Abstract
Background: Food choices are essential to successful glycemic control for people with diabetes. We compared the impact of three carbohydrate-rich meals on the postprandial glycemic response in adults with type 1 diabetes (T1D).
Methods: We performed a randomized crossover study in 12 adults with T1D (age 58.7 ± 14.2 years, baseline hemoglobin A1c 7.5% ± 1.3%) comparing the postprandial glycemic response to three meals using continuous glucose monitoring: (1) “higher protein” pasta containing 10 g protein/serving, (2) regular pasta with 7 g protein/serving, and (3) extra-long grain white rice. All meals contained 42 g carbohydrate; were served with homemade tomato sauce, green salad, and balsamic dressing; and were repeated twice in random order. After their insulin bolus, subjects were observed in clinic for 5 h. Linear mixed effects models were used to assess the glycemic response.
Results: Compared with white rice, peak glucose levels were significantly lower for higher protein pasta (−32.6 mg/dL; 95% CI −48.4 to −17.2; P < 0.001) and regular pasta (−43.2 mg/dL, 95% CI −58.7 to −27.7; P < 0.001). The difference between the two types of pastas did not reach statistical significance (−11 mg/dL; 95% CI −24.1 to 3.4; P = 0.17). Total glucose area under the curve was also significantly higher for white rice compared with both pastas (P < 0.001 for both comparisons).
Conclusions: This exploratory study concluded that different food types of similar macronutrient content (e.g., rice and pasta) generate significantly different postprandial glycemic responses in persons with T1D. These results provide useful insights into the impact of food choices on and optimization of glucose control. Clinical Trial Registry: clinicaltrials.gov NCT03362151.
Keywords: Type 1 diabetes, Nutrition, Pasta, Rice, High protein, Glycemic control, Continuous glucose monitoring, Carbohydrate choices
Introduction
As of 2017, >30 million Americans had diabetes.1 For this enormous subset of the U.S. population, deciding which foods to eat is highly consequential, as control of one's hour-to-hour and day-to-day blood glucose (BG) levels is known to affect both morbidity and mortality.2,3 A particularly challenging task relates to the choice of specific carbohydrates to be consumed at any given meal. However, the type and form of the carbohydrate foods consumed is important in addition to the quantity of carbohydrate. Each type of carbohydrate has a different effect on BG levels and average BG over time.4,5
Although the literature includes data regarding the glycemic index (GI) of various carbohydrate containing foods, the relative impact of commonly consumed carbohydrate-rich foods on BG has been only minimally studied in real-life settings.6,7 Importantly, the recent and increased availability of continuous glucose monitoring (CGM) technologies,8,9 along with recent published evidence that individuals with type 1 diabetes (T1D) and type 2 diabetes (T2D) can improve their BG management with CGM use as part of their ongoing diabetes management and education plan,10–12 has set the stage for a new line of clinical investigation in which the relative impact of different food choices (and their culinary preparation as part of complete meals) on glycemic control can be explored, in real-life settings, across populations and across a range of food choice options.
Among the more popular carbohydrate-rich food sources, namely potatoes, wheat, rice, corn, and pasta, it has been noted that regular (100% semolina aka “white”) pasta is a refined carbohydrate that has a relatively low GI compared with other popular carbohydrates.13 In addition, studies show that pasta produces a relatively low glycemic response.14,15 Potential mechanistic explanations include the compact texture and large particle size of digested pasta that may lower the rate of gastric emptying,16 or the presence of a protein matrix that entraps starch granules and slows digestion of starch (carbohydrate) in pasta.17
Although other macronutrients (e.g., fat and protein) included in a meal and not just carbohydrates can affect the postprandial glycemic profile,4,7,18,19 we focused this research to investigate the effect of different carbohydrates sources. We hypothesized that for individuals with T1D, the impact of pasta on BG is more favorable (i.e., lower peaks and lower BG over time) than that for white rice. We sought to explore this hypothesis in a controlled clinical study simulating a real-life meal setting.
Methods
Study design
We investigated the effect of three distinct carbohydrate-rich meals on postprandial glycemic response in adults with T1D in a randomized double-blinded crossover study. The study was conducted at the Sansum Diabetes Research Institute (SDRI), Santa Barbara, CA. After a screening visit, subjects were assigned to a sequence of six predetermined meals for lunch. After each meal session, subjects crossed over to the next meal session separated by at least 48 h. Specifically, we provided (1) a pasta made from semolina flour in combination with whole grain and legume flour containing 10 g protein/serving (aka “higher protein pasta”), (2) a pasta made from 100% semolina flour with 7 g protein/serving (aka “regular pasta”), or (3) extra-long grain white rice (Table 1). All meals were served with a healthy homemade tomato sauce (no sugar added), green salad, and balsamic dressing. The serving size for each type of meal was adjusted to contain 42 g of carbohydrates before adding the sauce, salad, and dressing. Each subject consumed each meal type twice, in random order.
Table 1.
Detailed Meal Composition for the Regular Pasta, Higher Protein Pasta, and White Rice
| 2 oz | Regular pasta | Higher protein pasta | White rice |
|---|---|---|---|
| Protein (g) | 7 | 10 | 5 |
| Carbohydrates (g) | 42 | 38 | 43 |
| Fat (g) | 1 | 2 | <1 |
| Dietary fiber (g) | 3 | 4 | <1 |
| Total calories (cal) | 200 | 190 | 170 |
| Ingredients | Semolina (wheat), durum wheat flour, vitamins/minerals: vitamin B3 (niacin), iron (ferrous sulfate), vitamin B1 (thiamine mononitrate), vitamin B2 (riboflavin), and folic acid | Semolina (wheat), grain legume flour blend (grains and legumes [lentils, chickpeas, flaxseed, barley, oats, and spelt], egg whites, and oat fiber), durum wheat flour, niacin, iron (ferrous sulfate), thiamine, mononitrate, riboflavin, and folic acid | Enriched long grain parboiled rice (long grain rice, iron [ferric orthophosphate], thiamin [thiamine mononitrate], and folate [folic acid]) |
The sequence of six meals for each subject was randomized to reduce temporal bias (Supplementary Fig. S1). Subjects calculated their respective meal insulin boluses based on the known amount of carbohydrates in the meals consumed and their own insulin to carbohydrate ratio. Meal boluses were given at the start of the study meals. After the meal insulin bolus, they were observed for 5 h postprandially at SDRI. At the end of the meal, subjects were asked to guess which type of pasta they ate, as this brand of higher protein/fiber pasta is made to look more like regular pasta than “whole wheat pasta,” which is made differently and is routinely far easier to differentiate visually and from a flavor/texture standpoint. The study protocol was approved by the Harvard T.H. Chan School of Public Health Institutional Review Board (IRB) and was registered on clinicaltrials.gov (NCT03362151).
Participants
Eligible subjects were recruited from the SDRI clinic population, and were between 18 and 75 years of age with T1D for at least 1 year, had a hemoglobin A1c (HbA1c) <10%, and used a rapid acting insulin analog (aspart, glulisine, or lispro) to bolus for their meal carbohydrate content by either injection or insulin pump (subjects could not be using any form of automated insulin delivery, to include hybrid closed-loop, predictive low-glucose suspend, or threshold suspend systems). Key exclusion criteria were pregnancy, gastrointestinal disease such as celiac disease, history of gastroparesis, multiple food allergies, any form of gluten sensitivity or wheat allergy, or allergies to any form of nuts and ingredients present in the study meals. Informed consent was obtained before all study procedures.
Before arriving at each lunch meal session, subjects were instructed to eat and bolus for their usual breakfast, perform their usual activity with no insulin boluses after 9 AM, and aim to have minimal insulin on board so as not to have any lingering effects on the provided lunch meal. If a subject arrived with persistent high BG, a significant amount of insulin on board, or was having glucose control issues that the investigator felt could affect subject safety or the lunch time meal response, then the study meal was rescheduled to a later date per protocol.
Postprandial period
During the six observed postprandial periods, subjects attended a hands-on cooking class given by a chef instructor from The Culinary Institute of America, as well as multiple educational sessions related to nutrition and healthy living. The purpose of this aspect of the study was (1) to provide additional nutrition information to individuals with diabetes about food choices and the science behind these diet-related choices, and (2) to enable the subjects to prepare all three components of this meal (i.e., pasta, homemade tomato sauce, and homemade salad/salad dressing) on their own, from scratch. Following ADA guidelines that show improved outcomes for patients with T1D and T2D with nutrition education,20 this educational component was added because it is the authors' contention that by enabling individuals to not only taste healthy, delicious food options, but also to prepare them effectively, quickly, and affordably, individuals will be more likely to incorporate these meals into their standard repertoire of foods eaten regularly at home. These sessions were included to educate and improve the adherence to the study and not to assess their effect over time.
Outcome measures
Postprandial glucose levels were measured with the Dexcom G4 (with SW505 algorithm update) or G5 CGM (Dexcom, Inc., San Diego, CA). Capillary BG measurements were collected using Contour® NEXT blood glucose meter (Ascensia Diabetes Care US, Inc., Parsippany, NJ) upon arrival at the center when the CGM was calibrated, at the start of the meal, at the end of the postprandial period and anytime during the study period that was determined necessary by the study physician or requested by the subject. CGM sensors were placed at least 24 h before meal sessions. Participants consumed the entire amount of the study meals and were treated for hypoglycemia if confirmatory capillary BG was <70 mg/dL.
The primary endpoint was the difference of glucose (peak mg/dL) from the start of the meal to the peak CGM glucose reading during the 5-h postprandial period. Secondary outcomes included postprandial glucose area under the curve (total AUC, mg/dL × min), time to peak glucose level (in minutes from intervention), and in instances of postprandial hypoglycemia, the percentage of the time that glucose was <70 mg/dL for the 5-h postprandial period. Since the study took place during lunch time and some effect of the morning meal could be evidenced in the glycemic response of the subsequent lunch meal,21 we computed total AUC and peak rather than incremental AUC and delta peak while controlling for the CGM value and the rate of glucose change at the start of the meal session as explained in the following sections and in Section 2 of the Supplementary Data.
Sample size calculation
Sample size was based on detecting a 25% reduction of peak glucose rise (maximum rise from baseline glucose) from the start of the meal to the peak CGM glucose reading (mg/dL) during the 5-h postprandial window, compared between white rice and each one of the two types of pastas. Furthermore, this pilot study was designed to provide exploratory information on the effect of the different types of pasta on postprandial glycemic response in individuals with T1D.
The standard deviation (SD) for peak rise for bread that accounts for both between- and within-subject variation is 16.2 mg/dL.22 Without information on the inter- versus intravariance ratio, we conservatively assumed the intravariance is 16.2 mg/dL. The peak rise for bread is 104.4 mg/dL.22 With 10 subjects each having 6 meals (2 meals for each of the 3 meal types), we computed to have 96% power to detect a 25% reduction in peak rise (27 mg/dL). After accounting for a conservative estimate of 20% dropout, we planned to enroll at least 13 subjects.
Statistical analysis
During the study, the subjects and the investigators were unaware of the type of pasta being served. In addition, the investigators conducting the data analysis were not present at the study sessions and were blinded during the analyses for all three meal types. All outcomes were evaluated using linear mixed effects models (LMEM) in which outcomes are included as dependent variables, whereas the meal type is included as a fixed effect. Further details are listed in the Supplementary Data.
Significance is reported at 0.05 level. Data are reported as mean ± SD. Subanalysis to test any differences in complementary metrics (meal insulin bolus, baseline CGM glucose level) was performed using a one-way ANOVA test. A Kolmogorov–Smirnov test was performed to examine if there were differences in the underlying distributions of AUC. Data analysis was performed using the intent-to-treat approach using Matlab 2016b (The MathWorks, Inc., MA) and R Language (R Development Core Team, 2010). Additional statistical methods are described in Section 2 of the Supplementary Data.
Results
Subject characteristics
Fourteen subjects were enrolled in the study. Three subjects withdrew from the study, one due to being hospitalized for an unrelated event before completing any study meals, and a second after completing only one study meal due to scheduling conflicts. These subjects were not included in the analysis. The third withdrew before the sixth study meal and that subject's data were included. The 12 subjects that were included in the analysis had a mean age of 58.7 ± 14.2 years and HbA1c of 7.5% ± 1.3%. Subject demographics are listed in Table 2.
Table 2.
Subject Demographics and Baseline Characteristics (Mean ± Standard Deviation) for n = 12 Subjects
| Characteristic | |
|---|---|
| Age (years) | 58.7 ± 14.2 |
| Gender | |
| Male | 4 |
| Female | 8 |
| Weight (kg) | 70.7 ± 13.8 |
| Body mass index (kg/m2) | 24.1 ± 3.3 |
| HbA1c (%) | 7.5 ± 1.3 |
| Duration of diabetes (years) | 33.9 ± 19.6 |
| Total daily insulin (U/day) | 31.9 ± 11.9 |
HbA1c, hemoglobin A1c.
Meal insulin bolus
Every participant gave a meal insulin bolus at the start of the meal session according to their usual calculation based on the amount of carbohydrates to be consumed and their insulin to carbohydrate ratio. The mean meal insulin bolus was 3.95 ± 1.7 U. There were no significant differences in insulin bolus size between meal types (P = 0.92).
Glucose peak
Baseline CGM values at the start of the study meal were not significantly different between the three meal types (145.7 ± 61.9 mg/dL for higher protein pasta, 125.6 ± 39.5 mg/dL for regular pasta, and 124.6 ± 26.4 mg/dL for white rice, P = 0.2).
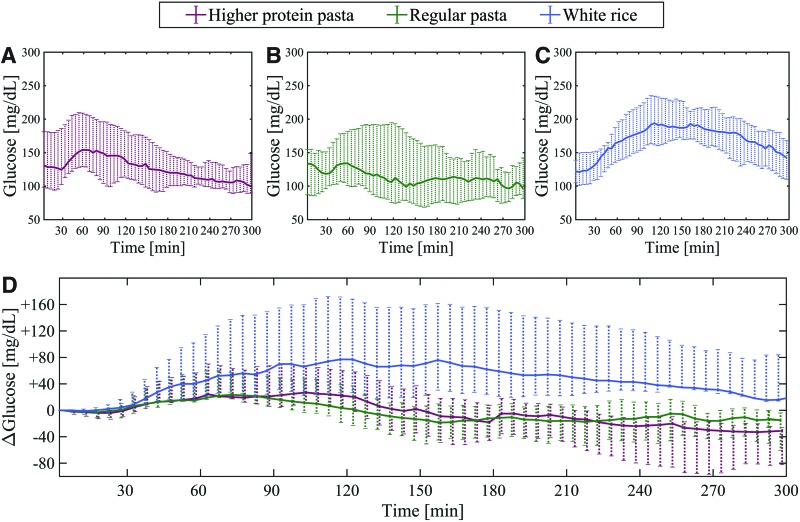
Compared with white rice, the higher protein pasta showed a decreased mean glucose peak of 32.6 mg/dL (95% CI −48.4 to −17.2; P < 0.001). Similarly, compared with white rice the regular pasta showed a decreased mean glucose peak of 43.2 mg/dL (95% CI −58.7 to −27.7; P < 0.001) (Table 3). The difference in glucose peak between regular pasta and higher protein pasta did not reach statistical significance (−11 mg/dL; 95% CI −24.1 to 3.4; P = 0.17). Median CGM values for the 5-h postprandial period are shown in Figure 1. Individual glucose peaks per subject per meal are plotted in Supplementary Figure S2.
Table 3.
Estimated Effects with 95% Confidence Interval of Three Meals on Peak Continuous Glucose Monitoring, Area Under the Curve, and Time to Peak Using Linear Mixed Effects Model
| Model parameters | Dependent variable | ||
|---|---|---|---|
| Peak glucose (CGM), mg/dL | Area under the curve (5 h), mg/dL × min | Time to peak, min | |
| β1 | 85.8 | 15465.9 | 116.5 |
| (38.6 to 133.4) | (7,264.5 to 23,703.4) | (77.3 to 155.7) | |
| P = 0.001 | P < 0.001 | P < 0.001 | |
| β2 | −32.6 | −6950.1 | −24.5 |
| (−48.4 to −17.2) | (−10,324.7 to −3,698.6) | (−43.8 to −5.3) | |
| P < 0.001 | P < 0.001 | P = 0.02 | |
| β3 | −43.2 | −9813.3 | −39.6 |
| (−58.7 to −27.7) | (−13,113.4 to −6,563.7) | (−59.1 to −20.2) | |
| P < 0.001 | P < 0.001 | P < 0.001 | |
Using white rice as the reference (β1), the estimated difference with higher protein pasta (β2) and with regular pasta (β3) are reported. The difference between regular pasta and higher protein pasta is estimated as β3 − β2.
The coefficient means (95% CI) and P-values were estimated using a linear mixed effects model (LMEM), including meal type, glucose at baseline, glucose rate of change at baseline (averaged for 15 min before the meal start), glucose at the end of the session, meal insulin bolus, total daily insulin per subject weight, hypoglycemic treatment, and period as fixed effects and subjects as random effects: 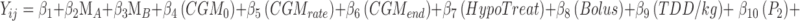 . The differences between meal effects were computed: A − C: β2, B − C: β3, and B − A: β3 − β2; where meal A (MA) is higher protein pasta, meal B (MB) is regular pasta, and meal C is white rice (reference meal).
. The differences between meal effects were computed: A − C: β2, B − C: β3, and B − A: β3 − β2; where meal A (MA) is higher protein pasta, meal B (MB) is regular pasta, and meal C is white rice (reference meal).
CGM, continuous glucose monitoring.
FIG. 1.
Top panel: median and IQR of CGM postprandial glucose profiles for the three meal types—higher protein pasta (A), regular pasta (B), and white rice (C). Bottom panel: the CGM profiles are corrected at baseline by subtracting the CGM value at the start of the meal at each CGM time point for each subject (D). White rice shows a higher glycemic response than the higher protein pasta or regular pasta. The meal effect (i.e., time required to return to baseline blood glucose level) decreased after ∼2 h of the meal start for both types of pasta, whereas the effect required up to 5 h to diminish for white rice. There were no significant differences between the two types of pasta. CGM, continuous glucose monitoring.
The effect of the three meal types on the time to glucose peak from the meal start was obtained by LMEM using the same model structure as the model for glucose peak (Supplementary Data, Section 2). Compared with white rice, the analysis indicated that higher protein pasta showed a peak glucose measurement 24 min earlier (95% CI −43.8 to −5.3; P = 0.02). Also compared with white rice, the time to peak for regular pasta was 40 min earlier (95% CI −59.1 to −20.2; P < 0.001) (Table 3). Regular pasta showed a peak 15 min earlier (95% CI −32.2 to 2.0; P = 0.12) than higher protein pasta. Summary descriptive statistics of these key outcomes are listed in Supplementary Table S1.
Area under the curve
Compared with white rice, the higher protein pasta showed a decreased total AUC by a mean difference of 6950.1 mg/dL × min (95% CI −10324.7 to −3698.6; P < 0.001) and the regular pasta a decrease in total AUC by a mean difference of 9813.3 mg/dL × min (95% CI −13113.4 to −6563.7; P < 0.001) (Table 3). There was no significant difference in the total AUC between regular pasta and higher protein pasta (−2863.3 mg/dL × min, 95% CI −3900.3 to 38.7; P = 0.07).
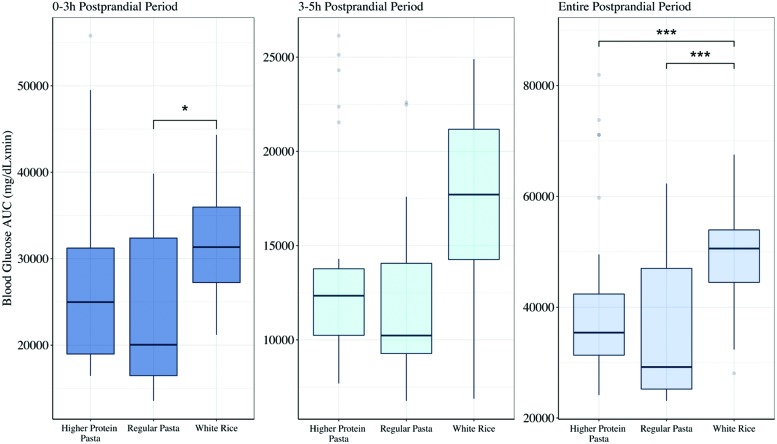
The effect of the three meal types on the 0–3 or 3–5 h postprandial period, defined as 3 h from the meal start and between 3 and 5 h, respectively, was also evaluated (Fig. 2). There was no statistically significant difference in AUC for the 0–3 h postprandial period between the two types of pastas (P = 0.26), nor was there any difference comparing higher protein pasta to white rice (P = 0.11). However, AUC of white rice was higher by a mean difference of 2312.3 mg/dL × min (95% CI 415.6–4208.9; P < 0.01) compared with the regular pasta. Furthermore, in the 3–5 h postprandial period, the regular pasta presented a lower AUC by a mean of 963.9 mg/dL × min (95% CI −2058.8 to 130.9; P = 0.09) compared with the higher protein pasta, and white rice had a higher AUC with a mean of 1188.6 mg/dL × min (95% CI −144.96 to 2522.08; P = 0.09) compared with the regular pasta, but these findings were not significant. During the 3–5 h postprandial period there was no significant difference between the higher protein pasta and white rice (P = 0.7).
FIG. 2.
Box-and-Whisker plot of postprandial blood glucose total AUC after the meals higher protein pasta, regular pasta, and white rice. The left subplot illustrates the total AUC during the 0–3 h postprandial period, the middle subplot shows the total AUC during the late postprandial period 3–5 h, and the right subplot shows the total AUC for the entire postprandial period 0–5 h. The three asterisks indicate statistical significance with P < 0.001, whereas the one asterisk shows P < 0.05 for differences between the total AUC of the three meal types as computed from the linear mixed effect model. AUC, area under the curve.
Furthermore, we examined if there is a difference in the underlying distribution of AUC for the two pasta meals to evaluate whether different pasta composition impacted BG responses differently. The interquartile range for AUC of the higher protein pasta in the entire postprandial period was 11040.1 mg/dL × min, whereas for the regular pasta it was twice as much, 21783.9 mg/dL × min. The distribution of AUC for the same postprandial period for the regular pasta was statistically different than that of the higher protein pasta (P = 0.02), whereas in the 0–3 h postprandial period the AUC distributions of both pastas, although not statistically significant, are still different (P = 0.06). In the 3–5 h period, the AUC distribution of the regular pasta was not statistically different than the higher protein pasta (P = 0.2).
Subanalysis
Within-subject variability and postprandial hypoglycemia are discussed in the Supplementary Data, Section 3.
Ability to distinguish the regular pasta versus higher protein pasta
Overall, subjects identified the correct pasta only 56% of the time. Subjects were able to correctly identify the regular semolina pasta for 75% of meals. However, subjects were only able to identify the higher protein pasta after 37.5% of meals, most often guessing it was regular pasta. In summary, subjects were unable to differentiate regular from higher protein pasta to a meaningful degree, reducing any potential biases that knowledge of the pasta type may have caused.
Discussion
This study suggests that for individuals with T1D, there is a significant difference in postprandial glycemic response to the consumption of white rice versus pasta when these are consumed in equal amounts of carbohydrate. These differences were observed when evaluating peak glucose levels, total AUC, time to peak glucose, and also time to return to baseline BG levels.
Our findings are consistent with studies examining the postprandial effects of pasta versus rice or other commonly consumed carbohydrates such as potato or white bread, where it has been observed that pasta, which has a lower GI, presents a lower AUC as compared with these other carbohydrate options that have a higher GI.4,14,15,22–25 A study that evaluated the postprandial glycemic response of three commercially available gluten-free pasta types in healthy and celiac subjects found that the gluten-free pasta made of rice flour produced a higher AUC compared with pasta containing corn or a mixture of corn and rice flour as the main ingredient.26 This is consistent with the finding that glycemic control in individuals with T1D can be improved with increased intake of high-fiber low-GI carbohydrate-containing foods.27
In our study, 5 h AUC was lowest for regular pasta compared with either higher protein pasta or white rice. It is noteworthy that there was no statistically significant difference in AUC for the 0–3 h postprandial period between higher protein pasta and white rice, but there was a significant difference between regular pasta and white rice. Differences in AUC for 3 h between meals with low and high GI has been reported in the literature for T1D.25,28 In the 3–5 h postprandial period, although not statistically significant, regular pasta had a lower AUC compared with white rice, indicating that the effect of white rice was still evident in the late postprandial phase compared with regular pasta. The higher likelihood of hypoglycemia occurrence after the consumption of regular pasta and lower AUC compared with higher protein pasta and white rice, although with a relatively small sample size in this study and few hypoglycemic events, suggests that less insulin may be required to compensate for its glycemic response (Subanalysis, Supplementary Data, Section 3). In other words, the insulin to carbohydrate ratio should be modified based on the source of carbohydrates (both low and high GI)28–30 and overall macronutrient content in the meal,4 not just the amount of carbohydrates.
We recognize limitations in our study. First, as the study session took place during lunch time, in our analysis we aimed to isolate the net effect of the study meals by controlling for possible confounders. However, we cannot rule out that there was a residual effect of breakfast on glucose profiles that intersects with the effect of the study meal.21 Second, we controlled for the effect of insulin during the study by having the participants receive identical meal insulin boluses based on the predetermined and known carbohydrate content of each meal. However, inappropriate insulin dosing due to suboptimal insulin to carbohydrate ratios could result in high intersubject variability that may only be partially associated with the effect of the meal. In addition, the study was powered to detect a difference in the postprandial glycemic response between rice and pasta. Future studies with larger number of subjects, or with pastas with greater differences in protein content, may be able to elucidate differences between the different types of pasta as well. It should also be noted that the participants of the study had a mean age of 58.7 ± 14.2 years, which is a relatively older adult population and can affect the results of our study since the metabolic rate is reduced with age.31 Finally, we recognize real-life meal sizes may vary significantly from the study meals in the amount of pasta versus the amount of rice that an individual may choose in a nonexperimental setting.
In the future, it may be useful to compare the postprandial glycemic responses of pasta (and/or white rice) with other commonly consumed carbohydrate food options, such as (1) potatoes—in various forms, (2) white bread—in various forms, (3) brown rice, (4) whole grain bread, and (5) other popular whole grains such as quinoa, barley, and so on. Importantly, this line of research should not be limited to studies of individual ingredient options, for example, pasta, white rice, white bread, and potatoes, eaten singularly, but rather to the relative effects of these commonly consumed foods within the context of an entire meal or spectrum of commonly consumed meals, as we know that the metabolic effects of carbohydrates are predictably altered when these foods are consumed in combination with other macronutrients.4 As such, it may be worth considering future experiments whereby certain popular restaurant meals from one restaurant or chain are compared with different meal choices from that same restaurant, or to meals from other restaurants (vs. home-cooked options) in terms of their comparative impact on blood sugar control.
In conclusion, this study suggests that there is a significant difference in postprandial glycemic response between rice and both types of pasta that agrees with earlier studies in individuals with T1D but is now verified for recent commercial products and the use of CGM to monitor glycemic profiles in real-life settings of complete meals. There is a possibility that pastas manufactured with different levels of fiber and protein may prove to be significantly different from one another, as similarly concluded by Anjana et al. for rice,32 but this will require additional research with larger numbers of subjects.
These initial observations set the stage for future research comparing the impact of individual food items as well as complete (reproducible) meals, either prepared at home or outside of the home, on the postprandial responses of individuals with T1D (as studied here) or with other types of diabetes in future studies, thereby contributing to their informed shopping, cooking, dining, and treating behaviors.
Supplementary Material
Acknowledgments
The authors thank all of the participants in this clinical trial. We also thank executive chef Paul DelleRose from The Culinary Institute of America for his extraordinary efforts in helping to prepare the study meals and teaching the study participants hands-on cooking classes, and Dr. Frank Hu for his thoughtful comments and discussion leading to the development of this study. This study was supported by an unrestricted gift from the Barilla Foundation to the Harvard John A. Paulson School of Engineering and Applied Sciences in support of collaborative nutrition research. The pasta used in the trial was provided by Barilla. Product support was provided by Dexcom, Inc. who provided research discount pricing on continuous glucose monitoring sensors, transmitters, and receivers (IIS-2017-045). This study was also conducted with statistical support from Harvard Catalyst, the Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and its affiliated academic health care centers.
Authors' Contributions
S.Z., J.M., S.D., J.E.P., M.C., F.J.D., E.D., and D.M.E. designed the research and analyzed the data. J.M., A.M., J.C., J.E.P., M.C., and C.A. conducted the research. S.Z., J.M., S.D., J.E.P., M.C., C.A., F.J.D., E.D., and D.M.E wrote the article. D.M.E. had primary responsibility for the final content. All authors read and approved the final article.
Disclaimers
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or the National Institutes of Health. The funders and device manufacturers had no influence on the design or conduct of the trial and were not involved in data collection or analysis, the writing of the article, or the decision to submit it for publication.
Author Disclosure Statement
D.M.E. has served as a consultant to and member of a scientific advisory committee of The Culinary Institute of America and is a scientific advisor to the Health and Wellness Advisory Committee of the Barilla Center for Food & Nutrition (Italy). J.E.P. has received product support to his institution as principal investigator from Dexcom, Inc. outside of the submitted study. No competing financial interests relevant to this project are reported for the rest of the authors.
Supplementary Material
References
- 1. Centers for Disease Control and Prevention (CDC): National Diabetes Statistics Report, 2017. Estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf (accessed September25, 2018)
- 2. Nathan DM, Genuth S, Lachin J, et al. : The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 3. Lu J, Ma X, Zhou J, et al. : Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care 2018;41:2370–2376 [DOI] [PubMed] [Google Scholar]
- 4. Bell KJ, Smart CE, Steil GM, et al. : Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015;38:1008–1015 [DOI] [PubMed] [Google Scholar]
- 5. Mohan V, Spiegelman D, Sudha V, et al. : Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in overweight Asian Indians: a randomized controlled trial. Diabetes Technol Ther 2014;16:317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeevi D, Korem T, Zmora N, et al. : Personalized nutrition by prediction of glycemic responses. Cell 2015;163:1079–1094 [DOI] [PubMed] [Google Scholar]
- 7. Bozzetto L, Giorgini M, Alderisio A, et al. : Glycaemic load versus carbohydrate counting for insulin bolus calculation in patients with type 1 diabetes on insulin pump. Acta Diabetologica 2015;52:865–871 [DOI] [PubMed] [Google Scholar]
- 8. Wong JC, Foster NC, Maahs DM, et al. : Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care 2014;37:2702–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ehrhardt NM, Chellappa M, Walker MS, et al. : The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol 2011;5:668–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vesco AT, Jedraszko AM, Garza KP, Weissberg-Benchell J: Continuous glucose monitoring associated with less diabetes-specific emotional distress and lower A1c among adolescents with type 1 diabetes. J Diabetes Sci Technol 2018;12:792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeSalvo DJ, Miller KM, Hermann JM, et al. : Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D Exchange and DPV Initiative. Pediatr Diabetes 2018;19:1271–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pounis G, Castelnuovo AD, Costanzo S, et al. : Association of pasta consumption with body mass index and waist-to-hip ratio: results from Moli-sani and INHES studies. Nutr Diabetes 2016;6:e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wolever TMS, Jenkins DJA, Kalmusky J, et al. : Glycemic response to pasta: effect of surface area, degree of cooking, and protein enrichment. Diabetes Care 1986;9:401–404 [DOI] [PubMed] [Google Scholar]
- 15. Hermansen K, Rasmussen OW, Arnfred J, et al. : Differential glycaemic effects of potato, rice and spaghetti in type 1 (insulin-dependent) diabetic patients at constant insulinaemia. Diabetologia 1986;29:358–361 [DOI] [PubMed] [Google Scholar]
- 16. Granfeldt Y, Bjorck I: Glycemic response to starch in pasta: a study of mechanisms of limited enzyme availability. J Cereal Sci 1991;14:47–61 [Google Scholar]
- 17. Kim EH, Petrie JR, Motoi L, et al. : Effect of structural and physicochemical characteristics of the protein matrix in pasta on in vitro starch digestibility. Food Biophys 2008;3:229–234 [Google Scholar]
- 18. Bell KJ, Toschi E, Steil GM, Wolper HA: Optimized mealtime insulin dosing for fat and protein in type 1 diabetes: application of a model-based approach to derive insulin doses for open-loop diabetes management. Diabetes Care 2016;39:1631–1634 [DOI] [PubMed] [Google Scholar]
- 19. Annan F: What matters for calculating insulin bolus dose? Diabetes Technol Ther 2016;18:213–215 [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association: 4. Lifestyle management: standards of medical care in diabetes-2018. Diabetes Care 2018;41:S38–S50 [DOI] [PubMed] [Google Scholar]
- 21. Meng H, Matthan NR, Ausman LM, Lichtenstein AH: Effect of prior meal macronutrient composition on postprandial glycemic responses and glycemic index and glycemic load value determinations. Am J Clin Nutr 2017;106:1246–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parillo M, Giacco R, Riccardi G, et al. : Different glycaemic responses to pasta, bread, and potatoes in diabetic patients. Diabet Med 1985;2:374–377 [DOI] [PubMed] [Google Scholar]
- 23. Huang M, Li J, Ha MA, et al. : A systematic review on the relations between pasta consumption and cardio-metabolic risk factors. Nutr Metab Cardiovasc Dis 2017;27:939–948 [DOI] [PubMed] [Google Scholar]
- 24. Kristensen M, Jensen MG, Riboldi G, et al. : Wholegrain vs. refined wheat bread and pasta. Effect on postprandial glycemia, appetite, and subsequent ad libitum energy intake in young healthy adults. Appetite 2010;54:163–169 [DOI] [PubMed] [Google Scholar]
- 25. Bozzetto L, Alderisio A, Giorgini M, et al. : Extra-virgin olive oil reduces glycemic response to a high-glycemic index meal in patients with type 1 diabetes: a randomized controlled trial. Diabetes Care 2016;39:518–524 [DOI] [PubMed] [Google Scholar]
- 26. Bacchetti T, Saturni L, Turco I, Ferretti G: The postprandial glucose response to some varieties of commercially available gluten-free pasta: a comparison between healthy and celiac subjects. Food Funct 2014;5:3014–3017 [DOI] [PubMed] [Google Scholar]
- 27. Nansel TR, Lipsky LM, Liu A: Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes. Am J Clin Nutr 2016;104:81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ryan RL, King BR, Anderson DG, et al. : Influence of and optimal insulin therapy for a low-glycemic index meal in children with type 1 diabetes receiving intensive. Diabetes Care 2008;31:1485–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bell KJ, King BR, Shafat A, Smart CE: The relationship between carbohydrate and the mealtime insulin dose in type 1 diabetes. J Diabetes Complications 2015;29:1323–1329 [DOI] [PubMed] [Google Scholar]
- 30. Parillo M, Annuzzi G, Rivellese AA, et al. : Treatment Effects of meals with different glycaemic index on postprandial blood glucose response in patients with Type 1 diabetes treated with continuous subcutaneous insulin infusion. Diabet Med 2010;28:227–229 [DOI] [PubMed] [Google Scholar]
- 31. Lazzer S, Bedogni G, Lafortuna CL, et al. : Relationship between basal metabolic rate, gender, age, and body composition in 8,780 white obese subjects. Obesity 2010;18:71–78 [DOI] [PubMed] [Google Scholar]
- 32. Anjana RM, Gayathri R, Lakshmipriya N, et al. : Effect of a novel high fiber rice diet on 24-hour glycemic responses in Asian Indians using continuous glucose monitoring: a randomized clinical trial. Diabetes Technol Ther 2019;21:177–182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.