Abstract
Significance: Vascular dysfunction plays a key role in the development of arteriosclerosis, heart disease, and hypertension, which causes one-third of deaths worldwide. Vascular oxidative stress and metabolic disorders contribute to vascular dysfunction, leading to impaired vasorelaxation, vascular hypertrophy, fibrosis, and aortic stiffening. Mitochondria are critical in the regulation of metabolic and antioxidant functions; therefore, mitochondria-targeted treatments could be beneficial.
Recent Advances: Vascular dysfunction is crucial in hypertension pathophysiology and exhibits bidirectional relationship. Metabolic disorders and oxidative stress contribute to the pathogenesis of vascular dysfunction and hypertension, which are associated with mitochondrial impairment and hyperacetylation. Mitochondrial deacetylase Sirtuin 3 (Sirt3) is critical in the regulation of metabolic and antioxidant functions. Clinical studies show that cardiovascular disease risk factors reduce Sirt3 level and Sirt3 declines with age, paralleling the increased incidence of cardiovascular disease and hypertension. An imbalance between mitochondrial acetylation and reduced Sirt3 activity contributes to mitochondrial dysfunction and oxidative stress. We propose that mitochondrial hyperacetylation drives a vicious cycle between metabolic disorders and mitochondrial oxidative stress, promoting vascular dysfunction and hypertension.
Critical Issues: The mechanisms of mitochondrial dysfunction are still obscure in human hypertension. Mitochondrial hyperacetylation and oxidative stress contribute to mitochondrial dysfunction; however, regulation of mitochondrial acetylation, the role of GCN5L1 (acetyl-CoA-binding protein promoting acetyltransferase protein acetylation) acetyltransferase, Sirt3 deacetylase, and acetylation of specific proteins require further investigations.
Future Directions: There is an urgent need to define molecular mechanisms and the pathophysiological role of mitochondrial hyperacetylation, identify novel pharmacological targets, and develop therapeutic approaches to reduce this phenomenon.
Keywords: mitochondria, oxidative stress, hyperacetylation, vascular dysfunction, hypertension, Sirt3 deacetylase
Introduction
Hypertension is a multifactorial disorder (52); however, in almost all experimental models of hypertension, production of reactive oxygen species (ROS: O2•− and hydrogen peroxide [H2O2]) is increased in multiple organs. In the brain, ROS promote neuronal firing, increasing sympathetic outflow (78, 132). In the kidney, ROS act in multiple sites to promote sodium resorption and volume retention (114). In the vasculature, ROS promote vasoconstriction and remodeling, increasing systemic vascular resistance (72). There are several sources of ROS contributing to hypertension, including the NADPH oxidase, uncoupled nitric oxide (NO) synthase, and the mitochondria (28) and we defined their interaction (25, 27). ROS overproduction leads to oxidative stress, which promotes target-organ-damage in hypertension (22); however, antioxidant therapy is not currently available and common antioxidants such as ascorbate and vitamin E are ineffective in preventing cardiovascular diseases and hypertension (47). These agents unlikely reach important sites of ROS production such as the mitochondria whereas therapies specifically targeted at mitochondria represent promising strategies to reduce target-organ-damage (95).
Mitochondrial dysfunction contributes to the pathogenesis of hypertension and cardiovascular disease (34, 95); however, despite the central role of mitochondria in human health and disease, there are no approved drugs that directly target mitochondria (118). Mitochondrial dysfunction is characterized by impaired adenosine triphosphate (ATP) production and increased oxidative stress, leading to cell dysfunction and apoptosis (36). Mitochondrial permeability transition pore (mPTP) plays a key role in mitochondrial dysfunction (48) and target-organ-damage in hypertension (36, 95). We have recently reported that depletion or inhibition of cyclophilin D (CypD), a regulatory subunit of mPTP opening (37), improves vascular function and attenuates hypertension (59). Previous studies implicated CypD in cell death (68, 111) and we showed that CypD is critical in the regulation of cytokine-induced vascular oxidative stress and endothelial dysfunction (59). Meanwhile, the precise regulation of CypD is elusive and specific CypD blockers are not available.
Experimental studies have shown an important role of mitochondrial ROS in the development of endothelial dysfunction, hypertension, and atherosclerosis (86, 94), and the overexpression of the key mitochondrial antioxidant, Mn-superoxide dismutase (SOD2), attenuates hypertension (30). SOD2 expression is regulated by peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (65), and mitochondrial deacetylase Sirtuin 3 (Sirt3) activates SOD2 by deacetylation of specific lysine residues near active center (55, 93, 131). Interestingly, human SOD2 expression is not changed with age but the activity of Sirt3 and SOD2 is diminished (12), suggesting that SOD2 inactivation by acetylation contributes to human hypertension. These data suggest Sirt3 depletion in endothelial dysfunction and hypertension. Indeed, we have found that hypertension is associated with diminished Sirt3 level, a profound increase in SOD2-K68 acetylation in humans with essential hypertension, and reduced SOD2 activity in the vasculature of hypertensive mice and that SOD2 mimetics improve vasorelaxation and reduce blood pressure (30, 31).
Sirt3 is a key node in the regulation of mitochondrial function (53). It activates mitochondrial metabolism by deacetylation of Krebs cycle (103), complex I (1, 90), and fatty acid β-oxidation enzymes (7, 56), and it maintains mitochondrial NADPH-GSH redox status by deacetylation of isocitrate dehydrogenase 2 (IDH2) (128). Sirt3 deacetylation is opposed by spontaneous acetylation of mitochondrial protein lysine residues with acetyl coenzyme A (Acetyl-CoA) (61, 91) and GCN5L1 (acetyl-CoA-binding protein promoting acetyltransferase protein acetylation) mediated acetyltransferase (97). An imbalance between mitochondrial acetylation and Sirt3 activity leads to mitochondrial hyperacetylation and contributes to impaired metabolism and oxidative stress (16, 24). Interestingly, hypertension and endothelial dysfunction are associated with mitochondrial hyperacetylation (31); however, molecular mechanisms and the pathophysiological role of mitochondrial hyperacetylation are not completely understood.
In this work, we reviewed specific pathways involved in the metabolic and redox regulation of mitochondrial functions. These data suggest a potential pathophysiological role of crosstalk between mitochondrial hyperacetylation and oxidative stress. We suggest that targeting of this feed-forward vicious cycle between metabolic disorders and mitochondrial oxidative stress can be beneficial for the treatment of vascular dysfunction and hypertension.
Mitochondrial Acetylation as a Major Post-Translational Modification
Mitochondrial metabolism generates high levels of Acetyl-CoA (0.1–1.5 mM), which is 3–50 times higher than cytosol and nucleus Acetyl-CoA concentrations (51). High concentration of Acetyl-CoA and high pH in mitochondrial matrix drive non-enzymatic acetylation of lysine residues (116). Acetylation can be more favorable at the active site lysine residues, with reduced pKa values suggesting that regulatory lysine residues can attract acetylation (43). Recently, Michael Murphy group showed that non-enzymatic N-acetylation of lysine residues in mitochondrial proteins frequently occurs via a proximal S-acetylated thiol intermediate (61). It is clear that metabolic disorders are associated with high levels of Acetyl-CoA and promote mitochondrial acetylation; however, these data do not exclude the possibility of enzyme-mediated protein acetylation in mitochondria. Indeed, Michael Sack group has described GCN5L1-mediated acetyltransferase, which plays a critical role in the acetylation of key mitochondrial enzymes such as SOD2 (97, 98). It has been proposed that GCN5L1-mediated acetylation counterbalances the Sirt3 deacetylase activity. Both enzymatic and non-enzymatic pathways account for protein acetylation as a major post-translational modification in the mitochondria, and ∼35% of all mitochondrial proteins are acetylated (4).
Mitochondrial Deacetylase Sirt3 in Regulation of Metabolic and Antioxidant Functions
Sirtuin family of nicotinamide adenine dinucleotide, oxidized form (NAD+)-dependent histone deacetylases catalyzes deacetylation of both histone and non-histone lysine residues and consists of seven isoforms (123). Mitochondria contain one known enzyme with deacetylase activity, Sirt3 (80). It plays a key role in the regulation of mitochondrial metabolism and the activity of mitochondrial antioxidants. Sirt3 activates a key fatty acid β-oxidation enzyme, long-chain acyl coenzyme A dehydrogenase (LCAD) (7, 56), Krebs cycle (103), nicotinamide adenine dinucleotide, reduced form (NADH) oxidase activity by complex I (1, 90), NADPH-producing IDH2 (128), and critical mitochondrial antioxidant SOD2 (110) by deacetylation of specific lysine residues (55, 56, 93, 131). Sirt3 can potentially activate AMP protein kinase via deacetylation/activation of LKB1 (serine/threonine liver kinase B1) (40), and it is conceivable that crosstalk between Sirt3 and adenosine monophosphate-activated protein kinase (AMPK) contributes to metabolic regulations. It has been suggested that both nuclear deacetylase Sirtuin 1 (Sirt1) and Sirt3 induce mitochondrial biogenesis via the PGC-1α pathway (79); however, the role of Sirt3 in mitochondrial biogenesis has not been confirmed. Of note, activation of different Sirtuin isoforms not only regulates distinct pathways but also may have opposing effects. For example, Sirt4 negatively affects fatty acid oxidation and Sirt4 depletion increases expression of Sirt1 and Sirt3 (130).
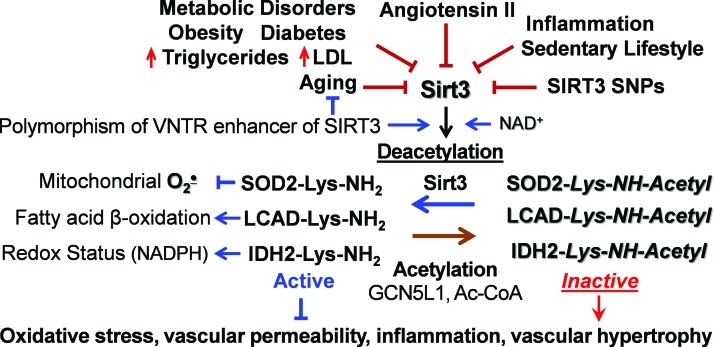
Sirt3 is associated with human longevity (5, 44). Variable number tandem repeat (VNTR) enhancer in Sirt3 gene is associated with human longevity, and two non-synonymous human SIRT3 single-nucleotide polymorphisms impact SIRT3 activity and stability (33). Activation of the angiotensin II/AT1R pathway reduces Sirt3 (15), and Sirt3 is downregulated in the metabolic syndrome, hyperlipidemia, diabetes, aging, and smoking (Fig. 1) (17, 75, 129). Reduced Sirt3 expression is associated with cell aging, as measured by diminished telomerase reverse transcriptase (hTERT) and increased senescence-associated β-galactosidase (SA-β-gal) (66, 121). Animal studies indicate that Sirt3 deficiency promotes tissue fibrosis (60) and cardiac hypertrophy (108), which are attenuated by Sirt3 activation (2, 107); however, the cell-specific role of Sirt3, its alterations in human pathological conditions, and Sirt3 targeting translational potential are not clear.
FIG. 1.
Multiple risk factors downregulate Sirt3, leading to mitochondrial dysfunction and oxidative stress. Sirt3 depletion causes mitochondrial hyperacetylation due to imbalance between acetylation and deacetylation pathways. This leads to SOD2 inactivation, inhibition of fatty acid β-oxidation, and altered redox status due to inactivation of SOD2, LCAD, and IDH2, which contribute to vascular dysfunction and hypertension. IDH2, isocitrate dehydrogenase 2; LCAD, long-chain acyl coenzyme A dehydrogenase; Sirt3, mitochondrial deacetylase Sirtuin 3; SOD2, mitochondrial manganese superoxide dismutase. Color images are available online.
Redox and Metabolic Regulations of Sirt3
Sirt3 requires NAD+ for its deacetylase activity, and “healthy” mitochondria have high NAD+ level and only a small fraction of reduced NADH form (122). Pathological conditions such as hypoxia and metabolic disorders are associated with (i) reduction of NAD+ to NADH diminishing NAD+ level, and (ii) depletion of NAD pool due to PPARγ activation (92). For example, inhibition of mitochondrial complex I NADH oxidase activity reduces NAD+ level, leading to Sirt3 inactivation and mitochondrial hyperacetylation (1). It has been suggested that NAD+ depletion contributes to Sirt3 inactivation in cardiovascular conditions and supplementation with NAD+ donors is beneficial due to Sirt3 activation (58); however, oral supplementation with NAD+ donors has limited pharmacological effect, potentially due to rapid liver metabolism (77).
Sirt3 activity depends on mitochondrial function and matrix pH (127); therefore, reduced membrane potential downregulates Sirt3 activity. Sirt3 is important for metabolic flexibility and Sirt3 depletion induces a switch of skeletal muscle substrate utilization from carbohydrate oxidation toward lactate production (62). Meanwhile, increased substrate utilization leads to high NADH/NAD+ ratio and elevated Acetyl-CoA, which inhibits Sirt3 activity and provides “on-demand” On/Off metabolic switch.
In recent years, it has become clear that Sirtuins can be inactivated by oxidative stress. For example, the activity of Sirt1 is regulated by reversible S-glutathionylation at Cys204 in the catalytic region (11). Sirt1 cysteine reaction with H2O2 drives S-glutathionylation, and glutaredoxin 2 activates Sirt1 by deglutathionylation of cysteine residue in the conserved catalytic region (11). Because the NAD+-dependent deacetylases are highly homologous, we hypothesized that Sirt3 might undergo redox inactivation in a manner similar to Sirt1. Indeed, incubation of human recombinant Sirt3 in the presence of H2O2 and reduced glutathione caused a dose-dependent inactivation of Sirt3, which was associated with Sirt3 S-glutathionylation (31). We reasoned that Sirt3 S-glutathionylation would be reduced in transgenic mCAT (mice expressing mitochondria-targeted catalase) mice with mitochondria-targeted expression of catalase. Indeed, angiotensin II-induced hypertension was associated with robust Sirt3 S-glutathionylation in the mitochondria of wild-type mice but scavenging of mitochondrial H2O2 in mCAT mice abrogates Sirt3 S-glutathionylation, prevents SOD2 inactivation by acetylation, diminishes mitochondrial superoxide, and attenuates hypertension (31). These data support the pathophysiological significance of Sirt3 S-glutathionylation in vascular dysfunction and hypertension.
It is important that glutaredoxins 1/2 in the mitochondrial intermembrane and thioredoxin-thioredoxin reductase in the matrix are critical for reversing mitochondrial protein S-glutathionylation in the vasculature and heart (42, 54). Acetylation of these proteins affects their activity and alters the redox maintenance (124). Further, hyperoxidation of cysteine residues into sulfenic, sulfinic, and sulfonic acids may cause irreversible loss of enzymatic activities and impair the recovery from oxidative stress (45).
Redox and Metabolic Regulations of CypD
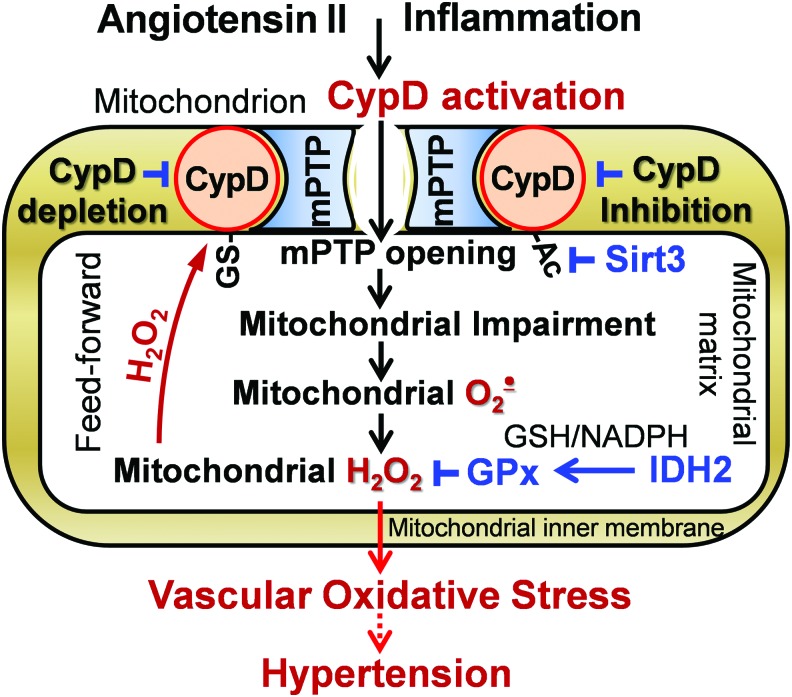
CypD plays a dual function in mitochondria: peptidyl prolyl cis-trans isomerase F and a regulatory subunit of the mPTP acting as a Ca2+ sensitizer for mPTP opening (83). Previous studies were focused on the important role of CypD in the regulation of cell death (68, 111), but recent data implicate CypD in the regulation of mitochondrial metabolism (71). CypD is exquisitely H2O2 sensitive via its cysteine 203 residue, which acts as a redox switch when it is S-glutathionylated (76). Further, CypD acetylation at lysine 166 promotes mPTP opening and mitochondrial Sirt3 deacetylates CypD-K166 (49). These data implicate both redox and metabolic regulations of CypD activity (Fig. 2).
FIG. 2.
CypD activation by S-glutathionylation and acetylation promotes mitochondrial impairment and oxidative stress, which contribute to vascular dysfunction and hypertension, and CypD depletion or CypD inhibition improves vascular function and diminishes hypertension. CypD, cyclophilin D; H2O2, hydrogen peroxide; mPTP, mitochondrial permeability transition pore. Color images are available online.
Recent data suggest a feed-forward regulation of CypD by oxidative stress. It has been shown that inhibition of CypD in isolated endothelial mitochondria reduces superoxide production and CypD deficiency attenuates superoxide production in leukocytes (32, 69). We tested whether mitochondrial H2O2 activates CypD by S-glutathionylation and this induces overproduction of mitochondrial ROS in the electron transport chain. Indeed, treatment of isolated aortic segments with H2O2 significantly increased mitochondrial superoxide and induced CypD S-glutathionylation whereas supplementation with the specific CypD inhibitor Sanglifehrin A or treatment with the complex I inhibitor rotenone blocked H2O2-induced mitochondrial superoxide production (59). Further, scavenging mitochondrial H2O2 by mitoEbselen (mitochondria-targeted glutathione peroxidase mimetic) or mitochondrial-targeted catalase in aorta isolated from mCAT mice completely prevented CypD S-glutathionylation and reduced mitochondrial superoxide. These data support the pathophysiological role of CypD redox modulation; however, specific molecular mechanisms of CypD S-glutathionylation and deglutathionylation are not clear.
David Sinclair group reported that global Sirt3 depletion induces acetylation of CypD at lysine 166 (K166), which promotes age-dependent mPTP opening, cardiac hypertrophy, and fibrosis (49). Authors suggested that age-associated Sirt3 depletion causes CypD hyperacetylation, which increases induction of the mPTP opening and the decline in cardiac function with age. CypD-K166-directed mutagenesis and Sirt3 overexpression in cardiomyoblasts prevented CypD acetylation, limited PTP opening, and reduced cell death in response to hypoxia-reoxygenation (9). Meanwhile, the effects of global Sirt3 depletion or Sirt3 overexpression are not limited by CypD acetylation, and further studies are necessary to define the specific regulation of CypD acetylation/deacetylation and its tissue-specific pathophysiological role.
Deacetylation of IDH2 in Regulation of Redox Status
Several mitochondrial enzymes maintain thiol redox status by NADPH-dependent reduction of glutathione, S-glutathionylated proteins, and protein disulfides such as glutathione reductase and thioredoxin reductase (63). Glutathione and thioredoxin systems serve parallel and non-redundant functions to maintain the dynamic mitochondrial redox balance and disruption of this redox organization is a common basis for disease (63, 64). Meanwhile, the maintenance of mitochondrial redox balance requires NADPH produced mainly by nicotinamide nucleotide transhydrogenase and IDH2 (74, 128). Depletion of either nicotinamide nucleotide transhydrogenase or IDH2 induces endothelial dysfunction and promotes hypertension (74, 88). Meanwhile, Sirt3-mediated deacetylation of IDH2 at lysine 413 is critical for IDH2 activity (128). Site-specific, genetic incorporation of N(ɛ)-acetyllysine into position 413 of IDH2 causes a dramatic 44-fold loss of activity.
Disruption of IDH2-mediated cellular redox balance enhances H2O2-induced apoptosis and hypertrophy (70). IDH2 deficiency increases mitochondrial superoxide, leading to mitochondrial dysfunction and diminished endothelial NO, which can be rescued by SOD2 mimetic mitoTEMPO (88). IDH2 functions as the principal source of NADPH for the mitochondrial GPx (Fig. 2) and thioredoxin antioxidant defense (50), and IDH2 depletion accelerates age-dependent hearing loss and renal dysfunction (73, 119). Meanwhile, IDH2 overexpression leads to “reductive stress” and contributes to genome instability and cancer (21). Therefore, Sirt3-mediated regulation of IDH2 plays an important role in cellular homeostasis.
Regulation of Mitochondrial Oxidative Stress
Mitochondrial oxidative stress is commonly defined as an imbalance between mitochondrial ROS production and antioxidant activity, which is associated with oxidative damage and cell dysfunction (29). It is frequently confused with redox signaling by thiol redox reactions serving as redox sensors in response to oxygen, metabolic, and oxidant fluxes (46, 101). Initial ROS production can induce specific cell signaling pathways mediated by protein phosphorylation and transcriptional factors such as NO synthase and NRF2 (transcription factor nuclear factor erythroid 2-related factor 2), which later provide a feed-back to downregulate the ROS production (13, 102). The dysregulation of redox signaling (wrong time-wrong place) leads to a feed-forward ROS-induced-ROS production and development of oxidative stress (25). Indeed, mitochondrial oxidative stress is associated with altered thiol redox status and scavenging of mitochondrial H2O2 reduces production of mitochondrial superoxide (28, 32). It has been suggested that both redox and metabolic alterations contribute to the development of mitochondrial oxidative stress (101).
Production of mitochondrial superoxide via reverse electron transport (RET) is of particular importance in vascular and cardiac oxidative stress (20, 84). Inhibition of mitochondrial complex II with malate reduces RET to complex I, inhibits superoxide production at complex I, and reduces endothelial oxidative stress (84, 87). Mitochondrial ROS promote T cell activation and prohypertensive immune response (29). RET is induced by high mitochondrial membrane potential and mitochondrial matrix alkalization, which can be associated with opening of the ATP-sensitive potassium channel (84) and CypD-mediated mPTP (59). Indeed, blocking the ATP-sensitive potassium channel and treatment with complex I and complex II inhibitors prevent endothelial oxidative stress (84). Further, CypD depletion or CypD inhibition prevents the rotenone-sensitive superoxide production (59). Interestingly, scavenging of mitochondrial H2O2 prevents CypD redox activation by S-glutathionylation, reduces mitochondrial superoxide production, and prevents cytokine-induced endothelial dysfunction (59) (Fig. 2). Meanwhile, the specific roles of redox and metabolic alterations of complex I and CypD in RET and mitochondrial dysfunction are still obscure.
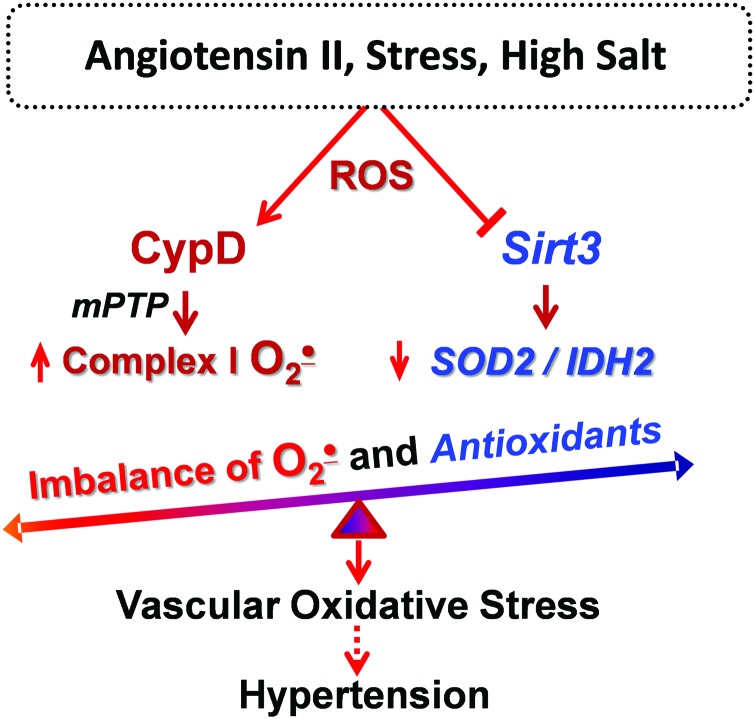
In recent years, it has become clear that metabolic conditions drive mitochondrial hyperacetylation, which induces mitochondrial oxidative stress and vascular dysfunction (41, 126). Hypertension is associated with metabolic impairment, and we showed that vascular oxidative stress and hypertension are associated with inactivation of key mitochondrial antioxidant enzyme, SOD2, due to SOD2 hyperacetylation. Treatment of mitochondrial lysate with recombinant Sirt3 deacetylates SOD2 and restores SOD2 activity (31). The impairment of Sirt3 in hypertension is likely mediated by Sirt3 depletion and Sirt3 S-glutathionylation (31). Sirt3 depletion in endothelial cells increases superoxide level in these cells and promotes endothelial dysfunction, whereas treatment of Sirt3-depleted mice after onset of hypertension with SOD2 mimetic mitoTEMPO rescues endothelial function and reduces hypertension (31). We suggest that Sirt3 inactivation results in imbalance between CypD-dependent superoxide production and SOD2/IDH2 activities, leading to mitochondrial oxidative stress, which contributes to vascular dysfunction and hypertension (Fig. 3).
FIG. 3.
An imbalance between mitochondrial ROS (O2•− and H2O2) and antioxidant activity (SOD2 and IDH2) leads to mitochondrial oxidative stress and contributes to vascular dysfunction and hypertension. ROS, reactive oxygen species. Color images are available online.
Fatty Acid β-Oxidation and Sirt3
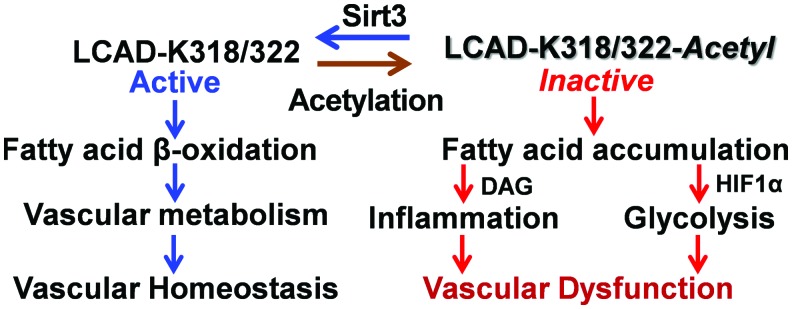
Fatty acids derived from triacylglycerols (fat) are important sources of energy and in tissues with high-energy requirement, such as the heart, more than 50% of ATP comes from fatty acid β-oxidation (81). Fatty acids are oxidized in peroxisomes and mitochondria. Mitochondria can oxidize fatty acids all the way to CO2 and H2O; however, peroxisomes are only able to chain-shorten fatty acids and the end products of peroxisomal β-oxidation must be transported to mitochondria for full oxidation (117). Fatty acids are transformed into fatty acyl-CoA and transported via Carnitine shuttle into mitochondrial matrix for β-oxidation. Sirt3 deacetylates and activates a key component of mitochondrial fatty acid β-oxidation, LCAD (7, 55, 56) (Fig. 4). K318 and K322 were identified as an Sirt3-targeted lysines (7). Medium-chain acyl-CoA dehydrogenase and acyl-CoA dehydrogenase 9 have lysines at positions equivalent to Lys-318/Lys-322, which were also efficiently deacetylated by Sirt3 (7).
FIG. 4.
The role of LCAD in vascular cell metabolism and potential effect of LCAD hyperacetylation. Hyperacetylation of LCAD contributes to mitochondrial dysfunction, increasing inflammation and metabolic switch to glycolysis, which promotes vascular dysfunction. Activation of the Sirt3 pathway deacetylates and activates LCAD, improving fatty acid β-oxidation and vascular metabolism, which support vascular homeostasis and protect from vascular dysfunction. Color images are available online.
Fatty acid β-oxidation is critical for endothelial and smooth muscle cells function (18, 125). Impaired fatty acid β-oxidation alters mitochondrial function and leads to accumulation of non-oxidized fatty acids, which promotes cell dedifferentiation and inflammation (14, 112). Fatty acid oxidation is critical for endothelial cell function, and disruption of fatty acid oxidation leads to phenotypic switch to endothelial-to-mesenchymal transition associated with vascular permeability and inflammation (19, 125) (Fig. 4). Fatty acids are important components of vascular smooth muscle cell function (100); dysregulation of long-chain fatty acid metabolism causes a shift toward glycolysis (109), downregulates expression of smooth muscle cell marker α-smooth muscle actin, and induces a phenotypic switch of smooth muscle cells (106). Multiple risk factors for cardiovascular disease and hypertension are associated with reduced Sirt3 expression and activity (39), and we suggest Sirt3 inactivation as a convergent mechanism that underlies the interplay of major risk factors leading to impaired fatty acid β-oxidation and mitochondrial dysfunction in these pathological conditions (Fig. 4).
Crosstalk Between Mitochondrial Dysfunction and Oxidative Stress
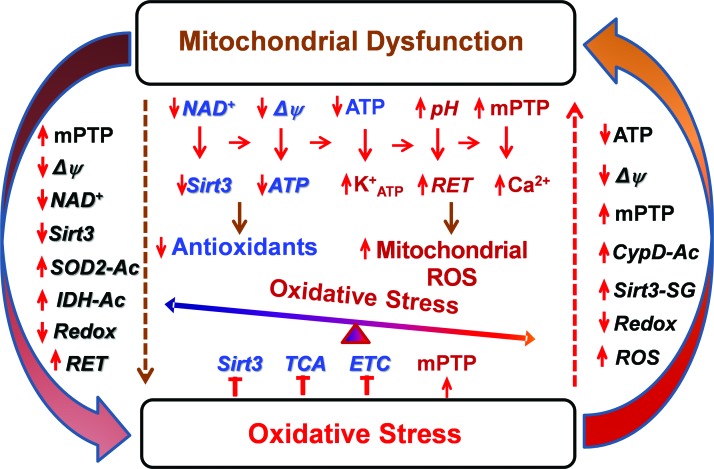
The sections described earlier provide an important insight into the molecular mechanisms of mitochondrial dysfunction and oxidative stress. It is interesting that oxidative stress is commonly associated with mitochondrial dysfunction and, vice versa, mitochondrial dysfunction causes ROS overproduction and development of oxidative stress. Indeed, enzymes that typically produce ROS are associated with metabolic regulation, and diseases associated with metabolic dysfunction involve changes in redox balance (38). Therefore, we suggest a crosstalk between mitochondrial dysfunction and oxidative stress (Fig. 5).
FIG. 5.
Crosstalk between mitochondrial dysfunction and oxidative stress. Mitochondrial dysfunction is associated with depletion of ATP and NAD+, diminished membrane potential (Δψ), increased matrix pH, mPTP opening, and increased ROS production via RET, which reduces Sirt3 activity, decreases antioxidants and redox state leading to development of oxidative stress. On the other hand, ROS overproduction directly inactivates Sirt3, Krebs cycle (TCA), and ETC and promotes mPTP opening, which leads to development of mitochondrial dysfunction. This feed-forward crosstalk results in the vicious cycle between mitochondrial dysfunction and oxidative stress. ATP, adenosine triphosphate; ETC, electron transport chain; NAD+, nicotinamide adenine dinucleotide, oxidized form; RET, reverse electron transport; TCA, tricarboxylic acid. Color images are available online.
Metabolic disorders and aging cause mitochondrial dysfunction associated with diminished respiration, increased mitochondrial uncoupling, altered membrane potential, and depletion of ATP and NAD+ (10, 23), which promote mPTP and ATP-sensitive potassium channel opening (84, 113), increase ROS production via RET, and reduce Sirt3 activity, decreasing redox state and antioxidant activity. Mitochondrial dysfunction may result from direct impairment by hyperacetylation and accumulation of toxic metabolites in certain metabolic diseases (96, 105). The activation of these pathways leads to development of oxidative stress (Fig. 5). On the other hand, oxidative stress inhibits Sirt3 and tricarboxylic acid (TCA) activity, reduces electron transfer by complex I, and promotes mPTP opening by S-glutathionylation of critical cysteine residues (31, 76, 82), leading to impaired mitochondrial metabolism and mitochondrial dysfunction. These data support a novel concept of crosstalk between mitochondrial dysfunction and oxidative stress. Meanwhile, the precise molecular mechanisms of this feed-forward vicious cycle and its pathophysiological significance in human diseases are still elusive. Further studies are required to define the role of individual pathways to specific human disease and develop mitochondria-targeted therapies.
Targeting Vicious Cycle Between Metabolic Disorders and Oxidative Stress
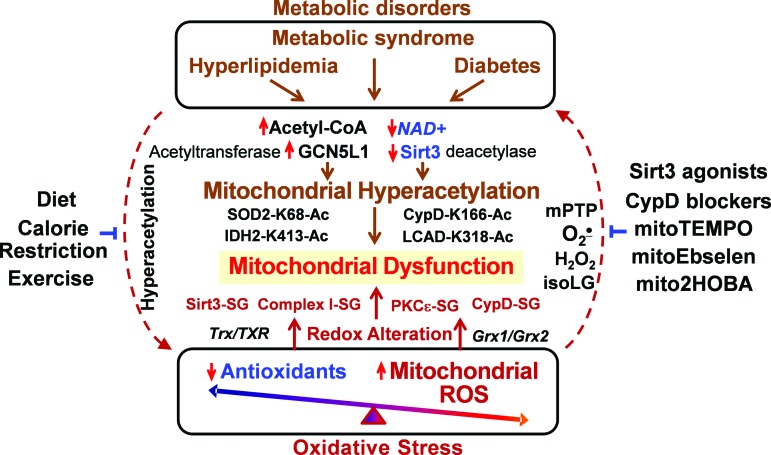
Both metabolic disorders and oxidative stress contribute to mitochondrial dysfunction, which plays an important role in multiple pathological conditions such as cardiovascular disease, hypertension, and neurodegeneration (38, 85). In the past decade, the focus of many studies was the role of oxidative stress in mitochondria dysfunction (26, 105); however, antioxidant therapy has not been developed. Mitochondria are an important source of superoxide and H2O2, which contribute to mitochondrial dysfunction and hypertension (28, 30, 120). This is likely mediated by S-glutathionylation/inactivation of key metabolic nodes, such as Sirt3 and complex I, redox activation of CypD-mediated mPTP opening (31, 76, 82), and impaired redox recovery by thioredoxin 2 (120). Oxidative stress triggers a vicious cycle where mitochondria are both the site and the target of oxidative damage (25). Oxidative modifications of mitochondrial metabolic targets such as Sirt3 lead to hyperacetylation of key mitochondrial metabolic and antioxidant enzymes such as LCAD, IDH2, CypD, and SOD2 (31), which promote metabolic disorders and disease progression (35, 99). On the other hand, metabolic disorders decrease NAD+/NADH ratio and increase acetyl-CoA/CoA ratio, leading to imbalance in mitochondrial protein acetylation/deacetylation and development of mitochondrial hyperacetylation (6), which contributes to mitochondrial dysfunction (91). This creates a vicious cycle between metabolic disorders and oxidative stress (Fig. 6).
FIG. 6.
Clinical translation: targeting vicious cycle between metabolic disorders and oxidative stress. Imbalance between acetylation and deacetylation leads to mitochondrial hyperacetylation, promotes mitochondrial dysfunction and oxidative stress, and, vice versa, oxidative stress contributes to redox impairment of key metabolic targets, resulting in mitochondrial dysfunction and metabolic impairment. Targeting of this feed-forward vicious cycle can be beneficial in the treatment of pathological conditions. Acetyl-CoA, acetyl coenzyme A; GCN5L1, acetyl-CoA-binding protein promoting acetyltransferase protein acetylation; mitoEbelson, mitochondria-targeted glutathione peroxidase mimetic; mitoTEMPO, mitochondria-targeted SOD2 mimetic. Color images are available online.
It is important to emphasize that metabolic disorders increase mitochondrial protein acetylation, which directly contributes to mitochondrial dysfunction (Fig. 6) in cardiovascular diseases and heart failure (57). The hyperacetylation of electron transport chain (complex I–V), TCA cycle enzymes, and LCAD inhibits mitochondrial bioenergetics (4). Mitochondrial hyperacetylation directly impairs mitochondrial dynamics (fusion/fission), protein synthesis, mitochondrial protein imports, calcium homeostasis, and cell signaling (3, 49, 89), which may occur without involvement of mitochondrial ROS. Meanwhile, we know that metabolic disorders are commonly associated with increased oxidative stress (8) and it is conceivable that targeting mitochondrial oxidative stress in metabolic disorders can improve mitochondrial function and alleviate these pathological conditions.
Recent studies show that treatment with mitochondria SOD2 mimetic mitoTEMPO, deacetylation of SOD2 by Sirt3 activators, and inhibition of mitochondrial ROS with CypD blockers or mitoEbselen can interrupt this vicious cycle and attenuate the mitochondrial dysfunction and disease progression (31, 59). On the other hand, calorie restriction and exercise increase Sirt3 activity, reduce mitochondrial acetylation, and inhibit mitochondrial oxidative stress (67, 93, 104, 115). Meanwhile, specific molecular targets that can be used for diagnostics and treatments in human disease are not clear, and further studies are warranted for the development of specific mitochondria-targeted therapies to break this vicious feed-forward cycle.
The direct links between the hyperacetylation of specific mitochondrial proteins, cell function, and hypertension have been supported by SOD2 hyperacetylation in human subjects with essential hypertension (31), mitochondrial hyperacetylation in pulmonary hypertension (35), and CypD acetylation in cardiac hypertrophy (49), which are in line with reduced Sirt3 level (39) and activity (12) in cardiovascular conditions, providing new insight into pathogenesis of mitochondrial dysfunction in cardiovascular conditions.
Conclusions
It is known that metabolic disorders increase risk of hypertension and cardiovascular disease. On the other hand, hypertension is frequently associated with metabolic abnormalities such as obesity, glucose intolerance, and dyslipidemia. These pathological conditions are associated with altered mitochondrial function and oxidative stress, suggesting the crosstalk between metabolic disorders and mitochondrial oxidative stress, which can be mediated by mitochondrial hyperacetylation. Metabolic disorders such as hyperglycemia and hyperlipidemia cause mitochondrial hyperacetylation, which leads to mitochondrial dysfunction and overproduction of mitochondrial ROS. On the other hand, mitochondrial oxidative stress in hypoxia and inflammation alter mitochondrial metabolism, causing the development of pathological conditions. We propose a novel crosstalk between mitochondrial hyperacetylation and oxidative stress. This crosstalk identifies potential novel targets for treatment of metabolic disorders and cardiovascular disease. There are many common conditions including aging, atherosclerosis, diabetes, heart failure, and neurodegenerative disorders in which mitochondrial dysfunction seems to play a role. It is conceivable that mitochondria-targeted interventions targeting the crosstalk between mitochondrial hyperacetylation and oxidative stress would be effective in these conditions.
Acknowledgments
This work was supported by funding from National Institutes of Health (R01HL124116) and the American Heart Association (16GRNT31230017).
Abbreviations Used
- Acetyl-CoA
acetyl coenzyme A
- ATP
adenosine triphosphate
- CypD
cyclophilin D
- GCN5L1
acetyl-CoA-binding protein promoting acetyltransferase protein acetylation
- H2O2
hydrogen peroxide
- IDH2
isocitrate dehydrogenase 2
- LCAD
long-chain acyl coenzyme A dehydrogenase
- mCAT
mice expressing mitochondria-targeted catalase
- mitoEbselen
mitochondria-targeted glutathione peroxidase mimetic
- mitoTEMPO
mitochondria-targeted SOD2 mimetic
- mPTP
mitochondrial permeability transition pore
- NAD+
nicotinamide adenine dinucleotide, oxidized form
- NADH
nicotinamide adenine dinucleotide, reduced form
- NO
nitric oxide
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- RET
reverse electron transport
- ROS
reactive oxygen species
- Sirt1
nuclear deacetylase Sirtuin 1
- Sirt3
mitochondrial deacetylase Sirtuin 3
- SOD2
mitochondrial manganese superoxide dismutase
- TCA
tricarboxylic acid
References
- 1. Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, and Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105: 14447–14452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akamata K, Wei J, Bhattacharyya M, Cheresh P, Bonner MY, Arbiser JL, Raparia K, Gupta MP, Kamp DW, and Varga J. SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget 7: 69321–69336, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park H-A, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jr, and Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A 111: 10580–10585, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson KA. and Hirschey MD. Mitochondrial protein acetylation regulates metabolism. Essays Biochem 52: 23–35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, Franceschi C, Passarino G, and De Benedictis G. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics 85: 258–263, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Berthiaume JM, Kurdys JG, Muntean DM, and Rosca MG. Mitochondrial NAD(+)/NADH redox state and diabetic cardiomyopathy. Antioxid Redox Signal 30: 375–398, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bharathi SS, Zhang Y, Mohsen AW, Uppala R, Balasubramani M, Schreiber E, Uechi G, Beck ME, Rardin MJ, Vockley J, Verdin E, Gibson BW, Hirschey MD, and Goetzman ES. Sirtuin 3 (SIRT3) protein regulates long-chain acyl-CoA dehydrogenase by deacetylating conserved lysines near the active site. J Biol Chem 288: 33837–33847, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhatti JS, Bhatti GK, and Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders—a step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis 1863: 1066–1077, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bochaton T, Crola-Da-Silva C, Pillot B, Villedieu C, Ferreras L, Alam MR, Thibault H, Strina M, Gharib A, Ovize M, and Baetz D. Inhibition of myocardial reperfusion injury by ischemic postconditioning requires sirtuin 3-mediated deacetylation of cyclophilin D. J Mol Cell Cardiol 84: 61–69, 2015 [DOI] [PubMed] [Google Scholar]
- 10. Bombicino SS, Iglesias DE, Mikusic IAR, D'Annunzio V, Gelpi RJ, Boveris A, and Valdez LB. Diabetes impairs heart mitochondrial function without changes in resting cardiac performance. Int J Biochem Cell Biol 81: 335–345, 2016 [DOI] [PubMed] [Google Scholar]
- 11. Bräutigam L, Jensen LD, Poschmann G, Nyström S, Bannenberg S, Dreij K, Lepka K, Prozorovski T, Montano SJ, Aktas O, Uhlén P, Stühler K, Cao Y, Holmgren A, and Berndt C. Glutaredoxin regulates vascular development by reversible glutathionylation of sirtuin 1. Proc Natl Acad Sci U S A 110: 20057–20062, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, and Chen D. SIRT3 reverses aging-associated degeneration. Cell Rep 3: 319–327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cai H, Li Z, Davis ME, Kanner W, Harrison DG, and Dudley SC., Jr Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol 63: 325–331, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Calder PC. Long chain fatty acids and gene expression in inflammation and immunity. Curr Opin Clin Nutr Metab Care 16: 425–433, 2013 [DOI] [PubMed] [Google Scholar]
- 15. Capettini LS, Montecucco F, Mach F, Stergiopulos N, Santos RA, and da Silva RF. Role of renin-angiotensin system in inflammation, immunity and aging. Curr Pharm Des 18: 963–970, 2012 [DOI] [PubMed] [Google Scholar]
- 16. Carrico C, Meyer JG, He W, Gibson BW, and Verdin E. The mitochondrial acylome emerges: proteomics, regulation by sirtuins, and metabolic and disease implications. Cell Metab 27: 497–512, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaudhry KN, Chavez P, Gasowski J, Grodzicki T, and Messerli FH. Hypertension in the elderly: some practical considerations. Cleve Clin J Med 79: 694–704, 2012 [DOI] [PubMed] [Google Scholar]
- 18. Chiong M, Cartes-Saavedra B, Norambuena-Soto I, Mondaca-Ruff D, Morales PE, García-Miguel M, and Mellado R. Mitochondrial metabolism and the control of vascular smooth muscle cell proliferation. Front Cell Dev Biol 2: 72, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho JG, Lee A, Chang W, Lee MS, and Kim J. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front Immunol 9: 294, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T, and Murphy MP. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515: 431–435, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clark O, Yen K, and Mellinghoff IK. molecular pathways: isocitrate dehydrogenase mutations in cancer. Clin Cancer Res 22: 1837–1842, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coats A. and Jain S. Protective effects of nebivolol from oxidative stress to prevent hypertension-related target organ damage. J Hum Hypertens 31: 376–381, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Conley KE, Marcinek DJ, and Villarin J. Mitochondrial dysfunction and age. Curr Opin Clin Nutr Metab Care 10: 688–692, 2007 [DOI] [PubMed] [Google Scholar]
- 24. D'Onofrio N, Vitiello M, Casale R, Servillo L, Giovane A, and Balestrieri ML. Sirtuins in vascular diseases: emerging roles and therapeutic potential. Biochim Biophys Acta 1852: 1311–1322, 2015 [DOI] [PubMed] [Google Scholar]
- 25. Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 51: 1289–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dikalov SI. and Dikalova AE. Contribution of mitochondrial oxidative stress to hypertension. Curr Opin Nephrol Hypertens 25: 73–80, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dikalov SI. and Nazarewicz RR. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal 19: 1085–1094, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassègue B, Griendling KK, Harrison DG, and Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal 20: 281–294, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dikalov SI. and Ungvari Z. Role of mitochondrial oxidative stress in hypertension. Am J Physiol Heart Circ Physiol 305: H1417–H1427, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, and Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107: 106–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dikalova AE, Itani HA, Nazarewicz RR, McMaster WG, Fessel JP, Flynn CR, Gamboa JL, Harrison DG, and Dikalov SI. Sirt3 impairment and SOD2 hyperacetylation in vascular oxidative stress and hypertension. Circ Res 121: 564–574, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doughan AK, Harrison DG, and Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction. Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 102: 488–496, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Dransfeld CL, Alborzinia H, Wölfl S, and Mahlknecht U. SIRT3 SNPs validation in 640 individuals, functional analyses and new insights into SIRT3 stability. Int J Oncol 36: 955–960, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Dromparis P. and Michelakis ED. Mitochondria in vascular health and disease. Annu Rev Physiol 75: 95–126, 2013 [DOI] [PubMed] [Google Scholar]
- 35. Egnatchik RA, Brittain EL, Shah AT, Fares WH, Ford HJ, Monahan K, Kang CJ, Kocurek EG, Zhu S, Luong T, Nguyen TT, Hysinger E, Austin ED, Skala MC, Young JD, Roberts LJ, 2nd, Hemnes AR, West J, and Fessel JP. Dysfunctional BMPR2 signaling drives an abnormal endothelial requirement for glutamine in pulmonary arterial hypertension. Pulm Circ 7: 186–199, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eirin A, Lerman A, and Lerman LO. Mitochondria: a pathogenic paradigm in hypertensive renal disease. Hypertension 65: 264–270, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elrod JW. and Molkentin JD. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ J 77: 1111–1122, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, and Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122: 877–902, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Freitas M, Rodrigues AR, Tomada N, Fonseca J, Magalhães A, Gouveia AM, and Neves D. Effects of aging and cardiovascular disease risk factors on the expression of sirtuins in the human corpus cavernosum. J Sex Med 12: 2141–2152, 2015 [DOI] [PubMed] [Google Scholar]
- 40. Fu J, Jin J, Cichewicz RH, Hageman SA, Ellis TK, Xiang L, Peng Q, Jiang M, Arbez N, Hotaling K, Ross CA, and Duan W. trans-(-)-epsilon-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington disease. J Biol Chem 287: 24460–24472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gao J, Zheng Z, Gu Q, Chen X, Liu X, and Xu X. Deacetylation of MnSOD by PARP-regulated SIRT3 protects retinal capillary endothelial cells from hyperglycemia-induced damage. Biochem Biophys Res Commun 472: 425–431, 2016 [DOI] [PubMed] [Google Scholar]
- 42. Gao XH, Qanungo S, Pai HV, Starke DW, Steller KM, Fujioka H, Lesnefsky EJ, Kerner J, Rosca MG, Hoppel CL, and Mieyal JJ. Aging-dependent changes in rat heart mitochondrial glutaredoxins—implications for redox regulation. Redox Biol 1: 586–598, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ghanta S, Grossmann RE, and Brenner C. Mitochondrial protein acetylation as a cell-intrinsic, evolutionary driver of fat storage: chemical and metabolic logic of acetyl-lysine modifications. Crit Rev Biochem Mol Biol 48: 561–574, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giblin W, Skinner ME, and Lombard DB. Sirtuins: guardians of mammalian healthspan. Trends Genet 30: 271–286, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Go YM, Chandler JD, and Jones DP. The cysteine proteome. Free Radic Biol Med 84: 227–245, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Go YM. and Jones DP. Thiol/disulfide redox states in signaling and sensing. Crit Rev Biochem Mol Biol 48: 173–181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Griendling KK. and Harrison DG. Out, damned dot: studies of the NADPH oxidase in atherosclerosis. J Clin Invest 108: 1423–1424, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gutiérrez-Aguilar M. and Baines CP. Structural mechanisms of cyclophilin D-dependent control of the mitochondrial permeability transition pore. Biochim Biophys Acta 1850: 2041–2047, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, and Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging 2: 914–923, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Han SJ, Choi HS, Kim JI, Park JW, and Park KM. IDH2 deficiency increases the liver susceptibility to ischemia-reperfusion injury via increased mitochondrial oxidative injury. Redox Biol 14: 142–153, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hansford RG. and Johnson RN. The steady state concentrations of coenzyme A-SH and coenzyme A thioester, citrate, and isocitrate during tricarboxylate cycle oxidations in rabbit heart mitochondria. J Biol Chem 250: 8361–8375, 1975 [PubMed] [Google Scholar]
- 52. Harrison DG. The Mosaic Theory revisited: common molecular mechanisms coordinating diverse organ and cellular events in hypertension. J Am Soc Hypertens 7: 68–74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. He W, Newman JC, Wang MZ, Ho L, and Verdin E. Mitochondrial sirtuins: regulators of protein acylation and metabolism. Trends Endocrinol Metab 23: 467–476, 2012 [DOI] [PubMed] [Google Scholar]
- 54. Hilgers RH, Kundumani-Sridharan V, Subramani J, Chen LC, Cuello LG, Rusch NJ, and Das KC. Thioredoxin reverses age-related hypertension by chronically improving vascular redox and restoring eNOS function. Sci Transl Med 9: pii: , 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, and Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hirschey MD, Shimazu T, Huang JY, Schwer B, and Verdin E. SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism. Cold Spring Harb Symp Quant Biol 76: 267–277, 2011 [DOI] [PubMed] [Google Scholar]
- 57. Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, Leone TC, Pagliarini DJ, Muoio DM, Bedi KC, Jr, Margulies KB, Coon JJ, and Kelly DP. Mitochondrial protein hyperacetylation in the failing heart. JCI insight 2: pii: , 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Houtkooper RH. and Auwerx J. Exploring the therapeutic space around NAD+. J Cell Biol 199: 205–209, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Itani HA, Dikalova AE, McMaster WG, Nazarewicz RR, Bikineyeva AT, Harrison DG, and Dikalov SI. Mitochondrial cyclophilin D in vascular oxidative stress and hypertension. Hypertension 67: 1218–1227, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jablonski RP, Kim SJ, Cheresh P, Williams DB, Morales-Nebreda L, Cheng Y, Yeldandi A, Bhorade S, Pardo A, Selman M, Ridge K, Gius D, Budinger GRS, and Kamp DW. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J 31: 2520–2532, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. James AM, Hoogewijs K, Logan A, Hall AR, Ding S, Fearnley IM, and Murphy MP. Non-enzymatic N-acetylation of lysine residues by AcetylCoA often occurs via a proximal S-acetylated thiol intermediate sensitive to glyoxalase II. Cell Rep 18: 2105–2112, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jing E, O'Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, Bain JR, Lee KY, Verdin EM, Newgard CB, Gibson BW, and Kahn CR. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes 62: 3404–3417, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jones DP. and Go YM. Redox compartmentalization and cellular stress. Diabetes Obes Metab 12 (Suppl 2): 116–125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jones DP. and Sies H. The redox code. Antioxid Redox Signal 23: 734–746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kang C. and Li Ji L. Role of PGC-1alpha signaling in skeletal muscle health and disease. Ann N Y Acad Sci 1271: 110–117, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Karnewar S, Neeli PK, Panuganti D, Kotagiri S, Mallappa S, Jain N, Jerald MK, and Kotamraju S. Metformin regulates mitochondrial biogenesis and senescence through AMPK mediated H3K79 methylation: relevance in age-associated vascular dysfunction. Biochim Biophys Acta 1864: 1115–1128, 2018 [DOI] [PubMed] [Google Scholar]
- 67. Kincaid B. and Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci 5: 48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kinnally KW, Peixoto PM, Ryu SY, and Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta 1813: 616–622, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kröller-Schön S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, Xia N, Hausding M, Mikhed Y, Zinssius E, Mader M, Stamm P, Treiber N, Scharffetter-Kochanek K, Li H, Schulz E, Wenzel P, Münzel T, and Daiber A. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal 20: 247–266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ku HJ. and Park JW. Downregulation of IDH2 exacerbates H2O2-mediated cell death and hypertrophy. Redox Rep 22: 35–41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lam MP, Lau E, Liem DA, and Ping P. Cyclophilin D and acetylation: a new link in cardiac signaling. Circ Res 113: 1268–1269, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee MY. and Griendling KK. Redox signaling, vascular function, and hypertension. Antioxid Redox Signal 10: 1045–1059, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lee SJ, Cha H, Lee S, Kim H, Ku HJ, Kim SH, Park JH, Lee JH, Park KM, and Park JW. Idh2 deficiency accelerates renal dysfunction in aged mice. Biochem Biophys Res Commun 493: 34–39, 2017 [DOI] [PubMed] [Google Scholar]
- 74. Leskov I, Neville A, Shen X, Pardue S, Kevil CG, Granger DN, and Krzywanski DM. Nicotinamide nucleotide transhydrogenase activity impacts mitochondrial redox balance and the development of hypertension in mice. J Am Soc Hypertens 11: 110–121, 2017 [DOI] [PubMed] [Google Scholar]
- 75. Li Y, Yu C, Shen G, Li G, Shen J, Xu Y, and Gong J. Sirt3–MnSOD axis represses nicotine-induced mitochondrial oxidative stress and mtDNA damage in osteoblasts. Acta Biochim Biophys Sin (Shanghai) 47: 306–312, 2015 [DOI] [PubMed] [Google Scholar]
- 76. Linard D, Kandlbinder A, Degand H, Morsomme P, Dietz KJ, and Knoops B. Redox characterization of human cyclophilin D: identification of a new mammalian mitochondrial redox sensor? Arch Biochem Biophys 491: 39–45, 2009 [DOI] [PubMed] [Google Scholar]
- 77. Liu L, Su X, Quinn WJ, 3rd, Hui S, Krukenberg K, Frederick DW, Redpath P, Zhan L, Chellappa K, White E, Migaud M, Mitchison TJ, Baur JA, and Rabinowitz JD. Quantitative analysis of NAD synthesis-breakdown fluxes. Cell Metab 27: 1067.e5–1080.e5, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lob HE, Schultz D, Marvar PJ, Davisson RL, and Harrison DG. Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension 61: 382–387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lomb DJ, Laurent G, and Haigis MC. Sirtuins regulate key aspects of lipid metabolism. Biochim Biophys Acta 1804: 1652–1657, 2010 [DOI] [PubMed] [Google Scholar]
- 80. Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr, Weissman S, Verdin E, and Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27: 8807–8814, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, and Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev 90: 207–258, 2010 [DOI] [PubMed] [Google Scholar]
- 82. Mailloux RJ. and Willmore WG. S-glutathionylation reactions in mitochondrial function and disease. Front Cell Dev Biol 2: 68, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, and Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005 [DOI] [PubMed] [Google Scholar]
- 84. Nazarewicz RR, Dikalova AE, Bikineyeva A, and Dikalov SI. Nox2 as a potential target of mitochondrial superoxide and its role in endothelial oxidative stress. Am J Physiol Heart Circ Physiol 305: H1131–H1140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Niemann B, Rohrbach S, Miller MR, Newby DE, Fuster V, and Kovacic JC. oxidative stress and cardiovascular risk: obesity, diabetes, smoking, and pollution: part 3 of a 3-part series. J Am Coll Cardiol 70: 230–251, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ohashi M, Runge MS, Faraci FM, and Heistad DD. MnSOD deficiency increases endothelial dysfunction in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 26: 2331–2336, 2006 [DOI] [PubMed] [Google Scholar]
- 87. Panov A, Schonfeld P, Dikalov S, Hemendinger R, Bonkovsky HL, and Brooks BR. The neuromediator glutamate, through specific substrate interactions, enhances mitochondrial ATP production and reactive oxygen species generation in nonsynaptic brain mitochondria. J Biol Chem 284: 14448–14456, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Park JB, Nagar H, Choi S, Jung SB, Kim HW, Kang SK, Lee JW, Lee JH, Park JW, Irani K, Jeon BH, Song HJ, and Kim CS. IDH2 deficiency impairs mitochondrial function in endothelial cells and endothelium-dependent vasomotor function. Free Radic Biol Med 94: 36–46, 2016 [DOI] [PubMed] [Google Scholar]
- 89. Parodi-Rullán RM, Chapa-Dubocq XR, and Javadov S. Acetylation of mitochondrial proteins in the heart: the role of SIRT3. Front Physiol 9: 1094, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Porter GA, Urciuoli WR, Brookes PS, and Nadtochiy SM. SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol 306: H1602–H1609, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pougovkina O, te Brinke H, Ofman R, van Cruchten AG, Kulik W, Wanders RJ, Houten SM, and de Boer VC. Mitochondrial protein acetylation is driven by acetyl-CoA from fatty acid oxidation. Hum Mol Genet 23: 3513–3522, 2014 [DOI] [PubMed] [Google Scholar]
- 92. Prolla TA. and Denu JM. NAD+ deficiency in age-related mitochondrial dysfunction. Cell Metab 19: 178–180, 2014 [DOI] [PubMed] [Google Scholar]
- 93. Qiu X, Brown K, Hirschey MD, Verdin E, and Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662–667, 2010 [DOI] [PubMed] [Google Scholar]
- 94. Rodriguez-Iturbe B, Sepassi L, Quiroz Y, Ni Z, Wallace DC, and Vaziri ND. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J Appl Physiol (1985) 102: 255–260, 2007 [DOI] [PubMed] [Google Scholar]
- 95. Rubattu S, Pagliaro B, Pierelli G, Santolamazza C, Castro SD, Mennuni S, and Volpe M. Pathogenesis of target organ damage in hypertension: role of mitochondrial oxidative stress. Int J Mol Sci 16: 823–839, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sack MN. Emerging characterization of the role of SIRT3-mediated mitochondrial protein deacetylation in the heart. Am J Physiol Heart Circ Physiol 301: H2191–H2197, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Scott I, Wang L, Wu K, Thapa D, and Sack MN. GCN5L1/BLOS1 links acetylation, organelle remodeling, and metabolism. Trends Cell Biol 28: 346–355, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Scott I, Webster BR, Li JH, and Sack MN. Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5L1. Biochem J 443: 655–661, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sheeran FL. and Pepe S. Mitochondrial bioenergetics and dysfunction in failing heart. Adv Exp Med Biol 982: 65–80, 2017 [DOI] [PubMed] [Google Scholar]
- 100. Shen H, Eguchi K, Kono N, Fujiu K, Matsumoto S, Shibata M, Oishi-Tanaka Y, Komuro I, Arai H, Nagai R, and Manabe I. Saturated fatty acid palmitate aggravates neointima formation by promoting smooth muscle phenotypic modulation. Arterioscler Thromb Vasc Biol 33: 2596–2607, 2013 [DOI] [PubMed] [Google Scholar]
- 101. Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem 289: 8735–8741, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sies H, Berndt C, and Jones DP. Oxidative stress. Annu Rev Biochem 86: 715–748, 2017 [DOI] [PubMed] [Google Scholar]
- 103. Sol EM, Wagner SA, Weinert BT, Kumar A, Kim HS, Deng CX, and Choudhary C. Proteomic investigations of lysine acetylation identify diverse substrates of mitochondrial deacetylase sirt3. PloS One 7: e50545, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, and Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143: 802–812, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Stepien KM, Heaton R, Rankin S, Murphy A, Bentley J, Sexton D, and Hargreaves IP. Evidence of oxidative stress and secondary mitochondrial dysfunction in metabolic and non-metabolic disorders. J Clin Med 6: pii: , 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sunaga H, Matsui H, Anjo S, Syamsunarno MR, Koitabashi N, Iso T, Matsuzaka T, Shimano H, Yokoyama T, and Kurabayashi M. Elongation of long-chain fatty acid family member 6 (Elovl6)-driven fatty acid metabolism regulates vascular smooth muscle cell phenotype through AMP-activated protein kinase/Kruppel-like factor 4 (AMPK/KLF4) signaling. J Am Heart Assoc 5: e004014, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sundaresan NR, Bindu S, Pillai VB, Samant S, Pan Y, Huang JY, Gupta M, Nagalingam RS, Wolfgeher D, Verdin E, and Gupta MP. SIRT3 blocks aging-associated tissue fibrosis in mice by deacetylating and activating GSK3beta. Mol Cell Biol 36: 678–692, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, and Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119: 2758–2771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sutendra G, Bonnet S, Rochefort G, Haromy A, Folmes KD, Lopaschuk GD, Dyck JR, and Michelakis ED. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med 2: 44ra58, 2010 [DOI] [PubMed] [Google Scholar]
- 110. Tao R, Vassilopoulos A, Parisiadou L, Yan Y, and Gius D. Regulation of MnSOD enzymatic activity by Sirt3 connects the mitochondrial acetylome signaling networks to aging and carcinogenesis. Antioxid Redox Signal 20: 1646–1654, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tavecchio M, Lisanti S, Lam A, Ghosh JC, Martin NM, O'Connell M, Weeraratna AT, Kossenkov AV, Showe LC, and Altieri DC. Cyclophilin D extramitochondrial signaling controls cell cycle progression and chemokine-directed cell motility. J Biol Chem 288: 5553–5561, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Toborek M, Lee YW, Garrido R, Kaiser S, and Hennig B. Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cells. Am J Clin Nutr 75: 119–125, 2002 [DOI] [PubMed] [Google Scholar]
- 113. Tonin AM, Amaral AU, Busanello EN, Gasparotto J, Gelain DP, Gregersen N, and Wajner M. Mitochondrial bioenergetics deregulation caused by long-chain 3-hydroxy fatty acids accumulating in LCHAD and MTP deficiencies in rat brain: a possible role of mPTP opening as a pathomechanism in these disorders? Biochim Biophys Acta 1842: 1658–1667, 2014 [DOI] [PubMed] [Google Scholar]
- 114. Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, and Harrison DG. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64: 1108–1115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Vargas-Ortiz K, Perez-Vazquez V, Diaz-Cisneros FJ, Figueroa A, Jiménez-Flores LM, Rodriguez-DelaRosa G, and Macias MH. Aerobic training increases expression levels of SIRT3 and PGC-1alpha in skeletal muscle of overweight adolescents without change in caloric intake. Pediatr Exerc Sci 27: 177–184, 2015 [DOI] [PubMed] [Google Scholar]
- 116. Wagner GR. and Payne RM. Widespread and enzyme-independent Nepsilon-acetylation and Nepsilon-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J Biol Chem 288: 29036–29045, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wanders RJ, Waterham HR, and Ferdinandusse S. Metabolic interplay between peroxisomes and other subcellular organelles including mitochondria and the endoplasmic reticulum. Front Cell Dev Biol 3: 83, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang W, Karamanlidis G, and Tian R. Novel targets for mitochondrial medicine. Sci Transl Med 8: 326rv3, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. White K, Kim MJ, Han C, Park HJ, Ding D, Boyd K, Walker L, Linser P, Meneses Z, Slade C, Hirst J, Santostefano K, Terada N, Miyakawa T, Tanokura M, Salvi R, and Someya S. Loss of IDH2 accelerates age-related hearing loss in male mice. Sci Rep 8: 5039, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Widder JD, Fraccarollo D, Galuppo P, Hansen JM, Jones DP, Ertl G, and Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of thioredoxin 2. Hypertension 54: 338–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, and Campisi J. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metab 23: 303–314, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Williamson DH, Lund P, and Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J 103: 514–527, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Winnik S, Auwerx J, Sinclair DA, and Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J 36: 3404–3412, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Wright DE, Altaany Z, Bi Y, Alperstein Z, and O'Donoghue P. Acetylation regulates thioredoxin reductase oligomerization and activity. Antioxid Redox Signal 29: 377–388, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Xiong J, Kawagishi H, Yan Y, Liu J, Wells QS, Edmunds LR, Fergusson MM, Yu ZX, Rovira II, Brittain EL, Wolfgang MJ, Jurczak MJ, Fessel JP, and Finkel T. A metabolic basis for endothelial-to-mesenchymal transition. Mol Cell 69: 689.e7–698.e7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yang L, Zhang J, Xing W, Zhang X, Xu J, Zhang H, Chen L, Ning X, Ji G, Li J, Zhao Q, and Gao F. SIRT3 deficiency induces endothelial insulin resistance and blunts endothelial-dependent vasorelaxation in mice and human with obesity. Sci Rep 6: 23366, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yang W, Nagasawa K, Münch C, Xu Y, Satterstrom K, Jeong S, Hayes SD, Jedrychowski MP, Vyas FS, Zaganjor E, Guarani V, Ringel AE, Gygi SP, Harper JW, and Haigis MC. Mitochondrial sirtuin network reveals dynamic SIRT3-dependent deacetylation in response to membrane depolarization. Cell 167: 985.e21–1000.e21, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yu W, Dittenhafer-Reed KE, and Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem 287: 14078–14086, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zeng H, Vaka VR, He X, Booz GW, and Chen JX. High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med 19: 1847–1856, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Zhong L. and Mostoslavsky R. Fine tuning our cellular factories: sirtuins in mitochondrial biology. Cell Metab 13: 621–626, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhu Y, Park SH, Ozden O, Kim HS, Jiang H, Vassilopoulos A, Spitz DR, and Gius D. Exploring the electrostatic repulsion model in the role of Sirt3 in directing MnSOD acetylation status and enzymatic activity. Free Radic Biol Med 53: 828–833, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, and Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002 [DOI] [PubMed] [Google Scholar]