHH syndrome
| Pt Gender (age at last evaluation) |
Genotype | Onset | Diagnosis | Previous coma | Previous lethargy | Seizures/ myoclonus | Intellectual disabilty | Adaptive functions | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Daily activity | Employment/ scholarization | |||||||||
|
1 male (53 years) |
79G > A/79G > A G27R/G27R |
2 yrs | 41 yrs | + | + | – | borderline | 1 | + (1) | [3, 12] |
|
2 male (44 years) |
535C > T/535C > T R179X/R179X |
12 yrs | 26 yrs | – | + | + | mild | 2 | none (2) | [3, 5] |
|
3 male (35 years) |
861insG/861insG S90X/S90X |
14 d | 2 yrs | + | + | + | severe | 3 | none (3) | [3, 5, 14] |
|
4 female (34 years) |
79G > A/79G > A G27R/G27R |
4 d | 3 mo | + | – | + | borderline | 1 | + (1) | [3, 5, 13, 14] |
|
5 female (34 years) |
824G > A/824G > A R275Q/R275Q |
3 yrs | 7 yrs | – | + | – | moderate | 2 | none (3) | [3, 5, 15] |
|
6 male (29 years) |
IVS5 + 1G > A/IVS5 + 1G/A (exon skipping) | 18 yrs | 21 yrs | + | + | + | borderline | 3 | none (3) | [3, 5] |
|
7a female (12 years) |
79G > A/823C > G G27R/R275G |
1 yr | 8 yrs | – | – | – | borderline | 1 | + (2) | [3] |
|
8a female (8 years) |
79G > A/823C > G G27R/R275G |
1 yr | 4 yrs (prospective) | – | – | – | borderline | 1 | + (2) | [3] |
|
9 female (8 years) |
68G > A/86C > G C23Y/P29R |
1 yr | 5 yrs | – | – | – | no (selective skills impairment) | 1 | + (2) | |
Legend: aSiblings. For adaptive functions: 1 = autonomous, 2 = assisted; 3 = dependent. yrs years, mo months, d days, + present, – absent
Neurological assessment
Spastic paraparesis was assessed according to Harding [16], and the severity of the motor phenotype was evaluated by the Spastic Paraplegia Rating Scale (SPRS) [17]. Central and peripheral somato-sensory signs, and cerebellar signs were evaluated as well.
Neurophysiological assessment
Long fibers tract assessment encompassed MEPs at upper and lower limbs for the corticospinal tract, and SSEPs at lower limbs for the afferent somato-sensory tracts. Neurophysiological assessments were completed by NCV at lower limbs.
MEPs recordings were performed at the abductor pollicis brevis (APB) muscle for the upper limbs, and at the tibialis anterior (TA) muscle or, in few cases, at the abductor hallucis (AH) muscle for the lower limbs. Assessments consisted in the recording of the central motor conduction time (CMCT) at the target muscle, in both conditions of rest and of slight tonic voluntary contraction (facilitation). CMCT values were considered abnormal if > 20 ms or with a side-to-side difference > 2 ms [18]. Normative CMCT values were obtained from literature data for adult patients [19] and from studies performed at our hospital on healthy age-matched subjects for pediatric ones. SSEPs were measured at the posterior tibial muscle by the central sensory conduction time (CSCT). Motor NCV was recorded at the popliteus sciaticus internus (PSI) or popliteus sciaticus externus (PSE) nerves, and sensory NCV at the sural nerve. In all the cases, single assessments encompassed side-to side recordings and mean values between right and left side were considered.
Neuroradiological assessment
MRI examinations were performed on a 1.5 or 3-Tesla MR scanner, and included sagittal T1w, axial T2w, FLAIR and diffusion weighted images. As comprehensive of all the required sequences, three brain MRI assessments performed in other institutions were considered as well. Images have been evaluated for the evidence of supra-tentorial, sub-tentorial, spinal cord involvement, and corpus callosum abnormalities. To assess the severity of atrophy in each area, and its progression over follow-up, a severity score was designed: - (absent/normal), + (mild), ++ (moderate), +++ (severe).
Results
Neurological assessment
Table 2 summarizes the neurological findings at follow-up. All patients showed variable signs of pyramidal dysfunction, and an overt spastic paraplegia was detected in 3/9 patients. The remaining ones showed milder symptoms, such as lower limbs hyperreflexia or bilateral clonus. Seven out of 9 patients had mild to moderate cerebellar signs. Notably, the two patients lacking cerebellar symptoms were the youngest in our cohort. At last examination, all patients were able to walk alone for few meters at least, except for patient #3 who was wheelchair-bound since the age of 20 years. Romberg’s test was assessed in 8/9 patients and resulted negative. Peripheral neuropathy was clinically evident only in patient #3. As assessed by SPRS, spasticity severity reached a median score of 17.8/54 (SD ±15.4), and no correlation was found when SPRS scores were matched with age at evaluation (p = > 0.05). Eight out of 9 patients had an intellectual disability of variable degree.
Table 2.
Main neurological, neurophysiological and neuroradiological findings at last follow-up in nine patients with HHH syndrome
| Pt (age at last evaluation) | Higher motor ability | Spastic paraplegia | Pyramidal signs | SPRS (max 52) | Cerebellar signs | Somato-sensory signs | Neuropathy signs |
Lower limb neurophysiological findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Romberg Discrimination touch Vibration | Pain Termic Protopatic touch | MEPs (CMCT) | SSEPs (CSCT) | NCV | ||||||||
| rest/ facilitation | motor | sensory | ||||||||||
| 1 (53 years) | run | – | + | 9 | + | – | – | – | NE/NE | delayed | normal | normal |
| 2 (44 years) | deambulant with assistance | + | + | 31 | + | – | – | – | delayed/delayed | normal | normal | normal |
| 3 (35 years) | wheelchair bound | + | + | 44 | + | nd | nd | + | nd | nd | NE | NE |
| 4 (34 years) | deambulant | – | + | 13 | + | – | – | – | NE/ normal | normal | normal | normal |
| 5 (34 years) | deambulant | – | + | 20 | + | – | – | – | NE/normal | normal | normal | normal |
| 6 (29 years) | deambulant with assistance | + | + | 37 | + | – | – | – | NE/delayed | normal | normal | normal |
| 7 (12 years) | run | – | + | 4 | + | – | – | – | normal/normal | normal | normal | normal |
| 8 (8 years) | run | – | + | 2 | – | – | – | – | delayed/delayed | normal | normal | normal |
| 9 (8 years) | run | – | + | 1 | – | – | – | – | NE/delayed | delayed | normal | normal |
Legend: CMCT Central motor conduction time, CSCT Central sensory conduction time, FU Follow-up, MEPs Motor evoked potentials, NCV Nerve conduction velocity, NE Not evocable, nd not done, SSEPs Somato-sensory evoked potentials, SPRS Spastic Paraplegia Rating Scale, + present, – absent
Clinical assessment of the somato-sensory system (performed in 8/9 patients) resulted invariable negative. However, beside some easily recognizable neurological signs (e.g. Romberg test), the ones depending on patient’s perception (e.g. discriminative and protopatic touch, sense of vibration, pain, heat) resulted often difficult to assess, because of the cognitive impairment of the patients themselves.
Neurophysiological assessment
One patient (#3) was excluded due to peripheral neuropathy, and neurophysiological assessment was finally performed in 8/9 patients.
Central motor pathway
Lower limb MEPs were recorded at TA muscle in 7/8 patients and at AH muscle in 1/8 patient (#6). One patient (#4) underwent recordings at both the muscles over its follow-up. Seven out of eight patients were tested at least twice (mean interval between first and last assessment 8.3 ± 6.9 years). Recordings at TA muscles showed mean CMCT 21.2 ± 4.8 ms at relaxed muscle (n.v. 15.9 ± 1.5) and 18.0 ± 4.4 ms at facilitation [n.v.12.5 ± 1.5 [19] for adults; 11.5 ± 1.6 for paediatric patients]. Longitudinal neurophysiological data for each patient are showed in Table 3. Mean TA CMCT values compared to healthy controls are shown in Fig. 1. Recordings at HA muscle showed no evocable response at relaxed muscle and a mean CMCT 21.0 ± 1.7 ms at facilitation [n.v. 16.9 ± 0.9 [20]]. At last follow-up, MEPs resulted pathological (absent or delayed) in 7/8 patients (87.5%) at rest, and in 5/8 (62.5%) at facilitation.
Table 3.
Longitudinal MEPs values in 9 patients with HHH syndrome
| Pt | CMCT (ms) at rest/facilitation | ||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| 1 | NE/23.5 | NE/NE | – | – | – |
| 2 | 19/18.6 | 19.5/18.9 | 20.3/19.45 | 23.4/20,25 | 26.35/21.25 |
| 3 | – | – | – | – | – |
| 4 | NE/14.5 | NE/15.0 | aNE/19.2 | aNE/20.0 | – |
| 5 | NE/12.1 | NE/12.3 | – | – | – |
| 6 | aNE/22.0 | aNE/22.9 | – | – | – |
| 7 | 16.6/12.5 | 21.8/12.8 | 15.9/11.6 | – | – |
| 8 | 27.6/22.8 | – | – | – | – |
| 9 | NE/25.4 | NE/17.45 | NE/21.95 | – | – |
Legend: recordings were performed at tibialis anterior muscle (TA), or at abductor halluces (AH) musclea. NE Not evocable, – not done
Fig. 1.
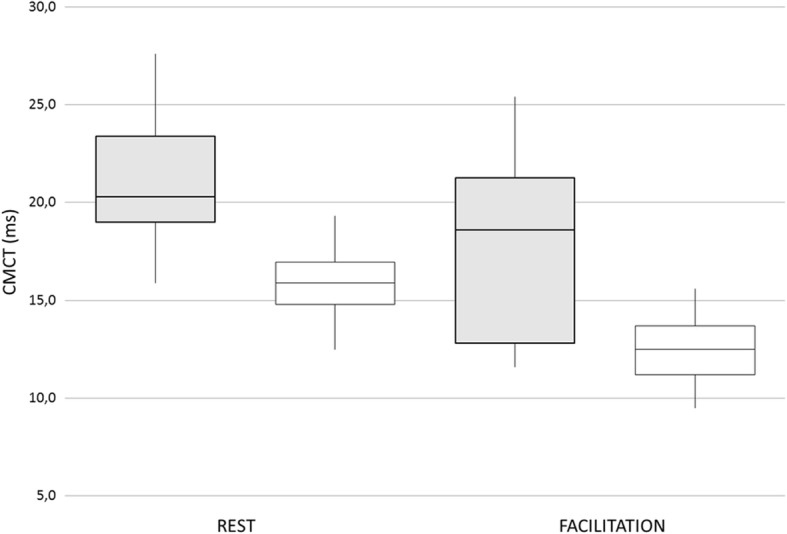
Lower limbs motor evoked potential (MEPs) in eight patients with HHH syndrome. Mean central motor conduction time (CMCT) at rest and during muscle facilitation (tibialis anterior muscle) in HHH patients (grey bars) in comparison with healthy controls (white bars). Values are expressed as milliseconds (ms)
Upper limb MEPs were recorded in 3/8 patients, with one patient tested twice at an interval of 5 years. Values resulted within the normal range with a mean CMCT 9.9 ± 1.7 ms [n.v. 7.9 ± 2.1; +3SD =14.2 [21]].
Central somato-sensory pathways
All patients but one (#5) were tested at least twice (mean interval between first and last assessment 7.1 ± 6.9 years). Mean CSCT was 19.8 ± 3.9 ms [n.v. 16.4 ± 1.4; +3SD = 20.6 [22, 23]]. Patient # 8 showed a progressively improving trend from infancy to adolescence, reaching borderline/normal values at 9 years. At last follow up, 2/9 patients showed abnormal responses (#1, 9). No correlation between SSEPs values and clinical findings were found.
Peripheral nerve conduction
Assessments included 14 PSI, 10 PSE and 16 sural nerve recordings in 8/9 patients. Six patients were tested at least twice (mean interval between first and last evaluation 6.7 ± 9.6 years). Eight out of 9 patients showed normal results [24]. Patient #3 showed progressively worsening NCVs with no evocable responses since the age of 18 years.
Neuroradiological findings
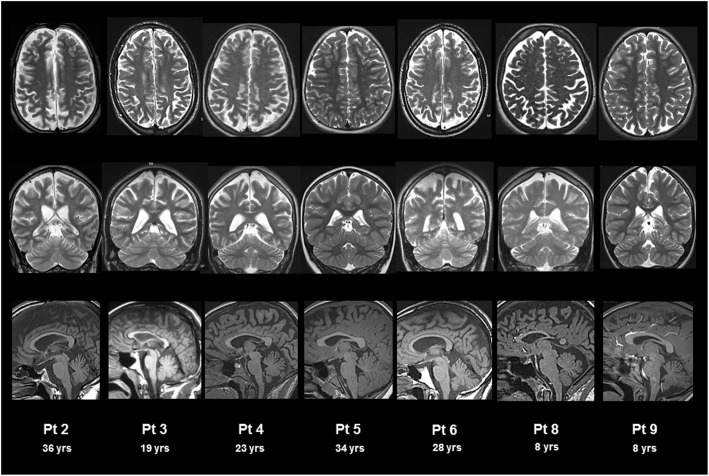
Assessments included 14 brain and 7 spinal cord MRI. All patients underwent at least one brain MRI. Brain neuro-radiological findings at follow-up are shown in Fig. 2. Atrophic changes were found in supratentorial and/or subtentorial regions in 7/9 patients. As shown in Table 4, atrophy involved supra-tentorial white matter in 6/9 patients, mild in 2 and moderate in 4. As for sub-tentorial regions, 6/9 patients showed mild atrophic changes of cerebellar vermis. Corpus callosum abnormalities were found in 5/9 patients with mild atrophy in 3 and moderate in 2. Four out of 9 patients (# 2, 4, 5, 6) underwent two or more brain MRI (mean age at baseline 19.4 ± 10.0 years; mean interval between first and last assessment 8.7 ± 2.9 years) with two patients (#2 and 5) showing a worsening trend over years.
Fig. 2.
Brain MRI in patients with HHH syndrome. T2 weighted axial (upper panel), coronal (middle panel), and T1 weighted sagittal (lower panel) brain MRI. Atrophy of variable degree is detected in supra-tentorial region (moderate in patients #2, 3, 5 and 6; mild in patient # 4; absent in patients #8, and 9), corpus callosum (moderate in patients #3 and 5; mild in patients # 2, 4 and 6; absent in patients # 8 and 9) and cerebellum (mild in patients #2, 4, 5, 6 and 9, absent in patients # 3, and 8)
Table 4.
Main neuroradiological findings over follow-up in 9 patients with HHH syndrome
| Pt | Age at MRI assessment (yrs) |
Brain atrophy | Spinal cord atrophy |
||
|---|---|---|---|---|---|
| Supratentorial | Corpus callosum | Subtentorial | |||
| 1 | 40 | + | – | + | n.d. |
| 2 | 27 | + | – | – | n.d. |
| 34 | ++ | + | – | n.d. | |
| 36 | ++ | + | + | ++ | |
| 3 | 19 | ++ | ++ | – | +++ |
| 4 | 18 | + | + | + | n.d. |
| 23 | + | + | + | + | |
| 5 | 25 | ++ | + | – | n.d. |
| 34 | ++ | ++ | + | – | |
| 6 | 28 | ++ | + | + | n.d |
| 7 | 12 | – | – | – | – |
| 8 | 8 | – | – | – | – |
| 9 | 8 | – | – | + | – |
Legend: +, mild; ++, moderate; +++, severe; absent, −. n.d not done
Neuroradiological findings at spinal cord are shown in Fig. 3. Spinal cord atrophy was detected in 3/7 patients (Table 4). In one patient (#3), the neuroradiological features were consistent with the particularly severe clinical and neurophysiological outcome.
Fig. 3.
Spinal cord MRI in patients with HHH syndrome. T1 weighted spinal cord MRI showed atrophy of variable degree (severe in patients #3, moderate in patient #2, mild in patient #4, and absent in patients #5, 8 and 9)
Discussion
This study reports on the longitudinal description of neurological, neurophysiological and neuroradiological patterns in HHH syndrome. In our cohort, pyramidal signs were invariably present, with an overt picture of spastic paraplegia detectable in one third of the cases. Other clinical features encompassed a cognitive impairment of variable degree, a frequent cerebellar dysfunction, and more rarely a secondary peripheral neuropathy, which enlarges the spectrum of the phenotypic disease severity. Characteristically, clinical signs of central sensory dysfunction were never observed. Neurophysiological data confirmed a prominent involvement of the primary motor descending pathway compared to the long fibers sensory tracts, and a less frequent involvement of peripheral nerve. Neuroradiological studies, despite not completed by diffusion tensor imaging techniques, confirmed the prominent affection of the regions anatomically corresponding to pyramidal tract (frontal subcortical white matter and, in the most severe cases, cortical gray matter as well). With regard to the final clinical outcome, our findings show heterogeneous profiles, which are apparently independent from the age at evaluation, from the age of treatment initiation, as well as from the length of the disease course, which is particularly difficult to assess in patients who never experienced overt hyperammonemia, which correspond to one third of our cohort.
The association of lower limbs spasticity with mild cerebellar signs, cognitive impairment, and possible neuropathy indicates that HHH syndrome should be listed among the hereditary spastic paraplegias (HSPs). In particular, given its composite phenotypic picture, HHH syndrome fulfills the diagnostic criteria of complex HSPs [25].
Nowadays, HSPs encompass more than 80 loci and over 60 genes [26, 27], nevertheless, many conditions remain undiagnosed, suggesting that new genetic determinants still remains to be identified.
With regard to neurophysiological findings in HHH syndrome, our data are consistent with the clinical picture, highlighting the predominant involvement of the descending motor tracts compared to the ascending sensory ones. MEPs recorded at lower limbs showed delayed or not evocable response in about 90% of our patients. This occurred in both adults and children, showing a progressive worsening trend over time. A slowing-down, up to the loss, of an evocable response of CMCT was mostly detectable at rest at first evaluation, but it progressively involved the response also at muscle contraction at follow-up. This is in line with the neurophysiological response to a progressive neuronal degeneration in which a pathological response at rest can be hided or mitigated in facilitation conditions, when higher threshold and faster pyramidal neurons are recruited [28, 29]. What follows is that the SPRS scale, which scores spastic paraplegia according to patient’s age and disease duration, is not an optimal tool to quantify the severity of pyramidal dysfunction in HHH syndrome.
Contrarily to MEPs, SSEPs resulted unaltered in the most of our patients. Accordingly, clinical signs of sensory defects were never recorded, not even in the two patients who showed mildly delayed SSEPs values.
Neuroradiological data also confirm a slow progression of the disease. Atrophy primarily affects the subcortical white matter and, in the most severe cases, mildly involving cortical gray matter as well, whilst cerebellum is less affected. These findings are in line with literature reports on brain lesions observed in urea cycle disorders, which selectively and primarily affects the deep white matter [30]. Since the disease course in HHH syndrome is usually stable, with low risk of hyperammonemia once diet and pharmacological therapy are started, brain lesions are milder if compared to other urea cycle disorders, presenting a more severe long-term course [31, 32]. Therefore, brain abnormalities detected in HHH syndrome are likely to be not only dependent on the severity and duration of hyperammonemia alone. In fact, the 4/9 patients in our cohort who experienced hyperammonemic coma showed heterogeneous MRI findings: #3 and #6 had the most severe clinical and neuroradiological outcome, while #1 and #4 had mild lesions, comparable to those of patients who never experienced severe hyperammonemia.
Concerning spinal cord, MRI changes suggest a correlation with the clinical outcome, with the most severe atrophic degeneration observed in patients with the higher SPRS score (patient #3 who is wheelchair bound, and patient #2 who hardly maintains autonomous gait, only for a few steps). Although spinal cord atrophy progression rate cannot be determined since patients underwent a single MRI study, the ongoing trend of neurophysiological assessments suggest a similar evolution.
Clinical, neurophysiological and neuroradiological findings in HHH syndrome resemble those observed in argininemia and pyrroline-5-carboxylate synthetase (P5CS) deficiency [33–36], two other disorders of aminoacid metabolism connected to the distal part of the urea cycle (Fig. 4).
Fig. 4.
Urea cycle and related pathways. The illustration shows the biochemical pathways connecting HHH syndrome, argininemia and pyrroline-5-carboxylate synthetase deficiency (gray boxes), the three disorders of aminoacid metabolism related to the distal part of the urea cycle sharing phenotypic similarities. AGAT, l-arginine:glycine amidinotransferase; ASL, argininosuccinate lyase; ASS, argininosuccinate synthetase; CPS, carbamyl-phosphate synthetase; GAA, guanidinoacetate; GAMT, guanidinoacetate N-methyltransferase; OAT, ornithine aminotransferase; ODC, ornithine decarboxylase; ORNT1, ornithine/citrulline antiporter; OTC, ornithine transcarbamylase; P5C, pyrroline-5-carboxylate; P5CR, pyrroline-5-carboxylate reductase; P5CS, pyrroline-5-carboxylate synthase
It is noteworthy that, as in HHH syndrome, these diseases are associated with spastic paraplegia, variable cognitive impairment and possible cerebellar signs, then suggesting possible common mechanisms connecting urea cycle-related pathways with the maintenance of the cortico-spinal tract integrity [37]. Possible shared mechanisms include abnormalities of arginine, creatine, polyamines, and proline metabolism, and dysregulation of the autophagy machinery, this last representing a known cause of some HSPs [38].
Conclusions
Our study highlights the progressive involvement of the corticospinal tract in HHH syndrome. With regard to neurophysiological data, corticospinal system dysfunction resulted clearly predominant if compared to central sensory system and to peripheral nervous system. MEPs showed a pathological pattern with a progressive worsening trend with age. Although the cause of the selective involvement of cortical spinal tract in HHH syndrome remains to be elucidated, the similarities with argininemia and P5CS deficiency, suggest possible common pathophysiological mechanisms.
The presence of pyramidal signs/lower limb spasticity associated with cerebellar signs and cognitive impairment, highlights the importance to list HHH syndrome among the complex form of HSPs.
Acknowledgements
We thank the Association “La Vita e’ un Dono” for supporting the fellowship of Dr. Giorgia Olivieri.
Abbreviations
- AH
Abductor halluces
- APB
Abductor pollicis brevis
- CMCT
Central motor conduction time
- HHH
Hyperornithinemia–hyperammonemia–homocitrullinuria
- MEPs
Motor evoked potentials
- NCV
Nerve conduction velocity
- P5CS
Pyrroline-5-carboxylate synthetase
- PSE
Popliteus sciaticus externus
- PSI
Popliteus sciaticus internus
- SPRS
Spastic paraplegia rating scale
- SSEPs
Somato-sensory evoked potentials
- TA
Tibialis anterior
Authors’ contributions
GO designed the study, collected clinical data, contributed to interpretation of the neurophysiological and neuro-radiological data and drafted the manuscript. SP and MDC contributed to acquisition and interpretation of the neurophysiological data. DD and DM contributed to interpretation of the neurophysiological and neuro-radiological data. DL contributed to acquisition and interpretation of the neuro-radiological data. EB contributed to interpretation of the data and revised the manuscript. CDV designed and supervised the study, contributed to interpretation of the data and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported with founds “Ricerca Corrente” by the Italian Ministry of Health and by the Association La Vita è un Dono.
Availability of data and materials
All data which have been generated and analyzed during this study are included in the article.
Ethics approval and consent to participate
Data reported in this study represent standard clinical practice and a formal approval by the ethics committee of the Bambino Gesù Children Hospital, Rome was not requested. All participants (or parents for minor patients) allowed the permission to participate in data collection.
Consent for publication
See above.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Häberle J, Boddaert N, Burlina A, Chakrapani A, Dixon M, Huemer M, et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012;7:32. doi: 10.1186/1750-1172-7-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Posset R, Garbade SF, Boy N, Burlina AB, Dionisi-Vici C, Dobbelaere D, et al. Transatlantic combined and comparative data analysis of 1095 patients with urea cycle disorders a successful strategy for clinical research of rare diseases. J Inherit Metab Dis. 2018. 10.1007/s10545-018-0222-z. [DOI] [PMC free article] [PubMed]
- 3.Martinelli D, Diodato D, Ponzi E, Monné M, Boenzi S, Bertini E, et al. The hyperornithinemia-hyperammonemia-homocitrullinuria syndrome. Orphanet J Rare Dis. 2015;10:29. doi: 10.1186/s13023-015-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemay JF, Lambert MA, Mitchell GA, Vanasse M, Valle D, Arbour JF, et al. Hyperammonemia-hyperornithinemia-homocitrullinuria syndrome: neurologic, ophthalmologic, and neuropsychologic examination of six patients. J Pediatr. 1992;121:725–730. doi: 10.1016/S0022-3476(05)81900-6. [DOI] [PubMed] [Google Scholar]
- 5.Salvi S, Santorelli FM, Bertini E, Boldrini R, Meli C, Donati A, et al. Clinical and molecular findings in hyperornithinemia-hyperammonemia-homocitrullinuria syndrome. Neurology. 2001;57:911–914. doi: 10.1212/WNL.57.5.911. [DOI] [PubMed] [Google Scholar]
- 6.Debray FG, Lambert M, Lemieux B, Soucy JF, Drouin R, Fenyves D, et al. Phenotypic variability among patients with hyperornithinaemia-hyperammonaemia-homocitrullinuria syndrome homozygous for the delF188 mutation in SLC25A15. J Med Genet. 2008;45:759–764. doi: 10.1136/jmg.2008.059097. [DOI] [PubMed] [Google Scholar]
- 7.Filosto M, Alberici A, Tessa A, Padovani A, Santorelli FM. Hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome in adulthood: a rare recognizable condition. Neurol Sci. 2013;34:1699–1701. doi: 10.1007/s10072-012-1266-8. [DOI] [PubMed] [Google Scholar]
- 8.Koike R, Fujimori K, Yuasa T, Miyatake T, Inoue I, Saheki T. Hyperornithinemia, hyperammonemia, and homocitrullinuria: case report and biochemical study. Neurology. 1987;37:1813–1815. doi: 10.1212/WNL.37.11.1813. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima M, Ishii S, Mito T, Takeshita K, Takashima S, Takakura H, et al. Clinical, biochemical and ultrastructural study on the pathogenesis of hyperornithinemia-hyperammonemia-homocitrullinuria syndrome. Brain Dev. 1988;10:181–185. doi: 10.1016/S0387-7604(88)80025-1. [DOI] [PubMed] [Google Scholar]
- 10.Tsujino S, Kanazawa N, Ohashi T, Eto Y, Saito T, Kira J, et al. Three novel mutations (G27E, insAAC, R179X) in the ORNT1 gene of Japanese patients with hyperornithinemia, hyperammonemia, and homocitrullinuria syndrome. Ann Neurol. 2000;47:625–631. doi: 10.1002/1531-8249(200005)47:5<625::AID-ANA10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 11.Miyamoto T, Kanazawa N, Kato S, Kawakami M, Inoue Y, Kuhara T, et al. Diagnosis of Japanese patients with HHH syndrome by molecular genetic analysis: a common mutation, R179X. J Hum Genet. 2001;46:260–262. doi: 10.1007/s100380170075. [DOI] [PubMed] [Google Scholar]
- 12.Tessa A, Fiermonte G, Dionisi-Vici C, Paradies E, Baumgartner MR, Chien YH, et al. Identification of novel mutations in the SLC25A15 gene in hyperornithinemia-hyperammonemia-homocitrullinuria (HHH) syndrome: a clinical, molecular, and functional study. Hum Mutat. 2009;30:741–748. doi: 10.1002/humu.20930. [DOI] [PubMed] [Google Scholar]
- 13.Haass C, Pedicino R, Sabetta G, Panero A, Colarizi P. Hyperornithinaemia, hyperammonemia and homocitrullinuria (HHH syndrome) with neonatal onset and favourable outcome. Ital J Pediatr. 1986;12:143–146. [Google Scholar]
- 14.Dionisi Vici C, Bachmann C, Gambarara M, Colombo JP, Sabetta G. Hyperornithinaemia-hyperammonaemia-homocitrullinuria syndrome: low creatine excretion and effect of citrulline, arginine, or ornithine supplement. Pediatr Res. 1987;22:364–367. doi: 10.1203/00006450-198709000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Morini C, Capozzi P, Boenzi S, Rizzo C, Santorelli FM, Dionisi-Vici C. Retinal degeneration. Ophthalmology. 2009;116:1593. doi: 10.1016/j.ophtha.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 16.Harding AE. Hereditary “pure” spastic paraplegia: a clinical and genetic study of 22 families. J Neurol Neurosurg Psychiatry. 1981;44:871–883. doi: 10.1136/jnnp.44.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schule R, Holland-Letz T, Klimpe S, Kassubek J, Klopstock T, Mall V, et al. The spastic paraplegia rating scale (SPRS): a reliable and valid measure of disease severity. Neurology. 2006;67:430–434. doi: 10.1212/01.wnl.0000228242.53336.90. [DOI] [PubMed] [Google Scholar]
- 18.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. committee. Clin Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantone M, Lanza G, Vinciguerra L, Puglisi V, Ricceri R, Fisicaro F, et al. Age, height, and sex on motor evoked potentials: translational data from a large italian cohort in a clinical environment. Front Hum Neurosci. 2019;13:185. doi: 10.3389/fnhum.2019.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Lazzaro V, Pilato F, Oliviero A, Saturno E, Dileone M, Tonali PA. Role of motor evoked potentials in diagnosis of cauda equine and lumbosacral cord lesions. Neurology. 2004;63:2266–2271. doi: 10.1212/01.WNL.0000147296.97980.CA. [DOI] [PubMed] [Google Scholar]
- 21.Eisen AA, Shtyble W. AAEM minimonograph #35: clinical experience with transcranial magnetic stimulation. Muscle Nerve. 1990;13:995–1011. doi: 10.1002/mus.880131102. [DOI] [PubMed] [Google Scholar]
- 22.Boor R, Li L, Goebel B, Reitter B. Subcortical somatosensory evoked potentials after posterior tibial nerve stimulation in children. Brain Dev. 2008;30:493–498. doi: 10.1016/j.braindev.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Cruccu G, Aminoff MJ, Curio G, Guerit JM, Kakigi R, Mauguiere F, et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol. 2008;119:1705–1719. doi: 10.1016/j.clinph.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Andary M, Buschbacher R, Del Toro D, Smith B, So Y, et al. Electrodiagnostic reference values for upper and lower limb nerve conduction studies in adult populations. Muscle Nerve. 2016;54:371–377. doi: 10.1002/mus.25203. [DOI] [PubMed] [Google Scholar]
- 25.Kara Kara E, Tucci A, Manzoni C, Lynch DS, Elpidorou M, Bettencourt C, et al. Genetic and phenotypic characterization of complex hereditary spastic paraplegia. Brain. 2016;139:1904–1918. doi: 10.1093/brain/aww111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morais S, Raymond L, Mairey M, Coutinho P, Brandão E, Ribeiro P, et al. Massive sequencing of 70 genes reveals a myriad of missing genes or mechanisms to be uncovered in hereditary spastic paraplegias. Eur J Hum Genet. 2017;25:1217–1228. doi: 10.1038/ejhg.2017.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesson C, Koht J, Stevarin G. Delving into the complexity of hereditary spastic paraplegias: how unexpected phenotypes and inheritance modes are revolutionizing their nosology. Hum Genet. 2015;134:511–538. doi: 10.1007/s00439-015-1536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennan E. The size-principle: a deterministic output emerges from a set of probabilistic connections. J Exp Biol. 1985;115:105–112. doi: 10.1242/jeb.115.1.105. [DOI] [PubMed] [Google Scholar]
- 29.Caramia MD, Desiato MT, Cicinelli P, Iani C, Rossini PM. Latency jump of “relaxed” versus “contracted” motor evoked potentials as a marker of cortico-spinal maturation. Electroencephalogr Clin Neurophysiol. 1993;89:61–66. doi: 10.1016/0168-5597(93)90086-5. [DOI] [PubMed] [Google Scholar]
- 30.Gropman AL, Summar M, Leonard JV. Neurological implications of urea cycle disorders. J Inherit Metab Dis. 2007;30:865–879. doi: 10.1007/s10545-007-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harding BN, Leonard JV, Erdohazi M. Ornithine carbamoyl transferase deficiency: a neuropathological study. Eur J Pediatr. 1984;141:215–220. doi: 10.1007/BF00572763. [DOI] [PubMed] [Google Scholar]
- 32.Dolman CL, Clasen RA, Dorovini-Zis K. Severe cerebral damage in ornithine transcarbamylase deficiency. Clin Neuropathol. 1988;7:10–15. [PubMed] [Google Scholar]
- 33.Jichlinski A, Clarke L, Whitehead MT, Gropman A. “Cerebral Palsy” in a patient with arginase deficiency. Semin Pediatr Neurol. 2018;26:110–114. doi: 10.1016/j.spen.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Coutelier M, Goizet C, Durr A, Habarou F, Morais S, Dionne-Laporte A, et al. Alteration of ornithine metabolism leads to dominant and recessive hereditary spastic paraplegia. Brain. 2015;138:2191–2205. doi: 10.1093/brain/awv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinelli D, Häberle J, Rubio V, Giunta C, Hausser I, Carrozzo R, et al. Understanding pyrroline-5-carboxylate synthetase deficiency: clinical, molecular, functional, and expression studies, structure-based analysis, and novel therapy with arginine. J Inherit Metab Dis. 2012;35:761–776. doi: 10.1007/s10545-011-9411-8. [DOI] [PubMed] [Google Scholar]
- 36.Zampatti S, Castori M, Fischer B, Ferrari P, Garavelli L, Dionisi-Vici C, et al. De Barsy syndrome: a genetically heterogeneous autosomal recessive cutis laxa syndrome related to P5CS and PYCR1 dysfunction. Am J Med Genet A. 2012;158A:927–931. doi: 10.1002/ajmg.a.35231. [DOI] [PubMed] [Google Scholar]
- 37.Panza E, Martinelli D, Magini P, Dionisi Vici C, Seri M. Hereditary spastic paraplegia is a common phenotypic finding in ARG1 deficiency, P5CS deficiency and HHH syndrome: three inborn errors of metabolism caused by alteration of an interconnected pathway of glutamate and urea cycle metabolism. Front Neurol. 2019;10:131. doi: 10.3389/fneur.2019.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebrahimi-Fakhari D. Congenital disorders of autophagy: what a pediatric neurologist should know. Neuropediatrics. 2018;49:18–25. doi: 10.1055/s-0037-1608652. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data which have been generated and analyzed during this study are included in the article.