Abstract
Objective: Analyze real-world usage and impact of a predictive low-glucose suspend (PLGS) insulin delivery system for maintenance of euglycemia and prevention of hypoglycemic events in people with insulin-dependent diabetes.
Methods: Retrospective analysis of Tandem Basal-IQ users who uploaded at least 21 days of PLGS usage data between August 31, 2018, and March 14, 2019 (N = 8132). Insulin delivery and sensor-glucose concentrations were analyzed. The times spent below 70 mg/dL, between 70 and 180 mg/dL, and above 180 mg/dL were assessed. Subgroup analyses were conducted to examine matched pre-/postoutcomes with experienced users (n = 1371) and performance over time for a mixed subgroup with >9 weeks of data (n = 3563).
Results: The mean age of patients was 32.4 years, 52% were female, 96% had type 1 diabetes, and 4% had type 2 diabetes. Mean duration on PLGS was 65 days. Algorithm introduction led to a 45% median relative risk reduction in sensor time <70 mg/dL, pre/post (% <70:2.0, 1.1), while the mean glucose remained stable (168 and 168 mg/dL). Mean frequency of hypoglycemic events decreased from one every 9 days to one every 30 days. Total daily insulin dose decreased from 43.4 to 42.3 U in the pre/post subgroup. Manual override of the system was low (4.5%). The number of daily suspensions remained stable (4.9).
Conclusions: Introduction of PLGS resulted in effective and sustained prevention of hypoglycemia without a significant increase in mean blood glucose and may be considered for people with type 1 diabetes at risk for hypoglycemia.
Keywords: Basal-IQ, Real-world, Predictive low-glucose suspend (PLGS), Insulin pump, Tandem, Hypoglycemia, Automated insulin delivery
Introduction
Hypoglycemia continues to be a dangerous and common, if not the most dangerous and common, sequelae for people living with insulin-dependent diabetes.1 Severe hypoglycemia can lead to significant morbidity and mortality, burdening both patients and the health care system. Each year in the United States, hypoglycemia is responsible for over 100,000 emergency room visits, approximately one-third of which result in hospitalization. A person who experiences mild biochemical hypoglycemia, defined as percent of time blood glucose is <70 mg/dL, is at increased risk for subsequent severe hypoglycemia and impaired glucose counter regulation, hypoglycemia awareness, and reduced quality of life.2 According to the Diabetes Control and Complications Trial data set, one or more measured blood glucose readings of <70 mg/dL in a single day can triple the individual's risk of a severe hypoglycemic event in the next 3 months.2 Beyond these clinical outcomes, the fear of hypoglycemia significantly affects the way individuals manage their diabetes and contributes to a lower overall quality of life.3,4
Interactive technology designed to prevent hypoglycemia is commercially available in the United States. For instance, real-time continuous glucose monitoring (CGM) serves to alert patients or caregivers of impending hypoglycemia, triggering preventative treatment, resulting in less severe outcomes. Recent evidence indicates CGM can decrease hypoglycemia by 13%–19%.5 However, CGM alerts and alarms only work to reduce hypoglycemia if (1) a person hears them and (2) the patient responds behaviorally by eating carbohydrates or taking glucose tabs and/or by manually reducing basal insulin delivery (for insulin pump users).6
Beyond CGM, predictive low-glucose suspend (PLGS) algorithms offer an additional and automated layer of hypoglycemia prevention. PLGS systems use CGM values to predict hypoglycemia and automatically suspend insulin delivery to help prevent hypoglycemia. This alleviates the burden of diabetes management7 and can be impactful for those with hypoglycemia unawareness, since in many cases no behavioral intervention is required to help avoid adverse outcomes. PLGS systems are different than threshold suspend systems8,9 in that PLGS systems predict future estimated blood glucose levels and suspend insulin to prevent hypoglycemia, whereas threshold suspend systems turn off insulin once estimated blood glucose levels are already low. Clinical research has demonstrated that PLGS systems significantly reduce hypoglycemia.10–18
One such PLGS system is the t:slim X2 insulin pump with Basal-IQ technology, which includes the t:slim X2 insulin pump and a PLGS algorithm embedded in the pump software (Tandem Diabetes Care, San Diego) that utilizes glucose values from a compatible CGM. The PLGS algorithm uses the last four sensor glucose values to predict sensor glucose values 30 min into the future. Insulin delivery suspends if the predicted glucose is <80 mg/dL or if the last observed glucose reading is below 70 mg/dL. Insulin delivery resumes in the following instances: sensor glucose rises, glucose is no longer predicted to drop below 80 mg/dL, no CGM data are available for 10 min, or an insulin suspension exceeds 120 min in any 150-min period.
The results of the PLGS for reduction of low glucose (PROLOG) trial demonstrated that PLGS significantly reduced hypoglycemia without increased mean glucose or hyperglycemia.10 During the PROLOG trial, those using the PLGS system experienced a 31% reduction in time spent <70 mg/dL, from 3.2% to 2.6%, compared to those using a sensor-augmented insulin pump (SAP).
Basal-IQ technology became commercially available in the United States in August 2018. Users of the system are able to upload pump data, including insulin suspensions, user overrides, sensor glucose readings, and other data, to Tandem's t:connect® diabetes management application. Uploading data to the diabetes management application helps patients review, track, and analyze their individual data. This study relied on deidentified data that users voluntarily uploaded into the diabetes management application.
Research Design and Methods
We conducted a retrospective analysis using deidentified data from the t:connect diabetes management application. The analysis included data from patients who were 6+ years old and who had at least 21 days of PLGS system use between August 31, 2018 and March 14, 2019 (N = 8123).
We also conducted two subanalyses to further evaluate the impact of the PLGS system. Subgroup A included experienced pump and CGM patients who used the PLGS system for at least 21 days, following a period of at least 21 days of using the same sensor-augmented t:slim X2 insulin pump without PLGS (n = 1371). Subgroup B comprised patients who had at least 9 weeks of PLGS system data (n = 3563). The majority of patients in subgroup B have no data before starting to use the PLGS system and some may have used an insulin pump for the first time.
The primary objective was to evaluate the impact of PLGS use on rates of hypoglycemia, defined as CGM-measured percentage of time spent <70 mg/dL. Other hypoglycemia outcomes that were measured included the percentage of time <54 mg/dL, the percentage of time <50 mg/dL, and the number of hypoglycemic events per day (defined according to Forlenza et al.10 as glucose values <54 mg/dL for at least 15 min, and ending glucose values >70 mg/dL for 30 min). The time in range was defined as 70–180 mg/dL glucose, and hyperglycemia outcomes were defined as the percentage of time spent at >180, >250, and >300 mg/dL glucose level. When the time in hypoglycemia is reduced, a rise in mean glucose is to be expected. Therefore, a corrected mean glucose was calculated by considering only CGM values >70 mg/dL for pre/post comparison. CGM metrics were assessed overall and separately for daytime (6 AM–10 PM) and nighttime (10 PM–6 AM). Insulin delivery was separated into delivery of basal, bolus, and total daily dose. The number of algorithm-driven suspensions per day, mean suspension duration, and the glucose values at suspension and resumption of insulin delivery were evaluated. Patient interaction was measured by (1) the percent of manual PLGS suspension overrides and (2) daily carbohydrates entered by the patient for each bolus.
Outcomes were aggregated by mean or median depending on their distribution. Means are reported with standard deviations, and medians are reported with interquartile ranges. For the pre/post analysis, paired t-tests and Wilcoxon-signed rank tests were performed where indicated. The effect size for skewed distributions was evaluated using the Hodges-Lehman estimator.19 Differences between age groups and pre/post samples were analyzed using t-tests. Trends in the usage over time were evaluated using linear regression and verified using the F-test and R2. The first day of usage was excluded from all regression analyses as an outlier. Regression analyses were conducted in python using the Statsmodels package. The pre/post analyses were performed in JASP.
Results
The analyzed cohort included 8123 individuals with insulin-dependent diabetes. Their age range was 6–90 years (mean age 32.4 years), 52% were female, 96% had type 1 diabetes, and 4% had type 2 diabetes. Mean number of days using PLGS was 65, and PLGS was enabled 98% of the time. The pre/post subgroup A (n = 1371) and subgroup B (n = 3563) were similarly composed (Table 1). Subgroup B included 689 patients from subgroup A who had used the PLGS system for at least 9 weeks.
Table 1.
Cohort Demographics
| n | Overall | Subgroup A | Subgroup B |
|---|---|---|---|
| 8123 | 1371 | 3563 | |
| Mean days of use | 65 (±35) | 50 (±19) | 63 |
| Age, mean, (SD) | 32.4 (±19) | 33.7 (±20) | 31.9 (±19) |
| Age, range | 6–90 | 6–87 | 6–87 |
| Under 18, n (%) | 2696 (33) | 491 (36) | 1220 (34) |
| 18–60, n (%) | 4729 (58) | 750 (55) | 2054 (58) |
| Over 60, n (%) | 698 (9) | 130 (10) | 289 (8) |
| Female sex, n (%) | 4211 (52) | 688 (50) | 1851 (52) |
| Type 1, n (%) | 7814 (96) | 1316 (96) | 3455 (97) |
| Type 2, n (%) | 309 (4) | 55 (4) | 108 (3) |
SD, standard deviation.
Hypoglycemia
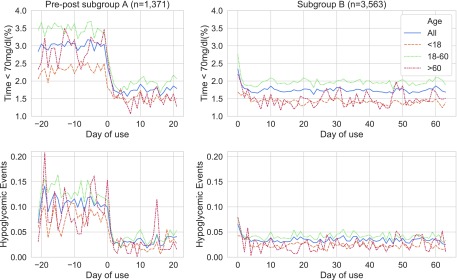
In the pre/post subgroup (Fig. 1), the introduction of PLGS reduced the median percent time <70 mg/dL to 1.1% from 2.0%, an absolute reduction of −0.91% (95% confidence interval [CI] −0.83 to −1.0, P < 0.001). This represents a 45% relative risk reduction (RRR) of hypoglycemia. The corresponding mean percent time <70 mg/dL dropped from 3.0% to 1.76%, a 1.23% absolute reduction (95% CI −1.43 to −1.03, P < 0.001). The subgroup B in Figure 1 shows that the mean time in hypoglycemia (<70 mg/dL) decreased sharply on the first day and remained low (1.74%) over time.
FIG. 1.
Hypoglycemia and hypoglycemic events in subgroup (A, B): the percent of sensor glucose time < 70 mg/dL, shown in the top row, pre- and post-PLGS, based on the day of use. Day 0 is the first day of using PLGS. The second row depicts the rate of hypoglycemic events per day, pre- and post-PLGS, based on the day of use.
The number of hypoglycemic events per day in subgroup A fell by 0.08, from 0.11 to 0.03 (95% CI −0.09 to 0.07, P < 0.001), a RRR of 71%. The median percent time spent at <54 and <50 mg/dL glucose level fell correspondingly to 0.13% and 0.07% (P < 0.001). The number of hypoglycemic events remained at 0.03 per day for subgroup B. In other words, patients using PLGS experienced on average one hypoglycemic event every 30 days instead of one event every 9 days. This is a skewed distribution in which 54% of PLGS users did not experience any hypoglycemic event during the analyzed period compared to 40% of users without PLGS.
Hypoglycemia outcomes for the overall sample (Table 2) improved or matched the pre/post subgroup performance (+0.1, P = 0.089). Median time <70 mg/dL was 1.01% (0.98% during the day, 0.84% at night) and the mean time <70 mg/dL was 1.68%. Overall time spent <54 and <50 mg/dL was 0.12% and 0.07%, respectively. Thirty-one percent of patients never had a CGM reading <50 mg/dL. Hypoglycemia outcomes were more favorable for patients >60 years old and comparable for the other two age groups.
Table 2.
Real-World Outcomes: Pre/Post Subgroup A; All patients; Age Specific
| Before PLGS | With PLGS | Effect size | Pa | All patients | <18 | 18–60 | >60 | |
|---|---|---|---|---|---|---|---|---|
| N | 1371 | 8123 | 2696 | 4729 | 698 | |||
| % Glucose <70 mg/dL | ||||||||
| Median | 2.02 (0.8, 4.0) | 1.1 (0.5, 2.3) | −0.91 (−0.83, −1.0) | <0.001 | 1.01 (0.41, 2.13) | 0.9 | 1.1 | 0.75 |
| Mean | 3.0 (± 3.2) | 1.76 (± 2.02) | −1.23 (−1.43, −1.03) | <0.001 | 1.66 (± 2.1) | 1.4 | 1.85 | 1.41 |
| Hypoglycemic events | 0.11 (± 0.23) | 0.03 (± 0.09) | −0.08 (−0.09, −0.07) | <0.001 | 0.03 (± 0.1) | 0.025 | 0.036 | 0.031 |
| Overall glucose control | ||||||||
| Mean glucose (mg/dL) | 168 (± 31) | 168 (± 31) | +0.1 (−2.20, 2.48) | 0.70 | 173 (± 32) | 188 | 167 | 164 |
| Corrected mean glucose | 171 (± 31) | 170 (± 31) | −1.1 (−0.4, −1.8) | 0.002 | 175 (± 32) | 189 | 169 | 165 |
| % Glucose 70–180 mg/dL | 60.6 (± 17) | 61.9 (± 18) | +1.3 (0.03, 2.65) | <0.001 | 59.1 (± 18) | 51 | 63 | 66 |
| Hypoglycemia | ||||||||
| % Glucose <54 mg/dL | 0.33 (0.1, 0.8) | 0.13 (0, 0.3) | −0.24 (−0.22, −0.28) | <0.001 | 0.12 (0.03, 0.3) | 0.12 | 0.13 | 0.08 |
| % Glucose <50 mg/dL | 0.19 (0, 0.5) | 0.07 (0, 0.2) | −0.16 (−0.14, −0.18) | <0.001 | 0.07 (0.02, 0.19) | 0.06 | 0.07 | 0.04 |
| Hyperglycemia | ||||||||
| % Glucose >180 mg/dL | 36.4 (23.0, 49.4) | 36.3 (22.0, 49.6) | −0.07 (−0.4, 0.3) | 0.72 | 38.8 (25.4, 52.4) | 48.1 | 34.7 | 32.2 |
| % Glucose >250 mg/dL | 10.1 (3.8, 18.5) | 8.9 (3.1, 17.9) | −0.61 (−0.41, −0.81) | <0.001 | 10.5 (4.3, 20.3) | 17.3 | 8.1 | 6.3 |
| % Glucose >300 mg/dL | 3.0 (0.7, 7.5) | 2.3 (0.5, 6.3) | −0.49 (−0.39, −0.61) | <0.001 | 3.0 (0.8, 7.7) | 6.1 | 2.0 | 1.5 |
| Insulin | ||||||||
| Total dose delivered (U) | 43.4 (29.7, 61.2) | 42.3 (29.6, 60.3) | −0.51 (−0.5, 0.03) | 0.076 | 44.3 (30.9, 62.0) | 40.5 | 46.6 | 39.9 |
| Basal (U) | 20.1 (13.5, 29.1) | 19.8 (13.4, 28.7) | −0.3 (−1.1, 0.5) | 0.11 | 20.2 (13.4, 28.7) | 16.8 | 23.1 | 19.6 |
| Bolus (U) | 22.0 (14.3, 32.8) | 21.2 (14.0, 33.0) | −0.16 (−1.13, 0.8) | 0.11 | 23.0 (15.1, 33.6) | 22.9 | 22.7 | 19.5 |
| PLGS suspensions | ||||||||
| No. per day | N/A | 5.1 (3.6, 7.2) | N/A | N/A | 4.86 (3.3, 6.8) | 4.89 | 4.9 | 4.2 |
| Duration (min) | N/A | 16:19 | N/A | N/A | 16:12 | 15:45 | 16:27 | 16:16 |
| Glucose at suspend | N/A | 105 (± 10) | N/A | N/A | 106 (± 11) | 111 | 104 | 103 |
| Glucose at resume | N/A | 96 (± 10) | N/A | N/A | 97 (± 10) | 100 | 96 | 96 |
| User interaction | ||||||||
| % Resume due to patient | N/A | 4.3 (± 4.5) | N/A | N/A | 4.5 (± 5.2) | 5.7 | 4.1 | 4.6 |
| Temporary basal rate | 67 (39, 104) | 98 (50, 140) | +17 (14, 20) | <0.001 | 85 (45, 128) | 98 | 80 | 87 |
| Carbohydrates per day | 147 (± 87) | 147 (± 90) | +0.2 | 0.98 | 149.3 (± 96.5) | 190 | 131 | 121 |
Data before and with PLGS (Predictive low-glucose suspend) are shown as median (quartiles) or as the mean (± SD).
Based on paired t-tests or Wilcoxon signed-rank test. Outcomes for the overall sample split by day and night can be found in supplementary table S1.
Glycemic control
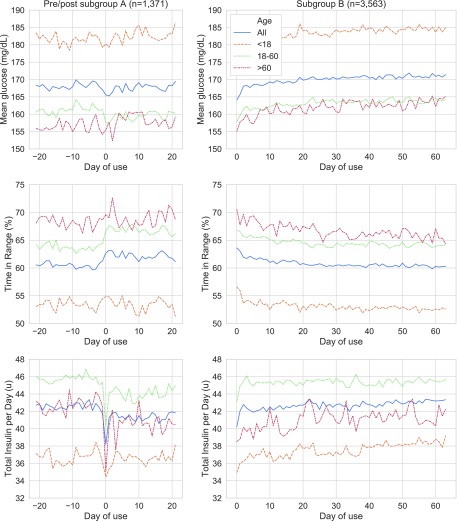
In subgroup A, the mean glucose remained at 168 mg/dL, while the corrected mean glucose revealed a small but significant reduction (−1.1 mg/dL, 95% CI −0.4 to −1.8, P = 0.002) after PLGS was introduced (Table 2). The time in range (70–180 mg/dL) improved accordingly by 1.34% from 60.6% to 61.9% (95% CI 0.03–2.65, P < 0.001).
In subgroup B, the time in range decreased gradually in the initial 3 weeks and then remained stable at 60.4% (Fig. 2). Correspondingly, the mean glucose increased slightly. The mean glucose increase was fitted to a logarithmic function of the days of usage. A significant regression equation was found (F(1, 62) = 275.5, P < 0.001), with an R2 of 82.1%. The overall mean glucose increased by 1.16 mg/dL with the logarithm of each day of use. As a result, the mean glucose at the end of the 9-week period was 4 mg/dL higher than after the initial rapid reversal in the first 2 days (172 and 168 mg/dL).
FIG. 2.
Glycemic outcomes and insulin delivery in subgroup (A) (left) and subgroup (B) (right). It should be noted that both subgroups may have differed in their glycemic control and insulin delivery before starting PLGS, but only SAP (Sensor augmented pump) data for subgroup A were available. For the intersection of both groups please refer to supplementary figure S1.
Glycemic control in the overall cohort was marked by significantly higher values of mean glucose (+5 mg/dL, P < 0.001) and lower time in range (−2.8%, P < 0.001) over time; however, the mean glucose and time in range varied significantly with patient age. Patients <18 years old demonstrated the highest starting and mean glucose (188 mg/dL), and patients >60 years old experienced the highest time in range (66%).
Insulin use
In the pre/post subgroup, median total daily insulin delivered fell by 0.51 U from 43.4 to 42.3 U (95% CI −0.5 to 0.03 U, P < 0.076). The bolus-to-basal insulin ratio remained unchanged. The mean carbohydrate intake documented in bolus requests remained constant.
In subgroup B with at least 9 weeks of PLGS usage, the median total daily insulin dose delivered increased by 1.26 U (95% CI 0.97 to 1.46 U, P < 0.001) from the day after starting PLGS over 9 weeks (Fig. 2), which was mainly attributable to a linear increase in basal insulin. A simple linear regression was calculated to predict the mean number of units of basal insulin delivered, based on the day after starting use of PLGS. A significant regression equation was found (F(1, 62) = 1140, P < 0.001), with an R2 of 95%. The mean basal insulin delivery across this subgroup rose by 0.014 U with each day of use after the first day. We found no changes in the frequency (mean of 5.0), duration (mean of 16:19 min) of suspend events, or manual suspend overrides (mean 4.5%). The carbohydrate intake reported in bolus requests did not change over time.
In the overall sample, the total insulin delivered for all patients was 44.3 U, which consisted of 48.5% basal and 51.5% bolus insulin. Patients <18 years old relied more on bolus insulin (57%) and required the least amount of insulin, with 40.5 total daily units. PLGS stopped insulin delivery an average of 4.86 times a day, and each suspension lasted an average of 16 min, 12 s. Insulin suspended at a mean CGM-measured glucose level of 106 mg/dL and resumed at 97 mg/dL. Patients >60 years old experienced fewer suspensions, and patients <18 years old had the shortest suspend durations. Patients manually stopped ongoing suspensions in 4.6% of cases. Patients <18 years old interrupted suspensions 36% more often and entered 45% more carbohydrates than adults.
Conclusions
This was the first study to examine the real-world impact of a PLGS system. Introduction of PLGS led to an immediate and sustained reduction in hypoglycemia and hypoglycemic events. Mean percent of time spent at <70 mg/dL glucose level observed in the real world was 1.44% lower than in the PLGS arm of the PROLOG clinical study.10 The observed 45% RRR of hypoglycemia with use of PLGS exceeded the reported benefits of the predictive low alarm of the Dexcom G6 sensor alone, which, in a recent study,5 achieved a RRR of hypoglycemia of 13%–19%. Similarly, Puhr et al. reported that use of the Dexcom G6 sensor alone resulted in a 33%–40% reduction in time <54 mg/dL,5 while PLGS reduced time spent at <54 mg/dL glucose level by 60.6%.
After introduction of PLGS, corrected mean glucose decreased slightly in the pre/post subgroup but gradually reverted to the mean. Subgroup B surprised with a concurrent increase in the mean glucose and total daily insulin delivery driven by a rising basal rate. Although not clinically significant and lacking historic baseline data, the observed association of decreased hypoglycemia with slightly higher mean glucose and insulin requirements might have a number of interpretations and contributing factors. One possibility is that once the user is confident that the PLGS system will prevent hypoglycemia, the basal rates are increased to promote glycemic control.
We hypothesize that behavioral changes due to trust in the system and reduced fear of hypoglycemia may be significant factors contributing to the observed findings in subgroup B. The fear of hypoglycemia is a major driver for patient caution.3,4,6 Recent research has also shown that for patients and caregivers, experiencing reduced hypoglycemia leads to increased confidence and trust in the technology, increased flexibility around mealtimes, and reduced diabetes distress.10 The observed low rate of patient-cancelled insulin suspensions supports this view, and more research is needed to verify this effect.
The strength of this retrospective analysis was the large sample size, the ability to analyze patients as they switched from SAP pump technology to the PLGS system, as well as the opportunity to follow a large cohort over time.
The limitations of the analysis include a potential self-selection of patients who upload their data to the diabetes management application, self-reported demographic data, and lack of a control arm. The lack of historic data in the subgroup B complicates interpretation as day zero readings reflect measurements after system initiation and cannot assess prior baselines. Analyzed outcomes were determined by the system back-end, and no clinician-facing or patient-reported outcomes were included. The PLGS system was designed to predict and help prevent hypoglycemia by monitoring glucose values from a connected CGM. Analyzing interruptions of CGM operation and connectivity were outside the scope of this analysis. Functionality to reduce hyperglycemia will complement future systems.
In conclusion, the PLGS significantly lowers hypoglycemia across age groups, and the effect is persistent over multiple weeks with real-world use. We observed a modest, counterintuitive increase in mean glucose and insulin delivery over time in select populations, which we speculate could be a product of system trust and a release in dietary discretion, perhaps driven by the reduced fear of hypoglycemia. The study results indicate that Basal-IQ technology might be a viable option for an ambulatory insulin-dependent population. Overall, these data support the use of algorithm-enhanced automated insulin delivery systems to improve health outcomes in patients with insulin-dependent diabetes. These may be appropriate for people with diabetes with difficult to control disease or those at particular risk for hypoglycemia.
Author Contributions
L.M. analyzed the data and wrote the article. S.H. wrote the article, organized the data, and edited the article. S.L. analyzed the data. E.S. wrote and edited the article. E.S. is the guarantor of this work and, as such, had full access to all the analyzed data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Supplementary Material
Author Disclosure Statement
Two authors, S.H. and S.L., are employees of Tandem Diabetes Care (San Diego, CA). Tandem Diabetes Care funded the PROLOG trial and the third-party data verification performed by University of California, San Diego School of Medicine and Design Lab Center for Health. E.S. and L.M. report grants from Dexcom outside of the submitted work. UC San Diego provided in-kind support for the final data analysis and article preparation.
Supplementary Material
References
- 1. Bronstone A, Graham C: The potential cost implications of averting severe hypoglycemic events requiring hospitalization in high-risk adults with type 1 diabetes using real-time continuous glucose monitoring. J Diabetes Sci Technol 2016;10:905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C: The association of biochemical hypoglycemia with the subsequent risk of a severe hypoglycemic event: analysis of the DCCT data set. Diabetes Technol Ther 2019;21:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martyn-Nemeth P, Schwarz Farabi S, Mihailescu D, et al. : Fear of hypoglycemia in adults with type 1 diabetes: impact of therapeutic advances and strategies for prevention—a review. J Diabetes Complications 2016;30:167–177 [DOI] [PubMed] [Google Scholar]
- 4. Wild D, von Maltzahn R, Brohan E, Christensen T, et al. : A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns 2007;68:10–15 [DOI] [PubMed] [Google Scholar]
- 5. Puhr S, Derdzinski M, Welsh JB, et al. : Real-world hypoglycemia avoidance with a continuous glucose monitoring system's predictive low glucose alert. Diabetes Technol Ther 2019;21:155–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pickup JC, Holloway MF, Samsi K: Real-time continuous glucose monitoring in type 1 diabetes: a qualitative framework analysis of patient narratives. Diabetes Care 2015;38:544–550 [DOI] [PubMed] [Google Scholar]
- 7. Forlenza GP, Messer LH, Berget C, et al. : Biopsychosocial factors associated with satisfaction and sustained use of artificial pancreas technology and its components: a call to the technology field. Curr Diab Rep 2018;18:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergenstal RM, Klonoff DC, Garg SK, et al. : Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 9. Bergenstal RM, Tamborlane WV, Ahmann A, et al. : Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med 2010;363:311–320 [DOI] [PubMed] [Google Scholar]
- 10. Forlenza GP, Li Z, Buckingham BA, et al. : Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care 2018;41:2155–2161 [DOI] [PubMed] [Google Scholar]
- 11. Abraham MB, Nicholas JA, Smith GJ, et al. : Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care 2017;41:dc171604. [DOI] [PubMed] [Google Scholar]
- 12. Lewis DM, Swain RS, Donner TW: Improvements in A1C and time-in-range in DIY closed-loop (OpenAPS) users. Diabetes 2018;67:352-OR [Google Scholar]
- 13. Stone MP, Agrawal P, Chen X, et al. : Retrospective analysis of 3-month real-world glucose data after the MiniMed 670G System Commercial Launch. Diabetes Technol Ther 2018;20:689–692 [DOI] [PubMed] [Google Scholar]
- 14. Zhong A, Choudhary P, McMahon C, et al. : Effectiveness of automated insulin management features of the MiniMed® 640G Sensor-Augmented Insulin Pump. Diabetes Technol Ther 2016;18:657–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Faulds ER, Dungan KM: Real-world implementation of hybrid closed-loop (HCL) insulin delivery. Diabetes 2018;67:49-LB [Google Scholar]
- 17. Weisman A, Bai J-W, Cardinez M, et al. : Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 18. Forlenza GP, Raghinaru D, Cameron F, et al. : Predictive hyperglycemia and hypoglycemia minimization: in-home double-blind randomized controlled evaluation in children and young adolescents. Pediatr Diabetes 2018;19:420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hodges JL, Lehmann EL: Estimates of location based on rank tests. Ann Math Stat 1963;598–611 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.